Abstract
Macrophages constitute a first line of pathogen defense by triggering a number of inflammatory responses and the secretion of various pro-inflammatory cytokines. Recently, we and others found that IκBζ, an atypical IκB family member and transcriptional coactivator of selected NF-κB target genes, is essential for macrophage expression of a subset of pro-inflammatory cytokines, such as IL-6, IL-12, and CCL2. Despite defective pro-inflammatory cytokine expression, however, IκBζ-deficient mice develop symptoms of chronic inflammation. To elucidate this discrepancy, we analyzed a regulatory role of IκBζ for the expression of anti-inflammatory cytokines and identified IκBζ as an essential activator of IL-10 expression. LPS-challenged peritoneal and bone marrow-derived macrophages from IκBζ-deficient mice revealed strongly decreased transcription and secretion of IL-10 compared with wild-type mice. Moreover, ectopic expression of IκBζ was sufficient to stimulate Il10 transcription. On the molecular level, IκBζ directly activated the Il10 promoter at a proximal κB site and was required for the transcription-enhancing trimethylation of histone 3 at lysine 4. Together, our findings show for the first time the IκBζ-dependent expression of an anti-inflammatory cytokine that is crucial in controlling immune responses.
Keywords: immunosuppressor, interleukin, macrophage, NF-κB, NF-κB transcription factor
Introduction
Macrophages constitute a heterogeneous group of phagocytes that fulfill pro- as well as anti-inflammatory responses (1, 2). A pro-inflammatory response of innate immune cells is indispensable for host defense. However, dysregulation of innate immunity can result in severe damage of the affected host. Weak immune responses lead to prolonged infection and persistence of pathogens, whereas overshooting responses promote chronic inflammation and autoimmune disease (3). Therefore, tight control of defense mechanisms is essential for host protection against self-destructive, excessive, and undue immune responses.
For macrophages, two distinct states of polarization have been defined. Classically activated (M1-polarized) macrophages exert their pro-inflammatory role as effector cells in cell-mediated immune responses, whereas alternatively activated (M2-polarized) macrophages are involved in immunosuppression, wound healing, and tissue regeneration (4). In contrast to M1 macrophages, the M2 counterparts secrete high amounts of anti-inflammatory cytokines, including the crucial immunosuppressive cytokine IL-10, thereby guaranteeing a balanced immune response (5).
IL-10 can inhibit various macrophage functions, such as nitric oxide synthesis and pro-inflammatory cytokine production, as well as the expression of major histocompatibility complex proteins and co-stimulatory receptors (6, 7). In contrast, the absence of IL-10 results in spontaneous development of inflammatory bowel disease and increased pathological alterations caused by uncontrolled responses to infectious pathogens (8, 9). IL-10 may also act as a negative feedback regulator of chronic infectious diseases by inhibiting IL-6, IL-12, and TNFα secretion, thereby keeping immune responses in check and preventing tissue damage (9). Furthermore, the administration of exogenous IL-10 has been shown to ameliorate inflammatory and autoimmune diseases in several animal models (8).
An important regulator for the expression of cytokines and other immune regulators is the transcription factor NF-κB. The NF-κB family consists of five members that bind as homo- or heterodimers at κB sites in the DNA of target genes (10). Depending on their transactivation activity, the NF-κB subunits can be divided into two subgroups. RelA (p65), RelB, and c-Rel possess a C-terminal transcription activation domain, whereas p50 (Nfkb1) and p52 (Nfkb2) lack a transcription activation domain. Based on these structural differences, NF-κB dimers containing at least one subunit with a transcription activation domain act as transcriptional activators, whereas p50/p50 or p52/p52 homodimers are assumed to function as transcriptional repressors.
Because various stimuli activate the NF-κB signaling pathway and a great diversity of target genes is regulated by NF-κB, a precise control of NF-κB activity is required to avoid misguided immune responses. In fact, NF-κB activation is controlled by a series of cytosolic and nuclear regulatory events, in which IκB proteins play a pivotal role (10, 11). In unstimulated cells, NF-κB is sequestered as an inactive complex bound to cytosolic IκB proteins such as IκBα, IκBβ, and IκBϵ. Various stimuli cause the phosphorylation of cytosolic IκBs, leading to their proteasomal degradation, which subsequently enables NF-κB to translocate to the nucleus and activate target genes.
Despite the presence of high-affinity binding sites, only a fraction of NF-κB target genes is generally activated in response to an inflammatory stimulus. It was suggested that NF-κB target genes can be categorized in two groups based on their kinetics of induction and the requirement of protein synthesis (12, 13). Although primary NF-κB response genes are rapidly induced, the expression of secondary target genes is delayed and requires the prior synthesis of additional NF-κB coregulators. A novel and emerging group of such NF-κB coregulators are so-called atypical IκB proteins, including Bcl-3, IκBNS, IκBζ, and IκBη (11, 14). Atypical IκBs differ markedly from classical cytosolic IκBs because they are mostly inducibly expressed and localized in the nucleus. Moreover, atypical IκBs do not exclusively act as inhibitors but can also activate the expression of secondary response genes.
The atypical IκB protein IκBζ has been recently implicated in differential NF-κB target gene expression in macrophages (15, 16) even though its physiological function remains largely unknown. The IκBζ-encoding Nfkbiz gene is rapidly induced as a primary NF-κB response gene by various inflammatory stimuli and, through association with the NF-κB subunit p50, is thought to exert its transcription-enhancing activity on secondary response genes mainly at the level of chromatin remodeling (12, 17, 18).
Recently, we showed that in macrophages, expression of CCL2 strictly depends on the presence of IκBζ and, consequently, that expression of CCL2 is abolished in macrophages from IκBζ-deficient mice (15). Furthermore, several other pro-inflammatory gene products, including IL-6, IL12p40, IL-17, IFNγ, and GM-CSF, have been found to be regulated by IκBζ (16, 19–23). Intriguingly, however, despite impaired expression of these pro-inflammatory cytokines, Nfkbiz−/− mice display a pro-inflammatory phenotype characterized by periocular inflammation, inflammatory skin alterations, and an M1 hyperpolarized macrophage state (15, 16, 24, 25). In view of the phenotype of Nfkbiz−/− mice, we therefore investigated a potential role of IκBζ for the regulation of IL-10 as an essential anti-inflammatory cytokine. Interestingly, although expression of Il10 has been found previously to be inhibited by the atypical IκB protein Bcl-3 (26, 27), our results establish IκBζ as a novel and essential transcriptional inducer of Il10 in macrophages. Our results therefore show for the first time that IκBζ is not only a pro-inflammatory mediator but also controls the activation of anti-inflammatory gene products.
Experimental Procedures
Animals
Nfkbiz−/− and control C57BL/6 mice were used at 6–8 weeks of age as described previously (23). Nfkbiz−/− mice were originally generated by injection of targeted AB2.2 ES cell (129 strain) clones into C57BL/6 murine blastocysts (24) and were backcrossed in a C57BL/6 background for more than 50 generations. Mouse work was performed in accordance with the German law guidelines of animal care as permitted by regional authorities (Regierungspräsidium Tübingen, application no. H6/12).
Culture of Peritoneal and Bone Marrow Macrophages
Female C57BL/6 mice were euthanized by CO2 asphyxiation. For isolation of peritoneal macrophages (PMΦ),3 the abdominal skin was removed, a catheter (24-gauge) was inserted into the peritoneal cavity, and 10 ml of ice-cold PBS was injected. After massage of the peritoneum, peritoneal fluids were aspirated and centrifuged at 500 × g for 10 min, and the resulting PMΦ were resuspended in 2 ml of macrophage medium containing DMEM/Ham's F-12, 10% FCS, and MycoZapPlusCL antibiotics (Lonza, Basel, Switzerland). Cells were seeded in 96-well plates and cultured for 2 h under standard conditions (5% CO2, 37 °C). Next, adherent PMΦ were washed four times with culture medium to remove non-adherent cells. To generate bone marrow-derived macrophages (BMMΦ), the femur and tibia were separated at the knee joint and rinsed with PBS. Bone marrow cells were singularized (40-μm cell strainer) and pelleted by centrifugation (500 × g, 10 min). Cells were resuspended in macrophage medium supplemented with M-CSF (30 ng/ml, Immunotools, Friesoythe, Germany), seeded in tissue culture flasks (3 × 106 cells/ml), and cultured under low-oxygen conditions (5% CO2, 5% O2). After 7 days of differentiation, cells were washed with PBS, scraped off, and cultured at a density of 2 × 105 cells/cm2 under low-oxygen conditions.
Activation and Pro-inflammatory Stimulation of PMΦ and BMMΦ
PMΦ and BMMΦ were cultured with murine IL-4 (100 ng/ml) or IFNγ (25 ng/ml, both from Immunotools) for 24 h to induce alternative or classical macrophage activation. Pro-inflammatory stimulation was achieved by culturing cells for the indicated time in the presence of 1 μg/ml LPS (Escherichia coli serotype O111:B4; Sigma-Aldrich, Taufkirchen, Germany). Recombinant murine IL-10 was obtained from Immunotools.
Culture of Raw264.7 Cells, Raw264.7/TetOn-IκBζ Cells, and MEFs
Mouse embryonic fibroblasts (MEFs) were isolated at embryonic day 10.5 according to standard procedures. MEFs and Raw264.7 cells were cultured in DMEM/high glucose, 10% FCS, and MycoZapPlusCL (Lonza). Raw264.7/TetOn-IκBζ cells were described previously (15) and cultured in the presence of 1 mg/ml neomycin (G418, Lonza). Cells were seeded at a density of 105 cells/cm2 24 h before the experiment. Optionally, 2 μg/ml doxycycline (Sigma-Aldrich) was added to promote ectopic IκBζ expression. For pro-inflammatory stimulation, cells were treated with 1 μg/ml LPS.
Transfections and Reporter Gene Assays
Raw264.7 or Raw264.7/TetOn-IκBζ cells were transfected with appropriate amounts of plasmids using jetPEI transfection reagent according to the instructions of the manufacturer (Polyplus, Illkirch, France). Expression plasmids for Nfkb1 (p50) and IκBζ have been described previously (15, 17). For reporter gene assays, the following luciferase promoter constructs were used: pGL2basic-Il10 and truncation mutants (28), pGL3basic-Lcn2 (29), and pGL3basic-Il6 and pGL3basic-Elam1 (16). Cells were trypsinized 24 h after transfection. Cells and quanta from each transfection were reseeded in four separate culture vessels for differential treatments. To induce ectopic IκBζ expression, Raw264.7/TetOn-IκBζ cells were cultured in the presence of 2 μg/ml doxycycline. After additional 16 h, doxycycline-treated and untreated cells were incubated with 1 μg/ml LPS. Cells were harvested 48 h after transfection, and luciferase assays were performed with the Dual-Luciferase reporter assay system (Promega Corp., Madison, WI).
Quantitative RT-PCR
Whole cell RNA was isolated using the RNeasy mini kit (Qiagen, Hilden, Germany) and reverse-transcribed (QuantiTect kit, Qiagen) according to the instructions of the manufacturer. Quantitative PCR (qPCR; LightCycler 480 II, Roche) was performed using SYBR Green/ROX qPCR Master Mix (Fermentas, Sankt Leon-Rot, Germany) as described in the two-step cycling protocol of the manufacturer (384-well plates, 10-μl reaction). The following primer pairs were used: Cxcl9, 5′-GAT TTG TAG TGG ATC GTG CCT C-3′ and 5′-GGA ACC CTA GTG ATA AGG AAT GC-3′; Gapdh, 5′-ACC ACA GTC CAT GCC ATC AC-3′ and 5′-CAC CAC CCT GTT GCT GTA GCC-3′; Gbp4, 5′-ATG GTG ATT CCC TTG TGG AAA G-3′ and 5′-AAG GAG TGA TAA AAC GCT GCT T-3′; Elam1, 5′-CTC ACT CCT GAC ATC GTC CTC-3′ and 5′-ACG TTG TAA GAA GGC ACA TGG-3′; Il6, 5′-AGT TGC CTT CTT GGG ACT GA-3′ and 5′-TCC ACG ATT TCC CAG AGA AC-3′; Il10, 5′-AGC CTT ATC GGA AAT GAT CCA GT-3′ and 5′-GGC CTT GTA GAC ACC TTG GT-3′; Nfkbia, 5′-CTC ACT CCT GAC ATC GTC CTC-3′ and 5′-ACG TTG TAA GAA GGC ACA TGG-3′; Nfkbiz, 5′-TAT CGG GTG ACA CAG TTG GA-3′ and 5′-TGA ATG GAC TTC CCC TTC AG-3′; Stat1, 5′-GCT GCC TAT GAT GTC TCG TTT-3′ and 5′-TGC TTT TCC GTA TGT TGT GCT-3′; and Tnfa, 5′-CCT CAG CCT CTT CTC CTT CCT-3′ and 5′-GGT GTG GGT GAG GAG CA-3′. Quantification of reverse-transcribed mRNA was performed using the second derivate maximum-based advanced relative quantification algorithm of the Roche LightCycler 480 software (V1.5).
Immunoblotting
Immunoblotting was performed as described previously (30) using anti-β-actin (A2228, Sigma-Aldrich, 1:10,000), rabbit anti-mouse Stat1 (9172, Cell Signaling Technology, Frankfurt, Germany, 1:2000), rabbit anti-mouse pStat1 (Tyr(P)701, clone 58D6, Cell Signaling Technology, 1:2000), and rabbit anti-IκBζ, which was produced as described previously (15).
Chromatin Immunoprecipitation
ChIP experiments were performed with the HighCell# ChIP kit (Diagenode, Seraing, Belgium) using anti-histone H3 (trimethyl-Lys4) antibodies (Bioss Antikörper, Aachen, Germany). ChIP efficiencies were determined by qPCR on a LightCycler 480 II (Roche) using Maxima Hot Start TaqDNA polymerase according to the two-step cycling protocol of the manufacturer (96-well plates, 20-μl volume). The following primer pairs spanning κB-binding sites of the murine Il6, Il10, and Tnfa gene promoters were used: Il6, 5′-CGA TGC TAA ACG ACG TCA CAT TGT GCA-3′ and 5′-CTC CAG AGC AGA ATG AGC TAC AGA CAT-3′; Il10, 5′-TAG AAG AGG GAG GAG GAG CC-3′ and 5′-TGT GGC TTT GGT AGT GCA AG-3′; and Tnfa, 5′-CCC CAG ATT GCC ACA GAA TC-3′ and 5′-CCA GTG AGT GAA AGG GAC AG-3′). Primers covering portions of the Gapdh promoter (5′-GGG GTT GCT GTG TCA CTA CCG-3′ and 5′-CAG AGA CCT GAA TGC TGC TTC C-3′) and Actb promoter (5′-TCG ATA TCC ACG TGA CAT CCA-3′ and 5′-GCA GCA TTT TTT TAC CCC CTC-3′) served as controls. The specificities of the primers were verified, and PCR efficiencies were determined. Samples were analyzed according to the instructions of the manufacturer. Relative promoter occupancies were calculated as described previously (15).
Measurement of Cytokine Concentrations
Concentrations of cytokines in cell culture supernatants were measured using the mouse cytometric bead array system (mouse anti-IL-6, anti-IL-10, and anti-TNFα) according to the instructions of the manufacturer (BD Biosciences). Before stimulation of cells in 96-well plates, the culture medium was exchanged with 200 μl of fresh medium per well. In the case of BMMΦ, cytokine concentrations in culture supernatants were directly compared and expressed as cytokine amounts per volume. To avoid mouse-specific differences in peritoneal cell counts, cytokine concentrations in supernatants of PMΦ were normalized to the protein content of cell lysates. To this end, 50 μl of 0.2 m NaOH was added to each well after aspiration of the supernatants, and protein concentrations in lysates were measured with the BCA protein assay (Thermo Scientific, Bonn, Germany).
Statistical Analysis
Values are expressed as mean ± S.D. or S.E. for the indicated numbers of independent experiments. For statistical comparisons, hypotheses were tested using an unpaired Student's t test.
Results
IκBζ Is Essential for Il10 Expression in Mouse Embryonic Fibroblasts and Peritoneal Macrophages
Although IκBζ transcriptionally induces several pro-inflammatory genes in cells of the monocyte lineage, Nfkbiz−/− mice exhibit features of chronic inflammation. To explore this discrepancy, we focused our analyses on IL-10 as one of the major anti-inflammatory mediators. Initially, we examined the gene expression profile of wild-type and Nfkbiz−/− MEFs by quantitative RT-PCR (qRT-PCR). As expected, LPS-challenged Nfkbiz−/− MEFs showed reduced expression of the IκBζ-dependent target gene Il6 (16) compared with wild-type MEFs (Fig. 1A). In contrast to Il6, expression of Tnfa, an IκBζ-independent NF-κB target gene (15, 16), was readily induced by LPS in wild-type and even more strongly in Nfkbiz−/− MEFs. Intriguingly, analysis of Il10 expression revealed strongly diminished transcript levels in Nfkbiz−/− MEFs compared with wild-type MEFs (Fig. 1A).
FIGURE 1.
IκBζ is essential for Il10 expression in mouse embryonic fibroblasts and peritoneal macrophages. A, MEFs were cultured in the presence of 1 μg/ml LPS for 5 h. Total RNA was isolated and subjected to qRT-PCR analysis to determine the expression levels of Tnfa, Il6, and Il10. Values are mean ± S.E. of four experiments. ctrl, control. B and C, naïve PMΦ (B) and PMΦ (C), classically primed with IFNγ, from wild-type and Nfkbiz−/− mice were either left untreated (ctrl) or cultured in the presence of 1 μg/ml LPS for 24 h before expression of Tnfa, Il6, and Il10 was analyzed by qRT-PCR. Values were normalized to Gapdh and are mean ± S.E. of four experiments. *, statistical significance comparing wild-type and Nfkbiz−/− cells; N.D., not detectable.
Because IL-10 is predominantly expressed by macrophages, we performed additional experiments in PMΦ from wild-type and Nfkbiz−/− mice. We initially assayed the expression of macrophage surface markers, such as F4/80, CD11b, CD11c, and Ly6G/C (supplemental Fig. 1), and classical macrophage functions, such as phagocytosis, migration, and oxidative burst (supplemental Fig. 2). These analyses did not reveal significant differences between the two genotypes, indicating that PMΦ are a suitable experimental system. Because IFNγ-primed PMΦ have been used previously in studies on IκBζ-dependent gene regulation (15), we next analyzed the gene expression profiles of naïve and IFNγ-primed wild-type and Nfkbiz−/− PMΦ. In line with published data (15), expression of Il6 was only slightly different in naïve PMΦ from both genotypes but strongly reduced in IFNγ-primed PMΦ from Nfkbiz−/− mice compared with wild-type cells. The expression levels of Tnfa did not significantly differ in wild-type and Nfkbiz−/− PMΦ (Fig. 1B). Importantly, compared with wild-type PMΦ, both naïve and IFNγ-primed PMΦ from Nfkbiz−/− mice revealed strongly reduced mRNA levels of Il10 (Fig. 1C).
IκBζ Deficiency Reduces IL-10 Secretion in Bone Marrow Macrophages
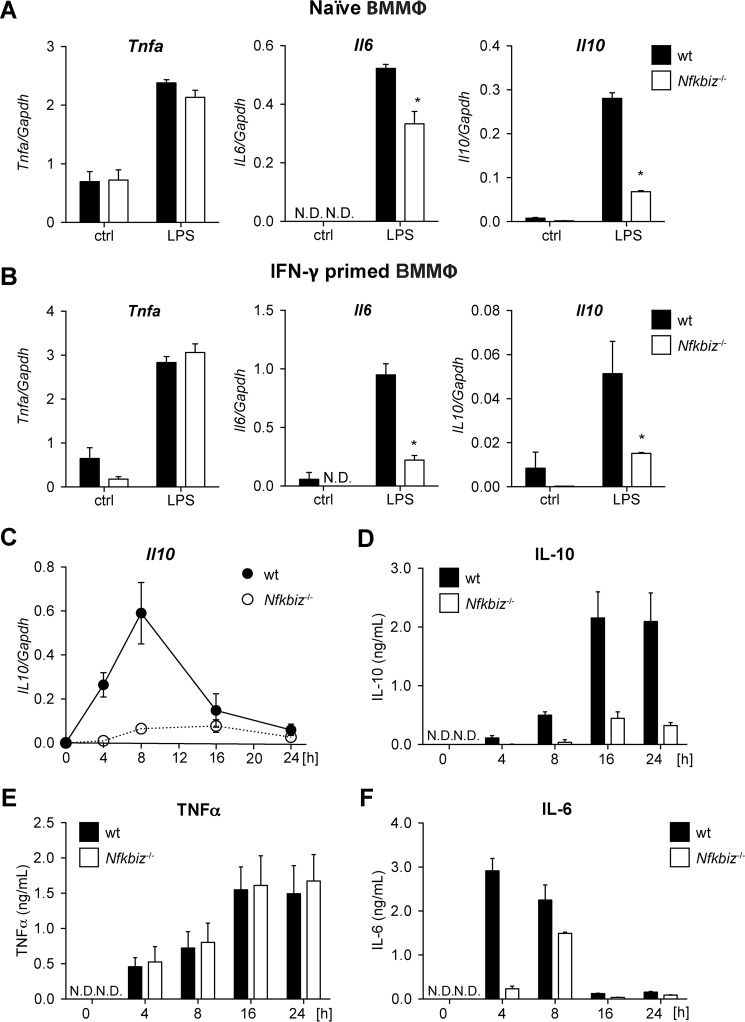
Having shown that Il10 expression is diminished in Nfkbiz−/− MEFs and PMΦ, we further analyzed IL-10 mRNA and protein expression in BMMΦ. Again, cells were left untreated (naïve) or pretreated with IFNγ before challenging with LPS. Gene expression analyses revealed comparable mRNA levels of Tnfa in naïve and IFNγ-primed BMMΦ, whereas IL6 expression was significantly reduced in the absence of IκBζ (Fig. 2, A and B). In line with the previous experiments, expression of Il10 mRNA was strongly reduced in Nfkbiz−/− BMMΦ compared with wild-type BMMΦ.
FIGURE 2.
IκBζ deficiency reduces IL-10 secretion in bone marrow macrophages. A and B, naïve (A) and classically activated (B) wild-type and Nfkbiz−/− BMMΦ were cultured in the presence of 1 μg/ml LPS. Total RNA was subjected to qRT-PCR analysis to determine the expression levels of Tnfa, Il6, and Il10. Values are mean ± S.E. from five experiments. ctrl, control. C, wild-type and Nfkbiz−/− BMMΦ were cultured with 1 μg/ml LPS. Total RNA was isolated after the indicated time points and subjected to qRT-PCR analysis to determine the levels of Tnfa, Il6, and Il10 mRNA expression. Values are mean ± S.E. from four experiments. D–F, IL-10 (D), TNFα (E), and IL-6 (F) concentrations in cell culture supernatants were assessed at the indicated time points of LPS stimulation. Values are mean ± S.E. from three experiments. *, statistical significance comparing wild-type and Nfkbiz−/− cells; N.D., not detectable.
We further analyzed the kinetic of Il10 expression in BMMΦ of both genotypes. LPS stimulation of wild-type BMMΦ induced Il10 mRNA expression already after 4 h, with maximal expression 8 h after addition of LPS (Fig. 2C). This time-dependent expression is comparable with that of Il6 and Ccl2 (15), which have been shown to be secondary response genes in the NF-κB signaling cascade. In line with the previous results, LPS-treated Nfkbiz−/− BMMΦ showed only marginally increased Il10 mRNA expression even 8 h after stimulation. In a similar setup, we analyzed cytokine concentrations in supernatants from LPS-challenged BMMΦ. The amount of TNFα detected in supernatants from LPS-challenged wild-type and Nfkbiz−/− BMMΦ was similar and served as a positive control (Fig. 2D). In line with the qRT-PCR analysis, the concentration of IL-10 increased in supernatants from LPS-challenged wild-type BMMΦ, reaching a maximum after 16 h, whereas, in supernatants from Nfkbiz−/− BMMΦ, only basal IL-10 levels were detected. TNFα levels were comparable in supernatants from BMMΦ of both genotypes (Fig. 2E), whereas the amount of IL-6 was significantly reduced in Nfkbiz−/− BMMΦ compared with wild-type cells (Fig. 2F). Thus, these data clearly indicate IκBζ-dependent regulation of Il10 mRNA expression and protein secretion in macrophages.
IκBζ-regulated IL-10 Expression Is Independent of Macrophage Polarization
The classical activation of macrophages induces M1 polarization, whereas alternative activation results in M2 polarization. Previous gene expression analysis revealed that the regulation of Il6 gene expression depends on the macrophage polarization state (15). Hence, we wondered whether IL-10 expression is also influenced by the polarization state. Therefore, we quantified the concentration of cytokines in supernatants from LPS-challenged naïve and M1- and M2-polarized BMMΦ. As a positive control, we analyzed the secretion of TNFα, which was readily detectable in supernatants from naïve and classically (M1) and alternatively (M2) activated wild-type and Nfkbiz−/− BMMΦ (Fig. 3, A–C). Interestingly, naïve Nfkbiz−/− macrophages secreted slightly more TNFα than wild-type BMMΦ (Fig. 3A). The concentrations of both IL-6 and IL-10 were significantly decreased in Nfkbiz−/− BMMΦ compared with wild-type BMMΦ in case of all three polarization states (Fig. 3, A–C). Thus, expression of IL-10 is not affected by the macrophage polarization state.
FIGURE 3.
IκBζ-regulated IL-10 expression is independent of macrophage polarization. A–C, BMMΦ were either left naïve (A), classically activated with 30 ng/ml IFNγ (B), or alternatively activated with 20 ng/ml IL-4 (C) for 24 h before challenging or not with 1 μg/ml LPS for an additional 24 h. Culture supernatants were analyzed for the concentration of TNFα, IL-6 and IL-10. Values are mean ± S.E. of six experiments. *, statistical significance comparing luciferase activity in the presence and absence of doxycycline; N.D., not detectable. ctrl, control.
IκBζ Overexpression Induces Il10 Promoter Activity and Gene Expression
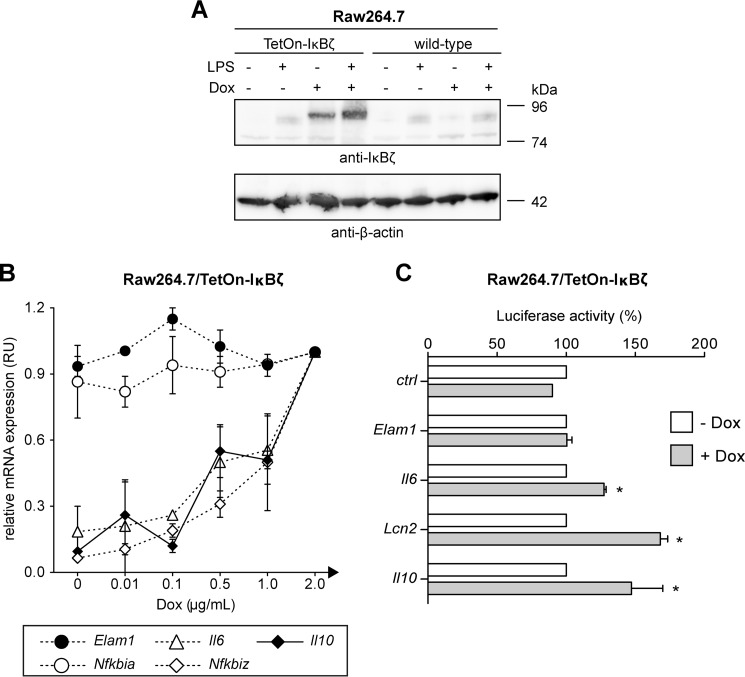
Because our investigations so far relied on knockout systems, we additionally employed the macrophage-like cell line Raw264.7, which was genetically modified to enable inducible doxycycline-dependent IκBζ expression. Treatment of the Raw264.7/TetOn-IκBζ cells with doxycycline resulted in the robust expression of IκBζ, which was further increased by stimulation with LPS (Fig. 4A).
FIGURE 4.
Ectopic expression of IκBζ induces Il10 expression. A, Raw264.7 cells were modified to enable the inducible doxycycline-dependent expression of IκBζ. Both Raw264.7 wild-type and the resulting Raw264.7/TetOn-IκBζ cells were cultured in the presence or absence of either 1 μg/ml LPS, 2 μg/ml doxycycline (Dox), or a combination thereof. After 24 h, induction of IκBζ expression was verified by immunoblot analysis. B, Raw264.7/TetOn-IκBζ cells were incubated in the presence of the indicated amounts of doxycycline. After 24 h, total RNA was isolated and subjected to qRT-PCR analysis for the expression of Il10, Il6, Nfkbiz, Nfkbia, and Elam1. Values are expressed as relative units (RU). Expression levels from cells treated with 2 μg/ml doxycycline were defined as 1 RU. Values are mean ± S.D. from three experiments. C, Raw264.7/TetOn-IκBζ cells were transfected with luciferase reporter gene constructs harboring the promoter region of the indicated genes or the empty vector control (ctrl). Cells were cultured in the presence of 2 μg/ml doxycycline for 48 h before luciferase assay was performed. Luciferase activity is given as percentage of the basal activity in the absence of doxycycline. Values are mean ± S.D. from three experiments. *, statistical significance comparing luciferase activity in the presence and absence of doxycycline.
We next investigated the impact of transgenic IκBζ expression on the induction of various IκBζ-independent and -dependent genes. Quantitative RT-PCR analyses confirmed a dose-dependent induction of Nfkbiz transcription in Raw264.7/TetOn-IκBζ by doxycycline (Fig. 4B). The expression of the IκBζ-independent NF-κB target genes Nfkbia and Elam1 remained unaffected by doxycycline, whereas expression of the IκBζ-dependent target genes Il6 and Lcn2 correlated with the concentration of doxycycline and the mRNA levels of Nfkbiz. Similar to Il6 and Lcn2, expression of Il10 was enhanced in the presence of doxycycline and induced Nfkbiz (Fig. 4B). Thus, expression of IκBζ is sufficient for induction of Il10 expression.
We further analyzed whether IκBζ directly activates the Il10 promoter. In a first set of experiments, we transfected Raw264.7/TetOn-IκBζ cells with Elam1, Lcn2, Il6, and Il10 reporter gene constructs and analyzed whether doxycycline-induced IκBζ expression results in increased luciferase activity. No reporter activity was induced from the empty vector backbone and the IκBζ-independent promoter of Elam1 (Fig. 4C). In contrast, IκBζ expression clearly induced luciferase activity from the Il6 and the Lcn2 promoters, which are both regulated by IκBζ (16, 31). Importantly, doxycycline-induced expression of IκBζ also strongly induced luciferase expression from the Il10 promoter construct (Fig. 4C).
IκBζ Targets the Proximal Promoter Region of the Il10 Genomic Locus
Analysis of the Il10 promoter reveals two NF-κB consensus sites that are located in distal (−1115 to −1106 bp) and proximal (−55 to −46 bp) promoter regions (Fig. 5A). Because these regions potentially serve as anchor points for IκBζ-mediated transcription, we used reporter constructs containing various truncated versions of the Il10 promoter. Upon transfection of Raw264.7 cells, LPS induced a strong activation of the full-length Il10 promoter (Fig. 5B). Truncations of the Il10 promoter and deletion of the distal NF-κB-binding site resulted only in a minor reduction of luciferase activity, indicating that the proximal NF-κB site is important for Il10 promoter activation.
FIGURE 5.
IκBζ regulates Il10 promoter activity. A, schematic of the murine Il10 promoter. The dark boxes indicate the positions of κB binding sites. B, Raw264.7 cells were transfected with the empty luciferase reporter gene vector (ctrl) or reporter gene constructs harboring the indicated regions of the Il10 promoter. Luciferase activity was analyzed after 24 h of incubation in the absence or presence of LPS. Values are mean ± S.D. from three experiments. *, statistical significance comparing luciferase activity in the presence and absence of LPS. AU, arbitrary units. C, Raw264.7 cells were transfected with a luciferase reporter construct of a truncated Il10 promoter (−158 to +64 bp) harboring the proximal κB binding site together with pcDNA4 expression vectors for Nfkb1 (p50), IκBζ, or a combination thereof. Luciferase activity was analyzed 24 h after transfection. Promoter activity obtained after transfection of the empty pcDNA4 vector was set as 1. Values are mean ± S.D. of three experiments. D, chromatin from LPS-treated (5 h) naïve wild-type and Nfkbiz−/− BMMΦ was subjected to ChIP assays applying an H3K4me3-specific antibody. The degree of H3K4 trimethylation of the Il10, Il6, and Tnfa promoter was determined via qPCR. ChIP analysis with an isotype control antibody served as a control. Values are mean ± S.D. from three experiments. *, statistical significance comparing wild-type and Nfkbiz−/− cells.
The recruitment of IκBζ to promoter regions is dependent on the DNA-binding subunit p50. To verify that the proximal NF-κB-binding site is responsible for IκBζ-mediated Il10 induction, we co-transfected Raw264.7 cells with the reporter construct of the proximal Il10 promoter region (−158 to +64 bp) together with expression vectors for IκBζ and p50. The single transfection of Nfkbiz did not result in luciferase activity (Fig. 5C). Furthermore, consistent with an inhibitory role of p50 homodimers (10), the sole expression of p50 even reduced reporter gene activity. However, upon co-transfection of IκBζ and p50, luciferase activity increased by 2- to 3-fold (Fig. 5C), indicating a direct activation of Il10 gene expression by the complex of IκBζ and p50.
Active gene transcription is associated with open chromatin and trimethylation of histone H3 at lysine 4 (H3K4me3) at promoter regions. We and others have previously shown that IκBζ is required for formation of the transcription preinitiation complex and H3K4 trimethylation at targeted loci (15, 19, 32). To further substantiate a role of the proximal Il10 promoter for IκBζ-mediated gene expression, we analyzed the degree of H3K4 trimethylation in the presence and absence of Nfkbiz. To this end, we employed qPCR-coupled ChIP analysis of the endogenous proximal Il10, Il6, and Tnfa promoter regions using an H3K4me3-specific antibody. Compared with unstimulated wild-type BMMΦ, LPS stimulation resulted in an ∼20-fold enrichment of H3K4 trimethylation at the proximal Il10 promoter (Fig. 5D). An even stronger enrichment was seen for the Il6 promoter, whereas H3K4 trimethylation of the proximal Tnfa promoter was barely affected. Importantly, compared with wild-type BMMΦ, no H3K4 trimethylation was detectable at the endogenous Il6 and Il10 promoters in IκBζ-deficient cells (Fig. 5D). The transcription-associated H3K4 trimethylation at the Il10 promoter was exclusively observed in the presence of IκBζ. Thus, we conclude that IκBζ regulates IL-10 expression by directly binding to the proximal region of the Il10 promoter together with p50.
IL-10 Partially Reverses the M1 Phenotype of Nfkbiz−/− BMMΦ
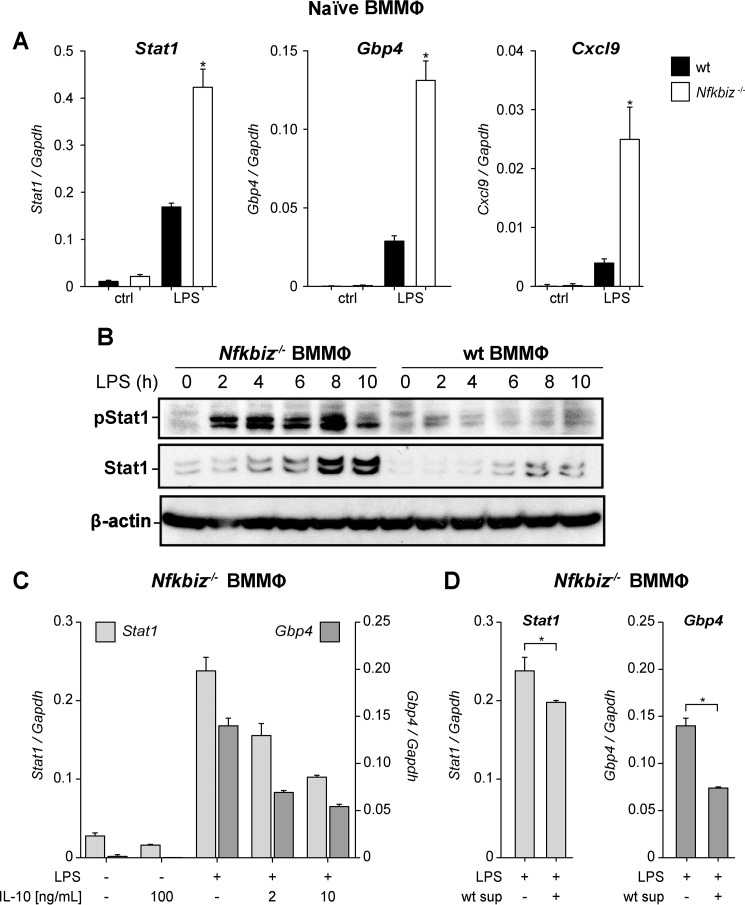
Our previous gene expression analysis comparing IκBζ-proficient and -deficient macrophages (GEO accession no. GSE43075, Ref. 15) revealed a strong up-regulation of several M1 markers in the absence of IκBζ, which is consistent with the pro-inflammatory phenotype of Nfkbiz−/− mice. Examples for the preferential M1 polarization are the elevated mRNA expression of Stat1, Gbp4, and Cxcl9 as well as the increased expression and phosphorylation of Stat1 protein in LPS-stimulated Nfkbiz−/− BMMΦ compared with the wild-type counterparts (Fig. 6, A and B).
FIGURE 6.
IL-10 partially attenuates the M1-hyperpolarized state of Nfkbiz-deficient bone marrow macrophages. A, BMMΦ from wild-type and Nfkbiz−/− mice were cultured in the presence or absence of 1 μg/ml LPS for 5 h. Cells were subsequently assayed for the indicated mRNA levels. Values were normalized to Gapdh and are mean ± S.D. from four experiments. *, statistical significance comparing wild-type and knockout cells; N.D., not detectable. ctrl, control. B, wild type and Nfkbiz−/− BMMΦ were stimulated with 1 μg/ml LPS for the indicated times. The levels of Stat1, phospho-Stat1 (Tyr701), and β-actin were analyzed by immunoblotting. An exemplary set of data is shown. C and D, Nfkbiz−/− BMMΦ were cultured in the presence of 1 μg/ml LPS or left untreated. After 4 h, the supernatants were replaced by medium supplemented with the indicated concentrations of IL-10 (C) or by supernatants from wild-type BMMΦ (wt) challenged for 24 h with LPS (D). Samples for qRT-PCR were prepared after 4 h of incubation and analyzed for relative mRNA expression of Stat1 and Gbp4. Values are mean ± S.D. from three experiments.
To investigate a potential role of IL-10 in this M1 polarization, we incubated Nfkbiz−/− BMMΦ in the presence of LPS and varying concentrations of IL-10 and examined the expression of the M1 markers Stat1 and Gbp4. Quantitative RT-PCR revealed a dose-dependent reduction of Stat1 and Gbp4 expression by exogenous IL-10 (Fig. 6C), whereas Cxcl9 expression remained largely unaffected (data not shown). Importantly, when Nfkbiz−/− BMMΦ were incubated with IL-10-proficient supernatants from LPS-treated wild-type BMMΦ, a significant reduction of Stat1 and Gbp4 expression was also detected (Fig. 6D). The reduction of Gbp4 expression roughly corresponded to the reduction observed with 2 ng/ml IL-10, a concentration similar to that present in supernatants of LPS-stimulated BMMΦ (Fig. 3A). Thus, these results not only show that IL-10 signaling is functional in Nfkbiz−/− BMMΦ but, moreover, indicate that the lack of IL-10 in Nfkbiz−/− BMMΦ at least partially contributes to their enhanced M1 polarization.
Discussion
Growing evidence suggests that the induction of NF-κB-regulated genes is not solely defined by the nuclear translocation of NF-κB but that different NF-κB target genes have individual expression profiles regarding kinetic, stimulus, or cell type, thereby ensuring a selectivity of an immune response. Several recent studies identified a subfamily of atypical IκB proteins as important “specifiers” that select particular κB-sites to be activated or repressed under certain conditions (14).
IκBζ is mostly regarded as a pro-inflammatory regulator, as demonstrated e.g. by its requirement for Th17 differentiation and expression of particular pro-inflammatory cytokines. Nevertheless, Nfkbiz−/− mice show a pro-inflammatory phenotype and M1 hyperpolarization of macrophages (15, 16, 24, 25), suggesting that so far unknown anti-inflammatory mediators might be controlled by IκBζ. In this study, we found that induction of the potent anti-inflammatory cytokine IL-10 by LPS but also by TLR2 agonists (data not shown) was strictly dependent on IκBζ and strongly reduced in Nfkbiz−/− mice. These results were supported by the finding that the doxycycline-inducible expression of ectopic IκBζ in Raw264.7 macrophages tightly correlated with increasing Il10 mRNA levels. Moreover, reporter analysis revealed that the proximal κB site of the Il10 promoter was responsible for IκBζ-mediated Il10 expression. The recruitment of IκBζ was associated with histone H3K4 trimethylation of the proximal promoter region as a marker of active gene transcription. Interestingly, in the absence of IκBζ, H3K4 trimethylation did not occur, which, along with other lines of evidence, suggests that chromatin remodeling is essential for IκBζ action. Thus, our results in knockout and overexpression models suggest that transcriptional regulation of Il10 directly depends on IκBζ.
So far, the expression of IL-10 in macrophages is known to be primarily regulated by transcription factors such as SP1, C/EBPβ, IRF1, and STAT3 (28, 33–35), whereas a role of different NF-κB proteins is relatively unknown. Because of the lack of a transcription activation domain, p50 NF-κB homodimers, which retain their ability to bind to κB sites, are thought to be transcriptional repressors. Interestingly, although not investigating atypical IκB proteins, earlier studies already showed that p50 homodimers bind to the proximal Il10 promoter and activate Il10 transcription in primary macrophages (36).
Moreover, in contrast to IκBζ, Bcl-3, a related atypical IκB protein that also requires p50 for co-regulation, negatively regulates Il10 transcription in macrophages (27, 37), although the exact role of Bcl-3 for Il10 expression is controversial (38). Bcl3 knockout mice show enhanced susceptibility to infection with Listeria monocytogenes, which is due to enhanced expression of IL-10, resulting in diminished levels of IL-12p70 and IFNγ. These results suggest that atypical IκB proteins, such as Bcl-3 and IκBζ, might regulate gene expression in an opposite manner, which is also underlined by the fact that IL-12p70 and IFNγ are direct IκBζ targets. Likewise, Bcl-3 and IκBζ have an antagonistic effect on CCL2 expression in macrophages. Although Bcl-3 inhibits the expression of CCL2, IκBζ promotes the expression of this chemokine (15, 38). Another example of such opposite gene regulation by atypical IκB proteins concerns IκBζ and IκBNS. For instance, although IκBζ is required for IL-6, IL-12p40, and G-CSF expression (15, 16), IκBNS apparently inhibits transcription of these cytokines (39, 40). Interestingly, our gene expression profiling suggest that atypical IκB proteins might also influence each other at the transcriptional level and, moreover, compete with each other for p50-mediated DNA binding. Together, these findings suggest that atypical IκB proteins form a complex network in controlling NF-κB responses.
In addition to transcription factor binding, previous studies suggested that Il10 expression is regulated by changes in the chromatin structure at the IL10 locus. The histone deacetylase HDAC11 has been found to inhibit IL-10 expression (41), whereas phosphorylation of histone H3 at serine 10 is needed for transcriptional activation of the Il10 promoter (42). A recent study found that IκBζ recruits the epigenetic modifier Tet2 to selective promoter regions independent of DNA methylation (43). IκBζ further mediates chromatin remodeling by recruiting the SWI/SNF complex to target genes, thereby enhancing promoter accessibility (32). The same mechanism presumably underlies the regulation of Il10 expression because we found that Il10 promoter accessibility and H3K4 trimethylation were reduced in Nfkbiz−/− cells. Thus, it will be interesting to explore whether Bcl-3 and IκBζ mediate their antagonistic effects at the Il10 promoter by recruiting distinct histone-modifying enzymes.
Although cells of the macrophage lineage are a major source of IL-10, several other cell types of the innate and adaptive immune system can express this cytokine (6, 7). Further studies are needed to explore whether the strict control of IL-10 expression by IκBζ is also relevant to other cell types. Our exemplary investigation of wild-type and Nfkbiz−/− MEFs indicates that the described mechanism is not restricted to macrophages. Interestingly, previous gene expression analysis revealed that IκBζ-deficient macrophages show a bias toward M1 polarization, evidenced by the increased expression of certain M1 markers (15). It is worth mentioning that p50-deficient mice also show exacerbated M1-driven inflammation and reduced M2 polarization of their macrophages (44), although several phenotypic alterations are distinct between Nfkbiz−/− and p50-deficient mice.
In functional studies, we found that the increased mRNA expression of Stat1 and Gpb4 could be partially reverted not only by IL-10 supplementation but also by IL-10-proficient supernatants from wild-type macrophages even though no reduction in Cxcl9 expression was observed. It was not the intention of our study to investigate the role of IκBζ-mediated IL-10 expression in macrophage polarization. Our results, however, indicate that decreased IL-10 expression contributes to at least some of the features of M1 polarization in Nfkbiz−/− mice. In line, IL-10-producing monocytes have been found to preferentially differentiate to M2 macrophages (45, 46).
Dysregulation of Il10 expression has been linked to several immune disorders. Transgenic mice overexpressing IL-10 in macrophages exhibit increased susceptibility to bacterial infections and septic shock (47). Excessive IL-10 secretion has also been linked to impaired tumor immune surveillance (48, 49). In contrast, the absence of IL-10 results in spontaneous inflammatory bowel disease (8), emphasizing its protective role in inflammatory and autoimmune conditions. We did not detect spontaneous colitis in Nfkbiz−/− mice, which might be caused by the genetic background because intestinal lesions have been reported to be least severe in C57BL/6 mice (50). It is, however, worth mentioning that Nfkbiz−/− mice exhibit an increased susceptibility to dextran sodium sulfate-induced colitis.4
In summary, we have uncovered an essential novel regulatory mechanism of Il10 gene regulation in macrophages. We demonstrate that IκBζ through p50-mediated recruitment to the proximal Il10 promoter and subsequent histone H3 modification, enables transcription of the Il10 locus. Because IL-10 plays a beneficial role in several inflammatory diseases, Nfkbiz−/− mice are an interesting model system for evaluating IκBζ as a potential therapeutic target in inflammatory diseases.
Author Contributions
Se. H., D. G. H., K. S. O., and F. E. designed the research. Se. H., D. G. H., W. S. L., and S. L. performed the research. Se. H., D. G. H., W. S. L., and F. E. analyzed the data. St. H., K. S. O., and F. E. wrote the paper.
Supplementary Material
Acknowledgments
We thank S. Gaffen, M. Morimatsu, S. Smale, I. Schmitz, and G. Totzke for reagents.
This work was supported by Deutsche Forschungsgemeinschaft Grants SFB685 and GRK1302 and Bundesminsterium für Bildung und Forschung Grant AID-NET. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Figs. 1 and 2.
S. Hörber, D. G. Hildebrand, S. Lorscheid, J. S. Frick, K. Schulze-Osthoff, and F. Essmann, unpublished results.
- PMΦ
- peritoneal macrophage(s)
- BMMΦ
- bone marrow-derived macrophage(s)
- MEF
- mouse embryonic fibroblasts
- qPCR
- quantitative PCR
- qRT-PCR
- quantitative RT-PCR
- H3K4me3
- trimethylation of histone H3 at lysine 4.
References
- 1.Gordon S., and Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 [DOI] [PubMed] [Google Scholar]
- 2.Ginhoux F., and Jung S. (2014) Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol 14, 392–404 [DOI] [PubMed] [Google Scholar]
- 3.Laskin D. L., Sunil V. R., Gardner C. R., and Laskin J. D. (2011) Macrophages and tissue injury: agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 51, 267–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray P. J., Allen J. E., Biswas S. K., Fisher E. A., Gilroy D. W., Goerdt S., Gordon S., Hamilton J. A., Ivashkiv L. B., Lawrence T., Locati M., Mantovani A., Martinez F. O., Mege J. L., Mosser D. M., et al. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sica A., and Mantovani A. (2012) Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore K. W., de Waal Malefyt R., Coffman R. L., and O'Garra A. (2001) Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 [DOI] [PubMed] [Google Scholar]
- 7.Saraiva M., and O'Garra A. (2010) The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10, 170–181 [DOI] [PubMed] [Google Scholar]
- 8.Kühn R., Löhler J., Rennick D., Rajewsky K., and Müller W. (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 [DOI] [PubMed] [Google Scholar]
- 9.O'Garra A., Vieira P. L., Vieira P., and Goldfeld A. E. (2004) IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J. Clin. Invest. 114, 1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oeckinghaus A., Hayden M. S., and Ghosh S. (2011) Crosstalk in NF-κB signaling pathways. Nat. Immunol. 12, 695–708 [DOI] [PubMed] [Google Scholar]
- 11.Hinz M., Arslan S. Ç., and Scheidereit C. (2012) It takes two to tango: IκBs, the multifunctional partners of NF-κB. Immunol. Rev. 246, 59–76 [DOI] [PubMed] [Google Scholar]
- 12.Smale S. T. (2011) Hierarchies of NF-κB target-gene regulation. Nat. Immunol. 12, 689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smale S. T. (2010) Selective transcription in response to an inflammatory stimulus. Cell 140, 833–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuster M., Annemann M., Plaza-Sirvent C., and Schmitz I. (2013) Atypical IκB proteins: nuclear modulators of NF-κB signaling. Cell Commun. Signal. 11, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildebrand D. G., Alexander E., Hörber S., Lehle S., Obermayer K., Münck N. A., Rothfuss O., Frick J. S., Morimatsu M., Schmitz I., Roth J., Ehrchen J. M., Essmann F., and Schulze-Osthoff K. (2013) IκBζ is a transcriptional key regulator of CCL2/MCP-1. J. Immunol. 190, 4812–4820 [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M., Yamazaki S., Uematsu S., Sato S., Hemmi H., Hoshino K., Kaisho T., Kuwata H., Takeuchi O., Takeshige K., Saitoh T., Yamaoka S., Yamamoto N., Yamamoto S., Muta T., et al. (2004) Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκBζ. Nature 430, 218–222 [DOI] [PubMed] [Google Scholar]
- 17.Totzke G., Essmann F., Pohlmann S., Lindenblatt C., Jänicke R. U., and Schulze-Osthoff K. (2006) A novel member of the IκB family, human IκB-ζ, inhibits transactivation of p65 and its DNA binding. J. Biol. Chem. 281, 12645–12654 [DOI] [PubMed] [Google Scholar]
- 18.Trinh D. V., Zhu N., Farhang G., Kim B. J., and Huxford T. (2008) The nuclear IκB protein IκBζ specifically binds NF-κB p50 homodimers and forms a ternary complex on κB DNA. J. Mol. Biol. 379, 122–135 [DOI] [PubMed] [Google Scholar]
- 19.Kayama H., Ramirez-Carrozzi V. R., Yamamoto M., Mizutani T., Kuwata H., Iba H., Matsumoto M., Honda K., Smale S. T., and Takeda K. (2008) Class-specific regulation of pro-inflammatory genes by MyD88 pathways and IκBζ. J. Biol. Chem. 283, 12468–12477 [DOI] [PubMed] [Google Scholar]
- 20.Okamoto K., Iwai Y., Oh-Hora M., Yamamoto M., Morio T., Aoki K., Ohya K., Jetten A. M., Akira S., Muta T., and Takayanagi H. (2010) IκBζ regulates T(H)17 development by cooperating with ROR nuclear receptors. Nature 464, 1381–1385 [DOI] [PubMed] [Google Scholar]
- 21.Kannan Y., Yu J., Raices R. M., Seshadri S., Wei M., Caligiuri M. A., and Wewers M. D. (2011) IκBζ augments IL-12- and IL-18-mediated IFN-γ production in human NK cells. Blood 117, 2855–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander E., Hildebrand D. G., Kriebs A., Obermayer K., Manz M., Rothfuss O., Schulze-Osthoff K., and Essmann F. (2013) IκBζ is a regulator of the senescence-associated secretory phenotype in DNA damage- and oncogene-induced senescence. J. Cell Sci. 126, 3738–3745 [DOI] [PubMed] [Google Scholar]
- 23.Johansen C., Mose M., Ommen P., Bertelsen T., Vinter H., Hailfinger S., Lorscheid S., Schulze-Osthoff K., and Iversen L. (2015) IκBζ is a key driver in the development of psoriasis. Proc. Natl. Acad. Sci. U.S.A. 112, E5825-E5833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiina T., Konno A., Oonuma T., Kitamura H., Imaoka K., Takeda N., Todokoro K., and Morimatsu M. (2004) Targeted disruption of MAIL, a nuclear IκB protein, leads to severe atopic dermatitis-like disease. J. Biol. Chem. 279, 55493–55498 [DOI] [PubMed] [Google Scholar]
- 25.Okuma A., Hoshino K., Ohba T., Fukushi S., Aiba S., Akira S., Ono M., Kaisho T., and Muta T. (2013) Enhanced apoptosis by disruption of the STAT3-IκB-ζ signaling pathway in epithelial cells induces Sjögren's syndrome-like autoimmune disease. Immunity 38, 450–460 [DOI] [PubMed] [Google Scholar]
- 26.Kuwata H., Watanabe Y., Miyoshi H., Yamamoto M., Kaisho T., Takeda K., and Akira S. (2003) IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-α production in macrophages. Blood 102, 4123–4129 [DOI] [PubMed] [Google Scholar]
- 27.Riemann M., Endres R., Liptay S., Pfeffer K., and Schmid R. M. (2005) The IκB protein Bcl-3 negatively regulates transcription of the IL-10 gene in macrophages. J. Immunol. 175, 3560–3568 [DOI] [PubMed] [Google Scholar]
- 28.Brightbill H. D., Plevy S. E., Modlin R. L., and Smale S. T. (2000) A prominent role for Sp1 during lipopolysaccharide-mediated induction of the IL-10 promoter in macrophages. J. Immunol. 164, 1940–1951 [DOI] [PubMed] [Google Scholar]
- 29.Shen F., Ruddy M. J., Plamondon P., and Gaffen S. L. (2005) Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-α-induced genes in bone cells. J. Leukocyte Biol. 77, 388–399 [DOI] [PubMed] [Google Scholar]
- 30.Graupner V., Alexander E., Overkamp T., Rothfuss O., De Laurenzi V., Gillissen B. F., Daniel P. T., Schulze-Osthoff K., and Essmann F. (2011) Differential regulation of the proapoptotic multidomain protein Bak by p53 and p73 at the promoter level. Cell Death Differ. 18, 1130–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowland J. B., Muta T., and Borregaard N. (2006) IL-1β-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IκB-ζ. J. Immunol. 176, 5559–5566 [DOI] [PubMed] [Google Scholar]
- 32.Tartey S., Matsushita K., Vandenbon A., Ori D., Imamura T., Mino T., Standley D. M., Hoffmann J. A., Reichhart J. M., Akira S., and Takeuchi O. (2014) Akirin2 is critical for inducing inflammatory genes by bridging IκB-ζ and the SWI/SNF complex. EMBO J. 33, 2332–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tone M., Powell M. J., Tone Y., Thompson S. A., and Waldmann H. (2000) IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J. Immunol. 165, 286–291 [DOI] [PubMed] [Google Scholar]
- 34.Brenner S., Prösch S., Schenke-Layland K., Riese U., Gausmann U., and Platzer C. (2003) cAMP-induced interleukin-10 promoter activation depends on CCAAT/enhancer-binding protein expression and monocytic differentiation. J. Biol. Chem. 278, 5597–5604 [DOI] [PubMed] [Google Scholar]
- 35.Ziegler-Heitbrock L., Lötzerich M., Schaefer A., Werner T., Frankenberger M., and Benkhart E. (2003) IFN-α induces the human IL-10 gene by recruiting both IFN regulatory factor 1 and Stat3. J. Immunol. 171, 285–290 [DOI] [PubMed] [Google Scholar]
- 36.Cao S., Zhang X., Edwards J. P., and Mosser D. M. (2006) NF-κB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J. Biol. Chem. 281, 26041–26050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wessells J., Baer M., Young H. A., Claudio E., Brown K., Siebenlist U., and Johnson P. F. (2004) BCL-3 and NF-κB p50 attenuate lipopolysaccharide-induced inflammatory responses in macrophages. J. Biol. Chem. 279, 49995–50003 [DOI] [PubMed] [Google Scholar]
- 38.Carmody R. J., Ruan Q., Palmer S., Hilliard B., and Chen Y. H. (2007) Negative regulation of toll-like receptor signaling by NF-κB p50 ubiquitination blockade. Science 317, 675–678 [DOI] [PubMed] [Google Scholar]
- 39.Hirotani T., Lee P. Y., Kuwata H., Yamamoto M., Matsumoto M., Kawase I., Akira S., and Takeda K. (2005) The nuclear IκB protein IκBNS selectively inhibits lipopolysaccharide-induced IL-6 production in macrophages of the colonic lamina propria. J. Immunol. 174, 3650–3657 [DOI] [PubMed] [Google Scholar]
- 40.Kuwata H., Matsumoto M., Atarashi K., Morishita H., Hirotani T., Koga R., and Takeda K. (2006) IκBNS inhibits induction of a subset of Toll-like receptor-dependent genes and limits inflammation. Immunity 24, 41–51 [DOI] [PubMed] [Google Scholar]
- 41.Villagra A., Cheng F., Wang H. W., Suarez I., Glozak M., Maurin M., Nguyen D., Wright K. L., Atadja P. W., Bhalla K., Pinilla-Ibarz J., Seto E., and Sotomayor E. M. (2009) The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat. Immunol. 10, 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucas M., Zhang X., Prasanna V., and Mosser D. M. (2005) ERK activation following macrophage FcγR ligation leads to chromatin modifications at the IL-10 locus. J. Immunol. 175, 469–477 [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q., Zhao K., Shen Q., Han Y., Gu Y., Li X., Zhao D., Liu Y., Wang C., Zhang X., Su X., Liu J., Ge W., Levine R. L., Li N., and Cao X. (2015) Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porta C., Rimoldi M., Raes G., Brys L., Ghezzi P., Di Liberto D., Dieli F., Ghisletti S., Natoli G., De Baetselier P., Mantovani A., and Sica A. (2009) Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor κB. Proc. Natl. Acad. Sci. U.S.A. 106, 14978–14983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasse A., Germann M., Pechkovsky D. V., Markert A., Verres T., Stahl M., Melchers I., Luttmann W., Müller-Quernheim J., and Zissel G. (2007) IL-10-producing monocytes differentiate to alternatively activated macrophages and are increased in atopic patients. J. Allergy Clin. Immunol. 119, 464–471 [DOI] [PubMed] [Google Scholar]
- 46.Makita N., Hizukuri Y., Yamashiro K., Murakawa M., and Hayashi Y. (2015) IL-10 enhances the phenotype of M2 macrophages induced by IL-4 and confers the ability to increase eosinophil migration. Int. Immunol. 27, 131–141 [DOI] [PubMed] [Google Scholar]
- 47.Lang R., Rutschman R. L., Greaves D. R., and Murray P. J. (2002) Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J. Immunol. 168, 3402–3411 [DOI] [PubMed] [Google Scholar]
- 48.Béguelin W., Sawh S., Chambwe N., Chan F. C., Jiang Y., Choo J. W., Scott D. W., Chalmers A., Geng H., Tsikitas L., Tam W., Bhagat G., Gascoyne R. D., and Shaknovich R. (2015) IL10 receptor is a novel therapeutic target in DLBCLs. Leukemia 29, 1684–1694 [DOI] [PubMed] [Google Scholar]
- 49.Ruffell B., Chang-Strachan D., Chan V., Rosenbusch A., Ho C. M., Pryer N., Daniel D., Hwang E. S., Rugo H. S., and Coussens L. M. (2014) Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26, 623–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berg D. J., Davidson N., Kühn R., Müller W., Menon S., Holland G., Thompson-Snipes L., Leach M. W., and Rennick D. (1996) Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4 TH1-like responses. J. Clin. Invest. 98, 1010–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.