Abstract
l-Dopachrome tautomerase (l-DCT), also called tyrosinase-related protein-2 (TRP-2), is a melanoma antigen overexpressed in most chemo-/radiotherapeutic stress-resistant tumor clones, and caveolin-1 (CAV1) is a main regulator of numerous signaling processes. A structural and functional relationship between DCT and CAV1 is first presented here in two human amelanotic melanoma cell lines, derived from vertical growth phase (MelJuSo) and metastatic (SKMel28) melanomas. DCT co-localizes at the plasma membrane with CAV1 and Cavin-1, another molecular marker for caveolae in both cell phenotypes. Our novel structural model proposed for the DCT-CAV1 complex, in addition to co-immunoprecipitation and mass spectrometry data, indicates a possible direct interaction between DCT and CAV1. The CAV1 control on DCT gene expression, DCT post-translational processing, and subcellular distribution is cell phenotype-dependent. DCT is a modulator of CAV1 stability and supramolecular assembly in both cell phenotypes. During autocrine stimulation, the expressions of DCT and CAV1 are oppositely regulated; DCT increases while CAV1 decreases. Sub-confluent MelJuSo clones DCThigh/CAV1low are proliferating and acquire fibroblast-like morphology, forming massive, confluent clusters as demonstrated by immunofluorescent staining and TissueFAXS quantitative image cytometry analysis. CAV1 down-regulation directly contributes to the expansion of MelJuSo DCThigh subtype. CAV1 involved in the perpetuation of cell phenotype-overexpressing anti-stress DCT molecule supports the concept that CAV1 functions as a tumor suppressor in early stages of melanoma. DCT is a regulator of the CAV1-associated structures and is possibly a new molecular player in CAV1-mediated processes in melanoma.
Keywords: antigen processing, caveolin, epithelial-mesenchymal transition (EMT), melanoma, structural model
Introduction
Cutaneous malignant melanoma remains the deadliest form of skin cancer worldwide (1). The skin melanocytes, normally located at the epidermis-dermis junction, start an uncontrolled proliferation process (radial growth phase, RGP), followed by dermis invasion (vertical growth phase, VGP).3 In a more advanced stage, melanoma cells migrate from this primary site to distant organs (liver, lung), populating them (metastasis) and eventually causing death (2). The mechanisms that specifically operate in tumor cell phenotypes determining the switch from the non-metastatic to metastatic stage are not entirely elucidated.
Tyrosinase-related protein-2 (TRP-2 or Tyrp2) is a member of the tyrosinase-related protein family, together with tyrosinase (TYR) and tyrosinase-related protein-1 (TRP-1). Tyrosinase-related proteins are expressed by both melanocytes and melanoma forming the enzymatic cluster responsible for pigment production (3).
TRP-2, also named dopachrome tautomerase (DCT), functions downstream of TYR by converting dopachrome into 5,6-dihydroxyindole-2-carboxylic acid (DHICA), the precursor of eumelanins with a potent hydroxyl radical-scavenging activity (4). In the absence of DCT, dopachrome is spontaneously converted into 5,6-dihydroxyindole, a more toxic intermediate than DHICA. The DCT-melanocyte protection via its enzymatic products also functions for the benefit of pigmented malignant melanoma. A DCT-mediated pathway that enables melanoma cells with more resistance to chemo-/radiotherapeutic or oxidative stress (5–8) has been reported. In a speculative proposed mechanism, the most stress-resistant pigmented melanoma cell lines, overexpressing endogenous DCT, will produce high amounts of the stable antioxidant DHICA, which may indirectly activate the ERK pathway (9). This theory, however, does not explain why in amelanotic melanoma cell lines (10) and specimens (11) DCT is well expressed despite the fact that its natural substrate, dopachrome, is absent due to TYR premature degradation.
DCT is frequently referred to as a significant tumor antigen in glioblastoma multiformis (12), a brain cancer with no melanin but sharing clinical characteristics and bad prognostics similar to melanoma. DCT has also been described as a regulator of neural progenitor cell proliferation (13) and found to be involved in a cellular antiapoptotic pathway in ASJ sensory neurons in Caenorhabditis elegans (14). All these data advocate for DCT anti-apoptotic activities outside melanogenic pathway. Despite this body of evidence about DCT, the molecular environment in which DCT operates and the regulatory mechanisms of its fate in melanoma are far from being understood.
Caveolin-1 (CAV1) is enriched in caveolae, invaginated plasma membrane subdomains defining a particular endocytic pathway, and in CAV1-scaffolds that correspond to non-caveolar, flat, and oligomerized domains. Both caveolae and CAV1-scaffolds are associated with lipid rafts, which are membrane domains with a very dynamic structure abundant in cholesterol, sphingolipids recruiting different molecular players of signaling platforms.
There are two opposite theories about CAV1 expression and function in tumor biology. One presents CAV1 as a tumor suppressor (15), and the other is associated with CAV1 overexpression in metastatic progression and poor prognostics (16). Either of these two can be true if one observes that CAV1 expression and stability are very dependent on numerous cellular and environmental factors that can eventually change the designation of CAV1 from tumor suppressor to tumor promotor.
In melanoma, CAV1 function is still ambiguous (17). Some studies associate CAV1 secreted in microvesicles with tumorigenicity (18), and others present CAV1 as a tumor suppressor by inhibiting Wnt-β-catenin-Tcf/Lef (19), Src/FAK (20) pathways, or attenuating tumor cell motility by disrupting glycosphingolipid GD3-mediated malignant signaling (21).
This study demonstrates for the first time a mutual structural and functional relationship between DCT and CAV1 in two human amelanotic melanoma cell lines, MelJuSo and SKMel28, representative for early malignant/VGP phenotype and metastatic phenotype, respectively. CAV1 is a modulator of DCT expression, processing, and subcellular distribution in early malignant cells, whereas DCT regulates CAV1 stability and assembly in supramolecular aggregates in both cell lines.
In the context of the acknowledged biological functions of DCT and CAV1, DCT-CAV1 cross-talk is involved in early phenotype switching and perpetuation of an anti-apoptotic cellular subtype as well as in the architecture of CAV1-associated structures and very likely in CAV1-mediated processes in melanoma.
Experimental Procedures
Primary Antibodies, Anti-DCT Antibodies
α-hDCT rabbit polyclonal antibodies were raised against the hDCT luminal domain (aa 27–439), obtained and characterized at the Institute of Biochemistry, Bucharest, Romania (22); D18 goat polyclonal antibodies with the epitope mapping near the N terminus of TRP-2 (DCT) of human origin were as specified in the manufacturer's data sheet (sc-10451, Santa Cruz Biotechnology); α-C-terminal rabbit polyclonal antibody was obtained against a sequence in the C-terminal domain of hDCT polypeptide (C-506METHLSSKRYTEEA519-COOH) (Sdix, Newark, DE).
Anti-CAV1 Antibodies
The anti-CAV1 (D46G3) XP® rabbit mAb, 3267S, was from Cell Signaling (α-CAV1-CS); anti-CAV1 7C8 antibody was from Santa Cruz Biotechnology (α-CAV1-sc) (sc-53564). Other primary antibodies are as follows: anti-Cavin-1 goat polyclonal C-20 (sc-82326) and anti-calnexin goat polyclonal C-20 (sc-6465) from Santa Cruz Biotechnology; purified mouse anti-actin Ab-5, (612656, BD Transduction Laboratories); anti-TRP1 (α-hPep1) and anti-TYR (α-Pep7h)-rabbit polyclonal antibodies obtained against a sequence in the C-terminal domain of hTRP1 and hTYR, respectively (23) (gift from V. Hearing, National Institutes of Health, Bethesda).
Secondary Antibodies
Donkey anti-goat IgG-HRP (sc-2020), goat anti-rabbit IgG-HRP (sc-2004), and rabbit anti-mouse IgG-HRP (sc-358914) were all from Santa Cruz Biotechnology; donkey anti-mouse, anti-rabbit, and anti-goat IgGs (H+L) coupled to Alexa Fluor 488, 594, and 647, were all from Life Technologies, Inc.
Chemicals
Trypsin-EDTA (0.05%, 25300, Gibco) was from Thermo Fisher; phenylmethylsulfonyl fluoride (PMSF) (P7626), paraformaldehyde, Tricine (T0377), Tween 20 (P7949), Coomassie SERVA Blue R (35051) were from Sigma; β-glycerophosphate (35675) was from Calbiochem; NaF (sc-24988), orthovanadate (sc-3540), DTT (sc-29089), nonfat dry milk, blotto (sc2325), and nitrocellulose membrane (sc-3724) were from Santa Cruz Biotechnology; protease inhibitor mixture (Complete, 11 697 498 001) was from Roche Applied Science; Nonidet P-40 (RIST1315) was from MP Biomedicals; BCA protein assay reagent was from Pierce; protein A-Sepharose 4B (101041) was from Invitrogen; saponin (from quillaja bark, 47036) was from Fluka); TRIzol was from Life Technologies, Inc.; and acrylamide (acrylamide/bisacrylamide solution 30%, 1006391000) was from Merck.
Cell Lines and Culture Conditions
Melanoma cell lines MelJuSo (MJS) (ATCC accession no. ACC74), SKMel28 (SK28) (ATCC accession no. HTB-72), and MNT-1 (a gift from Dr. P. G. Natali (University La Sapienza, Rome, Italy)) were grown in RPMI 1640 complete medium as described (22). Originally, the MJS line was indicated as representative of a very early stage of tumor differentiation (24) and by others as a VGP phenotype (25). SK28 line has been raised from a tumor that recurred after 4 years from the original tumor (26) and is indicated by others as metastatic (20, 27).
For time course experiments, cells were seeded at 10 × 104 cells in 25-cm2 flasks and grown for 48, 72, and 96 h (MJS) or 72, 96, and 120 h (SK28). These time periods were selected as representative for obtaining sub-confluent, semi-confluent, and confluent cultures visually estimated by the occupancy of culture flask surface as ∼20, 50, and 90%, respectively. Cells were harvested by trypsinization and counted. An equal number of cells was pelleted throughout the experiments and stored until further use at −80 °C. For immunofluorescence microscopy, 4 × 104 MJS cells and 2 × 104 SK28 cells were seeded on glass coverslips of 19 mm diameter in a 6-well plate and treated (si-ct, si-CAV1, and si-DCT) as described in each experiment and fixed at the indicated time points.
Western Blotting (WB) Analysis
Equal cell numbers pelleted for time course or silencing experiments were lysed in 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm EDTA, containing 1% Nonidet P-40, and protease inhibitor mixture (lysis buffer) by incubation on ice for 1 h. The soluble material (Nonidet P-40-soluble), representing total cell lysate, was separated from the insoluble material (Nonidet P-40-insoluble) by centrifugation, at 14,500 rpm, for 25 min at 4 °C. Equal protein amounts of Nonidet P-40-soluble fractions (determined by BCA assay) were mixed with SDS-PAGE sample buffer (50 mm Tris-HCl, pH 6.8, 2% SDS, 100 mm DTT, 4% glycerol, and 0.004% bromphenol blue, final concentration in samples), and Nonidet P-40-insoluble fractions were directly resuspended with sample buffer. All samples were thermally treated (+) (incubated at 100 °C, 5 min) or not (−) (incubated at 37 °C, 5 min), run in 10% SDS-PAGE, and blotted onto nitrocellulose membrane. Blots were blocked with blotto (5% nonfat dry milk in PBS + 0.2% Tween 20), overnight, at 4 °C. DCT and CAV1 detection was performed using the following antibodies: D18 (1:500); α-hDCT (1:750); α-C terminus (1:1000); α-CAV1-CS (1:2000); α-CAV1-sc (1:200), and all were incubated for 90 min with membranes at room temperature. For control protein loading, anti-actin (1:3000) or anti-calnexin (1:500) was used. Blots were washed with PBS + 0.1% Tween 20. Binding of the appropriate secondary antibody, donkey anti-goat IgG-HRP (1:3000), goat anti-rabbit IgG-HRP (1:10,000), and rabbit anti-mouse IgG-HRP (1:10,000), was followed by washing and detection with enhanced chemiluminescent substrate or SuperSignal West Femto chemiluminescent substrate (in case of CAV1 detection from SK28 samples) both from Thermo Scientific and Pierce.
Pulse-Chase Experiments
The protocol for the pulse-chase experiment was described in detail elsewhere (28). The pharmacological agent nystatin (N6261, Sigma) was added to the cells in a final concentration of 25 μg/ml during pulse (30 min) and maintained for the chase time period (3 h).
Glycolytic Treatments
Approximately 25 or 10 μg of total protein from Nonidet P-40-soluble fractions (determined by BCA assay) were set in reactions with either endoglycosidase H (EndoH) or peptide:N-glycosidase F (PNGase F) (New England Biolabs), according to a protocol described elsewhere (28).
Immunoprecipitation (IP) Experiment
MJS cells (passage 4) were seeded in ten 75-cm2 flasks at a density of 34 × 104 cells/flask. After 48 h, cells were washed with ice-cold PBS and scraped in ∼ 6.5 ml of lysis buffer (see “Western Blot (WB) Analysis”) containing protease and phosphatase inhibitors (protease inhibitor mixture, 1 mm PMSF, 1 mm β-glycerophosphate, 1 mm orthovanadate, 20 mm NaF). Cells were lysed for 1 h on ice, and lysates were centrifuged for 30 min, at 14,500 rpm, 4 °C; meanwhile protein A-Sepharose 4B beads were equilibrated in lysis buffer. An aliquot was used for a pre-clearing step (90 min, at 4 °C) of the lysate. Lysate was then divided over three tubes (∼1.8 ml containing 750 μg of total protein), and to each one of the following antibodies was added α-hDCT, α-C terminus, and anti-TYR (α-Pep7h). After 90 min of incubation, ∼30 μl of packed beads of pre-equilibrated protein A-Sepharose 4B were added, and incubation was continued overnight, at 4 °C, under rotation. The next day, the beads were washed five times, 10 min each with 1.3 ml of lysis buffer containing 0.2% Nonidet P-40, and bound proteins were eluted by boiling in 100 μl of reducing sample buffer. Cell lysates and 20 μl of immunoprecipitated material were analyzed by 10% SDS-PAGE for WB detection with anti-DCT or Coomassie staining and gel slicing for mass spectrometry analysis. For CAV1 detection by WB, samples were run on a 16% acrylamide-Tricine gel especially designed for small protein separation (29), and detection was done with α-CAV1-CS.
Immunofluorescence Microscopy
Melanoma cells treated with si-DCT, si-CAV1, or si-ct upon seeding on glass coverslips (631-0155, VWR International) and grown for variable time periods were fixed and permeabilized as described (22). Cells were labeled for DCT with α-hDCT (1:1000), D18 (1:100), for CAV1 with α-CAV1-sc (1:75), or α-CAV1-CS (1:200) and for Cavin-1 with α-Cavin-1 (1:100). Incubation with the primary antibodies was for 30 min at room temperature with the exception of D18 antibody done either for 30 min or overnight at 4 °C.
The pairs of antibodies α-hDCT/α-CAV1-sc and D18/α-CAV1-CS were counterstained with donkey anti-rabbit Alexa Fluor 594/donkey anti-mouse Alexa Fluor 488, and donkey anti-goat Alexa Fluor 594/donkey anti-rabbit Alexa Fluor 488, respectively. For triple staining, the appropriate secondary antibodies donkey anti-mouse, anti-rabbit, and anti-goat IgGs (H+L), coupled to Alexa 488, 594, and 647, respectively (1:400), were used to label the appropriate primary antibodies by 30 min of incubation. Cells mounted in ProLong antifade with DAPI (Life Technologies, Inc.) were analyzed with a Zeiss LSM 710 confocal microscope (Figs. 2, 3, 5, and 6), AxioImager.M2 with ApoTome.2 (Fig. 7E), or Tissue FAXSPlus System (Figs. 5D and 7F). Images were processed for publication in Adobe Photoshop, CSS, Version 12.0.
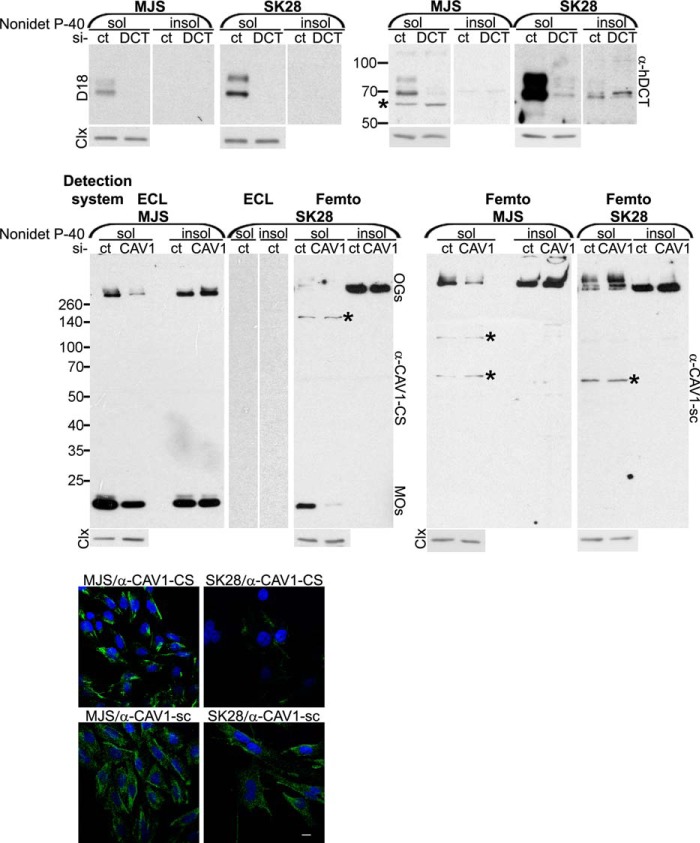
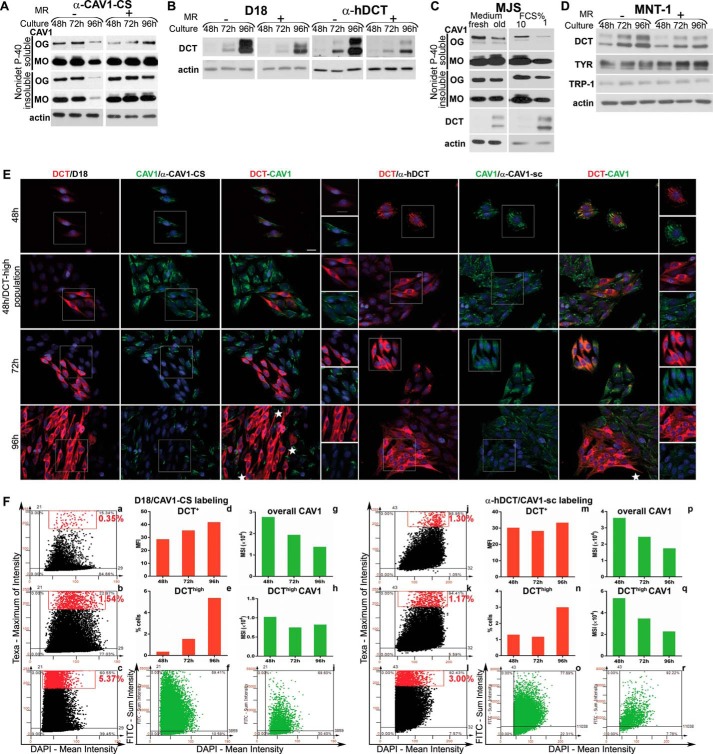
FIGURE 2.
Characterization of anti-DCT and anti-CAV1 antibodies in Western blot and immunofluorescence microscopy. MJS (72 h) and SK28 (96 h) melanoma cell lines transfected with siRNA control (si-ct), si-DCT (si-DCT), or si-CAV1(si-CAV1) were assessed for DCT and CAV1 expression in Western blot (WB) and immunofluorescence microscopy (IF) with D18 or α-hDCT and α-CAV1-CS or α-CAV1-sc, respectively. WBs were assessed with ECL or SuperSignal West Femto chemiluminescent substrate (Femto) detection systems. Both anti-DCT and anti-CAV1 antibodies recognize specifically in the two cell lines the products encoded by the DCT and CAV1 gene, respectively, different CAV1 constituents (monomers, oligomers, in Nonidet P-40-soluble (sol) and Nonidet P-40-insoluble (insol) fractions). Importantly, in both cell lines, an insoluble oligomeric CAV1 pool detected with both anti-CAV1 antibodies was resistant to CAV1 down-regulation. Unlike in MJS, the soluble oligomeric CAV1 detected preponderantly with α-CAV1-sc in SK28 cells was also resistant to si-CAV1 indicating that CAV1 aggregation is different in the two cell lines. The nonspecific DCT and CAV1 bands are indicated by stars. Calnexin (Clx) was used as loading control for Nonidet P-40-soluble fractions. The IF images were acquired using ×40 objective. Scale bar represents 10 μm. Each experiment is a representative one of three.
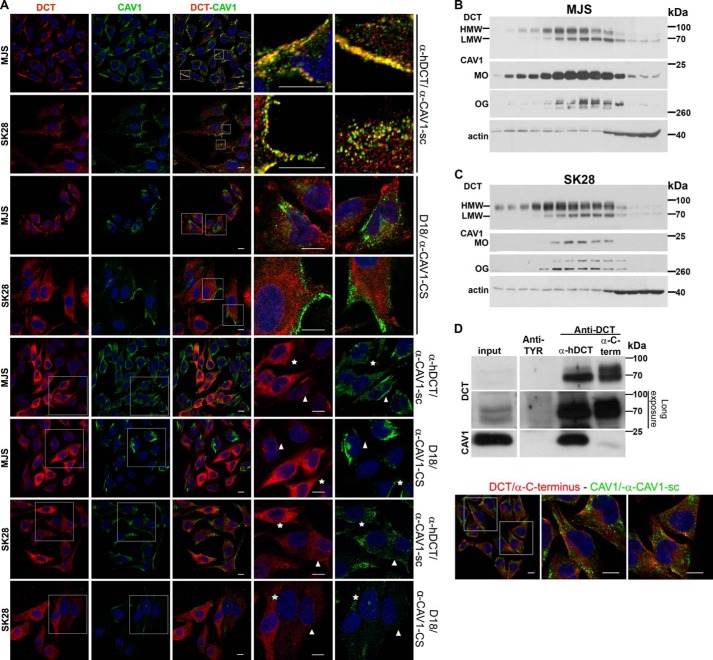
FIGURE 3.
DCT is a constituent of CAV1-associated structures in amelanotic melanoma cell lines. A, DCT and CAV1 distribution in MJS (72 h) and SK28 (96 h) cells by confocal IF microscopy assessed with anti-DCT and anti-CAV1 antibodies. DCT (red) and CAV1 (green) are distributed in common (yellow) (top and 2nd panels) or separate (red or green) structures (3rd and 4th panels) of MJS and SK28 cells. In SK28 cells for CAV1 detection with α-CAV1-CS, increased imaging parameters were used. The incubation with D18 antibody was done overnight in both cell lines. The MJS or SK28 cells overexpressing DCT are indicated by stars, and adjacent cells moderately expressing DCT are indicated by arrowheads. B and C, MJS and SK28 cellular fractions separated in a 4–25% Optiprep gradient ultracentrifugation were assessed for DCT and CAV1 in WB with D18/α-CAV1-CS antisera. CAV1 in SK28 samples is done with the Super Signal Femto enhancing system. D, identification of CAV1 as DCT interactor. The material immunoprecipitated with anti-DCT (α-hDCT or α-C-terminal) or anti-TYR (negative control) antisera from MJS (48 h) cell lysate and eluted from protein A-Sepharose beads analyzed in WB for DCT (D18) and CAV1 (α-CAV1-CS) (left panel) and mass spectrometry (see Table 1) is shown. DCT detection by immunofluorescence microscopy with α-C terminus (right panel) supports the existence of a DCT pool in CAV1-free membranes. The antibody combinations used for DCT/CAV1 detection and cell line are indicated at the right and left side, respectively, of each figure. The last two columns represent enlarged merged details or enlarged separate images. Images were acquired using ×40 oil immersion objective. Scale bar, 10 μm. Each experiment is a representative one of three.
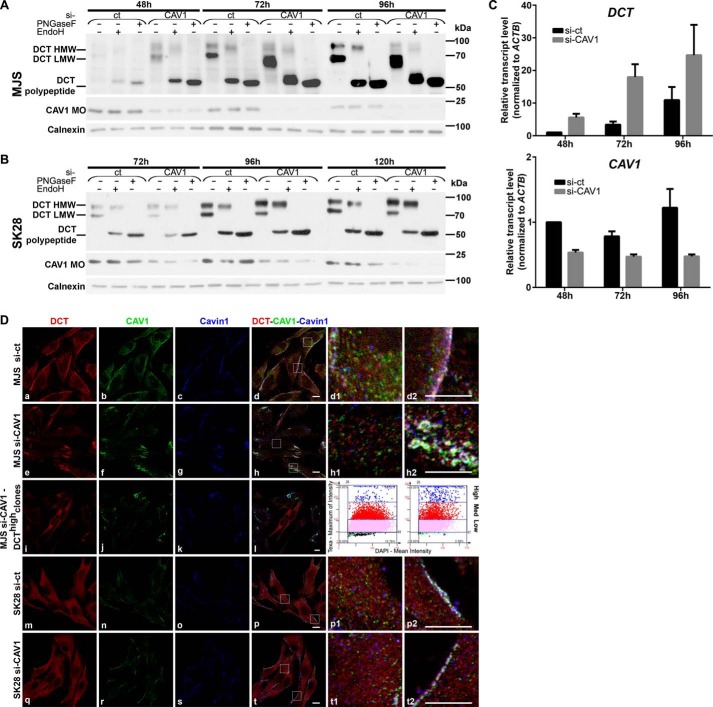
FIGURE 5.
CAV1 silencing has distinct impact on DCT expression, maturation, and subcellular distribution in early and metastatic melanoma cell phenotypes. A and B, DCT processing in si-CAV1 cells. MJS (A) and SK28 (B) cells sub-confluent (48 and 72 h), semi-confluent (72 and 96 h), and confluent (96 and 120 h) cultures in si-ct and si-CAV1 experiments were analyzed for DCT (with D18) by deglycosylation treatments with EndoH and PNGase F and CAV1 MOs (with α-CAV1-CS) in WB. Calnexin was used as loading control. C, DCT mRNA expression determined by real time RT-qPCR in MJS cells (panel DCT) upon silencing of the CAV1 gene (panel CAV1). Graphs show average of experiments (n = 6 replicates for each cell line and time point); error bars represent S.E. D, subcellular distribution of DCT, CAV1, Cavin-1 in MJS (72 h), and SK28 (96 h) si-ct and si-CAV1 cells assessed by triple staining DCT (red), CAV1 (green), and Cavin-1 (blue) in immunofluorescence confocal microscopy. The propensity of DCThigh cell clones upon si-CAV1 treatment assessed by TF quantitative image cytometry (3rd panel, blue dots on left scattergram, si-ct; on right scattergram, si-CAV1). Last columns represent enlarged details of merged images. Scale bar, 10 μm. Images were acquired using ×63 oil immersion objective. Each experiment is a representative one of three.
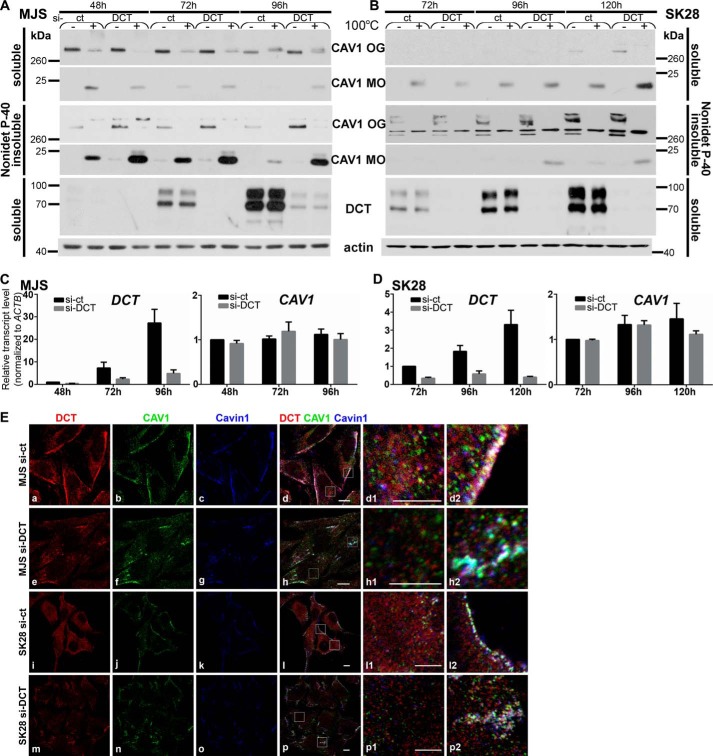
FIGURE 6.
DCT silencing affects CAV1 stability, assembly, and subcellular distribution in amelanotic melanoma cell phenotypes. A and B, CAV1 expression and assembly in si-DCT cells. MJS (A) and SK28 (B) sub-confluent (48 and 72 h), semi-confluent (72 and 96 h), and confluent (96 and 120 h) cultures in si-ct and si-DCT experiments analyzed for CAV1 MOs and OGs in Nonidet P-40-soluble and Nonidet P-40-insoluble fractions in thermally treated (+) or not (−) samples by WB. CAV1 was assessed with α-CAV1-CS and DCT with D18. Actin was used as loading control. C and D, impact of DCT gene silencing on CAV1 mRNA expression. DCT and CAV1 mRNA in si-ct and si-DCT MJS and SK28 cells were determined by real time RT-qPCR. Graphs show average of experiments (n = 6 replicates for each cell line and time point); error bars represent S.E. E, subcellular distribution of DCT (red), CAV1 (green), and Cavin-1 (blue) in MJS (72 h) and SK28 (96 h) si-ct and si-DCT cells assessed by immunofluorescence confocal microscopy. Last columns represent enlarged details of merged images. Scale bar, 10 μm in merged images and 5 μm in enlarged details. Images were acquired using ×63 oil immersion objective. Each experiment is a representative one of three.
FIGURE 7.
DCT and CAV1 expressions are oppositely modulated by environmental and cellular factors in MJS phenotype. A and B, MJS cells were plated and cultured for 48, 72, or 96 h, and in one experiment the culture medium was replenished with a fresh one every 24 h (MR+) or not changed during the indicated time periods (MR−). C, MJS cells cultured for 24 h were incubated with fresh culture medium (fresh) or with medium resulting from a 96-h (old) culture for an additional 24 h (left panel); MJS cells were cultured for 72 h in culture medium with 10 or 1% FCS (right panel). D, MJS and MNT cells were cultured and treated for the indicated time periods as described for A and B. For all experiments, the Nonidet P-40- soluble and Nonidet P-40-insoluble fractions of the MJS cell lysates were analyzed by WB for the expression of CAV1 (with α-CAV1-CS), DCT (with D18 or α-hDCT), TYR (with α-Pep7h), and TRP-1 (with αPep1h). Actin was used as loading control. Each experiment is a representative one of three. E, DCT (red) and CAV1 (green) distribution in cell populations during sub-confluent (48 h) to semi-confluent (72 h) and confluent (96 h) transition assessed by immunofluorescence microscopy. Last columns represent enlarged details of separate images. The antibody combinations for DCT and CAV1 immunostaining are indicated in each panel. Images were acquired using ×40 objective. Scale bar represents 20 μm. Each experiment is a representative one of three. F, image cytometry analysis of triple labeled (DCT/CAV1/nuclei with the two antibody combinations and DAPI) samples performed using the TF system. MFI and MSI were determined for DCT and for CAV1 expression, respectively. For each staining, DCT expression scattergrams for 48 h (upper left), 72 h (middle left), and 96 h (lower left) are gated to select cells with high marker expression (upper right quadrant represents cells positive for DCT, and the rectangle delineates the gated DCThigh cell subpopulation, shown as red dots). For the 96-h time point, CAV1 scattergrams of all cells (bottom middle) as compared with DCThigh cell subpopulation (bottom right) were generated. Values representing mean expression for DCT and CAV1 as well as % of cells in DCThigh subset were derived from Tissue Quest data statistics and are represented as graphs. One representative experiment of two performed is shown.
DCT and Cav1 Silencing
MJS (6 × 104) and SK28 (3 × 104) cells were seeded in 6-well plates and transfected with 7.5 nm CAV1 siRNA (h) (sc-29241, Santa Cruz Biotechnology) (si-CAV1), DCT siRNA (h) (sc-41661, Santa Cruz Biotechnology) (si-DCT), or control siRNA-A (sc-37007, Santa Cruz Biotechnology) (si-ct) designed as non-targeting, using Lipofectamine RNAiMAX (Invitrogen, Life Technologies, Inc.). Cells were harvested at the indicated time points, counted, and pelleted in equal cell numbers and analyzed by WB (thermally or glycolytically treated) as indicated. For RT-qPCR quantification, cells were directly lysed in TRIzol. Alternatively, si-CAV1, si-DCT, and si-ct cells (MJS or SK28) were analyzed by immunofluorescence microscopy.
Subcellular Fractionation of Melanoma Cells
Obtaining subcellular fractions from MJS and SK28 cells was performed after the protocol described for MJS in detail elsewhere (30), which represented an adaptation of the method described originally elsewhere (31). Briefly, MJS or SK28 cells resulting from four 175-cm2 flasks (cultured as semi-confluent cultures) were homogenized in Mg2+/Ca2+-containing base buffer and protease inhibitors on ice. The two postnuclear supernatants were mixed with a 50% Optiprep prepared in base buffer and laid at the bottom of a centrifuge tube. Subsequently, discontinuous 4–20% Optiprep gradient samples were prepared and laid one after the other with a syringe. Following centrifugation at 52,000 × g in SW41Ti Beckman rotor for 90 min at 4 °C, 14 fractions were collected, and equal volumes of fractions were analyzed for DCT and CAV1 by WB.
Mass Spectrometry (MS) Analysis
The samples containing the immune complexes from the IP experiment were run in SDS-PAGE, stained with Coomassie, and destained with a solution of acetic acid in methanol.
In-gel digestion was performed according to a previously described protocol with minor modifications (32). The resulting peptides were resuspended in 20 μl of mobile phase A (0.1% formic acid + 2% acetonitrile) and transferred into vials. For the analysis of the peptides, an Easy nano-LC II HPLC coupled on line to an Orbitrap Velos Pro mass spectrometer (Thermo Fisher Scientific) was used. A 2–30% gradient of mobile phase B (0.1% formic acid + 98% acetonitrile) was applied for 120 min to a chromatographic system composed of a 2-cm C18 (5 μm) precolumn (Thermo Fisher Scientific) connected on line to a 10-cm C18 (3 μm) analytical column (Thermo Fisher Scientific), coupled to a stainless steel emitter (Thermo Fisher Scientific) for electrospray ionization. The acquisition method involved a full scan between 300 and 1800 m/z with Orbitrap detection at a resolution of 30,000 for m/z 400, followed by 10 consecutive collision-induced dissociation fragmentation scans in the ion trap of the +2, +3, and higher charge states of most intense ions (top 10 method).
For peptide identification, raw files were searched with the SEQUEST HT algorithm integrated in Proteome Discoverer Version 1.4 against the human proteome (90,360 sequences as downloaded in May, 2015, from UNIPROT) using the following settings: 10 ppm for precursor mass tolerance and 0.5 Da for fragment ion tolerance, carbamidomethylation of Cys residues as a static modification, and oxidation of methionine as a dynamic modification. For peptide confidence estimation, all of the searches used the automated decoy database-searching algorithm available in Proteome Discoverer Version 1.4 with the percolator node (33). The results were filtered for a 1% false discovery rate at the Peptide Spectrum Match level, and only Peptide Spectrum Matches with a precursor mass tolerance of maximum 5 ppm were kept in the final report. At least two unique tryptic peptides were required for each protein group to assess the identification of the proteins.
Real Time RT-qPCR
Cells from 6-well plates were harvested in 1.0 ml of TRIzol (Life Technologies, Inc.) and stored at −80 °C before RNA purification in accordance with the manufacturer's recommendations. The SensiFAST SYBR No-ROX one-step kit (Bioline, London, UK) was used with 40 ng of total RNA template and 400 nmol/liter primers for the evaluation of the target genes and ACTB internal reference gene expression. Primers were from Eurofins MWG Operon GmbH (Ebersberg, Germany) and are listed in supplemental Table 1. In parallel, negative controls without RNA templates (no template controls) and without reverse transcriptase (no RT controls) were run with each assay using actin primers. Amplifications were performed in triplicate using the Rotor-Gene 6000 instrument from Corbett Life Science, a Qiagen Co. (Mortlake, Australia). After an initial reverse transcription at 42 °C for 10 min and polymerase activation at 95 °C for 10 min, temperature cycling was performed as specified in supplemental Table 1. The relative expressions of DCT and CAV1 were determined using calibrator values obtained for control samples at the first time point within the experimental interval. For every gene, mRNA signal was normalized to the actin internal control. Using comparative quantitative analysis (Rotorgene 6000 Software, Corbett), we determined the relative transcript level and represented the results using GraphPad Prism Version 6.0 (La Jolla, CA). Data represent the average of three independent experiments conducted in duplicate (n = 6).
Structural Bioinformatics Analysis
Amino acid sequences were extracted from NCBI RefSeq Protein Database (34): human CAV1 isoform α (NP_001744.2); human l-DCT isoform 1 precursor (NP_001913.2). The two sequences were subject to extensive bioinformatic analysis to guide structural modeling and pinpoint interacting segments. Location of transmembrane (TM) regions, secondary structure elements, and disordered regions was established based on a consensus prediction from several different methods, as described in the supplemental Material 1. Molecular modeling and visualization were accomplished using PyMOL Molecular Graphics System, Version 1.5.0.4.
Image Cytometry
Immunofluorescently stained samples were automatically scanned with Zeiss AxioImager. Z1 fluorescence inverted microscope controlled by TissueFAXS (TF) software Version 3.5.5 (Tissue Gnostics GmbH, Vienna, Austria). TF image cytometry system allows the quantitative analysis of immunofluorescently labeled antigens in cytologically fixed specimens (35, 36). Images were acquired at exposure times determined on negative and most brightly stained controls to ensure that fluorescent signals are within the dynamic range of the markers' expression. Image files were loaded onto the Tissue Quest analysis module Version 4.0.15 (Tissue Gnostics GmbH, Vienna, Austria) for cytometry analysis of DCT and CAV1 expression. We analyzed between 24,000 and 81,000 cells per coverslip for each time point and silencing treatment. Single cells were identified based on nuclei staining, and subpopulations were calculated as percentages of the total number of cells (DAPI+ cells) per region of interest. For DCT expression, Alexa Fluor 594 fluorescence emission pixel values were assessed by a maximum of intensity parameter, which defines the brightest pixel intensity of each cell. This was chosen to ensure best separation of DCThigh cell subpopulation. For CAV1 expression, Alexa Fluor 488 fluorescence was assessed by a sum intensity parameter, which defines the total integrated pixel intensity of each cell. This ensures the correct evaluation of CAV1 as it has a dotted scattered location throughout cytoplasm. For quantitative analysis, the means were calculated for each parameter and channel for analyzed cells and depicted on the graphs as mean of maximum fluorescence intensity (MFI) or mean sum intensity (MSI), respectively. Cutoffs and gates were set on a representative sample area in each series (72-h time point, untreated control) using the forward and backward gating functions of the software.
Results
DCT Is Well expressed and Fully Processed in Two Amelanotic Melanoma Cell Phenotypes
The DCT expression analyzed by WB showed two bands in both MJS and SK28 cells of ∼68 kDa (low molecular weight (LMW)) and 80 kDa (high molecular weight (HMW)) representing DCT glycoforms (Fig. 1A). The LMW band, totally EndoH-sensitive, was the DCT precursor synthesized in endoplasmic reticulum (ER), containing N-linked high mannose oligosaccharides whereas the HMW band, EndoH-resistant, was the mature DCT fully processed, containing complex oligosaccharides acquired in medial Golgi compartment. Following PNGase F digestion, the two bands co-migrated as one having the molecular weight corresponding to DCT polypeptide, however, in higher amounts in SK28 than in MJS (this difference is evident in lower exposure WB, data not shown). This indicated that in SK28 cells DCT expression was increased compared with MJS.
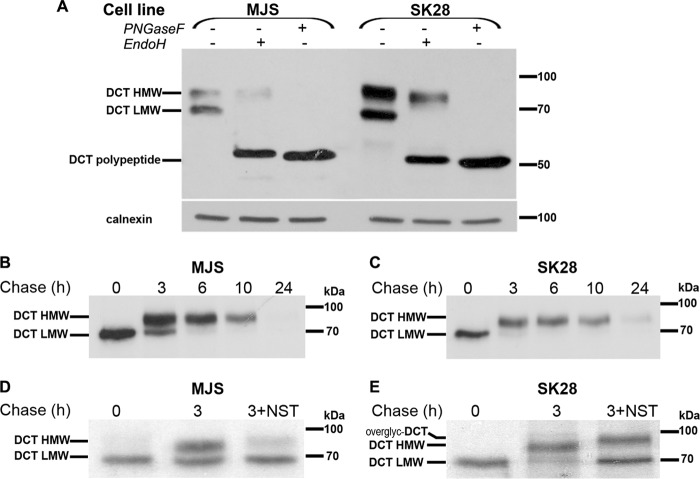
FIGURE 1.
DCT is fully processed in two human amelanotic melanoma cell lines and its maturation is dependent on the integrity of cholesterol-rich domains. A, MJS (72 h) and SK28 (96 h) cell lysates (25 μg total protein), comparatively analyzed for DCT expression and N-glycan processing by glycolytic treatments (EndoH and PNGase F) in WB. DCT-LMW band is a DCT precursor, containing high mannose oligosaccharides, and is totally EndoH-sensitive, whereas DCT-HMW band is DCT-mature, containing complex oligosaccharides, and is EndoH-resistant. Both high mannose and complex N-glycans are totally removed by PNGase F. B and C, kinetics of DCT maturation analyzed by pulse-chase experiments in MJS and SK28 cells. DCT precursor is gradually converted within 3 h into a mature DCT form that is further stable for ∼10 h. D and E, DCT maturation in the presence of cholesterol sequestrating agent NST results in formation of DCT precursor (MJS) or DCT overglycosylated (overglyc-DCT) (SK28). Each experiment is a representative one of three.
The kinetics of DCT maturation analyzed by pulse-chase and IP was similar in both amelanotic cell lines. The entire amount of DCT precursor synthesized within 30 min of pulse (0 chase) was converted into DCT mature glycoform within more than 3 h of chase in MJS cells (Fig. 1B) and in ∼3 h of chase in SK28 cells (Fig. 1C), which represented the time period in which DCT was processed and trafficked from ER along the Golgi complex. After conversion of the DCT-LMW form to the DCT-HMW form, no further change in electrophoretic mobility of slower running DCT-HMW occurred, indicating that mature DCT moved from the trans-Golgi network to a steady-state compartment or cycled between intracellular compartments. Between 6 and 10 h of chase, the intensities of DCT bands slightly decreased in both cell lines (Fig. 1, B and C) culminating within 24 h when no signal or a very faint band was detected in MJS (Fig. 1B) and SK28 (Fig. 1C), respectively. This demonstrated that beyond 10 h most of DCT synthesized within 30 min of pulse was degraded or left the cell being possibly secreted. By conducting the pulse-chase experiments in the presence of nystatin (NST), an agent known to sequestrate membrane cholesterol (37), in MJS cells only the DCT precursor was detected (Fig. 1D), whereas in SK28 cells, the complex DCT form had a higher apparent molecular weight than in the untreated cells (Fig. 1E). The opposite DCT glycosylation patterns induced by NST treatments in the two cell phenotypes were caused by an alteration in DCT traffic rather than in the Golgi glycosylation machinery. This alternative is supported by the presence of a high mannose DCT glycoform in addition to overglycosylated DCT at 3 h of chase in NST-treated SK28 cells, which does not appear in a DCT 3-h chase in SK28-untreated cells (Fig. 1E). In MJS, DCT was blocked before reaching the medial Golgi, whereas in SK28, DCT traffic was most probably only delayed along the Golgi resulting in slower maturation kinetics and a longer retention of DCT precursor in contact with the active medial or trans-Golgi glycosylation machinery. These data showed that DCT maturation is dependent on the integrity of cholesterol-rich domains (CRDs) that may have distinct composition in MJS and SK28 melanoma cell phenotypes.
DCT Is a Constituent of Caveolin-1-associated Membrane Microdomains
In the attempt to characterize more in-depth the membrane domains in which DCT accumulates along its biosynthetic pathway, we performed co-localization experiments of DCT with CAV1, the protein most acknowledged to be concentrated in CRDs (38). The DCT and CAV1 substructures were assessed using two different anti-DCT (α-hDCT and D18) and anti-CAV1 (α-CAV1-CS and α-CAV1-sc) antibodies characterized in Fig. 2. In WB, in both cell lines MJS and SK28, D18 and α-hDCT detected HMW- and LMW-DCT glycoforms, in Nonidet P-40 soluble fractions, which significantly decreased in si-DCT samples. In MJS, but not in SK28, the α-hDCT detected an additional band, migrating immediately below the LMW glycoform, which was not reduced by si-DCT and therefore, possibly, nonspecific (Fig. 2). The α-CAV1-CS antibody identified Nonidet P-40-soluble and Nonidet P-40-insoluble CAV1 in oligomeric (bands above 260 kDa) and monomeric (bands below 25 kDa) forms in MJS cells. In SK28 cells, assessed in identical detection conditions as MJS (ECL), no bands were seen. When SK28 samples were further incubated with an enhanced detection system (Femto), CAV1 monomers and oligomers appeared. This proved the extremely low expression of endogenous CAV1 in SK28 compared with MJS cells. The α-Cav-sc antibody revealed exclusively high molecular weight oligomeric CAV1 (soluble and insoluble) in both MJS and SK28 with enhanced detection system (Femto) indicating the affinity of this antibody, preponderantly, for a subpopulation of CAV1 oligomers.
Both α-CAV1-CS and α-CAV1-sc demonstrated specificity for the CAV1 gene product in the two cell lines (Fig. 2). Importantly, there was a CAV1 pool resistant to CAV1 down-regulation visible in si-CAV1 cells. This was in principle the insoluble CAV1 detectable in MJS and SK28 with both anti-CAV1 antibodies. However, the soluble CAV1 in SK28 seen by α-CAV1-sc was also not decreasing in si-CAV1 samples. A possible explanation is that CAV1 expressed at much lower levels in SK28 cells could be more stably oligomerized than in MJS, thus being more resistant to down-regulation. The two anti-CAV1 antibodies showed in IF (Fig. 2) comparable expression levels as the ones obtained in WB. In identical detection conditions, α-CAV1-CS immunostained intensely PM linear and cytoplasmic tubulo-vesicular or punctate structures in MJS and showed practically no staining in case of most SK28 cells or poor staining for isolated SK28 cells. With α-CAV1-sc the staining intensity was similar in MJS and SK28 and the distribution pattern showed PM structures in most MJS and occasionally in SK28 and cytoplasmic vesicular structures in both cell lines.
In MJS cells, DCT and CAV1 detected by confocal immunofluorescence microscopy with α-hDCT and α-CAV1-sc co-localized in tubules visible in cytoplasm or decorating segments of PM, whereas in SK28 they appeared together in cytoplasmic or at PM fine vesicles. In addition, DCT and CAV1 in separate structures were also seen (Fig. 3A, 1st and 2nd panels). With D18, the immunostaining of DCT after 30 min was negative in MJS and positive in SK28 (supplemental Fig. 2). This was due to the higher expression of DCT in SK28 cells versus MJS (Fig. 1A). After overnight incubation, DCT with D18 appeared in MJS spread in cytoplasm or in some cells at the PM (Fig. 3A, 3rd panel) and in SK28 homogeneously dispersed or concentrated in some areas within the cell body (Fig. 3A, 4th panel). To establish whether DCT/D18 and CAV1/α-CS pools were in common or separate structures and were due to very poor detection of CAV1 with α-CAV1-CS in this phenotype (Fig. 2), the images presented in Fig. 3A in SK28 cells were taken with increased imaging parameters.
Importantly, in neither MJS nor SK28, CAV1 and DCT co-localized when detected with α-CAV1-CS and D18, respectively, indicating the existence of a DCT pool in CAV1-free structures as well. The D18 epitope mapping near the N terminus of human TRP-2 (DCT) (as indicated by the manufacturer) may be hindered in the DCT pool associated with CAV1-positive membranes. The α-hDCT raised against an extended sequence within DCT luminal domain contains very likely more than one antibody population, thus being more sensitive. The topology of DCT at the PM, as type I membrane protein, is with the cytosolic tail in the cytoplasm and with the N terminus in the extracellular space where other epitopes than D18 are available and recognized by α-hDCT. MJS semi-confluent cultures were visibly heterogeneous with respect to cell morphology and DCT/CAV1 expression and distribution.
One population of polygonal cells expressed DCT moderately in common or separate substructures with CAV1 (shown in Fig. 3A, 1st and 3rd panels). In addition, MJS cells intensely fluorescent for DCT formed clusters observed with both α-hDCT and D18 antibodies. A slight tendency toward a more elongated phenotype was seen in this cell line along the experimental time frame (supplemental Material 2). In these DCThigh cells CAV1 immunostaining appeared redistributed from PM into granular cytoplasmic structures (with α-CAV1-sc) or was practically undetectable (with α-CAV1-CS) (Fig. 3A, 5th and 6th panels). This was a characteristic of MJS culture and was not observed in the SK28 phenotype. The SK28 cells overexpressing DCT compared with the ones expressing DCT moderately did not show visible differences in morphology or CAV1 immunostaining (Fig. 3A, last two bottom panels).
Additional supporting evidence for a possible DCT association with CAV1 structures was provided by fractionation of MJS and SK28 cellular homogenates in a 4–25% Optiprep gradient ultracentrifugation. According to the principle of separation of the method we used, the fractions containing the highest amounts of cholesterol and lipids (the lightest) were concentrated at the top, and the ones with the lowest amounts of these components (the heaviest) sedimented at the bottom of the gradient. In both MJS and SK28 cells, there was a clear partition along the gradient of the HMW/LMW DCT glycoforms, CAV1 OGs and constitutive MOs (Fig. 3, B and C).
In correlation with DCT maturation kinetics (Fig. 1, B and C) and with other data reported about CAV1 oligomerization along the secretory pathway, it was rational to assume that the bottom fractions (Fig. 3, B and C, 1st to 4th lanes from right to left) with DCT precursor, overlapping with CAV1 MOs (in MJS), contained the ER/cis-Golgi membranes; the middle fractions (Fig. 3, B and C, 5th to 9th lanes from right to left) enriched in DCT-mature complex glycoform overlapping with the highest amount of thermally resistant CAV1 OGs contained Golgi and post-Golgi membranes; the top fractions (Fig. 3, B and C, 10th to14th lanes from right to left) with DCT mature glycoform contained post-Golgi membranes and PM. The Golgi and post-Golgi membranes were expected to contain mainly CAV1 OGs, as it is known that the oligomerization process starts in ER and maturation of OGs continues along and post-Golgi. The presence of MOs in these fractions could be explained, in our opinion, as resulting from partial decomposition of thermally sensitive OGs. Thermal sensitivity of CAV1 complexes was reported by other studies (39) and by us, here, when CAV1 OGs were analyzed in conditions without (−) or with (+) thermal denaturation (Fig. 6). It is important to note that the ratio between thermally resistant OGs and MOs is in favor of MOs in MJS compared with SK28 where these two are similar (Fig. 3, B and C, middle fractions), which supports the idea that CAV1 OGs are more stable in SK28 than in MJS. Actin as a component of cytoskeleton was mainly concentrated on the heaviest fractions of the gradient.
A possible DCT-CAV1 interaction, indicated by DCT-CAV1 co-localization in cellular substructures (Fig. 3A) and distribution in ultracentrifugation fractions (Fig. 3, B and C), was further investigated and demonstrated by IP studies in the MJS cell line found abundantly expressing CAV1. The α-hDCT antiserum preponderantly immunoprecipitated the DCT precursor and retrieved significant amounts of CAV1 bound to DCT (Fig. 3D), whereas the CAV1 obtained after IP with an α-C-terminal antibody, which captured both DCT precursor and mature forms, was barely detectable (Fig. 3D).
This result may have two explanations as follows. (a) A DCT fraction, immunoprecipitated by α-C-terminal antibody, is present in CAV1-low or -free membranes. Indeed, when DCT was assessed with the α-C terminus in immunofluorescence microscopy, it appeared in distinct substructures other than CAV1 (Fig. 3D). (b) DCT is engaged in interaction with CAV1 via its cytosolic domain containing the α-C-terminal epitope and therefore cannot be retrieved by IP with this antiserum. This is supported also by the DCT-CAV1 structural model further described in Fig. 4.
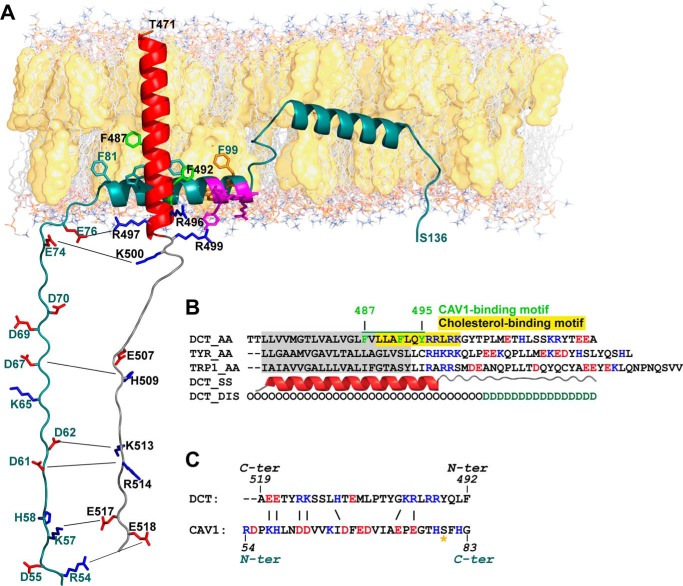
FIGURE 4.
In silico analysis of the interaction between CAV1 and DCT in the TM and cytosolic regions. A, schematic representation of the structural model of DCT (residues 471–519) (colored according to secondary structure elements: red for α-helix and gray for coil) interacting with CAV1 (residues 54–136) (colored dark cyan). DCT aromatic residues Phe-487, Phe-492, and Tyr-495 form the CAV1-binding motif. Phe-487 and Phe-492 have full side chains, green representation, and Tyr-495 is oriented toward the membrane and therefore is not visible. Amphipathic motif 96KYWFYR101 in CAV1 is colored magenta. The W/F/Y residues within this motif, colored here in orange, are membrane-facing and possibly interacting with cholesterol. B, sequence alignment of TM and cytosolic regions of proteins in the TRP family. DCT residues 487–495 forming the CAV1-binding motif are underlined in green, and aromatic residues (depicted also in A) are colored green. Cholesterol binding CRAC motif within DCT TM is highlighted in yellow. Predicted TM region in DCT is highlighted in gray. Residues in C-terminal cytosolic domain are colored according to net charge as follows: blue for positively charged (basic) and red for negatively charged (acidic) residues. C, charge complementarity between cytosolic domains of DCT and CAV1. Residues are colored according to charge; putative salt bridges are depicted by black lines connecting residues (same as in A). The possibly phosphorylated Ser-80 residue required for cholesterol binding is indicated by an orange star.
The MS analysis of the eluates obtained by IP with α-hDCT re-confirmed CAV1 presence (Table 1). Importantly, two proteins, Cavin-1 and Cavin-3 already known to localize to caveolae and essential for caveolae formation (40), were also identified as candidate components of DCT protein complexes by MS (Table 1).
TABLE 1.
CAV1 and CAV1-associated proteins identified as DCT interactors in MJS lysates using high performance liquid chromatography coupled with tandem mass spectrometry analysis
The Uniprot accession number from the human proteome, protein name, summed sequence coverage, number of unique peptides, and spectral counts in each of the three samples immunoprecipitated with anti-TYR antibody (α-hPep7) (negative control), α-hDCT (anti-hDCT luminal domain, aa 27–439), and α-C terminus (anti-hDCT C-terminus domain, aa 506–519) are given for each protein.
| Uniprot accession no. | Protein name (Alternative name) | Total sequence coverage | Total unique peptides | Spectral counts for IP with α-hPep7 antibody | Spectral counts for IP with α-hDCT antibody | Spectral counts for IP with α-C-terminus antibody | Protein mass |
|---|---|---|---|---|---|---|---|
| % | kDa | ||||||
| P40126 | l-Dopachrome tautomerase (tyrosinase-related protein 2) | 33.53 | 14 | 0 | 26 | 63 | 59.1 |
| Q03135 | Caveolin-1 (CAV1) | 24.16 | 3 | 0 | 6 | 3 | 20.5 |
| Q6NZI2 | Polymerase I and transcript release factor (Cavin-1) | 21.54 | 7 | 0 | 19 | 0 | 43.4 |
| Q969G5 | Protein kinase Cδ-binding protein (Cavin-3) | 30.27 | 7 | 0 | 19 | 0 | 27.7 |
In summary, these data showed that in both MJS and SK28 cells, expressing different CAV1 amounts, DCT was associated with CAV1-, non-CAV1-containing membranes, and possibly with Cavin-1- and Cavin-3-positive structures. The distribution of DCT glycoforms and CAV1 MOs/OGs in common subcellular fractions as well as DCT-CAV1 complexes at the PM suggested a possible DCT-CAV1 interaction that would start in the early steps of the secretory pathway and continues all along it.
Structural Model of the Interaction between DCT and CAV1
An in-depth understanding of the DCT-CAV1 interaction entails a detailed three-dimensional structure of the interacting partners. Our predictions were combined with published experimental data to model the complex between membrane-adjacent regions of the two partners, region 54–136 in CAV1 and region 470–519 in DCT.
A structural model of CAV1 was developed based on constraints from predicted structural elements (secondary structure, transmembrane segments, and hydropathy analysis) and published experimental data from several sources. This is the most extensive atomically detailed structural model of CAV1 (aa 54–136, underlined sequence in the supplemental material and dark cyan schematic structure in Fig. 4A), encompassing a cytosolic segment (aa 54–79), the caveolin scaffolding domain (CSD) (aa 82–101) containing the cholesterol-binding motif (generally referred as CRAC domain-Cholesterol Recognition Amino Acid Consensus) followed by the intramembrane U-shaped segment (aa 101–134). The cytosolic localization of segment (aa 54–79) in our model and CSD (aa 82 to 101) involvement in membrane attachment are supported by experiments of reconstitution of membrane binding in vitro, by various deletion mutants (41) showing that CAV1 segment (aa 1–101) behaves as an integral membrane protein, and the CAV1 segment (aa 1–81) behaves as a soluble protein. Lacking experimental structural information for the cytosolic segment (aa 54–79), our model is based exclusively on bioinformatic analysis. The consensus disorder prediction argues against an intrinsically disordered structure (5 of 7 methods predict an ordered structure), whereas the consensus secondary structure prediction indicates mainly a coiled-coil structure, with short stretches of extended β-strands (aa 66–72). Based on these clues, we assigned an extended conformation to cytosolic segment, yet keeping in mind that it might adopt multiple conformations. The CSD (aa 82–101) was predicted to adopt a helical structure, confirmed by structural experiments on isolated peptides, including chemical shift index combined with nuclear Overhauser effect (NOE) experiments on CAV1 (aa 82–109) (42), NMR analysis of CAV1 (aa 84–97) (43), solid state NMR, circular dichroism (CD), and FTIR spectroscopy on CAV1 (aa 82–109) (44). Importantly, although some of these structural studies indicate a partial β-strand structure, this corresponds only to short peptides, and helicity increases with peptide length. Therefore, we modeled the CSD as α-helix. The TM region location prediction showed an intrinsic preference for an interfacial localization rather than a membrane-embedded orientation. CD and NMR experiments on CAV1 (aa 94–102) (45) confirmed the presence of an amphipathic helix, stabilized by the 96KYWFYR101 motif. Within the amphipathic helix, there is also the 94VTKYWFYR101 fragment representing the CRAC motif in CAV1 (46). The one-letter amino acid code algorithm for CRAC is from the N to C terminus (L/V)X1–5(Y)X1–5(K/R), where X is any amino acid and Y is mandatory. The sequence Lys-96–Arg-101 (colored magenta in Fig. 4A) has two tyrosines flanked by two charged residues, one arginine and one lysine on the solvent-oriented face, and phenylalanine and tryptophan side chains on the membrane-oriented face. Despite this interfacial (not integral membrane) localization, truncation experiments (41) demonstrated that CSD is sufficient to anchor green fluorescent protein to membranes in vivo. The U-shaped intramembrane segment (aa 101–134) adopts a helix-break-helix structure, as revealed by CD and NMR spectroscopy into lyso-myristoylphosphatidylglycerol micelles (47). The break in the helix spans residues Gly-108–Pro-110, but only Pro-110 is critical for maintaining this structure (48).
In parallel, we built a structural model of DCT encompassing TM and C-terminal domains. Hydrophobicity pattern and secondary structure propensity placed the DCT TM segment in region 473–493, modeled as α-helix. Detailed sequence pattern analysis of the TM region identified motifs for CAV1 and cholesterol binding. The three aromatic residues (Phe-487, Phe-492, and Tyr-495) forming the CAV1-binding motif (depicted green in Fig. 4, A and B) are unique to DCT and not conserved in other TYR-related proteins (Fig. 4B), which may be an argument in favor of DCT interaction with CAV1. However, we should keep in mind that such interaction may occur even in the absence of ΦXXXXΦXXΦ motifs (Φ is aromatic and X is any residue). Within CAV1-binding motif of DCT, we could identify CRAC motif 489LLAFLQYRRLR499 as well (highlighted yellow in Fig. 4B). DCT C-terminal domain (aa 494–519) following the TM region is predicted to be generally disordered, with a low tendency to form a short helix (maximum 2 helical turns) in the middle, predicted only by 3 of 7 methods. In the absence of a well defined conformation, we assigned an extended structure to the C-terminal segment, similarly to CAV1 cytosolic segment, however keeping in mind that this is only one possible state in the large conformational space available.
The structural model of DCT interacting with CAV1, based on the analysis of individual models, advocates for an interaction between CAV1 and DCT mediated by two distinct regions, one within the membrane (hydrophobicity-driven interaction) and the second cytosolic (electrostatics-driven interaction). Within the membrane, interaction occurs between DCT residues forming a CAV1-binding motif (Fig. 4B), and CAV1 residues Trp-85, Phe-89, and Phe-92 forming the interfacial aromatic cluster in the CSD. The membrane-proximal cytosolic regions CAV1 (aa 54–79) and DCT (aa 494–519) have not only extended partially disordered structures but also atypical amino acid compositions, containing over 50% charged polar residues (CAV1: 6 positive, of which three are His, and 8 negative charges; DCT: 7 positive and 3 negative charges). Thus, CAV1 cytosolic segment carries a negative formal charge (−5) and DCT cytosolic segment carries a positive formal charge (+3). Peptide net charge may change with solvent pH, which varies in different intracellular compartments from 4.7 in lysosomes to 8 in mitochondria (49). However, charge complementarity between DCT and CAV1 cytosolic regions is maintained through the entire intracellular traffic, because theoretical calculations of isoelectric points indicate extremely high pI values (net positive charge) for DCT (9.82) and low pI values (net negative charge) for CAV1 (4.96). Moreover, complementary charged amino acids within the cytosolic region are located at neighboring positions, which would argue for an interaction mediated by salt bridges (indicated by thin lines in Fig. 4A).
The in silico analysis supported by experimental data described above strongly suggests that TM and cytosolic subdomains of DCT are involved in interaction with CAV1. Moreover, within the CAV1-binding domain of DCT has been identified the cholesterol-binding motif CRAC.
CAV1 Control on DCT Gene Expression, Processing, Subcellular Distribution, and Propagation of DCThigh Subtype Is Melanoma Cell Phenotype-specific
The data showing DCT-CAV1 co-localization (Fig. 3A) and interaction (Fig. 3D and Table 1) as well as the DCT-CAV1 structural model (Fig. 4) prompted us to investigate whether CAV1 would have any impact on DCT fate in melanoma cells.
Cells silenced for CAV1 were analyzed for DCT expression and maturation along the secretory pathway in both MJS and SK28 cell lines (Fig. 5). In si-ct-treated 48-h MJS cultures the DCT was nearly undetectable, whereas in si-CAV1 MJS cells, the intensities of DCT bands were significantly increased (Fig. 5A); this increase was further observed in si-CAV1 72- or 96-h cells and more evident when MJS DCT polypeptides resulted after PNGase F-deglycosylations were compared. In addition, in 72- and 96-h cells, the impact of si-CAV1 on DCT N-glycan maturation was dramatic. In si-CAV1 MJS cells, the DCT LMW-glycoform, EndoH-sensitive, was the dominating form, and the HMW band representing DCT with complex N-glycans was significantly less intense than in si-ct cells.
The same results were obtained whether DCT was assessed with α-hDCT (supplemental Fig. 1) indicating that this was an overall systemic effect of si-CAV1 on this protein, and it was not restricted to the DCT pool detected with D18 antiserum only. The demonstrated impact of si-CAV1 on DCT maturation along the secretory pathway together with ultracentrifugation results and DCT-CAV1 structural model strongly argue for a DCT-CAV1 direct interaction, which starts in ER and continues all the way up to the PM.
No visible change of DCT polypeptide amount was detected in SK28 si-CAV1 compared with si-ct cells. However, there was a slight change of the ratio between the two DCT glycoforms in favor of DCT-HMW in si-CAV1 cells (Fig. 5B).
The gene expression analysis was further performed in MJS cells where si-CAV1 had a significant impact on DCT protein expression. The real time qRT-PCR data (Fig. 5C) showed that DCT mRNA was always higher in si-CAV1-treated cells as compared with si-ct and was gradually increasing from 48 to 72 h and further to 96 h in both si-ct and si-CAV1 cells (Fig. 5C, top panel). Interestingly, despite the visible decrease of CAV1 protein in si-ct cells between 48 and 96 h (Fig. 5A), CAV1 transcript levels suffered no detectable changes in these cells (Fig. 5C, bottom panel).
Importantly, the significant DCT increase observed in both MJS and SK28 si-ct cells, although more attenuated in SK28 than in MJS, will be further addressed in Figs. 6 and 7. DCT-CAV1 co-localization at PM (Fig. 3A) and identification of Cavin-1, a component associated with caveolae formation and stability, as potential DCT interactor (Table 1), arouse our interest to investigate also DCT and Cavin-1 subcellular distribution in si-CAV1 cells.
The effect of si-CAV1 was visibly seen in MJS by the decrease of overall CAV1 (green) signal in MJS si-CAV1 cells compared with MJS si-ct cells (Fig. 5D, panels b, f, d1, and h1). In MJS si-ct cells, there was a pool of CAV1 in common structures with DCT and Cavin-1 distributed at PM (Fig. 5D, panels d and d2). In si-CAV1 cells, prominent round shaped vesicles containing residual CAV1, together with DCT and Cavin-1, were visible (Fig. 5D, panels h and h2). In accordance with the WB data (Fig. 2), this CAV1 pool may represent insoluble CAV1 preserved in silenced cells.
Another effect observed in si-CAV1 samples was the increase of MJS DCThigh clones, the cells with cytoplasmic DCT immunofluorescence (Fig. 5D, panel i), no CAV1 (Fig. 5D, panel j) or Cavin-1 (Fig. 5D, panel k), from 0.15% in si-ct (Figs. 5D, 3rd panel, left scattergram) to 1.1% in si-Cav1 samples (Fig. 5D, 3rd panel, right scattergram) as shown by TF image cytometry quantitative analysis. D18 antibody also detected the appearance of DCT-high clones (supplemental Fig. 2).
In SK28 CAV1 (green) signal was not visibly changed in si-CAV1 compared with si-ct cells (Fig. 5D, panels n, r and p1, t1). No obvious changes were seen in DCT (red) or Cavin-1 (blue) signals (Fig. 5D, compare panels m and q and o and s) and in morphology or distribution of CAV1-DCT-Cavin-1 structures in SK28 si-CAV1 compared with SK28 si-ct cells (Fig. 5D, panels p2 and t2). DCT labeling in si-CAV1 SK28 cells was similar with both anti-hDCT (Fig. 5) and D18 (supplemental Fig. 2) antibodies.
CAV1 is an important factor for maintaining the correct organization and distribution of the DCT-CAV1-Cavin-1-containing structures at the plasma membrane in MJS but not in SK28 phenotype. This is probably due to the very low amount of endogenous CAV1 in SK28 compared with MJS cells.
In summary these data demonstrate that CAV1 role of repressor for DCT gene and modulator of DCT processing and subcellular distribution is cell phenotype-dependent. CAV1 down-regulation also promotes the perpetuation of the morphologically distinct DCThigh MJS cellular subtype.
DCT Acts as a Modulator of CAV1 Stability and Assembly in Both Early and Metastatic Melanoma Cell Phenotypes
Considering the presence of DCT in CAV1-associated structures (Fig. 3) and the si-CAV1 effect (Fig. 5) on DCT, we anticipated that si-DCT would have an impact on CAV1 as well.
The analyzed samples included CAV1 MOs and OGs in Nonidet P-40-soluble cell fractions, and Nonidet P-40-insoluble cell fractions resulted from sub-confluent (MJS 48 h; SK28 72 h), semi-confluent (MJS 72 h; SK28 96 h), confluent (MJS 96 h; SK28 120 h) cultures. The samples were analyzed in conditions without (−) or with (+) thermal denaturation (see under “Experimental Procedures”) to observe variations, if any, in thermal stability of CAV1 OGs (Fig. 6, A and B, CAV1 panels). There was a clear impact of si-DCT on Nonidet P-40-soluble and Nonidet P-40-insoluble CAV1 in both cell lines, however more prominent in MJS than in SK28.
In sub-confluent (48 h) MJS cells, the amounts of both soluble OGs and MOs released upon thermal denaturation (+) were lower in si-DCT than in si-ct but higher in the insoluble fraction. In semi-confluent and confluent MJS si-DCT cells, both Nonidet P-40-soluble and Nonidet P-40-insoluble CAV1 OGs/MOs were higher than in si-ct cells (Fig. 6A).
Despite the very low expression of CAV1 in SK28 cells, the effect of si-DCT on CAV1 was present in this phenotype as well. Soluble OGs released upon non-thermal denaturation in confluent cells and the corresponding MOs released after thermal denaturation were higher in si-DCT than in si-ct samples (Fig. 6B, 120 h, Nonidet P-40-soluble, OGs, MOs). As in case of MJS, in SK28 cells the effect of si-DCT was more prominent in the Nonidet P-40-insoluble than in the Nonidet P-40-soluble fraction.
The analysis of si-ct-treated samples showed that in MJS-insoluble CAV1, OGs were more thermally sensitive than in SK28. In MJS confluent cells, compared with their sub-confluent counterparts, the soluble CAV1 OGs acquired thermo-resistance but were not converted into insoluble OGs, probably being more susceptible to degradation, whereas in SK28 cells the sub-confluent to confluent transition produced no visible effects on the dynamics of CAV1 assembly.
Upon DCT-silencing, CAV1 protein accumulation seen in both MJS and SK28 cells was not correlated with any significant variation in transcriptional levels (Fig. 6, C and D). This indicated that the increased CAV1 amount in si-DCT cells was due to the increased stability of CAV1 protein and not to CAV1 gene transcriptional regulation.
The DCT expression analysis confirmed the si-DCT effect on protein (Fig. 6, A and B) and mRNA (Fig. 6, C and D) levels. In si-ct samples, DCT expression was increased during sub-confluent to confluent transition in both cell lines, as has already been reported in si-CAV1 experiments (Fig. 6, A and B). The effect of si-DCT was visibly seen in both MJS and SK28 by the decrease of DCT (red) signal in MJS and SK28 si-DCT cells compared with si-ct cells (Fig. 6E, panels a, e; i, m; d1, h1; l1, and p1). The ordered PM structures containing DCT-CAV1-Cavin-1 in both MJS and SK28 si-ct cells (Fig. 6E, panels d2 and l2) appeared in si-DCT cells as aggregates positive for CAV1-Cavin-1 and residual DCT, more prominent in MJS (Fig. 6E, panel h2), and finely dispersed in SK28 (Fig. 6E, panel p2). The effects described in si-DCT MJS and SK28 cells for CAV1 pool detected with α-CAV1-sc (Fig. 6E) and for CAV1 pool detected with α-CAV1-CS (supplemental Fig. 2) were similar.
In conclusion, the si-DCT effect on CAV1 consists of higher amounts of soluble/insoluble CAV1 in both MJS and SK28, which are not correlated with any changes in CAV1 mRNA. Our interpretation is that without DCT CAV1 becomes redistributed in more stable membrane structures that favor the increase of the insoluble (more stable in time) CAV1 pool. From these data we propose DCT as being an important molecular player in CAV1 distribution (and possibly of other CAV1-asociated proteins, e.g. Cavin-1) between soluble and insoluble membrane fractions in MJS and SK28 melanoma cells.
DCT and CAV1 Expressions Are Oppositely Regulated by Autocrine Stimulation, the Selection Process of DCThigh/CAV1− Cell Phenotype
The DCT increase and CAV1 decrease in MJS si-ct samples, along the different time periods representing sub-confluent, semi-confluent, and confluent cultures were observed in two different experimental setups (Figs. 5 and 6). This represented a valuable indication that cellular processes, as increasing cell-cell contacts, concentration in the culture medium of secreted molecules as cytokines, growth factors, or nutrient deprivation, acted in vivo oppositely on DCT and CAV1 expression at least in some particular phenotypes. To gain some insights into these processes, several experimental approaches involving manipulation of cell culture parameters were performed on MJS cells. In a first experiment (Fig. 7A), two parallel samples were cultured for 48, 72, and 96 h in identical conditions. In one set, the medium was not changed during the indicated culture time periods (MR−), whereas in the other set the culture medium was replenished with a fresh one every 24 h (MR+). In MR− samples, CAV1 OGs and MOs decreased in both Nonidet P-40-soluble and Nonidet P-40-insoluble fractions, and in MR+ samples the CAV1 was constant (Fig. 7A). In the same fractions, the DCT detected with either α-hDCT or D18 (Fig. 7B) increased; however, this increase was more prevalent in MR− than in MR+ samples.
The second experiment (Fig. 7C) was done in MJS cells plated for 24 h and incubated in parallel with fresh medium (“fresh”) or with medium resulting from a 96-h culture (confluent) (“old”) for another 24 h. When cells were grown in medium resulting from a 96-h culture, DCT was also highly compared with cells grown in fresh medium, whereas Nonidet P-40-soluble CAV1 MOs were slightly increased at the expense of the Nonidet P-40-soluble CAV1 OGs decrease and Nonidet P-40-insoluble CAV1 was rather constant (Fig. 7C).
The third experiment (Fig. 7C) was performed growing cells for 72 h in complete medium containing 10 or 1% fetal calf serum (FCS) representing normal culture conditions or conditions mimicking low nutrients and medium growth factor deprivations, respectively. As seen in Fig. 7C in cells grown in 1% FCS compared with 10% FCS, the DCT increased, and the overall CAV1 (Nonidet P-40-soluble and Nonidet P-40-insoluble MOs and OGs) decreased, similar to what was obtained in MR− samples (Fig. 7A).
To establish whether the effects described above were restricted to DCT expressed as sole TRP member in MJS tumor cell phenotype, MNT-1, a pigmented cell line expressing all antigens from the TRP family, TYR, TRP1, and DCT, were assessed in an experiment shown in Fig. 7D similar to the one described in Fig. 7A. Surprisingly, in MNT-1 cells only DCT was increasing (Fig. 7E) in a similar manner as seen in MJS cells (Fig. 7, B and C), whereas neither TYR nor TRP-1 expressions were changed (Fig. 7D). The propagation of DCT and CAV1 expression within MJS cell populations during sub-confluent to confluent transition was explored by immunofluorescence microscopy with both DCT/CAV1 pairs of antibodies to detect the overall DCT and CAV1 pools (Fig. 7E).
In subconfluent cells at 48 h, DCT immunostaining with D18 was poor, and practically only CAV1-stained cells were detected, but with α-hDCT DCT was visible in tubular PM common structures with CAV1 in most cells (Fig. 7E). The cells with high DCT expression and low or redistributed CAV1 were visible even in sub-confluent culture at 48 h, as isolated cells (Fig. 7, 48 h, DCThigh population). The number of this DCThigh subset, detected with both α-hDCT and D18, was visibly increasing at 72 h and further at 96 h when they formed aggregates and giant clusters, respectively (Fig. 7E).
At 72 and 96 h in DCThigh cells, there was a clear and visible drop in the intensity of CAV1 immunostaining assessed with α-CAV1-CS antiserum, whereas CAV1 seen by α-CAV1-sc was redistributed in structures with granular morphology and gradually decreasing (Fig. 7E). It is very important to note that the morphology of DCT-expressing cells, assessed with either α-hDCT or D18, was changing from polygonal epithelium-like (DCTmedium) (Fig. 7E, 48 h) to elongated fibroblast-like shape (DCThigh) with long extensions marked in the figure with stars (Fig. 7E, 96 h).
These observations, however, could not explain whether the increase in DCT expression detected by WB was due to an overall increase in protein synthesis or to the high expression in specific DCThigh cell clones. To quantify DCT and CAV1 expression at single cell resolution, we next analyzed triple-labeled fluorescent subpopulations by TF image cytometry system (Fig. 7F).
Each cell was identified based on DAPI staining and interrogated for fluorescent signals associated with each of the two markers. For D18/CAV1-CS labeling, the analysis showed a progressive increase of identified DCT+ cells from 15.34% at 48 h to 22.97% at 72 h and to 60.55% at 96 h (Fig. 7F, panels a–c, upper right quadrants) in accordance with previous microscopy visualization (Fig. 7E). On samples stained with α-hDCT/CAV1-sc, the quantitation detected no significant differences in DCT+ cells propensity between time points, as the majority of cells were detected as positive from the beginning of data recording (∼92–98% DCT+ cells, Fig. 7F, panels j–l, upper right quadrants). However, when examining in more detail the heterogeneous MJS cell samples, an increase in both mean of MFI of DCT+ cells (all events in upper right quadrants in scattergrams panels a–c and j–l) and percentage of DCThigh cells from 48 to 96 h, irrespective of the antibody used (Fig. 7F, panels d and e, and 7F, panels m and n) was determined (corresponding to gated red dots in scattergrams panels a–c and j–l). Data dynamic range was increased for D18 antibody, where MFI increased from 28.64 to 41.81 between 48 and 96 h (Fig. 7F, panel d), as compared with 30.27–33.34 for α-hDCT (Fig. 7F, panel m). Although MFI associated with DCT expression showed a modest increase from 48 to 96 h (Fig. 7F, panels d and m), percent of DCThigh cells amplified up to 15-fold (Fig. 7F, panels e and n). These data suggested that the progressive rise in DCT protein expression determined by immunoblot (Fig. 7B) was due to the propensity of DCTmedium (data were not shown) and DCThigh cells at each time point rather than to the mean marker expression of individual cells.
Differences detected in CAV1 dynamics were similarly assessed to determine the timely change in overall protein expression and within the DCThigh cell subset. At 96 h, when DCThigh cell clusters were confluent, we measured 89.41% of total cells positive for CAV1 using α-CAV1-CS antibody (Fig. 7F, panel f). When gating on DCThigh subpopulation, 69.57% of those expressed Cav1 (Fig. 7F, panel i). With α-CAV1-sc antibody, 77.69% of cells were positive for CAV1 (Fig. 7F, panel o), whereas 91.81% of DCThigh cells were also positive for CAV1 (Fig. 7F, panel r). This supports the fact that α-CAV1-CS antibody recognizes a pool of CAV1, including monomers that decrease significantly with time, while α-CAV1-sc antibody has an affinity for oligomeric insoluble CAV1 (Fig. 7A) that is more stable and becomes redistributed into condensed structures with increased brightness (Fig. 7E).
Both anti-CAV1 antibodies detected a timely decrease in CAV1 overall MSI (Fig. 7F, panels g and p). When gating on the DCThigh subpopulation, the pool of molecules recognized by α-CAV1-CS antibody decreased from 48 to 72 h and reached a plateau (Fig. 7F, panel h). CAV1 molecules bound by α-CAV1-sc antibody were constantly decreasing in propensity within the DCThigh clusters from 48 to 96 h (Fig. 7F, panel q). Notably, the fluorescence intensity of CAV1 detected with α-CAV1-sc was enhanced as compared with overall average values at each time point. This was in accordance with the redistribution of these molecules from tubular elongated to more condensed dotted structures (Fig. 7E) during the experimental time frame.
The DCT and CAV1 expressions are distinctly and oppositely regulated by environmental and cellular factors. The expansion of a dormant cellular subset DCThigh/CAV1low present in sub-confluent cultures is emblematic for MJS phenotype during autocrine stimulation.
Discussion
This is the first report that associates DCT, previously known as melanogenic enzyme and melanoma antigen mediating a tumor stress resistance process, with CAV1, the well recognized protein in regulation of numerous signaling pathways. The structural and functional aspects of DCT-CAV1 cross-talk will be further discussed in the context of already acknowledged biological functions of DCT and CAV1 and in melanoma progression.
DCT-CAV1 Structural Relationship
The sessile detection of DCT, CAV1, and Cavin-1 at the PM (Fig. 4D and Table 1) at steady state in both MJS and in SK28 indicates their stability. Cavin-3 is also a potential DCT interactor identified in the MJS phenotype. Cavins are a class of scaffolding proteins, major regulators of CAV1 functions and organization having individual roles in caveolae morphologies and function in dependence with a certain cell type (50). There is much evidence about the CAV1 role outside caveolar structures (51), and a recent review suggests that cavins may also function independently of caveolins or caveolae (52). It would be important to establish whether DCT-CAV1-Cavin-1 indeed belongs to caveolae or whether DCT is regulating any cellular processes mediated by caveolae.
DCT was identified in CAV1-free membrane fractions or detected at PM in structures with no CAV1 as well. DCT either becomes associated with CAV1 and, in a late stage of its processing, a fraction is sorted in CAV1-free membranes or DCT pools are always sorted individually in CAV1-rich or -low/-free membranes.
An important result was DCT detection as fully processed and stable protein in two amelanotic phenotypes (Fig. 1). In SK28, as in other amelanotic cell lines shown to have alteration in pH homeostasis of the secretory pathway, TYR, also a member of TRP family and structurally related with DCT, is retained in ER as a partially glycosylated precursor and is prematurely degraded via proteasome machinery (53, 54). Previous reports from our group showed that monensin, known also to insert with high affinity into cholesterol-rich Golgi membranes (55), prevented DCT but not TRP1 maturation (56). We then anticipated that CRDs might have a role in DCT maturation, which has been demonstrated in this study.
The different sorting motifs in cytosolic tails of DCT, TYR, and TRP1 were suggested to be involved in distinct post-Golgi trafficking pathways for these antigens (57). The dependence of DCT processing on CRD integrity and its association with CAV1 in both MJS and SK28 cells could stand as a possible explanation for the different DCT and TYR maturation and stability along the amelanotic secretory pathway. TYR or TRP1 has no structural determinants for CAV1 binding (Fig. 4), which diminishes their chances to be sorted and trafficked in CAV1-associated membranes. TYR is transported in COPII vesicles (58), which function nonspecifically as selection and ER export machinery for most TM cargo. These data strengthen our previously advanced hypothesis that DCT, TYR, and TRP1 have different ER exit signals therefore being sorted and trafficked in vesicles with different characteristics along the early secretory pathway. This also supports the concept of a specific ER-Golgi transport (59). The mechanisms how caveolin and clathrin or COPI/II-coated vesicular transport function are fundamentally different. Vesicular carriers with caveolar coats are stable, and once assembled they remain attached on their own membranes and do not undergo cycles of assembly and disassembly during traffic (60). It is thus possible that pH alterations of amelanotic secretory pathway, thought to operate at ER-early Golgi interface (53), have less impact on DCT already recruited in ER-CAV1 free-cholesterol-rich-associated membranes than on TYR in COPII vesicles. However, we cannot exclude that a DCT pool, possibly the one in non-CAV1 membranes, is part of COPII cargo. The presence at steady state of DCT precursor, similar to TYR, in both amelanotic cells is in favor of this hypothesis.
The structural particularities of DCT may explain its ability to potentially interact with CAV1. The sequence pattern analysis has identified in the TM domain of DCT the same CBM already documented for other multiple signaling proteins (61, 62). In addition, DCT cytosolic subdomain with negatively charged residues would establish electrostatic interactions with the positively charged residues in CAV1 (Fig. 4).
The predicted involvement of these DCT residues is an indication that DCT could interfere with signaling molecules or pathways that involve CSD (CAV1 aa 82–101) (63), CAV1 oligomerization domain (CAV1 aa 61–101), or cholesterol binding. CAV1-Ser-80 phosphorylation required for cholesterol binding (64) is in the neighborhood of the caveolin-binding motif within DCT (Fig. 4B).
Aromatic residues found at the membrane-water interface in DCT TM segment (but not in TYR or TRP1 proteins) may have a role in stabilizing the TM region, through interaction with membrane components or with other TM segments. Specific roles of aromatic residues at the water-membrane interface, as anchors to stabilize the TM regions, have recently been reviewed (65, 66). These residues would have higher affinities for CRDs than aliphatic structures present in TM of TYR or TRP1 (Fig. 4B). Glycosphingolipid and cholesterol-enriched domains in the luminal leaflet of the Golgi are thought to play an essential role in the sorting of proteins toward the apical PM domain of epithelial cells (67). The DCT sorting in CRDs (Fig. 1, C and D) would be also contribute to its targeting to PM.
Possible scenarios of how DCT may exert its influence on Cav1 would be as follows. (a) DCT may regulate the formation of insoluble CAV1 OGs. If we admit that there is a DCT-CAV1 direct interaction (as the structural model suggests), DCT interacts with monomeric CAV1 in the early secretory pathway, “sequestrating” a pool of monomeric CAV1 in soluble (Nonidet P-40-soluble) but stable complexes. This is also supported by the fact that DCT is mainly found in Nonidet P-40-soluble fraction and is not detected in Nonidet P-40-insoluble material. The amount of CAV1-insoluble oligomers in si-DCT is higher than in si-ct. As CAV1-soluble oligomers are gradually transformed in insoluble ones by association with different membrane components, building the so-called raft structures (detergent-insoluble), we may consider that DCT regulates the formation of insoluble CAV1 oligomers. (b) DCT competes with CAV1 for cholesterol binding. In a non-interacting DCT-CAV1 situation, DCT (having cholesterol-binding motif CRAC, identified by bioinformatic analysis) can possibly compete with CAV1 (which has also CRAC motif) for cholesterol thus preventing CAV1 from entering into more insoluble membrane combinations. (c) DCT influences CAV1 via an interactor. DCT may exert its influence on CAV1 via an interactor, which may also be an important molecular player of the CAV1-associated membranes architecture. (d) DCT controls CAV1 translation. DCT directly or indirectly may repress the translation step in CAV1 synthesis.
DCT-CAV1 Functional Relationship
In the MJS phenotype CAV1 represents a regulator of the DCT gene at mRNA and protein levels (Fig. 5). This is the first report about a melanosomal protein/melanoma antigen regulated by CAV1 and a novel target gene for CAV1. Deciphering the molecular mechanisms that control the regulation of DCT at the transcriptional level by CAV1 is beyond this work and necessitates a more extended study. At this point we can only speculate that CAV1 could regulate DCT expression either directly, as shown for cyclin D or folate receptor promoters in ovarian carcinoma cells (68) or by different mechanisms or pathways with impact on DCT gene transcription. A well documented CAV1 mechanism is described for survivin, a member of the inhibitor apoptosis protein family (19), dramatically up-regulated in most human tumors and required for tumor survival (69). DCT has been also reported as an anti-apoptotic molecule and up-regulated in the most stress-resistant tumor clones (see Introduction). In this context, the DCT gene as a novel CAV1 molecular target advocates for the role of CAV1 as a tumor suppressor gene, at least in early malignant melanoma phenotypes, represented here by MJS cell line.
The minimal influence of CAV1 on DCT fate in SK28 cells is either due to poor CAV1 expression or to the fact that contributing molecular factors, which operate in MJS, are missing from the SK28 cellular phenotype. There is, however, a slight effect of CAV1 down-regulation on DCT maturation probably due to the perturbation of DCT trafficking along the Golgi network, an effect similar to the one induced by nystatin in the same cell line.
DCT-CAV1 Cross-talk in Early and Metastatic Melanoma Cell Phenotypes
The last part of our study is devoted to the integration of DCT-CAV1 relationship in a more in vivo context.
The reduced CAV1 expression in human metastatic melanoma cell lines and human tissue samples derived from metastatic lesions indicates that CAV1 loss is a late event in melanoma progression and may be involved in the metastatic process (20). Here, CAV1 is well expressed in MJS and barely detectable in SK28, which supports evidence that MJS indeed originates from a less malignant specimen than SK28. An emblematic characteristic of the MJS cells identified here is the switch of DCT and CAV1 expressions during the sub-confluent to confluent transition when cells proliferate intensely and exhaust severely the culture medium (Figs. 5–7).
CAV1 expression in breast cancer is modulated by transcriptional silencing via epigenetic changes rather than by mutation (70). In our system the nutrient and growth factor deprivations were the most critical parameters that acted on CAV1 expression, whereas DCT expression was responsive to any environmental changes. The paracrine and autocrine regulations are significant in melanoma progression (71). The VGP cells express a variety of growth factors for autocrine and paracrine stimulation (72) and can survive and proliferate in growth factor-free medium acquiring increased invasiveness through basement membranes.
CAV1 as a gene essential for regulation of numerous processes is maintained at constant levels in less stressful conditions (fresh medium) and decreased, but only at protein levels, in severe stressful conditions (exhausted medium or growth factor-minimal medium) (Fig. 7). Even SK28 metastatic cells resulting from natural selection processes express minimal levels of CAV1 protein that supports our above hypothesis of CAV1 preservation. Altogether these data show that DCT and CAV1 are independently regulated by autocrine factors in MJS phenotype during sub-confluent to confluent transition.
A key process in metastasis formation is the epithelial-mesenchymal transition (EMT). During EMT, tumor cells derived from epithelial cells can become more motile and invasive by acquiring characteristics of mesenchymal cells (73). In melanoma, the stepwise transformation of melanocytes to melanoma is very similar to the EMT process representative of epithelial cancers, and the EMT genes have been also involved in phenotype switching, which promotes melanoma metastasis (74). There is a phenotype switching in our system, too. In sub-confluent stage, 48 h after plating, MJS cells are interconnected by filamentous structures visible by an optical microscope and grow in groups of two or four cells (Fig. 7E) (epithelium-like stage). These cells express low DCT and high CAV1, and a significant DCT amount is in common structures with CAV1. A low percent is isolated in clones with DCThigh expression and low CAV1. During cell proliferation, the nutrient supply diminishes and the concentration of the autocrine secreted factors increases, and these may represent environmental signals able to trigger independently DCT increase and CAV1 decrease. The demonstrated increase of DCT expression by CAV1 down-regulation furthermore increases the percentage of DCThigh subset. In contrast, in the absence of DCT CAV1 structures become more stable (Fig. 6). This is in agreement with the data showing that DCT structural determinants could interfere with CAV1 oligomerization (Fig. 4) resulting in less stable CAV1 aggregates. The result of transition from the sub-confluent to confluent stage is the perpetuation of the MJS DCThigh/CAV1low subset from isolated dormant cells to large cell clusters with a fibroblast-like morphology (mesenchyme-like stage), displaying long cellular extensions similar to the ones observed in SK28 phenotype.
CAV1 is a significant molecular player in the EMT process. CAV1 low expression or absence is associated in other cancers with a bad prognosis (75), while others report that disappearance of CAV1 enables cells to undergo EMT (76).
In respect with these events, we consider MJS a more plastic tumor phenotype than SK28. In SK28 cells grown for 120 h, although there is a DCT increase, this is less pregnant than in MJS and is associated with no changes in CAV1 content or morphology of DCThigh clones. However, in SK28 cells DCT still controls stability of CAV1. SK28 as metastatic phenotype is probably committed to a final stage favoring cell survival in which the low CAV1 maintains well expressed DCT, an antigen associated with anti-apoptotic pathways.
All these data are in good agreement with the findings reported in our previous study (22). We showed there a specific tumor phenotype characterized by segregation of DCT and TYR expression. The DCT+/TYR− subset in the dermal-epidermal layer was positive for CAV1, whereas the one propagated in profound dermis was negative for CAV1. Moreover, the dermis DCT+/TYR−/CAV1− subset acquired expression/down-regulation and specific distribution for other markers associated with bad prognostic. This process is pretty much similar to what we observe in the present in vitro study. MJS is probably a tumor VGP phenotype from superficial/medium dermis expressing low-DCT and high-CAV1 in numerous isolated cells. Under environmental stressful conditions and/or autocrine regulation, MJS cells undergo a transformation process and become a new phenotype with CAV1 and DCT down- and up-regulated, respectively. Whether this new subtype would be associated with migratory and extended survival capacities remains to be further demonstrated.
Importantly, in that study, we did not identify in any of the investigated specimens a situation in which DCT remains expressed in the superficial tumor component, and TYR is preserved in profound dermis. This is now understandable considering the present data showing that in the MNT cell line, which expresses endogenously all tyrosinase-related proteins, only DCT expression is up-regulated by environmental stressors (Fig. 7D) and supports even more its pro-survival role in tumor cells.
In conclusion, this study demonstrates for the first time that there is a cross-talk between CAV1 and DCT in melanoma. CAV1 is one of the molecular regulators of DCT gene expression in early (VGP) melanoma phenotypes. In its turn DCT is a regulator of CAV1 assembly in both early and metastatic phenotypes and very likely of CAV1-modulated processes. However, it may be very well the case that the DCT role in CAV1-modulated processes to be distinct in the two phenotypes. Based on this study, we also postulate a role of DCT and CAV1 in the EMT program in cutaneous melanoma.
Author Contributions
I. L. P. planned, performed, and analyzed data (time course si-DCT, si-CAV1, WB, co-IP, IF microscopy, and subcellular fractionation). A. L. M. planned, performed, analyzed data, and wrote the manuscript (structural CAV1, DCT, Cav-DCT models, figures, and manuscript preparation). L. E. S. performed the experiments, analyzed data, and wrote the manuscript (mRNA determination and Tissue FAXS). P. R. A. performed the experiments (early setup experiments for si-CAV1, si-DCT, and time course). F. P. and C.V. A. M. performed the experiments, analyzed data, and wrote the manuscript (mass spectrometry). G. N. conceived, designed the experiments, analyzed data, and wrote the manuscript. All authors analyzed the results and approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We thank Dr. Simona Ghenea for RT-qPCR primers design and acknowledge the Tissue Gnostics Vienna and Iasi teams (Dr. R. Ecker, Dr. R. Rogojanu, Dr. D. Haisan, and C. Husanu) for Tissue FAXS training sessions and assistance in data acquisition and processing.
This work was supported by Grant Application 156, Exploratory Research Projects PN-II-ID-PCE-2011-3-0492-1, funded by Ministerul Educatiei si Cercetării Stiin̗tifice and in part by Academia Română Project 1/2011 of the Institute of Biochemistry.

This article contains supplemental Figs. S1 and S2, Table S1, and Materials 1 and 2.
- VGP
- vertical growth phase
- DCT
- dopachrome tautomerase
- TYR
- tyrosinase
- TRP
- tyrosinase-related protein
- CAV1
- caveolin-1
- MJS
- MelJuSo
- SK28
- SKMel28
- EndoH
- endoglycosidase H
- PNGase F
- peptide:N-glycosidase F
- PM
- plasma membrane
- ER
- endoplasmic reticulum
- TM
- transmembrane
- OG
- oligomer
- MO
- monomer
- CRD
- cholesterol-rich domain
- CSD
- caveolin-1 scaffolding domain
- CRAC
- cholesterol recognition amino acid consensus
- EMT
- epithelial-mesenchymal transition
- WB
- Western blot
- TF
- TissueFAXS
- MFI
- mean of maximum fluorescence intensity
- MSI
- mean of sum intensity
- qPCR
- quantitative PCR
- IP
- immunoprecipitation
- aa
- amino acid
- HMW
- high molecular weight
- LMW
- low molecular weight
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- h
- human
- DHICA
- 5,6-dihydroxyindole-2-carboxylic acid
- NST
- nystatin
- si-ct
- si-control.
References
- 1.Jemal A., Siegel R., Xu J., and Ward E. (2010) Cancer statistics 2010. CA Cancer J. Clin. 60, 277–300 [DOI] [PubMed] [Google Scholar]
- 2.Bastian B. C. (2014) The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu. Rev. Pathol. 9, 239–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slominski A., Tobin J. D., Shibahara S., and Wortsman J.P. (2004) Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 84, 1164–1167 [DOI] [PubMed] [Google Scholar]
- 4.Jiang S., Liu X. M., Dai X., Zhou Q., Lei T. C., Beermann F., Wakamatsu K., and Xu S. Z. (2010) Regulation of DHICA-mediated antioxidation by dopachrome tautomerase: implication for skin photoprotection against UVA radiation. Free Radic. Biol. Med. 48, 1144–1151 [DOI] [PubMed] [Google Scholar]
- 5.Chu W., Pak B. J., Bani M. R., Kapoor M., Lu S. J., Tamir A., Kerbel R. S., and Ben-David Y. (2000) Tyrosinase-related protein 2 as a mediator of melanoma specific resistance to cis-diamminedichloroplatinum(II): therapeutic implications. Oncogene 19, 395–402 [DOI] [PubMed] [Google Scholar]
- 6.Pak B. J., Li Q., Kerbel R. S., and Ben-David Y. (2000) TYRP2-mediated resistance to cis-diamminedichloroplatinum(II) in human melanoma cells is independent of tyrosinase and TYRP1 expression and melanin content. Melanoma Res. 10, 499–505 [DOI] [PubMed] [Google Scholar]
- 7.Pak B. J., Chu W., Lu S. J., Kerbel R. S., and Ben-David Y. (2001) Lineage-specific mechanism of drug and radiation resistance in melanoma mediated by tyrosinase-related protein 2. Cancer Metastasis Rev. 20, 27–32 [DOI] [PubMed] [Google Scholar]
- 8.Michard Q., Commo S., Belaidi J. P., Alleaume A. M., Michelet J. F., Daronnat E., Eilstein J., Duche D., Marrot L., and Bernard B. A. (2008) TRP-2 specifically decreases WM35 cell sensitivity to oxidative stress. Free Radic. Biol. Med. 44, 1023–1031 [DOI] [PubMed] [Google Scholar]
- 9.Pak B. J., Lee J., Thai B. L., Fuchs S. Y., Shaked Y., Ronai Z., Kerbel R. S., and Ben-David Y. (2004) Radiation resistance of human melanoma analysed by retroviral insertional mutagenesis reveals a possible role for dopachrome tautomerase. Oncogene 23, 30–38 [DOI] [PubMed] [Google Scholar]
- 10.Orlow S. J., Silvers W. K., Zhou B. K., and Mintz B. (1998) Comparative decreases in tyrosinase, TRP-1, TRP-2, and Pmel 17/silver antigenic proteins from melanotic to amelanotic stages of syngeneic mouse cutaneous melanomas and metastases. Cancer Res. 58, 1521–1523 [PubMed] [Google Scholar]
- 11.Smedley R. C., Lamoureux J., Sledge D. G., and Kiupel M. (2011) Immunohistochemical diagnosis of canine oral amelanotic melanocytic neoplasms. Vet. Pathol. 48, 32–40 [DOI] [PubMed] [Google Scholar]
- 12.Liu G., Khong H. T., Wheeler C. J., Yu J. S., Black K. L., and Ying H. (2003) Molecular and functional analysis of tyrosinase-related protein (TRP)-2 as a cytotoxic T lymphocyte target in patients with malignant glioma. J. Immunother. 26, 301–312 [DOI] [PubMed] [Google Scholar]
- 13.Jiao Z., Zhang Z. G., Hornyak T. J., Hozeska A., Zhang R. L., Wang Y., Wang L., Roberts C., Strickland F. M., and Chopp M. (2006) Dopachrome tautomerase (Dct) regulates neural progenitor cell proliferation. Dev. Biol. 296, 396–408 [DOI] [PubMed] [Google Scholar]
- 14.Sendoel A., Kohler I., Fellmann C., Lowe S. W., and Hengartner M. O. (2010) HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature 465, 577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shan T., Lu H., Ji H., Li Y., Guo J., Chen X., and Wu T. (2014) Loss of stromal caveolin-1 expression: a novel tumor microenvironment biomarker that can predict poor clinical outcomes for pancreatic cancer. PLoS ONE 9, e97239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savage K., Lambros M. B., Robertson D., Jones R. L., Jones C., Mackay A., James M., Hornick J. L., Pereira E. M., Milanezi F., Fletcher C. D., Schmitt F. C., Ashworth A., and Reis-Filho J. S. (2007) Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin. Cancer Res. 13, 90–101 [DOI] [PubMed] [Google Scholar]
- 17.Lobos-Gonzáles L., Aguilar L., Fernández G., Sanhueza C., and Quest A. F. (2011) in Advances in Malignant Melanoma-Clinical and Research Perspectives. (Armstrong A., ed) pp. 200–205, InTech, 10.5772/21829 [DOI] [Google Scholar]
- 18.Felicetti F., Parolini I., Bottero L., Fecchi K., Errico M. C., Raggi C., Biffoni M., Spadaro F., Lisanti M. P., Sargiacomo M., and Carè A. (2009) Caveolin-1 tumor-promoting role in human melanoma. Int. J. Cancer 125, 1514–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres V. A., Tapia J. C., Rodriguez D. A., Lladser A., Arredondo C., Leyton L., and Quest A. F. (2007) E-cadherin is required for caveolin-1-mediated down-regulation of the inhibitor of apoptosis protein survivin via reduced β-catenin-Tcf/Lef-dependent transcription. Mol. Cell. Biol. 27, 7703–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trimmer C., Whitaker-Menezes D., Bonuccelli G., Milliman J. N., Daumer K. M., Aplin A. E., Pestell R. G., Sotgia F., Lisanti M. P., and Capozza F. (2010) CAV1 inhibits metastatic potential in melanomas through suppression of the integrin/Src/FAK signaling pathway. Cancer Res. 70, 7489–7499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakashima H., Hamamura K., Houjou T., Taguchi R., Yamamoto N., Mitsudo K., Tohnai I., Ueda M., Urano T., Furukawa K., and Furukawa K. (2007) Overexpression of caveolin-1 in a human melanoma cell line results in dispersion of ganglioside GD3 from lipid rafts and alteration of leading edges, leading to attenuation of malignant properties. Cancer Sci. 98, 512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filimon A., Zurac S. A., Milac A. L., Sima L. E., Petrescu S. M., and Negroiu G. (2014) Value of dopachrome tautomerase detection in the assessment of melanocytic tumors. Melanoma Res. 24, 219–236 [DOI] [PubMed] [Google Scholar]
- 23.Jiménez M., Tsukamoto K., and Hearing V. J. (1991) Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J. Biol. Chem. 266, 1147–1156 [PubMed] [Google Scholar]
- 24.Ziegler-Heitbrock H. W., Munker R., Johnson J., Petersmann I., Schmoeckel C., and Riethmüller G. (1985) In vitro differentiation of human melanoma cells analyzed with monoclonal antibodies. Cancer Res. 45, 1344–1350 [PubMed] [Google Scholar]
- 25.Dalton L.E., Kamarashev J., Barinaga-Rementeria Ramirez I., White G., Malliri A., and Hurlstone A. (2013) Constitutive RAC activation is not sufficient to initiate melanocyte neoplasia but accelerates malignant progression. J. Invest. Dermatol. 133, 1572–1581 [DOI] [PubMed] [Google Scholar]
- 26.Carey T. E., Takahashi T., Resnick L. A., Oettgen H. F., and Old L. J. (1976) Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc. Natl. Acad. Sci. U.S.A. 73, 3278–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baruthio F., Quadroni M., Rüegg C., and Mariotti A. (2008) Proteomic analysis of membrane rafts of melanoma cells identifies protein patterns characteristic of the tumor progression stage. Proteomics 8, 4733–4747 [DOI] [PubMed] [Google Scholar]
- 28.Negroiu G., Dwek R. A., and Petrescu S. M. (2003) The inhibition of early N-glycan processing targets TRP-2 to degradation in B16 melanoma cells. J. Biol. Chem. 278, 27035–27042 [DOI] [PubMed] [Google Scholar]
- 29.Schägger H. (2006) Tricine-SDS-PAGE. Nat. Protoc. 1, 16–22 [DOI] [PubMed] [Google Scholar]
- 30.Popa I., Ganea E., and Petrescu S. M. (2014) Expression and subcellular localization of RAGE in melanoma cells. Biochem. Cell Biol. 92, 127–136 [DOI] [PubMed] [Google Scholar]
- 31.Macdonald J. L., and Pike L. J. (2005) A simplified method for the preparation of detergent-free lipid rafts. J. Lipid Res. 46, 1061–1067 [DOI] [PubMed] [Google Scholar]
- 32.Shevchenko A., Tomas H., Havlis J., Olsen J. V., and Mann M. (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 [DOI] [PubMed] [Google Scholar]
- 33.Käll L., Canterbury J. D., Weston J., Noble W. S., and MacCoss M. J. (2007) Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 4, 923–925 [DOI] [PubMed] [Google Scholar]
- 34.The NCBI Handbook (2002) National Library of Medicine, National Center for Biotechnology Information; 2002 Oct. Chapter 18, The Reference Sequence (RefSeq) Project. Bethesda, MD: Available from www.ncbi.nlm.nih.gov/books/NBK21091 [Google Scholar]
- 35.Sevenich L., Schurigt U., Sachse K., Gajda M., Werner F., Müller S., Vasiljeva O., Schwinde A., Klemm N., Deussing J., Peters C., and Reinheckel T. (2010) Synergistic antitumor effects of combined cathepsin B and cathepsin Z deficiencies on breast cancer progression and metastasis in mice. Proc. Natl. Acad. Sci. U.S.A. 107, 2497–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang G., Wu X., Jiang Z., Kasman I., Yao J., Guan Y., Oeh J., Modrusan Z., Bais C., Sampath D., and Ferrara N. (2012) Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 31, 3513–3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Récamier K. S., Hernández-Gómez A., González-Damián J., and Ortega-Blake I. (2010) Effect of membrane structure on the action of polyenes: I. Nystatin action in cholesterol- and ergosterol-containing membranes. J. Membr. Biol. 237, 31–40 [DOI] [PubMed] [Google Scholar]
- 38.Hayer A., Stoeber M., Bissig C., and Helenius A. (2010) Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic 11, 361–382 [DOI] [PubMed] [Google Scholar]
- 39.Monier S., Parton R.G., Vogel F., Behlke J., Henske A., and Kurzchalia T. (1995) VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol. Biol. Cell 6, 911–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L., and Pilch P. F. (2008) A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J. Biol. Chem. 283, 4314–4322 [DOI] [PubMed] [Google Scholar]
- 41.Schlegel A., Schwab R. B., Scherer P. E., and Lisanti M. P. (1999) A role for the caveolin scaffolding domain in mediating the membrane attachment of caveolin-1. The caveolin scaffolding domain is both necessary and sufficient for membrane binding in vitro. J. Biol. Chem. 274, 22660–22667 [DOI] [PubMed] [Google Scholar]
- 42.Le Lan C., Neumann J. M., and Jamin N. (2006) Role of the membrane interface on the conformation of the caveolin scaffolding domain: a CD and NMR study. FEBS Lett. 580, 5301–5305 [DOI] [PubMed] [Google Scholar]
- 43.Levin A. M., Coroneus J. G., Cocco M. J., and Weiss G. A. (2006) Exploring the interaction between the protein kinase A catalytic subunit and caveolin-1 scaffolding domain with shotgun scanning, oligomer complementation, NMR, and docking. Protein Sci. 15, 478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoop C. L., Sivanandam V. N., Kodali R., Srnec M. N., and van der Wel P. C. (2012) Structural characterization of the caveolin scaffolding domain in association with cholesterol-rich membranes. Biochemistry 51, 90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Lan C., Gallay J., Vincent M., Neumann J. M., de Foresta B., and Jamin N. (2010) Structural and dynamic properties of juxta-membrane segments of caveolin-1 and caveolin-2 at the membrane interface. Eur. Biophys. J. 39, 307–325 [DOI] [PubMed] [Google Scholar]
- 46.Epand R. M., Sayer B. G., and Epand R. F. (2005) Caveolin scaffolding region and cholesterol-rich domains in membranes. J. Mol. Biol. 345, 339–350 [DOI] [PubMed] [Google Scholar]
- 47.Lee J., and Glover K. J. (2012) The transmembrane domain of caveolin-1 exhibits a helix-break-helix structure. Biochim. Biophys. Acta 1818, 1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aoki S., and Epand R. M. (2012) Caveolin-1 hydrophobic segment peptides insertion into membrane mimetic systems: role of proline residue. Biochim. Biophys. Acta. 1818, 12–18 [DOI] [PubMed] [Google Scholar]
- 49.Casey J. R., Grinstein S., and Orlowski J. (2010) Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 11, 50–61 [DOI] [PubMed] [Google Scholar]
- 50.Hansen C. G., and Nichols B. J. (2010) Exploring the caves: Cavins, caveolins and caveolae. Trends Cell Biol. 20, 177–186 [DOI] [PubMed] [Google Scholar]
- 51.Lajoie P., Goetz J. G., Dennis J. W., and Nabi I. R. (2009) Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J. Cell Biol. 185, 381–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chidlow J. H. Jr., and Sessa W. C. (2010) Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc. Res. 86, 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halaban R., Cheng E., Zhang Y., Moellmann G., Hanlon D., Michalak M., Setaluri V., and Hebert D. N. (1997) Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the dedifferentiated phenotype of amelanotic melanoma cells. Proc. Natl. Acad. Sci. U.S.A. 94, 6210–6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watabe H., Valencia J. C., Yasumoto K., Kushimoto T., Ando H., Muller J., Vieira W. D., Mizoguchi M., Appella E., and Hearing V. J. (2004) Regulation of tyrosinase processing and trafficking by organellar pH and by proteasome activity. J. Biol. Chem. 279, 7971–7981 [DOI] [PubMed] [Google Scholar]
- 55.Bransburg-Zabary S., Nachliel E., and Gutman M. (1996) Utilization of monensin for detection of microdomains in cholesterol containing membrane. Biochim. Biophys. Acta 1285, 146–154 [DOI] [PubMed] [Google Scholar]
- 56.Negroiu G., Dwek R. A., and Petrescu S. M. (2005) Tyrosinase-related protein-2 and -1 are trafficked on distinct routes in B16 melanoma cells. Biochem. Biophys. Res. Commun. 328, 914–921 [DOI] [PubMed] [Google Scholar]
- 57.Setaluri V. (2000) Sorting and targeting of melanosomal membrane proteins: signals, pathways, and mechanisms. Pigment Cell Res. 13, 128–134 [DOI] [PubMed] [Google Scholar]
- 58.Wang N., and Hebert D. N. (2006) Tyrosinase maturation through the mammalian secretory pathway: bringing color to life. Pigment Cell Res. 19, 3–18 [DOI] [PubMed] [Google Scholar]
- 59.Watanabe R., and Riezman H. (2004) Differential ER exit in yeast and mammalian cells. Curr. Opin. Cell Biol. 16, 350–355 [DOI] [PubMed] [Google Scholar]
- 60.Tagawa A., Mezzacasa A., Hayer A., Longatti A., Pelkmans L., and Helenius A. (2005) Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J. Cell Biol. 170, 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okamoto T., Schlegel A., Scherer P. E., and Lisanti M. P. (1998) Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 273, 5419–5422 [DOI] [PubMed] [Google Scholar]
- 62.Smart E. J., Graf G. A., McNiven M. A., Sessa W. C., Engelman J. A., Scherer P. E., Okamoto T., and Lisanti M. P. (1999) Caveolins, liquid-ordered domains, and signal transduction. Mol. Cell. Biol. 19, 7289–7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trane A. E., Hiob M. A., Uy T., Pavlov D., and Bernatchez P. (2015) Caveolin-1 scaffolding domain residue phenylalanine 92 modulates Akt signaling. Eur. J. Pharmacol. 766, 46–55 [DOI] [PubMed] [Google Scholar]
- 64.Fielding P. E., Chau P., Liu D., Spencer T. A., and Fielding C. J. (2004) Mechanism of platelet-derived growth factor-dependent caveolin-1 phosphorylation: relationship to sterol binding and the role of serine-80. Biochemistry 43, 2578–2586 [DOI] [PubMed] [Google Scholar]
- 65.Madhusudan Makwana K., and Mahalakshmi R. (2015) Implications of aromatic-aromatic interactions: from protein structures to peptide models. Protein Sci. 24, 1920–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holt A., and Killian J. A. (2010) Orientation and dynamics of transmembrane peptides: the power of simple models. Eur. Biophys. J. 39, 609–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sprong H., Degroote S., Claessens T., van Drunen J., Oorschot V., Westerink B. H., Hirabayashi Y., Klumperman J., van der Sluijs P., and van Meer G. (2001) Glycosphingolipids are required for sorting melanosomal proteins in the Golgi complex. J. Cell Biol. 155, 369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanna E., Miotti S., Mazzi M., De Santis G., Canevari S., and Tomassetti A. (2007) Binding of nuclear caveolin-1 to promoter elements of growth-associated genes in ovarian carcinoma cells. Exp. Cell Res. 313, 1307–1317 [DOI] [PubMed] [Google Scholar]
- 69.Altieri D. C. (2008) Survivin, cancer networks and pathway-directed drug discovery. Nat. Rev. Cancer 8, 61–70 [DOI] [PubMed] [Google Scholar]
- 70.Chen S. T., Lin S. Y., Yeh K. T., Kuo S. J., Chan W. L., Chu Y. P., and Chang J. G. (2004) Mutational, epigenetic and expressional analyses of caveolin-1 gene in breast cancers. Int. J. Mol. Med. 14, 577–582 [PubMed] [Google Scholar]
- 71.Lázár-Molnár E., Hegyesi H., Tóth S., and Falus A. (2000) Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine 12, 547–554 [DOI] [PubMed] [Google Scholar]
- 72.Rodeck U., Melber K., Kath R., Menssen H. D., Varello M., Atkinson B., and Herlyn M. (1991) Constitutive expression of multiple growth factor genes by melanoma cells but not normal melanocytes. J. Invest. Dermatol. 97, 20–26 [DOI] [PubMed] [Google Scholar]
- 73.Lamouille S., Xu J., and Derynck R. (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li F. Z., Dhillon A. S., Anderson R. L., McArthur G., and Ferrao P. T. (2015) Phenotype switching in melanoma: implications for progression and therapy. Front. Oncol. 5, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jung A. C., Ray A. M., Ramolu L., Macabre C., Simon F., Noulet F., Blandin A. F., Renner G., Lehmann M., Choulier L., Kessler H., Abecassis J., Dontenwill M., and Martin S. (2015) Caveolin-1-negative head and neck squamous cell carcinoma primary tumors display increased epithelial to mesenchymal transition and prometastatic properties. Oncotarget. 6, 41884–41901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salem A. F., Bonuccelli G., Bevilacqua G., Arafat H., Pestell R. G., Sotgia F., and Lisanti M. P. (2011) Caveolin-1 promotes pancreatic cancer cell differentiation and restores membranous E-cadherin via suppression of the epithelial-mesenchymal transition. Cell Cycle 10, 3692–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.