Abstract
Exopolysaccharides play an important structural and functional role in the development and maintenance of microbial biofilms. Although the majority of research to date has focused on the exopolysaccharide systems of biofilm-forming bacteria, recent studies have demonstrated that medically relevant fungi such as Candida albicans and Aspergillus fumigatus also form biofilms during infection. These fungal biofilms share many similarities with those of bacteria, including the presence of secreted exopolysaccharides as core components of the extracellular matrix. This review will highlight our current understanding of fungal biofilm exopolysaccharides, as well as the parallels that can be drawn with those of their bacterial counterparts.
Keywords: bacterial adhesion, biofilm, carbohydrate biosynthesis, extracellular matrix, polysaccharide, exopolysaccharide
Introduction
Biofilms are communities of microorganisms growing within a self-produced extracellular matrix composed of proteins, extracellular DNA, lipids, and mono- and polysaccharides. Seminal studies in bacterial systems, as detailed in the other articles within this minireview compendium, have identified a critical role for exopolysaccharides in the establishment, structure, and function of biofilms. Emerging evidence suggests that biofilm formation is not restricted to bacteria and that pathogenic fungi, such as Aspergillus fumigatus and Candida albicans, also produce biofilms during colonization and infection. Fungal biofilms mediate adherence to host tissues and biomedical devices, and provide protection from host immune defenses and antifungal therapy.
C. albicans, the most common fungal pathogen of humans, forms extensive biofilms on medical devices and mucosal surfaces during infection (1, 2). Studies of the biofilm matrix have revealed a cooperative role of β-1,3-glucans with a mannan-glucan complex in the development and maintenance of biofilm structure (3, 4). Although similar polysaccharides are also found within the fungal cell wall, the synthesis of the matrix polysaccharides is governed by pathways independent of those that mediate cell wall synthesis. β-1,3-Glucans within the C. albicans biofilm matrix play an important role in sequestration of antifungal agents (5) and in masking fungal cells from recognition by neutrophils (6). In contrast, biofilms formed by the mold A. fumigatus have been reported during pulmonary infection (7) and are dependent on the exopolysaccharide galactosaminogalactan (GAG)3 (8). GAG is a partially deacetylated heteropolymer of α-1,4-linked galactose and N-acetyl galactosamine, which mediates adhesion between both fungi and also to other surfaces as well as protection against host immune defenses (8–11). GAG is synthesized by the protein products of a conserved cluster of five genes that is present in a number of plant and human fungal pathogens (11). Despite a lack of sequence homology with bacterial exopolysaccharide biosynthetic enzymes, there are striking similarities in the function and organization of these fungal exopolysaccharide biosynthetic proteins and the role of the resulting glycans in biofilm formation, drug resistance, and immune evasion. In this review, we will summarize our current understanding of the composition, biosynthesis, and function of fungal biofilm exopolysaccharides produced by the pathogenic fungi, A. fumigatus and C. albicans, and compare and contrast these mechanisms with those of common pathogenic bacteria.
A. fumigatus Biofilms
Biofilm formation by the filamentous fungus A. fumigatus has been recently described during growth in vitro and in vivo (7, 12). As with bacteria, the filamentous hyphae of A. fumigatus grow embedded within an extracellular polymeric substance (7, 12). This extracellular matrix mediates adherence to inorganic substrates and host cells and enhances resistance to host defenses and antifungal agents. This matrix is a heterogeneous substance composed of extracellular DNA, proteins, lipids and polyols, and exopolysaccharides including α-glucans, galactomannan and the glycan GAG (7, 13).
A. fumigatus Biofilm Exopolysaccharides
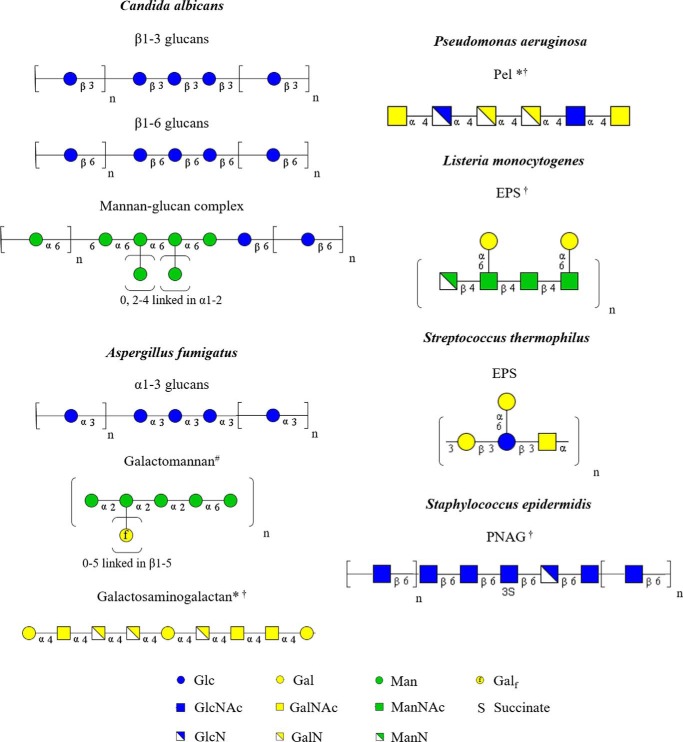
Of the three exopolysaccharides found within A. fumigatus biofilms, the role of GAG has been studied in the greatest detail. GAG is an α-1,4-linked heteropolysaccharide composed of galactose and partially deacetylated GalNAc that is absent in A. fumigatus spores but is produced in abundance by growing hyphae (8, 11, 14). GAG plays a critical role in the maintenance of the extracellular matrix of A. fumigatus biofilms (8). Strains deficient in GAG production fail to produce extracellular matrix and are unable to form adherent biofilms on plastic or host cells in vitro (8, 9, 15). As with other biofilm exopolysaccharides, GAG plays an important role in evading host defenses. As a result, strains deficient in GAG display attenuated virulence in mouse and invertebrate models of invasive aspergillosis (8, 9).
A functional role for the other polysaccharides found within the extracellular matrix of A. fumigatus biofilms has yet to be established. Galactomannan, which consists of a mannan core decorated with β-1,5-linked galactofuranose, is dispensable for biofilm formation, and galactofuranose-deficient mutants exhibit increased biofilm formation as a consequence of increased production of GAG (8, 10, 16). α-Glucans are abundant within the biofilm matrix; however, the role of this polysaccharide in biofilm formation has not been studied (13, 17–19).
Galactosaminogalactan Biosynthesis
Comparative transcriptomic studies of regulatory mutants deficient in GAG production identified a cluster of five co-regulated genes with predicted functions in polysaccharide synthesis and metabolism. Subsequent studies have confirmed a role for the protein products of many of these genes in the synthesis or processing of GAG. The presence of gene clusters resembling bacterial operons is relatively unusual in filamentous fungi, and often suggests that acquisition of these genes occurred through a horizontal gene transfer event. Bioinformatics analyses of available fungal genomes support this hypothesis, as the presence of a syntenic GAG gene cluster is found throughout a variety of taxonomically diverse fungal genomes (11).
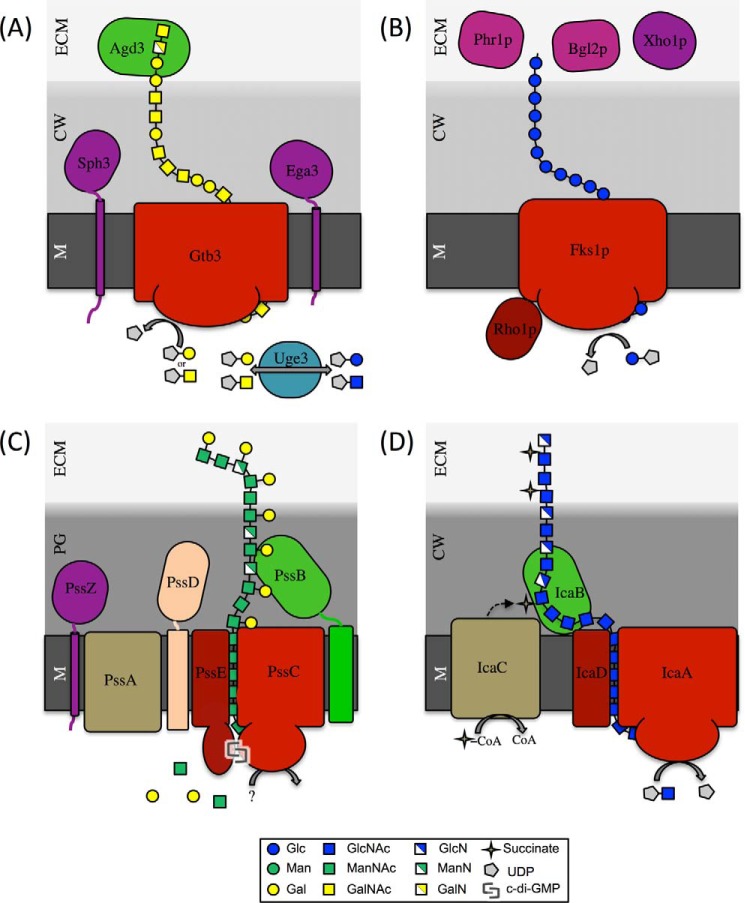
Through bioinformatics, gene deletion experiments, and the study of recombinant enzymes, a model for GAG biosynthesis is emerging (Fig. 1). This model shares some similarity with those described for the synthase-dependent production of hexosamine-containing polysaccharides found within bacterial biofilms (Table 1). As A. fumigatus contains only a single plasma membrane, the model of GAG biosynthesis is most similar to exopolysaccharide synthesis by Gram-positive bacteria such as Staphylococcus epidermidis (20) and Listeria monocytogenes (Fig. 1) (21).
FIGURE 1.
Schematic representation of the GAG, β-1,3-glucan, EPS, and PNAG polysaccharide synthase from A. fumigatus, C. albicans, L. monocytogenes, and S. epidermidis, respectively. In each panel, the glycosyltransferases and associated regulatory proteins are depicted in red and dark red, respectively; the deacetylase is in light green, the epimerase is in light blue, hydrolases are in purple, and other modifying enzymes are in beige. M, membrane; CW, cell wall; ECM, extracellular matrix; Glc, glucose; Gal, galactopyranose; Man, mannose; c-di-GMP, cyclic di-GMP.
TABLE 1.
Select microbial exopolysaccharides that contain hexosamine components
| Organism | Polymer | Operon | Structure |
|---|---|---|---|
| Aspergillus fumigatus | GAG | NAa | Partially deacetylated α-1,4-linked heteroglycan of Gal and GalNAc |
| Pseudomonas aeruginosa | Pel | pel | Partially deacetylated 1,4-heteroglycan of GlcNAc and GalNAc |
| Listeria monocytogenes | EPS | pss | β-1,4-Linked N-acetylmannosamine chain decorated with terminal α-1,6-linked Gal; possibly partially deacetylated |
| Streptococcus thermophilus | EPS | eps | Tetrasaccharide repeat with 1,3-linked β-Gal, β-Glc, and α-GalNAc with Gal α-1,6 linked to the central Glc |
| Staphylococcus epidermidisb | PNAG | ica | Partially deacetylated β-1,6-linked GlcNAc |
| Escherichia colib | PNAG | pga | Partially deacetylated β-1,6-linked GlcNAc |
a NA, not applicable.
b These species are included as Gram-positive and Gram-negative examples of the range of microorganisms that produce PNAG.
Amino Sugar Synthesis
Synthesis of GAG begins with the production of UDP-galactopyranose (Gal) and UDP-GalNAc from UDP-glucose and UDP-GlcNAc, respectively, by the bifunctional cytosolic epimerase Uge3 (8, 10). The activity and substrate specificity of this enzyme has been confirmed in vitro (10) and Uge3-deficient mutants produce no detectable GAG (8). The presence of an epimerase-encoding gene within the GAG biosynthetic gene cluster is unique to A. fumigatus. Although other bacteria such as Pseudomonas aeruginosa and Streptococcus thermophilus produce GalNAc-containing exopolysaccharides, the operons encoding the biosynthetic genes for these bacterial glycans do not include genes predicted to encode glucose epimerases (22, 23). In these bacteria, UDP-GalNAc is derived from other cellular metabolic pathways that are not specific to exopolysaccharide synthesis. This difference is best illustrated by experiments in which the entire S. thermophilus eps operon was expressed in Lactococcus lactis, which lacks endogenous GalNAc production (24). Although the operon was functional in L. lactis, the resulting polymer was altered in composition and was deficient in GalNAc, suggesting that this sugar is derived from other cellular processes and is not produced by enzymes encoded within the exopolysaccharide biosynthetic operon (24).
Glycan Chain Synthesis
Following synthesis by Uge3, UDP-Gal and UDP-GalNAc are predicted to be polymerized and exported from the cell through the action of a large transmembrane glycosyl transferase, Gtb3. Consistent with this model, disruption of gtb3 results in a complete absence of GAG production, although this protein has not yet been purified and studied in vitro. The ability of a single glycosyl transferase to display specificities for multiple donor and recipient sugars has also been reported in studies of the S. thermophilus EPS system (24). As described above, transfer of the eps operon to a GalNAc-deficient bacteria results in the substitution of galactose for GalNAc within the mature polymer (24), suggesting that either Gal or GalNAc can serve as a suitable substrate during polymer synthesis. This promiscuity of transferase activity suggests that the acetyl group of GalNAc is not part of the recognition signal for the binding of nucleotide sugars by these enzymes. Bioinformatics analysis suggests that the transmembrane and glycosyl transferase regions of Gtb3 comprise less than half of the protein, with the transferase region predicted to be a member of the GT4 family of proteins as defined by the Carbohydrate-Active Enzymes database (25). It is possible that the domains encoded by these uncharacterized regions may participate in post-translational regulation of GAG synthesis. This model would be similar to the bifunctional BcsA and WssB enzymes that are responsible for the synthesis of cellulose and acetylated cellulose, respectively (26–29). In addition to the transferase domain, these proteins also contain a cyclic di-GMP receptor domain. Binding of the nucleotide is required for cellulose synthesis. In contrast, other bacterial systems have a separate regulatory protein that is associated with a transmembrane synthase. Examples of these regulatory proteins include IcaD/PgaD in the poly-β1,6-N-acetylglucosamine (PNAG) systems (20, 30) and PelD in the P. aeruginosa Pel system (31). Further studies are required to elucidate the function of the other regions of Gtb3.
Post-synthetic Polymer Modification
The newly synthesized GAG polymer, consisting of α-1,4-linked Gal and GalNAc, is deacetylated by the secreted, cell wall-associated enzyme Agd3 (11). Deacetylation results in the production of a polycationic glycan that associates with negatively charged residues on the cell wall of A. fumigatus, as well as mediates adherence to other anionic surfaces including host cells and plastics (11). Agd3-deficient mutants of A. fumigatus do not form biofilms, and produce fully acetylated GAG, which fails to adhere to the surface of the organism and is shed into the culture supernatant (11). Deacetylation occurs in the extracellular space as co-culture of an Agd3-deficient mutant with culture supernatants from the GAG-deficient Δuge3 mutant restores the production of cell wall-associated GAG and biofilm formation (11). Deacetylation of N-acetyl hexosamine polymers by enzymes belonging to the carbohydrate esterase 4 superfamily, such as Agd3, is also common in bacterial systems. In S. epidermidis, IcaB mediates the deacetylation of GlcNAc within the PNAG polymer following synthesis of the nascent glycan (32). As with A. fumigatus GAG, deacetylation is required for the formation of adhesive PNAG, and fully acetylated PNAG fails to adhere to the cell wall of S. epidermidis and cannot support biofilm formation (32). Interestingly, deletion of the genes involved in de-N-acetylation of PNAG in Gram-negative species results in different phenotypes. Deletion of pgaB in Escherichia coli results in loss of polymer export (33), whereas deletion of bpsB in Bordetella bronchiseptica results in a weaker biofilm with significant loss of the complex biofilm architecture (34). The Gram-negative organism P. aeruginosa produces a heteropolymer of GlcNAc and GalNAc that is partially deacetylated (35). The pel operon contains a gene pelA that contains a carbohydrate deacetylase domain (36). Both deletion of pelA and mutation of catalytic residues that compromise deacetylase activity are associated with a loss of Pel-mediated biofilm production and an absence of detectable biofilm-associated Pel (36). In L. monocytogenes, the protein product of pssB has been hypothesized to mediate deacetylation of N-acetylmannosamine residues within the EPS of this Gram-positive organism (21). As with pelA, deletion of pssB resulted in a loss of cell aggregation and EPS production, suggesting that this putative deacetylase is also required for the production of functional polysaccharide in L. monocytogenes (21).
In addition to rendering GAG adhesive, deacetylation of the polymer plays an important role in other functions of this glycan, including virulence. Binding of GAG to the outer cell wall of A. fumigatus hyphae conceals β-1,3-glucan from recognition by the innate immune receptor Dectin-1 on dendritic cells, leading to reduced pro-inflammatory cytokine production by these cells (8). Cell wall-associated GAG also mediates resistance to killing by host neutrophil extracellular traps, likely through charge-mediated repulsion of cationic peptides embedded within the DNA matrix of these structures (9). Similar observations have been made with deacetylase-deficient mutants of S. epidermidis, in which loss of cell wall-associated PNAG results in enhanced susceptibility to neutrophil phagocytosis and to the cationic antimicrobial peptide LL-37 (32). Interestingly, a recent study suggests that the P. aeruginosa cationic exopolysaccharide Pel binds extracellular DNA within biofilms through charge-charge interactions (35). Whether GAG or other bacterial cationic polysaccharides play similar structural roles in their respective biofilms remains to be determined.
Glycoside Hydrolases
The GAG biosynthetic gene cluster contains two additional genes: sph3 and ega3. Although Ega3 is predicted to encode a membrane-anchored glycoside hydrolase, it has yet to be functionally characterized in vitro or in vivo. Recent work has found that Sph3 is a member of a novel glycoside hydrolase family, GH135 (15). Recombinant Sph3 cleaves purified and cell wall-associated GAG in vitro and displays no activity against chitosan, another hexosamine-containing polymer (15). Sph3 is required for GAG production (15), although the mechanism by which this enzyme participates in GAG synthesis remains unknown. This seeming contradiction in which hydrolases or other degrading enzymes mediate polymer production has also been reported in several bacterial exopolysaccharide systems. In L. monocytogenes, pssZ is predicted to encode a membrane-bound hydrolase that is required for exopolysaccharide production by this organism (21). Similarly, in several cellulose-producing organisms, the production of cellulose endoglucanase (37, 38) or carboxymethylcellulase (39) enhances the production of cellulose, suggesting that these cellulose-degrading enzymes play an important role in glycan maturation and/or export through the cell wall. Finally, in P. aeruginosa, alginate lyase (AlgL) is thought to form part of the transport complex of proteins that serve as a conduit to move the polysaccharide alginate through the periplasm, and also degrades any misdirected polymer within this space (40). Deletion of algL results in disruption of the conduit complex and retention of alginate within the periplasm, as well as the absence of AlgL-mediated polymer degradation, leading to cell lysis and death (40).
Regulation of A. fumigatus Biofilm Formation
Relatively little is known about the regulation of biofilm formation and exopolysaccharide synthesis by A. fumigatus. External stimuli that induce or suppress biofilm formation have yet to be identified. Production of GAG is dependent on the developmental regulatory proteins MedA (8, 41, 42), StuA (8), and SomA (43). Activation of the cell wall integrity pathway through deletion of the phosphatases SitA (44) or PtcB (45) has been linked to suppression of biofilm formation and extracellular matrix production. Although in bacteria, there is a well established role for cyclic di-GMP as a signaling molecule that stimulates exopolysaccharide production, there is no evidence that fungi, including A. fumigatus, produce this molecule.
Candida albicans Biofilms
The formation of biofilms by the yeast C. albicans plays a key role in the pathogenesis of both mucosal and catheter-related bloodstream infections in humans (46). Biofilm formation by C. albicans has been well studied in vitro and in animal models and is similar to the process that has been described for pathogenic bacteria (47). First, planktonic yeast cells adhere to an appropriate substrate such as an epithelial cell surface or an intravascular catheter. Adhesion and cell-cell aggregation are mediated by an array of glycosylphosphatidylinositol-linked cell wall glycoproteins including Eap1p (48), Hwp1p (49), and members of the agglutinin-like sequence (Als) family of proteins, particularly Als1p and Als3p (50–52). This initial adhesion and aggregation of yeast cells is further stabilized by the formation of amyloid structures between Als proteins (53–55). Following adhesion, C. albicans biofilms undergo maturation through reproduction of yeast cells and morphologic switching of yeast to produce filamentous hyphal and pseudohyphal structures. In parallel, these organisms elaborate an extracellular matrix consisting of DNA, proteins, lipids and mono- and polysaccharides. Mature biofilms may undergo a dispersal phase in which organisms shift morphology back to the yeast form, and are then released to disseminate through the environment.
The Exopolysaccharides of C. albicans Biofilm Matrix
A recent analysis of the composition of C. albicans biofilm matrix has revealed that three polysaccharides play a complex and cooperative function in the assembly and maintenance of the C. albicans biofilm matrix. Surprisingly, β-1,3-glucan, the predominant polysaccharide found within the C. albicans cell wall, and the glycan best linked with biofilm-mediated drug resistance, is only a minor component of the biofilm matrix (4). The most abundant polysaccharides within the extracellular matrix are an α-1,6-linked mannan with α-1,2-linked side chains (85%) and linear β-1,6-glucans (14%). These two polysaccharides were co-isolated, suggesting that they form a mannan-glucan complex (4). The components of the mannan-glucan complex exhibit structural features that are unique to the extracellular matrix and are not found within their cell wall counterparts. Mannans within the extracellular matrix are much longer (up to 12,000 residues) as compared with those within the cell wall (∼150) (4). Similarly, matrix β-1,6-glucans were found to be linear, in contrast with the highly branched structures found within the cell wall (4). Collectively, these observations suggest that in C. albicans, as has been established in bacteria (56, 57), the assembly of the cell wall and extracellular matrix are distinct processes. See Fig. 2 for graphical representations of matrix exopolysaccharides discussed in this review.
FIGURE 2.
Matrix exopolysaccharides discussed in this review. Shown is a graphical representation of exopolysaccharides with parentheses indicating repeating elements. Subscripts indicate possible number of residues in each element. Glc, glucose; Gal, galactopyranose; Man, mannose; Galf, galactofuranose; GalNAc, N-acetyl galactosamine; GalN, galactosamine. *, no specific arrangement of residues or polymer length has been defined. #, galactofuranose side chains of galactomannan can be linked to any of the indicated mannan residues. †, the degree of deacetylation of these polymers is not known.
β-1,3-Glucan Biosynthesis
The synthesis of β-1,3-glucan is the best studied of the C. albicans matrix polysaccharides. Production of β-1,3-glucan for both cell wall and matrix production in C. albicans is mediated by a protein complex composed of the transmembrane glucan synthase Fks1p (58–60) and the regulatory G-protein Rho1p (61, 62) (Fig. 1). Fks1p is predicted to be a member of the GT48 superfamily. The presence of a regulatory protein associated with a transmembrane synthase differs from the A. fumigatus GAG pathway but is common in bacterial exopolysaccharide pathways as described above. Activation of the C. albicans Fks1p-Rho1p complex leads to the production of β-1,3-glucan by Fks1p from intracellular UDP-glucose through the addition of glucose residues to the non-reducing end of the glycan (63, 64). The emerging polymer is then thought to be transported to the extracellular space via a channel formed by the transmembrane domains of Fks1p.
Delivery of β-1,3-glucan to the extracellular matrix is then governed, at least in part, by a novel glucan-modifying pathway composed of at least three proteins: two glycosyltransferases, Bgl2p and Phr1p, and a glucanase, Xog1p (65). Deletion of any of the genes encoding these proteins results in a 10-fold or more decrease in matrix β-1,3-glucan content and the formation of biofilms that are more easily disrupted (65). Consistent with the decrease in matrix β-1,3-glucan, biofilms formed by these mutants are less able to sequester fluconazole and exhibit increased sensitivity to this agent (65). Despite these dramatic changes in matrix composition, these mutants produce normal levels of β-1,3-glucan within their cell wall, suggesting that the activity of these enzymes was specific for matrix-associated β-1,3-glucan (65). Overexpression and double-deletion studies suggest that these enzymes play complementary roles in glucan delivery (65). The exact mechanism by which these three enzymes mediate delivery of β-1,3-glucan to the extracellular matrix remains to be determined; however, they may participate in the release and modification of cell wall glucans for transport and deposition within the extracellular matrix.
β-1,3-Glucan plays an important role in biofilm-mediated protection of C. albicans from immune defenses and antifungals. The production of β-1,3-glucan by C. albicans biofilms inhibits the production of reactive oxygen species (ROS) by neutrophils and protects fungal cells from neutrophil killing (6). Treatment with glucanase enhances neutrophil ROS production and renders biofilms more susceptible to killing, whereas the addition of a soluble β-glucan to early biofilms reduces ROS production and protects cells from neutrophil killing, suggesting a role for β-glucans in mediating protection against neutrophils (6). A similar role in suppressing oxidative burst and neutrophil killing has been reported for the P. aeruginosa exopolysaccharide PslG, through reducing complement binding and opsonization of bacteria within biofilms (66). β-1,3-Glucan directly enhances antifungal resistance by the binding and sequestration of fluconazole, preventing its intracellular penetration and antifungal activity (5, 67, 68). Sequestration of antimicrobials by bacterial exopolysaccharides has also been reported in P. aeruginosa. In this organism, binding of tobramycin by alginate has also been demonstrated; however, the significance of tobramycin sequestration in mediating tobramycin resistance is the subject of some debate (69).
Biosynthesis of the Mannan-Glucan Complex
The synthetic pathways governing production of the mannan-glucan complex are largely unknown. Targeted gene deletion studies have identified a number of candidate genes whose protein products are required for the synthesis of this glycan complex, although the function of these proteins has not been studied to date. Deletion of vig1 and kre5 genes was found to be required for β-1,6-glucan synthesis, whereas loss of alg11, mnn9, mnn11, van1, mnn4-4, pmr1, and vrg4 resulted in a reduction in the production of matrix α-mannan (3). Although the function of the protein products of these genes requires further investigation, this study provided insight into the contributions of the three major matrix polysaccharides to C. albicans biofilm formation. Loss of any one of the three polysaccharides was associated with a marked reduction in the extracellular matrix, lower levels of the other two polysaccharides, and enhanced susceptibility to fluconazole (3). Co-purification experiments suggested that the three polysaccharides physically associate in the extracellular matrix (3). Assembly of this polysaccharide matrix likely occurs in the extracellular space, as biofilm matrix formation was restored in mixed cultures of complementary mutant strains (3). Collectively, these findings suggest a model where the mannan-glucan complex and β-1,3-glucan are assembled after export from the cell and suggest that they play a cooperative role in the production of biofilm matrix. This cooperative function of multiple exopolysaccharides in the biofilm matrix is analogous to the roles of Pel and Psl in biofilm formation by P. aeruginosa (70). It has been suggested that these glycans function in a complementary fashion to govern the strength and viscosity of biofilm matrix in this bacteria.
Regulation of C. albicans Biofilm Formation
The regulation of C. albicans biofilm formation has been studied in greater detail than A. fumigatus. In C. albicans, biofilm formation and matrix synthesis are regulated by at least six transcription factors that govern adhesion, morphologic switching, and extracellular matrix production. Notable among these is Bcr1p, which positively regulates the expression of numerous adhesins during the initial phase of biofilm formation (71), and Zap1p, a transcriptional repressor of genes required for biofilm extracellular matrix production (72). As with bacteria, signaling molecules associated with fungal quorum sensing, such as tyrosol and farnesol, modulate biofilm formation in C. albicans (73). Tyrosol enhances early biofilm events including adhesion and filamentation (73). In contrast, farnesol is secreted later in biofilm development and inhibits adhesion, possibly facilitating biofilm dispersion (73).
Therapeutics Targeting Fungal Biofilms
A number of recent studies have suggested that, as with bacteria, targeting biofilm formation by fungi may be an attractive therapeutic strategy. Echinocandins inhibit Fks1p, the β-1,3-glucan synthase, and exhibit anti-biofilm activity in vitro and in animal models of catheter-associated C. albicans infection (74, 75). It has been hypothesized that the anti-biofilm activity of these agents may underlie their success in clinical trials for the treatment of Candida infections. These agents do not exhibit anti-biofilm activity against A. fumigatus, as they do not influence the synthesis of GAG. The use of therapeutic enzymes that degrade the biofilm matrix has been explored in both C. albicans and A. fumigatus. Therapy with recombinant DNase reduces C. albicans biofilm biomass and enhances the activity of amphotericin B, but not fluconazole, in vitro (76, 77). Similarly, recombinant DNase was found to disrupt A. fumigatus biofilms and to enhance the activity of several antifungal agents in vitro (78). No studies in animal models evaluating the effects of DNase on antifungal susceptibility have been reported to date. However, β-glucanase therapy enhanced the activity of neutrophils and antifungals against C. albicans biofilms both in vitro and in a rat catheter model of infection (6, 68), thus providing evidence that biofilm-degrading enzymes can be effective in vivo. The use of hydrolytic enzymes targeting GAG has not yet been explored as an anti-biofilm strategy against A. fumigatus; however, Sph3 has been demonstrated to hydrolyze both purified and cell wall-associated GAG (15), suggesting that this may be a promising avenue to explore in the future.
Conclusions
Biofilm formation by fungi is emerging as an important factor in the pathogenesis of human fungal disease, and as an important factor when considering the choice of therapeutic strategies for these diseases. Despite their evolutionary distance from bacteria, fungi utilize many of the same mechanisms during the synthesis and modification of biofilm exopolysaccharides as bacteria. Fungal glycans play a similar role in mediating protection against immune defenses and antimicrobial agents such as bacteria exopolysaccharides. The development of new anti-biofilm therapeutic agents holds great potential as a strategy for the treatment of fungal infections, either alone, or in combination with conventional antifungals.
Acknowledgments
We thank N. Bamford and F. Le Mauff for help with figure preparation.
This work was supported by Canada Institute of Health Research Grants 81361 (to P. L. H. and D. C. S.), 123306 (to D. C. S.), and 13337 and 43998 (to P. L. H.) and by Cystic Fibrosis Canada Grant 3019 (to P. L. H. and D. C. S.). This work is also supported by a Chercheur-Boursier Award from the Fonds de Recherche Quebec Santé (FRQS) (to D. C. S.). This is the first article in the Thematic Minireview series “Biofilms.” The authors declare that they have no conflicts of interest with the contents of this article.
- GAG
- galactosaminogalactan
- Gal
- galactopyranose
- PNAG
- poly-β1,6-N-acetylglucosamine
- EPS
- exopolysaccharide
- ROS
- reactive oxygen species
- AlgL
- alginate lyase
- ALS
- agglutinin-like sequence.
References
- 1.Dongari-Bagtzoglou A., Kashleva H., Dwivedi P., Diaz P., and Vasilakos J. (2009) Characterization of mucosal Candida albicans biofilms. PLoS One 4, e7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tchekmedyian N. S., Newman K., Moody M. R., Costerton J. W., Aisner J., Schimpff S. C., and Reed W. P. (1986) Special studies of the Hickman catheter of a patient with recurrent bacteremia and candidemia. Am. J. Med. Sci. 291, 419–424 [DOI] [PubMed] [Google Scholar]
- 3.Mitchell K. F., Zarnowski R., Sanchez H., Edward J. A., Reinicke E. L., Nett J. E., Mitchell A. P., and Andes D. R. (2015) Community participation in biofilm matrix assembly and function. Proc. Natl. Acad. Sci. U.S.A. 112, 4092–4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarnowski R., Westler W. M., Lacmbouh G. A., Marita J. M., Bothe J. R., Bernhardt J., Lounes-Hadj Sahraoui A., Fontaine J., Sanchez H., Hatfield R. D., Ntambi J. M., Nett J. E., Mitchell A. P., and Andes D. R. (2014) Novel entries in a fungal biofilm matrix encyclopedia. mBio 5, e01333–01314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nett J. E., Sanchez H., Cain M. T., and Andes D. R. (2010) Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J. Infect. Dis. 202, 171–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Z., Thompson A., Sobue T., Kashleva H., Xu H., Vasilakos J., and Dongari-Bagtzoglou A. (2012) Candida albicans biofilms do not trigger reactive oxygen species and evade neutrophil killing. J. Infect. Dis. 206, 1936–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loussert C., Schmitt C., Prevost M. C., Balloy V., Fadel E., Philippe B., Kauffmann-Lacroix C., Latgé J. P., and Beauvais A. (2010) In vivo biofilm composition of Aspergillus fumigatus. Cell. Microbiol. 12, 405–410 [DOI] [PubMed] [Google Scholar]
- 8.Gravelat F. N., Beauvais A., Liu H., Lee M. J., Snarr B. D., Chen D., Xu W., Kravtsov I., Hoareau C. M., Vanier G., Urb M., Campoli P., Al Abdallah Q., Lehoux M., Chabot J. C., et al. (2013) Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal β-glucan from the immune system. PLoS Pathog. 9, e1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee M. J., Liu H., Barker B. M., Snarr B. D., Gravelat F. N., Al Abdallah Q., Gavino C., Baistrocchi S. R., Ostapska H., Xiao T., Ralph B., Solis N. V., Lehoux M., Baptista S. D., Thammahong A., et al. (2015) The fungal exopolysaccharide galactosaminogalactan mediates virulence by enhancing resistance to neutrophil extracellular traps. PLoS Pathog. 11, e1005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee M. J., Gravelat F. N., Cerone R. P., Baptista S. D., Campoli P. V., Choe S. I., Kravtsov I., Vinogradov E., Creuzenet C., Liu H., Berghuis A. M., Latgé J. P., Filler S. G., Fontaine T., and Sheppard D. C. (2014) Overlapping and distinct roles of Aspergillus fumigatus UDP-glucose 4-epimerases in galactose metabolism and the synthesis of galactose-containing cell wall polysaccharides. J. Biol. Chem. 289, 1243–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee M. J., Geller A. M., Bamford N. C., Liu H., Gravelat F. N., Snarr B. D., Le Mauff F., Chabot J., Ralph B., Ostapska H., Lehoux M., Cerone R. P., Baptista S. D., Vinogradov E., Stajich J. E., Filler S. G., et al. (2016) Deacetylation of fungal exopolysaccharide mediates adhesion and biofilm formation. mBio 7, e00252–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mowat E., Butcher J., Lang S., Williams C., and Ramage G. (2007) Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J. Med. Microbiol. 56, 1205–1212 [DOI] [PubMed] [Google Scholar]
- 13.Fontaine T., Beauvais A., Loussert C., Thevenard B., Fulgsang C. C., Ohno N., Clavaud C., Prevost M. C., and Latgé J. P. (2010) Cell wall α1–3glucans induce the aggregation of germinating conidia of Aspergillus fumigatus. Fungal Genet. Biol. 47, 707–712 [DOI] [PubMed] [Google Scholar]
- 14.Fontaine T., Delangle A., Simenel C., Coddeville B., van Vliet S. J., van Kooyk Y., Bozza S., Moretti S., Schwarz F., Trichot C., Aebi M., Delepierre M., Elbim C., Romani L., and Latgé J. P. (2011) Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. 7, e1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bamford N. C., Snarr B. D., Gravelat F. N., Little D. J., Lee M. J., Zacharias C. A., Chabot J. C., Geller A. M., Baptista S. D., Baker P., Robinson H., Howell P. L., and Sheppard D. C. (2015) Sph3 is a glycoside hydrolase required for the biosynthesis of galactosaminogalactan in Aspergillus fumigatus. J. Biol. Chem. 290, 27438–27450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamarre C., Beau R., Balloy V., Fontaine T., Wong Sak Hoi J., Guadagnini S., Berkova N., Chignard M., Beauvais A., and Latgé J. P. (2009) Galactofuranose attenuates cellular adhesion of Aspergillus fumigatus. Cell. Microbiol. 11, 1612–1623 [DOI] [PubMed] [Google Scholar]
- 17.Maubon D., Park S., Tanguy M., Huerre M., Schmitt C., Prévost M. C., Perlin D. S., Latgé J. P., and Beauvais A. (2006) AGS3, an α(1–3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet. Biol. 43, 366–375 [DOI] [PubMed] [Google Scholar]
- 18.Beauvais A., Maubon D., Park S., Morelle W., Tanguy M., Huerre M., Perlin D. S., and Latgé J. P. (2005) Two α(1–3) glucan synthases with different functions in Aspergillus fumigatus. Appl. Environ. Microbiol. 71, 1531–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beauvais A., Bozza S., Kniemeyer O., Formosa C., Formosa C., Balloy V., Henry C., Roberson R. W., Dague E., Chignard M., Brakhage A. A., Romani L., and Latgé J. P. (2013) Deletion of the α-(1,3)-glucan synthase genes induces a restructuring of the conidial cell wall responsible for the avirulence of Aspergillus fumigatus. PLoS Pathog. 9, e1003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heilmann C., Schweitzer O., Gerke C., Vanittanakom N., Mack D., and Götz F. (1996) Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20, 1083–1091 [DOI] [PubMed] [Google Scholar]
- 21.Köseoğlu V. K., Heiss C., Azadi P., Topchiy E., Güvener Z. T., Lehmann T. E., Miller K. W., and Gomelsky M. (2015) Listeria monocytogenes exopolysaccharide: origin, structure, biosynthetic machinery and c-di-GMP-dependent regulation. Mol. Microbiol. 96, 728–743 [DOI] [PubMed] [Google Scholar]
- 22.Stingele F., Neeser J. R., and Mollet B. (1996) Identification and characterization of the eps (Exopolysaccharide) gene cluster from Streptococcus thermophilus Sfi6. J. Bacteriol. 178, 1680–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman L., and Kolter R. (2004) Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 51, 675–690 [DOI] [PubMed] [Google Scholar]
- 24.Stingele F., Vincent S. J., Faber E. J., Newell J. W., Kamerling J. P., and Neeser J. R. (1999) Introduction of the exopolysaccharide gene cluster from Streptococcus thermophilus Sfi6 into Lactococcus lactis MG1363: production and characterization of an altered polysaccharide. Mol. Microbiol. 32, 1287–1295 [DOI] [PubMed] [Google Scholar]
- 25.Lombard V., Golaconda Ramulu H., Drula E., Coutinho P. M., and Henrissat B. (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, D490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitney J. C., and Howell P. L. (2013) Synthase-dependent exopolysaccharide secretion in Gram-negative bacteria. Trends Microbiol. 21, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zogaj X., Nimtz M., Rohde M., Bokranz W., and Römling U. (2001) The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 39, 1452–1463 [DOI] [PubMed] [Google Scholar]
- 28.Saxena I. M., Brown R. M. Jr., Fevre M., Geremia R. A., and Henrissat B. (1995) Multidomain architecture of β-glycosyl transferases: implications for mechanism of action. J. Bacteriol. 177, 1419–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryjenkov D. A., Simm R., Römling U., and Gomelsky M. (2006) The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J. Biol. Chem. 281, 30310–30314 [DOI] [PubMed] [Google Scholar]
- 30.Wang X., Preston J. F. 3rd, and Romeo T. (2004) The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 186, 2724–2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitney J. C., Colvin K. M., Marmont L. S., Robinson H., Parsek M. R., and Howell P. L. (2012) Structure of the cytoplasmic region of PelD, a degenerate diguanylate cyclase receptor that regulates exopolysaccharide production in Pseudomonas aeruginosa. J. Biol. Chem. 287, 23582–23593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vuong C., Kocianova S., Voyich J. M., Yao Y., Fischer E. R., DeLeo F. R., and Otto M. (2004) A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 279, 54881–54886 [DOI] [PubMed] [Google Scholar]
- 33.Itoh Y., Rice J. D., Goller C., Pannuri A., Taylor J., Meisner J., Beveridge T. J., Preston J. F. 3rd, and Romeo T. (2008) Roles of pgaABCD genes in synthesis, modification, and export of the Escherichia coli biofilm adhesin poly-β-1,6-N-acetyl-d-glucosamine. J. Bacteriol. 190, 3670–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little D. J., Milek S., Bamford N. C., Ganguly T., DiFrancesco B. R., Nitz M., Deora R., and Howell P. L. (2015) The protein BpsB is a poly-β-1,6-N-acetyl-d-glucosamine deacetylase required for biofilm formation in Bordetella bronchiseptica. J. Biol. Chem. 290, 22827–22840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jennings L. K., Storek K. M., Ledvina H. E., Coulon C., Marmont L. S., Sadovskaya I., Secor P. R., Tseng B. S., Scian M., Filloux A., Wozniak D. J., Howell P. L., and Parsek M. R. (2015) Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. U.S.A. 112, 11353–11358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colvin K. M., Alnabelseya N., Baker P., Whitney J. C., Howell P. L., and Parsek M. R. (2013) PelA deacetylase activity is required for Pel polysaccharide synthesis in Pseudomonas aeruginosa. J. Bacteriol. 195, 2329–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koo H. M., Song S. H., Pyun Y. R., and Kim Y. S. (1998) Evidence that a β-1,4-endoglucanase secreted by Acetobacter xylinum plays an essential role for the formation of cellulose fiber. Biosci. Biotechnol. Biochem. 62, 2257–2259 [DOI] [PubMed] [Google Scholar]
- 38.Kawano S., Tajima K., Kono H., Erata T., Munekata M., and Takai M. (2002) Effects of endogenous endo-β-1,4-glucanase on cellulose biosynthesis in Acetobacter xylinum ATCC23769. J. Biosci. Bioeng. 94, 275–281 [DOI] [PubMed] [Google Scholar]
- 39.Nakai T., Sugano Y., Shoda M., Sakakibara H., Oiwa K., Tuzi S., Imai T., Sugiyama J., Takeuchi M., Yamauchi D., and Mineyuki Y. (2013) Formation of highly twisted ribbons in a carboxymethylcellulase gene-disrupted strain of a cellulose-producing bacterium. J. Bacteriol. 195, 958–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain S., and Ohman D. E. (2005) Role of an alginate lyase for alginate transport in mucoid Pseudomonas aeruginosa. Infect. Immun. 73, 6429–6436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al Abdallah Q., Choe S. I., Campoli P., Baptista S., Gravelat F. N., Lee M. J., and Sheppard D. C. (2012) A conserved C-terminal domain of the Aspergillus fumigatus developmental regulator MedA is required for nuclear localization, adhesion and virulence. PLoS One 7, e49959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gravelat F. N., Ejzykowicz D. E., Chiang L. Y., Chabot J. C., Urb M., Macdonald K. D., al-Bader N., Filler S. G., and Sheppard D. C. (2010) Aspergillus fumigatus MedA governs adherence, host cell interactions and virulence. Cell. Microbiol. 12, 473–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin C. J., Sasse C., Gerke J., Valerius O., Irmer H., Frauendorf H., Heinekamp T., Straßburger M., Tran V. T., Herzog B., Braus-Stromeyer S. A., and Braus G. H. (2015) Transcription factor SomA is required for adhesion, development and virulence of the human pathogen Aspergillus fumigatus. PLoS Pathog. 11, e1005205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bom V. L., de Castro P. A., Winkelströter L. K., Marine M., Hori J. I., Ramalho L. N., dos Reis T. F., Goldman M. H., Brown N. A., Rajendran R., Ramage G., Walker L. A., Munro C. A., Rocha M. C., Malavazi I., et al. (2015) The Aspergillus fumigatus sitA phosphatase homologue is important for adhesion, cell wall integrity, biofilm formation, and virulence. Eukaryot. Cell 14, 728–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winkelströter L. K., Bom V. L., de Castro P. A., Ramalho L. N., Goldman M. H., Brown N. A., Rajendran R., Ramage G., Bovier E., Dos Reis T. F., Savoldi M., Hagiwara D., and Goldman G. H. (2015) High osmolarity glycerol response PtcB phosphatase is important for Aspergillus fumigatus virulence. Mol. Microbiol. 96, 42–54 [DOI] [PubMed] [Google Scholar]
- 46.Ramage G., Mowat E., Jones B., Williams C., and Lopez-Ribot J. (2009) Our current understanding of fungal biofilms. Crit. Rev. Microbiol. 35, 340–355 [DOI] [PubMed] [Google Scholar]
- 47.Nobile C. J., and Mitchell A. P. (2006) Genetics and genomics of Candida albicans biofilm formation. Cell. Microbiol. 8, 1382–1391 [DOI] [PubMed] [Google Scholar]
- 48.Li F., Svarovsky M. J., Karlsson A. J., Wagner J. P., Marchillo K., Oshel P., Andes D., and Palecek S. P. (2007) Eap1p, an adhesin that mediates Candida albicans biofilm formation in vitro and in vivo. Eukaryot. Cell 6, 931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nobile C. J., Nett J. E., Andes D. R., and Mitchell A. P. (2006) Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot. Cell 5, 1604–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nobile C. J., Schneider H. A., Nett J. E., Sheppard D. C., Filler S. G., Andes D. R., and Mitchell A. P. (2008) Complementary adhesin function in C. albicans biofilm formation. Curr. Biol. 18, 1017–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheppard D. C., Yeaman M. R., Welch W. H., Phan Q. T., Fu Y., Ibrahim A. S., Filler S. G., Zhang M., Waring A. J., and Edwards J. E. Jr. (2004) Functional and structural diversity in the Als protein family of Candida albicans. J. Biol. Chem. 279, 30480–30489 [DOI] [PubMed] [Google Scholar]
- 52.Fu Y., Ibrahim A. S., Sheppard D. C., Chen Y. C., French S. W., Cutler J. E., Filler S. G., and Edwards J. E. Jr. (2002) Candida albicans Als1p: an adhesin that is a downstream effector of the EFG1 filamentation pathway. Mol. Microbiol. 44, 61–72 [DOI] [PubMed] [Google Scholar]
- 53.Otoo H. N., Lee K. G., Qiu W., and Lipke P. N. (2008) Candida albicans Als adhesins have conserved amyloid-forming sequences. Eukaryot. Cell 7, 776–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramsook C. B., Tan C., Garcia M. C., Fung R., Soybelman G., Henry R., Litewka A., O'Meally S., Otoo H. N., Khalaf R. A., Dranginis A. M., Gaur N. K., Klotz S. A., Rauceo J. M., Jue C. K., and Lipke P. N. (2010) Yeast cell adhesion molecules have functional amyloid-forming sequences. Eukaryot. Cell 9, 393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia M. C., Lee J. T., Ramsook C. B., Alsteens D., Dufrêne Y. F., and Lipke P. N. (2011) A role for amyloid in cell aggregation and biofilm formation. PLoS One 6, e17632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadovskaya I., Vinogradov E., Li J., Hachani A., Kowalska K., and Filloux A. (2010) High-level antibiotic resistance in Pseudomonas aeruginosa biofilm: the ndvB gene is involved in the production of highly glycerol-phosphorylated β-(1-→3)-glucans, which bind aminoglycosides. Glycobiology 20, 895–904 [DOI] [PubMed] [Google Scholar]
- 57.Bowen W. H., and Koo H. (2011) Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 45, 69–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orlean P. A. (1982) (1,3)-β-d-Glucan synthase from budding and filamentous cultures of the dimorphic fungus Candida albicans. Eur. J. Biochem. 127, 397–403 [DOI] [PubMed] [Google Scholar]
- 59.Mio T., Adachi-Shimizu M., Tachibana Y., Tabuchi H., Inoue S. B., Yabe T., Yamada-Okabe T., Arisawa M., Watanabe T., and Yamada-Okabe H. (1997) Cloning of the Candida albicans homolog of Saccharomyces cerevisiae GSC1/FKS1 and its involvement in β-1,3-glucan synthesis. J. Bacteriol. 179, 4096–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Douglas C. M., D'Ippolito J. A., Shei G. J., Meinz M., Onishi J., Marrinan J. A., Li W., Abruzzo G. K., Flattery A., Bartizal K., Mitchell A., and Kurtz M. B. (1997) Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41, 2471–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kondoh O., Tachibana Y., Ohya Y., Arisawa M., and Watanabe T. (1997) Cloning of the RHO1 gene from Candida albicans and its regulation of β-1,3-glucan synthesis. J. Bacteriol. 179, 7734–7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazur P., and Baginsky W. (1996) In vitro activity of 1,3-β-d-glucan synthase requires the GTP-binding protein Rho1. J. Biol. Chem. 271, 14604–14609 [DOI] [PubMed] [Google Scholar]
- 63.Shematek E. M., and Cabib E. (1980) Biosynthesis of the yeast cell wall. II. Regulation of β-(1 leads to 3)glucan synthetase by ATP and GTP. J. Biol. Chem. 255, 895–902 [PubMed] [Google Scholar]
- 64.Shematek E. M., Braatz J. A., and Cabib E. (1980) Biosynthesis of the yeast cell wall. I. Preparation and properties of β-(1 leads to 3)glucan synthetase. J. Biol. Chem. 255, 888–894 [PubMed] [Google Scholar]
- 65.Taff H. T., Nett J. E., Zarnowski R., Ross K. M., Sanchez H., Cain M. T., Hamaker J., Mitchell A. P., and Andes D. R. (2012) A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS Pathog. 8, e1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mishra M., Byrd M. S., Sergeant S., Azad A. K., Parsek M. R., McPhail L., Schlesinger L. S., and Wozniak D. J. (2012) Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell. Microbiol. 14, 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitchell K. F., Taff H. T., Cuevas M. A., Reinicke E. L., Sanchez H., and Andes D. R. (2013) Role of matrix β-1,3 glucan in antifungal resistance of non-albicans Candida biofilms. Antimicrob. Agents Chemother. 57, 1918–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nett J., Lincoln L., Marchillo K., Massey R., Holoyda K., Hoff B., VanHandel M., and Andes D. (2007) Putative role of β-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 51, 510–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nichols W. W., Dorrington S. M., Slack M. P., and Walmsley H. L. (1988) Inhibition of tobramycin diffusion by binding to alginate. Antimicrob. Agents Chemother. 32, 518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colvin K. M., Irie Y., Tart C. S., Urbano R., Whitney J. C., Ryder C., Howell P. L., Wozniak D. J., and Parsek M. R. (2012) The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 14, 1913–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nobile C. J., Andes D. R., Nett J. E., Smith F. J., Yue F., Phan Q. T., Edwards J. E., Filler S. G., and Mitchell A. P. (2006) Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2, e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nobile C. J., Nett J. E., Hernday A. D., Homann O. R., Deneault J. S., Nantel A., Andes D. R., Johnson A. D., and Mitchell A. P. (2009) Biofilm matrix regulation by Candida albicans Zap1. PLoS Biol. 7, e1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alem M. A., Oteef M. D., Flowers T. H., and Douglas L. J. (2006) Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilm development. Eukaryot. Cell 5, 1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bachmann S. P., VandeWalle K., Ramage G., Patterson T. F., Wickes B. L., Graybill J. R., and López-Ribot J. L. (2002) In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46, 3591–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lazzell A. L., Chaturvedi A. K., Pierce C. G., Prasad D., Uppuluri P., and Lopez-Ribot J. L. (2009) Treatment and prevention of Candida albicans biofilms with caspofungin in a novel central venous catheter murine model of candidiasis. J. Antimicrob. Chemother. 64, 567–570 [DOI] [PubMed] [Google Scholar]
- 76.Martins M., Henriques M., Lopez-Ribot J. L., and Oliveira R. (2012) Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms. Mycoses 55, 80–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martins M., Uppuluri P., Thomas D. P., Cleary I. A., Henriques M., Lopez-Ribot J. L., and Oliveira R. (2010) Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia 169, 323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rajendran R., Williams C., Lappin D. F., Millington O., Martins M., and Ramage G. (2013) Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryot. Cell 12, 420–429 [DOI] [PMC free article] [PubMed] [Google Scholar]