Abstract
Background
The combination of galunisertib, a transforming growth factor (TGF)-β receptor (R)1 kinase inhibitor, and lomustine was found to have antitumor activity in murine models of glioblastoma.
Methods
Galunisertib (300 mg/day) was given orally 14 days on/14 days off (intermittent dosing). Lomustine was given as approved. Patients were randomized in a 2:1:1 ratio to galunisertib + lomustine, galunisertib monotherapy, or placebo + lomustine. The primary objective was overall survival (OS); secondary objectives were safety, pharmacokinetics (PKs), and antitumor activity.
Results
One hundred fifty-eight patients were randomized: galunisertib + lomustine (N = 79), galunisertib (N = 39), and placebo + lomustine (N = 40). Baseline characteristics were: male (64.6%), white (75.3%), median age 58 years, ECOG performance status (PS) 1 (63.3%), and primary glioblastoma (93.7%). The PKs of galunisertib were not altered with lomustine, and galunisertib had a median half-life of ∼8 hours. Median OS in months (95% credible interval [CrI]) for galunisertib + lomustine was 6.7 (range: 5.3–8.5), 8.0 (range: 5.7–11.7) for galunisertib alone, and 7.5 (range: 5.6–10.3) for placebo + lomustine. There was no difference in OS for patients treated with galunisertib + lomustine compared with placebo + lomustine [P (HR < 1) = 26%]. Median progression-free survival of ∼2 months was observed in all 3 arms. Among 8 patients with IDH1 mutation, 7 patients were treated with galunisertib (monotherapy or with lomustine); OS ranged from 4 to 17 months. Patients treated with galunisertib alone had fewer drug-related grade 3/4 adverse events (n = 34) compared with lomustine-treated patients (10% vs 26%). Baseline PS, post-discontinuation of bevacizumab, tumor size, and baseline levels of MDC/CCL22 were correlated with OS.
Conclusions
Galunisertib + lomustine failed to demonstrate improved OS relative to placebo + lomustine. Efficacy outcomes were similar in all 3 arms.
Clinical Trial Registration
NCT01582269, ClinicalTrials.gov.
Keywords: antitumor activity, Bayesian design, galunisertib monohydrate (LY2157299), pharmacokinetics, Phase II randomized study, safety
Glioblastoma is the most common brain cancer in adults; despite aggressive treatment with surgery and chemoradiation, the median survival remains approximately 12–15 months from initial diagnosis.1 Genetic mutations and alterations have been associated with growth of malignant glioma, tumor cell motility, angiogenesis, epithelial-mesenchymal transition, and escape from immune surveillance.2 Transforming growth factor-beta (TGF-β) signaling appears to be connected with some of the recognized genetic mutations in glioma, in particular those related to neuronal and stem cell development.3–7
In addition to a dysregulation of TGF-β signaling in the tumor tissue or its surrounding microenvironment, elevated TGF-β ligand levels have been reported in glioblastoma patients.8 Elevated plasma TGF-β1 levels are also hypothesized to be associated with increased numbers of regulatory T cells in patients with glioblastoma.9
Based on these biological observations, the small molecule inhibitors of the TGF-β signaling pathway SD-208 and SX-007 were developed and demonstrated antitumor effect in syngeneic models such as SMA-560 gliomas in VM/Dk mice.10,11
Consistent with these observations, the small molecule inhibitor galunisertib (LY2157299 monohydrate) showed antitumor effect in glioblastoma animal models.5,6 In particular, glioma-initiating cells expressing CD44high/Id1high were reduced not only in animal models but also in one patient who participated in the first-in-human dose (FHD) study with monotherapy galunisertib.12 Furthermore, patients who progressed after their first- and second-line treatments for glioblastoma showed partial response (PR) and complete response (CR) in 16.6% while on galunisertib monotherapy.13 Preclinical studies suggested that the combination of lomustine and galunisertib may have synergistic or at least additive antitumor effects.14 In the FHD study, an additional cohort of patients received the combination of lomustine and galunisertib. In this cohort, a CR/PR rate of 7.7% was observed; stable disease (SD) was seen in 15.4% of patients who received > 6 cycles of treatment. The combined clinical benefit as defined by CR/PR/SD ≥ 6 cycles was comparable to the monotherapy of galunisertib (approximately 20%). Given these observations of preclinical and clinical antitumor activity, the present Phase II study was initiated to evaluate the overall survival (OS), safety, pharmacokinetics (PKs), and biomarker activity of galunisertib in combination with lomustine in patients with recurrent glioblastoma.
Methods
Experimental Design
This was a multinational (Supplemental Table 1), 3-arm, randomized Bayesian augmented control Phase II study of galunisertib + lomustine or galunisertib monotherapy compared with lomustine + placebo in patients with relapsed or progressed glioblastoma (NCT01582269).
Patients
Patients ≥ 18 years of age eligible for enrollment were diagnosed with recurrent intracranial glioblastoma (WHO grade IV) confirmed by histological evaluation. All patients had Eastern Cooperative Oncology Group performance status (ECOG PS) ≤1. Patients must have had evidence of tumor progression as determined by Response Assessment in Neuro Oncology (RANO) criteria following at least 12 weeks after the end of standard chemoradiotherapy.15,16 Patients were eligible for enrollment (i) after completing one prior regimen (all patients were considered at first relapse); (ii) were required to have adequate hematologic, hepatic, and renal function; and (iii) had discontinued all previous therapies including chemotherapy (excluding palliative care for cancer) at least 4 weeks prior to study enrollment. Exclusion criteria included medically uncontrolled cardiovascular illness, medically significant electrocardiogram abnormalities, and serious pre-existing medical conditions. Patients were excluded if they were enrolled in a clinical trial investigating galunisertib and/or vascular endothelial growth factor receptor (VEGFR) inhibitors. Patients were also excluded if they had prior treatment for glioblastoma with nitrosourea (lomustine) and/or bevacizumab.
The study was conducted according to the principles of good clinical practice, applicable laws and regulations, and the Declaration of Helsinki. Each institution's review board approved the study, and all participants signed an informed consent document before study participation.
Treatment: Dose and Dose Levels
One cycle of treatment was defined as 28 days in all treatment arms. This study adopted intermittent treatment as described in the FHD study based on a PK/pharmacodynamic (PD) model.17 Participants were treated orally with galunisertib 300 mg/day (150-mg tablets twice a day, morning and evening) for 14 days followed by 14 days off in a 28-day cycle. The first dose of lomustine was given as 100 mg/m2 after 7 days of galunisertib treatment and thereafter (at the discretion of the investigator) was given orally once every 6 weeks at 100–130 mg/m2 (See Supplemental Fig. 1). Galunisertib had not yet been evaluated to determine whether food intake may change the PK profile; therefore, participants were instructed to take galunisertib on an empty stomach and to wait at least one hour (preferably 2 hours) after taking galunisertib before eating a meal.
Randomization and Blinding
Participants were randomized with a 2:1:1 allocation ratio to galunisertib + lomustine, galunisertib, or placebo + lomustine. Randomization used a dynamic allocation method18 to minimize imbalance according to the following factors: baseline ECOG PS (0,1), age (≤60 y, >60 y), and type of glioblastoma (primary or secondary at study entry). Participants and investigators were blinded to the galunisertib or placebo assignment for those patients who received lomustine.
Sample Size Determination
The primary objective was to compare OS between galunisertib + lomustine (combination arm) and placebo + lomustine (control arm) using a Bayesian augmented control design (See Supplemental Information TEXT 1).19,20
The planned enrollment was 155 participants with the final analysis to occur after 135 events had been observed, which would result in 80% power for a true OS hazard ratio (HR) of 0.667 with approximately a 14% type 1 error. Additional information on the Bayesian statistical methodology, along with information on the 3 planned interim analyses, are provided in Supplemental Information TEXT 1.21
Bioanalytical Methods
Pharmacokinetic Methods
Human plasma samples obtained during this study were analyzed for galunisertib using validated LC-MS/MS methods (BPLY215A and BPLY215B). The lower limit of quantification was 0.050 ng/mL, and the upper limit of quantification was 10.000 ng/mL for BPLY215A. The lower limit of quantification was 5.000 ng/mL, and the upper limit of quantification was 1 000.000 ng/mL for BPLY215B. Additional information on the PK methods, assay accuracy, and assay precision are provided in Supplemental Information TEXT 2.
Biomarker and Pharmacodynamic Methods
Tumor tissue samples were evaluated for the presence of the isocitrate dehyrogenase (IDH1) (R132H) mutation and T-cell infiltrates as determined by CD3 immunohistochemistry staining. The process of evaluation was based on the standard immunohistochemistry staining developed at the Neuropathology Department of the University Clinic of Heidelberg, Germany.22
Plasma samples from participants were analyzed for TGF-β1 levels by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, DB100B). Platelet factor 4 levels were also assessed to determine possible platelet activation, although it was expected that glioma patients will have elevated platelet factor 4 levels as part of their tumor-associated intravascular coagulopathy. Based on the observation from the FHD study, chemokine (c-c motif) ligand-22/macrophage-derived chemokine (CCL22/MDC) levels were determined at baseline using the multi-analyte immunoassay panel developed by Myriad/RBM.23 Whole blood samples were used to determine the levels of T cell subsets such as CD4+ and CD8+, CD4+CD25+CD127−/LOFOXP3+ by standard flow cytometry. In addition, percentage of FOXP3 and CD3 were determined in whole blood using an epigenetic T cell assay (Epiontis).24 All of these markers are associated with TGF-β-dependent immune regulation and have been previously associated with supporting tumor growth in glioblastoma.
Statistical Analysis
Safety
Safety was evaluated in participants who received at least one dose of study medication and was based on summaries of adverse events (AEs) (Common Terminology Criteria of Adverse Events [CTCAE] version 4.0), possible relatedness to study drug, dose-limiting toxicities, laboratory changes (including monthly brain natriuretic peptide and troponin I levels), changes in ECOG PS, electrocardiogram, and echocardiography/Doppler (every 2 cycles and reviewed by a central cardiologist). In addition, standard chemistry, hematology, and urinalysis panels were performed. All concomitant medications were documented throughout the patient's participation in the study.
Efficacy
The primary efficacy endpoint was OS, analyzed using a Bayesian exponential-likelihood model. Secondary efficacy analyses of OS and progression-free survival (PFS) used Kaplan-Meier estimates and the log-rank test. Overall response rates by RANO criteria were calculated by dividing the total number of responders (CR or PR) by the number of randomized patients with 95% confidence intervals (CIs). Imaging was performed, as recommended by RANO criteria, every 2 months. In exploratory analyses, changes in tumor size were evaluated for each participant with measureable lesions and the fold change from baseline at cycle 2 displayed visually.
Potential clinical and laboratory prognostic factors as measured at baseline were evaluated for their impact on OS. Clinical characteristics included the factors used in randomization and tumor location (frontal, other), baseline tumor burden, time since diagnosis, sex, and prior surgical resection (complete, partial/none). Whether the participant received bevacizumab post discontinuation therapy was also included as a covariate. Laboratory units for blood CD3+, FOX P3, CD4+, CD8+, CD4+CD25+CD127−/LOFOX P3+, neutrophils, lymphocytes, monocytes and eosinophils, plasma MDC/CCL22 and TGF-β1, and serum S100β, LDH and C-Reactive Protein (high sensitivity) parameters are indicated on tables/figures as percentage, cells/μL, GI/L, U/L, pg/mL. Continuous variables and laboratory parameters were split into 2 groups at the median for high versus low comparison. Univariate Cox models were first used to select covariates with P ≤ .05. For these covariates, a multivariate Cox model was used to make stepwise selection with both entry and exit P = .05 in order to identify independent prognostic factors.
Pharmacokinetic Analyses
Plasma samples collected from participants who received galunisertib in monotherapy and in combination treatment were first graphically analyzed and then pooled for analyses using nonlinear mixed-effect modeling (NONMEM, version 7.3). The analysis was performed to characterize the PKs of galunisertib and to identify demographic patient factors that may influence galunisertib disposition in patients with relapsed or progressive glioblastoma.
Pharmacodynamic Methods
Tumor tissue biomarker analysis was performed to identify a potential predictive biomarker using a multi-marker approach.25
Health Outcomes
Patient-reported symptoms were assessed using the MD Anderson Symptom Inventory-Brain Tumor Module (MDASI-BT), and neurocognitive function was assessed using the Hopkins Verbal Learning Test-Revised (HVLT-R), Trail Making Test, and the Controlled Oral Word Association (COWA) test for each treatment arm. Each neurocognitive test was converted to a standardized score using published healthy control data26–31 and summarized descriptively.
Results
Patient Disposition and Characteristics
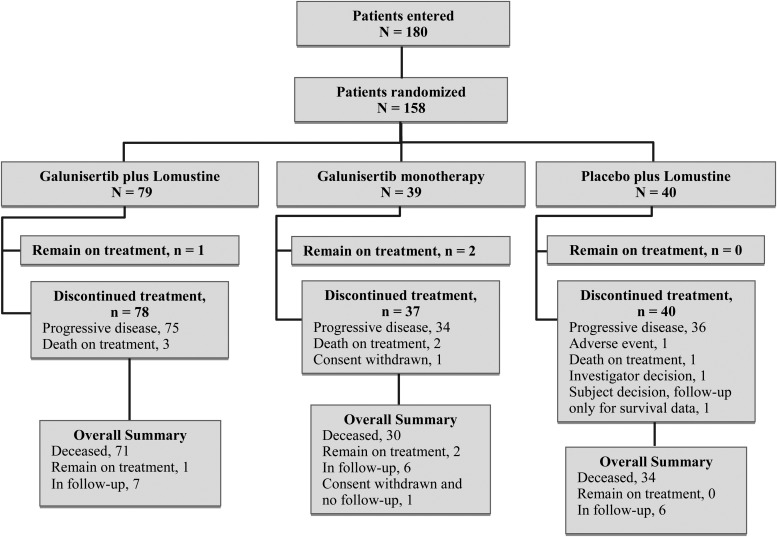
This Phase II study was conducted between May 2012 and July 2014 at 34 centers in 10 countries (See Supplemental Table 1). Figure 1 shows a flow chart of patient disposition. Briefly, 158 eligible patients were randomized to 3 treatment arms: galunisertib + lomustine (N = 79), galunisertib monotherapy (N = 39), and placebo + lomustine (N = 40).
Fig. 1.
Patient disposition: 180 patients were entered into the study, and 158 were randomized to 3 arms in a 2:1:1 ratio.
The participant baseline demographic and disease characteristics are shown in Table 1. At baseline, most participants were male (64.6%) and white (75.3%) with a median age of 58.0 (23–74) years. Most participants (93.7%) had primary glioblastoma, and all participants (100%) were WHO grade IV at study entry, with ECOG PS of 0–1 (99.4%). All participants (100%) in each treatment arm had received prior therapy. The randomization was successful in ensuring good balance across the treatment groups for major prognostic factors of age, ECOG PS, and glioblastoma type (primary/secondary).
Table 1.
Patient baseline characteristics
| Characteristics | Galunisertib + Lomustine N = 79 | Galunisertib N = 39 | Placebo + Lomustine N = 40 |
|---|---|---|---|
| Mean age, years (SD) | 57.5 (9.3) | 56.6 (10.9) | 56.9 (10.2) |
| Age group, n (%) | |||
| ≤60 y | 45 (57.0) | 23 (59.0) | 22 (55.0) |
| >60 y | 34 (43.0) | 16 (41.0) | 18 (45.0) |
| Sex, n (%) | |||
| Male | 58 (73.4) | 21 (53.8) | 23 (57.5) |
| Race, n (%) | |||
| White | 60 (75.9) | 30 (76.9) | 29 (72.5) |
| Asian | 1 (1.3) | 2 (5.1) | 1 (2.5) |
| Black or African American | 2 (2.5) | 0 (0.0) | 0 (0.0) |
| Missing | 16 (20.3) | 7 (17.9) | 10 (25.0) |
| ECOG PS, n (%) | |||
| 0 | 28 (35.4) | 15 (38.5) | 14 (35.0) |
| 1 | 51 (64.6) | 24 (61.5) | 25 (62.5) |
| Glioblastoma, n (%) | |||
| Primary | 74 (93.7) | 36 (92.3) | 38 (95.0) |
| Secondary | 5 (6.3) | 3 (7.7) | 2 (5.0) |
| Basis for initial pathological diagnosis, n (%) | |||
| Histopathologicala | 78 (98.7) | 38 (97.4) | 40 (100.0) |
| Initial histopathological grade, n (%) | |||
| WHO grade IV | 74 (93.7) | 35 (89.7) | 38 (95.0) |
| Study entry histopathological grade, n (%) | |||
| WHO grade IV | 79 (100) | 39 (100) | 40 (100) |
| Study entry diagnosis, n (%) | |||
| Glioblastoma | 78 (98.7) | 37 (94.9) | 37 (92.5) |
| Giant cell glioblastoma | 1 (2.5) | ||
| Gliosarcoma | 1 (1.3) | 1 (2.6) | 2 (5.0) |
| Other | 1 (2.6) | ||
| Prior therapy, n (%) | |||
| Radiotherapy | 79 (100.0) | 39 (100.0) | 40 (100.0) |
| Systemic therapy | 79 (100.0) | 39 (100.0) | 40 (100.0) |
| One regimen | 79 (100.0) | 39 (100.0) | 39 (97.5) |
| Median MDC, pg/mL (range) | 245 (24, 920) | 207 (31, 879) | 260 (59, 1220) |
| IDH1 R132H, n(%) | |||
| Positive | 3 (3.8) | 4 (10.3) | 1 (2.5) |
| Negative | 55 (69.6) | 28 (71.8) | 29 (72.5) |
| Not evaluable/Not done | 21 (26.6) | 7 (17.9) | 10 (25.0) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, Performance Status; SD, standard deviation; WHO, World Health Organization.
aTwo patients did not have initial pathologic diagnosis reported to be based on histopathology. This was due to missing information for one patient and cytological diagnosis for the other patient. However, at the time of study enrollment and prior to treatment, all patients had histopathologically diagnosed glioblastoma.
At the time of database lock, 135 participants had died, 19 had discontinued treatment and remained in follow-up for OS, one had discontinued the study with no follow-up for OS, and 3 remained on treatment. The most common reason for treatment discontinuation was progressive disease (n = 145). The median number of cycles of galunisertib was 2.0 (range: 1–19 cycles) for the galunisertib + lomustine arm and 3.0 (range: 1–23 cycles) in the galunisertib monotherapy arm. The median number of doses of lomustine was 2.0 (range: 1–8 doses) in the galunisertib + lomustine arm and 2.0 (range: 1–6 doses) in the placebo + lomustine arm.
Efficacy Outcomes
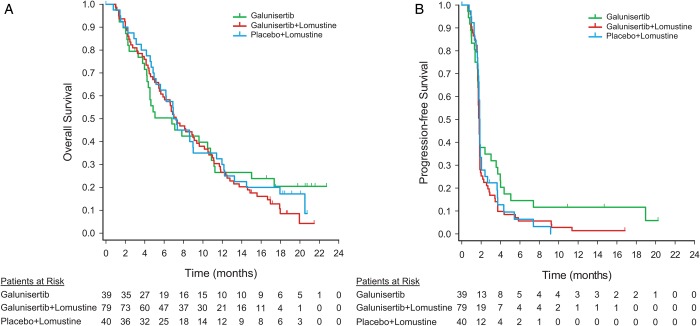
The primary outcome measure was not met in this study, and the posterior probability for detecting a HR of <1 for OS of the combination arm versus the control arm was 0.262. Two analyses of OS were conducted. In the primary Bayesian analysis, the median OS in months (95% CrI) for galunisertib + lomustine was 6.7 (5.3–8.5), [HR (galunisertib + lomustine):(placebo + lomustine) 1.13 (0.78–1.65)], 8.0 (5.7–11.7) for galunisertib alone [HR (galunisertib):(placebo + lomustine) 0.93 (0.58–1.49)], 7.5 (5.6–10.3) for placebo + lomustine (Table 2). In the secondary Kaplan-Meier analyses, the median OS was 7.2 months (95% CI: 5.7–9.4) for galunisertib + lomustine arm, 6.8 (95% CI: 4.2–10.8) for galunisertib monotherapy, and 7.1 (95% CI: 5.2–9.0) for the placebo + lomustine (Fig. 2A).
Table 2.
Primary endpoint: overall survival using Bayesian analysis
| Galunisertib + Lomustine N = 79 | Galunisertib N = 39 | Placebo + Lomustine N = 40 | Primary Comparison: Galunisertib + Lomustine vs Placebo + Lomustine | |
|---|---|---|---|---|
| Deaths | 71 | 30 | 34 | |
| Censoring rate (%) | 10.1 | 23.1 | 15.0 | |
| OS (months), median (95% CrI)a | 6.7 (5.3, 8.5) | 8.0 (5.7, 11.7) | 7.5 (5.6, 10.3) | |
| Hazard ratio (95% CrI) | 1.13 (0.78, 1.65) | |||
| Posterior probability P (HR < 1) | 0.26 |
a 95% CrI = 95% Credible Interval. The denominator for response rates is the number of randomized patients per arm. All randomized patients had measureable disease at baseline.
Fig. 2.
Efficacy endpoints: (A) Kaplan-Meier curve for overall survival; (B) Kaplan-Meier curve for progression-free survival (based on investigator review). Patients at risk denote the number of patients who were event-free at the beginning of the period.
The secondary efficacy outcome measures included PFS, response rates, and change in tumor size. The median PFS was 1.8 months (95% CI: 1.7–1.8) for galunisertib + lomustine, 1.8 months (95% CI: 1.6–3.0) for galunisertib monotherapy, and 1.9 months (95% CI: 1.7–1.9) for placebo + lomustine (Fig. 2B). Based on Kaplan-Meier estimates at 6 months, the probability of being progression-free was 0.06 (95% CI: 0.02–0.13) for galunisertib + lomustine, 0.15 (95% CI: 0.05–0.28) for galunisertib monotherapy, and 0.06 (95% CI: 0.01–0.18) for the placebo + lomustine arm.
Table 3 provides response rates across the 3 treatment arms. One patient (1.3%) had a CR in the galunisertib + lomustine arm, and 2 patients (5.1%) had PR in the galunisertib monotherapy arm. Clinical responses were noted in 21.5% of participants in the combination therapy arm (galunisertib + lomustine), 30.8% in monotherapy arm (galunisertib), and 30.0% of participants in the control arm (placebo + lomustine).
Table 3.
Best overall response per RANO criteria
| Best Overall Response n (%) | Galunisertib + Lomustine N = 79 | Galunisertib N = 39 | Placebo + Lomustine N = 40 |
|---|---|---|---|
| Complete response (CR) | 1 (1.3) | 0 | 0 |
| Partial response (PR) | 0 | 2 (5.1) | 0 |
| Stable disease (SD) | 16 (20.3) | 10 (25.6) | 12 (30.0) |
| Progressive disease (PD) | 50 (63.3) | 21 (53.8) | 26 (65.0) |
| Unevaluable/no lesion measures post baseline | 12 (15.2) | 6 (15.4) | 2 (5.0) |
| Number of clinical benefit responders (CR + PR + SD) | 17 (21.5) | 12 (30.8) | 12 (30.0) |
Abbreviation: RANO, Response Assessment in Neuro-oncology.
The denominator for response rates is the number of randomized patients per arm. All randomized patients had measureable disease at baseline.
A waterfall plot illustrating the change in tumor size for all participants in the 3 treatment groups at cycle 2 is represented in Supplemental Fig. 2.
In univariate analysis of baseline clinical characteristics, ECOG PS (0), sum of target tumor size (small), and time since initial diagnosis (long) were prognostic factors for improved OS (See Supplemental Fig. 3A). In addition, a significant improvement in OS was observed for participants who received bevacizumab post discontinuation of therapy (42% of participants in the galunisertib + lomustine arm, 36% in the galunisertib monotherapy arm, and 40% in the placebo + lomustine arm) (See Supplemental Fig. 3A). However, sex, tumor location, glioblastoma type, age group, and extent of prior surgery resection were not found to be significant prognostic markers for OS in this study. In univariate analysis of baseline laboratory parameters, high baseline values of CD3+ (%), FOX P3 (%), MDC (pg/mL), CD4+CD25+CD127−/LOFOX P3+(cells/μL), and eosinophils (GI/L) were found to be prognostic for improved OS, with high baseline neutrophils (GI/L) being associated with poorer OS (See Supplemental Fig. 3B). We also observed that about half of the participants had lymphocyte counts in the low reference ranges. Interestingly, we detected correlations between certain laboratory immune markers (See Supplemental Fig. 3C). For example, CD3% was correlated with lymphocyte counts or FOXP3%. The chemokine MDC/CCL22 was correlated with CD3%, FOXP3%, CD4+CD25+CD127−/LOFOXP3+, and eosinophils. By contrast, MDC levels were negatively correlated with neutrophil counts or the neutrophil/lymphocyte ratio (See Supplemental Fig. 3C). Overall, this suggested that MDC may represent an important factor for immune cells, including regulatory T cells.
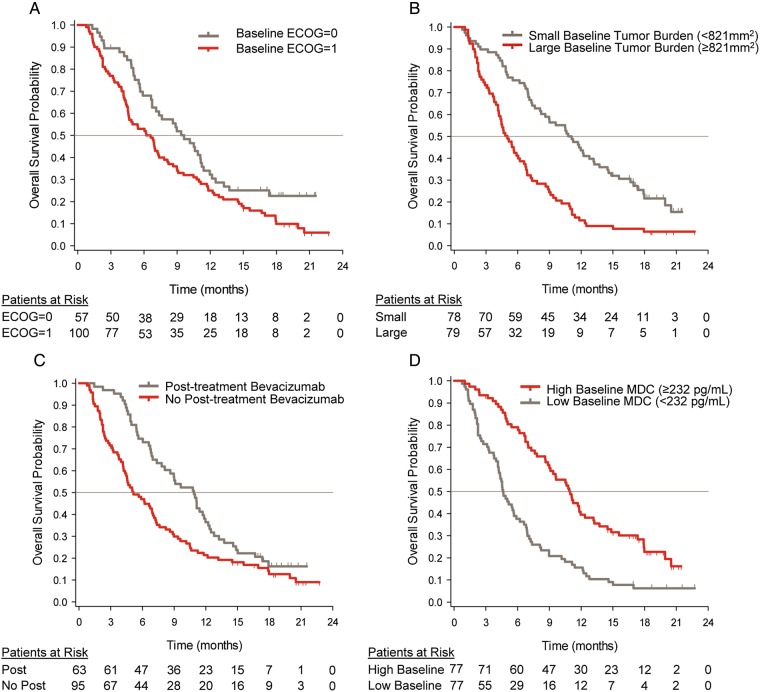
To determine whether interdependence existed between the single variates, an analysis using multivariate stepwise regression hazards models was performed. One hundred and seventeen participants had evaluable data for the identified prognostic factors and were included in the analysis. ECOG PS = 0, receiving bevacizumab post discontinuation therapy, small baseline tumor burden, and high baseline MDC were independent prognostic factors for OS. The continuous markers of tumor burden and MDC were cut into 2 groups at the median. The median baseline tumor burden was 821 mm2, thus baseline tumor burden (low) indicates baseline tumor burden < 821 mm2. The median baseline MDC was 232 pg/mL (range: 24–1220), thus baseline MDC (high) indicates baseline MDC ≥ 232 pg/mL. Adjustment for the significant prognostic factors in Cox proportional hazards models for OS did not alter the study conclusions regarding the primary outcome measure.
Median OS was 11.0 months for participants with baseline concentrations above the median compared with 4.6 months for participants with baseline concentrations below the median, log-rank P value < .0001. (Fig. 3).
Fig. 3.
Kaplan-Meier curves for overall survival by baseline prognostic factors. (A) ECOG PS; (B) tumor burden; (C) bevacizumab; (D) MDC levels. “Patients at risk” denote the number of patients who were event-free at the beginning of the period. Continuous factors (baseline tumor burden and baseline MDC levels) were split into 2 groups at the median. The median baseline tumor burden was 821 mm2, and the median baseline MDC was 232 pg/mL.
TGF-β1 levels at baseline were not found to be a significant prognostic marker for OS. The median level of TGF-β1 in plasma at baseline was 2225 pg/mL (range: 25–28203 pg/mL). Based on previous observations in treating HCC patients with galunisertib,32 we used the cutoff of 3400 pg/mL. The number of participants with baseline TGF-β1 > 3400 pg/mL was 18 (22.8%) galunisertib + lomustine, 9 (23.1%) galunisertib monotherapy, and 11 (27.5%) for the placebo + lomustine arm. Median OS (95% CI) in the subset of participants with baseline TGF-β1 > 3400 was 7.0 months (3.9, 10.5), 11.2 months (1.8, not calculated), and 4.9 months (2.3, 11.4) for each treatment arm, respectively. Median OS (95% CI) in the subset of participants with baseline TGF-β1 ≤ 3400 was 7.2 months (5.7, 10.6), 4.7 months (3.8, 10.8), and 7.2 months (5.6, 12.0) for each treatment arm, respectively.
Because of previous observations,23 we evaluated the OS in participants with IDH1 mutation. A total of 8 tumors were identified as IDH1 R132H-positive among the 3 treatments arms: 4 in galunisertib monotherapy (range of OS: 4–17 mo), 3 in galunisertib + lomustine (range of OS: 10–17 mo), and 1 in placebo + lomustine (OS: 3 mo). Three of the 7 participants treated with galunisertib or galunisertib + lomustine had SD, and another had PR.
Looking across other markers in tumor tissue or plasma, no strong predictive markers were identified with any of the treatment arms.
Safety Outcomes
All participants were evaluated for safety and tolerability. A total of 144 (91.1%) of the 158 participants experienced at least one treatment-emergent adverse event (TEAE) during this study, defined as an AE that first occurred or worsened in severity after starting treatment. There were 91.0% in the galunisertib + lomustine arm, 92.5% in the galunisertib monotherapy arm, and 89.7% in the placebo + lomustine arm. A total of 95 participants (60.1%) experienced treatment-related TEAEs, defined as TEAEs judged by the investigator to be possibly related to the study drug. There were 59.0% treatment-related TEAEs in the galunisertib + lomustine arm, 57.0% in the galunisertib monotherapy arm, and 66.7% in the placebo + lomustine group. Overall, 86 participants (54.4%) had grade 3 or 4 events; only 34 participants (21.5%) had grade 3 or 4 events that were considered by the investigator to be related to study drug. Prevalence of drug-related grade 3/4 AEs was 10% in the galunisertib alone arm, compared with 26% each for the galunisertib + lomustine and the placebo + lomustine arms.
Drug-related TEAEs occurring in ≥ 10% of the participants in any treatment arm are presented in Table 4. Most drug-related TEAEs were of mild or moderate severity. The most common study drug-related grade 3 or 4 events were hematologic events and fatigue (Table 4). Drug-related TEAEs occurring in ≥ 10% of the participants by each CTCAE grade are presented in Supplemental Table 2. Most laboratory and nonlaboratory events were grades 1 and 2.
Table 4.
Study drug-related treatment-emergent adverse events by common terminology criteria for adverse events term
| Event | Galunisertib + Lomustine N = 78 | Galunisertib N = 40 | Placebo + Lomustine N = 39 |
|---|---|---|---|
| TEAEs in ≥ 10% of Patients | |||
| Anemia | 2 (2.5) | 0 (0.0) | 4 (10.0) |
| Vomiting | 7 (8.9) | 4 (10.3) | 5 (12.5) |
| Nausea | 6 (7.6) | 5 (12.8) | 4 (10.0) |
| Diarrhea | 3 (3.8) | 2 (5.1) | 5 (12.5) |
| Fatigue | 13 (16.5) | 5 (12.8) | 10 (25.0) |
| Platelet count decreased | 22 (27.8) | 2 (5.1) | 15 (37.5) |
| Neutrophil count decreased | 10 (12.7) | 0 (0.0) | 6 (15.0) |
| Lymphocyte count decreased | 9 (11.4) | 1 (2.6) | 2 (5.0) |
| White blood cell count decreased | 7 (8.9) | 0 (0.0) | 5 (12.5) |
| TEAEs by CTCAE Grade 3/4 | |||
| Fatigue | 3 (3.8) | 1 (2.6) | 1 (2.5) |
| Platelet count decreased | 6 (7.6) | 0 (0.0) | 5 (12.5) |
| Neutrophil count decreased | 6 (7.6) | 0 (0.0) | 2 (5.0) |
| Lymphocyte count decreased | 7 (8.9) | 1 (2.6) | 0 (0.0) |
| White blood cell count decreased | 4 (5.1) | 0 (0.0) | 3 (7.5) |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; TEAE, treatment-emergent adverse event.
Six participants died while on study treatment (3 from disease progression and 3 from AEs not related to study treatment). There were 67 (42.4%) participants who experienced SAEs; 11 participants (7.0%) experienced SAEs that were related to study treatment.
No cardiac toxicity was observed.
Pharmacokinetics
Galunisertib concentrations were determined from all evaluable participants (n = 114) in both the galunisertib monotherapy arm and the combination arm (galunisertib + lomustine). The observed galunisertib plasma concentration by study arms (monotherapy vs combination with lomustine) on day 1 and at steady state (day 14) were comparable (plots not shown). There were also comparable concentrations across study arms (See Supplemental Fig. 4) and dosing days. These suggest no galunisertib exposure alteration in the presence of lomustine as well as time-linear PKs within the studied dose range of 150 mg twice daily.
Galunisertib was rapidly absorbed into the systemic circulation and typically reached maximum concentrations within 1 hour, with mean population clearance of galunisertib of 38 L/h and steady-state volume of distribution (Vss) of 175 L. Using all available PK data from this study (114 participants, 796 concentration observations), the between-patient variance was estimated to be 36% on the population apparent clearance. None of the demographic patient characteristics (specifically age, sex, weight, body mass index, smoking, and alcohol behaviors) were found to be significant for inclusion in the final population PK model of galunisertib based on the data from 114 participants. Specifically, the observed median age (minimum-maximum ranges) was 58.5 years (23–74), weight on enrollment 80 kg (39–126), and body mass index of 22.9 kg/m2 (12.6–34.3). Alcohol consumption of all participants was 27.2%, no alcohol consumption 71.9%, and 0.9% unknown. The percentage of participants who smoked was 36.8%, unknown 0.9%, and no smoking 62.3%. Exploratory plots (not shown) and covariate search analysis confirmed that these covariates did not significantly influence any of the PK parameters.
A visual predictive check was performed to ensure that the model was predicting the individual concentration time profiles, PK parameters, and exposure estimates reasonably well. Supplemental Fig. 4 shows the observed concentration data plotted with the median, 5th, 20th, 80th, and 95th percentile prediction intervals at steady state (day 14) following a 150-mg twice-daily dose.
Health Outcomes
There were no notable differences among the 3 treatment arms in regard to the health outcomes including the neurocognitive tests and MDASI-BT module (See Supplemental Fig. 5).
Discussion
Consistent with the FHD study,13 participants treated with galunisertib alone had fewer hematologic toxicities (eg, reduction in platelet, white blood, and lymphocyte counts) compared with participants receiving either lomustine alone or the combination with lomustine. However, the additive or synergistic antitumor effect of the combination of lomustine and galunisertib seen in preclinical models did not translate into increased survival for participants. Although in vitro and animal in vivo data suggested a potential synergistic antitumor effect of combining galunisertib with lomustine,14 the median OS of 7 months for this combination was similar to either lomustine or galunisertib alone.
In order to increase the statistical power and the exposure of patients to the novel therapies, we used historic information to augment the control by using a Bayesian design (see Supplemental Information TEXT 1).19,20 To utilize such historical information, all participants in this study were required to have had only one prior treatment with standard chemoradiation, no prior bevacizumab, and be at first relapse.
Outcomes of the lomustine control arm in this study were comparable to historical data, with median OS of 7.1 months compared with 7.1 months and 9.8 months in the 2 prior studies19,20; most recent lomustine data in a similar study population21 reported median OS of 8.6 months and median PFS of 1.5 months, which were comparable to the findings in this study.
The median OS was 2 months less compared with patients treated with bevacizumab in second-line therapy.33 While participants who were able to receive bevacizumab after they progressed on this study had a better outcome, the post-study treatment use of bevacizumab was equally distributed across the 3 arms and was administered to approximately 40% of all participants.
The PFS and response rates for all 3 treatment arms in this current study were similar, indicating rapid progression for most of the glioblastoma participants in this study. PFS rates at 6 months for the control arm were 6% in this study, compared with 19% and 24% in the 2 historical studies.19,20 Overall response rates for the lomustine control arm were 0% in this current study compared with 9% and 4% in the 2 previous studies.19,20
Despite OS, PFS, and response rates being similar across all 3 treatment arms in the current study, it is perhaps noteworthy that participants on galunisertib monotherapy had a higher censoring rate for OS (23%) compared with the other 2 arms with lomustine (10% and 15%, respectively). Perhaps there is a small subset of patients who benefit from galunisertib monotherapy because the monotherapy is able to preserve important T cell subsets.
Another factor in this study was the novelty of this agent for most investigators and the difficulty finding patients whose tumors were progressing more slowly. As observed in the FHD study,13 responses to galunisertib tended to occur later in the cycles after the tumor size of patients stabilized. Because of its presumed mechanism of action in reverting epithelial-mesenchymal transition and influencing immune responses,34 it was recommended to include patients with slowly progressing tumors, as they are often observed in secondary glioblastoma. However, the selection of patients with slowly progressing glioblastoma is difficult, and there are no objective criteria that can help identify such patients. Perhaps one such group includes patients with secondary glioblastoma or with IDH1 mutations.
Previous observations have suggested that IDH1-mutated glioblastoma may respond to galunisertib;23 however, this mutation was present in only 8 of the 120 participants with evaluable tumor tissue. Unfortunately, the remainder of participants could not be assessed for IDH1 mutation due to insufficient tissue for analysis, which may have potentially increased the sample size for this subgroup and impacted the results. While such numbers did not allow a helpful comparison across the arms, for the 7 participants who received galunisertib either as monotherapy or in combination with lomustine, the median OS was approximately 11 months (range: 4–17 mo). Similarly, we also had a small number of participants with secondary glioblastoma (6%) in this study. This low number of either glioblastoma with IDH1 mutation or secondary glioblastoma contrasts with the patient population enrolled in the FHD study, in which 20% of participants had secondary glioblastoma in the monotherapy arm.13 Hence, exposure to galunisertib was short and perhaps may have reduced the opportunity for exerting its activity on rapidly growing tumors.
Galunisertib plasma PKs in both monotherapy and combination arms were similar to those in the FHD studies,13,17 with median (20th–80th percentiles) steady-state exposure of 4560 (2650–7570) ng*h/mL and within the predefined therapeutic window. We also obtained 6 cerebrospinal fluid (CSF) samples from participants. The measured galunisertib concentrations varied from approximately 12 to 208 ng/mL, well above the lower quantitation limit of 0.1 ng/mL. Although patients with glioblastoma often have a disrupted blood-brain-barrier, these data suggest that galunisertib can be detected in the CSF and perhaps crosses the blood-brain-barrier.
As expected, high plasma LDH,35 S100β,36 and neutrophil counts37 were associated with poor outcome. Improved prognosis was associated with post-discontinuation bevacizumab therapy, an ECOG PS of zero, small tumors, high baseline levels of MDC, high baseline eosinophil counts, high CD4+CD25+CD127−/LOFOXP3+, and high percentage in CD3+ and FOX P3 as detected by an epigenetic T cell assay. Based on the multivariate analysis, the chemokine MDC appeared to be an independent prognostic factor. High plasma levels of MDC were associated with better OS in the FHD study,23 and this study confirms this observation. The key role of MDC and OS was recently reported for a cohort of glioblastoma patients compared with subjects without cancer and should be considered in future studies as a potential prognostic marker.38 Patients with a phenotype of regulatory T cells (ie, high CD4+CD25+CD127−/LOFOXP3+ and MDC levels) appear to have longer OS, which contradicts the general view that regulatory T cells are responsible for downregulating immune responses in glioma and are thus associated with poor OS.39 However, the functional role of these regulatory T cells was not assessed, and thus we do not know whether they have antitumor activity. We also did not determine whether these CD4+ T cells are Th1- or Th2-like. Based on the high counts of eosinophils in this study and the role of MDC in inducing Th2-like CD4+ T cells,40 it is likely that most of the CD4+ T cells are Th2-like.41 While such Th2-like T cells are known to induce PD-L2 in inflammatory macrophages,42 their role in aiding escape of immune surveillance in glioma is still under investigation.43
In summary, galunisertib + lomustine failed to demonstrate improved OS relative to placebo + lomustine. We observed that galunisertib monotherapy had a similar median OS as lomustine with lesser toxicity. Safety, in particular cardiovascular safety, was unremarkable. The PK profile of galunisertib was unchanged across both treatment arms and comparable to previous PK assessments. Health outcome analyses were performed for neurocognitive function and the MDASI-BT questionnaire. There were no notable differences among the 3 treatment arms for either outcome measurement.
Financial Disclosures
Dr. Brandes has received traveling grants for ASCO, SNO, and ESMO meetings from Eli Lilly and Company, Roche, and Pfizer. Dr. Wick has received research grants from Apogenix, Boehringer Ingelheim, Eli Lilly and Company, Immatics, MSD, and Roche as well as honoraria for lectures or advisory board participation from MSD and Roche. He is or has been the coordinating investigator for sponsored clinical trials evaluating APG101 (Apogenix), bevacizumab (Roche), galunisertib (Eli Lilly and Company), temozolomide (MSD), and temsirolimus (Pfizer). Dr. Steinbach has received a grant from Merck as well as honoraria for lectures, travel, or advisory board participation from Roche, Medac, and Mundipharma. Dr. Carpentier has served as a consultant for Roche. Drs. Capper, Cher, Chinot, Kesari, Rodon, Sepúlveda-Sanchez, Specenier, and Wheeler have no financial disclosures.
Drs. Gueorguieva, Desaiah, and Guba and Ms. Cleverly, Ms. Smith, and Mr. Miles are employees of Eli Lilly and Company (Indianapolis, Indiana) and may hold company stock. Dr. Lahn is a former employee of Eli Lilly and Company and holds company stock.
Supplementary Material
Acknowledgments
This study was sponsored by Eli Lilly and Company (Indianapolis Indiana). The study team thanks the participants and families for their willingness to participate in this study. Further, we thank all site staff and investigators at the institutions (See Supplemental Table 1) and the trial personnel at Eli Lilly and Company, Quintiles, and ICON.
The data from this study were presented in part at ASCO 2013 and ASCO 2015.
References
- 1.Stupp R, Pavlidis N, Jelic S, ESMO Guidelines Task Force. ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of malignant glioma. Ann Oncol. 2005;16(Suppl 1):i64–i65. [DOI] [PubMed] [Google Scholar]
- 2.Agnihotri S, Burrell KE, Wolf A et al. Glioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategies. Arch Immunol Ther Exp. 2013;61(1):25–41. [DOI] [PubMed] [Google Scholar]
- 3.Golestaneh N, Mishra B. TGF-b, neuronal stem cells and glioblastoma. Oncogene. 2005;24(37):5722–5730. [DOI] [PubMed] [Google Scholar]
- 4.Schneider T, Sailer M, Ansorge S, Firsching R, Reinhold D. Increased concentrations of transforming growth factor beta1 and beta2 in the plasma of patients with glioblastoma. J Neurooncol. 2006;79(1):61–65. [DOI] [PubMed] [Google Scholar]
- 5.Bruna A, Darken RS, Rojo F et al. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11(2):147–160. [DOI] [PubMed] [Google Scholar]
- 6.Peñuelas S, Anido J, Prieto-Sánchez RM et al. TGF-β Increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15(4):315–327. [DOI] [PubMed] [Google Scholar]
- 7.Tritschler I, Gramatzki D, Capper DD et al. Modulation of TGF-beta activity by latent TGF-beta-binding protein 1 in human malignant glioma cells. Int J Cancer. 2009;125(3):530–540. [DOI] [PubMed] [Google Scholar]
- 8.Wild-Bode C, Weller M, Wick W. Molecular determinants of glioma cell migration and invasion. J Neurosurg. 2001;94(6):978–984. [DOI] [PubMed] [Google Scholar]
- 9.Fecci PE, Mitchell DA, Whitesides JF et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. [DOI] [PubMed] [Google Scholar]
- 10.Uhl M, Aulwurm S, Wischhusen J et al. SD-208, a novel transforming growth factor β receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64(21):7954–7961. [DOI] [PubMed] [Google Scholar]
- 11.Tran TT, Uhl M, Ma JY et al. Inhibiting TGF-β signaling restores immune surveillance in the SMA-560 glioma model. Neuro Oncol. 2007;9(3):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anido J, Sáez-Borderías A, Gonzàlez-Juncà A et al. TGFß receptor inhibitors target the CD44high/Id1high glioma-initiating cell population in human glioblastoma. Cancer Cell. 2010;18(6):655–668. [DOI] [PubMed] [Google Scholar]
- 13.Rodon J, Carducci MA, Sepulveda-Sánchez JM et al. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. 2015;21(3):553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons S, Sawyer S, Yan L et al. The combination of the small molecule TGFβR1 inhibitor LY2157299 monohydrate with CCNU substantially blocks SMAD phosphorylation and significantly suppresses human glioblastoma xenograft growth. Paper presented at: AACR-NCI-EORTC 2011; AACR. Mol Cancer Ther. 2011;10(Suppl 11): C201. [Google Scholar]
- 15.Stupp R, Brada M, van den Bent MJ et al. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii93–iii101. [DOI] [PubMed] [Google Scholar]
- 16.Brandes AA, Tosoni A, Spagnolli F et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 2008;10(3):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gueorguieva I, Cleverly AL, Stauber A et al. Defining a therapeutic window for the novel TGF-β inhibitor LY2157299 monohydrate based on a pharmacokinetic/pharmacodynamic model. Br J Clin Pharmacol. 2014;77(5):796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han B, Enas NH, McEntegart D. Randomization by minimization for unbalanced treatment allocation. Stat Med. 2009;28(27):3329–3346. [DOI] [PubMed] [Google Scholar]
- 19.Wick W, Puduvalli VK, Chamberlain MC et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batchelor TT, Mulholland P, Neyns B et al. The efficacy of cediranib as monotherapy and in combination with lomustine compared to lomustine alone in patients with recurrent glioblastoma: a Phase III randomized study. Neuro Oncol. 2010;12:iv69–iv78. [Google Scholar]
- 21.Wick W, Brandes AA, Gorlia T et al. Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 Trial. Neuro Oncol. 2015;17(suppl 5):v1. [Google Scholar]
- 22.Preusser M, Capper D, Hartmann C. IDH testing in diagnostic neuropathology: review and practical guideline article invited by the Euro-CNS research committee. Clin Neuropathol. 2011;30(5):217–230. [DOI] [PubMed] [Google Scholar]
- 23.Rodón J, Carducci M, Sepulveda-Sánchez JM et al. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-β receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest New Drugs. 2015;33(2):357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baron U, Floess S, Wieczorek G et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur J Immunol. 2007;37(9):2378–2389. [DOI] [PubMed] [Google Scholar]
- 25.Tahara H, Sato M, Thurin M et al. Emerging concepts in biomarker discovery; the US-Japan Workshop on Immunological Molecular Markers in Oncology. J Transl Med. 2009;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong TS, Mendoza T, Gning I et al. Validation of the M. D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neurooncol. 2006;80(1):27–35. [DOI] [PubMed] [Google Scholar]
- 27.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test—Revised: Normative data and analysis of inter-form and test–retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 28.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–1309. [DOI] [PubMed] [Google Scholar]
- 29.Reitan RM. Trail Making Test. Manual for Administration and Scoring. South Tucson, AZ: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- 30.Ruff RM, Light RH, Parker SB. Benton controlled oral word association test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338. [PubMed] [Google Scholar]
- 31.Wefel J, Cloughesy T, Zazzali JL et al. Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(6):660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannelli G, Faivre S, Santoro A. Evaluation of LY2157299 monohydrate, a TGF-β receptor I kinase inhibitor, in patients with advanced hepatocellular carcinoma: Phase 2 study results of safety, efficacy and PK/PD. Presented at: International Liver Congress 2014; April 9-13, 2014; London, England Abstract A-627-0008-03078. [Google Scholar]
- 33.Wong ET, Gautam S, Malchow C, Lun M, Pan E, Brem S. Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw. 2011;9(4):403–407. [DOI] [PubMed] [Google Scholar]
- 34.Herbertz S, Sawyer JS, Stauber AJ et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther. 2015;9:4479–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumann F, Leukel P, Doerfelt A et al. Lactate promotes glioma migration by TGF-beta2- dependent regulation of matrix metalloproteinase-2. Neurol Oncol. 2009;11(4):368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanner AA, Marchi N, Vincent Fazio V et al. Serum S100β:A noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003;97:2806–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bambury RM, Teo MY, Power DG et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013;114(1):149–154. [DOI] [PubMed] [Google Scholar]
- 38.Zhou M, Bracci PM, McCoy LS et al. Serum macrophage-derived chemokine/CCL22 levels are associated with glioma risk, CD4T cell lymphopenia and survival time. Int J Cancer. 2015;137(4):826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8(3):261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani A, Gray PA, Van Damme J, Sozzani S. Macrophage-derived chemokine (MDC). J Leukoc Biol. 2000;68(3):400–404. [PubMed] [Google Scholar]
- 41.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98. [DOI] [PubMed] [Google Scholar]
- 42.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100(9):5336–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580–6587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.