Abstract
Podocytes are key components of the kidney blood filtration barrier, and their ability to withstand hemodynamic strain is proposed to be closely tied to their unique and flexible cytoarchitecture. However, the mechanisms that control such mechanotransduction are poorly understood. We have previously established that tyrosine phosphorylation of the transmembrane protein nephrin promotes recruitment of the Nck1/2 cytoskeletal adaptor proteins and downstream actin remodeling. We now reveal that Nck integrates nephrin with the Hippo kinase cascade through association with the adaptor protein WTIP. Using mutational analysis, we show that Nck sequesters WTIP and its binding partner Lats1 to phosphorylated nephrin, resulting in decreased phospho-activation of Lats1. We further demonstrate that, coincident with nephrin dephosphorylation in a transient model of podocyte injury in mice, Lats1 is rapidly activated, and this precedes significant down-regulation of the transcription regulator Yap. Moreover, we show reduced levels of Yap protein in mice with chronic disruption of nephrin phospho-signaling. Together, these findings support the existence of a dynamic molecular link between nephrin signaling and the canonical Hippo pathway in podocytes, which may facilitate the conversion of mechanical cues to biochemical signals promoting podocyte viability.
Keywords: Hippo pathway, kidney, phosphotyrosine, podocyte, signal transduction, Nck, WTIP, nephrin
Introduction
Podocytes are specialized terminally differentiated epithelial cells of the kidney blood filtration barrier (1). Their cell bodies extend interdigitating actin-rich projections, known as foot processes, that are connected via a unique intercellular junction known as the slit diaphragm. Integration of the slit diaphragm with the underlying actin cytoskeleton is proposed to endow the podocyte with a flexible cytoarchitecture that can dynamically adapt to the contractile demands of mechanical strain (2, 3). Accordingly, a hallmark of kidney disease is remodeling of the foot process actin cytoskeleton, leading to simplification of podocyte structure (effacement), disruption of filtration (proteinuria), and podocyte loss (1).
A major component of the podocyte slit diaphragm is the transmembrane protein nephrin. The extracellular region of nephrin interacts with adjacent nephrin molecules, forming a structural filter, whereas the intracellular tail coordinates signaling cascades that regulate actin dynamics as well as podocyte survival (4). Nephrin is phosphorylated on intracellular tyrosine residues by the Src family kinase (SFK)4 Fyn, and phosphorylation of three of these tyrosines, in particular Tyr-1176, Tyr-1193, and Tyr-1217, creates docking sites for the Nck1/2 cytoskeletal adaptor proteins (5, 6). Nephrin phosphorylation on these sites is reduced in renal diseases associated with podocyte effacement (7, 8), and this phosphorylation is essential in mice for stabilization and restoration of foot process morphology (9).
Similarly, Nck proteins are critically required for the maintenance of mature podocyte structure as well as the initial development of foot processes (5, 7). Nck proteins provide a dynamic link between nephrin and the actin cytoskeleton. Nck binds via its Src homology 2 (SH2) domain to phosphorylated tyrosine residues on nephrin and via its three tandem SH3 domains to effector molecules, which contain proline-rich motifs such as N-WASp (5, 6, 10). Despite the central importance of Nck in podocyte biology, the downstream signaling pathways controlling its function remain to be fully elucidated.
In a search for novel effectors of Nck signaling within podocytes, we have identified Wilms tumor-interacting protein (WTIP). WTIP is a Lim domain- and poly-proline-containing scaffold protein of the Ajuba family that is expressed in podocytes, where it has been shown to regulate F-actin assembly (11, 12). WTIP is also an evolutionarily conserved negative regulator of the Hippo pathway, a kinase cascade terminating with Lats1/2 kinases that phosphorylate the transcriptional regulators Yap and Taz, leading to their nuclear exclusion and subsequent degradation (13, 14). The Hippo pathway is regulated by signals from the physical environment, including mechanical stretch, matrix stiffness, and cell geometry (15, 16). In podocytes, activation of this pathway is associated with apoptosis (17, 18), with podocyte-specific deletion of Yap in mice resulting in foot process effacement, proteinuria, and podocyte depletion (19).
In this study, we demonstrate that Nck bridges the interaction of WTIP and Lats1 with phosphorylated nephrin, resulting in reduced Hippo signaling. We further show that Lats1 is transiently activated in a reversible mouse model of podocyte injury coincident with nephrin dephosphorylation and that Lats1 activation precedes phosphorylation and subsequent degradation of Yap. Last, we demonstrate reduced expression of Yap in mice with chronic loss of nephrin tyrosine phosphorylation. Together, we have uncovered a novel role for Nck in connecting phosphorylated nephrin with the Hippo pathway through WTIP, which may have implications in mechanotransduction and podocyte survival.
Experimental Procedures
Plasmids
Constructs encoding human GFP-tagged CD16/7-nephrin and FLAG-tagged Nck1 and Nck2 and mutants thereof have been described previously (5, 20). GFP-tagged CD16/7-nephrinY3F-3xSH3 was constructed by cloning the three Nck2 SH3 domains in-frame with the C terminus of CD16/7-nephrinY3F. Human Nck1 3xSH3 and 3xSH3* were previously cloned into pGEX-4T-1 as GST fusion constructs. Mouse WTIP (BC054125) and WTIPΔPRR, which lacks 351 base pairs (128–479, inclusive), resulting in deletion of 117 amino acids of the PRR of WTIP, were cloned into pEGFP-C1 and pCDNA3.1-HA. FLAG-tagged Lats1 was provided by Dr. Clark Wells (Indiana University) and described previously (21).
Antibodies
The following antibodies were obtained commercially: rabbit anti-GFP (Abcam, 290), mouse anti-GAPDH (clone 1D4, G041, Applied Biological Materials Inc.), mouse anti-CD16 (sc-19620, Santa Cruz Biotechnology), mouse anti-FLAG clone M2 (F3165, Sigma-Aldrich), rabbit anti-Lats1 (A300-477A, Bethyl), rabbit anti-Ser(P)-909 Lats1 (9157, Cell Signaling Technology), rabbit anti-Yap (D8H1XP(R), Cell Signaling Technology), and rabbit anti- Ser(P)-127 Yap (4911, Cell Signaling Technology). Mouse anti-HA (clone 12CA5) was obtained from the Sunnybrook Hybridoma Bank (Toronto, ON, Canada). Rabbit anti-nephrin was provided by Dr. Tomoko Takano (McGill University) and described previously (22). Phospho-specific anti-nephrin antibodies were generated and validated previously (7). Secondary horseradish peroxidase-conjugated goat anti-mouse and goat anti-rabbit secondary antibodies (Bio-Rad) were used for immunoblot detection.
Tissue Culture
HEK293T cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were grown in DMEM (Sigma-Aldrich) supplemented with 10% FBS, 200 units/ml penicillin, and 200 μg/ml streptomycin (Invitrogen) and maintained at 37 °C and 5% CO2. Transient transfection was performed using PEI for 48 h.
Cell Lysis, Immunoprecipitation, and Western Blotting
Cells expressing CD16/7-nephrin were starved in serum-free medium overnight and stimulated with 1 μg/ml anti-CD16 antibody for 10 min at 37 °C. For inhibition of SFKs, cells were treated with 10 μm PP2 (Sigma-Aldrich) for 3 h prior to CD16 stimulation, and CD16 stimulation was performed in the continued presence of PP2. Cells were lysed in phospholipase C lysis buffer supplemented with protease inhibitors by vortexing and sonicating on ice. Immunoprecipitation was performed overnight at 4 °C with rotation, followed by washing three times with phospholipase C buffer. Immunocomplexes were eluted from the beads in 2× SDS loading buffer by boiling at 100 °C for 2 min. For Western blotting, proteins from total lysates and immunoprecipitates were resolved using 10% SDS-PAGE gels, transferred to a PVDF membrane, blocked for 30 min in Tris-buffered saline with Tween 20 (TBST) containing 5% nonfat milk powder or BSA, and incubated overnight at 4 °C with primary antibody. Detection was performed using ECL Western blotting substrate (Pierce), and membranes were exposed to film (Pierce). Values used for densitometry were obtained using ImageLab v2.0 analysis software (Bio-Rad).
GST Pulldown Assay
Expression of GST fusion proteins was induced in Escherichia coli DH5α using 0.5 mm isopropyl 1-thio-β-d-galactopyranoside for 3 h. Fusion proteins were purified using glutathione-SepharoseTM 4B (GE Healthcare). 5 μg of each GST fusion protein was incubated for 3 h at 4 °C with lysates from HEK293T cells and processed as described for immunoprecipitation.
Animals
NephrinY3F knockin mice were generated by homologous recombination targeting mouse Tyr-1191, Tyr-1208, and Tyr-1232 for mutation to Phe as described previously (9). Male and female animals on the C57BL/6N background were used between 5–8 weeks of age, which is prior to the onset of significant renal injury.
Nephrotoxic Serum Injury Model
Male C57BL/6N mice (475, Charles River Laboratories Canada) aged 8–10 weeks were injected with 0.04 mg/g of γ2 subclass sheep anti-rat nephrotoxic serum (a gift from Dr. David Salant, Boston University) via tail vein injection. Prior to injection, mice were warmed in an empty cage under a heat lamp for 15 min to induce tail vein dilation. Spot urine samples were collected prior to and at various times after injection. At 0, 6, or 24 h post-injection, mice were euthanized with CO2, and both kidneys were removed and immediately processed for glomerular isolation.
Study Approval
Animal studies were approved by the University of Guelph Animal Care Committee and carried out in accordance with Canadian Council on Animal Care protocols.
Glomerular Isolation and Lysis
Kidneys were dissected, and cortices were separated from the medulla, minced, and digested by incubation with 1 mg/ml type 4 collagenase (Worthington Biochemical) in PBS at 37 °C with 140 rpm agitation for 30 min. Digested cortices were passed through 100-mm sterile nylon cell strainers with chilled PBS to obtain glomeruli. Red blood cells were lysed in sterile Ack lysis buffer (150 mm NH4Cl, 10 mm KHCO3, and 0.1 mm EDTA), and the remaining enriched glomeruli were lysed in phospholipase C lysis buffer supplemented with fresh protease inhibitors as described above. Lysed glomeruli were further subjected to sonication for 10 s on ice before proceeding to separating soluble protein from the cell pellet through centrifugation.
Semiquantitative Real-time RT-PCR
For mRNA isolation, kidney cortices were dissected from mice injected for 0 or 24 h with NTS and immediately flash-frozen and stored at −80 °C. mRNA was isolated using the Ambion RNA isolation kit (Thermo Fisher Scientific) and treated with DNase I (Invitrogen), and concentrations were measured using a Nanodrop (Thermo Fisher Scientific). cDNA synthesis was performed on 1 μg of RNA with the Superscript II kit (Invitrogen). Real-time RT-PCR reactions were carried out using PerfeCta SYBR Green FastMix, Rox (Quanta Bioscience) using the StepOnePlus real-time PCR system (Thermo Fisher Scientific). Standard curves were generated for all primer pairs used, and melt curves were examined to verify the presence of a single amplicon. “No template” controls were included in all runs. Expression differences between time 0 and 24 h samples were calculated using the ΔCt method and corrected for primer efficiency using StepOnePlus v2.3 software (Thermo Fisher Scientific). Differences were normalized to the reference gene β2-microglobulin. The following primer pairs were used: β2-microglobulin, GCTATCCAGAAAACCCCTCA (forward) and CCGTTCTTCAGCATTTGGAT (reverse); nephrin, CTAGTTTCCCCCAAGGTGCT (forward) and GTCCACCCTGGATGAAGATG (reverse); Yap1, TTTCGGCAGGCAATACGGAA (forward) and AGGGATCGGAACTATTGGTTGT (reverse); CCND1, GCGTACCCTGACACCAATCT (forward) and CACAGACCTCCAGCATCCAG (reverse); and amphiregulin, ACAGCGAGGATGACAAGGAC (forward) and GCCAATAGCTGCGAGGATGA (reverse).
Results
Nck1 and Nck2 Interact with WTIP
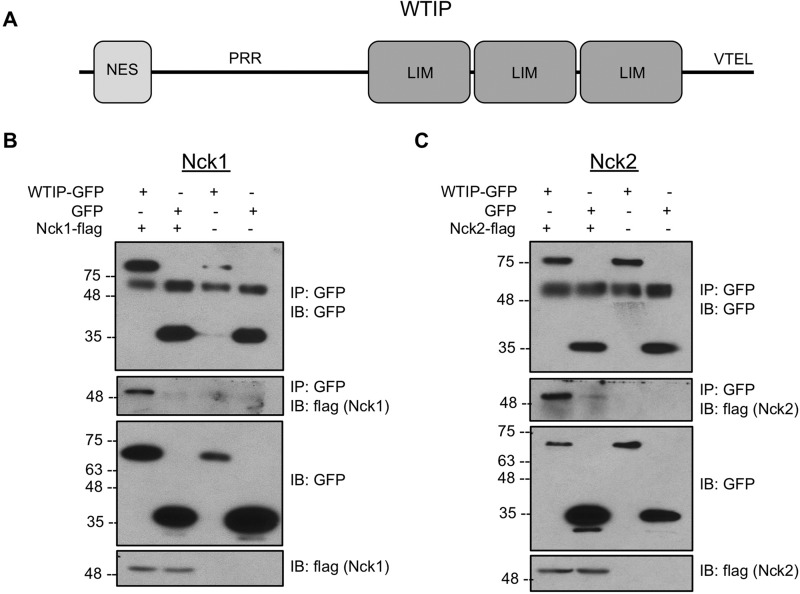
WTIP was identified as a putative Nck interaction partner based on the presence of an extensive proline-rich region (PRR) (Fig. 1A), including the minimal consensus motif required for Nck SH3 domain binding, PXXP (where P is proline and X is any amino acid) (23). Therefore, to validate that Nck and WTIP interact, FLAG-tagged Nck1 or Nck2 was transiently coexpressed with GFP-tagged WTIP or GFP alone in HEK293T cells, and cell lysates were harvested for immunoprecipitation of GFP (Fig. 1, B and C). The immunoblot for GFP and FLAG reveals a specific interaction between both Nck1 and Nck2 with WTIP-GFP but not GFP alone.
FIGURE 1.
Nck and WTIP interact. A, schematic of WTIP protein domains and motifs, including an N-terminal nuclear export signal (NES), the internal PRR, three tandem C-terminal LIM domains, and a terminating PDZ domain binding sequence, VTEL (valine, threonine, glutamate, leucine). B and C, lysates from HEK293T transiently co-expressing WTIP-GFP or GFP alone with Nck1-FLAG (B) or Nck2-FLAG (C) were immunoprecipitated (IP) with GFP and immunoblotted (IB) for GFP and FLAG. WTIP-GFP, but not GFP, was able to co-immunoprecipitate Nck1-FLAG and Nck2-FLAG.
Nck and WTIP Interact via the SH3 domains of Nck and the PRR of WTIP
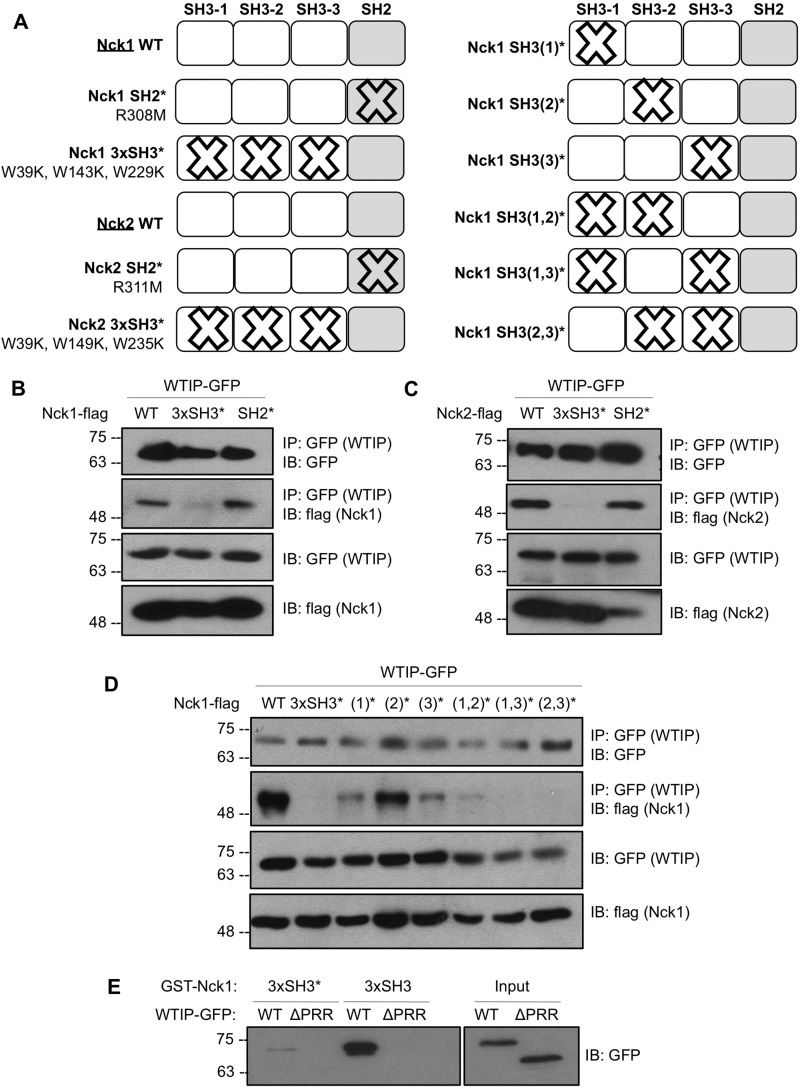
We hypothesized that the proline-binding SH3 domains of Nck would facilitate the interaction with WTIP. To test this, Nck1 and Nck2 variants with point mutations (Fig. 2A) inactivating the proline-binding functions of all three SH3 domains of Nck (3xSH3*) or a point mutation that inactivates the phosphotyrosine binding function of the SH2 domain (SH2*) were coexpressed with WTIP-GFP in HEK293T cells, and lysates were harvested for binding assays. Co-immunoprecipitation of WTIP-GFP and Nck1-FLAG is detected for Nck1 WT and SH2*, but the interaction is obstructed by the 3xSH3* mutation (Fig. 2B). Similarly, the interaction between WTIP-GFP and Nck2-FLAG is also impaired by the 3xSH3* mutation (Fig. 2C). Therefore, at least one of the Nck SH3 domains is required to bind WTIP, whereas the Nck SH2 domain is not required.
FIGURE 2.
Nck and WTIP interact via Nck SH3 domains and the WTIP PRR. A, schematic of Nck1 and Nck2 variants used in binding assays to map the interaction between Nck and WTIP, depicting point mutations and nomenclature used for inactivation of SH2 and single or multiple SH3 domains. B and C, lysates from HEK293T transiently co-expressing WTIP-GFP with Nck1-FLAG or Nck2-FLAG WT and variants with inactivated SH2 or SH3 domains were immunoprecipitated (IP) for GFP and immunoblotted (IB) for GFP and FLAG. The interaction between Nck1/2 and WTIP was abolished by inactivation of all three SH3 domains. D, lysates from HEK293T transiently co-expressing WTIP-GFP with Nck1-FLAG WT and variants with single, double-, or triple-inactivated SH3 domains were immunoprecipitated for GFP and immunoblotted for GFP and FLAG. The interaction between Nck1 and WTIP is most substantially reduced by inactivation of the first and third Nck1 SH3 domains. E, lysates from HEK293T cells overexpressing WTIP-GFP or the deletion variant WTIPΔPRR-GFP (Input) were incubated with immobilized GST fusion proteins corresponding to the three WT Nck1 SH3 domains (3xSH3) or three inactivated Nck1 SH3 domains (3xSH3*). Pulldown complexes were immunoblotted for GFP, demonstrating loss of binding between Nck1 SH3 domains and WTIPΔPRR and between Nck1 3xSH3* and full-length WTIP.
Because there are three potential Nck SH3 domains capable of binding WTIP, we next sought to isolate which of these domains is most critical for the Nck-WTIP interaction by performing binding assays between WTIP-GFP and all possible Nck1 variants with one, two, or three SH3 domains inactivated (Fig. 2A). Immunoprecipitation of GFP (WTIP) and immunoblot for FLAG (Nck1 variants) reveals that the first and third SH3 domains of Nck are primarily responsible for binding to WTIP (Fig. 2D).
Furthermore, to verify the role of the PRR of WTIP in binding Nck, a deletion variant of WTIP was generated that lacks the internal PRR, denoted as WTIPΔPRR. A pulldown assay of wild-type WTIP and WTIPΔPRR using GST fused to either the three wild-type Nck1 SH3 domains (3xSH3) or three mutated Nck1 SH3 domains (3xSH3*) reveals that the PRR of WTIP is required for the interaction between Nck and WTIP (Fig. 2E).
Nck Recruits WTIP to Tyrosine-phosphorylated Nephrin
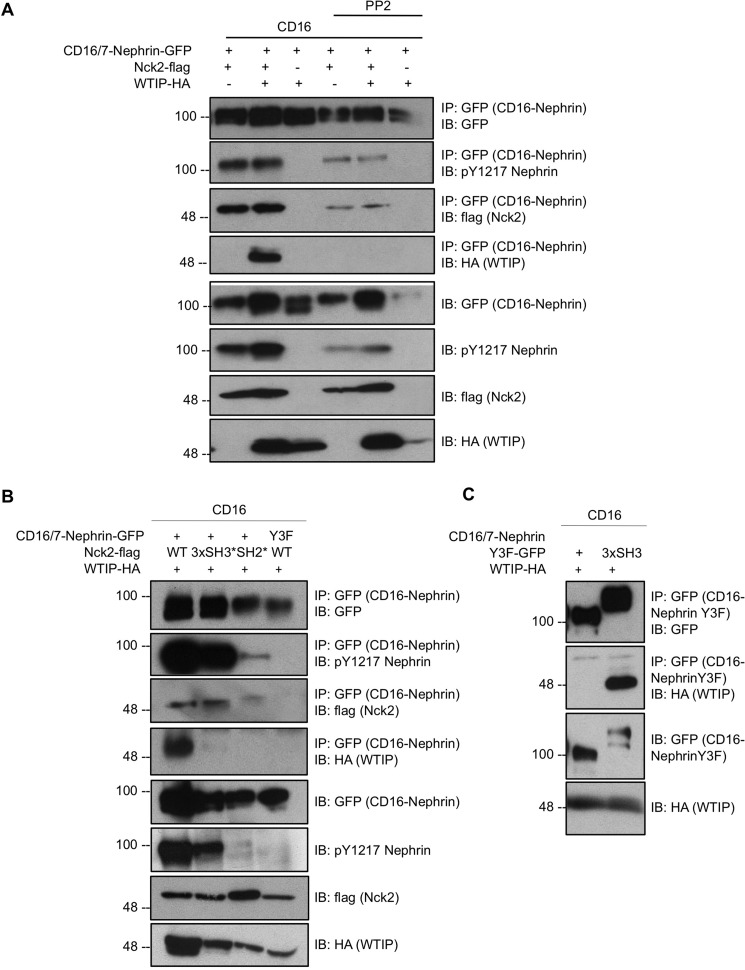
A prominent function of Nck in podocytes is connecting nephrin at the slit diaphragm to the underlying actin cytoskeleton. Thus, we next investigated whether Nck could recruit WTIP to nephrin. To test this, chimeric CD16/7-nephrin was used to facilitate inducible phosphorylation of the nephrin intracellular domain upon CD16 clustering (5). Cells transiently coexpressing CD16/7-nephrin-GFP, Nck2-FLAG, and WTIP-HA were stimulated with CD16 in the presence or absence of the SFK inhibitor PP2, and lysates were harvested for immunoprecipitation of GFP (CD16/7-nephrin). As reported previously (24), CD16/7-nephrin phosphorylation induced by CD16 stimulation is suppressed in the presence of PP2 and enhanced by overexpression of Nck2 (Fig. 3A). Accordingly, Nck recruitment to nephrin is suppressed in the presence of PP2 (Fig. 3A). Notably, we demonstrate that WTIP-HA coimmunoprecipitation with CD16/7-nephrin-GFP occurs primarily when CD16/7-nephrin has been clustered and Nck2-FLAG is overexpressed above endogenous levels (Fig. 3A), suggesting that Nck can mediate the recruitment of WTIP to nephrin upon nephrin phosphorylation by SFKs.
FIGURE 3.
Nck recruits WTIP to phospho-nephrin. A, lysates from HEK293T cells transiently coexpressing CD16/7-nephrin-GFP, Nck2-FLAG, and WTIP-HA were stimulated with anti-CD16 antibody in the presence or absence of the SFK inhibitor PP2, immunoprecipitated (IP) for GFP (CD16/7-nephrin), and immunoblotted (IB) for GFP, phospho-nephrin Tyr-1217, FLAG, and HA. Complex formation between the nephrin intracellular domain and WTIP is phospho- and Nck-dependent. B, lysates from HEK293T cells transiently coexpressing CD16/7-nephrin-GFP, Nck2-FLAG, WTIP-HA, and variants as indicated were stimulated with anti-CD16 antibody, immunoprecipitated for GFP (CD16/7-nephrin), and immunoblotted for GFP, phospho-nephrin Tyr-1217, FLAG, and HA. Recruitment of WTIP to the nephrin intracellular domain is abolished by mutations that disrupt nephrin-Nck interaction (Y3F and SH2*) or Nck-WTIP interaction (3xSH3*). C, the three Nck2 SH3 domains were genetically conjugated to the tail of CD16/7-nephrinY3F, and this construct (CD16/7-nephrinY3F-3xSH3) was coexpressed with WTIP in HEK293T cells. Cells were stimulated with anti-CD16 antibody, and lysates were immunoprecipitated for GFP (CD16/7-nephrinY3F) and immunoblotted for GFP and HA. WTIP binding to the intracellular domain of nephrinY3F is rescued by fusion of the Nck2 SH3 domains.
To further validate that Nck mediates recruitment of WTIP to phosphorylated nephrin, Nck mutants that disrupt upstream and downstream interactions were coexpressed with CD16/7-nephrin and WTIP-HA. Nck2 3xSH3* is able to bind the intracellular tail of nephrin but is unable to bind WTIP. By contrast, Nck2 SH2* is able to bind WTIP but is unable to be recruited to the intracellular tail of nephrin. In addition, we employed a variant of CD16/7-nephrin in which the three Nck-binding tyrosines are mutated to phenylalanine (Y3F) to disrupt Nck recruitment to nephrin. Transfected cells were stimulated with anti-CD16 antibody and harvested for immunoprecipitation of GFP (CD16/7-nephrin). Coimmunoprecipitation of CD16/7-nephrin-GFP and WTIP-HA only occurs when the nephrin tyrosine residues are present and phosphorylated and when Nck2 contains all functional SH3 and SH2 domains (Fig. 3B).
Last, to verify the phospho- and Nck dependence of WTIP recruitment to nephrin, we generated a construct in which the Nck2 SH3 domains were directly fused to the intracellular tail of CD16/7-nephrinY3F (CD16/7-nephrinY3F-3xSH3). Coexpression of this construct with WTIP-HA rescues binding of WTIP to CD16/7-nephrinY3F (Fig. 3C), demonstrating that Nck links phosphorylated nephrin with WTIP.
WTIP Connects Nephrin to the Hippo Pathway Kinase Lats1
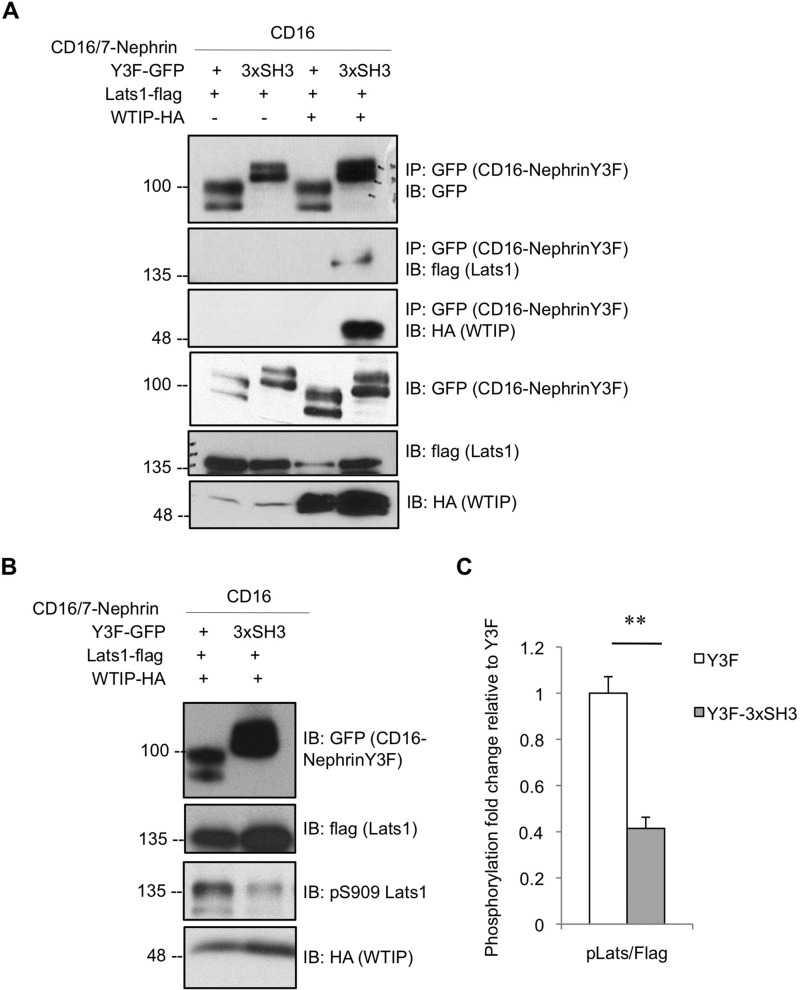
WTIP negatively regulates the Hippo pathway through binding and inhibiting Lats kinases, which in turn promotes stabilization of Yap (13). To first assess the interplay between WTIP, Lats and nephrin, we coexpressed CD16/7-nephrinY3F or CD16/7-nephrinY3F-3xSH3 fusion construct with WTIP-HA and Lats1-FLAG in HEK293T cells and harvested lysates for immunoprecipitation of GFP (CD16/7-nephrin). Immunoblotting for HA and FLAG demonstrates that Lats1 is recruited to nephrin coincident with WTIP (Fig. 4A). Furthermore, assessment of lysates from cells coexpressing CD16/7-nephrinY3F-GFP or CD16/7-nephrinY3F-3xSH3-GFP with WTIP-HA and Lats1-FLAG by immunoblot for phospho-Lats (Ser-909-active kinase) suggests that complex formation between nephrin, WTIP, and Lats1 coincides with a significant decrease in Lats1 phosphorylation (Fig. 4, B and C), implying reduced Hippo pathway activation.
FIGURE 4.
WTIP links nephrin to Lats1 inhibition. A, lysates from HEK293T cells coexpressing CD16/7-nephrinY3F or CD16/7-nephrinY3F-3xSH3 with Lats1-FLAG in the presence or absence of WTIP-HA were stimulated with anti-CD16 antibody, immunoprecipitated (IP) for GFP, and immunoblotted (IB) for GFP, FLAG, and HA. Lats1 binds the CD16/7-nephrinY3F-3xSH3 fusion protein but not CD16/7-nephrinY3F alone upon coexpression of WTIP. B, immunoblot of equivalent lysates from the third and fourth lanes of A with phospho-Lats1 Ser-909 shows a decrease in Lats1 activation upon complex formation between CD16/7-nephrinY3F-3xSH3, WTIP, and Lats1. C, densitometry of B, showing a statistically significant 2.4-fold reduction of Lats1 phosphorylation on Ser-909 coincident with complex formation between CD16/7-nephrinY3F-3xSH3, WTIP, and Lats1 (n = 4). **, p < 0.01.
Nephrin Dephosphorylation Is Associated with Activation of Lats1 and Inhibition of Yap
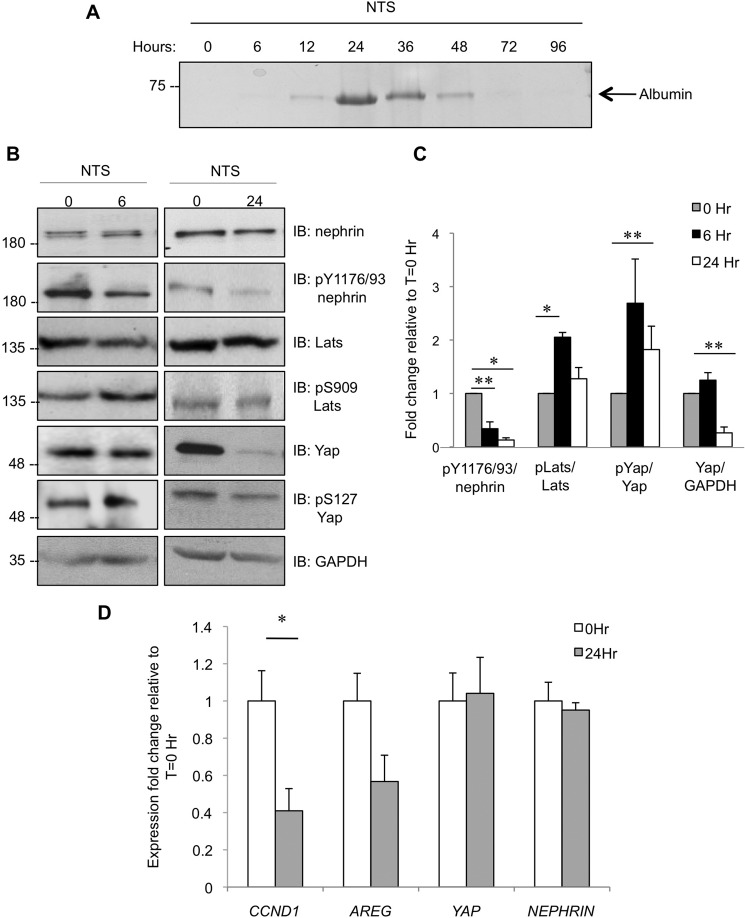
We next explored whether changes in components of the Hippo pathway might correlate with altered nephrin signaling induced by podocyte injury. Here, we employed the γ2 nephrotoxic serum (NTS) nephritis model, in which injection of C57BL/6 mice with sheep anti-rat glomerular antiserum induces nephrotic-range proteinuria within 24 h after injection, which resolves by 72–96 h post-injection (Fig. 5A) (25). Glomeruli were isolated from mice at 0, 6, or 24 h following NTS injection and processed for immunoblotting and subsequent densitometric quantitation (Fig. 5, B and C). At 6 h post-NTS injection, which precedes marked proteinuria, there is significantly reduced nephrin phosphorylation on Tyr-1176/1193 compared with uninjected controls. This reduction in nephrin phosphorylation is coincident with a significant increase of Lats1 phosphorylation on Ser-909 and a concomitant rise in Yap phosphorylation on Ser-127. By 24 h post-NTS injection, when proteinuria is maximal, nephrin phosphorylation remains significantly lower than in control animals. Intriguingly however, although phospho-Lats1 Ser-909 has returned back to baseline at this time point, phospho-Yap Ser-127 remains significantly elevated, and there is a marked reduction in total Yap levels, consistent with the notion that phosphorylation of Yap on Ser-127 triggers its degradation (14).
FIGURE 5.
The Hippo signaling pathway is activated in a podocyte injury model. A, Coomassie-stained SDS-PAGE gel of urine collected from mice injected with NTS at the indicated time points. Proteinuria peaked at 24 h post-injection, and animals recovered by 72–96 h post-NTS injection. B, representative immunoblots (IB) of glomerular lysates from mice injected with NTS at 0, 6, or 24 h for phospho- and total nephrin, Lats1, and Yap as well as GAPDH as a loading control. C, densitometry of B, showing statistically significant phospho-dynamics for nephrin, Lats1, and Yap as well as changes in total Yap levels in glomeruli of mice injected with NTS (n = 3, except for Yap/GAPDH at 24 h, where n = 7). *, p < 0.05; **, p < 0.01. D, quantitative RT-PCR results comparing transcript levels of cyclin D1 (CCND1), amphiregulin (AREG), Yap, and nephrin in kidney cortices from mice injected with NTS for 24 h or uninjected controls (n = 2, except for CCND1, where n = 3). *, p < 0.05.
To determine whether this loss of Yap protein affected its transcriptional activity, we isolated mRNA from the kidney cortex of NTS-injected mice and controls and used quantitative PCR to examine transcript levels of several Yap target genes. At 24 h post-injection, we noted a marked decrease in expression of amphiregulin (AREG) and cyclin D1 (CCND1) (Fig. 5D). As expected, no significant changes were observed in nephrin or Yap mRNA levels. Overall, the NTS model of transient podocyte injury demonstrates temporally regulated Lats1 activation and Yap down-regulation coincident with nephrin dephosphorylation.
Loss of Nephrin-Nck Signaling Leads to Reduced Yap Protein
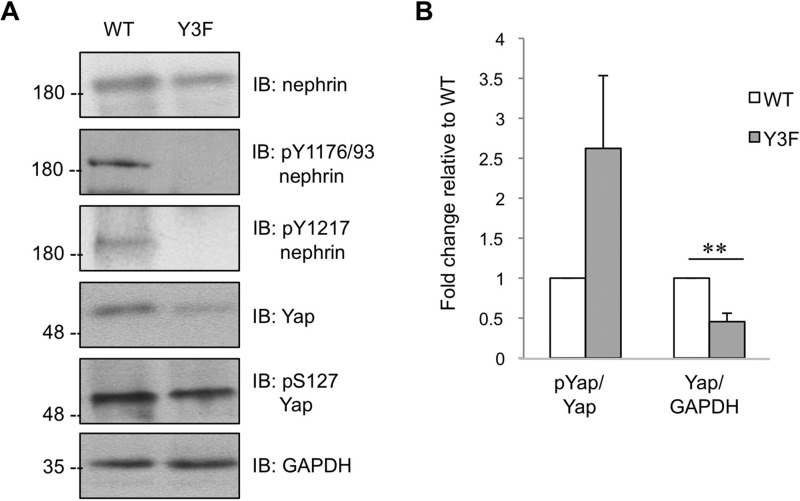
The striking reduction in glomerular Yap expression in the NTS model prompted us to examine whether this protein would be similarly altered in mice with chronic loss of tyrosine phosphorylated nephrin. Here, we used homozygous nephrinY3F mice, which harbor knockin mutations in the three Nck binding sites and thus cannot initiate nephrin-Nck signaling, leading to foot process effacement and proteinuria (9). Compared with WT littermates, which retain nephrin phosphorylation on the Nck binding sites, glomeruli from nephrinY3F mice display significantly reduced total Yap protein as well as elevated Yap phosphorylation on Ser-127 relative to total Yap (Fig. 6, A and B). We thus conclude that nephrin phospho-signaling directly regulates stabilization of the key Hippo pathway effector Yap.
FIGURE 6.
Reduced Yap expression in glomeruli of nephrinY3F mice. A, representative immunoblot (IB) of phospho- and total nephrin and Yap in glomeruli isolated from nephrinY3F (Y3F) mice and control (WT) littermates. B, densitometry of A, showing a 2.6-fold increase in phospho-Yap/Yap in nephrinY3F glomeruli compared with wild-type controls and a 2.2-fold decrease in Yap/GAPDH (n = 5). **, p < 0.01.
Discussion
In summary, we have identified a novel Nck binding partner, WTIP, and showed that, through its interaction with Nck, it is recruited to the critical podocyte slit diaphragm molecule nephrin. To date, the association of nephrin and Nck has been ascribed functional significance primarily as a signaling interaction upstream of N-WASp and actin assembly (5, 26). Here, we extend this interactome to reveal that Nck is capable of using its SH2 and SH3 domains to bridge phospho-nephrin to WTIP. WTIP was first identified through its association with the podocyte-specific transcription factor WT1 (11). In cultured podocytes, WTIP is localized within the cytoplasm at cell-cell adhesion sites, where it is proposed to modulate actin dynamics (27), similar to Nck. However, in response to injury, WTIP translocates to the nucleus, where it suppresses WT1-dependent transcriptional activation of target genes such as amphiregulin (11, 12). The physiological significance of WTIP as a negative regulator of WT1 is unclear, largely because of limited information regarding the genes controlled by WT1. Recently however, a comprehensive genome-wide WT1 transcriptional network analysis has revealed that WT1 is involved in driving the expression of numerous Hippo constituents in podocytes (28), implicating a central role for WT1 in Hippo signaling.
WTIP associates with the core Hippo kinases Lats1/2 (13), presumably via its LIM domains (13, 29), and we have now shown that Nck-mediated binding of the WTIP-Lats1 complex to nephrin sequesters Lats1 from phospho-activation. These findings are consistent with a previous report showing that Lats1 is not highly active in cultured podocytes (18). However, dephosphorylation of nephrin on the Nck binding sites, as observed here upon nephrotoxic serum-mediated injury in mice, triggers rapid activation of Lats1 and subsequent phosphorylation and degradation of Yap, coincident with maximal proteinuria. This reduction in Yap protein expression is accompanied by a decrease in Yap target gene expression, which also includes amphiregulin, further solidifying the link between WT1 and the Hippo pathway.
Activation of the Hippo pathway in cultured podocytes through enhanced Lats1 kinase activity and inhibition of Yap is associated with increased susceptibility to apoptotic stimuli (17, 18). Moreover, podocyte-specific deletion of Yap in mice leads to foot process effacement, podocyte depletion, and proteinuria, and Yap protein is decreased in the glomeruli of patients with focal segmental glomerulosclerosis (FSGS) (19). Intriguingly, we observe decreased Yap protein levels in glomeruli of homozygous nephrinY3F mice, which have disrupted phospho-nephrin signaling and comparable adult-onset renal damage (9). These data provide strong in vivo evidence identifying nephrin as a novel upstream regulator of the Hippo pathway, and they suggest that this relationship may be important for preservation of podocytes and filtration barrier function.
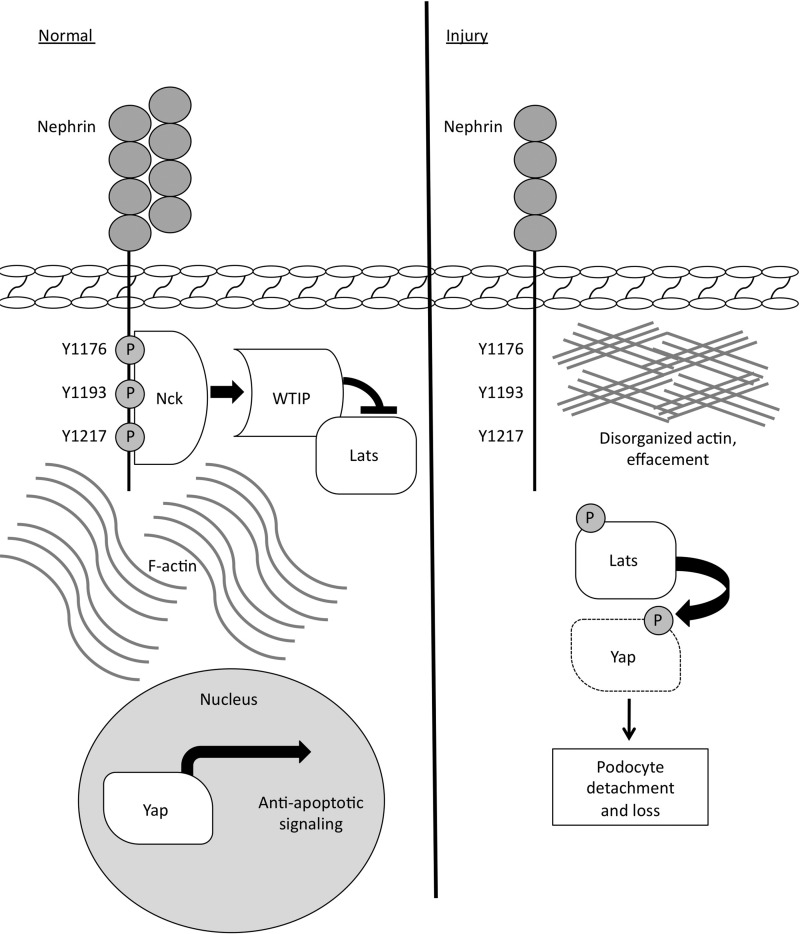
Hippo pathway activation is reciprocally regulated by the actin cytoskeleton (16), and there is evidence in podocytes that actin disassembly induces phosphorylation of Lats and Yap (18). In addition, TEAD transcription factors, which are downstream of Yap, are involved synergistically with WT1 in regulating the expression of cytoskeleton-regulating genes in podocytes (28). Furthermore, from our nephrinY3F mice, we posit that nephrin tyrosine phosphorylation and dynamic recruitment of actin-regulating molecules such as Nck facilitate adaptive tethering to the underlying actin cytoskeleton to promote podocyte viability (9). Together with the findings presented here, a model is emerging in which phosphorylated nephrin and Nck recruit WTIP and Lats1 to the slit diaphragm, where Lats1 is suppressed and Yap is active within the nucleus (Fig. 7). This mechanism of Lats1 inhibition is similar to the model proposed in Drosophila, wherein the homologues of WTIP and Lats1 are recruited to cadherin at the membrane (30). Nephrin dephosphorylation leads to disassembly of the Nck-WTIP-Lats1 complex, prompting Lats activation, Yap phosphorylation, and degradation. Nephrin thus serves as a central regulator to stabilize the podocyte actin cytoskeleton and promote anti-apoptotic signaling.
FIGURE 7.
Schematic of the molecular model proposed in this study. In healthy podocytes, phosphorylated nephrin binds Nck, which regulates F-actin and recruits WTIP and Lats1. Here, Lats1 kinase is inhibited, thus Yap is not phosphorylated and remains active in the nucleus, where it promotes anti-apoptotic signaling. In response to specific and injurious stimuli that induce nephrin dephosphorylation and actin disorganization, the complex of Nck, WTIP, and Lats1 is disassembled, and Lats1 kinase becomes activated and, in turn, phosphorylates Yap. Yap is subsequently retained in the cytosol and targeted for degradation, thereby increasing podocyte susceptibility to apoptosis.
In closing, it has been widely hypothesized that nephrin must be involved in sensing and responding to mechanical signals induced by fluctuating glomerular hemodynamic forces (31). We now present WTIP as a molecular bridge, integrating nephrin phospho-signaling at the slit diaphragm with the Hippo kinase cascade. These findings support the burgeoning role of Hippo signaling in mechanosensing, and they have implications for our understanding of podocyte plasticity and how integrity of the kidney filtration barrier is maintained.
Author Contributions
A. K. C. conceived and coordinated the study and wrote the paper in collaboration with N. J. A. K. C. performed and analyzed the experiments shown in Figs. 1–6. C. E. M. performed the experiments shown in Figs. 4–6. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Drs. David Salant, Clark Wells, and Tomoko Takano for reagents. We also acknowledge Dr. Terry Van Raay for helpful discussions and Dr. Laura New, Rachael McNeilly, Olivia Anderson, and Megan Fortino for assistance with the experiments (University of Guelph). We also thank Jenn Randall, Martha Smith, and the University of Guelph Central Animal Facility staff for expertise with animal husbandry and experiments.
This work was supported by grants from the Kidney Foundation of Canada (to N. J.). The authors declare that they have no conflicts of interest with the contents of this article.
- SFK
- Src family kinase
- SH
- Src homology
- WTIP
- Wilms tumor-interacting protein
- PRR
- proline-rich region
- NTS
- nephrotoxic serum.
References
- 1.Greka A., and Mundel P. (2012) Cell biology and pathology of podocytes. Annu. Rev. Physiol. 74, 299–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kriz W., and Lemley K. V. (2015) A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J. Am. Soc. Nephrol. 26, 258–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endlich N., and Endlich K. (2012) The challenge and response of podocytes to glomerular hypertension. Semin. Nephrol. 32, 327–341 [DOI] [PubMed] [Google Scholar]
- 4.New L. A., Martin C. E., and Jones N. (2014) Advances in slit diaphragm signaling. Curr. Opin. Nephrol. Hypertens. 23, 420–430 [DOI] [PubMed] [Google Scholar]
- 5.Jones N., Blasutig I. M., Eremina V., Ruston J. M., Bladt F., Li H., Huang H., Larose L., Li S. S., Takano T., Quaggin S. E., and Pawson T. (2006) Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440, 818–823 [DOI] [PubMed] [Google Scholar]
- 6.Verma R., Kovari I., Soofi A., Nihalani D., Patrie K., and Holzman L. B. (2006) Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J. Clin. Invest. 116, 1346–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones N., New L. A., Fortino M. A., Eremina V., Ruston J., Blasutig I. M., Aoudjit L., Zou Y., Liu X., Yu G. L., Takano T., Quaggin S. E., and Pawson T. (2009) Nck proteins maintain the adult glomerular filtration barrier. J. Am. Soc. Nephrol. 20, 1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchida K., Suzuki K., Iwamoto M., Kawachi H., Ohno M., Horita S., and Nitta K. (2008) Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int. 73, 926–932 [DOI] [PubMed] [Google Scholar]
- 9.New L. A., Martin C. E., Scott R. P., Platt M. J., Keyvani Chahi A., Stringer C. D., Lu P., Samborska B., Eremina V., Takano T., Simpson J. A., Quaggin S. E., and Jones N. (2016) Nephrin tyrosine phosphorylation is required to stabilize and restore podocyte foot process architecture J. Am. Soc. Neph. 10.1681/ASN.2015091048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H., Zhu J., Aoudjit L., Latreille M., Kawachi H., Larose L., and Takano T. (2006) Rat nephrin modulates cell morphology via the adaptor protein Nck. Biochem. Biophys. Res. Commun. 349, 310–316 [DOI] [PubMed] [Google Scholar]
- 11.Srichai M. B., Konieczkowski M., Padiyar A., Konieczkowski D. J., Mukherjee A., Hayden P. S., Kamat S., El-Meanawy M. A., Khan S., Mundel P., Lee S. B., Bruggeman L. A., Schelling J. R., and Sedor J. R. (2004) A WT1 co-regulator controls podocyte phenotype by shuttling between adhesion structures and nucleus. J. Biol. Chem. 279, 14398–14408 [DOI] [PubMed] [Google Scholar]
- 12.Kim J. H., Konieczkowski M., Mukherjee A., Schechtman S., Khan S., Schelling J. R., Ross M. D., Bruggeman L. A., and Sedor J. R. (2010) Podocyte injury induces nuclear translocation of WTIP via microtubule-dependent transport. J. Biol. Chem. 285, 9995–10004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das Thakur M., Feng Y., Jagannathan R., Seppa M. J., Skeath J. B., and Longmore G. D. (2010) Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr. Biol. 20, 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varelas X. (2014) The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141, 1614–1626 [DOI] [PubMed] [Google Scholar]
- 15.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., and Piccolo S. (2011) Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 [DOI] [PubMed] [Google Scholar]
- 16.Low B. C., Pan C. Q., Shivashankar G. V., Bershadsky A., Sudol M., and Sheetz M. (2014) YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 588, 2663–2670 [DOI] [PubMed] [Google Scholar]
- 17.Campbell K. N., Wong J. S., Gupta R., Asanuma K., Sudol M., He J. C., and Mundel P. (2013) Yes-associated protein (YAP) promotes cell survival by inhibiting proapoptotic dendrin signaling. J. Biol. Chem. 288, 17057–17062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wennmann D. O., Vollenbröker B., Eckart A. K., Bonse J., Erdmann F., Wolters D. A., Schenk L. K., Schulze U., Kremerskothen J., Weide T., and Pavenstädt H. (2014) The Hippo pathway is controlled by angiotensin II signaling and its reactivation induces apoptosis in podocytes. Cell Death Dis. 5, e1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartzman M., Reginensi A., Wong J. S., Basgen J. M., Meliambro K., Nicholas S. B., D'Agati V., McNeill H., and Campbell K. N. (2016) Podocyte-specific deletion of Yes-associated protein causes FSGS and progressive renal failure. J. Am. Soc. Nephrol. 27, 216–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blasutig I. M., New L. A., Thanabalasuriar A., Dayarathna T. K., Goudreault M., Quaggin S. E., Li S. S., Gruenheid S., Jones N., and Pawson T. (2008) Phosphorylated YDXV motifs and Nck SH2/SH3 adaptors act cooperatively to induce actin reorganization. Mol. Cell Biol. 28, 2035–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler J. J., Johnson D. E., Heller B. L., Bringman L. R., Ranahan W. P., Conwell M. D., Sun Y., Hudmon A., and Wells C. D. (2013) Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases. Proc. Natl. Acad. Sci. U.S.A. 110, 17368–17373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H., Lemay S., Aoudjit L., Kawachi H., and Takano T. (2004) SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J. Am. Soc. Nephrol. 15, 3006–3015 [DOI] [PubMed] [Google Scholar]
- 23.Ren R., Mayer B. J., Cicchetti P., and Baltimore D. (1993) Identification of a ten-amino acid proline-rich SH3 binding site. Science 259, 1157–1161 [DOI] [PubMed] [Google Scholar]
- 24.New L. A., Keyvani Chahi A., and Jones N. (2013) Direct regulation of nephrin tyrosine phosphorylation by Nck adaptor proteins. J. Biol. Chem. 288, 1500–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoppe J. M., and Vielhauer V. (2014) Induction and analysis of nephrotoxic serum nephritis in mice. Methods Mol. Biol. 1169, 159–174 [DOI] [PubMed] [Google Scholar]
- 26.Banjade S., and Rosen M. K. (2014) Phase transitions of multivalent proteins can promote clustering of membrane receptors. eLife 3, 10.7554/eLife.04123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J. H., Mukherjee A., Madhavan S. M., Konieczkowski M., and Sedor J. R. (2012) WT1-interacting protein (Wtip) regulates podocyte phenotype by cell-cell and cell-matrix contact reorganization. Am. J. Physiol. Renal Physiol. 302, F103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kann M., Ettou S., Jung Y. L., Lenz M. O., Taglienti M. E., Park P. J., Schermer B., Benzing T., and Kreidberg J. A. (2015) Genome-wide analysis of Wilms' tumor 1-controlled gene expression in podocytes reveals key regulatory mechanisms. J. Am. Soc. Nephrol. 26, 2097–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe Y., Ohsugi M., Haraguchi K., Fujimoto J., and Yamamoto T. (2006) LATS2-Ajuba complex regulates γ-tubulin recruitment to centrosomes and spindle organization during mitosis. FEBS Lett. 580, 782–788 [DOI] [PubMed] [Google Scholar]
- 30.Rauskolb C., Sun S., Sun G., Pan Y., and Irvine K. D. (2014) Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell 158, 143–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simons M., and Huber T. B. (2008) It's not all about nephrin. Kidney Int. 73, 671–673 [DOI] [PubMed] [Google Scholar]