Abstract
Nanostructured electrochemical sensors often suffer from irreversible aggregation and poor adhesion to the supporting materials, resulting in reduced sensitivity and selectivity over time. We describe a versatile method for fabrication of a H2O2 sensor by immobilizing copper nanoparticles (Cu NPs; 20 nm) on graphene oxide (GO) sheets via in-situ reduction of copper(II) on a polydopamine (PDA) coating on a glassy carbon electrode. The PDA film with its amino groups and catechol groups acts as both a reductant and an adhesive that warrants tight bonding between the Cu NPs and the support. The modified electrode, best operated at a working voltage of −0.4 V (vs. Ag/AgCl), has a linear response to H2O2 in the 5 μM to 12 mM concentration range, a sensitivity of 141.54 μA∙mM‾1∙cm‾2, a response time of 4 s, and a 1.4 μM detection limit (at an S/N ratio of 3). The sensor is highly reproducible and selective (with minimal interference to ascorbic acid and uric acid). The method was applied to the determination of H2O2 in sterilant by the standard addition method and gave recoveries between 97% and 99%.
Introduction
Rapid, low-cost and reliable detection of hydrogen peroxide (H2O2) is of particular significance due to its wide application in food industry, environmental protection, and medical field [1–4]. Most previous studies indicate that H2O2 is actually a universal metabolic intermediate in organism and is closely associated with the metabolic function of human body [5, 6]. Moreover, bioaccumulation of H2O2 produces oxidative stress and results in severely detrimental damage to cells [7]. Nowadays many analytical methods have been employed for the determination of hydrogen peroxide, such as chemiluminescence, spectrophotometry, titrimetry and electrochemistry [8–11]. Among them, electrochemical sensors are attracted much attention due to its excellent properties of low-cost, simplicity, high sensitivity and handing convenience [12].
Hydrogen peroxide sensors based on the nanoparticles of metal or metal oxides have been intensively explored recently [13–15]. The electrocatalytic activities of the nanoparticles can produce remarkable voltammetric responses towards H2O2. But the irreversible aggregation will occur due to the high surface energy of free nanoparticles and will significantly decrease the analytical performance [16, 17]. A variety of supporting materials have been employed to disperse the nanoparticles. And graphene is the most frequently employed substrate to integrate the nanoparticles by procedures such as hydrothermal deposition and chemical vapor deposition [18, 19]. The sensitivity and selectivity of H2O2 determination depend on the immobilized nanoparticles. Therefore, the adhesion of the immobilized nanoparticles to the substrate is a key issue for H2O2 sensors in practical applications.
Herein, we present a versatile approach to immobilize nanoparticles on graphene sheets via in situ reduction of metal ions on the polydopamine (PDA) coating that can provide a tight bonding between the nanoparticles and the supporting materials. Graphene oxide (GO) was coated with a PDA film through simple dip-coating in an aqueous solution of dopamine, which can generate a nanofilm on most inorganic and organic materials in alkaline media by self-polymerization [20–23]. Dopamine is a chemical agent that contains catechol and amine groups. The PDA film composed of cross-linking with amine and catechol groups can serve as a reductant as well as an adhesive agent [24–28]. Copper nanoparticles (Cu NPs) were produced on the PDA film by in situ chemical reduction of copper ions and were integrated to the graphene sheets by the adhesive PDA coating. The GO/PDA/Cu NPs composite was characterized by transmission electron microscope (TEM) and X-ray photoelectron spectroscopy (XPS). The electrochemical behaviors were studied by cyclic voltammetry (CV) and amperometric i-t curve. The electrochemical sensor fabricated by GO/PDA/Cu NPs exhibited high sensitivity towards H2O2 determination with excellent reproducibility. The sensor also displayed good selectivity with minimal interference from the coexisting species such as ascorbic acid (AA) and uric acid (UA) in biological fluids and can be applied to real sample analysis.
Materials and Methods
Reagents
Hydrogen peroxide (H2O2, 30%) was purchased from Nangjing Chemical Reagent. Dopamine hydrochloride, tris(hydroxymethyl)aminomethane (Tris, 99%), ascorbic acid (AA), uric acid (UA) and polyvinylpyrrolidone (PVP, MW = 5000) were purchased from Sigma-Aldrich and used without further purification. Graphene oxide (GO, 1 mg/mL) was obtained from Institute of Coal Chemistry of Chinese Academy of Science. Other analytical grade chemicals were purchased from Sinopharm Group Co. Ltd. and also used without further purification. All aqueous solutions were prepared with deionized (DI) water and prepared just prior to use.
Instruments
The microstructure and composition of GO/PDA and GO/PDA/Cu NPs were characterized by TEM (JEM-2010 UHR, JEOL) and XPS (ESCALAB 250, Thermo Fisher Scientific) using the Al Kα radiation (1486.6 eV, 15 kV, 150 W) at a vacuum of 2×10−9 mbar. Electrochemical measurement was performed at room temperature in phosphate buffer (PBS) on the CHI 660e potentiostat (CH Instruments Inc., Shanghai, China). A simple three-electrode configuration used in the measurement consisted of an Ag/AgCl electrode as the reference electrode, a Pt wire as the counter electrode, and a glassy carbon electrode (GCE, 3 mm in diameter) modified with GO/PDA/Cu NPs composite as the working electrode.
Preparation of GO/PDA and GO/PDA/Cu NPs
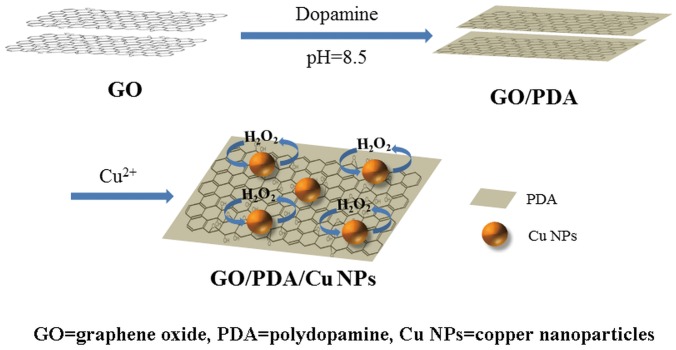
GO powder was obtained by drying the purchased GO aqueous solution (1 mg/mL) at 80°C under vacuum. An amount of 20 mg GO powder was dispersed in 4 mL Tris buffer (10 mM, pH = 8.5) and ultra-sonicated in ice bath for 5 min. Then added 16 mL Tris buffer in the GO solution and continued ultra-sonicated for 5min to obtain a homogeneous dispersion of GO. The GO solution was separated by centrifugation before washing with Tris buffer for three times, and then dispersed in 2 mg/mL dopamine solution under stirring for 24 h at room temperature. GO/PDA composite was obtained by centrifugation and dried in vacuum at 60°C. An amount of 10 mg of GO/PDA composite was added into the fresh prepared 10 mM copper sulfate solution, followed by addition of sodium citrate (0.1 M) and PVP (1 wt.%) under stirring for 12 h. After centrifugation, the GO/PDA/Cu NPs sediment was washed with DI water and dried in vacuum [29]. The fabrication procedures can be depicted in Fig 1.
Fig 1. Scheme of the GO/PDA/Cu NPs sensor fabricated by immobilizing Cu NPs on GO sheets via in-situ reduction of copper(II) on a PDA coating.
The GCE was polished with 0.05 μm α-Al2O3 power, and then sonicated in ethanol and DI water for several times to obtain a clean surface. The GO/PDA/Cu NPs composite was dripped onto the cleaned GCE surface. For comparison, GO/PDA composite was also dripped onto the GCE surface. The electrodes were dried at room temperature prior to electrochemical test.
Results and Discussion
Morphology and composition of GO/PDA/Cu NPs composite
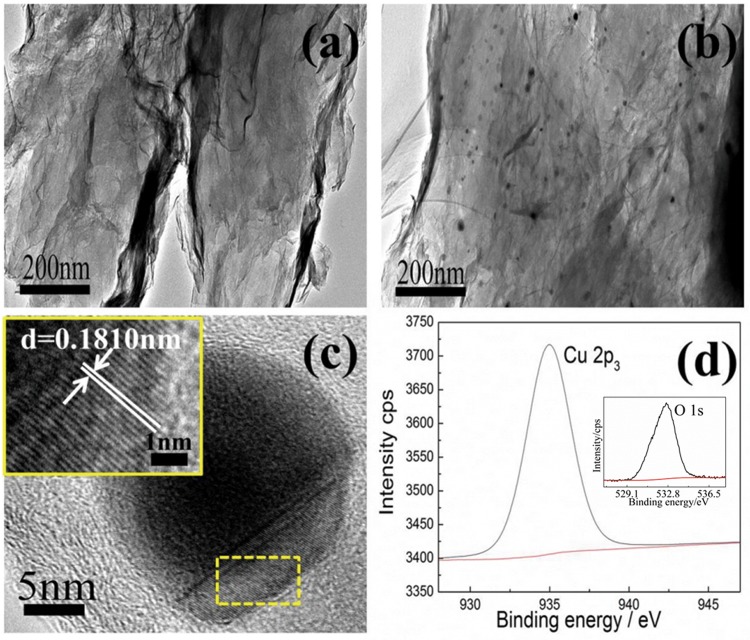
The morphology of GO/PDA and GO/PDA/Cu NPs was investigated by TEM. The TEM images revealed multilayer structures of GO/PDA composite without any particles (Fig 2a). Spherical nanoparticles with an average diameter of 20±4 nm (estimation from 100 nanoparticles) uniformly deposited on the surface of GO/PDA were observed in Fig 2b. As can be seen from HR-TEM images (inset in Fig 2c), the spacing of the lattice fringes (0.1810 nm) corresponds to the lattice planes Cu (1, 1, 1) [30], suggesting the deposition of Cu NPs on the GO/PDA sheets. Chemical composition of the nanoparticles was also analyzed by XPS (Fig 2d). The Cu 2p3 single peak for Cu NPs at 934.92 eV should be attributed to copper zero or copper (I). The O 1s peak at 532.67 eV for Cu NPs indicates the existence of copper oxide [31]. The results further confirm that the formation of the GO/PDA/Cu NPs composite.
Fig 2. TEM images of GO/PDA (a), GO/PDA/Cu NPs (b), HR-TEM images of GO/PDA/Cu NPs (c) and the Cu2p and O 1s (inset) XPS spectra of GO/PDA/Cu NPs (d).
Electrochemical behaviors of the GO/PDA/Cu NP sensor
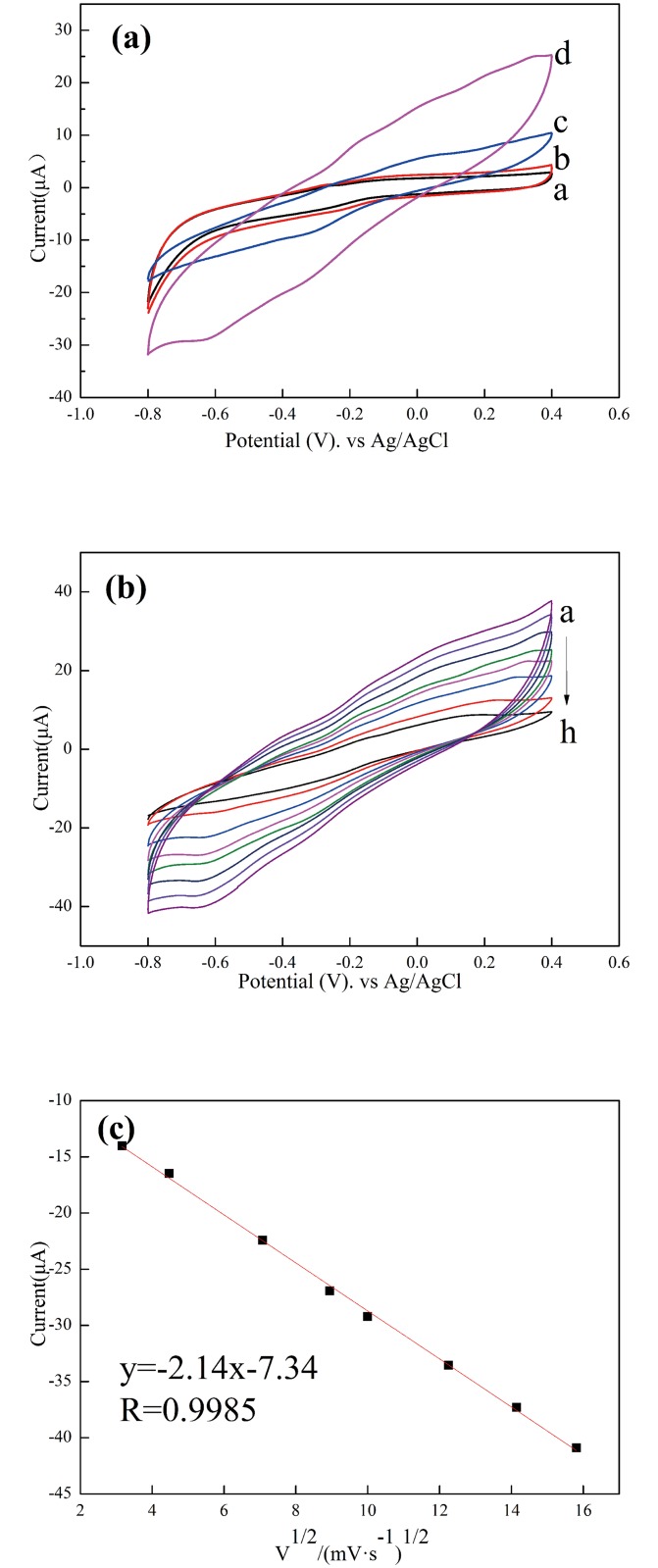
Cyclic voltammograms (CV) were measured to investigate the electrocatalytic property of the GO/PDA/Cu NPs composite in PBS buffer, as shown in Fig 3a. No obvious current response changes can be observed between the CV of GO/PDA in the absence (a) and presence (b) of 10 mM H2O2. Compared the GO/PDA/Cu NPs without H2O2 (c), the GO/PDA/Cu NPs with H2O2 (d) shows a steep increase of current response. There are no obvious current peaks associated with CuNPs or PDA, as can be seen from Fig 3a in the absence of H2O2. While in the presence of H2O2, a peak current at -0.65 V associated with the reduction of H2O2 was observed at the GO/PDA/Cu NPs sensor. The odd shape of the CVs should be attributed to the impedence of the PDA film that produced an ohmic shape when the current was significantly enhanced in the presence of H2O2 at the GO/PDA/Cu NPs sensor [32–33]. These results indicate that the GO/PDA/Cu NPs composite exhibits good electrocatalytic performance for the reduction of H2O2. Thus it can be considered as for a promising H2O2 sensor. The electrocatalytic detection of H2O2 should be attributed to the reduction of H2O2 on the GO/PDA/Cu NPs surface. The possible reaction can be summarized as the following equation [14, 34]:
Fig 3.
(a) Cyclic voltammograms of GO/PDA sensor (a and b) and GO/PDA/Cu NPs/GCE sensor (c and d) in the absence (a and c) and presence (b and d) of 10 mM H2O2 at a scanning rate of 100 mV/s in 0.1 M pH 7.2 PBS. (b) Cyclic voltammograms of the GO/PDA/Cu NP sensor in 0.1 M pH 7.2 PBS containing 10 mM H2O2 at different scan rates (V) of 10, 20, 50, 80, 100, 150, 200 and 250 mV/s (from a to h). (c) Plots of the response peak current vs. the square root of the scan rate (V1/2). Results are presented as mean ±SD (error bar) of triplicate experiments.
Fig 3b shows the cyclic voltammograms of GO/PDA/Cu NPs sensor in 0.1 M pH 7.2 PBS containing 10 mM H2O2 at different scan rates of 10~250 mV/s. As can be seen, the response currents are proportional to the square root of the scan rate with the correlation coefficient of 0.9985 (Fig 3c), indicating that the electrocatalytic reaction on the modified electrode is a diffusion-controlled process instead of a surface-controlled one [35].
Determination of H2O2 by the GO/PDA/Cu NP sensor
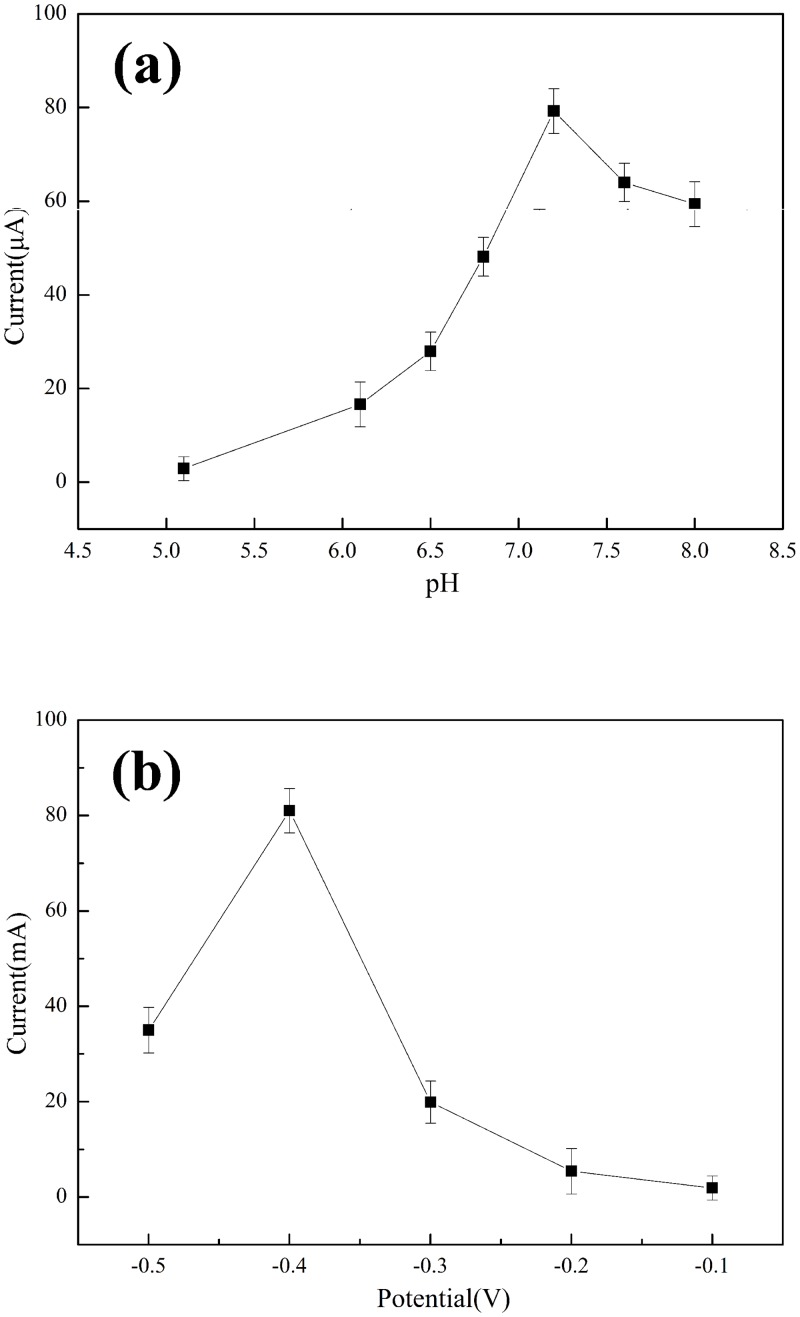
The pH value and applied potential are essential parameters to electrocatalytic process of GO/PDA/Cu NP sensor. Hence, effects of pH (Fig 4a) and applied potential (Fig 4b) on the response current of 10 mM H2O2 at GO/PDA/Cu NP sensor were studied. In Fig 4a, the current response increases when the pH increases from 5.1 to 7.2, and then begins to decrease when the pH is further increasing. It indicates that pH 7.2 is optimal condition for the electrocatalytic reaction, which is close to the pH in the environment of human body fluids. In Fig 4b, it is found that the current response increases with the increase of applied potential in the range of −0.1 V and −0.4 V. It suggests that the current response of the GO/PDA/Cu NP sensor has reached high electrocatalytic activity at the applied potential of −0.4 V. For a higher sensitivity, −0.4 V was chosen as the optimal applied potential for the detection of hydrogen peroxide.
Fig 4. Effects of pH value at an applied potential −0.4 V (vs. Ag/AgCl) (a) and applied potential (b) on the response current of 10 mM H2O2 on the GO/PDA/Cu NP sensor.
Results are presented as mean ±SD (error bar) of triplicate experiments.
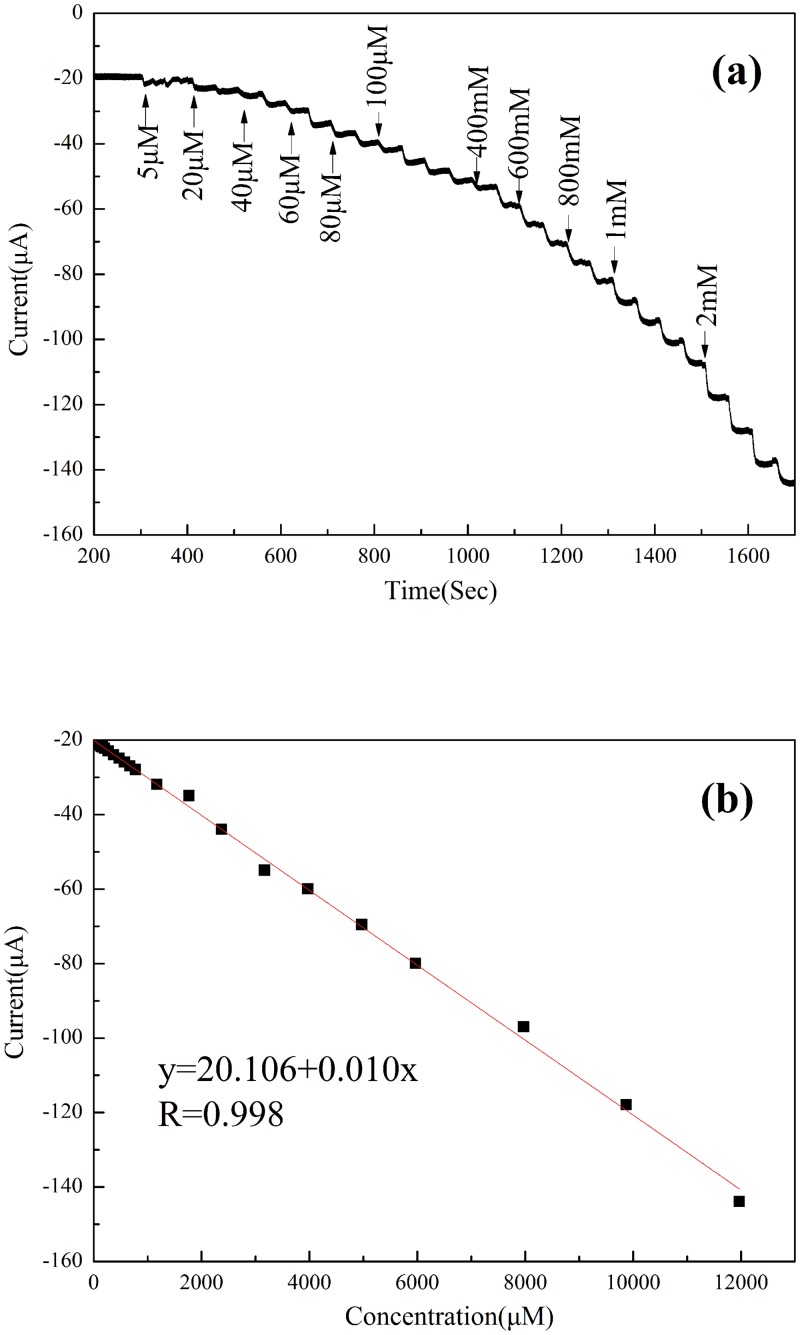
The electrocatalytic responses of the GO/PDA/Cu NP sensor to hydrogen peroxide were further evaluated by amperometric current-time response upon successive addition of H2O2 into 0.1 M pH 7.2 PBS with an applied potential −0.4 V under a stirring condition (Fig 5a). By increasing the concentration of H2O2, the current response increases gradually as shown in Fig 5b. A linear relationship between hydrogen peroxide concentration (x) and current response (y) is obtained as a function of hydrogen peroxide in the concentration range of 5 μM to 12 mM with the calibration equation of y = 20.106+0.010x (R = 0.998). The estimated sensitivity for the hydrogen peroxide sensor is 141.54 μA·cm−2·mM−1, and the detection limit is calculated to be 1.4 μM at an S/N = 3 with a response time of 4 s.
Fig 5.
(a) Amperometric responses of the GO/PDA/Cu NP sensor upon successive addition of H2O2 into 0.1 M pH 7.2 PBS at an applied potential −0.4 V under a stirring condition. (b) Calibration curve of the GO/PDA/Cu NP sensor versus H2O2 concentration. Results are presented as mean ±SD (error bar) of triplicate experiments.
Reproducibility, stability and selectivity
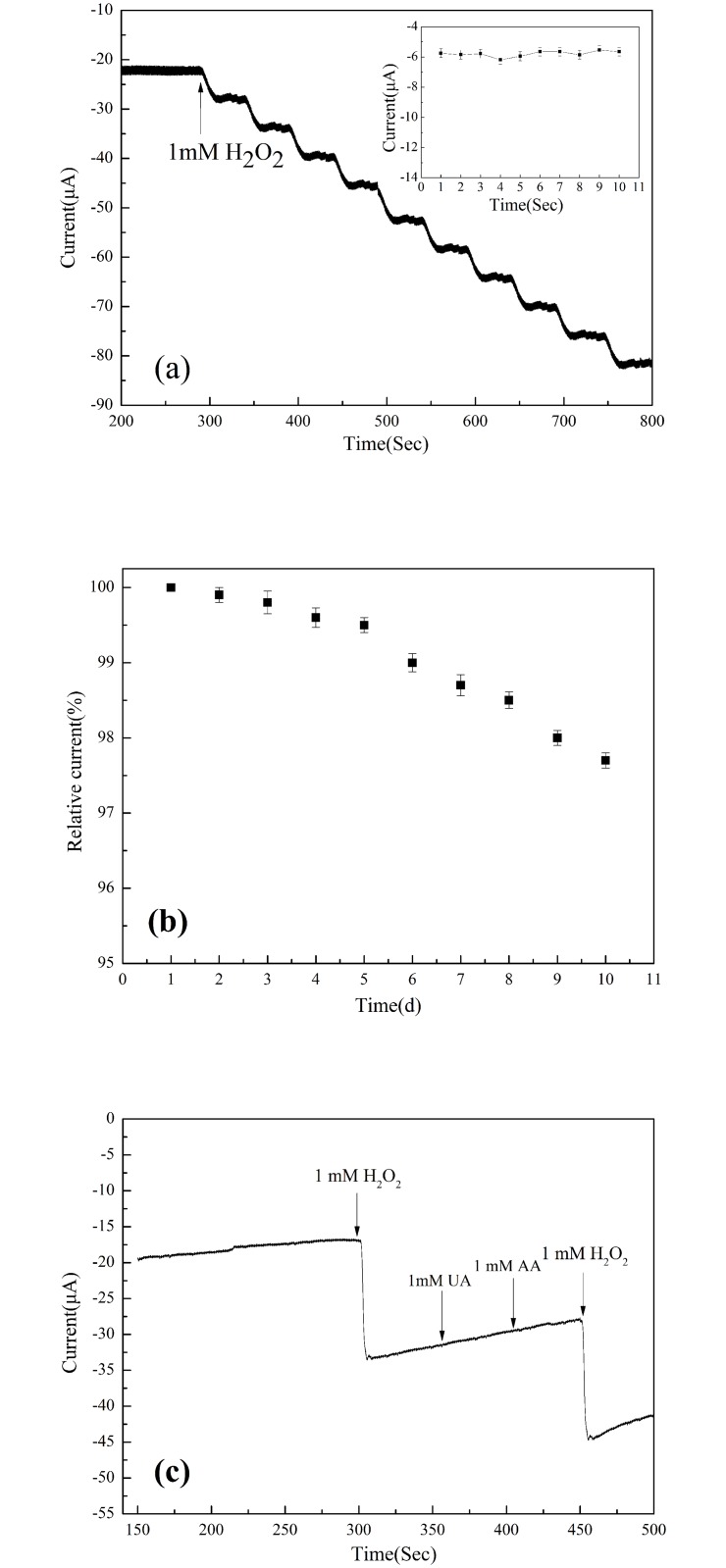
In order to investigate the reproducibility of the GO/PDA/Cu NP sensor, amperometric responses for successive injection of 1 mM H2O2 into 0.1 M pH 7.2 PBS with continuous stirring at an applied potential of −0.4 V were examined and the results are shown in Fig 6a. The inset of Fig 6a shows the amperometric response for different injections. The relative standard deviation (RSD) is calculated to be 2.5%,indicating good reproducibility of the GO/PDA/Cu NP sensor. Also, the stability of the GO/PDA/Cu NP sensor was evaluated by successive monitoring after storing in desiccator for 10 days, as seen in Fig 6b. It can be seen that only 2.3% of the current signal diminished from the original current response, indicating that the sensor is considerably stable. Thus, the GO/PDA/Cu NP sensor has good reproducibility and stability towards hydrogen peroxide determination.
Fig 6.
(a) Amperometric responses for successive injection of 1 mM H2O2 into 0.1 M pH 7.2 PBS with continuous stirring at an applied potential of −0.4 V with the inset showing the amperometric response for different injections. (b) Long-term stability of the sensor in 10 days. (c) Amperometric responses of the GO/PDA/Cu NP sensor upon the successive addition of 1 mM H2O2, UA, AA and H2O2 into 0.1 M pH 7.2 PBS at an applied potential −0.4 V under a stirring condition. Results are presented as mean ±SD (error bar) of triplicate experiments.
To evaluate the selectivity of the GO/PDA/Cu NP sensor, UA and AA were employed as interfering species by successive addition of 1 mM H2O2, UA, AA and H2O2 into 0.1 M pH 7.2 phosphate buffer with an applied potential −0.4 V under a stirring condition (Fig 6c). Compared to the current response of H2O2, the current responses of UA and AA were negligible, revealing that these substances don’t interfere the detection of hydrogen peroxide.
Real sample analysis
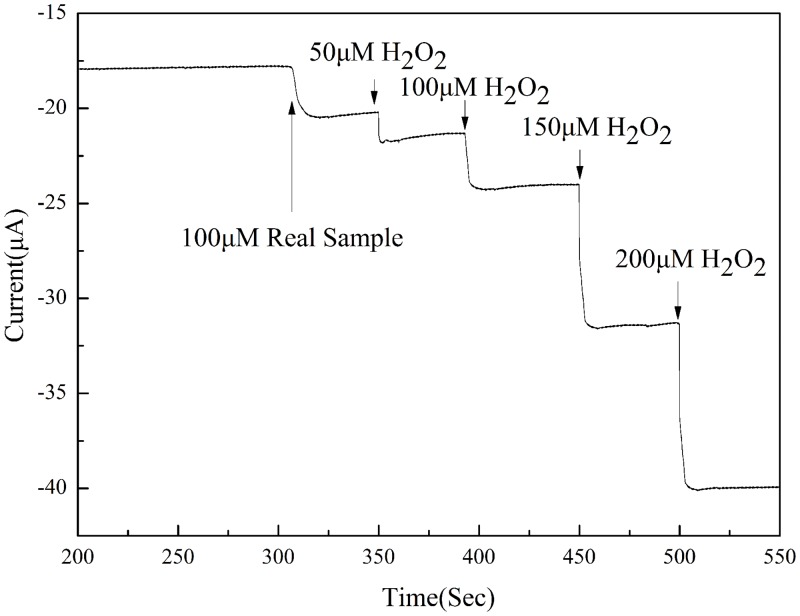
To test the reliability of the GO/PDA/Cu NP sensor as a non-enzymatic electrochemical hydrogen peroxide sensor, amperometric measurement of H2O2 in sterilant obtained from a local supermarket was carried out. Before measurement, the sterilant sample was diluted 10000-fold with phosphate buffer. In Fig 7, amperometric responses of the GO/PDA/Cu NP sensor upon the successive addition of 100 μM real sample, 50 μM H2O2, 100 μM H2O2, 150 μM H2O2 and 200 μM H2O2 into 0.1 M pH 7.2 PBS with an applied potential −0.4 V under a stirring condition are displayed. Then contact lens solution with 1000-fold dilution and milk sample with 50-fold dilution are also examined. The concentration of H2O2 was calculated by standard addition method [36] and they are summarized in Table 1. The recovery values for the samples were 95%-99%. According to the obtained results, the GO/PDA/Cu NP sensor exhibits excellent electrocatalytic performance in practical analysis.
Fig 7. Amperometric responses of the GO/PDA/Cu NP sensor upon successive addition of 100 μM real sample, 50 μM H2O2, 100 μM H2O2, 150 μM H2O2 and 200 μM H2O2 into 0.1 M pH 7.2 PBS with an applied potential −0.4 V under a stirring condition.
Table 1. Amperometric determination of H2O2 in sterilant (1), contact lens solution (2), and the milk sample (3).
| Sample | Value found in real sample (μM) | Added H2O2 (μM) | Found H2O2 (μM) | Recovery (%) |
|---|---|---|---|---|
| 1 | 100 | - | - | - |
| - | - | 50 | 49 | 98 |
| - | - | 100 | 99 | 99 |
| - | - | 150 | 146 | 97 |
| - | - | 200 | 198 | 99 |
| 2 | 150 | - | - | - |
| - | - | 50 | 49.5 | 99 |
| - | - | 100 | 97 | 97 |
| - | - | 150 | 147 | 98 |
| - | - | 200 | 192 | 96 |
| 3 | 10 | - | - | - |
| - | - | 20 | 19.6 | 98 |
| - | - | 50 | 48 | 96 |
| - | - | 70 | 66.5 | 95 |
| - | - | 100 | 97 | 97 |
Conclusions
We present a versatile approach to fabricate H2O2 sensor by immobilization of Cu NPs on the sheets of GO via in situ reduction of copper ions on the PDA coating that can provide a tight bonding between the Cu NPs and GO. In this process, the PDA coating acts as a reductant as well as an adhesive agent. The GO/PDA/Cu NPs composite exhibits high sensitivity towards H2O2 determination with excellent reproducibility. The sensor also shows good selectivity with minimal interference from the coexisting species such as AA and UA in biological fluids. The recovery values estimated from the standard addition method imply the potential applications of the GO/PDA/Cu NP sensor in practical analysis.
Acknowledgments
We acknowledge the help of Miss Zeng Y on material characterization.
Data Availability
All relevant data are within the paper.
Funding Statement
R.S.C. acknowledges support by the National Natural Science Foundation of China under award number 21105077, the Open Funds of Key Laboratory of Analytical Chemistry for Biology and Medicine of Ministry of Education under contract number ACBM2014001, and the Open Funds of Key Laboratory of Inorganic Coating Materials of Chinese Academy of Sciences KLICM-2013-05. F.L. thanks the National Natural Science Foundation of China under award number 21372183, Hubei Provincial Natural Science Foundation of China 2013CFB328, Wuhan Applied Basic Research Programs of China 2015060101010069, and the Open Funds of the State Key Laboratory of Electroanalytical Chemistry SKLEAC201609. S.M.L. acknowledges support by the Thousand Youth Talents Program.
References
- 1.Zhang MY, Sheng QL, Nie F, Zheng JB. Synthesis of Cu nanoparticles-loaded Fe3O4@carbon core-shell nanocomposite and its application for electrochemical sensing of hydrogen peroxide. Journal of Electroanalytical Chemistry 2014; 730:10–15. [Google Scholar]
- 2.Mahmoudian MR, Alias Y, Basirun WJ, Woi PM, Sookhakian M. Facile preparation of MnO2 nanotubes/reduced graphene oxide nanocomposite for electrochemical sensing of hydrogen peroxide. Sensors and Actuators B: Chemical 2014; 201:526–534. [Google Scholar]
- 3.Heli H, Pishahang J. Cobalt oxide nanoparticles anchored to multiwalled carbon nanotubes: Synthesis and application for enhanced electrocatalytic reaction and highly sensitive nonenzymatic detection of hydrogen peroxide. Electrochimica Acta 2014; 123:518–526. [Google Scholar]
- 4.Ensafi AA, Abarghoui MM, Rezaei B. Electrochemical determination of hydrogen peroxide using copper/porous silicon based non-enzymatic sensor. Sensors and Actuators B: Chemical 2014; 196:398–405. [Google Scholar]
- 5.Lorestani F, Shahnavaz Z, Mn P, Alias Y, Manan NSA. One-step hydrothermal green synthesis of silver nanoparticle-carbon nanotube reduced-graphene oxide composite and its application as hydrogen peroxide sensor. Sensors and Actuators B: Chemical 2015; 208:389–398. [Google Scholar]
- 6.Liu MM, Liu R, Chen W. Graphene wrapped Cu2O nanocubes: non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosensors & Bioelectronics 2013; 45:206–212. [DOI] [PubMed] [Google Scholar]
- 7.Karuppiah C, Palanisamy S, Chen SM, Veeramani V, Periakaruppan P. A novel enzymatic glucose biosensor and sensitive non-enzymatic hydrogen peroxide sensor based on graphene and cobalt oxide nanoparticles composite modified glassy carbon electrode. Sensors and Actuators B: Chemical 2014; 196:450–456. [Google Scholar]
- 8.Lin YQ, Li LB, Hu LL, Liu KY, Xu YA. Multifunctional poly(dopamine)-assisted synthesis of silver nano particles/carbon nanotubes nanocomposite: Toward electrochemical sensing of hydrogen peroxide with enhanced sensitivity. Sensors and Actuators B: Chemical 2014; 202:527–535. [Google Scholar]
- 9.Tahirovic A, Copra A, Omanovic-Miklicanin E, Kalcher K. A chemiluminescence sensor for the determination of hydrogen peroxide. Talanta 2007; 72 (4):1378–1385. 10.1016/j.talanta.2007.01.072 [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Wei WZ, Zeng JX, Liu XY, Zeng XD. Fabrication of a copper nanoparticle/chitosan/carbon nanotube-modified glassy carbon electrode for electrochemical sensing of hydrogen peroxide and glucose. Microchimica Acta 2007; 160 (1–2):253–260. [Google Scholar]
- 11.Gnana kumar G, Justice Babu K, Nahm KS, Hwang YJ. A facile one-pot green synthesis of reduced graphene oxide and its composites for non-enzymatic hydrogen peroxide sensor applications. RSC Advances 2014; 4 (16):7944. [Google Scholar]
- 12.Zhang TT, Yuan R, Chai YQ, Li WJ, Ling SJ. A novel nonenzymatic hydrogen peroxide sensor based on a polypyrrole nanowire-copper nanocomposite modified gold electrode. Sensors 2008; 8 (8):5141–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin ZJ, Wu JJ, Yang ZS. Amperometric sensors based on Ni/Al and Co/Al layered double hydroxides modified electrode and their application for hydrogen peroxide detection. Biosensors & Bioelectronics 2011; 26 (5):1970–1974. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Fu RZ, Yuan JJ, Wu SY, Zhang JL, Wang HY. Highly sensitive hydrogen peroxide sensor based on a glassy carbon electrode modified with platinum nanoparticles on carbon nanofiber heterostructures. Microchimica Acta 2015; 182 (13–14):2241–2249. [Google Scholar]
- 15.Kivrak H, Alal O, Atbas D. Efficient and rapid microwave-assisted route to synthesize Pt–MnOx hydrogen peroxide sensor. Electrochimica Acta 2015; 176:497–503. [Google Scholar]
- 16.Mane GP, Talapaneni SN, Anand C, Varghese S, Iwai H, Ji Q, et al. Preparation of highly ordered nitrogen-containing mesoporous carbon from a gelatin biomolecule and its excellent sensing of acetic acid. Advanced Functional Materials 2012; 22 (17):3596–3604. [Google Scholar]
- 17.Zhang C, Ni HW, Chen RS, Zhan WT, Zhang BW, Lei R, et al. Enzyme-free glucose sensing based on Fe3O4 nanorod arrays. Microchimica Acta 2015; 182 (9–10):1811–1818. [Google Scholar]
- 18.Yang W, Xu XW, Li Z, Yang F, Zhang LQ, Li YF, et al. Construction of efficient counter electrodes for dye-sensitized solar cells: Fe2O3 nanoparticles anchored onto graphene frameworks. Carbon 2016; 96:947–954. [Google Scholar]
- 19.Qiu HW, Huo YY, Li Z, Zhang C, Chen PX, Jiang SZ, et al. Surface-enhanced Raman scattering based on controllable-layer graphene shells directly synthesized on Cu nanoparticles for molecular detection. Chemphyschem 2015; 16 (14):2953–2960. 10.1002/cphc.201500502 [DOI] [PubMed] [Google Scholar]
- 20.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007; 19 (318):426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu HL, Liu XK, Wang DY. Interfacial basicity-guided formation of polydopamine hollow capsules in pristine O/W emulsions—toward understanding of emulsion template roles. Chemistry of Materials 2011; 23 (23):5105–5110. [Google Scholar]
- 22.Chuah YJ, Koh YT, Lim K, Menon NV, Wu Y, Kang Y. Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized mesenchymal stem cell adhesion and multipotency. Scientific Reports 2015; 5:18162 10.1038/srep18162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su L, Yu Y, Zhao YS, Liang F, Zhang XJ. Strong antibacterial polydopamine coatings prepared by a shaking-assisted method. Scientific Reports 2016, 6:24420 10.1038/srep24420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng T, Zhang XL, Niu HY, Ma YR, Li WH, Cai YQ. In situ growth of gold nanoparticles onto polydopamine-encapsulated magnetic microspheres for catalytic reduction of nitrobenzene. Applied Catalysis B: Environmental 2013; 134–135:26–33. [Google Scholar]
- 25.Yan YH, Zheng ZF, Deng CH, Li Y, Zhang XM, Yang PY. Hydrophilic polydopamine-coated graphene for metal ion immobilization as a novel immobilized metal ion affinity chromatography platform for phosphoproteome analysis. Analytical Chemistry 2013; 85 (18):8483–8487. 10.1021/ac401668e [DOI] [PubMed] [Google Scholar]
- 26.Wu MY, Xiao XC, Vukmirovic N, Xun SD, Das PK, Song XY, et al. Toward an ideal polymer binder design for high-capacity battery anodes. Journal of the American Chemical Society 2013; 135 (32):12048–12056. 10.1021/ja4054465 [DOI] [PubMed] [Google Scholar]
- 27.Rong JP, Ge MY, Fang X, Zhou CW. Solution ionic strength engineering as a generic strategy to coat graphene oxide (GO) on various functional particles and its application in high-performance lithium-sulfur (Li-S) batteries. Nano Letters 2014; 14 (2):473–479. 10.1021/nl403404v [DOI] [PubMed] [Google Scholar]
- 28.Murali S, Chang JL, Zen JM. Bismuth oxide nanoparticles as a nanoscale guide to form a silver–polydopamine hybrid electrocatalyst with enhanced activity and stability for the oxygen reduction reaction. RSC Advances 2015; 5 (6):4286–4291. [Google Scholar]
- 29.Liu HY, Xu SM, He ZM, Deng AP, Zhu JJ. Supersandwich cytosensor for selective and ultrasensitive detection of cancer cells using aptamer-DNA concatamer-quantum dots probes. Analytical Chemistry 2013; 85 (6):3385–3392. 10.1021/ac303789x [DOI] [PubMed] [Google Scholar]
- 30.Freire RLH, Kiejna A, Silva JLFD. Adsorption of Rh, Pd, Ir, and Pt on the Au(111) and Cu(111) surfaces: A density functional theory investigation. Journal of Physical Chemistry C 2014; 118 (33):19051–19061. [Google Scholar]
- 31.Tahir D, Tougaard S. Electronic and optical properties of Cu, CuO and Cu2O studied by electron spectroscopy. Journal of Physics Condensed Matter: an Institute of Physics Journal 2012; 24 (17):175002. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Niu LH, Zhang ZJ, Zhao J, Chou LJ. Electrochemical Behavior of a Polydopamine Nanofilm. Analytical Letters 2015; 48 (13): 2031–2039. [Google Scholar]
- 33.Zaino LP. Contento NM, Branagan SP, Bohn PW. Coupled Electrokinetic Transport and Electron Transfer at Annular Nanoband Electrodes Embedded in Cylindrical Nanopores. ChemElectroChem 2014; 1 (9): 1570–1576. [Google Scholar]
- 34.Butwong N, Zhou L, Ng-eontae W, Burakham R, Moore E, Srijaranai S, et al. A sensitive nonenzymatic hydrogen peroxide sensor using cadmium oxide nanoparticles/multiwall carbon nanotube modified glassy carbon electrode. Journal of Electroanalytical Chemistry 2014; 717–718:41–46. [Google Scholar]
- 35.Alegret S, Merkoci A. Electrochemical Sensor Analysis. Elsevier: Amsterdam; 2007. [Google Scholar]
- 36.Harris DC. Quantitative Chemical Analysis. W.H. Freeman: New York; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.