Abstract
Wnt signaling plays a critical role in embryonic development, tissue homeostasis, and cancer development. Dishevelled (Dvl) is an essential and central component in Wnt signaling, and its stability and activity is tightly regulated. It has been shown that Dvl can be degraded via both the proteasome and autophagy-lysosome pathways. Here we report that receptor for activated C kinase 1 (RACK1) negatively regulates Dishevelled stability and Wnt signaling. RACK1 interacts with Dvl proteins and promotes their lysosomal degradation, and this effect is enhanced by autophagy induction. RACK1 also interacts with LC3 and enhances the association of LC3 with Dvl2, thereby leading to degradation of Dvl proteins through autophagy. These findings reveal a novel regulatory function of RACK1 in Wnt signaling by modulating Dvl stability.
Keywords: autophagy, lysosome, protein degradation, protein stability, Wnt signaling, Dishevelled, RACK1
Introduction
Wnt proteins regulate embryonic development and tissue homeostasis, and deregulation of Wnt signaling is closely associated with a variety of human diseases such as cancer (1, 2). In the canonical Wnt pathway, Wnt ligands initiate signal transduction through interacting with their cell surface receptors Frizzled and low-density lipoprotein receptor-related protein 5/6 (LRP5/6). The downstream mediator Dishevelled (Dvl)4 is then recruited to Frizzled, and subsequent recruitment of Axin to LRP5/6 leads to disruption of the β-catenin destruction complex, which contains Axin, adenomatous polyposis coli, glycogen synthase kinase 3-β (GSK3β), and casein kinase 1-α (CK1α), and thus β-catenin accumulation. β-Catenin works together with T cell factor to regulate the expression of Wnt target genes in the nucleus. In addition, Wnt ligands can also transduce their signals via β-catenin-independent pathways such as the planar cell polarity pathway and the Ca2+-dependent Wnt pathway (3, 4).
Dvl proteins (Dvl1, 2, and 3) are a central component to relay Wnt signaling from the receptors to downstream effectors in both canonical and non-canonical Wnt pathways, and their activity and stability are tightly controlled (5). Dvl proteins can be degraded through either the proteasomal or the autophagosome-lysosomal pathways. Several E3 ubiquitin ligases have been identified to target Dvl proteins for proteasomal degradation, such as KLHL12-Cullin-3 (6), NEDL1 (7), Itchy (8), and NEDD4L (9). We have also demonstrated that Dvl proteins can be degraded via autophagy after ubiquitinated by von Hippel-Lindau tumor suppressor (pVHL) (10). Dapper1, a Dvl-binding protein, acts as a scaffold protein to enhance the interaction between pVHL and Dvl and facilitates autophagic degradation of Dvl proteins (11, 12).
Receptor for activated C kinase 1 (RACK1) is a scaffold protein with seven WD40 domains that mediate protein-protein interactions (13, 14). Via associating with PKC, Src, and other proteins, RACK1 regulates several cellular signaling pathways (13, 14). In particular, RACK1 has been reported to promote Wnt/planar cell polarity signaling by interacting with van Gogh-like 2 (Vangl2) or tyrosine-protein kinase-like 7 (PTK7) while inhibiting canonical Wnt signaling by stabilizing the β-catenin destruction complex in gastric cancer cells (15–17).
In this study, we showed that RACK1 promoted Dvl degradation by enhancing the Dvl-LC3 interaction and thereby Dvl recruitment to the autophagosome. Together with the previous reports, this shows that RACK1 can modulate Wnt signaling via various mechanisms.
Experimental Procedures
Cell Culture and Transfection
Human HEK293T cells and normal rat kidney (NRK) cells were cultured in DMEM supplemented with 10% FBS (Hyclone) at 37 °C in a humidified 5% CO2 incubator. Transfection was accomplished with Lipofectamine 2000 (Invitrogen) or VigoFect (Vigorous Biotechnology, Beijing, China), following the instructions of the manufacturers.
Constructs
The complete coding sequence of RACK1 was amplified and cloned from human cDNA and inserted into pcDNA3.1(+) or pCMV5 with a FLAG tag, HA tag, or Myc tag at the 5′ terminus to make the constructs expressing FLAG-RACK1, HA-RACK1, and Myc-RACK. RACK1 with an LC3-interacting region mutation was generated by PCR-based mutagenesis as described previously (18). Constructs expressing shRNA targeting human RACK1 were purchased from Sigma, and lentivirus-mediated RNA interference was performed following the manual. Other plasmids have been described previously (10–12, 19).
Antibodies and Reagents
Anti-FLAG (M2) antibody was purchased from Sigma, anti-RACK1 from BD Biosciences, anti-Dvl2 from Cell Signaling Technology, anti-p62 and anti-LC3 from MBL, and other antibodies were from Santa Cruz Biotechnology. MG132 was purchased from Selleck, cycloheximide from Inalco, and other chemicals from Sigma.
Reporter Assay
The Topflash luciferase reporter was reported previously (20), and the reporter assay was conducted following the protocol described previously (21). HEK293T cells cultured in a 48-well plate were transfected with Renilla, Topflash, and other indicated plasmids for 24 h, and then the cells were treated with Wnt3a conditioned medium or rapamycin for the indicated time. The cells in each well were lysed in 100 μl of passive lysate buffer, and 20 μl of lysate was loaded into a 96-well white plate for measurement of luciferase and Renilla activity using a luciferase assay system (Promega). Reporter assays were conducted with three biological repeats, and the luciferase activity is presented as means ± S.D. after normalizing to Renilla activity. Statistical analysis was performed with two-tailed unpaired Student's t test, and p < 0.05 was considered statistically significant.
Immunoprecipitation, Immunoblotting, Immunofluorescence, and Statistical Analysis
These proceeded as described previously (18). Immunofluorescence images were captured by laser-scanning microscope (FV1200, Olympus), and analyzed by FluoView (v. 4.1) and ImageJ (22). Briefly, HEK293T cells cultured in a 12-well plate were lysed in 100 μl of TNE buffer (50 mm Tris, 0.5% Nonidet P-40, 1 mm EDTA, 150 mm NaCl, pH 7.5), and 10 μl of lysate was used. Cells in a 6-well plate or 10-cm dish were harvested and lysed with 200 μl to 1 ml TNE buffer for exogenous or endogenous immunoprecipitation, respectively. Total cell lysates (20 μl) or precipitated samples were separated on SDS-PAGE gels and then transferred to nitrocellulose membranes (Pall). The membranes were incubated with primary antibodies at 4 °C overnight and then incubated with secondary antibodies at room temperature for 1 h. The specific proteins were detected using a chemiluminescence immunoblotting kit (Millipore). The immunoblotting film was scanned and quantified by ImageJ. Three independent experiments were used for quantification analyses. Two-tailed unpaired Student's t test was performed, and p < 0.05 was considered statistically significant. All experiments were independently repeated three times, and a representative one is shown.
Results
RACK1 Inhibits Dvl-mediated Wnt Signaling
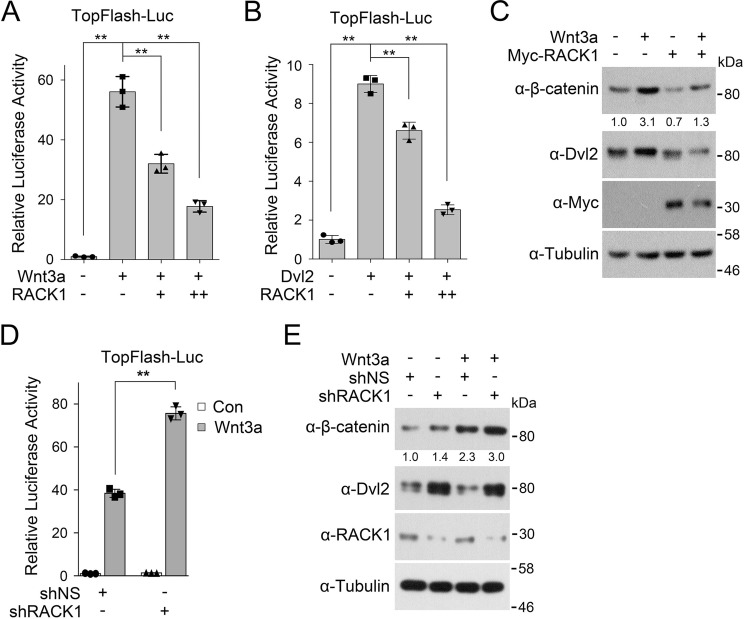
An early study reported that RACK1 could inhibit Wnt/β-catenin signaling by promoting the interaction between GSK3β and β-catenin and thus β-catenin degradation (17). We confirmed the inhibitory effect of RACK1 on Wnt signaling. The Topflash luciferase reporter, which contains three T cell factor response elements (CCTTTGATC) upstream of a c-fos promoter (20), is widely used as a transcriptional reporter for Wnt/β-catenin signaling. We observed that RACK1 attenuated Wnt-induced or Dvl2-induced Topflash activity (Fig. 1, A and B), which acts upstream of GSK3β and β-catenin. In agreement with this, overexpression of RACK1 led to a decrease in β-catenin level regardless of the presence of Wnt3a (Fig. 1C). These results were supported by RACK1 knockdown experiments. Depletion of RACK1 up-regulated the Wnt3a-stimulated reporter (Fig. 1D) and resulted in the accumulation of β-catenin (Fig. 1E). These data together suggest that RACK1 could negatively regulate Wnt signaling at the Dvl level.
FIGURE 1.
RACK1 attenuates Dvl-mediated Wnt signaling. A, after transfection with Topflash luciferase with or without Myc-RACK1 for 24 h, HEK293T cells were treated with control medium or Wnt3a conditioned medium (CM) overnight, and then luciferase activity was measured. The pRL-TK Renilla reporter was co-transfected to normalize transfection efficiency. Myc-RACK1 was transfected with gradients of 50 or 100 ng. B, HEK293T cells were transfected with Topflash luciferase, Myc-RACK1, and FLAG-Dvl2 as indicated for 24 h and harvested for luciferase activity determination. C, HEK293T cells were transfected with or without Myc-RACK1. After 24 h, the cells were treated with Wnt3a CM for 6 h and then harvested for immunoblotting. Tubulin served as a loading control. D, HEK293T cells were transfected with Topflash luciferase (Luc) for 24 h after being infected by a lentivirus expressing nonspecific or RACK1 shRNA. Cells were then treated with control (Con) medium or Wnt3a CM overnight, and then luciferase activity was measured. The pRL-TK Renilla reporter was co-transfected to normalize transfection efficiency. E, HEK293T cells were infected by a lentivirus expressing nonspecific or RACK1 shRNA. After 48 h, the cells were treated with Wnt3a CM for 6 h and then harvested for IB. The protein levels of β-catenin were quantified and normalized against tubulin, and the values are shown below the bands. All reporter assays were performed in triplicate, and the data are represented as mean ± S.D. after being normalized against Renilla activity. Statistical analysis was performed with two-tailed unpaired Student's t test (**, p < 0.01).
RACK1 Accelerates Dvl Degradation
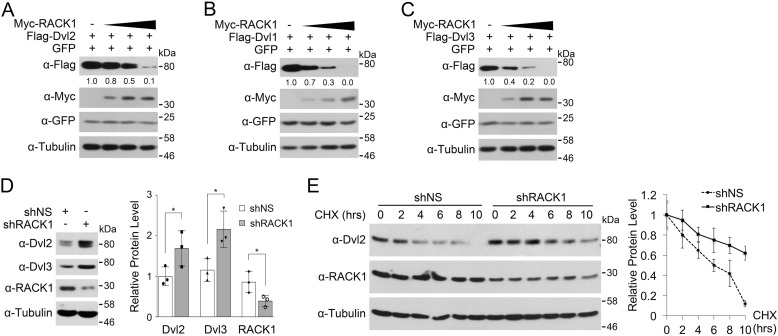
The data in Fig. 1, C and D, also indicate that RACK1 could induce Dvl2 degradation. To further assess this issue, we coexpressed RACK1 with Dvl2 in HEK293T cells. As shown in Fig. 2A, RACK1 overexpression decreased the protein level of Dvl2 in a dose-dependent manner. Furthermore, RACK1 could also reduce the protein levels of Dvl1 and Dvl3 (Fig. 2, B and C). Consistent with these data, RACK1 knockdown led to an increase in the protein level of Dvl2 and Dvl3 (Fig. 2D). It has been reported that RACK1 can bind to ribosomes and affect mRNA translation and protein synthesis (23). To exclude the effect of RACK1 in Dvl protein synthesis, the cells were treated with cycloheximide to block translation. The result showed that RACK1 knockdown stabilized Dvl2 protein and prolonged the half-life of Dvl2 (Fig. 2E).
FIGURE 2.
RACK1 promotes Dvl degradation. A–C, HEK293T cells were transfected with FLAG-Dvl1, Dvl2, or Dvl3 and Myc-RACK1 after 24 h and then harvested for immunoblotting. Myc-RACK1 was transfected with concentration gradients of 0.25, 0.5, and 1.0 μg each. GFP was co-transfected as a transfection control. The protein levels of FLAG-Dvl1, Dvl2, and Dvl3 were quantified and normalized against tubulin, and the values are shown below the bands. D, HEK293T cells were infected by a lentivirus expressing nonspecific (NS) or RACK1 shRNA for 48 h and harvested for immunoblotting. Three independent experiments were conducted for quantification analyses. The protein levels of Dvl2, Dvl3, and RACK1 were normalized against tubulin, and the data are represented as mean ± S.D. in the right panel. Two-tailed unpaired t test was used in statistical analyses (*, p < 0.05). E, HEK293T cells infected by a lentivirus expressing nonspecific or RACK1 shRNA were treated with cycloheximide (CHX, 20 μg/ml) for the indicated time. Three independent experiments were conducted for quantification analyses. The protein levels of Dvl2 were normalized against tubulin, and the data are represented as mean ± S.D. in the right panel.
RACK1 Interacts with Dvl2
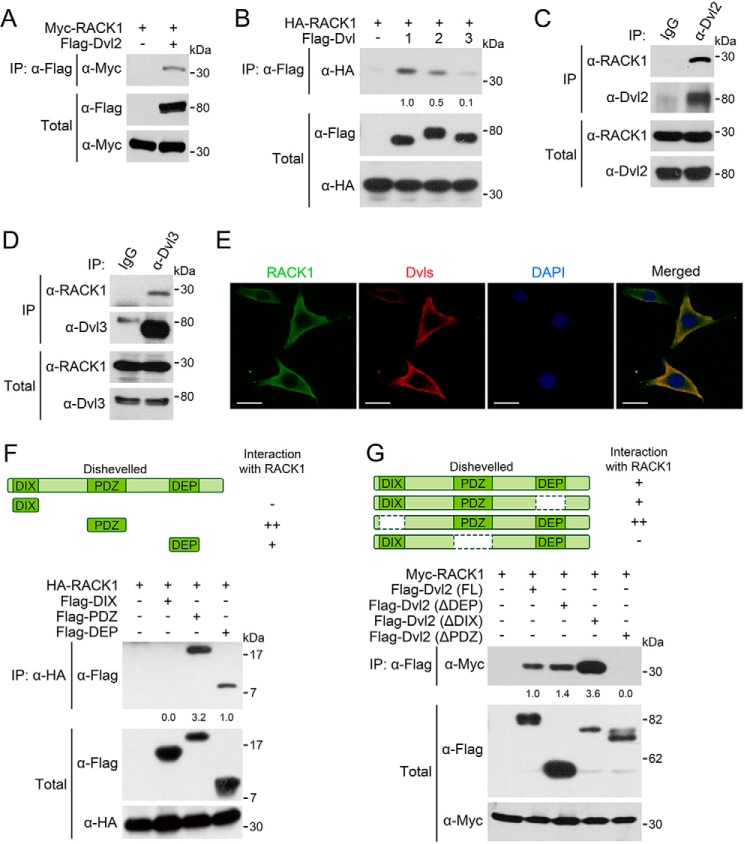
To investigate how RACK1 promotes Dvl2 degradation, we examined whether RACK1 and Dvl2 could interact with each other. An immunoprecipitation experiment revealed an interaction between RACK1 and Dvl2 when they were exogenously expressed in HEK293T cells (Fig. 3A). RACK1 also associated with Dvl1 and Dvl3 when they were overexpressed (Fig. 3B). The interaction between RACK1 and Dvl2 or Dvl3 was also detected at the endogenous level (Fig. 3, C and D). The two proteins were colocalized in the cytoplasm, as revealed by immunofluorescence (Fig. 3E).
FIGURE 3.
RACK1 interacts with Dvl proteins. A, HEK293T cells were harvested 24 h after being transfected with FLAG-Dvl2 and Myc-RACK1 for anti-FLAG IP followed by IB. The total cell lysates were analyzed by IB with anti-FLAG and anti-Myc antibodies. B, HEK293T cells were harvested 24 h after transfected with FLAG-Dvl1/Dvl2/Dvl3 and HA-RACK1. C and D, HEK293T cells were harvested for IP with anti-Dvl2/Dvl3 antibodies or anti-IgG serum as a control. E, NRK cells were fixed and stained with the indicated antibodies for immunofluorescence. DAPI stained the nucleus. Scale bars = 10 μm. F and G, HEK293T cells were transfected with HA/Myc-RACK1 and truncated Dvl2 fragments. After 24 h, the cells were harvested for anti-HA/FLAG IP followed by IB. The protein levels of precipitated proteins were quantified and normalized against their corresponding total proteins levels, and the values are shown below the bands. All the experiments were independently repeated three times, and a representative one is shown.
Dvl proteins have three important and conserved domains (DEP domain, PDZ domain, and DIX domain) that mediate their aggregation or interactions with other proteins (5). To map the domains mediating the interaction between RACK1 and Dvl2, the truncation mutants of Dvl2 were coexpressed with RACK1. Although RACK1 interacted with both the PDZ domain and DEP domain (Fig. 3F), deletion of the PDZ domain abolished the binding of Dvl2 to RACK1 (Fig. 3G), indicating that the interaction between RACK1 and Dvl2 is mainly mediated by the PDZ domain of Dvl2.
RACK1 Facilitates Dvl Degradation via the Autophagy-Lysosome Pathway
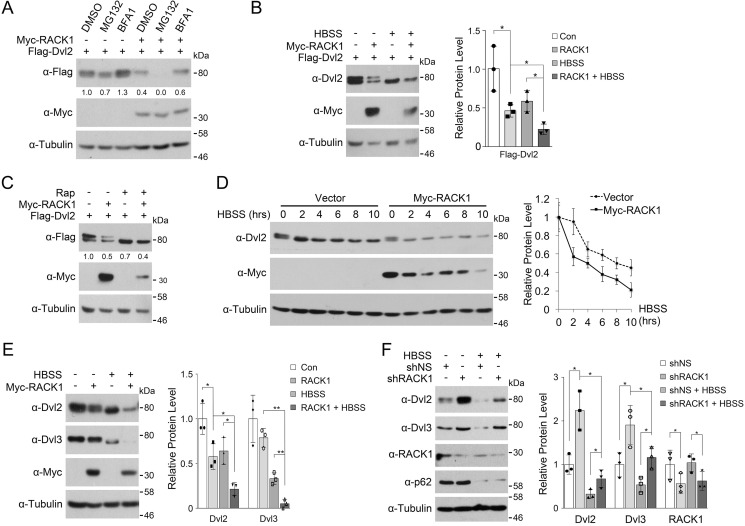
Dvl proteins have been shown to be degraded through either the proteasome or lysosome. To delineate which pathway is responsible for RACK1-mediated Dvl degradation, cells transfected with RACK1 and Dvl2 were treated with the proteasome inhibitor MG132 or the lysosome inhibitor bafilomycin A1 (BFA1). As shown in Fig. 4A, RACK1-facilitated degradation of Dvl2 was attenuated by BFA1 but not MG132, suggesting that RACK1 promotes Dvl2 degradation through the lysosome pathway. In fact, MG132 accelerated RACK1-induced Dvl degradation, which is in agreement with reports showing that this chemical can promote autophagy (24, 25).
FIGURE 4.
RACK1 facilitates starvation-induced Dvl2 degradation. A, HEK293T cells transfected with FLAG-Dvl2 and Myc-RACK1 as indicated were treated with the proteasome inhibitor MG132 (10 μg/ml) or the lysosome inhibitor BFA1 (0.2 μm) for 6 h before being harvested for immunoblotting. The protein levels of FLAG-Dvl2 were quantified and normalized against tubulin, and the values are shown below the bands. B, HEK293T cells were transfected with FLAG-Dvl2 and Myc-RACK1. After 24 h, they were starved in HBSS for 6 h before being harvested for immunoblotting. Three independent experiments were conducted for quantification analyses. The protein levels of FLAG-Dvl2 were quantified and normalized against tubulin, and the data are represented as mean ± S.D. in the right panel. Two-tailed unpaired t test was used in statistical analyses (*, p < 0.05). Con, control. C, HEK293T cells were transfected with Myc-RACK1 and FLAG-Dvl2. After 24 h, the cells were treated with rapamycin (Rap, 2 mm) for 6 h before being harvested for immunoblotting. The protein levels of FLAG-Dvl2 were quantified and normalized against tubulin, and the values are shown below the bands. D, HEK293T cells were transfected with Myc-RACK1. After 24 h, the cells were starved in HBSS for the indicated times before being harvested for immunoblotting. Three independent experiments were conducted for quantification analyses. The protein levels of Dvl2 were quantified and normalized against tubulin, and the data are represented as mean ± S.D. in the right panel. E, HEK293T cells were transfected with Myc-RACK1. After 24 h, they were starved in HBSS for 6 h before being harvested for immunoblotting. Three independent experiments were conducted for quantification analyses. The protein levels of Dvl2 and Dvl3 were quantified and normalized against tubulin, and the data are represented as mean ± S.D. in the right panel. Two-tailed unpaired t test was used in statistical analyses (*, p < 0.05 ; **, p < 0.01). F, HEK293T cells were infected by a lentivirus expressing nonspecific or RACK1 shRNA. After 48 h, cells were starved in HBSS for 6 h before being harvested for immunoblotting. p62 served as an autophagy marker. Three independent experiments were conducted for quantification analyses. The protein levels of Dvl2, Dvl3, and RACK1 were quantified and normalized against tubulin, and the data are represented as mean ± S.D. in the right panel. Two-tailed unpaired t test was used in statistical analyses (*, p < 0.05).
We have reported that Dvl proteins can degrade via starvation-induced autophagy (10). To address whether RACK1 promotes Dvl degradation through autophagy, HEK293T cells transfected with RACK1 and Dvl2 were deprived of nutrients by being treated with Hanks' balanced salt solution (HBSS) to induce autophagy. As shown in Fig. 4B, either RACK1 coexpression or HBSS treatment reduced the Dvl2 protein level, and both together had the most dramatic effect. The autophagic degradation of Dvl2 was also confirmed with the target of rapamycin (mTOR) inhibitor rapamycin, which can trigger autophagy (26) (Fig. 4C). RACK1 overexpression also accelerated the turnover of endogenous Dvl2 (Fig. 4D). RACK1 overexpression or nutrient starvation decreased the endogenous protein level of both Dvl2 and Dvl3, and both together showed the most remarkable effect (Fig. 4E). The promoting effect of RACK1 on Dvl degradation was also supported by loss-of-function experiments showing that RACK1 knockdown led to a higher level of endogenous Dvl2 and Dvl3 proteins both under nutrition-rich and starvation conditions (Fig. 4F).
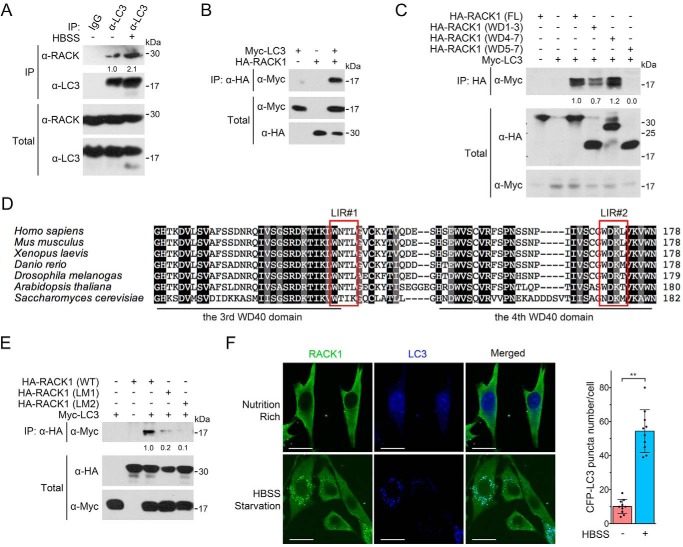
RACK1 Interacts with LC3
It was reported that loss of RACK1 resulted in attenuated autophagy and glycogen store in Drosophila, and RACK1 was partially colocalized with the Drosophila LC3 homolog Atg8 (27). We observed that RACK1 interacted with LC3 with endogenous or exogenously expressed proteins (Fig. 5, A and B) and that nutrient deprivation enhanced this interaction.
FIGURE 5.
RACK1 associates with LC3. A, HEK293T cells were starved in HBSS before being harvested for anti-LC3 immunoprecipitation followed by immunoblotting. BFA1 was added to HBSS to block LC3 degradation in starvation-induced autophagy. The total cell lysates were analyzed by IB. The protein levels of precipitated RACK1 were quantified and normalized against precipitated LC3, and the values are shown below the bands. B, HEK293T cells were transfected with Myc-LC3 and HA-RACK1 for 24 h before being harvested for IP followed by IB. The total cell lysates were also analyzed by IB. C, HEK293T cells were transfected with Myc-LC3 and HA-tagged wild-type or truncated RACK1 mutants. After 24 h, cells were harvested for IP-IB analysis. The total cell lysates were also analyzed by IB. The protein levels of precipitated Myc-LC3 were quantified and normalized to the corresponding total Myc-LC3 fragments, and the values are shown below the bands. FL, full-length. D, the amino acid sequences of RACK1 of human (P63244), mouse (P68040), frog (Q5U275), zebrafish (O42248), fruit fly (B4NZ46), Arabidopsis (O24456 and Q9LV28), and budding yeast (P38011) were aligned by ClustalX. The conserved LIR motif is boxed. E, the WXXL sequence of LIRs in human RACK1 was mutated to AXXA to generate HA-RACK1(LM1) and RACK1(LM2) mutants. HEK293T cells were transfected with Myc-LC3 and HA-RACK1/HA-RACK1(LM1/2). After 24 h, cells were harvested for IP and IB analyses. The protein levels of precipitated Myc-LC3 were quantified and normalized to total Myc-LC3, and the values are shown below the bands. F, NRK cells stably expressing CFP-LC3 were starved in HBSS for 2 h and then subjected to anti-RACK1 immunofluorescence. The CFP-LC3 puncta in each cell (n = 10 cells from three different slides) were counted, and the number of puncta per cell is represented as the mean ± S.D. in the right panel. Two-tailed unpaired Student's t test was used in statistical analyses (**, p < 0.01). Scale bars = 10 μm.
Domain mapping experiments showed that the N-terminal region containing the #1–3 WD40 domain of RACK1 had a weak binding ability with LC3 (Fig. 5C). The C-terminal region containing the #4–7 WD40 domain retained full interaction, as wild-type RACK1, whereas deletion of the #4 WD40 domain eliminated the interaction with LC3. These results suggest that RACK1 interacts with LC3 mainly through its #3 and #4 WD40 domains. It has been reported that LC3 recognizes the LC3-interacting region (LIR) motif in its binding proteins, which is a four-amino acid sequence (WXXL, where X represents any amino acid) (28). Two LIR motifs were found in human RACK1: one in the #3 WD40 domain (W132NTL135) and the other in the #4 WD40 domain (W170DKL173) (Fig. 5D). To investigate the significance of these LIR motifs, we generated mutant RACK1(LM1) with (W132A, L135A) and RACK1(LM2) with (W170A, L173A). Immunoprecipitation analysis revealed that RACK1(LM2) lost its binding ability to LC3, and RACK1(LM1) also exhibited a weak binding to LC3 (Fig. 5E), indicating that both LIR motifs are important to mediate the interaction between RACK1 and LC3. Nutrition starvation induces autophagosome formation, which can be shown by the formation of LC3 puncta (29, 30). The immunofluorescence results showed that RACK1 and LC3 were seldom colocalized under nutrition-rich conditions but colocalized under starvation conditions (Fig. 5F), suggesting that RACK1 associates with LC3 in autophagosomes.
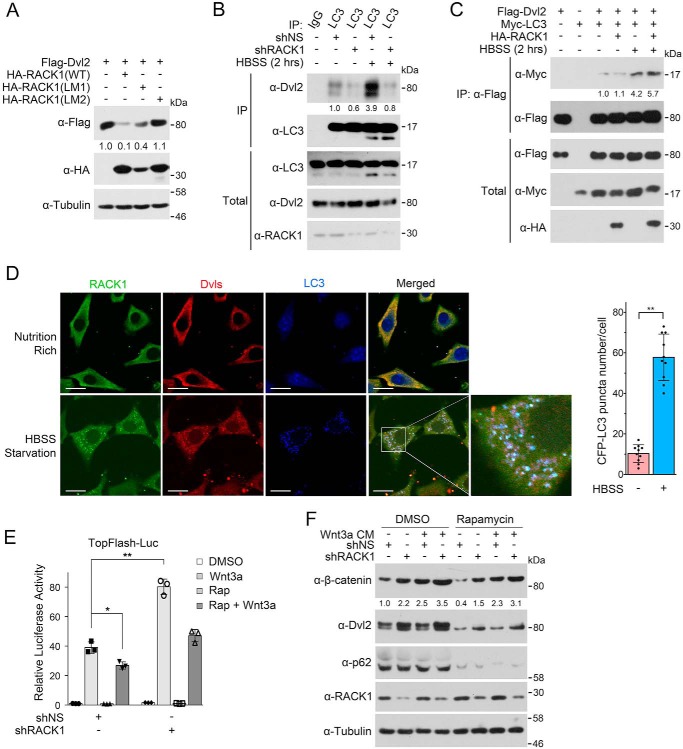
RACK1 Enhances the Recruitment of Dvl to Autophagosomes via LC3
The above data showing that RACK1 could bind to both Dvl and LC3 prompted us to ask whether RACK1 could enhance the interaction between Dvl and LC3 and thus promote Dvl recruitment to autophagosomes. Because the LIR motifs of RACK1 are critical for the LC3 interaction, we examined whether this motif was important for RACK1 to mediate Dvl degradation. Indeed, the RACK1(LM1) mutant was less efficient to induce Dvl2 degradation, and RACK1(LM2) lost the ability to promote Dvl2 degradation (Fig. 6A), indicating that RACK1-induced Dvl degradation depends on the association between RACK1 and LC3. In accordance with the above data, RACK1 knockdown dramatically reduced the endogenous interaction between LC3 and Dvl2 (Fig. 6B), and overexpressed RACK1 enhanced the interaction between Dvl2 and LC3 upon starvation, although it had no obvious effect under nutrient-rich condition (Fig. 6C). The immunofluorescence experiment also revealed the colocalization of RACK1, Dvl, and LC3 upon starvation (Fig. 6D). These results together indicate that RACK1 facilitates the recruitment of Dvl to LC3-positive autophagosomes by increasing the Dvl-LC3 interaction.
FIGURE 6.
RACK1 enhances the recruitment of Dvl to autophagosome via LC3. A, HEK293T cells were transfected with FLAG-Dvl2 and HA-RACK1 or HA-RACK1 (LM1/2), and cells were then harvested for IB. Tubulin served as a loading control. The protein levels of FLAG-Dvl2 were quantified and normalized against tubulin, and the values are shown below the bands. B, HEK293T cells were infected by a lentivirus expressing nonspecific or RACK1 shRNA. After 48 h, cells were treated with BFA1 (0.2 μm) for 12 h and then treated within HBSS plus BFA1 for another 2 h. Then cell lysates were subjected to IP-IB analysis, and the total cell lysates were analyzed by IB. The protein levels of precipitated Dvl2 were quantified and normalized against precipitated LC3, and the values are shown below the bands. C, HEK293T cells were transfected with FLAG-Dvl2, Myc-LC3, and HA-RACK1 for 24 h and then starved in HBSS for another 2 h. BFA1 (0.2 μm) was added to HBSS for preventing Dvl2 degradation. Then cell lysates were subjected to IP-IB analysis, and the total cell lysates were analyzed by IB. The precipitated Myc-LC3 was quantified and normalized against precipitated FLAG-Dvl2, and the values are shown below the bands. D, NRK cells stably expressing CFP-LC3 were treated with HBSS containing 0.2 μm BFA1 for 2 h and then fixed and stained with anti-Dvl and anti-RACK1 antibodies for immunofluorescence. The CFP-LC3 puncta in each cell (n = 10 cells from three different slides) were counted, and the number of puncta per cell is represented as the mean ± S.D. in the right panel. Two-tailed unpaired Student's t test was used in statistical analyses (**, p < 0.01). Scale bars = 10 μm. E, the experiment was conducted similarly as in Fig. 1D, and rapamycin (Rap, 2 μm) was added to induce autophagy. Luc, luciferase. F, HEK293T cells were infected by a lentivirus expressing nonspecific or RACK1 shRNA. After 48 h, the cells were treated with Wnt3a CM and/or rapamycin (2 μm) for 6 h and then harvested for IB. p62 was used as an autophagy marker. The protein levels of β-catenin were quantified and normalized to tubulin, and the values are shown below the bands.
Finally, we assessed the effect of RACK1 on autophagy-mediated inhibition of Wnt/β-catenin signaling. Consistent with our early report (10), rapamycin attenuated Wnt signaling, as shown in both Topflash reporter activity (Fig. 6E) and β-catenin accumulation (Fig. 6F), and RACK1 knockdown attenuated the inhibitory effect of rapamycin-induced autophagy on Wnt signaling.
Discussion
As a critical mediator of Wnt signaling, the activity and stability of Dvl are strictly regulated. Dvl proteins can undergo degradation via either the proteasome or the autophagy-lysosome pathway (5, 8–10, 31). We have shown that Dvl recruitment to autophagosomes could be facilitated by Dapper1 (12, 19). In this study, we identified RACK1 as a novel regulator to control Dvl stability by enhancing the interaction between Dvl and LC3 and thus enhancing their autophagic degradation.
RACK1 may act as a negative regulator of Wnt signaling via multiple mechanisms. It has been reported RACK1 stabilizes the β-catenin disruption complex and attenuates Wnt signaling in gastric tumors (17). RACK1 can associate with GSK3β and Axin and strengthen the interaction of GSK3β and β-catenin, therefore accelerating β-catenin degradation via the proteasome pathway (17). Our results demonstrate that RACK1 could also accelerate Dvl turnover and thereby prevent β-catenin accumulation. We observed that RACK1 could interact with both Dvl and LC3 and promote Dvl recruitment to the autophagosome and its degradation in the lysosome. Interestingly, RACK1 can interact with pVHL (32) and Dapper1 (data not shown), both of which facilitate Dvl recruitment to autophagosome via ubiquitination or protein-protein interaction (10, 12). It remains unclear at this moment whether RACK1 has any functional interactions with pVHL or Dapper in promoting Dvl degradation. Nonetheless, identification of multiple RACK1-interacting proteins in the Wnt pathway is consistent with its role as a multifaceted scaffold protein (14).
RACK1 has been implicated in regulating autophagy. Nutrient deprivation induced RACK1 expression, and loss of RACK1 attenuated starvation-induced autophagy in Drosophila, suggesting that RACK1 is a critical regulator of autophagy in response to starvation (27). Moreover, a recent study indicated that RACK1 could positively regulate autophagy by promoting formation of the Atg14L-Beclin 1-Vps34-Vps15 complex upon phosphorylation by 5′-adenosine monophosphate-activated protein kinase (AMPK) in the liver (33). In accordance with these observations, our results demonstrate the function of RACK1 in autophagy by facilitating substrate recruitment into the autophagosome. RACK1 binds to LC3 through the conserved LIR motif, which exists in autophagy adaptors and many other LC3-interacting proteins (34). Because RACK1 is an evolutionally conserved protein from yeast to human, and this LIR motif is also conserved in RACK1 from different species, its function in regulating autophagy could be evolutionally conserved.
The function of RACK1 in tumorigenesis and metastasis is complex because of the various proteins with which it interacts and the multiple signaling pathways in which it is involved. RACK1 expression varies in different cancer types (13). It is up-regulated in most cancer types, such as hepatocellular carcinoma (35, 36), non-small-cell lung cancer (37), and glioma (38), but decreased in gastric cancer (17). Deregulation of Wnt signaling has been observed in multiple tumors, especially in colon cancer, and we have reported that Dvl expression is negatively correlated with autophagy in the late stage of colon cancer development (10). Interestingly, RACK1 can inhibit colon cancer cell growth (39, 40) and might induce cell apoptosis (41, 42). Whether autophagic degradation of Dvl mediated by RACK1 is also involved in this process needs further investigation. Therefore, aberrant autophagy-mediated degradation of Dvl proteins may contribute to cancer development in some tissues. However, the pathophysiological role of RACK1-mediated Dvl degradation needs future clarification.
Author Contributions
M. C., H. X., W. C., and Y. G. C. designed the study, and M. C. and Y. G. C. wrote the paper. M. C., H. X., and W. C. obtained most of the data, and W. L., H. C., B. L., B. M., and X. Y. were involved in some experiments. All authors analyzed the results and approved the final version of the manuscript.
This work was supported by 973 Program Grant 2013CB933700 and National Natural Science Foundation of China Grants 31330049 (to Y. G. C.) and 31501144 (to H. X.). The authors declare that they have no conflicts of interest with the contents of this article.
- Dvl
- Dishevelled
- RACK1
- receptor for activated C kinase 1
- VHL
- von Hippel-Lindau
- NRK cell
- normal rat kidney
- BFA1
- bafilomycin A1
- HBSS
- Hanks' balanced salt solution
- LIR
- LC3-interacting region
- CM
- conditioned medium
- IB
- immunoblot
- IP
- immunoprecipitation.
References
- 1.Clevers H., and Nusse R. (2012) Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 [DOI] [PubMed] [Google Scholar]
- 2.MacDonald B. T., Tamai K., and He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devenport D. (2014) The cell biology of planar cell polarity. J. Cell Biol. 207, 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De A. (2011) Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim. Biophys. Sin. 43, 745–756 [DOI] [PubMed] [Google Scholar]
- 5.Gao C., and Chen Y. G. (2010) Dishevelled: the hub of Wnt signaling. Cell Signal. 22, 717–727 [DOI] [PubMed] [Google Scholar]
- 6.Angers S., Thorpe C. J., Biechele T. L., Goldenberg S. J., Zheng N., MacCoss M. J., and Moon R. T. (2006) The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-β-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8, 348–357 [DOI] [PubMed] [Google Scholar]
- 7.Miyazaki K., Fujita T., Ozaki T., Kato C., Kurose Y., Sakamoto M., Kato S., Goto T., Itoyama Y., Aoki M., and Nakagawara A. (2004) NEDL1, a novel ubiquitin-protein isopeptide ligase for dishevelled-1, targets mutant superoxide dismutase-1. J. Biol. Chem. 279, 11327–11335 [DOI] [PubMed] [Google Scholar]
- 8.Wei W., Li M., Wang J., Nie F., and Li L. (2012) The E3 ubiquitin ligase ITCH negatively regulates canonical Wnt signaling by targeting dishevelled protein. Mol. Cell. Biol. 32, 3903–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding Y., Zhang Y., Xu C., Tao Q. H., and Chen Y. G. (2013) HECT domain-containing E3 ubiquitin ligase NEDD4L negatively regulates Wnt signaling by targeting dishevelled for proteasomal degradation. J. Biol. Chem. 288, 8289–8298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao C., Cao W., Bao L., Zuo W., Xie G., Cai T., Fu W., Zhang J., Wu W., Zhang X., and Chen Y. G. (2010) Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat. Cell Biol. 12, 781–790 [DOI] [PubMed] [Google Scholar]
- 11.Zhang L., Gao X., Wen J., Ning Y., and Chen Y. G. (2006) Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. J. Biol. Chem. 281, 8607–8612 [DOI] [PubMed] [Google Scholar]
- 12.Ma B., Liu B., Cao W., Gao C., Qi Z., Ning Y., and Chen Y. G. (2015) The Wnt signaling antagonist Dapper1 accelerates Dishevelled2 degradation via promoting its ubiquitination and aggregate-induced autophagy. J. Biol. Chem. 290, 12346–12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J. J., and Xie D. (2015) RACK1, a versatile hub in cancer. Oncogene 34, 1890–1898 [DOI] [PubMed] [Google Scholar]
- 14.Adams D. R., Ron D., and Kiely P. A. (2011) RACK1, A multifaceted scaffolding protein: structure and function. Cell Commun. Signal. 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wehner P., Shnitsar I., Urlaub H., and Borchers A. (2011) RACK1 is a novel interaction partner of PTK7 that is required for neural tube closure. Development 138, 1321–1327 [DOI] [PubMed] [Google Scholar]
- 16.Li S., Esterberg R., Lachance V., Ren D., Radde-Gallwitz K., Chi F., Parent J. L., Fritz A., and Chen P. (2011) Rack1 is required for Vangl2 membrane localization and planar cell polarity signaling while attenuating canonical Wnt activity. Proc. Natl. Acad. Sci. U.S.A. 108, 2264–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng Y. Z., Yao F., Li J. J., Mao Z. F., Hu P. T., Long L. Y., Li G., Ji X. D., Shi S., Guan D. X., Feng Y. Y., Cui L., Li D. S., Liu Y., Du X., Guo M. Z., Xu L. Y., Li E. M., Wang H. Y., and Xie D. (2012) RACK1 suppresses gastric tumorigenesis by stabilizing the β-catenin destruction complex. Gastroenterology 142, 812–823.e15 [DOI] [PubMed] [Google Scholar]
- 18.Yan X., Lin Z., Chen F., Zhao X., Chen H., Ning Y., and Chen Y. G. (2009) Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-β signaling. J. Biol. Chem. 284, 30097–30104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma B., Cao W., Li W., Gao C., Qi Z., Zhao Y., Du J., Xue H., Peng J., Wen J., Chen H., Ning Y., Huang L., Zhang H., Gao X., Yu L., and Chen Y. G. (2014) Dapper1 promotes autophagy by enhancing the Beclin1-Vps34-Atg14L complex formation. Cell Res. 24, 912–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., and Clevers H. (1997) Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275, 1784–1787 [DOI] [PubMed] [Google Scholar]
- 21.Biechele T. L., and Moon R. T. (2008) Assaying β-catenin/TCF transcription with β-catenin/TCF transcription-based reporter constructs. Methods Mol. Biol. 468, 99–110 [DOI] [PubMed] [Google Scholar]
- 22.Schneider C. A., Rasband W. S., and Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson J., Sengupta J., Frank J., and Nissen P. (2004) Regulation of eukaryotic translation by the RACK1 protein: a platform for signalling molecules on the ribosome. EMBO Rep. 5, 1137–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada M., Strnad P., Toivola D. M., and Omary M. B. (2008) Autophagy modulates keratin-containing inclusion formation and apoptosis in cell culture in a context-dependent fashion. Exp. Cell Res. 314, 1753–1764 [DOI] [PubMed] [Google Scholar]
- 25.Tumbarello D. A., Waxse B. J., Arden S. D., Bright N. A., Kendrick-Jones J., and Buss F. (2012) Autophagy receptors link myosin VI to autophagosomes to mediate Tom1-dependent autophagosome maturation and fusion with the lysosome. Nat. Cell Biol. 14, 1024–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell R. C., Yuan H. X., and Guan K. L. (2014) Autophagy regulation by nutrient signaling. Cell Res. 24, 42–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdi B., Nagy P., Zvara A., Varga A., Pircs K., Ménesi D., Puskás L. G., and Juhász G. (2012) Loss of the starvation-induced gene Rack1 leads to glycogen deficiency and impaired autophagic responses in Drosophila. Autophagy 8, 1124–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noda N. N., Kumeta H., Nakatogawa H., Satoo K., Adachi W., Ishii J., Fujioka Y., Ohsumi Y., and Inagaki F. (2008) Structural basis of target recognition by Atg8/LC3 during selective autophagy. Genes Cells 13, 1211–1218 [DOI] [PubMed] [Google Scholar]
- 29.Ohsumi Y. (2014) Historical landmarks of autophagy research. Cell Res. 24, 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizushima N., Yoshimori T., and Ohsumi Y. (2011) The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132 [DOI] [PubMed] [Google Scholar]
- 31.Sharma J., Mulherkar S., Mukherjee D., and Jana N. R. (2012) Malin regulates Wnt signaling pathway through degradation of dishevelled2. J. Biol. Chem. 287, 6830–6839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He X., Wang J., Messing E. M., and Wu G. (2011) Regulation of receptor for activated C kinase 1 protein by the von Hippel-Lindau tumor suppressor in IGF-I-induced renal carcinoma cell invasiveness. Oncogene 30, 535–547 [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y., Wang Q., Qiu G., Zhou S., Jing Z., Wang J., Wang W., Cao J., Han K., Cheng Q., Shen B., Chen Y., Zhang W. J., Ma Y., and Zhang J. (2015) RACK1 promotes autophagy by enhancing the Atg14L-Beclin 1-Vps34-Vps15 complex formation upon phosphorylation by AMPK. Cell Rep. 13, 1407–1417 [DOI] [PubMed] [Google Scholar]
- 34.Johansen T., and Lamark T. (2011) Selective autophagy mediated by autophagic adapter proteins. Autophagy 7, 279–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Y., Wang W., Wang J., Feng J., Wang Q., Jin J., Lv M., Li X., Li Y., Ma Y., Shen B., and Zhang J. (2013) Receptor for activated C kinase 1 promotes hepatocellular carcinoma growth by enhancing mitogen-activated protein kinase kinase 7 activity. Hepatology 57, 140–151 [DOI] [PubMed] [Google Scholar]
- 36.Ruan Y., Sun L., Hao Y., Wang L., Xu J., Zhang W., Xie J., Guo L., Zhou L., Yun X., Zhu H., Shen A., and Gu J. (2012) Ribosomal RACK1 promotes chemoresistance and growth in human hepatocellular carcinoma. J. Clin. Invest. 122, 2554–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi S., Deng Y. Z., Zhao J. S., Ji X. D., Shi J., Feng Y. X., Li G., Li J. J., Zhu D., Koeffler H. P., Zhao Y., and Xie D. (2012) RACK1 promotes non-small-cell lung cancer tumorigenicity through activating sonic hedgehog signaling pathway. J. Biol. Chem. 287, 7845–7858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng R., Jiang B., Ma J., Ma Z., Wan X., Liu H., Chen Z., Cheng Q., and Chen R. (2013) Forced downregulation of RACK1 inhibits glioma development by suppressing Src/Akt signaling activity. Oncol. Rep. 30, 2195–2202 [DOI] [PubMed] [Google Scholar]
- 39.Mamidipudi V., Dhillon N. K., Parman T., Miller L. D., Lee K. C., and Cartwright C. A. (2007) RACK1 inhibits colonic cell growth by regulating Src activity at cell cycle checkpoints. Oncogene 26, 2914–2924 [DOI] [PubMed] [Google Scholar]
- 40.Mamidipudi V., Zhang J., Lee K. C., and Cartwright C. A. (2004) RACK1 regulates G1/S progression by suppressing Src kinase activity. Mol. Cell. Biol. 24, 6788–6798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subauste M. C., Sansom O. J., Porecha N., Raich N., Du L., and Maher J. F. (2010) Fem1b, a proapoptotic protein, mediates proteasome inhibitor-induced apoptosis of human colon cancer cells. Mol. Carcinog. 49, 105–113 [DOI] [PubMed] [Google Scholar]
- 42.Mamidipudi V., and Cartwright C. A. (2009) A novel pro-apoptotic function of RACK1: suppression of Src activity in the intrinsic and Akt pathways. Oncogene 28, 4421–4433 [DOI] [PubMed] [Google Scholar]