Abstract
Most colon cancer cases are initiated by truncating mutations in the tumor suppressor, adenomatous polyposis coli (APC). APC is a critical negative regulator of the Wnt signaling pathway that participates in a multi-protein “destruction complex” to target the key effector protein β-catenin for ubiquitin-mediated proteolysis. Prior work has established that the poly(ADP-ribose) polymerase (PARP) enzyme Tankyrase (TNKS) antagonizes destruction complex activity by promoting degradation of the scaffold protein Axin, and recent work suggests that TNKS inhibition is a promising cancer therapy. We performed a yeast two-hybrid (Y2H) screen and uncovered TNKS as a putative binding partner of Drosophila APC2, suggesting that TNKS may play multiple roles in destruction complex regulation. We find that TNKS binds a C-terminal RPQPSG motif in Drosophila APC2, and that this motif is conserved in human APC2, but not human APC1. In addition, we find that APC2 can recruit TNKS into the β-catenin destruction complex, placing the APC2/TNKS interaction at the correct intracellular location to regulate β-catenin proteolysis. We further show that TNKS directly PARylates both Drosophila Axin and APC2, but that PARylation does not globally regulate APC2 protein levels as it does for Axin. Moreover, TNKS inhibition in colon cancer cells decreases β-catenin signaling, which we find cannot be explained solely through Axin stabilization. Instead, our findings suggest that TNKS regulates destruction complex activity at the level of both Axin and APC2, providing further mechanistic insight into TNKS inhibition as a potential Wnt pathway cancer therapy.
Keywords: ADP-ribosylation, axin, beta-catenin (β-catenin), colon cancer, Wnt signaling, Adenomatous Polyposis Coli, Tankyrase, XAV939, destruction complex
Introduction
The Wnt pathway helps direct a myriad of normal developmental and adult homeostasic processes in metazoans, but is also misregulated in several human diseases such as cancer (1, 2). Wnt signaling is regulated through the activity of a multi-protein “destruction complex” that promotes proteolysis of the transcriptional co-activator β-catenin (βcat)3 by stimulating phosphorylation of the βcat phosphodegron (3). Core components of the destruction complex include the scaffold protein Axin, the tumor suppressor adenomatous polyposis coli (APC), and the kinases CK1 and GSK3.
While Wnt signaling plays essential roles during development, it is inappropriately activated in a number of cancers, most notably colorectal cancer. Truncating mutations in the tumor suppressor adenomatous polyposis coli (APC) are the initiating mutational event in more than 80% of all colon cancer cases, and these mutations hyperactivate βcat signaling (4). Thus small molecule inhibitors of the Wnt pathway should provide an effective therapeutic strategy. Among these strategies, inhibition of oncogenic βcat activity would appear to be the most direct approach, as studies have demonstrated that the accumulation of βcat is what initiates oncogenesis, and that tumors have a continued reliance on oncogenic βcat signaling (5). Indeed, recent promising antagonists have been identified that specifically disrupt βcat binding to TCF or the CBP transcriptional coactivator (6, 7).
In addition to these approaches, small molecule inhibitors of the Wnt pathway have been identified that regulate βcat signaling through Axin stabilization. These molecules (XAV939, WIKI4, and IWR-1 among others) have been shown to stabilize Axin by inhibiting the activity of the poly(ADP-ribose) polymerase enzyme Tankyrase (TNKS) (8, 9). TNKS-mediated ADP-ribosylation (PARylation) of Axin promotes an interaction with the RNF146 E3 ubiquitin ligase (10–13), thereby stimulating Axin destruction. TNKS inhibition thereby results in the assembly of more functional destruction complexes that can target βcat for degradation. Importantly, TNKS inhibition antagonizes βcat signaling even in colon cancer cells with mutated APC (9, 14), consistent with the finding that overexpressed Axin can stimulate βcat destruction in colon cancer cell lines (15). The effectiveness of TNKS inhibition as a cancer therapeutic is being actively studied with promising results (14, 16–18); however, others have cautioned that the therapeutic value of TNKS inhibition may be limiting due to LEF1 and B9L shielding of βcat in the nucleus, which protects βcat from Axin-mediated destruction (19).
Here we report the surprising finding that the effects of TNKS inhibition on Wnt signaling cannot be explained fully by Axin stabilization. Instead, we explore a novel protein interaction we identified in Drosophila between TNKS and another component of the βcat destruction complex, the fly APC homolog APC2. Our findings suggest that TNKS antagonizes destruction complex activity at the level of both Axin and APC2, providing additional insight into the mechanism of TNKS inhibition and its potential as a therapeutic strategy.
Experimental Procedures
DNA Cloning
DNA constructs for expression of Drosophila or human versions of APC2, Axin/Axin2, and TNKS were generated using the approach outlined previously (20). Briefly, full-length genes or gene fragments were PCR amplified and either TOPO-TA cloned into the pCR8/GW/TOPO Gateway entry vector (Life Technologies) or BP cloned into the pDONR-Zeo entry vector (Life Technologies). Entry vectors were then LR cloned (Life Technologies) into the appropriate destination vector. For cell culture experiments, modified ECFP-N1 destination vectors (Clontech) were used containing either an N-terminal GFP or Flag tag followed by a Gateway cassette. pCDNA3.1/nV5-pDEST was used for V5-tagged constructs. For transgenic fly lines, APC2 and APC2ΔTBD were cloned into a modified pUAStattB vector (Basler laboratory, GenBankTM accession number EF362409) containing the endogenous APC2 promoter, an N-terminal GFP tag, and the Gateway cassette. All transgenic flies were generated by the PhiC31 approach at BestGene Inc. (Chino Hills, CA) using the BL 9723 line. Additional cloning details are available upon request.
Yeast Two-hybrid Analysis
Yeast Two-Hybrid (Y2H) analysis was performed using the Matchmaker System (Clontech) as previously described (21). Briefly, the pGBKT7 and pGADT7 yeast vectors were engineered to be Gateway compatible, and APC2 entry vectors LR cloned into pGBKT7-W, whereas Armadillo, Axin, and TNKS were LR cloned into pGADT7-W. pGBKT7-W constructs were transformed into the Y2HGold yeast strain and pGADT7-W into Y187 using the SC Easy Transformation kit (Life Technologies). Selection was on -Trp or -Leu plates respectively (Sigma Aldrich). The appropriate transformed yeast colonies were then mated in 2× YPAD media for 24 h and plated on double selection -Leu -Trp plates. β-galactosidase assays were performed using the yeast β-galactosidase assay kit (Thermo Scientific, Pierce). The initial Y2H screen was performed by Hybrigenics (Paris, France) using a Drosophila embryonic cDNA library.
Cell Culture and Transfections
SW480 and HCT116 cells were cultured at 37 °C and 5% CO2 in DMEM-H supplemented with 10% heat inactivated fetal bovine serum (FBS) and 1× Pen/Strep/Glutamine (Gibco). For transient transfections, SW480 cells were plated at a density of 2.5 × 105 cells per well in 6-well plates and grown overnight. HCT116s were plated at 7.0 × 105 cells per well. DNA constructs were transfected using Lipfectamine 2000 (Life Technologies) according to the manufacturer's instructions.
Immunoprecipitations
For immunoprecipitations, transfected SW480 cells were lysed in RIPA buffer (50 mm Tris pH 7.4, 150 mm NaCl, 1% Igepal, and 0.25% Na deoxycholate) supplemented with protease inhibitors (SigmaFAST Protease Inhibitor tablet, Sigma Aldrich), 1 mm sodium fluoride, and 0.5 mm sodium orthovanadate. Cell debris was pelleted, and lysates incubated with rabbit anti-GFP antibody (Abcam, cat. number Ab290 at 1:500) for 20 min at 4 °C. Protein G-Sepharose beads (Sigma Aldrich) were washed three times with RIPA buffer, and the lysates incubated with the beads for an additional 20 min at 4 °C. Beads were then washed extensively with RIPA buffer and proteins denatured by adding 2× Laemmli Buffer (Bio-Rad).
GST Pulldowns
The Drosophila APC2 SAMP1 fragment or SAMP1ΔTBD fragment were LR cloned into the pDEST15 destination vector (Life Technologies). The resulting bacterial expression vectors were transformed into BL21Ai cells (Life Technologies) and induction of recombinant proteins performed in LB medium using 0.2% l-arabinose (Sigma Aldrich) for 3 h at 37 °C. Bacterial cells were ruptured by sonication in 1× PBS, and recombinant protein purified using the GST Bulk Kit (GE Healthcare) according to the manufacturer's instructions. Protein purity was verified by SDS-PAGE and Coomassie Blue staining (Bio-Rad). SW480 cells were transfected with Flag-tagged TNKS, and cell lysates prepared in RIPA buffer. Lysates were pre-cleared with glutathione beads (GE Healthcare) and incubated with 10 μg of the appropriate recombinant protein at 4 °C for 1 h. Beads were washed extensively with RIPA buffer and samples analyzed via SDS-PAGE.
Immunoblotting
Protein samples were prepared using 2× Laemmli solution (Bio-Rad), boiled for 5 min, and resolved by SDS-PAGE using 4–20% TGX gradient gels (Bio-Rad). 7.5% gels were used to resolve endogenous Axin2 from GFP-tagged Axin2. Proteins were transferred to nitrocellulose using the TurboTransfer system (Bio-Rad). Membranes were probed with mouse anti-GFP (Clontech, clone JL-8, 1:1000), mouse-anti Flag (Sigma Aldrich, clone M2, 1:1000) mouse anti-β-catenin (BD Transduction, 1:1000), mouse-anti APC (Abcam, clone Ab58 1:1000), rabbit-anti Axin2 (Cell Signaling, clone D48G4 1:500), rabbit anti-GST (Genscript, 1:1000), and mouse anti-tubulin (Sigma Aldrich, DM1A, 1:5000). HRP-conjugated secondary antibodies (Santa Cruz Biotechnology) were used at 1:5000. Signal was detected using SuperSignal West Dura Chemiluminescent Substrate (Pierce) and imaged on a Fluor Chem Q imager (Protein Simple).
In Vitro ADP-ribosylation ELISA
An N-terminal fragment of Drosophila Axin (amino acids 1–280) or a C-terminal fragment of Drosophila APC2 (20R2-Stop) were LR cloned into the pDEST17 destination vector (Life Technologies). Vectors were transformed into BL21Ai cells, and expression performed as described above. Purification of recombinant protein was performed using the HIS GraviTrap and HIS Buffer kits (GE Healthcare) according to the manufacturer's instructions. Recombinant proteins were coated on an ELISA plate (BSA was used as a negative control) and the Tankyrase1 (Parp5A) Colorimetric Activity Assay kit (Trevigen) used to monitor in vitro PARylation of Drosophila Axin and APC2. 10 μm XAV939 was included in some reactions to establish that ELISA signal was dependent on TNKS activity.
Immunofluorescence
Cells were plated on coverslips, transfected 24 h post-plating, and fixed 24 h later with 4% formaldehyde in 1× phosphate-buffered saline (1× PBS) for 10–15 min. Cells were then washed three times with 1× PBS, blocked for 15 min in 1× PBTN (1× PBS containing 1% normal goat serum and 0.1% Triton X-100), and antibody stained. Primary antibodies were mouse anti-β-catenin (BD Transduction Laboratories, cat. 610153, 1:1000), rat anti-Flag (Novus Biologicals, 1:1000), and mouse anti-V5 (Life Technologies, 1:1000). Secondary antibodies were goat anti-mouse Alexa 568 or 647 (Life Technologies, 1:500).
Flow Cytometry to Quantify βcat or Axin2 Protein Levels in Transfected SW480 Cells
Cells were trypsinized, washed with 1× PBS, and then fixed in 10% formaldehyde/1× PBS for 20 min. Cells were permeabilized with 1× Perm/Wash reagent (BD Biosciences), and then antibody stained in 1× Perm/Wash with mouse anti-β-catenin (BD Transduction, 1:200) or rabbit anti-Axin2 (Cell Signaling, 1:500) followed by goat anti-mouse or goat anti-rabbit Alexa 647 (Life Technologies, 1:1000). Cells were analyzed on an Accuri C6 Flow Cytometer, and the mean fluorescence intensity determined in GFP-positive cells. At least 5,000 transfected cells were measured per sample, and at least three independent experiments were performed. The mean fluorescence intensity was first normalized to untransfected cells for each sample to account for staining variability. Values were then normalized to the GFP only control.
TOP/FOP Luciferase Reporter Assay
Luciferase assays were performed using the Dual Glo Luciferase System (Promega) according to the manufacturer's protocol. Briefly, SW480 or HCT116 cells were transiently co-transfected with 2 μg of the relevant APC2 construct, 1 μg of pRL, and 1 μg of either TOP or FOP Flash Luciferase reporter. After 24 h, cells were lysed in a hypotonic 0.1× PBS solution and subjected to a 5 min freeze-thaw at −80 °C. Cells were scraped and cellular debris pelleted. Luciferase activity of each lysate was measured using a Perkin Elmer EnSpire plate reader and normalized to Renilla signal. All samples were measured in triplicate per experiment, and at least three independent experiments were performed. None of the constructs displayed significant FOP Flash activity.
Drosophila Genetics, Embryonic Lethality, and Cuticle Patterning
APC2 transgene function was determined by crossing the transgene into the APC2g10 single mutant background and assessing embryonic viability and cuticle patterning.
APC2 Transgene
APC2g10 males and females were crossed to obtain embryos maternally and zygotically deficient for endogenous APC2, but expressing the APC2 transgene. Embryonic viability and cuticle patterning experiments were performed as previously described (21).
Results
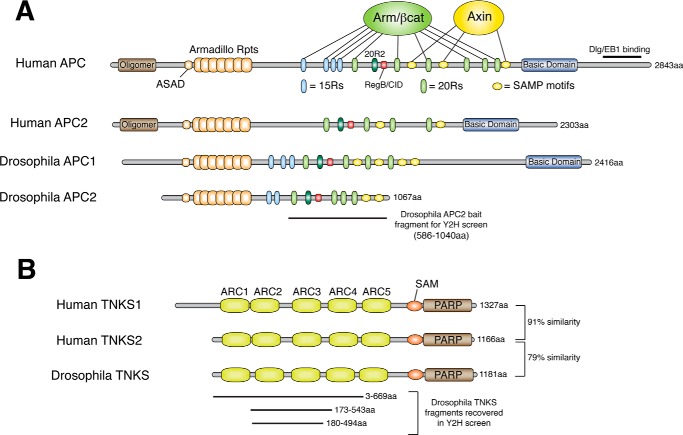
The fruit fly Drosophila melanogaster has proven to be an excellent model system to study APC biology. Both flies and humans have two related APC proteins that share a number of core features including a set of N-terminal Armadillo repeats, 15 amino acid repeats (15Rs) and 20 amino acid repeats (20Rs) that both bind βcat, and a series of SAMP repeats that bind Axin (Fig. 1A). We and others have focused on Drosophila APC2 as a model to investigate conserved features of how APC proteins contribute to destruction complex function (20–23).
FIGURE 1.
Schematics of APC and TNKS proteins. A, humans and flies have two APC proteins that share a core region of binding domains and motifs. These consist of Armadillo repeats, 15 amino acid repeats (15Rs), 20 amino acid repeats (20Rs), the catenin inhibitory domain (CID, called Region B in Drosophila), and SAMP motifs. The 15Rs and 20Rs all bind βcat (except 20R2) and SAMP motifs bind Axin. C-terminal sequences in APCs are more divergent, with some containing a basic domain and an EB1 binding site. B, humans have two TNKS proteins (TNKS1 and TNKS2), whereas flies have a single closely-related TNKS. TNKS proteins consist of five sets of ankyrin repeat complexes (ARCs) that individually bind substrate, a SAM domain that mediates oligomerization, and a catalytic PARP domain. A yeast two-hybrid (Y2H) screen using a fragment of Drosophila APC2 encompassing 20R1-SAMP2 recovered 10 clones of Drosophila TNKS, all of which encoded for regions of the ARCs. 5 of these clones were fully sequenced, and 3 represent unique clones.
TNKS Binds Drosophila APC2 at a Conserved C-terminal Motif
To identify novel APC interactors, we performed a yeast two-hybrid (Y2H) screen using a C-terminal fragment of Drosophila APC2 encompassing 20R1 to SAMP2 (amino acids 586–1040) and a fly embryonic cDNA library. This screen recovered over 200 clones of Armadillo (fly βcat) as a positive control, but also recovered 10 clones of the PARP enzyme Tankyrase (TNKS). TNKS proteins have an N-terminal set of five ankyrin repeat complexes (ARCs) that bind substrate, a SAM domain that mediates oligomerization, and the catalytic PARP domain (24). All recovered fragments of Drosophila TNKS encompassed the ARC region (Fig. 1B), consistent with the hypothesis that APC2 may be a novel TNKS substrate.
Prior studies have proposed a consensus TNKS binding motif of RxxPxG with the R at position 1 and the G at position 6 being required, and a need for a small hydrophobic residue at position 4 (24). In addition, a second non-consensus TNKS binding motif on Axin was revealed through the Axin/TNKS crystal structure (25), which may be a weaker affinity site since it cannot be detected by yeast two-hybrid or co-IP approaches. We therefore scanned the Drosophila APC2 sequence for a consensus TNKS motif and identified an RPQPSG sequence immediately downstream of SAMP1 (Fig. 2A). We confirmed that this sequence is both necessary and sufficient for TNKS binding by unbiased Y2H mapping using sequentially shorter fragments of APC2 or a large APC2 fragment that precisely deleted only the six amino acids (Fig. 2B). Moreover, the APC2/TNKS interaction could also be detected using coIP and GST pulldown approaches (Fig. 2, C and D). Surprisingly, deletion of the TNKS binding motif from APC2 (APC2ΔTBD) reduced, but did not eliminate the ability to coIP TNKS from SW480 cell extracts (Fig. 2C); however, it did eliminate the interaction via GST pulldown (Fig. 2D). We hypothesize that the residual binding observed in coIP is likely due to an indirect interaction, perhaps through Axin.
FIGURE 2.
APC2 is a novel TNKS binding partner and substrate. A, protein alignment comparing putative TNKS binding sites on APC2 proteins from various species. Drosophila APC2s have a single consensus TNKS-binding motif, whereas human APC2 has a consensus motif and a second putative motif that matches the non-consensus TNKS binding site in human Axin. B, unbiased Y2H mapping of the TNKS binding site on Drosophila APC2. An RPQPSG motif on APC2 is both necessary and sufficient for TNKS binding. C, Drosophila APC2 co-immunoprecipitates with TNKS when co-expressed in SW480 cells. Deletion of the RPQPSG motif reduces, but does not eliminate the interaction. D, binding assay using a recombinant GST fragment of Drosophila APC2 with epitope-tagged TNKS expressed in SW480 cells. E, ELISA-based PARylation assay to test for poly ADP-ribosylation of recombinant Drosophila Axin and APC2. Treatment with the TNKS inhibitor XAV939 reduces the signal, demonstrating the specificity of the assay. F and G, yeast two-hybrid analysis of human APC2 and APC1 fragments. A short fragment of human APC2 encompassing 20R5 to the Tankyrase binding domain (TBD) binds both human TNKS1 and TNKS2. Deletion of the TBD greatly reduces this interaction. No interaction between fragments of human APC1 and TNKS could be detected.
Both mammals and Drosophila have two APC family members that arose by independent gene duplications. Protein alignments revealed that variants of the RPQPSG motif exist in APC2 proteins from a wide range of species (Fig. 2A), but no TNKS-binding site could be detected in APC1 proteins. Y2H studies confirmed that hAPC2 binds both hTNKS1 and hTNKS2 (Fig. 2F). Surprisingly, a TNKS-binding motif appears to be absent in APC2 from some non-mammalian vertebrates (chicken, frog, zebrafish); however, this is also true for a number of known TNKS-binding proteins such as TRF1, NuMA, and IRAP, at least some of which still interact with TNKS in these species (26). In addition to a consensus TNKS binding site, some vertebrate APC2 proteins contain a second putative TNKS binding motif that appears to match the non-consensus TNKS binding site identified in Axin (Fig. 2A). Consistent with the apparent lack of a TNKS motif in APC1 proteins, we were unable to detect a direct interaction between fragments of hAPC1 and TNKS in Y2H studies (Fig. 2G). It has been previously reported that hTNKS1 coIPs with hAPC1 (27), suggesting that either TNKS binds APC1 via a non-consensus motif we were unable to identify, or that TNKS is in a complex with APC1 but lacks direct binding.
APC2 Is a Novel TNKS Substrate
TNKS proteins are PARP enzymes that stimulate the covalent addition of Poly ADP-ribose (PAR) chains onto substrate proteins. To test the hypothesis that APC2 is a TNKS substrate, we utilized an ELISA based assay to detect PARylation of recombinant proteins. PARylation of both Drosophila Axin and APC2 was readily detected in this assay, but no PARylation was observed for BSA as a negative control (Fig. 2E). Addition of the TNKS inhibitor XAV939 also decreased Axin and APC2 PARylation, demonstrating the specificity of the assay. Together, these studies have identified a novel protein interaction between APC2 and TNKS, and further demonstrated that APC2 is a direct TNKS substrate.
APC2 Can Recruit TNKS into the β-Catenin Destruction Complex
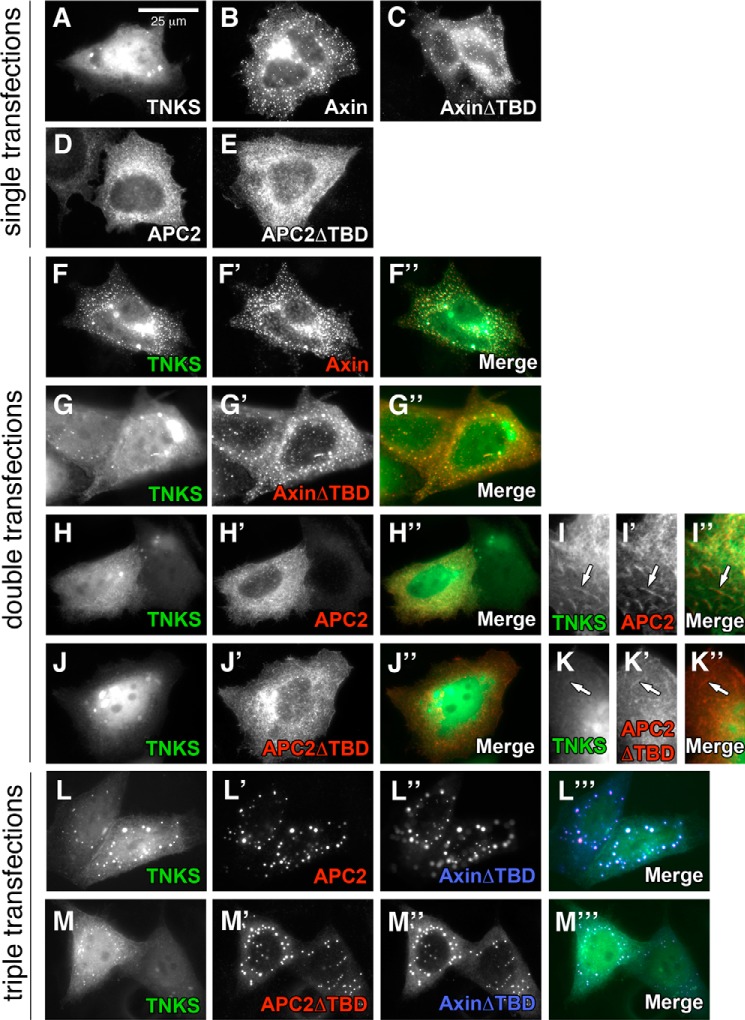
To begin to assess if the APC2/TNKS interaction impacts Wnt signaling, we first investigated whether TNKS localizes to the βcat destruction complex. When expressed in cultured cells, Axin forms pronounced cytoplasmic puncta (called degradasomes) (28), which are believed to represent functional destruction complexes (19). We therefore sought to determine if TNKS can be recruited into Axin degradasomes. SW480 cells were co-transfected with Drosophila GFP-TNKS, Flag-Axin, and/or V5-APC2 individually or in combinations. When expressed alone, GFP-TNKS was diffuse throughout the cytosol with some nuclear enrichment (Fig. 3A); however, when co-transfected with Flag-Axin it nearly completely co-localized with Axin puncta, demonstrating Axin-mediated recruitment of TNKS into degradasomes (Fig. 3F). Deletion of the Tankyrase binding domain on Axin (AxinΔTBD) greatly reduced TNKS recruitment into degradasomes (Fig. 3G). Importantly, recruitment of human TNKS into Axin degradasomes has also been recently reported (29).
FIGURE 3.
APC2 can recruit TNKS into Axin degradasomes. SW480 cells were transiently transfected with epitope-tagged versions of Drosophila APC2, Axin, and TNKS and localization assessed by immunofluorescence. A–E, individually expressed proteins. TNKS has diffuse localization in both the cytosol and nucleus (A), whereas Axin and AxinΔTBD form discrete cytosolic puncta (B, C). APC2 and APC2ΔTBD are largely nuclear excluded and have a fibrous, cytoskeletal localization (D, E). F–K, TNKS co-expressed with Axin (F), AxinΔTBD (G), APC2 (H, I), or APC2ΔTBD (J, K). When co-expressed, TNKS co-localizes with Axin puncta (F), and this localization was greatly reduced when TNKS was co-expressed with AxinΔTBD (G). When expressed with APC2, nuclear TNKS signal was diminished (H) and co-localization of TNKS and APC2 on cytoskeletal filaments could be observed (I, arrow). L and M, triple transfected SW480s expressing TNKS, AxinΔTBD, and either APC2 (L) or APC2ΔTBD (M). TNKS co-localization in Axin degradasomes was observed with full-length APC2 (L), and this was diminished with APC2ΔTBD (M). Scale bar, 25 μm.
Given that TNKS binds both Axin and APC2, we sought to determine if APC2 and TNKS co-localize. When expressed individually, APC2 was largely nuclear excluded and had a fibrous appearance (Fig. 3D), consistent with reports that APC2 binds actin filaments (30, 31). When TNKS and APC2 were co-expressed, nuclear TNKS was diminished, and instead fibrous-looking TNKS localization could be detected in these cells that co-localized with APC2 signal (Fig. 3, H and I). Deletion of the Tankyrase binding domain on APC2 (APC2ΔTBD) greatly reduced co-localization as indicated by enriched nuclear localization of TNKS (Fig. 3J) and less TNKS on APC2 labeled filaments (Fig. 3K). These findings suggest that APC2 and TNKS can interact on actin filaments, and may have implications for a role for TNKS in cytoskeletal regulation.
To establish if APC2 can recruit TNKS into Axin degradasomes, we assessed TNKS localization in cells triple transfected with epitope-tagged TNKS, AxinΔTBD, and APC2. Importantly, APC2 was readily recruited into AxinΔTBD degradasomes, and TNKS co-localized with these APC2/AxinΔTBD degradasomes (Fig. 3L). TNKS recruitment into degradasomes was decreased, although not eliminated, by expression of the APC2ΔTBD mutant (Fig. 3M), consistent with our observation that APC2ΔTBD can still coIP residual TNKS (Fig. 2C). Overall, these studies suggest that both APC2 and Axin are able to recruit TNKS into the β-catenin destruction complex, and that the APC2/TNKS interaction may therefore impact βcat signaling.
The Tankyrase Binding Domain Is Not Required for APC2 to Rescue β-Catenin Destruction in Transfected SW480 Cells
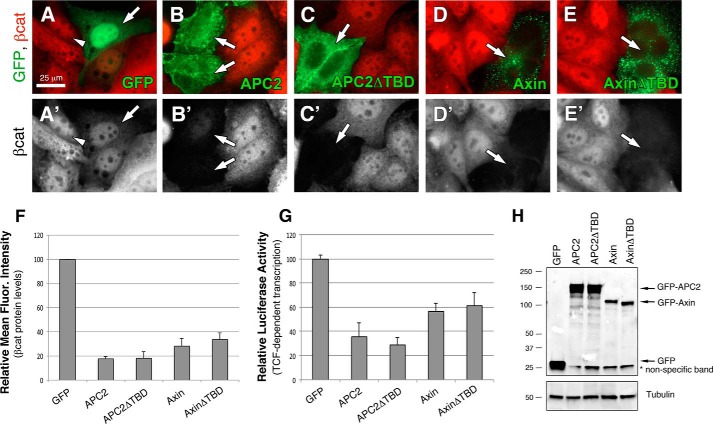
To investigate the importance of the APC2/TNKS interaction in regulating βcat signaling, we first performed functional studies in the colon cancer line SW480, which harbors a truncating mutation in APC1. SW480 cells were transfected with GFP, GFP-APC2, or GFP-APC2ΔTBD, and βcat protein levels assessed by immunofluorescence and flow cytometry. In immunofluorescence experiments, βcat levels are high in untransfected SW480 cells, and nuclear enrichment of βcat is readily detectable (Fig. 4A, arrowhead). Transfection of GFP as a negative control did not affect βcat levels or localization (Fig. 4A, arrow). However, exogenous Drosophila APC2 greatly diminished both cytosolic and nuclear βcat staining (Fig. 4B, arrows), as did APC2ΔTBD (Fig. 4C, arrow). Exogenous Axin has previously been shown to stimulate βcat destruction in SW480 cells, therefore we also expressed Drosophila Axin and AxinΔTBD as controls, both of which diminished βcat staining (Fig. 4, D and E, arrows).
FIGURE 4.
Deleting the TNKS binding site does not alter the activity of exogenous APC2 or Axin proteins. A–E, GFP-tagged APC2 or Axin constructs were transiently transfected into SW480 cells, and relative βcat protein levels assessed via immunofluorescence. Arrows indicate transfected cells, and arrowhead emphasizes nuclear βcat. F, relative mean fluorescence intensity of βcat protein in transfected SW480 cells as determined by flow cytometry. APC2ΔTBD was not significantly different than APC2, and AxinΔTBD was not statistically different than Axin in unpaired Student's t-tests (p > 0.05). G, TOPFlash reporter assay to determine the level of βcat-stimulated transcription. APC2ΔTBD was not statistically different than WT APC2 (p > 0.05), nor was AxinΔTBD different than Axin (p > 0.05). H, representative immunoblot of transfected cells from panel G. Scale bar, 25 μm.
To quantify the activity of these APC2 and Axin proteins in βcat destruction, we turned to flow cytometry. βcat protein levels were measured in transfected (GFP positive) SW480 cells and the values normalized to the GFP control (Fig. 4F). Expression of Drosophila APC2 diminished βcat protein levels to ∼20% of the GFP control, and no enhancement of βcat destruction was observed with APC2ΔTBD. This could imply that the APC2/TNKS interaction has no bearing on βcat regulation; however, the same effect was observed with Axin where TNKS is known to play an important role: Drosophila Axin reduced βcat levels to ∼30% that of the GFP control, but no enhancement was detected with AxinΔTBD (Fig. 4F). We further measured βcat-stimulated transcriptional output using the well-established TOPFlash luciferase assay (32). APC2 reduced transcriptional activity to ∼30% that of the GFP control, and APC2ΔTBD did not result in a statistically significant further enhancement (Fig. 4G). Similarly, AxinΔTBD failed to result in a further enhancement over wild type Axin, and AxinΔTBD protein did not accumulate to higher levels than Axin as would be predicted for deletion of the TBD on endogenous Axin (Fig. 4H). We reasoned that these differences may be due to protein overexpression.
The Effects of TNKS Inhibition on βcat Signaling Cannot Be Explained Solely by Axin Stabilization
We next set out to investigate if TNKS regulates βcat signaling through Axin, APC2, or both. siRNA knockdown of Axin in combination with TNKS inhibition was previously shown to diminish the effect of TNKS inhibition in SW480 cells (9), theoretically providing an experimental approach to parse the relative contribution of Axin and APC2. However, since APC2 and Axin work together in a complex, their functions are interdependent, siRNA knockdown of one results in the de facto inactivation of the other. This argues that a siRNA approach can establish that TNKS regulates the destruction complex in general, but cannot differentiate relative contributions of Axin or APC2. Therefore, to address this issue, we used an alternative approach looking at the effect of Axin or APC2 expression in combination with inhibition of endogenous TNKS.
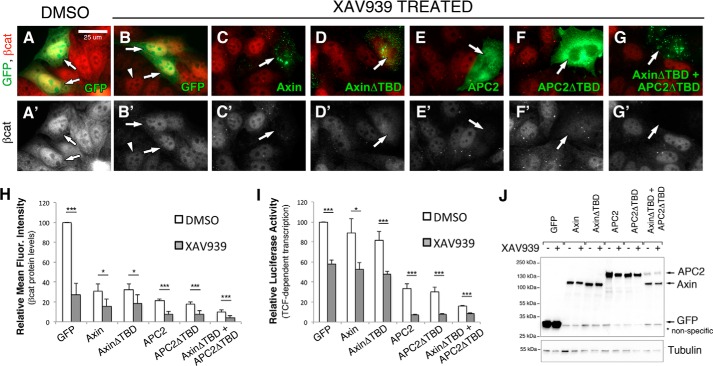
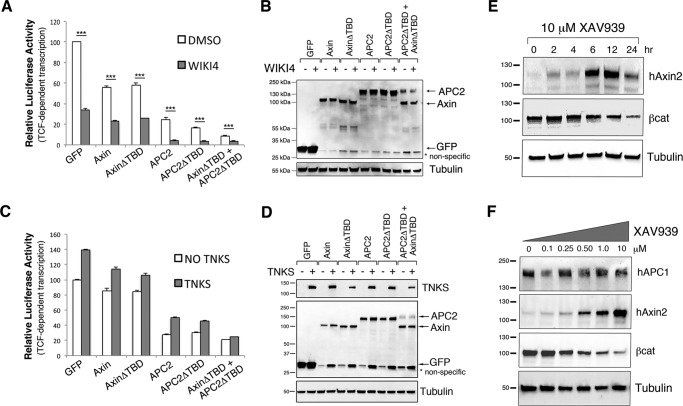
TNKS inhibition is thought to affect βcat signaling through Axin protein stabilization, so overexpression of Axin is predicted to mimic TNKS inhibition if Axin is the only component of the destruction complex regulated by TNKS. To test this hypothesis, SW480 cells were again transfected with Axin or AxinΔTBD, only this time they were additionally treated with the well-characterized TNKS inhibitor XAV939 (9, 19). We hypothesized that deletion of the TBD would obviate the effects of TNKS inhibition. In immunofluorescence studies, XAV939 treatment diminished cytosolic βcat in untransfected cells, but nuclear βcat was still detectable as previously reported (19) (Fig. 5B, arrowhead), verifying the effectiveness of our inhibitor treatment. All Axin and APC2 proteins further diminished both cytosolic and nuclear βcat following XAV939 treatment (Fig. 5, C–F).
FIGURE 5.
TNKS inhibition antagonizes destruction complex activity through an additional mechanism(s) other than Axin stabilization. A–G, SW480 cells were transiently transfected with GFP-tagged APC2 or Axin proteins, treated with DMSO (A) or the TNKS inhibitor XAV939 (B–G) for 24 h, and processed for immunofluorescence. Arrows indicate transfected cells, and arrowheads point to residual nuclear βcat staining after XAV939 treatment. H, relative mean fluorescence intensity of βcat protein levels in transfected SW480 cells treated with either XAV939 or DMSO as a control. Four independent experiments were conducted, normalized to GFP DMSO, and averaged. I, TOPFlash reporter assay to determine βcat-stimulated transcriptional output in transfected SW480 cells treated with XAV939 or DMSO as a control. XAV939 treatment further reduced βcat protein levels and transcriptional output in cells transfected with Axin, AxinΔTBD, APC2, and APC2ΔTBD (all p < 0.05). Six independent experiments were conducted, normalized to GFP DMSO, and averaged. J, representative immunoblot of transfected cells from panel H. Scale bar, 25 μm. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To quantify the effects of XAV939 treatment on βcat protein levels and transcriptional output, we again turned to flow cytometry and TOPFlash assays. In TOPFlash experiments, XAV939 alone reduced βcat transcriptional output to ∼60% that of the DMSO treated GFP control (Fig. 5I). In contrast, Axin overexpression alone reduced βcat protein levels to only ∼85%, suggesting that TNKS inhibition is more effective at reducing βcat transcriptional output than Axin overexpression (Fig. 5I, compare GFP XAV939 versus Axin DMSO). Furthermore, the combined effect of Axin overexpression and XAV939 treatment only approximated XAV939 treatment alone (Fig. 5I, compare GFP XAV939 versus Axin XAV939), suggesting that additional Axin stabilization does not enhance the effect of TNKS inhibition for transcriptional output. The same trend was observed for AxinΔTBD (which is predicted to achieve maximal Axin protein levels). No difference in Axin or AxinΔTBD protein levels were detected after XAV939 treatment by immunoblot (Fig. 5J). These trends in TOPFlash experiments were also observed using flow cytometry to measure βcat protein levels (Fig. 5H), although here the combined Axin overexpression and XAV939 treatment did display a cooperative effect (compare Axin DMSO versus Axin XAV939). Collectively, these findings argue that TNKS antagonizes destruction complex activity by an additional mechanism(s) other than destabilizing Axin protein levels. In further support of this conclusion, we observed a separation of Axin protein levels and βcat destruction when monitoring the effects of XAV939 treatment over time in SW480 cells. At 24 h of XAV939 treatment, Axin protein levels began decreasing again whereas βcat levels continued to drop (Fig. 6E), providing further evidence that XAV939 influences βcat through more than just stabilizing Axin.
FIGURE 6.
Alternative approaches to investigate the mechanism of TNKS inhibition on βcat signaling. A, TOPFlash reporter assay to determine βcat-stimulated transcriptional output in transfected SW480 cells treated with WIKI4 or DMSO as a control. WIKI4 treatment further reduced βcat transcriptional output in cells transfected with Axin, AxinΔTBD, APC2, and APC2ΔTBD (all p < 0.001). B, representative immunoblot from lysates from panel A. C, TOPFlash assay measuring the effects of TNKS overexpression on transfected SW480 cells. The same trend was observed in three independent experiments. D, representative immunoblot from lysates from panel C. E, immunoblot detecting Axin2 and βcat levels in SW480 cells treated with 10 μm XAV939 for different time durations up to 24 h. F, immunoblot detecting APC1, Axin2, and βcat levels in SW480 cells treated with increasing concentrations of XAV939 for 24 h. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
TNKS Antagonizes Destruction Complex Activity through Both Axin and APC2
Given our finding that TNKS also binds and PARylates APC2, we hypothesized that the additional effect of XAV939 treatment could involve APC2. To test this hypothesis, we treated APC2 or APC2ΔTBD transfected cells with XAV939 and assessed βcat outputs as before. In APC2 and APC2ΔTBD transfected cells, XAV939 treatment further reduced βcat protein levels (Fig. 5H) and transcriptional output (Fig. 5I). If TNKS antagonizes the destruction complex through PARylation of both Axin and APC2, then co-expression of AxinΔTBD and APC2ΔTBD should be impervious to TNKS inhibition. Indeed, a further reduction in βcat protein levels (Fig. 5H) and transcriptional output (Fig. 5I) was observed; however, a statistically significant difference between DMSO- or XAV939-treated cells transfected with AxinΔTBD and APC2ΔTBD was still measured, suggesting that additional unknown components are also likely involved. As a side-note, we repeatedly observed that APC2 protein levels were reduced when co-expressed with Axin regardless of XAV939 treatment (Figs. 5J and 6, B and D) consistent with a prior report that Axin promotes APC ubiquitination and destruction (33).
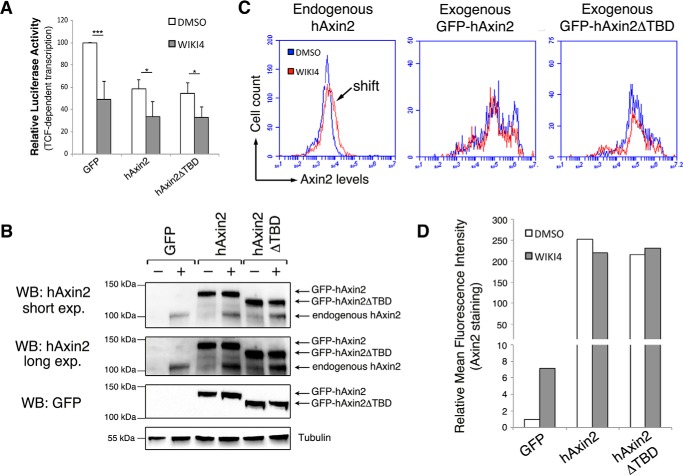
To ensure that the observed effects of XAV939 treatment relate to TNKS inhibition, we performed parallel experiments using the structurally distinct TNKS inhibitor WIKI4 (Fig. 6, A and B) (34, 35), and also performed the complimentary experiment of co-expressing TNKS in Axin or APC2 transfected cells and monitoring βcat transcriptional activity (Fig. 6, C and D). Our findings were consistent with those with XAV939 treatment. Additionally, we conducted TOPFlash experiments with human Axin2 in combination with TNKS inhibition (Fig. 7, A and B) and used this as a means to quantify the relative abundance of endogenous versus overexpressed hAxin2 protein using flow cytometry. These studies revealed that TNKS inhibition elevates endogenous hAxin2 ∼7-fold, whereas overexpressed hAxin2 is nearly 250-fold higher (Fig. 7, C and D), arguing against the likelihood of simple additive effects. Collectively, these studies further support the idea that TNKS inhibition involves mechanisms in addition to Axin stabilization.
FIGURE 7.
Quantification of endogenous and exogenous Axin. A, TOPFlash reporter activity was measured in SW480 cells transfected with hAxin2 and treated with DMSO or WIKI4. WIKI4 treatment further reduced βcat transcriptional output in cells transfected with hAxin2 and hAxin2ΔTBD (both p < 0.05). B, representative immunoblot of hAxin2 from lysates from panel A. Densitometry analysis indicated that endogenous Axin2 levels increase ∼7-fold following WIK4 treatment. Saturation of the signal prevented reliable measurement of exogenous Axin2 levels. C, flow cytometry was used to measure the mean fluorescence intensity of hAxin2 in SW480 cells treated with DMSO or WIKI4 and additionally transfected with GFP, GFP-hAxin2, or GFP- hAxin2ΔTBD. A shift in the mean fluorescence intensity of GFP-transfected cells treated with DMSO versus WIKI4 is indicative of endogenous hAxin2 stabilization. Exogenous hAxin2 levels were ∼250-fold higher than endogenous Axin2. D, graphical representation of the average mean fluorescence intensity in panel C across three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
The Effects of TNKS Inhibition on βcat Signaling May Be Partially GSK3 Independent
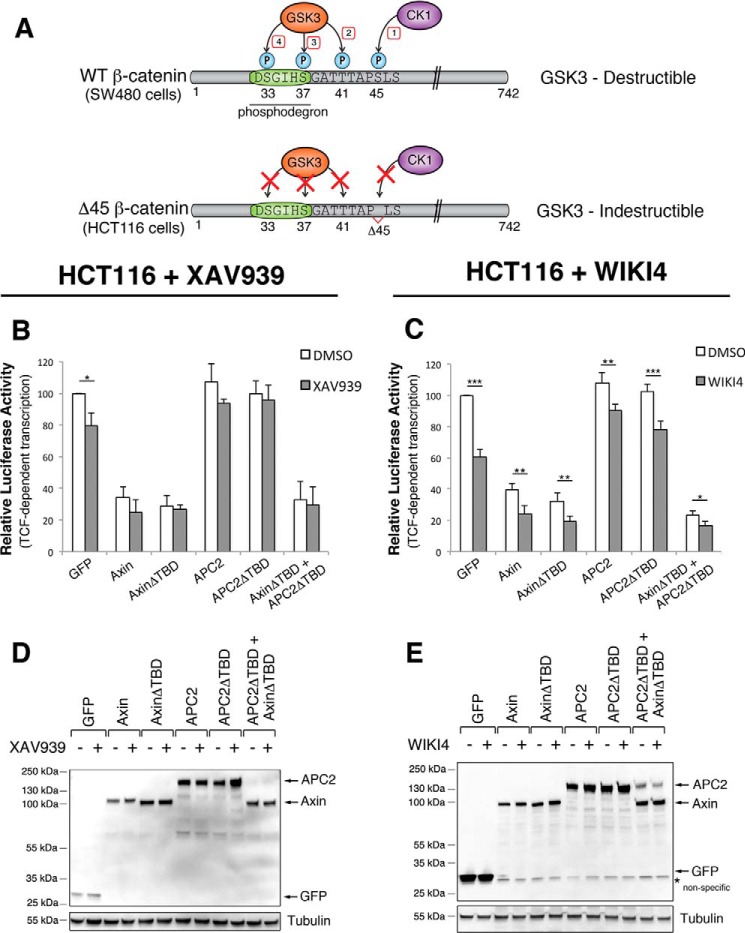
Our studies on XAV939 treated cells doubly transfected with AxinΔTBD and APC2ΔTBD (Figs. 5, H, I, and 6A) suggest that TNKS inhibition may operate via an additional mechanism that is APC and Axin independent. We wondered if this mechanism involved GSK3, as GSK3 independent mechanisms of βcat destruction have been reported (36, 37). To test this hypothesis, we turned to HCT116 cells, which are a colorectal cancer line expressing GSK3-indestructible βcat (Fig. 8A) (38, 39). TOPFlash experiments with XAV939 (Fig. 8, B and D) or WIKI4 (Fig. 8, C and E) treatment revealed that HCT116 cells are sensitive to TNKS inhibition as previously reported (18). A statistically significant difference was also observed in DMSO versus WIKI4-treated HCT116s co-transfected with both AxinΔTBD and APC2ΔTBD (Fig. 8C), arguing that the additional mechanism could be GSK3 independent.
FIGURE 8.
TNKS inhibition may partially impact βcat signaling through a GSK3-independent mechanism. A, schematic of the βcat phosphodegron in SW480 and HCT116 cells. HCT116 cells contain a hyperactive βcat with Ser-45 deleted, which is the CK1 priming site for GSK3 phosphorylation of the phosphodegron. B and C, TOPFlash reporter assays in HCT116 cells treated with (B) XAV939 or (C) WIKI4. HCT116 cells are sensitive to TNKS inhibition, indicating that at least some of the effect of TNKS inhibition on βcat signaling is independent of GSK3 phosphorylation of the phosphodegron. D and E, representative immunoblots of the experiments presented in panels B and C. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Blocking the Interaction with TNKS Does Not Globally Alter APC2 Protein Levels in Drosophila Embryos
Several studies have established that PARylation of Axin by TNKS promotes an interaction with the E3 ubiquitin ligase RNF146, thus resulting in ubiquitination and degradation of Axin (10–13). We hypothesized that TNKS binding to APC2 could mediate a similar response when APC2 is expressed at a physiological level. To test this hypothesis, we first treated SW480 cells with increasing concentrations of XAV939 and monitored protein levels by immunoblot. While Axin2 levels increased and βcat levels decreased as expected (Fig. 6F), we were unable to identify an antibody against human APC2 that detected a band at the appropriate molecular weight. Interestingly, human APC1 levels were unaffected by XAV939 treatment, consistent with the lack of a detectable Tankyrase binding motif in APC1 (Fig. 6F).
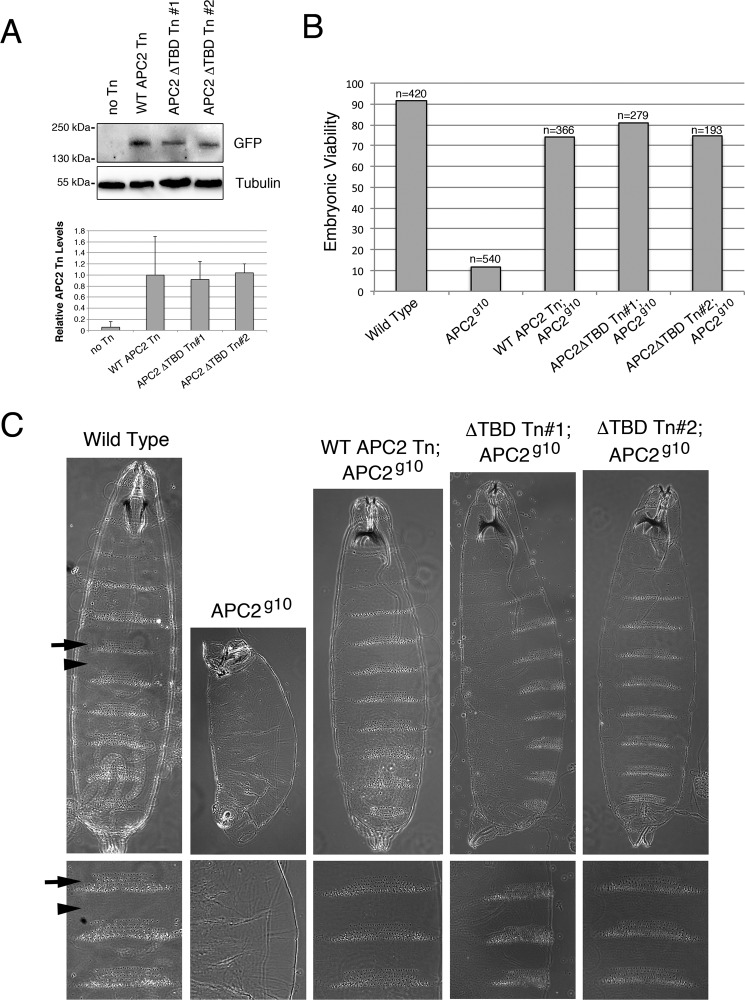
As an alternative approach to monitor APC2 protein levels, we generated transgenic flies expressing either GFP-APC2 or GFP-APC2ΔTBD. In both instances, APC2 proteins were driven by the endogenous APC2 promoter and transgenic lines made by the PhiC31 approach (which targets transgenes to the same genomic location thereby avoiding issues associated with position affect variation). We previously established that this approach results in consistent transgene expression that approximates endogenous APC2 levels (20). We monitored APC2 and APC2ΔTBD transgene levels in Drosophila embryos by immunoblot, and quantified APC2 protein levels by normalizing to tubulin. These studies revealed no alteration in APC2ΔTBD protein levels from two different transgenic lines as compared with the wild type APC2 transgene (Fig. 9A). Thus PARylation does not appear to globally destabilize APC2, although one limitation of this experiment is that PARylation and its effects could be cell type specific as has been previously reported for different colorectal cell lines (14).
FIGURE 9.
The APC2/TNKS interaction is not required for proper Wnt regulation in the Drosophila embryo. Transgenic flies were generated expressing GFP-tagged APC2 or APC2ΔTBD at the same genomic location using the PhiC31 approach. Transgenes were driven by the endogenous APC2 promoter to mimic physiological expression levels. A, immunoblot of lysates from transgenic embryos comparing protein levels between APC2 and APC2ΔTBD. GFP signal was quantified by densitometry and averaged across three independent experiments. B, embryonic viability of APC2 mutant Drosophila embryos expressing APC2 transgenes as determined by the number of fertilized eggs that hatch into larvae. C, cuticle analysis of Drosophila APC2 or APC2ΔTBD transgenes expressed in the APC2g10 maternal/zygotic mutant background. Arrows, denticle bands. Arrowheads, naked cuticle.
The APC2/TNKS Interaction Is Not Required for Wnt Regulation in Drosophila Embryos
APC2ΔTBD transgenic fly lines also gave us the ability to evaluate the importance of the APC2/TNKS interaction when all proteins are expressed at physiological levels. We therefore crossed the APC2 and APC2ΔTBD transgenes into the APC2-null background (APC2g10) and assessed rescue of APC2 mutant phenotypes. Maternally/zygotically-null APC2 mutants are embryonic lethal and display phenotypes consistent with hyperactive Wg/Wnt signaling including denticle patterning of the embryonic ventral epidermis. In these experiments, transgenic APC2ΔTBD behaved like wild type APC2 as APC2ΔTBD rescued embryonic viability to a similar rate as wild type APC2 (Fig. 9B), and cuticle patterning appeared completely normal in these animals (Fig. 9C). This indicates that the APC2/TNKS interaction is not required to regulate βcat signaling at a physiological level in the Drosophila embryo, and is consistent with a recent report that TNKS null flies are adult viable with no obvious Wg/Wnt activation phenotypes (40). Thus the importance of TNKS regulation of the destruction complex appears to be context dependent, with the human colon epithelium potentially being uniquely sensitive to TNKS activity.
Discussion
Inhibition of the PARP enzyme TNKS is garnering attention as a promising colon cancer therapeutic that blunts oncogenic βcat signaling through stabilization of Axin (41, 42). TNKS normally functions to promote Axin degradation through direct PARylation and subsequent ubiquitination of Axin by the PAR-dependent E3 ubiquitin ligase RNF146. Axin is thought to be the limiting component of the βcat destruction complex (43), so Axin stabilization is predicted to result in an increased number of functional destruction complexes that stimulate rapid βcat turnover. Somewhat surprisingly, TNKS inhibition can effectively reduce βcat protein levels in colon cancer cell lines with truncating mutations in APC, prompting the question of how increased Axin levels can promote βcat destruction in the absence of functional APC? It seems plausible that the answer may lie in the fact that colon cancer cells do not lack full APC activity, but rather express full-length APC2 in addition to truncated APC1. Overexpression of exogenous human APC2 in colon cancer cells rescues βcat destruction and reduces oncogenic signaling in SW480 cells (44), suggesting that APC2 levels or activity is somehow limiting in colorectal cancer. Moreover, overexpressed Axin fails to rescue Wg/Wnt activation phenotypes in Drosophila embryos null for APC activity (28), further supporting the idea that over-expressed Axin cooperates with residual APC activity to abate oncogenic signaling in colon cancer cells. Here we provide further mechanistic insight into the effects of TNKS inhibition on Wnt signaling, suggesting that additional mechanism(s) are involved other than Axin stabilization.
In this work, we identified TNKS as a novel binding partner of Drosophila APC2. Both Drosophila and human APC2 have a consensus TNKS binding motif that is necessary and sufficient for TNKS binding in Y2H and GST-pulldown studies (Fig. 2). Importantly, we show that TNKS stimulates PARylation of APC2 (Fig. 2E), and that APC2 can recruit TNKS into Axin degradasomes (Fig. 3, L and M). These findings argue that the APC2/TNKS interaction could have a physiologically relevant impact on Wnt signaling regulation. To test this hypothesis, we investigated the relative contribution of Axin and/or APC2 on βcat signaling upon TNKS inhibition. We reasoned that an siRNA approach to answer this question would be difficult to interpret since prior studies have reported difficulty in achieving limiting levels of APC via siRNA knockdown (44, 45), and because the function of Axin and APC are interdependent (meaning that knockdown of one impacts the other). Instead, we evaluated the relative importance of Axin and APC2 upon inhibition of endogenous TNKS.
Overexpression of Axin is predicted to mimic TNKS inhibition if the sole consequence of TNKS activity on the destruction complex is to stabilize Axin. However, we instead observed that XAV939 could further decrease βcat signaling in cells overexpressing Axin (Fig. 5I, 6A, 7A). This suggests that TNKS antagonizes destruction complex function through an additional mechanism(s) that operates in conjunction with Axin destabilization. Complementary results were obtained using a similar approach in which we overexpressed TNKS (Fig. 6, C and D). Collectively, these findings argue that TNKS modulates βcat signaling through more than Axin protein stability. We suggest this additional role at least partially involves PARylation of APC2, as co-expression of AxinΔTBD and APC2ΔTBD reduces the effects of TNKS inhibition or overexpression (Fig. 5, H and I). However, a remaining statistically significant difference suggests that additional mechanism(s) are also likely involved.
A GSK3-independent mechanism of βcat destruction has been previously reported (36, 37). Therefore, we investigated if the additional mechanism of TNKS inhibition was independent of GSK3-mediated phosphorylation of βcat's phosphodegron. HCT116 cells were sensitive to the TNKS inhibitors XAV939 and WIKI4, arguing that some of the effect of TNKS inhibition on βcat signaling could be GSK3 independent (Fig. 8). One mechanism to destroy βcat independent of GSK3-phosphorylation involves the E3-ubiquitin ligase Siah. In the future, it will be interesting to investigate if Siah contributes to the observed effect of TNKS inhibition, especially since Siah up-regulation in HCT116 cells is associated with increased βcat destruction (46).
Our work comparing APC2 and APC2ΔTBD transgenes in flies revealed that APC2ΔTBD protein did not accumulate to higher levels than APC2 (Fig. 9A), suggesting that TNKS does not globally destabilize APC2. Similarly no differences between APC2 and APC2ΔTBD were detected when assessing rescue of Wnt activation phenotypes in APC2 null mutant embryos (Fig. 9, B and C), consistent with a recent report that TNKS null flies are adult viable with no Wg/Wnt phenotypes (40). Thus TNKS regulation of destruction complex activity does not appear to be a universally important mechanism in all cell types, but may be uniquely critical in the colon epithelium. Future studies are ongoing to directly test this hypothesis.
How TNKS antagonizes destruction complex activity through PARylation of APC2 is still an open question. Although we were unable to detect global APC2 stabilization in Drosophila embryos, it is possible that stabilization occurs specifically in colon epithelial cells, which would suggest that TNKS may inactivate the destruction complex through simultaneous destabilization of Axin and APC2. In colorectal cancer, this would imply that TNKS inhibition could blunt oncogenic βcat signaling through up-regulation of human APC2 above a hypothetical threshold. It also remains possible that the effect of APC2 PARylation on Wnt signaling is independent of APC2 protein stabilization. In fact, co-expression of AxinΔTBD and APC2ΔTBD promoted an even greater decrease in βcat protein levels and transcriptional output than overexpression of APC2ΔTBD itself (Fig. 5, H and I), even though APC2ΔTBD protein levels were greatly diminished when co-expressed with AxinΔTBD (Fig. 5J). So perhaps APC2 protein levels are not critical for the effect. Alternatively, RNF146 has been demonstrated to stimulate K11, K48, and K63 ubiquitination on target proteins (10), and prior work established that human APC1 receives both K48 and K63 ubiquitination, with K63 being predominant (47). We have verified that Drosophila APC2 is also K48 and K63 ubiquitinated when expressed in SW480 cells,4 suggesting that TNKS could promote K63 ubiquitination of APC2 as a regulatory mechanism that antagonizes destruction complex activity. Investigating these and other possibilities further will be critical to elucidate the full therapeutic value of TNKS inhibition in colon cancer.
Author Contributions
H. E. C. and J. G. performed initial yeast two-hybrid work for the screen that identified TNKS. C. N. F. mapped the TNKS binding site on APC2. P. R. and R. V. K. investigated TNKS recruitment into degradasomes. RJY performed GST pulldowns. C. N. F., S. C., and C. M. P. conducted Y2H investigating binding of human TNKS and APC/APC2. A. V. A. F. and K. D. J. performed the PARylation assays. A. M. T. performed co-IPs. H. H. A. and B. D. G. did TOPFlash and flow cytometry experiments in SW480 cells. B. K. D. provided intellectual contributions and reagents. K. D. J. provided technical assistance on several experiments. D. M. R. conceived the idea for the project, performed several experiments, analyzed all data, and wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank M. Peifer for helpful comments on the manuscript and R. Moon and H. Clevers for reagents. The initial yeast two-hybrid screen was performed by Hybrigenics, Paris, France.
This work was supported by National Institutes of Health Grant R15 GM107796 (to D. M. R.), startup funds from Franklin & Marshall College (to D. M. R.), and an Undergraduate Science Education Award from the Howard Hughes Medical Institute to Franklin & Marshall College (52007538). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
J. Tran and D. Roberts, unpublished data.
- βcat
- β-catenin
- APC
- adenomatous polyposis coli
- PARP
- poly(ADP-ribose) polymerase
- TNKS
- Tankyrase
- Y2H
- yeast two-hybrid.
References
- 1.Clevers H., and Nusse R. (2012) Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 [DOI] [PubMed] [Google Scholar]
- 2.Cadigan K. M., and Peifer M. (2009) Wnt signaling from development to disease: insights from model systems. Cold Spring Harbor Perspect. Biol. 1, a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stamos J. L., and Weis W. I. (2013) The β-catenin destruction complex. Cold Spring Harbor Perspect. Biol. 5, a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polakis P. (2012) Wnt signaling in cancer. Cold Spring Harbor Perspect. Biol. 4, a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholer-Dahirel A., Schlabach M. R., Loo A., Bagdasarian L., Meyer R., Guo R., Woolfenden S., Yu K. K., Markovits J., Killary K., Sonkin D., Yao Y. M., Warmuth M., Sellers W. R., Schlegel R., Stegmeier F., Mosher R. E., and McLaughlin M. E. (2011) Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. U.S.A. 108, 17135–17140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado E. R., Yang J., So J., Leimgruber S., Kahn M., Ishitani T., Shin D., Mustata Wilson G., and Monga S. P. (2014) Identification and characterization of a novel small-molecule inhibitor of β-catenin signaling. Am. J. Pathol. 184, 2111–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emami K. H., Nguyen C., Ma H., Kim D. H., Jeong K. W., Eguchi M., Moon R. T., Teo J. L., Oh S. W., Kim H. Y., Moon S. H., Ha J. R., and Kahn M. (2004) A small molecule inhibitor of β-catenin/CREB-binding protein transcription [corrected]. Proc. Natl. Acad. Sci. U.S.A. 101, 12682–12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen B., Dodge M. E., Tang W., Lu J., Ma Z., Fan C. W., Wei S., Hao W., Kilgore J., Williams N. S., Roth M. G., Amatruda J. F., Chen C., and Lum L. (2009) Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S. M., Mishina Y. M., Liu S., Cheung A., Stegmeier F., Michaud G. A., Charlat O., Wiellette E., Zhang Y., Wiessner S., Hild M., Shi X., Wilson C. J., Mickanin C., Myer V., Fazal A., Tomlinson R., Serluca F., Shao W., Cheng H., Shultz M., Rau C., Schirle M., Schlegl J., Ghidelli S., Fawell S., Lu C., Curtis D., Kirschner M. W., Lengauer C., Finan P. M., Tallarico J. A., Bouwmeester T., Porter J. A., Bauer A., and Cong F. (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620 [DOI] [PubMed] [Google Scholar]
- 10.Callow M. G., Tran H., Phu L., Lau T., Lee J., Sandoval W. N., Liu P. S., Bheddah S., Tao J., Lill J. R., Hongo J. A., Davis D., Kirkpatrick D. S., Polakis P., and Costa M. (2011) Ubiquitin ligase RNF146 regulates tankyrase and Axin to promote Wnt signaling. PloS one 6, e22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DaRosa P. A., Wang Z., Jiang X., Pruneda J. N., Cong F., Klevit R. E., and Xu W. (2015) Allosteric activation of the RNF146 ubiquitin ligase by a poly(ADP-ribosyl)ation signal. Nature 517, 223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Liu S., Mickanin C., Feng Y., Charlat O., Michaud G. A., Schirle M., Shi X., Hild M., Bauer A., Myer V. E., Finan P. M., Porter J. A., Huang S. M., and Cong F. (2011) RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nat. Cell Biol. 13, 623–629 [DOI] [PubMed] [Google Scholar]
- 13.Zhou Z. D., Chan C. H., Xiao Z. C., and Tan E. K. (2011) Ring finger protein 146/Iduna is a poly(ADP-ribose) polymer binding and PARsylation dependent E3 ubiquitin ligase. Cell Adh. Migr. 5, 463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau T., Chan E., Callow M., Waaler J., Boggs J., Blake R. A., Magnuson S., Sambrone A., Schutten M., Firestein R., Machon O., Korinek V., Choo E., Diaz D., Merchant M., Polakis P., Holsworth D. D., Krauss S., and Costa M. (2013) A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 73, 3132–3144 [DOI] [PubMed] [Google Scholar]
- 15.Behrens J., Jerchow B. A., Wurtele M., Grimm J., Asbrand C., Wirtz R., Kühl M., Wedlich D., and Birchmeier W. (1998) Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 280, 596–599 [DOI] [PubMed] [Google Scholar]
- 16.Bao R., Christova T., Song S., Angers S., Yan X., and Attisano L. (2012) Inhibition of tankyrases induces Axin stabilization and blocks Wnt signalling in breast cancer cells. PloS one 7, e48670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casás-Selves M., Kim J., Zhang Z., Helfrich B. A., Gao D., Porter C. C., Scarborough H. A., Bunn P. A. Jr., Chan D. C., Tan A. C., and DeGregori J. (2012) Tankyrase and the canonical Wnt pathway protect lung cancer cells from EGFR inhibition. Cancer Res. 72, 4154–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waaler J., Machon O., Tumova L., Dinh H., Korinek V., Wilson S. R., Paulsen J. E., Pedersen N. M., Eide T. J., Machonova O., Gradl D., Voronkov A., von Kries J. P., and Krauss S. (2012) A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 72, 2822–2832 [DOI] [PubMed] [Google Scholar]
- 19.de la Roche M., Ibrahim A. E., Mieszczanek J., and Bienz M. (2014) LEF1 and B9L shield beta-catenin from inactivation by Axin, desensitizing colorectal cancer cells to tankyrase inhibitors. Cancer Res. 74, 1495–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts D. M., Pronobis M. I., Poulton J. S., Waldmann J. D., Stephenson E. M., Hanna S., and Peifer M. (2011) Deconstructing the sscatenin destruction complex: mechanistic roles for the tumor suppressor APC in regulating Wnt signaling. Mol. Biol. Cell 22, 1845–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamulla R. J., Kane E. G., Moody A. E., Politi K. A., Lock N. E., Foley A. V., and Roberts D. M. (2014) Testing models of the APC tumor suppressor/β-catenin interaction reshapes our view of the destruction complex in Wnt signaling. Genetics 197, 1285–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunttas-Tatli E., Roberts D. M., and McCartney B. M. (2014) Self-association of the APC tumor suppressor is required for the assembly, stability, and activity of the Wnt signaling destruction complex. Mol. Biol. Cell 25, 3424–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pronobis M. I., Rusan N. M., and Peifer M. (2015) A novel GSK3-regulated APC:Axin interaction regulates Wnt signaling by driving a catalytic cycle of efficient β-catenin destruction. Elife 4, e08032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riffell J. L., Lord C. J., and Ashworth A. (2012) Tankyrase-targeted therapeutics: expanding opportunities in the PARP family. Nat. Rev. Drug Discov. 11, 923–936 [DOI] [PubMed] [Google Scholar]
- 25.Morrone S., Cheng Z., Moon R. T., Cong F., and Xu W. (2012) Crystal structure of a Tankyrase-Axin complex and its implications for Axin turnover and Tankyrase substrate recruitment. Proc. Natl. Acad. Sci. U.S.A. 109, 1500–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsiao S. J., and Smith S. (2008) Tankyrase function at telomeres, spindle poles, and beyond. Biochimie 90, 83–92 [DOI] [PubMed] [Google Scholar]
- 27.Tran H., Bustos D., Yeh R., Rubinfeld B., Lam C., Shriver S., Zilberleyb I., Lee M. W., Phu L., Sarkar A. A., Zohn I. E., Wertz I. E., Kirkpatrick D. S., and Polakis P. (2013) HectD1 E3 ligase modifies adenomatous polyposis coli (APC) with polyubiquitin to promote the APC-axin interaction. J. Biol. Chem. 288, 3753–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendoza-Topaz C., Mieszczanek J., and Bienz M. (2011) The Adenomatous polyposis coli tumour suppressor is essential for Axin complex assembly and function and opposes Axin's interaction with Dishevelled. Open Biol. 1, 110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorvaldsen T. E., Pedersen N. M., Wenzel E. M., Schultz S. W., Brech A., Liestol K., Waaler J., Krauss S., and Stenmark H. (2015) Structure, dynamics, and functionality of Tankyrase inhibitor-induced degradasomes. Mol. Cancer Res. 13, 1487–1501 [DOI] [PubMed] [Google Scholar]
- 30.Yu X., and Bienz M. (1999) Ubiquitous expression of a Drosophila adenomatous polyposis coli homolog and its localization in cortical actin caps. Mech. Dev. 84, 69–73 [DOI] [PubMed] [Google Scholar]
- 31.McCartney B. M., Dierick H. A., Kirkpatrick C., Moline M. M., Baas A., Peifer M., and Bejsovec A. (1999) Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J. Cell Biol. 146, 1303–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., and Clevers H. (1997) Constitutive transcriptional activation by a β-catenin-Tcf complex in APC-/- colon carcinoma. Science 275, 1784–1787 [DOI] [PubMed] [Google Scholar]
- 33.Choi J., Park S. Y., Costantini F., Jho E. H., and Joo C. K. (2004) Adenomatous polyposis coli is down-regulated by the ubiquitin-proteasome pathway in a process facilitated by Axin. J. Biol. Chem. 279, 49188–49198 [DOI] [PubMed] [Google Scholar]
- 34.Haikarainen T., Venkannagari H., Narwal M., Obaji E., Lee H. W., Nkizinkiko Y., and Lehtiö L. (2013) Structural basis and selectivity of tankyrase inhibition by a Wnt signaling inhibitor WIKI4. PloS one 8, e65404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James R. G., Davidson K. C., Bosch K. A., Biechele T. L., Robin N. C., Taylor R. J., Major M. B., Camp N. D., Fowler K., Martins T. J., and Moon R. T. (2012) WIKI4, a novel inhibitor of tankyrase and Wnt/ss-catenin signaling. PloS one 7, e50457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J., Stevens J., Rote C. A., Yost H. J., Hu Y., Neufeld K. L., White R. L., and Matsunami N. (2001) Siah-1 mediates a novel β-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol. Cell 7, 927–936 [DOI] [PubMed] [Google Scholar]
- 37.Matsuzawa S. I., and Reed J. C. (2001) Siah-1, SIP, and Ebi collaborate in a novel pathway for β-catenin degradation linked to p53 responses. Mol. Cell 7, 915–926 [DOI] [PubMed] [Google Scholar]
- 38.Liu C., Li Y., Semenov M., Han C., Baeg G. H., Tan Y., Zhang Z., Lin X., and He X. (2002) Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837–847 [DOI] [PubMed] [Google Scholar]
- 39.Amit S., Hatzubai A., Birman Y., Andersen J. S., Ben-Shushan E., Mann M., Ben-Neriah Y., and Alkalay I. (2002) Axin-mediated CKI phosphorylation of β-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 16, 1066–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng Y., Li X., Ray L., Song H., Qu J., Lin S., and Lin X. (2014) The Drosophila tankyrase regulates Wg signaling depending on the concentration of Daxin. Cell Signal. 26, 1717–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fearon E. R. (2009) PARsing the phrase “all in for Axin”- Wnt pathway targets in cancer. Cancer Cell 16, 366–368 [DOI] [PubMed] [Google Scholar]
- 42.Haikarainen T., Krauss S., and Lehtio L. (2014) Tankyrases: structure, function and therapeutic implications in cancer. Curr. Pharm. Des. 20, 6472–6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee E., Salic A., Krüger R., Heinrich R., and Kirschner M. W. (2003) The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLos Biol. 1, E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneikert J., Vijaya Chandra S. H., Ruppert J. G., Ray S., Wenzel E. M., and Behrens J. (2013) Functional comparison of human adenomatous polyposis coli (APC) and APC-like in targeting β-catenin for degradation. PloS one 8, e68072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandra S. H., Wacker I., Appelt U. K., Behrens J., and Schneikert J. (2012) A common role for various human truncated adenomatous polyposis coli isoforms in the control of β-catenin activity and cell proliferation. PloS one 7, e34479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gwak J., Song T., Song J. Y., Yun Y. S., Choi I. W., Jeong Y., Shin J. G., and Oh S. (2009) Isoreserpine promotes β-catenin degradation via Siah-1 up-regulation in HCT116 colon cancer cells. Biochem. Biophys. Res. Commun. 387, 444–449 [DOI] [PubMed] [Google Scholar]
- 47.Tran H., and Polakis P. (2012) Reversible modification of adenomatous polyposis coli (APC) with K63-linked polyubiquitin regulates the assembly and activity of the β-catenin destruction complex. J. Biol. Chem. 287, 28552–28563 [DOI] [PMC free article] [PubMed] [Google Scholar]