Abstract
Background/Aims
C-reactive protein (CRP) is an easily measured index of disease activity, but its ability to predict clinical course is controversial. We therefore designed a study to determine whether the CRP level at Crohn’s disease (CD) diagnosis is a valuable indicator of the disease phenotype, activity, and clinical course.
Methods
We retrospectively analyzed 705 CD patients from 32 institutions. The patients were classified into two groups according to CRP level. The patients’ demographic and clinical characteristics and their use of immunosuppressive or biological agents were recorded. Disease location and behavior, hospitalization, and surgery were analyzed.
Results
A high CRP was associated with younger age, steroid use, colonic or ileocolonic location, high CD activity index, and active inflammation at colonoscopy (p<0.001). As the disease progressed, patients with high CRP were more likely to exhibit strictures (p=0.027). There were significant differences in the use of 5-aminosalicylic acid, antibiotics, corticosteroids, azathioprine, and infliximab (p<0.001, p<0.001, p<0.001, p<0.001, and p=0.023, respectively). Hospitalization was also more frequent in patients with high CRP.
Conclusions
The CRP level at diagnosis is useful for evaluating the phenotype, activity, and clinical course of CD. Closer follow-up strategies, with early aggressive treatment, could be considered for patients with high CRP.
Keywords: Crohn disease, C-reactive protein, Clinical course
INTRODUCTION
The clinical manifestation of Crohn’s disease (CD) is heterogeneous, and the natural course is unpredictable, with phenotypic changes over time.1–5 The development of prognostic markers would enable early treatment for severe CD. Although several clinical indices and serological and fecal markers, such as calprotectin, and genetic biomarkers, such as nucleotide-binding oligomerization domain-containing protein 2, have been evaluated for the prediction of clinical course, a definitive predictive marker has not been fully established.6–10
C-reactive protein (CRP) is a surrogate marker of acute inflammation induced by infection, stress, tissue necrosis, trauma, and malignancies.11 CRP is synthesized principally by hepatocytes in response to stimulation by interleukin-1, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α).12 Recent studies have suggested that CRP is also produced by peripheral lymphocytes, inflamed kidneys, alveolar macrophage, and adipocytes in quantities insufficient to affect plasma CRP levels.13,14 CRP functions independent of physiological and pathological conditions and plasma CRP levels in patients with CD.11 Therefore, CRP production by the liver may be the only factor determining the plasma CRP level, suggesting the potential of CRP as a valuable marker to diagnose and monitor CD patients.
Estimating CRP may be useful to evaluate disease activity because it requires an inexpensive and easily accessible test; in addition, compared to other inflammatory markers, CRP has a short half-life of 19 hours.15 Several studies have demonstrated that CRP levels are associated with the clinical, radiographic, endoscopic, and histological activity in CD.16–18 However, whether CRP levels can predict the clinical course is not known. Higher CRP levels at the time of diagnosis or relapse have been associated with more frequent and severe short- and medium-term relapses.11,19 A retrospective study from Portugal reported that high CRP in CD predicts nonresponse to infliximab.20 However, these data were from a single-center study with a small number of subjects, thus limiting the generalizability of the results. There is lack of reports that provide conclusive evidence for predicting aggressive clinical course and establishing treatment plan.
We therefore conducted a multicenter cohort study of a large number of CD subjects to determine if the CRP level at diagnosis is a valuable predictive marker of disease phenotype, activity, and clinical course.
MATERIALS AND METHODS
1. Study population
We reviewed a retrospective clinical database that included patients diagnosed with CD between July 1982 and December 2008 who were enrolled in the Crohn’s Disease Clinical Network and Cohort (CONNECT) study. The gastroenterology clinics of 32 institutions in Korea participated in this cohort study, which was performed by the Korean Association for the Study of Intestinal Diseases.21,22 The institutions comprised 30 university hospitals and two community hospitals. Patients were eligible for the study if they had a measurable CRP level at the time of diagnosis, they did not have coexisting infectious colitis, they were followed up for longer than 6 months (to identify long-term clinical outcomes), and the status of their CD was evaluated before enrollment and at the end of the study. We excluded patients with incomplete clinical data (n=100), those for whom CRP level at diagnosis was unavailable (n=490), and those with lack of medical record from the referral hospitals (n=79), and those who were followed up for less than 6 months (n=8).
Patients were classified into two groups based on CRP >20 mg/L and ≤20 mg/L.
The diagnosis of CD was confirmed in all cases by previously established guidelines based on clinical history, endoscopic and radiological examinations, and histopathological findings.23
2. Data collection and outcome measurement
Clinical data at initial presentation of CD and subsequent follow-up visits were retrieved for analysis from the electronic medical record system. The collected data included age, gender, length of follow-up, disease location, and behavior at the time of diagnosis according to the Montreal classification. Disease location at final follow-up was categorized as colorectum, jejunum, ileum, or esophagogastroduodenum. The development of intestinal strictures, perianal fistula, and other fistulas and the time required to develop these lesions were assessed. Other fistulas were defined as abnormal tracks formed between the gut and gut, skin, an abscess cavity, or other hollow structures including the bladder, urethra, uterus, or vagina. We also recorded complications including abscesses, perforations, intestinal cancers, and primary sclerosing cholangitis.
Clinical disease activity at the time of diagnosis was assessed using the Crohn’s disease activity index (CDAI), with a score of >150 indicating active disease. Among the typical endoscopic features of CD, a cobblestone appearance and longitudinal ulcers were reviewed because these lesions can indicate increased CD activity. We recorded medication use during follow-up period, including 5-aminosalicylic acid (5-ASA), antibiotics, budesonide, and corticosteroids. The use of immunosuppressants, including azathioprine, 6-mercaptopurine, methotrexate, and TNF blockers such as infliximab and adalimumab, was also reviewed. Time from CD diagnosis to first use of azathioprine or TNF blockers was calculated to assess the length of time before these drugs were required to treat severe disease. Disease-related hospitalization (including readmissions) and surgery data were assessed because these can indicate a more severe course in CD.24 The collected data regarding hospitalization included the total number of hospital admissions and the readmission rate. In this analysis, readmission was defined as two or more admission of a discharged patient for further inpatient care as a new episode.
The proportion of patients who underwent surgery was recorded, and additional data including the total number of surgeries, resurgeries, and survival rates were obtained. These patients were analyzed according to the causative lesion for surgery or resurgery: intestinal or perianal lesion. Resurgery was defined as surgery at the same lesion site or at another site for a relapsed or uncontrolled lesion or to repair temporary structures from a previous surgery such as ileostomy repair. This study protocol was reviewed and approved by the Institutional Review Board of each participating medical center.
3. Statistical analysis
Continuous variables are presented as means±standard deviation when appropriate. Categorical variables were summarized as frequency and percentage. We performed univariate analyses using an independent t-test for continuous variables and the Pearson chi-square test, Fischer exact test, or linear by linear association for categorical variables. Kaplan-Meier method and log-rank test were used to determine the cumulative probability of stricture, perianal fistula, other fistula, use of azathioprine and TNF blocker, and risk of surgery according to the CRP level at diagnosis. Binary logistic regression analysis was performed using a forward stepwise selection method to evaluate risk factors affecting hospital admission of patients with CD. A multivariate analysis was performed if the univariate analysis yielded p<0.25. A p-value <0.05 was considered to indicate statistical significance. All analyses were performed using IBM SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
1. Demographic and clinical characteristics of patients
A total of 705 CD patients with measurable CRP levels were ultimately included in this analysis. The CRP value at the time of diagnosis was used as the index CRP level. The patients were classified into two groups by their index CRP level, with a cutoff at 20 mg/L: 373 had a CRP >20, and 332 had a CRP ≤20 mg/L. The patients were 7 to 77 years of age at the time of CD diagnosis, and the mean follow-up duration was 7.1 years. The demographic, clinical and laboratory details of the two groups are summarized in Table 1. There were no significant differences between the groups in gender, family history of inflammatory bowel disease (IBD), history of perianal disease, or history of taking anti-tuberculosis medications. Body mass index (BMI) was significantly lower in the high CRP group. Patients with high CRP were younger at diagnosis than those with low CRP (p<0.001) and had lower hemoglobin, higher CDAI, and elevated erythrocyte sedimentation rate (ESR). There was a significant difference in lesion location at time of diagnosis (p<0.001). Lesions in the high CRP group more frequently involved the colon (24.4%) and ileocolon (56.4%), while the low CRP group had more frequent terminal ileal (32.9%) and ileocolonic (48.4%) involvement. The CRP level did not distinguish disease behavior (p=0.503); the nonstricturing and nonpenetrating phenotype (B1) was the most frequent disease behavior type in both groups. Location and behavior at diagnosis were unavailable in 137 and 192 patients, respectively. Corticosteroid use at the time of diagnosis was significantly higher in the high CRP group compared with the low CRP group (41.2% vs 25.3%, p<0.001).
Table 1.
Baseline Characteristics of Patients with Crohn’s Disease according to the C-Reactive Protein Level at Diagnosis
| Variable | CRP >20 (n=373) | CRP ≤20 (n=332) | p-value |
|---|---|---|---|
| Age, yr | 25.4±10.1 | 28.6±14.2 | <0.001 |
| Sex, male/female | 285/88 | 238/94 | 0.153 |
| Time to follow-up, mo | 86.4±44.1 | 83.5±43.9 | 0.379 |
| BMI, kg/m2 | 19.1±3.3 | 20.9±4.2 | <0.001 |
| Smoking status | 0.020 | ||
| Never smoker | 245 (77.8) | 168 (70.0) | |
| Current smoker | 46 (14.6) | 36 (15.0) | |
| Ex-smoker | 16 (5.1) | 31 (12.9) | |
| Stop smoking after treatment | 8 (2.5) | 5 (2.1) | |
| Family history of IBD | 9 (2.4) | 7 (2.1) | 0.447 |
| Hb, g/dL | 11.9±1.9 | 12.8±2.1 | <0.001 |
| Hct, % | 36.0 | 38.1 | <0.001 |
| ESR, mm/hr | 47.5±29.2 | 23.6±21.5 | <0.001 |
| CDAI at diagnosis | 217.5±110.2 | 164.6±89.2 | <0.001 |
| Location at diagnosis* | <0.001 | ||
| L1 (ileal) | 51 (17.5) | 91 (32.9) | |
| L2 (colonic) | 71 (24.4) | 48 (17.3) | |
| L3 (ileocolonic) | 164 (56.4) | 134 (48.4) | |
| L4 (upper GI) | 5 (1.7) | 4 (1.4) | |
| Behavior at diagnosis* | 0.503 | ||
| B1 (inflammatory) | 176 (71.3) | 193 (72.6) | |
| B2 (stricturing) | 32 (13.0) | 39 (14.7) | |
| B3 (penetrating) | 39 (15.8) | 34 (12.8) | |
| Steroid use at diagnosis | 154 (41.2) | 84 (25.3) | <0.001 |
| History of anti-TB medication | 67 (18.0) | 70 (21.1) | 0.296 |
| History of perianal disease | 108 (29.0) | 81 (24.4) | 0.173 |
Data are presented as mean±SD or number (%).
CRP, C-reactive protein; BMI, body mass index; IBD, inflammatory bowel disease; Hb, hemoglobin; Hct, hematocrit; ESR, erythrocyte sedimentation rate; CDAI, Crohn’s disease activity index; GI, gastrointestinal; TB, tuberculosis.
Location and behavior at the time of diagnosis were unavailable in 137 and 192 patients, respectively.
2. Disease manifestation
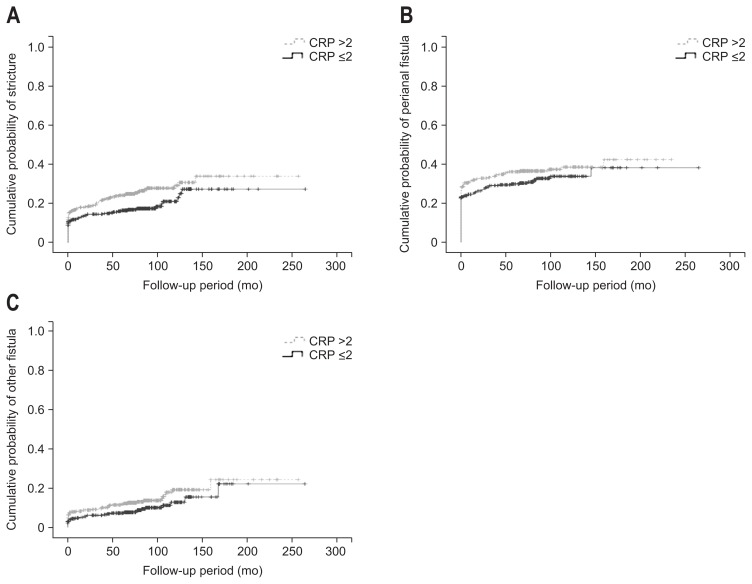
Colorectal involvement during the course of the disease was more frequent in the high CRP group (p<0.001). There were no statistically significant differences in jejunal, ileal, and esophagogastroduodenal involvement between the two groups. Endoscopic findings of longitudinal ulcer and cobblestone appearance were more frequent in the high CRP group (p<0.001 and p<0.001, respectively). Stricture during the course of the disease was more frequent in the high compared to the low CRP group (p=0.027). There were no significant differences in the development of fistulas, intestinal perforations, abscesses, intestinal cancers, or primary sclerosing cholangitis between the groups (Table 2). Using the Kaplan-Meier method, significant difference was found in the cumulative probability of stricture according to the CRP level at diagnosis (p=0.019). However, cumulative probability of perianal fistula and other fistula did not differ (Fig. 1).
Table 2.
Disease Manifestation according to the C-Reactive Protein Level at Diagnosis
| Variable | CRP >20 (n=373) | CRP ≤20 (n=332) | p-value |
|---|---|---|---|
| Involved location | |||
| Colorectum | 322 (86.3) | 222 (66.9) | <0.001 |
| Jejunum | 49 (13.1) | 61 (18.4) | 0.651 |
| Ileum | 310 (83.1) | 270 (81.3) | 0.917 |
| Esophagogastroduodenum | 36 (9.7) | 27 (8.1) | 0.324 |
| Endoscopic findings | |||
| Longitudinal ulcer | 255 (68.4) | 148 (44.6) | <0.001 |
| Cobblestone appearance | 160 (42.9) | 57 (17.2) | <0.001 |
| Histological finding | |||
| Granuloma | 148 (39.7) | 110 (33.1) | 0.657 |
| Stricture | 0.027 | ||
| Yes, at diagnosis | 49 (13.5) | 33 (10.0) | |
| Yes, during disease course | 46 (12.7) | 29 (8.8) | |
| Perianal fistula | 0.137 | ||
| Yes, at diagnosis | 105 (28.6) | 77 (23.3) | |
| Yes, during disease course | 35 (9.5) | 28 (8.5) | |
| Other fistula | 0.145 | ||
| Yes, at diagnosis | 27 (7.4) | 10 (3.1) | |
| Yes, during disease course | 28 (7.7) | 22 (6.7) | |
| Abdominal abscess | 0.068 | ||
| Yes, at diagnosis | 20 (5.5) | 14 (4.3) | |
| Yes, during disease course | 31 (8.5) | 17 (5.3) | |
| Perforation | 0.103 | ||
| Yes, at diagnosis | 14 (3.8) | 14 (4.3) | |
| Yes, during disease course | 6 (1.6) | 12 (3.7) | |
| Intestinal cancer* | 0.659 | ||
| Yes, at diagnosis | 1 (0.3) | 3 (0.9) | |
| Yes, during disease course | 2 (0.5) | 0 | |
| Primary sclerosing cholangitis | 0.472 | ||
| No | 364 (100) | 324 (99.7) | |
| Yes, during disease course | 0 | 1 (0.3) | |
Data are presented as number (%).
CRP, C-reactive protein.
Includes small bowel and colon cancer.
Fig. 1.
(A) Kaplan-Meier curve showing the cumulative probability of stricture according to the C-reactive protein (CRP) level (log-rank test, p=0.019). (B) Kaplan-Meier curve showing the cumulative probability of perianal fistula according to the CRP level (log-rank test, p=0.157). (C) Kaplan-Meier curve showing the cumulative probability of other fistula according to the CRP level (log-rank test, p=0.086).
3. Use of medications
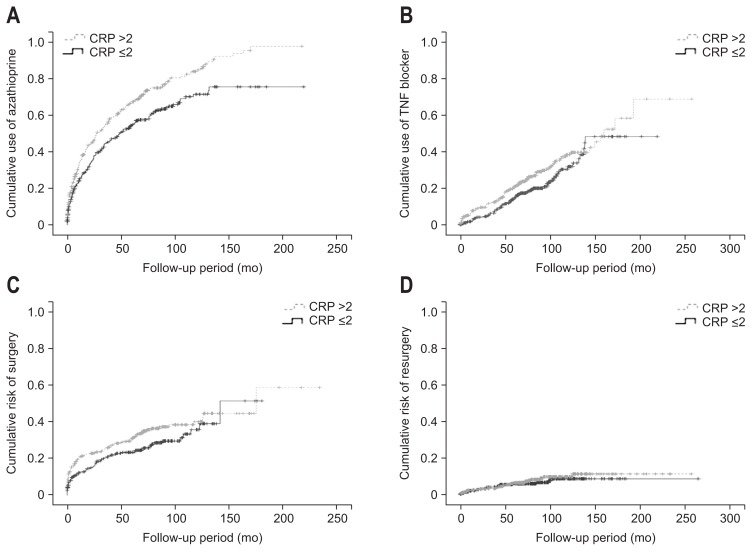
Medication use was investigated according to the index CRP level (Table 3). There were significant differences between the two groups in the use of 5-ASA-based agents, antibiotics, corticosteroids, azathioprine, and biological agents (p<0.001, p<0.001, p<0.001, p<0.001, and p=0.042, respectively). Of these medications, more subjects with high CRP had used infliximab (p=0.023), but there was no significant difference in adalimumab use between the two groups. The cumulative use of azathioprine and TNF blocker was significantly higher in high CRP group than in low CRP group (Fig. 2).
Table 3.
Use of Medications according to the C-Reactive Protein Level at Diagnosis
| Variable | CRP >20 (n=373) | CRP ≤20 (n=332) | p-value |
|---|---|---|---|
| Use of medications | |||
| 5-ASA | 368 (98.7) | 309 (93.1) | <0.001 |
| Antibiotics | 239 (64.1) | 153 (46.1) | <0.001 |
| Budesonide | 42 (11.3) | 32 (9.6) | 0.494 |
| Corticosteroid | 263 (70.5) | 164 (49.7) | <0.001 |
| Azathioprine | 281 (75.3) | 194 (58.4) | <0.001 |
| MTX | 4 (1.1) | 6 (1.8) | 0.407 |
| Infliximab | 102 (27.4) | 66 (19.9) | 0.023 |
| Adalimumab | 21 (5.6) | 15 (4.5) | 0.516 |
| TNF blocker | 111 (29.8) | 76 (22.9) | 0.042 |
| TPN | 77 (20.6) | 59 (17.8) | 0.796 |
| Time to use of azathioprine, mo | 28.8±34.0 | 28.6±31.3 | 0.950 |
| Time to use of TNF blockers, mo | 52.2±41.1 | 61.8±43.1 | 0.114 |
Data are presented as number (%) or mean±SD.
CRP, C-reactive protein; ASA, aminosalicylic acid; MTX, methotrexate; TNF, tumor necrosis factor; TPN, total parenteral nutrition.
Fig. 2.
(A) Kaplan-Meier curve showing the cumulative use of azathioprine according to the C-reactive protein (CRP) level (log-rank test, p<0.001). (B) Kaplan-Meier curve showing the cumulative use of a tumor necrosis factor blocker according to the CRP level (log-rank test, p=0.041). (C) Kaplan-Meier curve showing cumulative risk of surgery according to the CRP level (log-rank test, p=0.067). (D) Kaplan-Meier curve showing cumulative risk of resurgery according to the CRP level (log-rank test, p=0.489).
4. Hospital admission, surgery, and survival
The total numbers of hospital admissions and readmissions were also analyzed according to the index CRP level. The total number of hospital admissions differed significantly between the patients with high or low CRP (3.2±4.2 vs 2.3±3.7, p=0.011). In addition, the rate of readmission was higher in the high CRP group (p=0.048). Regarding CD-related surgeries, there were no significant differences in the total number of surgeries or the proportion of patients who underwent surgery for intestinal or perianal lesions between the two groups, nor did they differ in the proportion of repeat surgeries for intestinal or perianal lesions. The cumulative risk of surgery and resurgery did not differ according to the CRP level at diagnosis (Fig. 2). Only two patients died during the follow-up period: one from cardiac arrest of unknown cause and the other from intestinal bleeding associated with CD (Table 4).
Table 4.
Hospital Admission, Surgery, and Survival according to the C-Reactive Protein Level at Diagnosis
| Variable | CRP >20 (n=373) | CRP ≤20 (n=332) | p-value |
|---|---|---|---|
| Total no. of admission | 3.10±4.20 | 2.30±3.70 | 0.011 |
| Readmission | 193 (51.7) | 147 (44.3) | 0.048 |
| Patients performed surgery | 179 (48.0) | 146 (44.0) | 0.286 |
| Total no. of surgeries | 1.50±0.84 | 1.50±0.81 | 0.823 |
| Surgery of intestinal lesion | 82 (22.0) | 74 (22.3) | 0.922 |
| Surgery of perianal lesion | 109 (29.2) | 76 (22.9) | 0.056 |
| Resurgery of intestinal lesion | 15 (4.0) | 9 (2.7) | 0.338 |
| Resurgery of perianal lesion | 26 (7.0) | 25 (7.5) | 0.775 |
| Dead | 1 (0.3) | 1 (0.3) | 0.932 |
Data are presented as mean±SD or number (%).
CRP, C-reactive protein.
5. Predictors of hospitalization
We performed logistic regression analysis to identify factors that predicted hospitalization (Table 5). In univariate analysis, high index CRP, younger age, use of a TNF blocker, high CDAI at diagnosis, and a stricturing phenotype were all significant risk factors for hospitalization. Variables including high CRP (hazard ratio [HR], 2.03; 95% confidence interval [CI], 1.10 to 3.82; p=0.028), use of a TNF blocker (HR, 22.90; 95% CI, 3.10 to 172.10; p=0.002), and a stricturing phenotype (HR, 11.31; 95% CI, 2.62 to 48.81; p=0.001) were also significant risk factors for hospitalization in the multivariate analysis.
Table 5.
Multivariate Logistic Regression Analysis to Evaluate Potential Predictors for Hospitalization
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| CRP, >20 mg/L | 2.30 | 1.63–3.26 | <0.001 | 2.03 | 1.10–3.82 | 0.028 |
| Age | 0.98 | 0.97–0.99 | 0.004 | 0.99 | 0.97–1.02 | 0.761 |
| Use of TNF blocker | 7.16 | 3.88–13.23 | <0.001 | 22.90 | 3.10–172.10 | 0.002 |
| CDAI at diagnosis | 1.00 | 1.00–1.01 | 0.021 | 1.00 | 1.00–1.01 | 0.255 |
| Stricturing phenotype | 3.86 | 2.23–6.69 | <0.001 | 11.31 | 2.62–48.81 | 0.001 |
HR, hazard ratio; CI, confidence interval; CRP, C-reactive protein; TNF, tumor necrosis factor; CDAI, Crohn’s disease activity index.
DISCUSSION
In this study, CD patients with CRP >20 mg/L were more likely to be younger and exhibit colonic or ileocolonic involvement. The CRP level was a useful index of clinical and endoscopic disease activity. During follow-up, high CRP was associated with more aggressive treatments, such as immunosuppressants and biological agents, and with the total number of hospital admissions and readmissions.
CRP was described as a marker for the differential diagnosis of CD and ulcerative colitis,15 and the association between CRP and disease activity has been investigated. Several recent studies have reported a correlation between the index CRP and clinical relapses, the subsequent need for aggressive treatment, and response to treatment.19,25 However, research supporting the CRP level at the time of diagnosis as a predictor of the clinical course is lacking.
In a recent study by the Brisbane IBD research group, the low CRP group had a strikingly lower BMI and more pure ileal disease (predisposing them to intestinal resections) than the high CRP group.26 A retrospective study in Germany also reported disease with an exclusively ileal distribution in the low CRP group.27 Although we could not reproduce the correlation between a low BMI and prior intestinal resection, we confirmed that patients with low CRP tend to develop ileal disease, while those with high CRP have a higher frequency of colonic involvement. Our results are consistent with those of previous studies.16,18,28 Although the exact mechanism underlying the predominance of ileal lesions in the low CRP group is not understood, a study has indicated that ileal disease has a tendency to evolve more locoregional inflammatory disease, as evidenced by a high rate of fat wrapping in surgical specimens, than systemic disease and, consequently, does not elevate CRP.26
Studies of the Montreal classification of CD behavior based on the CRP level have yielded conflicting results. In a prospective population-based study, noninflammatory behavior including stricturing or penetrating disease was associated with a high CRP at the time of diagnosis.25 By contrast, a retrospective database-based study concluded that there was no significant difference in disease behavior based on the index CRP level during a clinical relapse.19 However, little information is available regarding changes in disease behavior during the course of the disease among these patients. In our study, there was no significant difference in disease behavior between the two groups at the initial presentation of CD, and inflammatory disease was the predominant phenotype in both groups. In addition, we found that intestinal strictures occurred more frequently in high CRP patients throughout the disease course. Our findings suggest that high CRP patients have a higher likelihood of fibrostenotic changes because they have a chronic inflammatory profile. We therefore believe that CRP can be a valuable tool to predict further stricture formation, which is a major indication for surgery.
We also observed that high CRP was significantly correlated with a cobblestone appearance and longitudinal ulcers at colonoscopy, which suggest active inflammation. In addition, the CDAI score of high CRP patients was higher than the score of those with low CRP. These results correspond with those of a retrospective study of 147 American patients that demonstrated that an elevated CRP was associated with clinical disease activity, endoscopic inflammation, and several other IBD biomarkers.16
CRP can be used to predict subsequent need for azathioprine and biological agents as well as clinical relapse.19 A retrospective study in the Netherlands provided additional evidence for the role of CRP in predicting a more severe clinical course of CD, such as number of relapses, severity of relapse, and cumulative days of prednisone use.25 In accord with previous studies, we determined that patients with high CRP received more aggressive medical treatment, such as immunosuppressants or biological agents. Our multivariate analysis also indicated that a high CRP level is an independent predictor for hospitalization. Interestingly, the CRP groups did not differ in their need for surgery or repeat surgery. These results coincide well with those of a previous study that reported that patients with low CRP more often have purely ileal disease, with an increased requirement for intestinal resection.26 Accordingly, we can postulate that the surgery rate in patients with low CRP would be similar to that of those with a high CRP, in whom more aggressive treatment would be required.
In the present study, we observed a correlation of CRP levels with clinical progression to severe disease. Although the precise mechanisms underlying this correlation have not been fully elucidated, some plausible explanation for these results can be inferred from some published reports. Mesenteric fat, rather than the intestinal wall, is likely important for the production of CRP in CD patients.29 The mesenteric fat hyperplasia observed in CD but not UC has been suggested to contribute to the synthesis of inflammatory mediators such as TNF-α and IL-6, which promote damage to the intestinal mucosa. In addition, mesenteric fat hyperplasia could impair gut barrier function by disturbing innate immunity to the gut flora.30 Based on these findings, the elevation of CRP at baseline could reflect the degree of mesenteric fat hyperplasia. Accordingly, increased production of inflammatory cytokines via the accumulation of mesenteric adipocytes might induce a severe, ongoing, inflammatory process, and direct assessment of this inflammatory activity could facilitate the prediction of a more severe clinical course.
Several studies have presented different CRP cutoff values for CD patients,19,25,31 but a definitive cutoff value for predicting disease activity and clinical course has not been established. In a study of French patients, high CRP (>20 mg/L) was emphasized as a valuable diagnostic marker for predicting a van Hees index ≥150.32 The GETAID group emphasized that CRP >20 mg/L and ESR >15 mm/hr were the only meaningful laboratory markers predictive of relapse.31 Furthermore, a multicenter randomized controlled study indicated that high CRP (>20 mg/L) was correlated with relapse after azathioprine withdrawal.33 These results determined our selection of 20 mg/L as the optimal CRP cutoff value for categorizing the disease manifestations and clinical course of CD.
This study improves upon previous studies by employing a multicenter cohort from 32 institutions of CD patients with measurable CRP. This design minimizes bias that might arise from a single center case-control study. We also enrolled a significant proportion of potential cases, ultimately including 705 patients and thereby substantially increasing the statistical power. Furthermore, uniform findings based on the CRP level were reproduced in this study despite the heterogeneity of the studied population. The classification of disease phenotype, activity and clinical course by CRP level may therefore be applicable to the patients with CD in clinical practice.
Our study is limited by its retrospective nature, which limits our ability to provide a causal explanation for the relationship between CRP levels and clinical outcomes.
This study includes missing values on potentially important covariates, which could be unavoidable in retrospective and observational study. However, unavailable data is mostly due to insufficient data collection and work-up or loss to follow-up before electronic medical record was introduced in the early 2000s. In addition, there is a possibility of poor quality assurance concerning admission, treatment, and follow-up and patient enrollment with relatively severe disease state due to characteristics of participating secondary or tertiary care center, which could limit reliability of the study. However, these potential confounders were minimized by the retrieval of clinical data from electronic medical record systems at all participating hospitals and accurate recording of the time to occurrence of any event or use of medication. Unfortunately, we could not investigate clinical data on the frequency or severity of relapses, improvement of subjective symptoms, and treatment response, particularly for biological agents, due to the lack of medical records and the observational study design. In conclusion, CRP at the time of diagnosis appears to be useful in predicting disease phenotype, clinical and endoscopic activity, and the clinical course of CD. Early aggressive treatment with intensive follow-up management strategy may be warranted in patients with high CRP. In addition, the integration of CRP levels with other predictive indices will improve the reliability of the identification of the natural history of CD. A further prospective study that includes a large number of subjects could provide definitive evidence of the correlation of CRP levels with multiple clinical outcomes.
ACKNOWLEDGEMENTS
This study was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2013-E63004-01).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Mazor Y, Maza I, Kaufman E, et al. Prediction of disease complication occurrence in Crohn’s disease using phenotype and genotype parameters at diagnosis. J Crohns Colitis. 2011;5:592–597. doi: 10.1016/j.crohns.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Jung YS, Song CS, Kim ER, et al. Seasonal variation in months of birth and symptom flares in Korean patients with inflammatory bowel disease. Gut Liver. 2013;7:661–667. doi: 10.5009/gnl.2013.7.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang CW, Wong JM, Tung CC, Shih IL, Wang HY, Wei SC. Intestinal stricture in Crohn’s disease. Intest Res. 2015;13:19–26. doi: 10.5217/ir.2015.13.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu PJ. Inflammatory bowel disease in Asia: the challenges and opportunities. Intest Res. 2015;13:188–190. doi: 10.5217/ir.2015.13.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotze PG, Spinelli A, da Silva RN, et al. Conventional versus biological therapy for prevention of postoperative endoscopic recurrence in patients with Crohn’s disease: an International, multicenter, and observational study. Intest Res. 2015;13:259–265. doi: 10.5217/ir.2015.13.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Incà R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008;103:2007–2014. doi: 10.1111/j.1572-0241.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 7.Cotterill L, Payne D, Levinson S, et al. Replication and meta-analysis of 13,000 cases defines the risk for interleukin-23 receptor and autophagy-related 16-like 1 variants in Crohn’s disease. Can J Gastroenterol. 2010;24:297–302. doi: 10.1155/2010/480458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtenstein GR. Emerging prognostic markers to determine Crohn’s disease natural history and improve management strategies: a review of recent literature. Gastroenterol Hepatol (NY) 2010;6:99–107. [PMC free article] [PubMed] [Google Scholar]
- 9.Van Limbergen J, Russell RK, Nimmo ER, Satsangi J. The genetics of inflammatory bowel disease. Am J Gastroenterol. 2007;102:2820–2831. doi: 10.1111/j.1572-0241.2007.01527.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee KM. Fecal biomarkers in inflammatory bowel disease. Intest Res. 2013;11:73–78. doi: 10.5217/ir.2013.11.2.73. [DOI] [Google Scholar]
- 11.Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:661–665. doi: 10.1097/00054725-200409000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Jürgens M, Mahachie John JM, Cleynen I, et al. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:421–427.e1. doi: 10.1016/j.cgh.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Mendall MA, Strachan DP, Butland BK, et al. C-reactive protein: relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. Eur Heart J. 2000;21:1584–1590. doi: 10.1053/euhj.1999.1982. [DOI] [PubMed] [Google Scholar]
- 14.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–767. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- 15.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solem CA, Loftus EV, Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–712. doi: 10.1097/01.MIB.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 17.Cellier C, Sahmoud T, Froguel E, et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease: a prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231–235. doi: 10.1136/gut.35.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakatos PL, Kiss LS, Palatka K, et al. Serum lipopolysaccharide-binding protein and soluble CD14 are markers of disease activity in patients with Crohn’s disease. Inflamm Bowel Dis. 2011;17:767–777. doi: 10.1002/ibd.21402. [DOI] [PubMed] [Google Scholar]
- 19.Koelewijn CL, Schwartz MP, Samsom M, Oldenburg B. C-reactive protein levels during a relapse of Crohn’s disease are associated with the clinical course of the disease. World J Gastroenterol. 2008;14:85–89. doi: 10.3748/wjg.14.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magro F, Rodrigues-Pinto E, Santos-Antunes J, et al. High C-reactive protein in Crohn’s disease patients predicts nonresponse to infliximab treatment. J Crohns Colitis. 2014;8:129–136. doi: 10.1016/j.crohns.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Cheon JH, Kim YS, Ye BD, et al. Crohn’s Disease Clinical Network and Cohort (CONNECT) Study: the first step toward nationwide multicenter research of Crohn’s disease in Korea. Intest Res. 2014;12:173–175. doi: 10.5217/ir.2014.12.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JW, Im JP, Cheon JH, Kim YS, Kim JS, Han DS. Inflammatory bowel disease cohort studies in Korea: present and future. Intest Res. 2015;13:213–218. doi: 10.5217/ir.2015.13.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter MJ, Lobo AJ, Travis SP IBD Section, British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl 5):V1–V16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein CN, Loftus EV, Ng SC, Lakatos PL, Moum B. Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61:622–629. doi: 10.1136/gutjnl-2011-301397. [DOI] [PubMed] [Google Scholar]
- 25.Kiss LS, Papp M, Lovasz BD, et al. High-sensitivity C-reactive protein for identification of disease phenotype, active disease, and clinical relapses in Crohn’s disease: a marker for patient classification? Inflamm Bowel Dis. 2012;18:1647–1654. doi: 10.1002/ibd.21933. [DOI] [PubMed] [Google Scholar]
- 26.Florin TH, Paterson EW, Fowler EV, Radford-Smith GL. Clinically active Crohn’s disease in the presence of a low C-reactive protein. Scand J Gastroenterol. 2006;41:306–311. doi: 10.1080/00365520500217118. [DOI] [PubMed] [Google Scholar]
- 27.Thalmaier D, Dambacher J, Seiderer J, et al. The +1059G/C polymorphism in the C-reactive protein (CRP) gene is associated with involvement of the terminal ileum and decreased serum CRP levels in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;24:1105–1115. doi: 10.1111/j.1365-2036.2006.03093.x. [DOI] [PubMed] [Google Scholar]
- 28.Yang DH, Yang SK, Park SH, et al. Usefulness of C-reactive protein as a disease activity marker in Crohn’s disease according to the location of disease. Gut Liver. 2015;9:80–86. doi: 10.5009/gnl13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut. 2012;61:78–85. doi: 10.1136/gutjnl-2011-300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peyrin-Biroulet L, Chamaillard M, Gonzalez F, et al. Mesenteric fat in Crohn’s disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577–583. doi: 10.1136/gut.2005.082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consigny Y, Modigliani R, Colombel JF, et al. A simple biological score for predicting low risk of short-term relapse in Crohn’s disease. Inflamm Bowel Dis. 2006;12:551–557. doi: 10.1097/01.ibd.0000225334.60990.5b. [DOI] [PubMed] [Google Scholar]
- 32.Chamouard P, Richert Z, Meyer N, Rahmi G, Baumann R. Diagnostic value of C-reactive protein for predicting activity level of Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:882–887. doi: 10.1016/j.cgh.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Treton X, Bouhnik Y, Mary JY, et al. Azathioprine withdrawal in patients with Crohn’s disease maintained on prolonged remission: a high risk of relapse. Clin Gastroenterol Hepatol. 2009;7:80–85. doi: 10.1016/j.cgh.2008.08.028. [DOI] [PubMed] [Google Scholar]