Abstract
Background
Despite aggressive standard of care (SOC) treatment, survival of malignant gliomas remains very poor. This Phase II, prospective, matched controlled, multicenter trial was conducted to assess the safety and efficacy of aglatimagene besadenovec (AdV-tk) plus valacyclovir (gene-mediated cytotoxic immunotherapy [GMCI]) in combination with SOC for newly diagnosed malignant glioma patients.
Methods
Treatment cohort patients received SOC + GMCI and were enrolled at 4 institutions from 2006 to 2010. The preplanned, matched-control cohort included all concurrent patients meeting protocol criteria and SOC at a fifth institution. AdV-tk was administered at surgery followed by SOC radiation and temozolomide. Subset analyses were preplanned, based on prognostic factors: pathological diagnosis (glioblastoma vs others) and extent of resection.
Results
Forty-eight patients completed SOC + GMCI, and 134 met control cohort criteria. Median overall survival (OS) was 17.1 months for GMCI + SOC versus 13.5 months for SOC alone (P = .0417). Survival at 1, 2, and 3 years was 67%, 35%, and 19% versus 57%, 22%, and 8%, respectively. The greatest benefit was observed in gross total resection patients: median OS of 25 versus 16.9 months (P = .0492); 1, 2, and 3-year survival of 90%, 53%, and 32% versus 64%, 28% and 6%, respectively. There were no dose-limiting toxicities; fever, fatigue, and headache were the most common GMCI-related symptoms.
Conclusions
GMCI can be safely combined with SOC in newly diagnosed malignant gliomas. Survival outcomes were most notably improved in patients with minimal residual disease after gross total resection. These data should help guide future immunotherapy studies and strongly support further evaluation of GMCI for malignant gliomas.
Clinical trial registry
ClinicalTrials.gov NCT00589875.
Keywords: gene therapy, glioblastoma, immuno-oncology, immunotherapy, malignant glioma
Malignant gliomas, most of which are glioblastoma multiforme (GBM), represent a major unmet medical need.1 Current standard of care (SOC), which includes surgical resection followed by radiation and temozolomide (TMZ), results in a median survival <15 months.2 Extent of resection is associated with survival; however, due to the infiltrative nature of malignant gliomas, complete resection is not possible and progression occurs in almost all patients.3,4 New therapies are clearly needed for this devastating disease.
Gene-mediated cytotoxic immunotherapy (GMCI) is a potential new treatment for GBM. It includes the local delivery of aglatimagene besadenovec (AdV-tk), an adenoviral vector containing the herpes simplex virus thymidine kinase gene, followed by an antiherpetic prodrug such as valacyclovir.5 The prodrug is phosphorylated and functions as a nucleotide analog inhibiting DNA replication or repair and inducing cell death by necrosis and apoptosis, which elicits immune-activating danger signals, stimulates antigen presenting cell maturation, and CD8+ T-cell expansion.6–11 The immune response is critical for efficacy as evidenced by decreased local and systemic antitumor effects in immune-deficient mice compared with immune-competent mice and elimination of effects upon CD8-depletion of treated mice.6,8,10 In addition, GMCI treatment leads to protection against tumor rechallenge, and this protection can be transferred to naïve mice via CD8+ T cells.9,11–13
DNA-damaging chemotherapies and radiation further enhance AdV-tk-induced oncolysis and immune effects.14–17 Synergy with tumor debulking has also been shown,18,19 likely due to decrease of tumor-derived immune inhibitory effects such as myeloid-derived suppressor cells (MDSCs), regulatory T-cells (Tregs), and check-point inhibitory molecules.19,20 Since most differentiated cells do not actively replicate or express mutated tumor-associated antigens (TAAs), quiescent cells in the treatment area, such as neurons, are less susceptible to AdV-tk cytotoxicity and antitumor immune effects. Thus, GMCI causes tumor cytotoxicity and consequent release of TAAs. At the same time, the delivered virions, and the TK protein, which functions as a superantigen, stimulate a highly immunogenic microenvironment. Surgery and radiation diminish the tumor immune-inhibitory impact. This spatiotemporal union of TAA and immune stimulation results in a potent, cell-based antitumor immune response.5
Immunotherapies, including oncolytic viral approaches, have recently shown encouraging clinical results in multiple tumor types.20–25 Initial studies with GMCI in brain tumors were done in recurrent disease to establish a safe dose range and biological activity.26 Studies in preimmunized mice indicate that the immune response to adenovirus does not significantly impact tumor transduction after intratumoral vector delivery.27 Pre-existing antibody titers were evaluated in the first Phase I clinical study in recurrent malignant glioma and did not correlate with response.26 Subsequently, GMCI was brought to upfront disease to target minimal residual disease and capitalize on potential synergies with SOC.28 Since the combination with SOC could have unexpected toxicity, dose escalation was re-evaluated in a phase 1b study. No dose-limiting toxicities (DLTs) were detected. Tumor tissue evaluated from 4 participants had significant CD3+ T cell infiltration that was found to be CD8+ and CD4− in one case evaluated for these T cell markers; some subjects had gradual resolution of radiographic enhancement over months to years, consistent with immunotherapy responses.28 Reported here is the expansion Phase II trial of GMCI + SOC compared with SOC alone as a multicenter, matched controlled study.
Materials and Methods
Study Design and Treatment
The phase 1b study evaluated 12 participants at 3 vector dose levels (3 × 1010, 1 × 1011, and 3 × 1011 vector particles (vp) .28 The second phase added 36 evaluable participants to the third dose (3 × 1011 vp) to expand safety and efficacy assessment. Institutional review boards at participating treatment institutions (Ohio State University, City of Hope, University of Chicago, and Houston Methodist Hospital) approved the protocol and consent documents. Informed consent was obtained from each patient before enrollment.
Vector administration was previously described.28 Briefly, 1 mL was divided in up to 10 sites in the resection bed. Care was taken to avoid injection into eloquent areas, ventricles, and subarachnoid space. Valacyclovir (2 g 3 times per day × 14 days) was started 1–3 days after vector injection. Intravenous acyclovir (10 mg/kg) was substituted when patients were unable to take oral medication. Prodrug doses were adjusted for renal impairment based on calculated creatinine clearance. SOC radiation therapy (XRT) was initiated 4–13 days (mean 7 days) after vector injection (Supplementary Appendix Fig. SA1). TMZ was administered after completion of valacyclovir during and after radiation per SOC dosing. Participants in the control cohort underwent the same SOC surgical resection followed by XRT and TMZ.
Patient Selection
Patients ≥18 years of age with presumed malignant glioma, planning to undergo SOC surgery, radiation, and TMZ were considered eligible. Pathologic confirmation at surgery was required. Tumor sites were supratentorial and amenable to injection. Additional inclusion criteria included KPS ≥ 70, platelets >100 000/uL, WBC > 3000/uL, serum creatinine <2 mg/dL, and AST <3 times upper limit of normal.
To preclude selection bias, a matched control cohort that included all concurrent participants (between 2006 and 2010) with newly diagnosed malignant glioma was pre-planned to be selected from a site that did not enroll participants in the clinical trial (Brigham and Women's Hospital). Participants were identified from the neurosurgical database and only excluded if they did not meet protocol criteria or did not receive the same SOC.
Clinical Assessments
Adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. DLTs were defined as any grade 3 or 4 toxicity requiring interruption in radiation for more than 7 days. Total resection was defined as removal of >95% of the enhancing tumor. Progression was defined according to Macdonald criteria.29
Quality of Life Assessment
The Functional Assessment of Cancer Therapy-Brain (FACT-Br) questionnaire, version 4, was used to assess patient-reported outcomes.30 Clinicians assessed KPS.
Statistical Methods
Primary efficacy analysis was planned on the null hypothesis of no improvement in survival over the matched control SOC group with planned subset analyses of significant disease prognostic factors. Stopping rules were in place for toxicity. Progression-free survival (PFS) and overall survival (OS) were measured from date of surgery; estimates were determined using the Kaplan-Meier method. and differences were assessed by the log-rank (Mantel-Cox) test using GraphPad Prism version 5.0. Survival rates at fixed time points and baseline participant demographics were compared using a 2-tailed Fisher exact probability test.
Results
Participants and Treatment
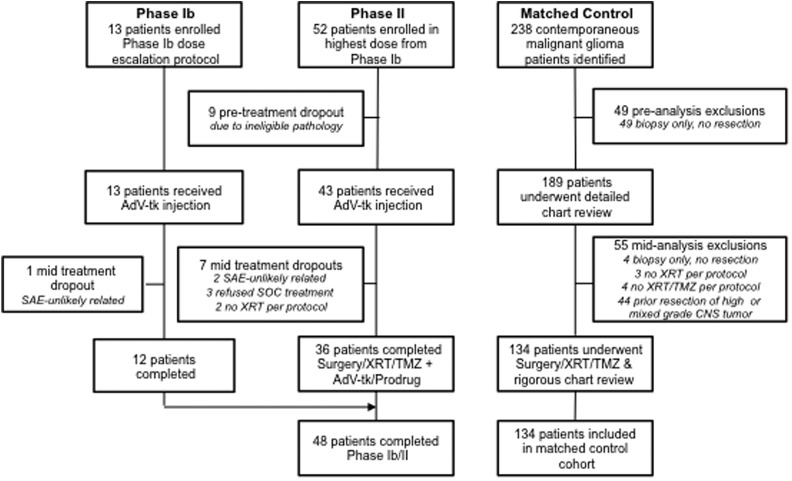
From 2006 to 2010, 65 participants were enrolled. and 48 completed therapy per protocol at 4 clinical sites. Participants enrolled but withdrawn are explained in the CONSORT diagram (Fig. 1). For the preplanned matched control cohort, all 238 malignant glioma partcipants treated at a fifth site during the same period were assessed, and 134 patients met criteria. Those excluded are explained in the CONSORT diagram (Fig. 1). Baseline characteristics for the evaluable participants in the GMCI + SOC and SOC cohorts were well matched (Table 1). The grade of disease (GBM vs anaplastic astrocytoma or anaplastic oligodendroglioma AA or AO) and the extent of resection (subtotal vs total) are known prognostic factors and were preplanned subgroups for analysis. In the GMCI + SOC, 90% were GBM versus 95% in SOC, and 40% underwent total resections versus 35% in the SOC group. These differences were not statistically significant; nonetheless, the subsets were independently analyzed. During the study, clinical assessment of MGMT methylation was not routine or standardized. However, data from 23 (48%) and 85 (63%) participants in the GMCI + SOC and SOC groups, respectively, showed no significant difference in composition between the groups.
Fig 1.
Enrollment. CONSORT diagram for the phase 1b/2 study-group patients treated with gene-mediated cytotoxic immunotherapy plus standard of care (GMCI + SOC) (left) and patients who received SOC therapy alone in the matched control cohort (right). Serious adverse events (SAEs) leading to mid-treatment dropout in the GMCI + SOC group were surgical complications and cardiac arrhythmia, deemed unlikely related to protocol treatment. Abbreviations: AdV-tk, aglatimagene besadenovec; SOC, standard of care; TMZ, temozolomide; XRT, radiation therapy.
Table 1.
Baseline patient demographics of gene-mediated cytotoxic immunotherapy + standard of care group and standard of care matched control group
| GMCI + SOC N = 48 | SOC N = 134 | Comparison (P value) | |
|---|---|---|---|
| AGE | |||
| <50 y | 13 (27%) | 26 (19%) | .306 |
| ≥50 y | 35 (73%) | 108 (81%) | |
| Median (range) | 57 (32–72) | 60 (24–90) | |
| KPS | |||
| 90–100 | 32 (67%) | 81 (60%) | .491 |
| 70–80 | 16 (33%) | 53 (40%) | |
| Median | 90 | 90 | |
| Tumor Histology | |||
| WHO IV (GBM) | 43 (90%) | 128 (95%) | .162 |
| WHO III (AA, AO) | 5 (10%) | 6 (5%) | |
| Extent of Resection | |||
| Total | 19 (40%) | 47 (35%) | .602 |
| Subtotal | 29 (60%) | 87 (65%) | |
Safety
No DLTs were observed. Adverse events > grade 1 that occurred in ≥5% of participants are summarized in Table 2. Events are shown per treatment period: 0–3 weeks after surgery/AdV-tk injection during which time valacyclovir was administered for 14 days and radiation was initiated; subsequent 4–8 weeks includes events during the remainder of radiation and concomitant TMZ. Most observed adverse events were expected complications of the underlying disease, surgery, radiation, or TMZ. The most common events considered possibly related to GMCI were fatigue, fever, and headache. One participant developed grade 4 hemiparesis (neuropathy-motor in CTCAE) and baseline speech impairment worsening to grade 3 immediately after surgery, both of which subsequently improved. The only other possibly related grade 3 events were insomnia, headache, and wound complication.
Table 2.
Adverse events higher than grade 1 reported in ≥5% of patients in the phase 1b/2 study
| Adverse Event | 0–3 wk (n = 56) No. Patients (%) |
4–8 wk (n = 53) No. Patients (%) |
||
|---|---|---|---|---|
| Grade > 1 | Grade > 2 | Grade > 1 | Grade > 2 | |
| Constitutional Symptoms- any | 10 (18) | 1 (2) | 10 (19) | – |
| Fatigue | 4 (7) | – | 6 (11) | – |
| Insomnia | 4 (7) | 1 (2) | 4 (8) | – |
| Dermatology/skin- any | – | – | 5 (9) | 1 (2) |
| Gastrointestinal- any | 7 (13) | – | 5 (9) | – |
| Anorexia | 4 (7) | – | 1 (2) | – |
| Constipation | 1 (2) | – | 3 (6) | – |
| Infection- any | 3 (5) | – | 2 (4) | 1 (2) |
| Urinary tract infection | 3 (5) | – | 1 (2) | – |
| Neurology- any | 26 (46) | 9 (16) | 12 (23) | 3 (6) |
| Mood alteration–depression | 4 (7) | 1 (2) | 2 (4) | – |
| Neuropathy–Motor | 6 (11) | 3 (5) | 1 (2) | – |
| Seizure | 3 (5) | 1 (2) | 3 (6) | 1 (2) |
| Speech impairment | 5 (9) | 1 (2) | 3 (6) | – |
| Ocular- any | 3 (5) | 1 (2) | – | – |
| Pain- any | 10 (18) | 2 (4) | 4 (8) | 1 (2) |
| Headache | 9 (16) | 2 (4) | 2 (4) | 1 (2) |
| Vascular- any | 3 (5) | 2 (4) | 3 (6) | 3 (6) |
| Thrombosis Embolism | 3 (5) | 2 (4) | 3 (6) | 3 (6) |
| Laboratory | ||||
| Elevated AST/ALT | 5 (9) | 3 (5) | 2 (4) | – |
| Low calcium | 3 (5) | – | 1 (2) | – |
| Low hemoglobin | 3 (5) | 1 (2) | 2 (4) | – |
| Low lymphocytes | 8 (14) | 2 (4) | 17 (32) | 7 (13) |
Abbreviations: ALT, alanine amino transferase; AST, aspartate aminotransferase.
Events during the 0–3 week period occurring after surgery and AdV-tk, aglatimagene besadenovec injection, during the 14 days of prodrug administration, and up to 2 weeks of radiation. Events during the 4–8 week period occurred during the follow-on radiation period and concomitant temozolomide administration. Grading is based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0
Survival
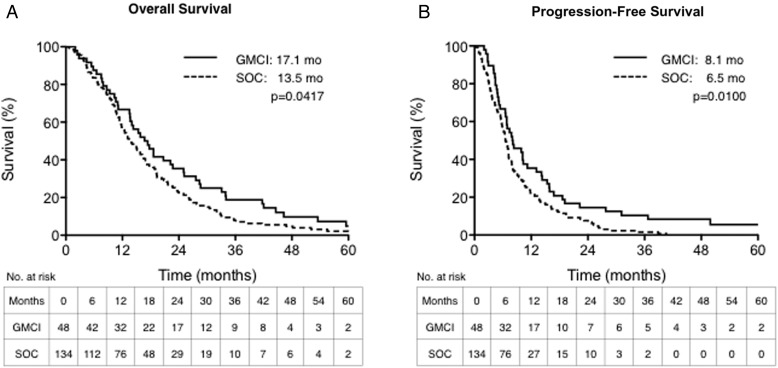
Accrual was completed in 2010 with 3 participants still alive at last follow-up, 43–88 months after surgery/AdV-tk injection. Median OS was 17.1 and 13.5 months for GMCI + SOC and SOC, respectively (hazard ratio [HR]: 0.72, 95% confidence interval [CI]: 0.52–0.99, P = .0417; Fig. 2A). The median PFS was 8.1 and 6.5 months, respectively (HR: 0.66, 95% CI: 0.48–0.91, P = .0100; Fig. 2B). The 1, 2, and 3 year OS rates were 66.7%, 35.4%, and 18.8%, for GMCI + SOC compared with 56.7%, 21.6%, and 7.5% for SOC, respectively (Table 3).
Fig. 2.
Overall and progression-free survival outcomes. Kaplan-Meier curves of overall survival (OS) and progression-free survival (PFS) are presented for the study group (red) and matched control group (blue). The study group had significantly higher median OS (panel A) and PFS (panel B) compared with the matched control group (P = .0417 and P = .0100, respectively).
Table 3.
Overall survival rates for gene-mediated cytotoxic immunotherapy + standard of care compared with standard of care alone for the whole study population and prognostic subgroups
| Study Cohort |
N | Survival Rate (95% CI) |
Median OS (mo) | Hazard Ratio (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Group | Subgroup | Treatment | 12 mo | 24 mo | 36 mo | |||
| All Histologies (GBM, AA and AO) | All resections | GMCI | 48 | 66.7 (53.3–80.0) | 35.4 (21.9–48.9) | 18.8a (7.7–29.8) | 17.1 | 0.72 (0.52–0.988) P = .0417 |
| SOC | 134 | 56.7 (48.3–65.1) | 21.6 (14.7–28.6) | 7.5 (3.0–11.9) | 13.5 | |||
| Gross total resection | GMCI | 19 | 89.5 (75.7–100) | 52.6 (30.2–75.1) | 31.6a (10.7–52.5) | 25.0 | 0.59 (0.35–0.998) P = .0492 | |
| SOC | 47 | 63.8 (50.1–77.6) | 27.7 (14.9–40.4) | 6.4 (0.0–13.4) | 16.9 | |||
| Subtotal resection | GMCI | 29 | 51.7 (33.5–69.9) | 24.1 (8.6–39.7) | 10.3 (0.0–21.4) | 13.5 | 0.85 (0.56–1.30) P = .4584 | |
| SOC | 87 | 52.9 (42.4–63.4) | 18.4 (10.3–26.5) | 8.0 (2.3–13.8) | 12.5 | |||
| GBM only | All resections | GMCI | 43 | 62.8 (48.3–77.2) | 32.6 (18.6–46.6) | 14.0 (3.6–24.3) | 16.7 | 0.81 (0.58–1.13) P = .2073 |
| SOC | 128 | 57.8 (49.3–66.4) | 21.9 (14.7–29.0) | 7.0 (2.6–11.5) | 13.7 | |||
| Gross total resection | GMCI | 18 | 88.9 (74.4–100) | 55.6a (32.6–78.5) | 33.3a (11.6–55.1) | 25.1 | 0.50 (0.29–0.86) P = .0120 | |
| SOC | 44 | 63.6 (49.4–77.9) | 27.3 (14.1–40.4) | 4.5 (0.0–10.7) | 16.3 | |||
| Subtotal resection | GMCI | 25 | 44.0 (24.5–63.5) | 16.0 (1.6–30.4) | 0.0 (NA) | 10.6 | 1.36 (0.83–2.21) P = .2231 | |
| SOC | 84 | 54.8 (44.1–65.4) | 19.0 (10.7–27.4) | 8.3 (2.4–14.2) | 12.8 | |||
Abbreviations: AA, anaplastic astrocytoma; AO, anaplastic oligodendroglioma; CI, confidence interval; GBM, glioblastoma multiforme; GMCI, gene-mediated cytotoxic immunotherapy; OS, overall survival; SOC, standard of care.
aStatistically significant (P < .05) between 2 groups using 2-tailed Fisher exact probability test.
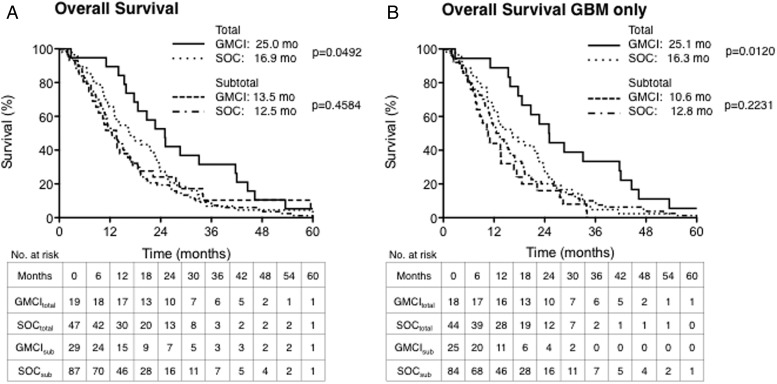
Planned analyses of GMCI survival impact based on pathological grade at diagnosis and the extent of resection were performed (Fig. 3). GMCI survival impact on the entire study population and GBM-only subset were similar (Table 3 and Supplementary Appendix Fig. SA2). However, the effect of GMCI was significantly different based on the extent of resection. The median OS for gross total resection (GTR) participants was 25.0 and 16.9 months (a difference of 8.1 months) for GMCI + SOC and SOC, respectively (HR: 0.59, 95% CI: 0.35–0.998, P = .0492) and only 1 month for those with subtotal resections, 13.5 versus 12.5 months, (P = .4584). Similarly, in the GBM-only subset, the median OS after GTR was 25.1 versus 16.3 months for GMCI + SOC and SOC, respectively (HR: 0.50, 95% CI: 0.29–0.86, P = .0120), and 10.6 versus 12.8 (P = .2231) in the subtotal resection subgroup. The survival rates at 1, 2 and 3 years were also consistently improved in the GMCI + SOC-treated GTR groups but not in those with subtotal resection (Table 3). For the entire GTR patient population, the survival rate was nearly doubled at 2 years (52.6% vs 27.7%, P = .08) and reached a 5-fold improvement at 3 years (31.6% vs 6.4%, P < .05). For the GBM-only GTR participants, the survival rate improvement was significant at 2 and 3 years with >2-fold (55.6% vs 27.3%, P < .05) and >7-fold (33.3% vs 4.5%, P = .006), respectively. Median PFS improved from 7.2 to 11.2 months in the GTR population, similarly from 7.2 to 12.7 in the GBM-only GTR population, but no differences were seen in the subtotal resection groups.
Fig. 3.
Overall survival (OS) outcomes by prognostic subgroups. Kaplan-Meier curves of OS by surgical extent groups: total resection (solid or dotted lines) and subtotal resection (dashed lines); and by diagnostic stage: all malignant gliomas (panel A) or glioblastoma multiforme (GBM)-only patients (panel B). Patients treated with gene-mediated cytotoxic immunotherapy plus standard of care (GMCI + SOC) were compared with those in the same matched control subgroups. There was no significant difference between the study and control groups in participants who had undergone subtotal surgical resections. However, in patients who underwent gross total resection, in both the all malignant gliomas and GBM-only subgroups, the GMCI + SOC-treated participants had significantly higher median OS compared with the respective matched control subgroups (P = .0492 and P = .0120, respectively).
Other Clinical Outcomes
The FACT-Br questionnaire scores on each of the general subscales, the brain–specific subscale, and the total scores were maintained throughout treatment without decrease, suggesting well-being preservation throughout treatment (Supplementary Appendix Fig. SA3). Participants who completed the questionnaire more than 2 years after treatment had an average total score of 158 compared with an average score of 144 for participants completing it at baseline. In the 43 GMCI + SOC participants with evaluable data, KPS was maintained at 70 or higher for a median time of 12 months. These data were not powered for statistical analysis.
Discussion
The current trial was designed to capitalize on the good safety profile and synergy that GMCI has shown with surgery,18,19 radiation,15,16 and TMZ.14,17 The study showed a convincing difference in OS and PFS between participants who received the study drug and those in the matched control cohort.
The protocol was designed using a matched control group with a hybrid of cluster randomization and Zelen's design,31 rather than a patient-by-patient randomization. This is a potential confounding factor. However, in this well-characterized disease, it allowed efficient group comparison while minimizing unnecessary participant discomfort that may result from placebo randomization. On post hoc analyses, the 2 groups were well matched with respect to major prognostic factors such as age and KPS scores. During the study period, validated MGMT promoter methylation or IDH1/2 mutation analyses were not clinically available. Based on MGMT methylation status reported on a small number of participants from both cohorts, no significant difference was detected between the groups. The cohort equivalence was further supported by similarity of outcomes in the subtotal resection subsets. This study methodology warrants consideration as an alternative when a sufficiently powered randomized study cannot be performed.
Between 2006 and 2010, 48 evaluable patients were enrolled in the treatment cohort at 4 clinical sites. There were 134 evaluable patients in the preplanned matched control group, which included all concurrent malignant glioma patients who met the study criteria and SOC at a fifth site that did not enroll patients in the treatment group (Brigham and Women's Hospital). The trial did not require stable or decreasing dexamethasone use, time without progression after surgery, or any other pretreatment or posttreatment selection for participants with good prognostic factors.
The safety and efficacy results of this study were positive. There were few possibly related or unexpected adverse events and no DLTs observed. The treatment group showed a 28% decrease in risk of death (HR: 0.72) and a 3.6 month increase in median OS. GMCI was safely combined with SOC for newly diagnosed GBM with little to no added toxicity and significantly improved survival relative to SOC alone. A previous study with sitimagene ceradenovec, a vector similar to AdV-tk, reported more modest benefits.32 It is not possible to assign a cause and effect to a specific difference; the improved results could be due to physical differences in the products (the vectors and manufacturing process are similar but distinct from each other) or differences in clinical design, vector delivery, participant SOC, etc. For example, the current study took advantage of the synergy between radiation and GMCI 16; in the previous study, radiation was administered several weeks after the prodrug, and thus there would have been no treatment overlap. This study demonstrated that multifaceted interactions in the analysis of complex biologics make inference from one product or study to another difficult.
The protocol had preplanned subset analyses based on prognostic characteristics. GMCI showed improved survival of the entire study population and the WHO grade IV (GBM)-only subset (Table 3). There were too few AA or AO participants to allow direct evaluation of GMCI in WHO grade III disease. However, GMCI's potential in malignant glioma does not seem limited by histological grade. As expected in malignant glioma patients, survival and PFS were more favorable in younger participants from both groups, but the study was not powered to evaluate the potential impact of this variable between the 2 groups.
The extent of resection did seem to have an impact on response. A significant improvement was observed in participants who underwent GTR, whereas the difference was not detected in participants with subtotal resections. This important finding may have implications for any immunotherapy approach in GBM. Although there were some long-term survivors in the GMCI + SOC subtotal resection subset, there were no significant survival differences in this subset; almost all of the GMCI-mediated improvements were in participants with total resection, independent of tumor grade (Table 3). In participants with GTR, the risk of death decreased by 41%, and median OS increased 8.1 months. This was also true for the GBM-only GTR subgroup; the risk of death decreased by 50%, and median OS increased by 8.8 months. TMZ, the last approved drug for first-line therapy in malignant gliomas, yielded a 2.5-month improvement in the total population and a 4.6-month improvement in the GTR subset.2 The magnitude of the improvements is remarkable for this devastating disease.
The impact observed from the extent of resection may be due to immunosuppressive features in malignant gliomas such as increased expression of programmed death-ligand 1 (PD-L1), inhibitory cytokines, high numbers of MDSCs and Tregs, microvesicle shedding, and other tumor environment modulators.20,22,33 Debulking with surgery as well as radiation and TMZ may diminish the impact of these factors.17,20,34–36 This effect has been shown with GMCI in various tumor models.18,19 GMCI likely stimulates an immune response in both GTR and subtotal resection participants; however, if the residual tumor burden is too large, the tumor-mediated immunosuppression may mask the GMCI effect. GTR may improve the odds in favor of the immune system by reducing the quantity and frequency of immunosuppressive factors. The extent of resection should be considered an important factor in future trials. Addition of checkpoint inhibitors may be particularly important in patients with subtotal resection due to immune inhibitory factors such as PD-L1, which may be expressed by residual tumor cells. These factors are being considered in the design of the next trials.
As has been pointed out by others, the impact of immunotherapies may be delayed and not fully captured by just median survival analysis. In this study, the survival benefit increased with time. One year after treatment, 90% of GTR participants in the treatment cohort were alive versus 64% in the control group, a 1.4-fold improvement. The difference was almost 2-fold (53% vs 28%) by 2 years and nearly 5-fold (32% vs 6%) by 3 years. This delayed differential is consistent with what may be expected from immune-mediated therapies.37–39 Approximately 25% of participants had evidence of pseudoprogression after completing radiation, which subsequently (and gradually) resolved (as previously described).28 Some cases were symptomatic and responded to corticosteroids. One participant, believed to be recurrent at 38 months, had a biopsy to confirm progression prior to being enrolled in another trial. This individual was found to have necrosis, not recurrence and was still progression-free at last follow-up (64 months).
There have been a number of recent successes in immuno-oncology. An antigen-specific vaccine against EGFRvIII (rindopepimut, Celldex Therapeutics), present in 25%–30% of GBMs, has shown significant improvement in PFS and OS.40 Unfortunately, subsequent tumor immune-escape by downregulation of EGFRvIII has also been observed.41 Since GMCI induces an in situ polyclonal immune response to whatever tumor-associated antigens are released by the patient's own tumor, immune escape may be less likely than with single antigen vaccines. In pancreatic cancer patients, GMCI was shown to induce CD8+ T cell infiltration with a concomitant increase in PD-L1 expression.42 Immune checkpoint inhibitors (ICIs) are another immunotherapic approach that has recently resulted in impressive, durable responses. Three of these ICI products have recently been approved for melanoma and non–small cell lung cancer.43–45 Although not limited to a specific antigen, however, only a subset of patients respond to ICIs. Responders are likely those patients with significant pre-existing antitumor immunity.46,47 Combining GMCI, a polyclonal, immune activating approach, may provide synergies with other immunotherapies such as anti-PD-1/PD-L1 and perhaps improve responses, even in patients with subtotal resections.
In summary, this study showed that GMCI could be a significant new tool in the armamentarium against GBM. It has improved survival compared with a prespecified matched control cohort and compared favorably to historical results, even from randomized studies with patient eligibility criteria determined after recovery from surgery and thus is likely to preselect a more favorable group.2,3 There have been a number of immunotherapy reports in malignant gliomas, but to our knowledge, this is the first study to demonstrate the correlation between maximum debulking and survival. It will be important to further evaluate the generalizability and immune mechanisms behind this observation. This study was conducted prior to routine evaluation of molecular signature subgroups such as IDH mutation or MGMT promoter methylation. Although these would not be expected to differ between the study groups or significantly affect immunotherapy approaches, it is a potential study-confounding factor. A prospectively randomized study will be required to rigorously assess the value of this promising and safe new therapeutic approach and the possible impact of prognostic factors, including molecular signatures.
Supplementary Material
Funding
Advantagene, Inc. was the sponsor of the trial. This study was also supported in part by grant number R44CA107745 from the National Cancer Institute.
Supplementary Material
Acknowledgments
We would like to thank the patients and their families for participation in this study, Robert Wiemann for his assistance with clinical database queries, and Joanne O′Hara for administrative support.
Conflict of interest statement. A.M., L.A., and E.A. are employees and shareholders of Advantagene, Inc.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(17):492–507. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 3.Kreth F-W, Thon N, Simon M et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol. 2013;24(12):3117–3123. [DOI] [PubMed] [Google Scholar]
- 4.Grabowski MM, Recinos PF, Nowacki AS et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 5.Aguilar LK, Guzik BW, Aguilar-Cordova E. Cytotoxic immunotherapy strategies for cancer: mechanisms and clinical development. J Cell Biochem. 2011;112(8):1969–1977. [DOI] [PubMed] [Google Scholar]
- 6.Vile RG, Nelson JA, Castleden S, Chong H, Hart IR. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994;54(23):6228–34. [PubMed] [Google Scholar]
- 7.Vile RG, Castleden S, Marshall J, Camplejohn R, Upton C, Chong H. Generation of an anti-tumour immune response in a non-immunogenic tumour: HSVtk killing in vivo stimulates a mononuclear cell infiltrate and a Th1-like profile of intratumoural cytokine expression. Int J Cancer. 1997;71(2):267–274. [DOI] [PubMed] [Google Scholar]
- 8.Kuriyama S, Kikukawa M, Masui K et al. Cancer gene therapy with HSV-tk/GCV system depends on T-cell-mediated immune responses and causes apoptotic death of tumor cells in vivo. Int J Cancer. 1999;83(3):374–380. [DOI] [PubMed] [Google Scholar]
- 9.Hall SJ, Mutchnik SE, Chen SH, Woo SL, Thompson TC. Adenovirus-mediated herpes simplex virus thymidine kinase gene and ganciclovir therapy leads to systemic activity against spontaneous and induced metastasis in an orthotopic mouse model of prostate cancer. Int J Cancer. 1997;70(2):183–187. [DOI] [PubMed] [Google Scholar]
- 10.Gagandeep S, Brew R, Green B et al. Prodrug-activated gene therapy: involvement of an immunological component in the “bystander effect”. Cancer Gene Ther. 1996;3(2):83–88. [PubMed] [Google Scholar]
- 11.Agard C, Ligeza C, Dupas B et al. Immune-dependent distant bystander effect after adenovirus-mediated suicide gene transfer in a rat model of liver colorectal metastasis. Cancer Gene Ther. 2001;8(2):128–136. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Cruet MJ, Trask TW, Chen SH et al. Adenovirus-mediated gene therapy of experimental gliomas. J Neurosci Res. 1994;39(4):506–511. [DOI] [PubMed] [Google Scholar]
- 13.Predina JD, Judy B, Aliperti LA et al. Neoadjuvant in situ gene-mediated cytotoxic immunotherapy improves postoperative outcomes in novel syngeneic esophageal carcinoma models. Cancer Gene Ther. 2011;18(12):871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rainov NG, Fels C, Droege JW, Schäfer C, Kramm CM, Chou TC. Temozolomide enhances herpes simplex virus thymidine kinase/ganciclovir therapy of malignant glioma. Cancer Gene Ther. 2001;8(9):662–668. [DOI] [PubMed] [Google Scholar]
- 15.Nestler U, Wakimoto H, Siller-Lopez F et al. The combination of adenoviral HSV TK gene therapy and radiation is effective in athymic mouse glioblastoma xenografts without increasing toxic side effects. J Neurooncol. 2004;67(1-2):177–188. [DOI] [PubMed] [Google Scholar]
- 16.Chhikara M, Huang H, Vlachaki MT et al. Enhanced therapeutic effect of HSV-tk + GCV gene therapy and ionizing radiation for prostate cancer. Mol Ther. 2001;3(4):536–542. [DOI] [PubMed] [Google Scholar]
- 17.Fridlender ZG, Sun J, Singhal S et al. Chemotherapy delivered after viral immunogene therapy augments antitumor efficacy via multiple immune-mediated mechanisms. Mol Ther. 2010;18(11):1947–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sukin SW, Chhikara M, Zhu X et al. In vivo surgical resection plus adjuvant gene therapy in the treatment of mammary and prostate cancer. Mol Ther. 2001;3(4):500–506. [DOI] [PubMed] [Google Scholar]
- 19.Predina JD, Kapoor V, Judy BF et al. Cytoreduction surgery reduces systemic myeloid suppressor cell populations and restores intratumoral immunotherapy effectiveness. J Hematol Oncol. 2012;5(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reardon DA, Freeman G, Wu C et al. Immunotherapy advances for glioblastoma. Neuro Oncol. 2014;16(11):1441–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2(i):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fecci PE, Heimberger AB, Sampson JH. Immunotherapy for primary brain tumors: No longer a matter of privilege. Clin cancer Res. 2014;20(22):5620–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguilar LK, Arvizu M, Aguilar-Cordova E, Chiocca EA. The spectrum of vaccine therapies for patients with glioblastoma multiforme. Curr Treat Options Oncol. 2012;13(4):437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andtbacka RHI, Kaufman HL, Collichio F et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol. 2015;33(25):2780–2788. [DOI] [PubMed] [Google Scholar]
- 26.Trask TW, Trask RP, Aguilar-Cordova E et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1(2):195–203. [DOI] [PubMed] [Google Scholar]
- 27.Vlachaki MT, Hernandez-Garcia A, Ittmann M et al. Impact of preimmunization on adenoviral vector expression and toxicity in a subcutaneous mouse cancer model. Mol Ther. 2002;6(3):342–348. [DOI] [PubMed] [Google Scholar]
- 28.Chiocca EA, Aguilar LK, Bell SD et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29(27):3611–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macdonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–80. [DOI] [PubMed] [Google Scholar]
- 30.Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75(5):1151–1161. [DOI] [PubMed] [Google Scholar]
- 31.Zelen M. A new design for randomized clinical trials. N Engl J Med. 1979;300(22):1242–1245. [DOI] [PubMed] [Google Scholar]
- 32.Westphal M, Ylä-Herttuala S, Martin J et al. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(9):823–833. [DOI] [PubMed] [Google Scholar]
- 33.Nawaz M, Fatima F, Zanetti BR et al. Microvesicles in Gliomas and Medulloblastomas An Overview. J Cancer Ther. 2014;5(February):182–191. [Google Scholar]
- 34.Raychaudhuri B, Rayman P, Ireland J et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(6):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7):718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heimberger AB, Sun W, Hussain SF et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol. 2008;10(1):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheeler CJ, Black KL, Liu G et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68(14):5955–5964. [DOI] [PubMed] [Google Scholar]
- 38.Madan RA, Gulley JL, Schlom J et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14(14):4526–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoos A, Eggermont AMM, Janetzki S et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuster J, Lai RK, Recht LD et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol. 2015;17(6):854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampson JH, Heimberger AB, Archer GE et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguilar LK, Shirley LA, Chung VM et al. Gene-mediated cytotoxic immunotherapy as adjuvant to surgery or chemoradiation for pancreatic adenocarcinoma. Cancer Immunol Immunother. 2015;64(6):727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 44.Postow MA, Chesney J, Pavlick AC et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borghaei H, Paz-Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tumeh PC, Harview CL, Yearley JH et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.