Abstract
Background
Glioblastoma is one of the most malignant brain tumors in adults and has a dismal prognosis. In a previous report, we reported that CD40, a TNF-R-related cell surface receptor, and its ligand CD40L were associated with glioma outcomes. Here we attempted to activate CD40 signaling in the tumor and determine if it exerted therapeutic efficacy.
Methods
CD40 expression was examined in 3 mouse glioma cell lines (GL261, NSCL61, and bRiTs-G3) and 5 human glioma cell lines (U87, U251, U373, T98, and A172). NSCL61 and bRiTs-G3, as glioma stem cells, also expressed the glioma stem cell markers MELK and CD44. In vitro, we demonstrated direct antitumor effects of an anti-CD40 agonistic monoclonal antibody (FGK45) against the cell lines. The efficacy of FGK45 was examined by local convection-enhanced delivery of the monoclonal antibody against each glioma model.
Results
CD40 was expressed in all mouse and human cell lines tested and was found at the cell membrane of each of the 3 mouse cell lines. FGK45 administration induced significant, direct antitumor effects in vitro. The local delivery of FGK45 significantly prolonged survival compared with controls in the NSCL61 and bRiTs-G3 models, but the effect was not significant in the GL261 model. Increases in apoptosis and CD4+ and CD8+ T cell infiltration were observed in the bRiTs-G3 model after FGK45 treatment.
Conclusions
Local delivery of FGK45 significantly prolonged survival in glioma stem cell models. Thus, local delivery of this monoclonal antibody is promising for immunotherapy against gliomas.
Keywords: CD40, convection-enhanced delivery, glioma, immunotherapy
Glioblastoma (GBM) is the most common malignant brain tumor in adults and is classified as a grade IV glioma by the World Health Organization. The standard therapy comprises maximal surgical resection with adjuvant radiotherapy and temozolomide administration. Despite the current therapeutic interventions, GBM prognosis is still dismal, and patients' median survival ranges from 12 to 15 months.1,2 Some immunotherapy studies have demonstrated efficacy in establishing tumor-specific immunity against mouse glioma models; only a few of them, however, have demonstrated clinical efficacy. In a previous study, we reported that CD40 and its ligand CD40L were associated with glioma outcomes.3 CD40 is a member of the tumor necrosis factor (TNF) receptor superfamily and is expressed on antigen-presenting cells such as macrophages and dendritic cells, human glioma tissue, and some human and mouse glioma cell lines.3,4 In the same study, we demonstrated that vaccination with FGK45, an anti-CD40 agonistic monoclonal antibody (mAb), markedly prolonged survival of the mouse glioma model.3 Although it was also effective against the glioma stem cell models, its efficacy was compromised to some extent when compared with normal glioma models. In this study, we further attempted to potentiate the CD40-based immunotherapeutic strategy against glioma stem cell models. It has been suggested that immunotherapy enhances its therapeutic activity if there is tumor death at the tumor site,5 and it has been demonstrated that CD40 induces apoptotic cell death in CD40-positive transformed cells.6 On the basis of these results, we attempted to develop effective CD40-based immunotherapy involving local delivery of the CD40 molecule.
Materials and Methods
Animals and Cell Lines
Six to 8-week-old C57BL/6 female mice and severe combined immunodeficiency (SCID) mice were purchased from SLC Japan, Inc. They were kept under pathogen-free conditions in accordance with the protocols reviewed and approved by the Institute for Animal Experimentation, Tohoku University Graduate School of Medicine. The mouse glioma cell line GL261 was kindly provided by Dr. Masaki Toda at Keio University (Tokyo, Japan).7 The mouse glioma stem cell lines NSCL61 and bRiTs-G3 were kindly provided by Dr. Toru Kondo at RIKEN (Kobe, Japan)8 and Dr. Hideyuki Saya at Keio University (Tokyo, Japan),9 respectively. The cell lines were used for mouse intracranial tumor models. Five human glioma cell lines (U87, U251, U373, T98, and A172) were obtained from DS PHARMA Biomedical (Osaka, Japan), the Health Science Research Resources Bank (Osaka, Japan), ATCC, DS PHARMA Biomedical, and RIKEN BRC (Tsukuba, Ibaraki, Japan), respectively. GL261, U87, U251, U373, T98, and A172 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum and 100 U/mL penicillin/streptomycin, and cultures were placed in a humidified incubator with 5% CO2 at 37°C. NSCL61 cells were cultured in DMEM/F12 supplemented with 10 ng/mL basic fibroblast growth factor, 10 ng/mL epidermal growth factor, and 100 U/mL penicillin/streptomycin, and cultures were placed in a humidified incubator with 5% CO2 at 37°C. bRiTs-G3 cells were cultured in DMEM/F12 supplemented with recombinant 20 ng/mL human epidermal growth factor, 20 ng/mL recombinant human basic fibroblastic growth factor, B27 supplement without vitamin A, 200 ng/mL heparin sulfate, and 100 U/mL penicillin/streptomycin.
Refining FGK45
FGK45 was refined using FGK45 hybridoma cells from 7 to 8-week-old BALB/c male nude mice. Pristane (0.5 mL) was administered to the mice twice, and 1 × 106 hybridoma cells were implanted into the peritoneum. One to 2 weeks later, ascetic fluid was obtained for FGK refining. This sample was eluted in the HiTrap Protein A HP Columns (GE Healthcare) by elusion buffer. FGK45 concentration was calculated using Nano Drop 2000 (Thermo Fisher Scientific), and FGK45 was stored at −80°C until use.
Western Blotting
The cell lines in culture were harvested at 70% confluence. Each cell culture was treated with radio-immunoprecipitation assay buffer (catalog no. 182-02451; WAKO) and used as the whole-cell lysate sample. Protein concentrations were determined by comparison with known concentrations of bovine serum albumin using a commercial kit (catalog no. 23225, Thermo Fisher Scientific). Equal quantities of the samples (10 µg) were loaded per lane and analyzed by SDS-PAGE on a 4%–15% Mini-PROTEAN Gel (catalog no. 456-1085, Bio-Rad Laboratories). The primary antibodies were used at a 1:5000 dilution of rabbit monoclonal anti-beta actin antibody (molecular weight, 45 kDa; catalog no. 4967; Cell Signaling Technology), a 1:2000 dilution of a rabbit polyclonal anti-CD40 antibody (molecular weight, 35–45 kDa; catalog no. ab13545, Abcam), a 1:1000 dilution of a rabbit polyclonal anti-MELK antibody (molecular weight, 73 kDa; catalog no. sc-292832, Santa Cruz Biotechnology), and a 1:1000 dilution of a rabbit polyclonal anti-CD44 antibody (molecular weight, 82–95 kDa; catalog no. ab41478, Abcam), and cleaved caspase-3 (Asp175) antibody (1:2000, molecular weight, 17–19 kDa; catalog no. 9661, Cell Signaling Technology). After incubation with horseradish peroxidase-conjugated anti-rabbit IgG (catalog no. 7074; Cell Signaling Technology), the antigen was detected by ECL Western Blotting Substrate (catalog no. 32106; Thermo Fisher Scientific). Images were scanned with a ChemiDoc MP imaging system (Bio-Rad Laboratories).
Immunofluorescent Staining
For immunocytochemistry, GL261, NSCL61, bRiTs-G3, and U87 cells were cultured on 4-well chamber slides, washed with phosphate-buffered saline (PBS), and fixed with 4% paraformaldehyde in PBS for 15 minutes. They were then washed with PBS and incubated for 1 hour in a blocking solution (PBS containing 3% bovine serum albumin and 0.3% Triton X-100). For immunohistochemistry, the animals were euthanized and perfused with PBS, followed by 4% paraformaldehyde in PBS. The animal brains were post-fixed overnight in the same fixative at 4°C. For cryosectioning, fixed tissues were cryoprotected in 10% sucrose in PBS overnight at 4°C and in 20% sucrose in PBS overnight at 4°C, and then embedded in Tissue-Tek OCT compound (Funakoshi Inc.). Cryostat sections (7 µm) were cut and affixed to glass slides. Cells or tissue sections were subsequently incubated overnight at 4°C in an appropriate mixture of primary antibodies: anti-CD40 (1:100; catalog no. ab13545, Abcam), anti-CD4 (1:500); or anti-CD8a (1:200, eBioscience,). After 3 washes with PBS, cells or tissue sections were incubated for 1 hour with a 1:500 dilution of the secondary antibodies: Alexa Fluor 488-conjugated goat anti-rabbit IgG, Alexa Fluor 568-conjugated goat anti-rabbit IgG, and anti-rat IgG Alexa 546. After 3 washes with PBS, samples were covered with Vectashield mounting medium with DAPI. To detect apoptotic cells, sections were stained with the TUNEL method using a Click-it TUNEL Alexa Fluor 488 or 598 imaging assay for microscopy and HCS (catalog no. C10245 and C10246, respectively; Thermo Fisher Scientific), according to the manufacturer's instructions. The samples were examined by confocal (C2si, Nikon) or fluorescence microscopy (BZ-9000, Keyence).
Cell Viability Assay
GL261, NSCL61, and bRiTs-G3 cells were seeded into a 96-well plate (1 × 104 cells/well) and cultured in 200 µL medium. A Cell Counting Kit-8 (CCK-8) assay (Dojindo Laboratories) was used to measure the cytotoxicity of FGK45 on GL261, NSCL61, and bRiTs-G3 cells. GL261 cells were examined after 72 hours, and NSCL61 and bRiTs-G3 cells were examined after 48 hours. Sample absorbance at 450 nm was measured using a SpectraMax190 microplate reader (Molecular Devices). The mean absorbance of 5 wells for each cell line and for each treatment was calculated.
Brain Tumor Models
GL261, NSCL61, and bRiTs-G3 cell lines were used for mouse intracranial tumor models. After anesthesia using isoflurane, the animals were placed in a stereotactic apparatus (NARISHIGE Inc.,). A burr hole was drilled into the skull (0.5 mm forward, 2.5 mm lateral from the bregma, and 3.5 mm depth from the dura). Subsequently, 2 × 106 GL261 cells or 1 × 104 NSCL61 and bRiTs-G3 cells in 2 µL PBS were injected into the right striatum using a 2-μl Hamilton syringe (Hamilton Company) with a 26-gauge needle.
Direct Injection and Convection-enhanced Delivery Treatment
For the FGK45 kinetic distribution studies, we used 3 mice each for convection-enhanced delivery (CED) infusion and direct injection. CED of CD40 mAb (10 µg in 10 µL of PBS) was performed with a rate-controllable microinfusion pump (Bioanalytical Systems, Inc.,) with adjusted rates for 10 µL total infusion volume as follows: 0.2 µL/minute for 15 minutes, 0.5 µL/minute for 10 minutes, 0.8 µL/minutes for 2.5 minutes, and the pump stopped for 5 minutes. The direct injection of CD40 mAb (10 µg in 10 µL of PBS) was performed using a Hamilton syringe with a 26-gauge needle into the mouse brain through a burr hole at depth of 3 mm below the skull surface. In addition, this experiment was repeated in the bRiTs-G3 brain tumor model. Infusion was performed 5 days after tumor cell implantation.
Animal Experiments
All animal experiments were reviewed and approved by the Animal Care and Use Committee, Tohoku University School of Medicine, and performed in accordance with institutional ethical guidelines.
Therapeutic Studies in Mouse Models
C57BL6 mice received intracranial implantation of GL261 (2 × 106), NSCL61 (1 × 104), or iRTs-G3 (1 × 104) cells in 2 µL of PBS. The coordinates for implantation were 0.5 mm forward, 2.5 mm lateral from the bregma, and 3.5 mm deep from the dura using the stereotactic frame (David Kopf Instruments), stereotaxic injector (Stoelting Co.), and 10-μl Hamilton syringe (Hamilton Company) under anesthesia on day zero. Five days after tumor implantation, 10 µg of FGK45 (in 10 µL PBS [n = 8]) or 10 µg of rat IgG in 10 µL PBS (control group, n = 8) was administered by the CED method to the same coordinates as those mentioned earlier.
Vaccination Therapy
Heavily irradiated tumor cells were used as tumor lysates. Irradiation of 7000 rad was administered for 1 × 104 NSCL61 and bRiTs-G3 cells. To observe the additive effects of triggering CD40, 100 µg FGK45 or rat IgG (control) was added to subcutaneous lysate-based vaccinations. Vaccinations were administered twice at 5-day intervals.
Statistical Analyses
For the in vitro study, data were collected from 3 independent experiments; for the animal survival study, data were collected from 8 mice in each group. Significance was determined using the Mann-Whitney U test for comparison between 2 groups. Comparison between >3 groups was determined using 1-way analysis of variance. The log-rank test was used for analysis of the Kaplan–Meier survival curves. All statistical analyses were performed with GraphPad Prism 5.0.3. All statistical studies were 2-sided, and P < .05 represented significance.
Results
CD40 Expression in Mouse and Human Glioma Cell Lines
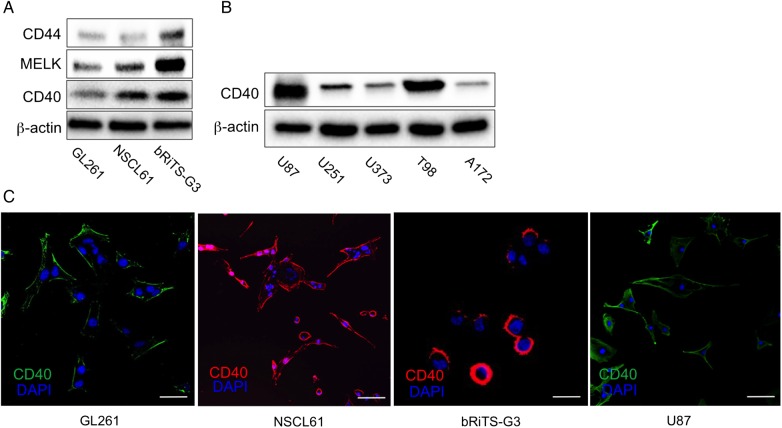
CD40 expression was assessed in 3 mouse glioma cell lines (GL261, NSCL61, and bRiTs-G3) and 5 human glioma cell lines (U87, U251, U373, T98, and A172). CD40 expression was detected in all mouse glioma cell lines (Fig. 1A). All human glioma cell lines also expressed CD40. U87 and T98 expressions were exceptionally high (Fig. 1B). MELK and CD44 (glioma stem cell markers) were also expressed in NSCL61 and bRiTs-G3 cell lines, confirming the stemness of these cell lines (Fig. 1A). GL261 cells, although not the stem cell lines, also expressed these markers at an almost similar level as NSCL61. This may be because GL261 is a well-established cell line. CD40 expression was found at cell membranes in all mouse glioma cell lines and in U87 (Fig. 1C).
Fig. 1.
Expression of CD40 in mouse and human glioma cell lines. (A) CD40 expression was found in all mouse glioma cell lines. NSCL61 and bRiTs-G3 cells showed relatively higher levels of CD40 expression than GL261 cells. Glioma stem cell markers, MELK, and CD44 were also expressed in these cells. (B) CD40 expression was also found in human glioma cell lines. (C) Cells were examined by immunocytochemistry for CD40 (B: green; C, D: red). Nuclei were counterstained with DAPI (blue). CD40 expression was found at cell membranes. Scale bars, 20 µm.
CD40 mAb Directly Induced Antitumor Effects
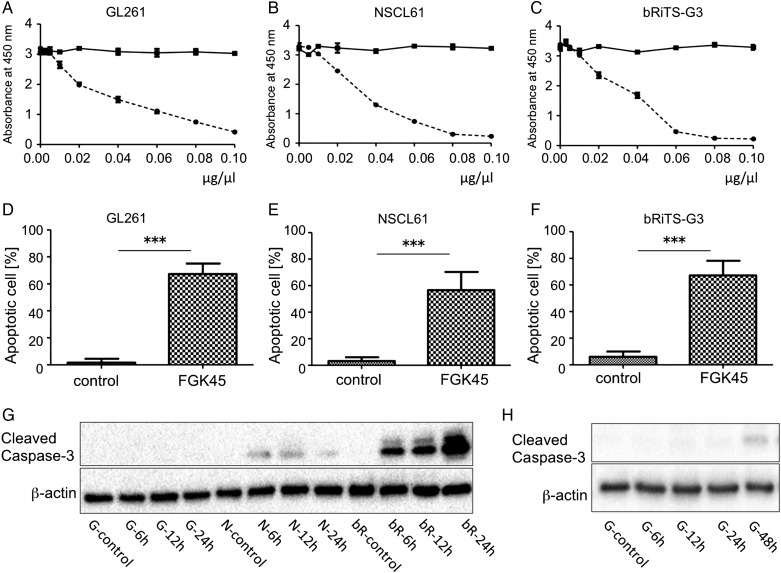
Antitumor effects of FGK45 were tested in vitro. Cell proliferation was evaluated using the WST-8 assay to observe the effects of FGK45 on the 3 mouse glioma cell lines. We found that the FGK45 dose-dependently inhibited the proliferation in all mouse glioma cell lines (Fig. 2; A: GL261; B: NSCL61; C: bRiTs-G3).
Fig. 2.
Antitumor effects of FGK45 on tumor cell lines in vitro. Antitumor effects of FGK45 or IgG (control) on GL261 (A), NSCL61 (B), and bRiTs-G3 (C) cells were determined by the WST-8 assay. Data were obtained 72 hours after FGK45 treatment (A: GL261) and 48 hours after the treatment (B: NSCL61, C: bRiTs-G3). Each point indicates the mean value of 5 independent runs. Bars; mean ± SD. The absorbance of cells treated with FGK45 was significantly lower than those treated with IgG (A, B, and C). The reductions in cellular proliferation were dose-dependent. Cells after FGK45 treatment were subjected to TUNEL staining. This examination was performed 48 hours after FGK45 administration for GL261 and 24 hours after the administration for NSCL61 and bRiTs-G3 (D: GL261, E: NSCL61, F: bRiTs-G3). Compared with the IgG-treated control group, FGK45 administration induced marked apoptotic cell death (***P < .0001). (G and H) Detection of cleaved caspase-3. Each cell line was treated with 0.05 µg/µL IgG (control) or FGK45. IgG-treated cells were harvested after 24 hours for NSCL61 and bRiTs-G3 cell lines and after 48 hhours for GL261 cell line. FGK45-treated cells were harvested after 6, 12, and 24 hours in NSCL61 and bRiTs-G3 cell lines, (G) and after 6, 12, 24, and 48 hours in GL261 (H). Cleaved caspase-3 was detected after 6 to 24 hours in NSCL61 and bRiTs-G3 cell lines; however, it was detected only after 48 hours in GL261 cell line. G: GL261, N: NSCL61, bR: bRiTs-G3.
To investigate the mechanism of the antitumor effects of FGK45 following FGK45 treatment, cells were stained with TUNEL staining. This examination was performed 48 hours after FGK45 administration for GL261 and 24 hours after FGK45 administration for NSCL61 and bRiTs-G3 (Fig. 2; D: GL261; E: NSCL61; F: bRiTs-G3, Supplementary material, Fig. S1). In comparison with the IgG-treated control group, FGK45 administration induced marked apoptotic cell death in these cells (***P < .0001). This result suggests that FGK45 reduced the proliferation of mouse glioma cell lines and induced cellular apoptosis. TUNEL staining was performed at different time points for each cell line as activation of caspase-3 occurred at different time points. Each cell line was treated with IgG (control) or FGK45 at the concentration of 0.05 µg/µL. Cells were harvested after 6, 12, and 24 hours of treatment and, with GL261 cell line, cells harvested after 48 hours of treatment were also evaluated (Fig. 2G and H). In NSCL61 and bRiTs-G3 cell lines, cleaved caspase-3 was detected after 6–24 hours of treatment; however, it was detected after 48 hours (Fig. 2H) in the GL261 cell line (Fig. 2G).
Local Delivery of Anti-CD40 Antibody in Mouse Brain Parenchyma
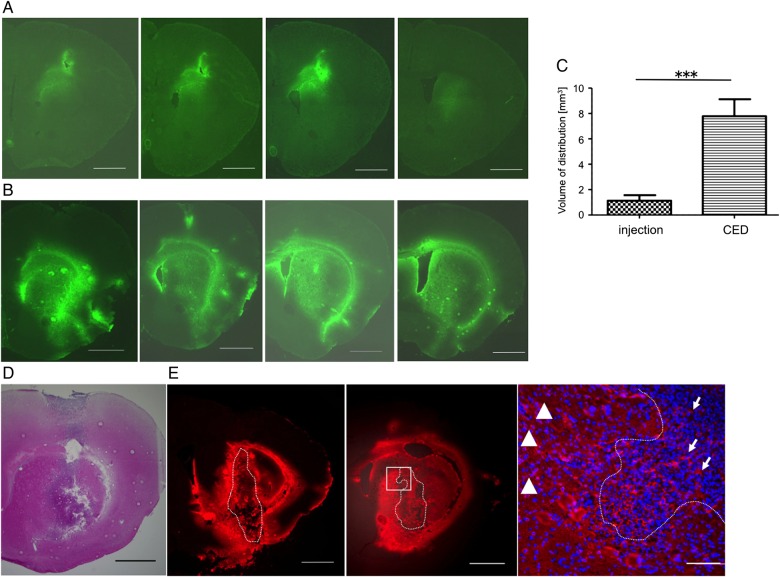
To our knowledge, local delivery of CD40 mAb to the brain parenchyma has not yet been performed. At first, we assessed the most efficient method of local delivery into the mouse brain. We compared the direct injection method with CED (Fig. 3A–C). In mouse brains, FGK45 administration via CED (10 µL) produced more extensive and diffuse distribution than direct injection (Fig. 3A and B). The volume of distribution (Vd) was calculated to be 1.126 ± 0.1354 mm3 for direct injection and 7.788 ± 0.4316 mm3 immediately after CED (Fig. 3C). In the brain tumor model (Fig. 3D), CED achieved robust distribution in the tumor bed as well as in the surrounding brain parenchyma (Fig. 3E).
Fig. 3.
Distribution of FGK45 in mouse brain parenchyma (A, B, and C) and in mouse brain tumor model (D and E). After direct injection (A) or convection-enhanced delivery (CED) (B) of FGK45, fluorescent staining for FGK45 (green) detected the distribution of FGK45 in mouse brain parenchyma. Sequential sections from representative mouse brain are shown. The mean Vd of CED (n = 5, 7.788 ± 0.4316 mm3) was significantly robust compared with that of direct injection (n = 5, 1.126 ± 1.126 mm3) (C). Bars indicate mean ± SE. Student t test ***P < .0001. In bRiTs-G3 model 5 days after tumor cell implantation (D, H&E stain), extensive and diffuse distribution of FGK45 (red) after CED were detected in tumor bed as well as in surrounding brain parenchyma (E, left and middle; sequential slices with 100 µm interval). Enlarged image of the white rectangular box in middle panel (E, right). Dotted lines indicate tumor margin. Scale bars in A, B, D, and E (left and middle) indicate 1000 µm, and in E (right) indicate 100 µm. Arrows indicate FGK45 distribution inside the tumor, and arrowhead depicts that in surrounding brain parenchyma.
Efficacy of Local Delivery of FGK45 in Mouse Glioma Models
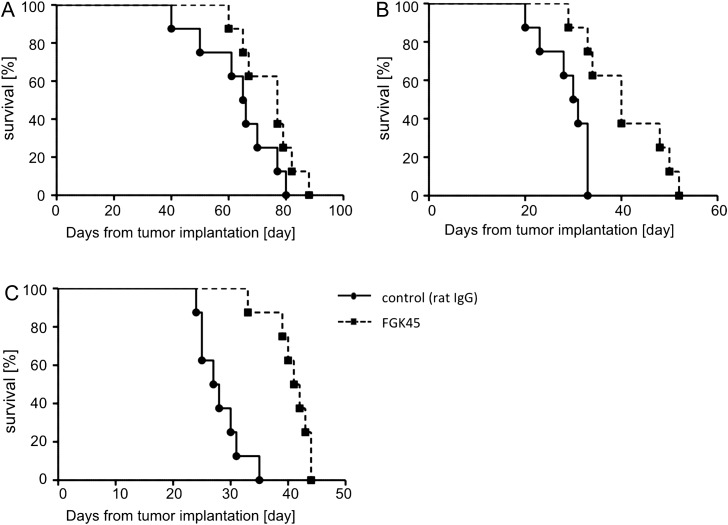
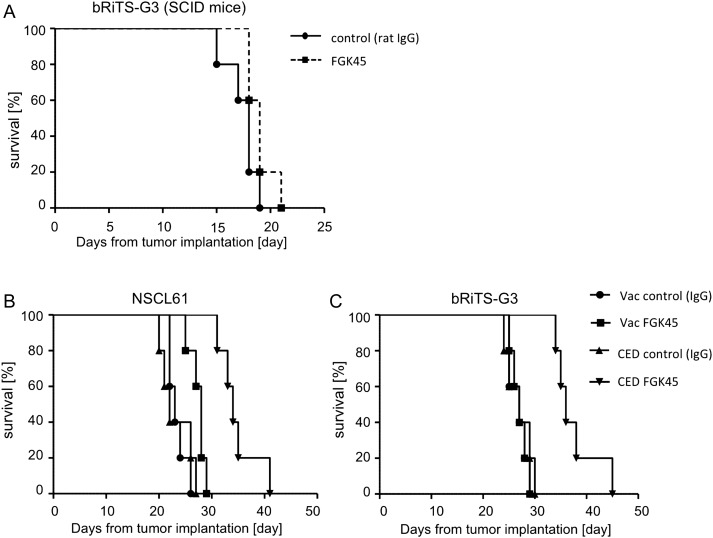
To investigate the efficacy of local delivery of the anti-CD40 agonistic antibody against intracranial mouse glioma models, an intracranial glioma model was established using GL261, NSCL61, and bRiTs-G3 cell lines. Five days after cell implantation, 10 µg of FGK45 in 10 µL PBS or 10 µg IgG in 10 µL PBS were administered by CED to mice glioma models (each group, n = 8). FGK45-treated mice survived significantly longer than IgG-treated mice in NSCL61 (P = .0072, Fig. 4B) and bRiTs-G3 (P = .0006, Fig. 4C) models, although not significantly in the GL261 model (P = .1241, Fig. 4A). This indicated that the local delivery of FGK45 may induce significant antitumor effects in mouse glioma models.
Fig. 4.
Efficacy of local delivery of FGK45 in mouse glioma cell lines. Each group of C57BL6 mice (n = 8) was implanted with GL261 (A), NSCL61 (B), and bRiTs-G3 (C) and treated with CED of 10 µg FGK45 (in 10 µL PBS) on day 5. Control mice received CED of 10 µg IgG (in 10 µL PBS). Survival analysis of GL261 model mice treated with FGK45 or IgG (control) in the GL261 (A), NSCL61 (B), and bRiTs-G3 models (C). Local delivery of FGK45 significantly prolonged survival compared with controls in the NSCL61 (P= .0072) and bRiTs-G3 (P = .0006) models, but the effect was not significant in the GL261 model (P = .1241).
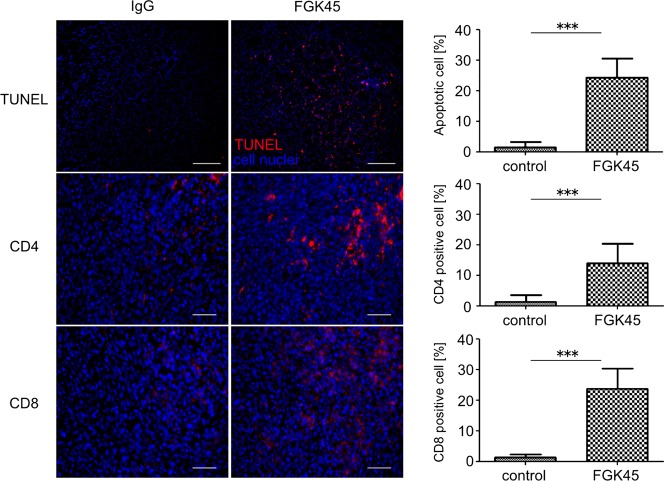
To investigate the mechanism underlying the antitumor effects of FGK45, brain sections from the bRiTs-G3 model mice (n = 5) were stained with TUNEL staining 2 days after treatment (Fig. 5). The number of total and TUNEL-positive cells (red) and the average ratio of TUNEL-positive cells/total cells were calculated. In comparison with IgG sections, CED of FGK45 induced a marked number of apoptotic cells (Fig. 5, ***P < .0001). This suggested that local delivery of FGK45 induced apoptosis in vivo as well. TUNEL assay was also examined in GL261 and NSCL61 models (Supplementary material, Fig. S2). In the GL261 model, local delivery of FGK45 resulted in few apoptotic cell deaths, while local delivery of FGK45 obviously induced apoptosis in the in the NSCL61 model.
Fig. 5.
Antitumor efficacy of local delivery of FGK45 in the bRiTs-G3 model. The mechanism underlying antitumor efficacy of local delivery of FGK45 was evaluated in the bRiTs-G3 model. Two days after treatment, TUNEL staining revealed an increased number of apoptotic cells in the FGK45-treated group. TUNEL (red) staining in the bRiTs-G3 model, counterstained with DAPI (blue). Scale bars, 100 µm. The number of cells was counted under ×400 magnification. Bars indicate mean ± SD (***P < .05). Immunohistochemistry for mouse CD4 and CD8-positive T cells (red) in the bRiTs-G3 model was performed using brains harvested 7 days after treatment. Nuclei were counterstained with DAPI (blue). The number of cells positively stained for CD4 or CD8 was counted under ×400 magnification. Bars indicate mean ± SD (***P < .05). Scale bars, 50 µm.
Subsequently, brain sections from bRiTs-G3 model mice (n = 5) were evaluated 7 days after treatment for lymphocyte infiltration. They were immunohistochemically stained with anti-CD4 and -CD8 antibodies (red [Fig. 5; CD4, ***P = .0003; CD8, ***P < .0001]). The infiltration of CD4 and CD8T cells in the FGK45-localized delivery group was significantly higher than that in the IgG-treated group. This suggested that localized delivery of FGK45 induced infiltration of immune cells.
Next, we examined, using immunocompromised SCID mice, whether the in vivo efficacy was due to direct effects on tumor cells or immunopotentiating effects of the antibodies on infiltrating immune cells or both (Fig. 6A). In bRiTs-G3 model developed with SCID mice, CED of FGK45 resulted in slightly longer survival, but the difference was not significant (P = .1021).
Fig. 6.
Efficacy of local delivery of FGK45 in bRiTs-G3 models developed with severe combined immunodeficiency (SCID) mice and comparison of efficacy of vaccination therapy and local delivery of FGK45 in NSCL61 and bRiTs-G3 models. (A) SCID mice (n = 5) were implanted with bRiTs-G3 cells and treated with convection-enhanced delivery (CED) of 10 µg FGK45 (in 10 µL PBS) on day 5. Control mice received CED of 10 µg IgG (in 10 µL PBS). FGK45-treated SCID mice survived slightly longer than IgG-treated SCID mice, but the difference was not significant (P = .1021). (B) Groups of mice treated with IgG (100 µg, control) or FGK45 (100 µg) added to tumor lysate-based vaccination (n = 5 for each group) or those treated with local delivery of IgG (10 µg, control) or FGK45 (10 µg) (each group, n = 5) were compared. Local delivery of FGK45 was more effective than vaccination therapy of FGK45 in both models (B: NSCL61, P = .0040, C: bRiTs-G3, P = .0018).
Efficacy of FGK45 Added to Vaccines or Delivered Locally
The efficacy of FGK45 added to tumor lysate-based vaccines was compared with that of FGK45 delivered locally. Survival assay was performed using NSCL61 and bRiTs-G3 mouse glioma models. In both models, survival of mice receiving IgG or FGK45 together with tumor lysate-based vaccinations (n = 5 for each group) and those receiving intratumoral CED of IgG or FGK45 (n = 5 for each group) were compared. The mice that received intratumoral delivery of FGK45 survived significantly longer than those that received subcutaneous vaccination of FGK45 added to cell lysates in NSCL61 (P= .0040, Fig. 6B) and bRiTs-G3 (P= .0018, Fig. 6C) models.
Discussion
CD40 has been previously shown to be expressed by glioma cells in vitro and in vivo.3,4 In a prior report, we demonstrated that higher expression of CD40/CD40L correlated with better survival for GBM patients.3 On the other hand, CD40 is known to mediate cell death in certain transformed cell lines including neuroblastoma cells10 and other tumor cells of mesenchymal and epithelial origin.11,12 Although the biological significance of aberrant CD40 expression in cancer cell lines and the mechanism by which CD40 triggers the death receptor-dependent apoptotic pathway have remained enigmatic,4 it can be hypothesized that local activation of CD40 signaling has some therapeutic potential against cancers. In vitro, the agonistic antibody to CD40 induced antitumor effects when added to glioma cell cultures and glioma-initiating cells. TUNEL-positive cells were seen, which indicated the cytocidal effects (Fig. 2). Detection of activated caspase-3 indicated that FGK45 induced apoptosis in NSCL61 and in bRiTs-G3 cell lines earlier and more effectively than in GL261 (Fig. 2G and H). This efficacy correlated with the cellular expression levels of CD40 (Fig. 1A).
In a previous study, we demonstrated that augmentation in CD40 signaling enhanced the efficacy of vaccinations against glioma models.3 In those experiments, we added the agonistic antibody to CD40 into subcutaneous cell lysate-based vaccines. When cell lysate-based vaccines were administered subcutaneously, the efficacy of CD40 augmentation was more pronounced when the agonistic antibody was administered at the same subcutaneous sites rather than via intraperitoneal systemic administration. However, it was suggested that the efficacy of immunostimulation improves when cytotoxic treatment is given concurrently to tumor sites.5 Therefore, we tested the efficacy of local intratumoral administration of CD40 agonistic antibodies by CED (Figs 4 and 5). CED directly delivers agents into the tumor and surrounding parenchyma based on continuous positive-pressure infusion.13 CED can achieve large volumes of distribution as the diffusion is not limited by concentration gradients.14 Importantly, CED provides direct access to the tumor bed, resulting in high local concentrations of drugs with minimal systemic absorption.15
Single intratumoral CED of CD40 agonistic antibodies demonstrated significant efficacy in NSCL61 and bRiTS-G3 cells, the glioma stem cell tumor models. In addition, local delivery of FGK45 was more effective than when added to vaccination therapy in glioma stem models NSCL61 and bRiTs-G3 (Fig. 6B and C). Usually these models are more resistant to treatment as they represent glioma stem cells. However, the efficacy of this treatment was less in the standard GL261 mouse glioma model. This again could be due to lower CD40 expression in GL261 cells than in NSCL61 and bRiTS-G3 cells. In the glioma-initiating cell-like cell tumor models, which showed susceptibility to CED of the CD40 agonistic antibody, apoptosis and marked infiltration of CD4/CD8-positive cells were observed inside the tumor, which was indicative of both the cytocidal and immunostimulative effects of CD40 stimulation therapy. The slight difference in apoptosis induction might have resulted in a big difference in the efficacy in vivo. In addition, we performed in an vivo study using immunocompromised SCID mice (Fig. 6A). In the immunocompromised host, local delivery of FGK45 failed to provide a significant survival benefit compared with control IgG delivery. Thus, these results indicate the importance of both local effect, inducing apoptosis, and immunopotentiating effects.
In this study, the concentration of FGK45 tested was 1 µg/µL. This concentration was selected because it was the available highest concentration with our method of FGK45 refining. At 1 µg/µL concentration, CED of FGK45 can be performed safely with negligible tissue toxicity and without weight loss or neurological deficit (Supplementary material, Fig. S3). Therefore, we used this concentration for in vivo study. As 1 µg/µL concentration was nontoxic, higher concentration of FGK45 or repeated local delivery may yield better efficacy.
The toxicity of the systemic anti-CD40 agonistic antibody has already been tested in clinical settings.16 In that study, cytokine-release syndrome (CRS) was observed. CRS was clinically evident within minutes to hours after infusion, manifested by varying combinations of chills, rigors, fever, rash, nausea, vomiting, muscle aches, and back pain.17,18 Because of these adverse effects, the maximum tolerated dose of the anti-CD40 agonistic antibody was low, resulting in insufficient efficacy.16 Intratumoral delivery of mAb is proposed as an approach to circumvent these adverse effects. It has been reported that local (intratumoral) injection of CD40 mAb induces antitumor effects and leads to systemic antitumor immunity in mouse melanomas,19 mesothelioma models,20 or other cell lines.21 Our study demonstrated that this local delivery strategy can be also applied to gliomas that reside in immunoprivileged sites. Of note, van Mierlo et al reported that a subsequent i.v. injection of the CD40 agonistic mAb 2 weeks after the first i.v. injections led to a shock-like syndrome in all mice tested, resulting in 50% mortality that was probably related to induction of a cytokine burst of CD40 cells.21 However, they did not observe this toxic effect after multiple intratumoral injections of anti-CD40 mAb. These findings indicate that local anti-CD40 treatment is to be preferred over systemic injection.
Local CED of the anti-CD40 agonistic antibody was not only effective but also presented an improved safety profile against gliomas. One limitation to this strategy is the fact that cytocidal effects may depend on the cellular expression levels of CD40; therefore, this strategy may not be effective for tumors without CD40 expression or tumors expressing low CD40 levels. However, the efficacy against glioma-initiating cell-like cell tumor models of note suggests that even cancer stem cells can be killed if they express CD40. Another limitation is that this study used mouse physiology. As expression of CD40 is also present in human gliomas, clinical trials relating to human physiology are required.
Supplementary Material
Funding
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (#26293319 to R.S.).
Supplementary Material
Acknowledgments
We thank Antonius G. Rolink (Basel University, Basel, Switzerland) for providing the FGK45 hybridoma cells, Masahiro Toda (Keio University School of Medicine, Tokyo, Japan) for providing the GL261 cells, Toru Kondo (Institute for Genetic Medicine, Hokkaido, Japan) for providing the NSCL61 cells, and Hideyuki Saya (Keio University School of Medicine, Tokyo, Japan) for providing the bRiTS-G3 cells.
Conflict of interest statement. None declared.
References
- 1.Stupp R, Hegi ME, Mason WP et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2.Hegi ME, Diserens AC, Gorlia T et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. New Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 3.Chonan M, Saito R, Shoji T et al. CD40/CD40L expression correlates with the survival of patients with glioblastomas and an augmentation in CD40 signaling enhances the efficacy of vaccinations against glioma models. Neuro Oncol. 2015;17(11):1453–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wischhusen J, Schneider D, Mittelbronn M et al. Death receptor-mediated apoptosis in human malignant glioma cells: modulation by the CD40/CD40L system. J Neuroimmunol. 2005;162(1–2):28–42. [DOI] [PubMed] [Google Scholar]
- 5.Ali S, King GD, Curtin JF et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65(16):7194–7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallman C, Johnson PW, Packham G. Differential regulation of cell survival by CD40. Apoptosis. 2003;8(1):45–53. [DOI] [PubMed] [Google Scholar]
- 7.Iizuka Y, Kojima H, Kobata T, Kawase T, Kawakami Y, Toda M. Identification of a glioma antigen, GARC-1, using cytotoxic T lymphocytes induced by HSV cancer vaccine. Int J Cancer. 2006;118(4):942–949. [DOI] [PubMed] [Google Scholar]
- 8.Hide T, Takezaki T, Nakatani Y, Nakamura H, Kuratsu J, Kondo T. Sox11 prevents tumorigenesis of glioma-initiating cells by inducing neuronal differentiation. Cancer Res. 2009;69(20):7953–7959. [DOI] [PubMed] [Google Scholar]
- 9.Osuka S, Sampetrean O, Shimizu T et al. IGF1 receptor signaling regulates adaptive radioprotection in glioma stem cells. Stem Cells. 2013;31(4):627–640. [DOI] [PubMed] [Google Scholar]
- 10.Airoldi I, Lualdi S, Bruno S et al. Expression of costimulatory molecules in human neuroblastoma. Evidence that CD40 + neuroblastoma cells undergo apoptosis following interaction with CD40L. Br J Cancer. 2003;88(10):1527–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hess S, Engelmann H. A novel function of CD40: induction of cell death in transformed cells. J Exp Med. 1996;183(1):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eliopoulos AG, Dawson CW, Mosialos G et al. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr Virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene. 1996;13(10):2243–2254. [PubMed] [Google Scholar]
- 13.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91(6):2076–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg. 1999;90(2):315–320. [DOI] [PubMed] [Google Scholar]
- 15.Yun J, Rothrock RJ, Canoll P, Bruce JN. Convection-enhanced delivery for targeted delivery of antiglioma agents: the translational experience. J Drug Deliv. 2013;2013:107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19(5):1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruter J, Antonia SJ, Burris HA, Huhn RD, Vonderheide RH. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol Ther. 2010;10(5):983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vonderheide RH, Flaherty KT, Khalil M et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25(7):876–883. [DOI] [PubMed] [Google Scholar]
- 19.Van De Voort TJ, Felder MA, Yang RK, Sondel PM, Rakhmilevich AL. Intratumoral delivery of low doses of anti-CD40 mAb combined with monophosphoryl lipid a induces local and systemic antitumor effects in immunocompetent and T cell-deficient mice. J Immunother. 2013;36(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackaman C, Lew AM, Zhan Y et al. Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. Int Immunol. 2008;20(11):1467–1479. [DOI] [PubMed] [Google Scholar]
- 21.van Mierlo GJ, den Boer AT, Medema JP et al. CD40 stimulation leads to effective therapy of CD40(-) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci USA 2002;99(8):5561–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.