Abstract
Background
There is strong concern about the costs associated with adding tumor-treating fields (TTF) therapy to standard first-line treatment for glioblastoma (GBM). Hence, we aimed to determine the cost-effectiveness of TTF therapy for the treatment of newly diagnosed patients with GBM.
Methods
We developed a 3-health-state Markov model. The perspective was that of the French Health Insurance, and the horizon was lifetime. We calculated the transition probabilities from the survival parameters reported in the EF-14 trial. The main outcome measure was incremental effectiveness expressed as life-years gained (LYG). Input costs were derived from the literature. We calculated the incremental cost-effectiveness ratio (ICER) expressed as cost/LYG. We used 1-way deterministic and probabilistic sensitivity analysis to evaluate the model uncertainty.
Results
In the base-case analysis, adding TTF therapy to standard of care resulted in increases of life expectancy of 4.08 months (0.34 LYG) and €185 476 per patient. The ICER was €549 909/LYG. The discounted ICER was €596 411/LYG. Parameters with the most influence on ICER were the cost of TTF therapy, followed equally by overall survival and progression-free survival in both arms. The probabilistic sensitivity analysis showed a 95% confidence interval of the ICER of €447 017/LYG to €745 805/LYG with 0% chance to be cost-effective at a threshold of €100 000/LYG.
Conclusion
The ICER of TTF therapy at first-line treatment is far beyond conventional thresholds due to the prohibitive announced cost of the device. Strong price regulation by health authorities could make this technology more affordable and consequently accessible to patients.
Keywords: brain tumor, cost-effectiveness analysis, glioblastoma, temozolomide, tumor-treating fields
Glioblastoma (GBM) is the type of glioma with the highest grade of malignancy (grade IV). It represents the most frequent and aggressive form of brain tumor in adults,1 with a median survival of 3 months without treatment. GBM is characterized by its capacity to systematically recur over time, even in patients with complete surgical resection.2 In order to increase progression-free survival (PFS) and overall survival (OS), different strategies of adjuvant therapy have been tested. To date, the standard therapy is radiotherapy combined with temozolomide (TMZ).3 This therapy consists of concomitant radiotherapy and TMZ followed by TMZ alone for 6 cycles. This radiochemotherapy protocol was shown to improve median OS from 12.1 to 14.6 months.4 During the last decade, newly tested adjuvant strategies such as the addition of bevacizumab5,6 have failed to demonstrate a benefit on OS.
Based on the preliminary results of the EF-14 trial, there has been a marked interest in tumor-treating fields (TTF) therapy as a front-line regimen. TTF therapy consists of a medical device that creates low-intensity and intermediate-frequency electric fields inducing an antimitotic effect on cancer cells. This device employs a transducer that is applied on a shaved scalp and connected to an electric generator and battery. The electric fields cause mitotic disorder by disrupting mitotic spindle formation during metaphases and thereby causing dielectrophoretic movement on organelles during cytokinesis.7
The EF-14 trial was a Phase III randomized, controlled trial in which the addition of TTF therapy to the radiochemotherapy protocol was shown to improve median PFS and median OS by 3.1 months and 4.9 months, respectively.8 Those results led to a new US Food and Drug Administration indication for first-line use in GBM. However, concerns were rapidly raised over the cost of TTF therapy, which is anticipated to be US$20 000/month.9 Here we aimed to determine the cost-effectiveness of TTF therapy added to standard therapy for newly diagnosed patients with GBM.
Methods
Model, Population, and Treatment
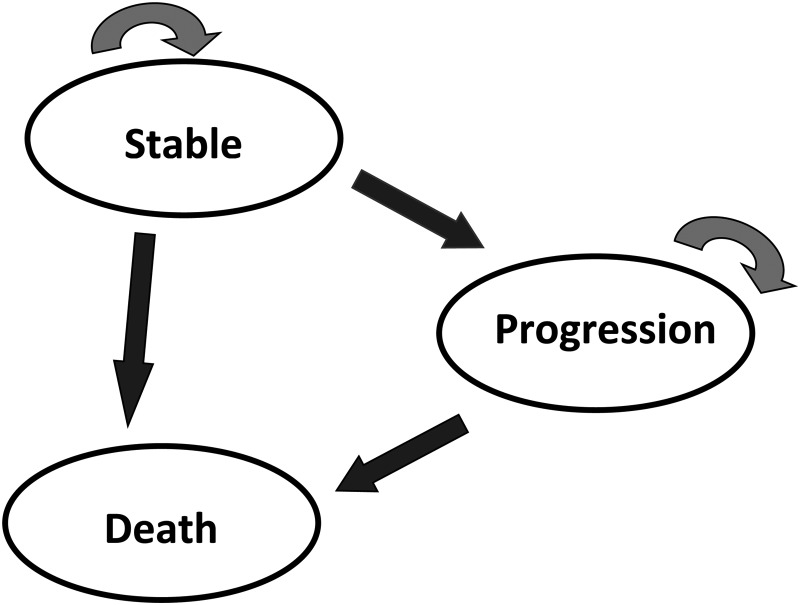
We constructed a Markov model with Tree Age Pro 2015, R1.2 (Williamstown, MA: Tree Age Software, Inc.). This type of model is widely used in health economic evaluations10 and has already been implemented for GBM.11–13 We used this model to measure and compare the medical cost and health outcomes for the 2 following strategies: standard of care alone with radiochemotherapy and addition of TTF therapy to standard of care. The Markov decision model included 3 mutually exclusive health states (Fig. 1): stable disease, progressive disease, and death. The target population was a hypothetical cohort of 1000 people with the same characteristics as those in the EF-14 trial8 (main inclusion criteria: newly diagnosed grade IV astrocytoma, Karnofsky Performance status score ≥70). The whole cohort was entered in the model and started the simulation in the stable-disease state. We assumed that all patients had previously undergone radiotherapy plus TMZ. We chose a cycle length of one month and a lifetime horizon. In each cycle, patients had a given probability of staying in the same health state or moving to the progression state or death state. Any patient could stay in only one health state at a time, and no backward transitions were permitted to stable disease.
Fig. 1.
The Markov model
The stable-disease state describes the time of radiochemotherapy protocol in which patients had TMZ alone for 6 cycles or in combination with TTF therapy for up to 24 cycles, to the first relapse. The progression state represents the time from the first relapse until advancement to the death state. Death is modeled as the absorbing state.
We conducted the analysis from the perspective of the French Health Insurance.
Transition Probabilities
Similarly to Messali et al.,13 we calculated the monthly transition probabilities (values associated with the arrows in Fig. 1) with the Declining Exponential Approximation of Life Expectancies method (DEALE method)14,15 using median PFS and OS from the EF-14 trial. The median OSs were those reported in the prespecified analysis, which evaluated only eligible patients who received the assigned treatment.8 The DEALE method assumes that patients have a constant hazard of death throughout the time and that patients’ survival describes a decreasing exponential curve. The transition probabilities calculated accordingly are presented in Table 1.
Table 1.
Transition probabilities for the Markov model
| Monthly Transition Probabilities |
||
|---|---|---|
| State Transition | TMZ Alone | TTF Therapy + TMZ |
| Stable disease to progression | 0.15910 | 0.09301 |
| Stable disease to death | 0.04346 | 0.03325 |
| Progression to death | 0.05800 | 0.05041 |
Abbreviations: TMZ, temozolomide; TTF, tumor-treating fields
Direct Costs and Effectiveness
The healthcare resource utilization inputs for each strategy were derived from a literature search conducted from 2010 to 2015 and focused on the management of GBM in French settings. We identified one study in which the patterns of care and associated direct costs of newly diagnosed patients with GBM were assessed from diagnosis to death or last follow-up date.16,17 Patients had similar baseline characteristics compared with our model hypothetical cohort since a large majority received the radiochemotherapy protocol. In this study, costs were estimated from the perspective of the French Health Insurance and were calculated using rates from year 2014. Indirect costs had not been included. Medical and nonmedical costs included chemotherapy drugs (at front-line and at tumor recurrences), hospital stays with the Diagnosis Related Group (DRG) tariffs extracted from the French hospital information system, specialized medical visits for outpatient services (oncologist or neurosurgeon), outpatient procedures (imaging, laboratory test), and medicalized transportation. The costs of surgery and concomitant radiotherapy and TMZ were ignored because the randomization occurred at the time when TMZ was supposed to be given alone. The cost of TMZ for the stable-disease state was calculated monthly for up to 6 cycles. Other direct medical costs were assumed to be provided equally throughout the stable and progressive-disease states for both strategies. Chemotherapy hospital stays were included only in the progressive state as this state corresponded to the administration of intravenous chemotherapies after relapse. Total costs for this study were divided by the mean duration of survival (reported at 20.1 months) to obtain monthly costs except costs for chemotherapies at relapse, which were divided by the time from relapse to death or last follow-up date (found at 7.9 months).
For TTF therapy, we considered the utilization of Optune (Novocure Inc.). To date, there is no regulated tariff for this medical device, but the cost reported by the company is €21 000 per month (corresponding to the provision of the device plus additional support). Similarly to the EF-14 trial, this cost was included in the stable-disease state for a maximum of 24 months. Patients could be kept on TTF therapy up to the second relapse. Knowing that the time to first progression was 7.1 months and that the median duration of TTF therapy was 9 months, we assumed that the device was used an average of 2 months in progressive disease (eg, until the second relapse). Hence, we input the cost of TTF therapy only for the first 2 cycles in the progressive state.
The costs related to chemotherapies (drugs and hospitalizations) at recurrence were applied monthly and equally among progressive-disease state cycles in the conventional strategy. For the TTF group, these costs were applied from the third cycle in the progressive state (eg, the first 2 cycles being for a month of TTF therapy).
Input costs are summarized in Table 2.
Table 2.
Input monthly costs for both strategies in Eurosa
| Stable Disease |
Progression |
||||
|---|---|---|---|---|---|
| Months 1–6 | Months 7–24 | Subsequent Months | Months 1–2 | Subsequent Months | |
| TMZ alone strategy | |||||
| Costs other than drugs | |||||
| Chemotherapy hospital stays | 0 | 0 | 0 | 390 | 390 |
| Other hospital staysb | 1133 | 1133 | 1133 | 1133 | 1133 |
| Transports | 315 | 315 | 315 | 315 | 315 |
| Imaging | 50 | 50 | 50 | 50 | 50 |
| Medical visits | 25 | 25 | 25 | 25 | 25 |
| Biologic exams | 9 | 9 | 9 | 9 | 9 |
| Drugs costs | |||||
| Adjuvant TMZ | 789 | 0 | 0 | 0 | 0 |
| Chemotherapy at reccurencec | 0 | 0 | 0 | 1650 | 1650 |
| TOTAL | 2321 | 1532 | 1532 | 3572 | 3572 |
| TTF therapy strategy | |||||
| Costs other than drugs and devices | |||||
| Chemotherapy hospital stays | 0 | 0 | 0 | 0 | 390 |
| Other hospital staysb | 1133 | 1133 | 1133 | 1133 | 1133 |
| Transports | 315 | 315 | 315 | 315 | 315 |
| Imaging | 50 | 50 | 50 | 50 | 50 |
| Medical visits | 25 | 25 | 25 | 25 | 25 |
| Biologic exams | 9 | 9 | 9 | 9 | 9 |
| Drugs and devices costs (€) | |||||
| Adjuvant TMZ | 789 | 0 | 0 | 0 | 0 |
| TTF therapy | 21 000 | 21 000 | 0 | 21 000 | 0 |
| Chemotherapy at reccurencec | 0 | 0 | 0 | 0 | 1650 |
| TOTAL | 23 321 | 22 532 | 1532 | 22 532 | 3572 |
Abbreviations: TMZ, temozolomide; TTF, tumor-treating fields.
aExcept for the cost of TTF therapy, all monthly costs were derived from a French cohort study after conversion of total costs to monthly costs.
bOther hospital stays include long-term care, home care,and rehabilitation.
cIncludes TMZ and other chemotherapies commonly used (eg, bevacizumab).
Our effectiveness outcome was life expectancy after each cycle. Hence, the effectiveness input was one month regardless if patients were in the stable or progressive state, and zero for the death state. We did not use quality adjusted-life-year (QALY) because of the lack of relevant published data on health-state utilities associated with GBM.
Analysis
We calculated the incremental cost-effectiveness ratio (ICER) expressed as monetary costs per life-years gained (LYG). We applied a 4% annual discount rate to costs and outcomes according to French national guidelines.10 Our threshold limit was arbitrarily chosen at €100 000/LYG. We performed 1-way deterministic sensitivity analysis for all parameters in order to assess the impact that a fixed change in each parameter has on the ICER. We applied±20% on costs,±50% on discount rate, and±2 weeks on median PFS and OS. Since we anticipated finding a high ICER, we also conducted a threshold sensitivity analysis in which the range of cost of TTF therapy varied between €2000 and €21 000/month. This was aimed at exploring the cost of TTF therapy that could reach a more acceptable ICER. Finally, we conducted a probabilistic sensitivity analysis using a second-order Monte Carlo analysis with symmetric triangular distributions for each parameter. The principle of triangular distribution is to apply for each parameter a likeliest, minimum, and maximum value. The likeliest values corresponded to those that were applied for the base-case analysis. The ranges for sensitivity analyses (minimum-maximum) that we applied were the same as those in the one-way sensitivity analyses. The multivariate probabilistic analysis was performed running 1000 patients in 10 000 Monte Carlo iterations.
Results
Base Case
The base-case analysis showed a life expectancy of 22.08 months in the TTF therapy strategy and 18 months in the conventional strategy (incremental effectiveness: 4.08 life-months gained or 0.34 LYG). The total costs of TTF therapy and conventional therapy strategies were €243 141 and €57 665, respectively (incremental cost: €185 476). This analysis resulted in an ICER of €549 909/LYG. After applying a 4% annual rate discount, the ICER was estimated at €596 411/LYG (incremental cost: €180 431; incremental effectiveness: 0.3 LYG).
Sensitivity Analysis
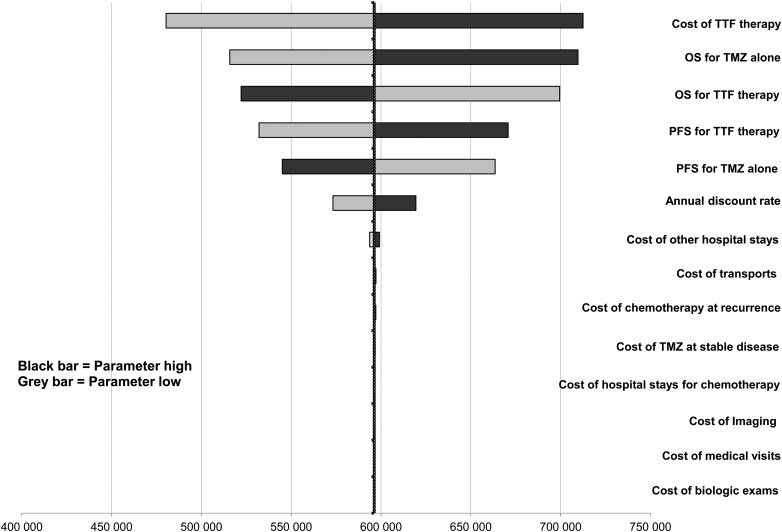
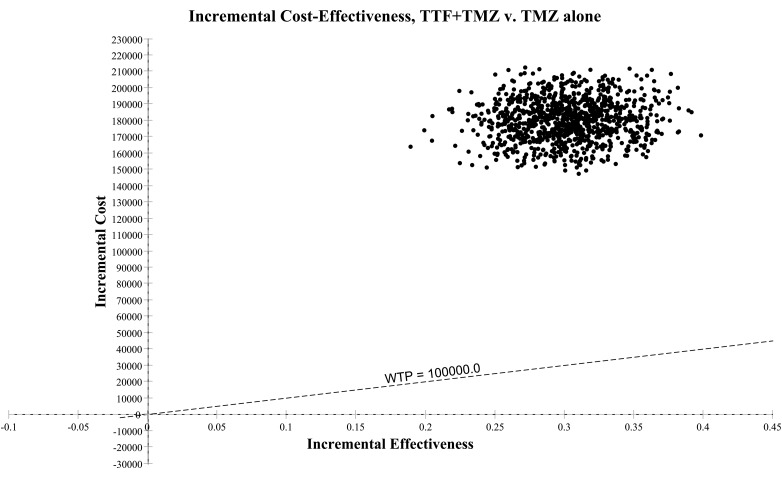
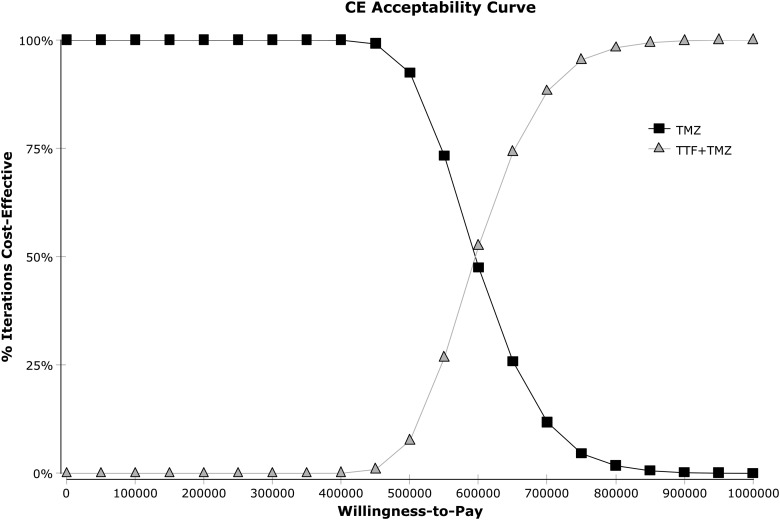
One-way sensitivity analyses on the ICER were represented in a tornado diagram based on assumptions previously described (Fig. 2). The parameters with the most influence on the ICER were the cost of TTF therapy, followed by OS and PFS in both arms. The diagram shows that the variation of each parameter in every case exceeds the threshold limit (€100 000/LYG). The threshold sensitivity analysis on the cost of TTF therapy showed that at a cost of €10 000/month, the ICER would be €292 353/LYG. At a cost of €2000/month (price discounted by approximately 90%), the ICER would be €71 220/LYG. At a monthly cost of €3000, the ICER would fall below the threshold of €100 000/LYG (€98 862/LYG). The probabilistic sensitivity analysis is represented by a Monte Carlo diagram in Fig. 3. The 95% confidence interval of the ICER was estimated at €447 017/LYG to €745 805/LYG. At a threshold of €100 000/LYG, the probability of TTF therapy being cost-effective is 0% (Fig. 4). Fig. 4 also shows the probability of TTF being cost-effective for other threshold limits than €100 000/LYG.
Fig. 2.
Tornado diagram (calculated with discounted incremental cost-effectiveness ratio (ICER) expressed as €/life-years gained [LYG]). One-way sensitivity analysis in a tornado diagram. Variations on variables were ±2 weeks for survival parameters, ±20% for cost, and ±50% discount rate.
Fig. 3.
Monte Carlo diagram (calculated with discounted incremental cost-effectiveness ratio [ICER] expressed as €/life-years gained [LYG]). ICER scatterplots per year with a willingness- to- pay (WTP) equal to €100 000/LYG. Abbreviations: TMZ, temozolomide; TTF, tumor-treating fields.
Fig. 4.
Cost effectiveness acceptability curve (calculated with discounted incremental cost-effectiveness ratio [ICER] expressed as €/life-years gained [LYG]). The willingness-to-pay corresponds to a given threshold ICER expressed as €/LYG. Abbreviations: TMZ, temozolomide; TTF, tumor-treating fields.
Discussion
We evaluated the cost-effectiveness of adding TTF therapy to standard of care using a 3-health-state Markov model. The model had the same illustrative structure compared with other recently published studies on GBM.11,13 Our effectiveness results are consistent with those reported in the EF-14 trial (for the TTF group, life expectancy was 22.08 months in our model and median OS of 20.5 months in the trial; for the TMZ alone group, life expectancy was 18 months in our model and median OS of 15.6 months in the trial), leading to a comparable incremental effectiveness (4.08 months in our model and 4.9 months in the trial).
The slight difference can be explained by the fact that life expectancy and median survival are mathematically different. Indeed, life expectancy corresponds to the arithmetic mean of the actual survival times of all individuals, whereas median survival is the length of time from the randomization in which half of the patients in a group of patients are still alive.
The results are also consistent for evaluation of costs. In the conventional strategy (TMZ alone starting from the second stage of the radiochemotherapy protocol), total undiscounted costs were estimated at €57 665. This excludes costs of surgery and concomitant radiotherapy and TMZ. The costs were similar to those reported in a French pharmacoepidemiologic study16 in which total costs from diagnosis to death were €70 201 including €18 500 for surgery and concomitant radiotherapy-TMZ. Furthermore, the incremental undiscounted cost of €185 476 obtained in our model is consistent with the utilization of TTF therapy at a cost of €21 000/month for 9 months.
Following the emergence of costly strategies for the treatment of GBM, a growing body of literature on cost-effectiveness studies has been published over the last years. Two were aimed at assessing cost-effectiveness of fluorescence-guided surgery with 5-ALA18,19 compared with conventional surgery and reported ICERs of €6700/LYG and €4550/additional complete resection achieved, respectively. Others have evaluated the cost-effectiveness of TMZ as adjuvant therapy.13,20 In the economic evaluation conducted with the trial validating the radiochemotherapy protocol, the incremental cost of TMZ was €9402, and the incremental effectiveness was 0.252 LYG, leading to an ICER of €37 361/LYG.20 More recently, Messali et al.13 reported an ICER of US$8 875/QALY for TMZ used at the same disease stage. There is also a growing concern on cost issues with bevacizumab for both front-line and late stage of GBM.11,16
This study is, to our knowledge, the first to report the cost-effectiveness of TTF therapy added to standard of care in newly diagnosed GBM patients. We found an ICER of €596 411/LYG, which is far beyond conventional thresholds even if we take rare diseases into account. Although cost-effectiveness and cost-utility studies are conceptually different (the first evaluates an ICER and uses outcome measures such as life expectancy; the second evaluates an incremental cost-utility ratio [ICUR] and uses QALY as an outcome measure), one can remark that the ICER of TTF therapy has a similar magnitude as the ICUR of bevacizumab for first-line treatment (US$439 764/QALY).11 However, the factors explaining such substantial ICER/ICUR are different. The addition of bevacizumab to standard of care induces a significant incremental cost but, most importantly, it has null incremental effectiveness (no improvement of median OS) and a very modest increase of QALYs. Consequently, regardless of any cost matters, the use of bevacizumab as front-line therapy appears to be unjustified because it has no impact on patient survival.
Conversely, the high ICER for TTF therapy is mainly due to a dramatic increase in cost and not a lack of effectiveness in terms of OS improvement. As shown in the tornado diagram, the cost of TTF therapy was the parameter with the highest influence on ICER. In France, cost-effectiveness evaluation does not have a key role in decision-making for reimbursement of health technologies since reimbursement is mainly decided on the basis of clinical effectiveness. However, assuming that a positive opinion is provided for reimbursement, it is almost certain that pricing negotiation with the manufacturer would not lead to an agreement and consequently restrict the access to patients. In countries where cost-effectiveness is a major component of decision-making, such as the United Kingdom, the ICER would be far beyond the conventional threshold and would lead to a non-recommendation of the technology. Consequently, the likelihood of having the technology refunded by sickness funds is very small, although it is the first treatment for GBM to demonstrate a clinically significant benefit to OS since 2005. Very little is known about factors that might explain the anticipated cost of the technology. Health economic evaluations have been extensively applied to anticancer therapies. Recently, the ICER of cetuximab versus bevacizumab as first-line treatment for metastatic colorectal cancer (mCRC) was estimated at US$97 223/LYG.21 In another study, the ICUR of bevacizumab in addition to chemotherapy in first- and second-line treatment for mCRC was estimated at US$571 240/QALY and US$364 083/QALY, respectively.22 A high level of ICUR was also found for bevacizumab added to chemotherapy in advanced non–small cell lung cancer (incremental QALYs: 0.13; incremental cost: US$72 000; ICUR: US$ 560 000/QALY).23 Recently approved therapies for advanced melanoma such ipilimumab and nivolumab have dramatically changed the management of the disease, but these drugs are very expensive (approximately €60 000–80 000/patient). However, the associated incremental effectiveness is notable, which leads to more acceptable ICERs/ICURs. Barzey et al.24 estimated that ipilimumab as second-line treatment for advanced melanoma had an ICER of US$78 218/LYG (incremental effectiveness: 1.88 LYG; incremental cost: US$146 716) versus best supportive care. More recently, Bohensky et al.25 found that the ICER of nivolumab compared with ipilimumab for the treatment of Braf wild-type advanced melanoma in Australia was AUD$48 851/LYG (incremental effectiveness: 1.58 LYG; incremental cost:AUD$77 119). Based on these examples, ICERs higher than €500 000/LYG are rarely encountered.
With a cost of €21 000 per month and a median of 9 months of treatment, TTF therapy would be one of the most expensive treatments using a medical device. A price halved by 2 would still exceed conventional benchmarks (€292 353/LYG). A decrease of monthly price to €3000 per month would lead to a more acceptable ICER.
To our knowledge, no data from the EF-14 trial are currently available to compare the clinical outcome of patients who continued TTF therapy after the first progression with those who discontinued after the first progression. Hence, the impact on the ICER of early TTF therapy discontinuation after the first progression cannot be measured.
Our study has several limitations. First, we conducted a cost-effectiveness evaluation and not a cost-utility study using QALYs as the outcome measure. To our knowledge, there is only one study by Garside et al.26 that reported estimates of health-state utilities associated with GBM. In that study, health-related utilities were elicited from only 36 healthy volunteers among the general UK population, which is very unlikely to be representative for the French population. Consequently, we found it irrelevant to conduct a cost-utility analysis based on these data, and we preferred to use a cost-effectiveness analysis with OS as an outcome measure, which is a valid option according to evaluation guidelines.10 Second, our cost-effectiveness study is based on a model that is not as accurate as an economic evaluation alongside a clinical trial. To our knowledge, no cost-effectiveness evaluation has been conducted within the EF-14 trial. The model can be considered a simplistic representation of the GBM pathway as we assumed having only one state of progression. In the EF-14 trial, patients could receive TTF therapy up to the second progression/recurrence. Hence, a 4-health-state model with a second progression state could have been more appropriate. Furthermore, no backward transition from progression state to stable disease was possible. We used these assumptions due to lack of available information from the EF-14 trial. The third limitation is that transition probabilities were derived from only one Phase III trial. In the EF-14 trial, the survival estimates in the radiochemotherapy-alone arm may, however, be considered as robust because PFS and OS were very similar to those published by Stupp et al.4 Survival estimates for the TTF therapy will have to be confirmed from registries or prospective studies based on real-life evidence. This is of particular importance since our sensitivity analysis revealed that PFS and OS in the TTF strategy also had a strong influence on the ICER. We also used a simplified distribution type (symmetric triangular) for the Monte Carlo simulation, but we assume this had no impact on our conclusions given the magnitude of ICER. Finally, the healthcare resource utilization inputs for each strategy were mainly taken from a study that had a limited sample size (217 patients) and included only direct costs.
In conclusion, although our work has inherent limitations due to modeling and limited available information, our study emphasizes that the current cost of TTF results in an ICER that is much too high to be considered cost-effective. Strong regulation on its price by health authorities could make this technology more affordable and accessible to patients.
Funding
None declared.
Acknowledgments
The authors would like to thank Peter Auguste and Dr G.J. Melendez-Torres (University of Warwick, England) for revising the manuscript.
Conflict of interest statement. None for FBA, ML, GA, and XA. FD reported receiving travel and accommodation from Novocure to attend a scientific conference. JH reported receiving trial support from Novocure and serving on an advisory board for Novocure.
References
- 1.Bauchet L, Mathieu-Daude H, Fabbro-Peray P et al. Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol. 2010;12(7):725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11(9):504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis ESMO Guidelines Working Group. . High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii93–ii101. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 5.Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert MR, Dignam JJ, Armstrong TS et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner SG, Gergel T, Wu H, Lacroix M, Toms SA. The effect of field strength on glioblastoma multiforme response in patients treated with the NovoTTF-100A system. World J Surg Oncol. 2014;12:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stupp R, Taillibert S, Kanner A et al. Tumor treating fields (TTFields): A novel treatment modality added to standard chemo- and radiotherapy in newly diagnosed glioblastoma—First report of the full dataset of the EF14 randomized phase III trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 9.American Society of Clinical Oncology. Tumor Treating Fields Device Extends Glioblastoma Survival, but Cost Questions Raised. 2015. Available at http://am.asco.org/tumor-treating-fields-device-extends-glioblastoma-survival-cost-questions-raised (last accessed May 2016).
- 10.Haute Autorité de santé. Choices in Methods for Economic Evaluation: a methodological guide. 2012. Available at http://www.hassante.fr/portail/upload/docs/application/pdf/2012-10/choices_in_methods_for_economic_evaluation.pdf (last accessed May 2016).
- 11.Kovic B, Xie F. Economic Evaluation of Bevacizumab for the First-Line Treatment of Newly Diagnosed Glioblastoma Multiforme. J Clin Oncol. 2015;33(20):2296–2302. [DOI] [PubMed] [Google Scholar]
- 12.Martikainen JA, Kivioja A, Hallinen T, Vihinen P. Economic evaluation of temozolomide in the treatment of recurrent glioblastoma multiforme. Pharmacoeconomics. 2005;23(8):803–815. [DOI] [PubMed] [Google Scholar]
- 13.Messali A, Hay JW, Villacorta R. The cost-effectiveness of temozolomide in the adjuvant treatment of newly diagnosed glioblastoma in the United States. Neuro Oncol. 2013;15(11):1532–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”). I. Validation of the method. Am J Med. 1982;73(6):883–888. [DOI] [PubMed] [Google Scholar]
- 15.Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (the “DEALE”). II. Use in medical decision-making. Am J Med. 1982;73(6):889–897. [DOI] [PubMed] [Google Scholar]
- 16.Diebold G, Ducray F, Henaine AM et al. Management of glioblastoma: comparison of clinical practices and cost-effectiveness in two cohorts of patients (2008 versus 2004) diagnosed in a French university hospital. J Clin Pharm Ther. 2014;39(6):642–648. [DOI] [PubMed] [Google Scholar]
- 17.Henaine AM, Paubel N, Ducray F et al. Current trends in the management of glioblastoma in a French University Hospital and associated direct costs. J Clin Pharm Ther. 2016;41(1):47–53. [DOI] [PubMed] [Google Scholar]
- 18.Esteves S, Alves M, Castel-Branco M, Stummer W. A pilot cost-effectiveness analysis of treatments in newly diagnosed high-grade gliomas: the example of 5-aminolevulinic Acid compared with white-light surgery. Neurosurgery. 2015;76(5):552–562; discussion 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slof J, Diez Valle R, Galvan J. Cost-effectiveness of 5-aminolevulinic acid-induced fluorescence in malignant glioma surgery. Neurologia. 2015;30(3):163–168. [DOI] [PubMed] [Google Scholar]
- 20.Lamers LM, Stupp R, van den Bent MJ et al. Cost-effectiveness of temozolomide for the treatment of newly diagnosed glioblastoma multiforme: a report from the EORTC 26981/22981 NCI-C CE3 Intergroup Study. Cancer. 2008;112(6):1337–1344. [DOI] [PubMed] [Google Scholar]
- 21.Shankaran V, Ortendahl JD, Purdum AG et al. Cost-Effectiveness of Cetuximab as First-line Treatment for Metastatic Colorectal Cancer in the United States. Am J Clin Oncol. 2015. Sep 22. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein DA, Chen Q, Ayer T et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis. J Clin Oncol. 2015;33(10):1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value Health. 2011;14(6):836–845. [DOI] [PubMed] [Google Scholar]
- 24.Barzey V, Atkins MB, Garrison LP, Asukai Y, Kotapati S, Penrod JR. Ipilimumab in 2nd line treatment of patients with advanced melanoma: a cost-effectiveness analysis. J Med Econ. 2013;16(2):202–212. [DOI] [PubMed] [Google Scholar]
- 25.Bohensky M, Pasupathi K, Gorelik A, Kim H, Harrison JP, Liew D. A Cost Effectiveness Analysis of Nivolumab Compared to Ipilimumab for the Treatment of Braf Wild-Type Advanced Melanoma in Australia. Value Health. 2015;18(7):A340. [DOI] [PubMed] [Google Scholar]
- 26.Garside R, Pitt M, Anderson R et al. The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation. Health Technol Assess. 2007;11(45):iii–iiv, ix–221. [DOI] [PubMed] [Google Scholar]