Abstract
Background/Aims
To evaluate the adjuvant effects of N-acetylcysteine (NAC) on first-line sequential therapy (SQT) for Helicobacter pylori infection.
Methods
Patients with H. pylori infections were randomly assigned to receive sequential therapy with (SQT+NAC group, n=49) or without (SQT-only group, n=50) NAC. Sequential therapy consisted of rabeprazole 20 mg and amoxicillin 1 g for the first 5 days, followed by rabeprazole 20 mg, clarithromycin 500 mg and metronidazole 500 mg for the remaining 5 days; all drugs were administered twice daily. For the SQT+NAC group, NAC 400 mg bid was added for the first 5 days of sequential therapy. H. pylori eradication was evaluated 4 weeks after the completion of therapy.
Results
The eradication rates by intention-to-treat analysis were 58.0% in the SQT-only group and 67.3% in the SQT+NAC group (p=0.336). The eradication rates by per-protocol analysis were 70.0% in the SQT-only group and 80.5% in the SQT+NAC group (p=0.274). Compliance was very good in both groups (SQT only/SQT+NAC groups: 95.2%/100%, p=0.494). There was no significant difference in the adverse event rates between groups (SQT-only/SQT+NAC groups: 26.2%/26.8%, p=0.947).
Conclusions
The H. pylori eradication rate was numerically higher in the SQT+NAC group than in the SQT-only group. As our data did not reach statistical significance, larger trials are warranted.
Keywords: Eradication, Helicobacter pylori, N-acetylcysteine, Sequential therapy
INTRODUCTION
Standard triple therapy consisting of proton pump inhibitors (PPIs), amoxicillin, and clarithromycin, has long been recommended as first-line therapy for Helicobacter pylori infection.1,2 However, the eradication rate of this triple therapy has been decreasing because of increasing antibiotic resistance;3,4 in fact, it is now reported to be <80%.5 Sequential therapy is one of the promising alternative regimens to standard triple therapy. Early meta-analyses reported that the eradication rate of sequential therapy is >90%.6–8 Therefore, this regimen is currently recommended as the alternative first-line treatment for H. pylori infection by European guidelines.9 However, a recent meta-analysis concluded that although this regimen appears to be superior to standard triple therapy for H. pylori infection in Asian adults, its pooled efficacy is lower than what was reported in earlier European studies.10 Therefore, it remains controversial whether sequential therapy (SQT) could replace standard triple therapy in Asia.
Adjuvant agents to the eradication regimen have been continuously studied to improve the efficacy of H. pylori eradication therapy.11 One of these adjuvants consists of a material that destroys biofilm since several studied demonstrated that H. pylori forms biofilm that likely helps it survive on the gastric mucosa epithelium.12,13 Among several candidates for antibio-film therapeutic agents, N-acetylcysteine (NAC) has received attention.5 NAC, a compound that has mucolytic and antioxidant functions, has been widely used for respiratory and otolaryngologic diseases. In a mouse model, NAC was reported to inhibit the growth of H. pylori,14 while the addition of NAC to amoxicillin showed comparable eradication rates to standard triple therapy.15 A Turkish study in humans suggested that the addition of NAC could increase the eradication rate of dual therapy consisting of a PPI and clarithromycin.16 In addition, a resent Italian study demonstrated that NAC pretreatment before a culture-guided antibiotic regimen is effective for overcoming H. pylori antibiotic resistance in patients with a history of multiple eradication failure.17
The key theoretical basis of sequential therapy is the effect of amoxicillin on the bacterial cell wall. Amoxicillin, which is administrated in the first half of the regimen, damages the H. pylori cell wall to overcome the antibiotic resistance and increase the eradication rate by two mechanisms. First, the injured cell wall could help the other antibiotics penetrate the H. pylori strain. Second, H. pylori with damaged cell walls cannot develop an efflux channel for clarithromycin.18,19 Therefore, we hypothesized that the addition of NAC to the first half of sequential therapy could increase the eradication rate by destroying the biofilm and weakening the cell wall together with amoxicillin. To test this hypothesis, we performed a randomized open-labeled pilot study comparing the eradication rates of H. pylori using sequential therapy with and without NAC.
MATERIALS AND METHODS
1. Patients
Between July 2013 and January 2014, patients with H. pylori infection were enrolled in this randomized open-labeled pilot study at Seoul National University Bundang Hospital in South Korea. H. pylori infection was defined based on the results of at least one of the following three tests: (1) a positive 13C-urea breath test (UBT) results; (2) histological evidence of H. pylori in the stomach by modified Giemsa staining; and (3) a positive rapid urease test (CLO test; Delta West, Bentley, Australia) result by gastric mucosal biopsy. Because there was a report that NAC administration induced gastric ulcers in rats, patients with active peptic ulcer disease were excluded.20 Patients with a history of the use of PPIs, histamine-2 receptor antagonists, or antibiotics within the previous 2 months were also excluded. All patients were provided informed consent and this study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB number: B-1304/198-005).
2. Study design
Patients were randomly assigned to the SQT-only or SQT+NAC group using a computer-generated table in blocks of four. The SQT-only group received 10-day sequential therapy (rabeprazole 20 mg and amoxicillin 1 g for the first 5 days, followed by rabeprazole 20 mg, clarithromycin 500 mg and metronidazole 500 mg for the remaining 5 days; all drugs were administrated twice daily). For the SQT+NAC group, NAC 400 mg bid was added for the first 5 days of sequential therapy. Patients were instructed not to take antibiotics for at least 4 weeks and PPIs for at least 2 weeks before testing for H. pylori infection to minimize the chance of false negative results.
Four weeks after the completion of eradication therapy, H. pylori infection was assessed by UBT. However, modified Giemsa staining and the rapid urease test were performed in patients with gastric ulcer or gastric neoplasia for whom a follow-up endoscopic examination was necessary. At this time, compliance was evaluated by direct questioning and patients were interviewed for adverse events. Noncompliance was defined as drug intake <85%. Because smoking is known to increase the treatment failure rate for H. pylori eradication,21 smoking history was also evaluated.
3. 13C-urea breath test
After a 4-hour fasting, each patient was administrated 100 mg of 13C-urea powder (UBiTkit™; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) dissolved in 100 mL of water. A second breath sample was collected 20 minutes later. The samples were analyzed using an isotope-selective nondispersive infrared spectrometer (UBiT-IR300®; Otsuka Pharmaceutical Co., Ltd.). The cutoff value for H. pylori eradication was 2.5‰.
4. Statistical analysis
Eradication rates of H. pylori were determined on intention-to-treat (ITT) and per-protocol (PP) bases. All enrolled patients were included in the ITT analysis. Patients who were lost to follow-up, were noncompliant, or dropped out due to adverse events were excluded from the PP analysis.
SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. Continuous variables were analyzed using Student t-test and categorical variables were assessed using the chi-square test or Fisher exact test. All results were considered to indicate statistical significance when the p-values were <0.05. The statistical methods of this study were reviewed by Medical Research Collaborating Center at Seoul National University Bundang Hospital.
RESULTS
1. Patients’ clinical characteristics
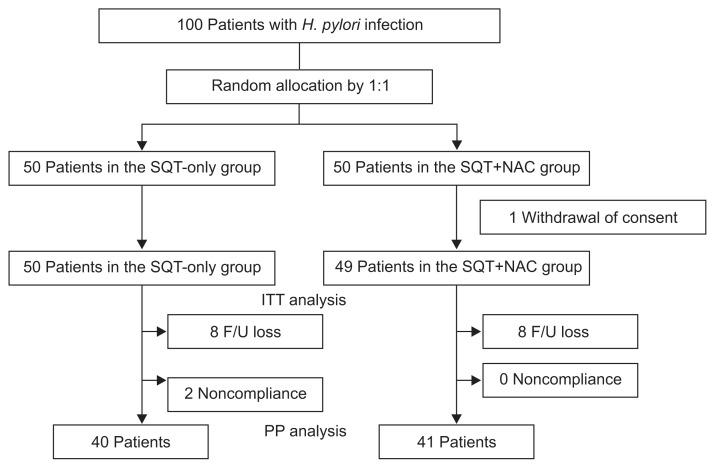
Fig. 1 shows a schematic diagram of this study. One hundred patients with H. pylori infection consented to participate in this study and were randomly allocated to the SQT-only group or the SQT+NAC group in a 1:1 ratio; one patient in the SQT+NAC group withdrew consent. As a result, a total of 99 patients were enrolled in this study (SQT-only group: 50 patients; SQT+NAC group: 49 patients). There was no significant difference in baseline demographics between the two groups (Table 1). About half of the patients were diagnosed with nonulcer dyspepsia in both groups. H. pylori infection was diagnosed by both histology and rapid urease test in 75.6% (75/99) of patients. In the remaining patients, a single method among histology, rapid urease test, and UBT was used for the detection of H. pylori.
Fig. 1.
Flow schematic of the study included intention-to-treat and per-protocol analyses.
H. pylori, Helicobacter pylori; SQT-only, sequential therapy only; SQT+NAC, sequential therapy+N-acetylcysteine; ITT, intention-to-treat; F/U, follow-up; PP, per-protocol.
Table 1.
Clinical Characteristics of the Patients
| Characteristic | SQT-only (n=50) | SQT+NAC (n=49) | p-value |
|---|---|---|---|
| Male sex | 23 (46.0) | 25 (51.0) | 0.617 |
| Age, yr | 58.8±12.7 | 57.2±11.1 | 0.529 |
| Disease | 0.067 | ||
| Peptic ulcer disease | 5 (10.0) | 9 (18.4) | |
| Gastric dysplasia or cancer | 11 (22.0) | 2 (4.1) | |
| Family history of gastric cancer | 2 (4.0) | 3 (6.1) | |
| Chronic atrophic gastritis | 7 (14.0) | 11 (22.4) | |
| Nonulcer dyspepsia | 25 (50.0) | 24 (49.0) | |
| Smoking | 0.778 | ||
| Yes | 5 (11.4) | 6 (13.3) | |
| No | 39 (88.6) | 39 (86.7) | |
| Drop out | |||
| Follow-up loss | 8 (16.0) | 8 (16.3) | |
| Noncompliance | 2 (4.0) | 0 | |
| Discontinued therapy because of adverse events | 0 | 0 | |
Data are presented as number (%) or mean±SD.
SQT-only, sequential therapy only; SQT+NAC, sequential therapy+N-acetylcysteine.
2. H. pylori eradication rates
Table 2 shows eradication rates of H. pylori in both groups. The eradication rates by ITT analysis were 58.0% (95% confidence interval [CI], 43.8 to 72.2) and 67.3% (95% CI, 53.7 to 81.0) in the SQT-only and SQT+NAC groups, respectively (p=0.336). The eradication rates by PP analysis after the exclusion of 16 patients who were lost to follow-up and two noncompliant patients were 70.0% (95% CI, 55.2 to 84.8) and 80.5% (95% CI, 67.8 to 93.2) in the SQT-only and SQT+NAC groups, respectively (p=0.274). We additionally compared the eradication rates of both groups divided by peptic ulcer disease and nonulcer dyspepsia (Table 3). However, there was still no significant difference in the ITT and PP eradication rates between two groups.
Table 2.
Helicobacter pylori Eradication rates
| SQT-only (n=50) | SQT+NAC (n=49) | p-value | |
|---|---|---|---|
| ITT analysis | |||
| Eradication rate, % | 58.0 (29/50) | 67.3 (33/49) | 0.336 |
| 95% CI | 43.8–72.2 | 53.7–81.0 | |
| PP analysis | |||
| Eradication rate, % | 70.0 (28/40) | 80.5 (33/41) | 0.274 |
| 95% CI | 55.2–84.8 | 67.8–93.2 | |
SQT-only, sequential therapy only; SQT+NAC, sequential therapy+N-acetylcysteine; ITT, intention-to-treat; CI, confidence interval; PP, per-protocol.
Table 3.
Helicobacter pylori Eradication Rates according to Disease Categories
| SQT-only | SQT+NAC | p-value | |
|---|---|---|---|
| PUD | |||
| ITT analysis | |||
| Eradication rate, % | 100 (5/5) | 66.7 (6/9) | 0.258 |
| 95% CI | 34.0–99.4 | ||
| PP analysis | |||
| Eradication rate, % | 100 (5/5) | 100 (6/6) | |
| 95% CI | |||
| NUD | |||
| ITT analysis | |||
| Eradication rate, % | 48.0 (12/25) | 58.3 (14/24) | 0.571 |
| 95% CI | 28.0–68.0 | 38.1–78.5 | |
| PP analysis | |||
| Eradication rate, % | 63.2 (12/19) | 70.0 (14/20) | 0.741 |
| 95% CI | 40.9–85.5 | 49.4–90.6 | |
SQT-only, sequential therapy only; SQT+NAC, sequential therapy+N-acetylcysteine; PUD, peptic ulcer disease; ITT, intention-to-treat; CI, confidence interval; NUD, nonulcer dyspepsia; PP, per-protocol.
3. Compliance and adverse events
Compliance was very good in both groups (SQT-only group, 95.2%; SQT+NAC group, 100%; p=0.494). The adverse event rates were 26.2% and 26.8% in the SQT-only and SQT+NAC groups, respectively (p=0.947) (Table 4). The most common adverse event was epigastric soreness in the SQT-only group and diarrhea in the SQT+NAC group. No patient in either group discontinued eradication therapy because of adverse events. Two patients in the SQT-only group complained of more than one kind of adverse events. One of the two, a 41-year-old woman visited the emergency room in the last half of the eradication therapy because of severe adverse events. She complained of diarrhea, a metallic taste, and dizziness. However, because there were no abnormal laboratory findings, she was discharged with symptomatic medication and completed the remaining eradication regimen.
Table 4.
Adverse Events
| SQT-only (n=42) | SQT+NAC (n=41) | p-value | |
|---|---|---|---|
| Total, n (%) | 11 (26.2) | 11 (26.8) | 0.947 |
| Nausea or vomiting | 3 | 2 | |
| Diarrhea | 1 | 3 | |
| Dyspepsia | 2 | 1 | |
| Epigastric soreness | 4 | 0 | |
| Abdominal pain or discomfort | 1 | 1 | |
| Dizziness | 1 | 1 | |
| Metallic taste | 1 | 1 | |
| Thirsty | 1 | 1 | |
| Abdominal distension | 0 | 1 | |
| Regurgitation | 1 | 0 |
SQT-only, sequential therapy only; SQT+NAC, sequential therapy+N-acetylcysteine.
DISCUSSION
To our knowledge, this is the first study to evaluate the adjuvant effect of NAC on sequential therapy for H. pylori infection. We hypothesized that the addition of NAC, a mucolytic agent, to sequential therapy would increase the eradication rate by decreasing the viscosity of the gastric mucus and destroying the biofilm formed by H. pylori. The results showed that the eradication rate in the SQT+NAC group was approximately 10% higher than the eradication rate in the SQT-only group on the ITT and PP analyses. However, these features did not reach statistical significance. A similar phenomenon was reported in the recent Italian study.22 Although Karbasi et al.22 suggested that the addition of NAC to triple therapy consisting of a PPI, ciprofloxacin, and bismuth increases the eradication rate by 9.3%, they failed to demonstrate statistical significance. Until now, all studies including the present study, regarding the adjuvant effect of NAC on eradication therapy for H. pylori infection were small pilot studies.16,17,22 Therefore, the results of this study should not be interpreted as a failure of this regimen. Instead, it could be used to determine the sample size needed to achieve the planned power for testing our hypothesis. Based on the results of the present study, if we hypothesize that the eradication rate of sequential therapy is 75% and the addition of NAC could increase the eradication rate by 10%, in the setting of an alpha of 0.05 and power of 80%, the estimated number of subjects needed is 250 in each group.23 Therefore, larger studies are required to obtain conclusive results.
The eradication rate in SQT-only group in this study was unexpectedly lower than those of previous studied performed in Korea. We do not know the exact reason for this discrepancy; however, we can speculate about it. An earlier Korean study of sequential therapy between 2008 and 2009 reported high eradication rates (85.9%/92.6% by ITT/PP analyses).24 However, subsequent studies performed in our institution suggest decreasing efficacy of sequential therapy in Korea. The eradication rate of sequential therapy by ITT/PP analyses were 79.3%/81.9% between 2009 and 2010,25 75.6%/76.8% between 2011 and 2012,26 and 58.0%/70.0% between 2013 and 2014 in this study.27 These findings imply that resistance to antibiotics in H. pylori treatment is still increasing and that sequential therapy might already be suboptimal in some regions. Therefore, more studies are strongly required to develop a more effective regimen. In addition, the low eradication rate in the SQT-only group by ITT analysis could be explained in part by the high drop-out rate in this group. Although controversy persists, H. pylori eradication therapy tends to be more effective in patients with peptic ulcer disease than in those with nonulcer dyspepsia.28,29 Therefore, the very low proportion of patients with peptic ulcer disease among those in this study also might have negatively influenced the efficacy of sequential therapy. In the present study, the PP eradication rate in patients with peptic ulcer disease was very high in both SQT-only and SQT+NAC groups. However, the absolute number of patients with peptic ulcer disease was too small to allow us to draw significant conclusions in this subgroup.
One recent meta-analysis that evaluated the efficacy of sequential therapy in Asian adults reported that the rate of adverse events of this regimen was 22.6%.10 The results of our study are consistent with previous data (adverse event rate in the SQT-only group, 26.2%). Furthermore, the addition of NAC did not increase the adverse events of sequential therapy; this finding is also similar to the previous study.16 NAC is generally very safe and classified as an over-the-counter drug. In this study, we excluded patients with active peptic ulcer disease based on a report that the administration of NAC induced gastric ulcers in rats. However, this report is a very old one; it was published in the 1980s. We could not find a subsequent article reporting this adverse event in humans.30 In the present study, we used 800 mg/day of NAC in the SQT+NAC group based on the previous human studies which used 60016,22 or 1,20017 mg/day of NAC. Because NAC was very well tolerated in this study, to maximize its effect, we are considering a study in which the dose of NAC is 1,200 mg/day and the duration of administration is extended to the entire period of sequential therapy. In addition, to add NAC in the standard triple therapy also would be worth to try, because surprisingly, no one tested this basic hypothesis in human.
This study has several limitations. First, although the total number of enrolled patients fulfills a recommendation for sample size calculation in pilot studies that is to take more than 30 patients31 and the total number of patients is higher than that of previous studies, it is still a pilot study. However, our findings will be helpful in the design of new studies in this area. Second, we could not performed culture for H. pylori due to a shortage of manpower and cost issues. Therefore, we could not investigate the antibiotic resistance in each patient. However, we believe that the randomized allocation of the subjects to both groups would have overcome this weakness to some extent. Third, due to the cost issues of placebo administration in the SQT-only group, this study had an open-labeled design.
In conclusion, the eradication rate for H. pylori infection was numerically higher in the SQT+NAC group than in the SQT-only group. However, our data did not reach statistical significance, indicating that larger trials are needed.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587–1600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 2.Chey WD, Wong BC Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004;48:4843–4847. doi: 10.1128/AAC.48.12.4843-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim N, Kim JM, Kim CH, et al. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol. 2006;40:683–687. doi: 10.1097/00004836-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–331. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–931. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 7.Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069–3079. doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 8.Tong JL, Ran ZH, Shen J, Xiao SD. Sequential therapy vs. standard triple therapies for Helicobacter pylori infection: a meta-analysis. J Clin Pharm Ther. 2009;34:41–53. doi: 10.1111/j.1365-2710.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection: the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 10.Yoon H, Lee DH, Kim N, et al. Meta-analysis: is sequential therapy superior to standard triple therapy for Helicobacter pylori infection in Asian adults? J Gastroenterol Hepatol. 2013;28:1801–1809. doi: 10.1111/jgh.12397. [DOI] [PubMed] [Google Scholar]
- 11.Bang CS, Kim YS, Park SH, et al. Additive effect of pronase on the eradication rate of first-line therapy for Helicobacter pylori infection. Gut and Liver. 2015;9:340–345. doi: 10.5009/gnl13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cammarota G, Sanguinetti M, Gallo A, Posteraro B. Review article: biofilm formation by Helicobacter pylori as a target for eradication of resistant infection. Aliment Pharmacol Ther. 2012;36:222–230. doi: 10.1111/j.1365-2036.2012.05165.x. [DOI] [PubMed] [Google Scholar]
- 13.Makipour K, Friedenberg FK. The potential role of N-acetylcysteine for the treatment of Helicobacter pylori. J Clin Gastroenterol. 2011;45:841–843. doi: 10.1097/MCG.0b013e31822be4d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huynh HQ, Couper RT, Tran CD, Moore L, Kelso R, Butler RN. N-acetylcysteine, a novel treatment for Helicobacter pylori infection. Dig Dis Sci. 2004;49:1853–1861. doi: 10.1007/s10620-004-9583-2. [DOI] [PubMed] [Google Scholar]
- 15.Tran CD, Kritas S, Campbell MA, Huynh HQ, Lee SS, Butler RN. Novel combination therapy for the eradication of Helicobacter pylori infection in a mouse model. Scand J Gastroenterol. 2010;45:1424–1430. doi: 10.3109/00365521.2010.506245. [DOI] [PubMed] [Google Scholar]
- 16.Gurbuz AK, Ozel AM, Ozturk R, Yildirim S, Yazgan Y, Demirturk L. Effect of N-acetyl cysteine on Helicobacter pylori. South Med J. 2005;98:1095–1097. doi: 10.1097/01.smj.0000182486.39913.da. [DOI] [PubMed] [Google Scholar]
- 17.Cammarota G, Branca G, Ardito F, et al. Biofilm demolition and antibiotic treatment to eradicate resistant Helicobacter pylori: a clinical trial. Clin Gastroenterol Hepatol. 2010;8:817–820.e3. doi: 10.1016/j.cgh.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353–1357. doi: 10.1136/gut.2007.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami K, Fujioka T, Okimoto T, Sato R, Kodama M, Nasu M. Drug combinations with amoxycillin reduce selection of clarithromycin resistance during Helicobacter pylori eradication therapy. Int J Antimicrob Agents. 2002;19:67–70. doi: 10.1016/S0924-8579(01)00456-3. [DOI] [PubMed] [Google Scholar]
- 20.Koo MW, Ogle CW, Cho CH. Effects of verapamil, carbenoxolone and N-acetylcysteine on gastric wall mucus and ulceration in stressed rats. Pharmacology. 1986;32:326–334. doi: 10.1159/000138188. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Matsuo K, Ito H, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006;119:217–224. doi: 10.1016/j.amjmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Karbasi A, Hossein Hosseini S, Shohrati M, Amini M, Najafian B. Effect of oral N-acetyl cysteine on eradication of Helicobacter pylori in patients with dyspepsia. Minerva Gastroenterol Dietol. 2013;59:107–112. [PubMed] [Google Scholar]
- 23.Sahai H, Khurshid A. Formulae and tables for the determination of sample sizes and power in clinical trials for testing differences in proportions for the two-sample design: a review. Stat Med. 1996;15:1–21. doi: 10.1002/(SICI)1097-0258(19960115)15:1<1::AID-SIM134>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Kim YS, Kim SJ, Yoon JH, et al. Randomised clinical trial: the efficacy of a 10-day sequential therapy vs. a 14-day standard proton pump inhibitor-based triple therapy for Helicobacter pylori in Korea. Aliment Pharmacol Ther. 2011;34:1098–1105. doi: 10.1111/j.1365-2036.2011.04843.x. [DOI] [PubMed] [Google Scholar]
- 25.Oh HS, Lee DH, Seo JY, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol. 2012;27:504–509. doi: 10.1111/j.1440-1746.2011.06922.x. [DOI] [PubMed] [Google Scholar]
- 26.Lim JH, Lee DH, Choi C, et al. Clinical outcomes of two-week sequential and concomitant therapies for Helicobacter pylori eradication: a randomized pilot study. Helicobacter. 2013;18:180–186. doi: 10.1111/hel.12034. [DOI] [PubMed] [Google Scholar]
- 27.Chung KH, Lee DH, Jin E, et al. The efficacy of moxifloxacin-containing triple therapy after standard triple, sequential, or concomitant therapy failure for Helicobacter pylori eradication in Korea. Gut and Liver. 2014;8:605–611. doi: 10.5009/gnl13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gisbert JP, Marcos S, Gisbert JL, Pajares JM. Helicobacter pylori eradication therapy is more effective in peptic ulcer than in non-ulcer dyspepsia. Eur J Gastroenterol Hepatol. 2001;13:1303–1307. doi: 10.1097/00042737-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Chung SJ, Lee DH, Kim N, et al. Eradication rates of helicobacter pylori infection with second-line treatment: non-ulcer dyspepsia compared to peptic ulcer disease. Hepatogastroenterology. 2007;54:1293–1296. [PubMed] [Google Scholar]
- 30.Sadowska AM, Verbraecken J, Darquennes K, De Backer WA. Role of N-acetylcysteine in the management of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:425–434. doi: 10.2147/copd.2006.1.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10:307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]