Abstract
Introduction
A major limitation of current liposomal cancer therapies is the inability of liposome therapeutics to penetrate throughout the entire tumor mass. This inhomogeneous distribution of liposome therapeutics within the tumor has been linked to treatment failure and drug resistance. Both liposome particle transport properties and tumor microenvironment characteristics contribute to this challenge in cancer therapy. This limitation is relevant to both intravenously and intratumorally administered liposome therapeutics.
Areas covered
Strategies to improve the intratumoral distribution of liposome therapeutics are described. Combination therapies of intravenous liposome therapeutics with pharmacologic agents modulating abnormal tumor vasculature, interstitial fluid pressure, extracellular matrix components, and tumor associated macrophages are discussed. Combination therapies using external stimuli (hyperthermia, radiofrequency ablation, magnetic field, radiation, and ultrasound) with intravenous liposome therapeutics are discussed. Intratumoral convection-enhanced delivery (CED) of liposomal therapeutics is reviewed.
Expert opinion
Optimization of the combination therapies and drug delivery protocols are necessary. Further research should be conducted in appropriate cancer types with consideration of physiochemical features of liposomes and their timing sequence. More investigation of the role of tumor associated macrophages in intratumoral distribution is warranted. Intratumoral infusion of liposomes using CED is a promising approach to improve their distribution within the tumor mass.
Keywords: Convection enhanced delivery, drug delivery, imaging, intratumoral administration, intratumoral distribution, liposomes, nanoparticles, tumor penetration
1. Introduction
One of the major challenges for effective liposomal cancer therapy is the inability to deliver the therapeutic agent throughout the entire tumor mass. This problem is not unique to liposomes but is a common problem limiting the effectiveness of all types of therapeutic agents including small molecules, biologic agents and other nanoparticle-based drug delivery systems [1–5]. Both anatomic and physiological barriers must be overcome by intravenously administered liposomes [3, 6]. First, intravenously administered liposomes need to evade clearance by the reticuloendothelial system (RES) organs. Some of the avenues taken by researchers to evade the RES have been modification of the particle size, surface charge, shape, and pegylation of the liposome formulation [7, 8]. For those liposomes reaching the tumor, early fluorescent imaging studies revealed the liposomes could extravasate through leaky, poorly formed tumor vasculature into the tumor interstitium by a passive targeting mechanism, but moved only a short distance from the blood vessels [9]. The accumulated liposomes were retained in the tumor due to the poor lymphatic drainage. Maeda termed this process the enhanced permeability and retention (EPR) effect [10]. Recently, the role of the EPR effect as a universal feature of liposome accumulation in tumors has been revisited [11]. Positron emission tomography imaging studies in different types of canine spontaneous tumors demonstrated copper-64 (64Cu)-liposome uptake was more likely in carcinomas compared with sarcomas, indicating liposome cancer therapeutics may need to be designed to work in a particular tumor type [12, 13]. Moreover, liposome formulations exhibiting increased microvascular permeability and retention may not necessarily have better tumor penetrability and deliver liposomal drug to all tumor cells within the tumor. This inhomogeneous tumor coverage by liposomal drugs is a leading cause of treatment failure and why the use of liposomes to overcome drug resistance has had limited success [4, 14].
In some cases, loco-regional delivery of liposomal agents by direct intratumoral injection or using convection enhanced delivery (CED) has been proposed as a strategy to by-pass the RES and tumor vasculature barriers. This option could benefit a subset of patients with cancers that spread locally or regionally rather than systemically by treating the primary tumor site to control local cancer invasion [15, 16]. With loco-regional administration, access to the tumor is critical so these methods are used with superficial tumors or by placement of an interventional catheter using image guidance. Less prevalent with liposomal chemotherapeutics, intratumoral administration gained favor to deliver cationic liposome-based gene therapies to shift the organ distribution and increase tumor liposome concentrations [17, 18]. Intratumoral administration also has been investigated for delivery of therapeutic beta-emitting radionuclides as a means to decrease non-target tissue radiation exposure observed after intravenous injection [16]. This review will discuss intratumoral injection as a strategy to improve the accumulation and distribution of liposomes within the tumor. Methods to achieve a homogeneous tumor distribution of liposomes delivered loco-regionally will be discussed.
The inhomogeneous intratumoral distribution of liposomes can be attributed to both the tumor’s architecture and its macromolecular/nanoparticle transport properties [4]. Abnormal blood vessel and defective lymphatic networks as well as increased contractility of tumor stroma cells contribute to the elevated interstitial fluid pressures (IFP) observed in tumors [2, 4, 19]. High tumor IFP shifts the fluid flow from the tumor center to the tumor periphery, preventing effective penetration of the liposomes within the tumor microenvironment to reach all tumor cells [4]. Penetration of liposomes through the tumor interstitium is also hindered by the dense extracellular matrix (ECM) from increased levels of ECM components such as collagen and glycosaminoglycans (hyluronan) [19, 20].
Both intravenously and intratumorally administered liposome cancer therapies face additional barriers once distributed within the tumor. Intratumoral liposomes must have the capability to release their drug cargo in sufficient amounts. One strategy to increase intratumoral drug release utilizes tumor-responsive liposome formulations that are triggered to release their encapsulated drugs once reaching the tumor by interacting with the local tumor environment’s unique physiology which differs from normal tissue. These include liposome formulations that are responsive to lower pH, increased redox potential and protein/enzyme levels (phospholipidase A2 and matrix metalloproteinase cleavage) in the tumor [21, 22]. The prolific tumor-responsive liposome literature is beyond the scope of this article but has been recently reviewed [21–23]. A different strategy highlighted in this article is the use of liposome formulations responsive to external stimuli (hyperthermia, radiofrequency ablation, ultrasound, magnetic field, and radiotherapy) [3, 21–27]. By applying an external stimulus to the local tumor region, the liposomes located within the tumor are triggered to release their drug payload. The free drug can readily diffuse (depending on the tissue binding affinity of free drug) and be taken up by tumor cells [2, 7, 25]. A limiting factor with this strategy is there must be sufficient drug levels available to kill all tumor cells. One distinct advantage of liposomes containing beta-emitting therapeutic radionuclides is that they do not have to have a mechanism for releasing the radionuclide from the liposomes because the therapeutic beta radiation emitted from the liposome-delivered therapeutic radionuclide extends through many cell diameters (1–4 mm on average) from the deposited site of the liposome, thereby eradicating a micro-field region of the tumor. This extended range of therapeutic beta radiation also means that the liposomes do not have to reach all the cells in a tumor to deliver effective treatment and so the requirements of a homogeneous distribution of liposomes in the tumor are less rigorous than for liposomes carrying chemotherapeutics or nucleic acid-based agents [16, 28].
Once reaching the tumor, liposome formulations carrying chemotherapeutics and nucleic acid agents which act on targets in the cytoplasm or nucleus must be able to cross cellular and subcellular membranes [7, 21]. The design of liposome formulations containing cell-penetrating peptides to assist with intracellular and organelle delivery has been recently reviewed [21, 22]. This review will summarize and evaluate the strategies being investigated for improving the intratumoral distribution of liposome cancer therapeutics (Figure 1). Many of these strategies could also be applied to other types of nanoparticle therapeutics. Several reviews concerning nanoparticle drug delivery strategies to improve intratumoral distribution have been published [3, 5, 13, 19, 21, 29–32]. The approach of intravenous liposome cancer therapies combined with pharmacologic agents that can modify the tumor microenvironment or improve tumor penetrability will be described. Strategies using external stimuli methods (hyperthermia, radiofrequency ablation, ultrasound, magnetic field, and radiotherapy) to improve intratumoral accumulation and drug release of intravenous liposome therapies will be discussed. As an alternative strategy, loco-regional direct intratumoral injection or infusion using convection enhanced delivery (CED) methods will be described.
Figure 1.
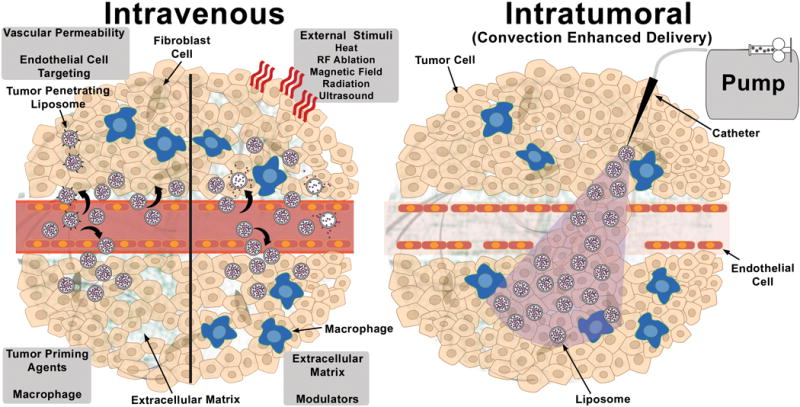
Schematic diagram describing the strategies for improving intratumoral accumulation and distribution of liposomal therapeutics administered by intravenous (Left Panel) and intratumoral (Right Panel) routes. Intravenous panel (Clockwise beginning with upper right quadrant) depicts liposome penetration into the tumor as well as intravascular and intratumoral drug release after applying an external stimuli (heat, radiofrequency (RF) ablation, magnetic field, radiation or ultrasound), or administering a pharmacologic agent to modulate the extracellular matrix (lower right quadrant), prime the tumor or macrophage association (lower left quadrant), increase vascular permeability or target angiogenic endothelial cells (upper left quadrant). The penetration of liposomes modified with tumor penetrating peptides is also depicted. Intratumoral panel depicts intratumoral dispersion and retention of the liposomal therapeutic following convection enhanced delivery through a catheter connected to a syringe pump.
2. Techniques for Monitoring Intratumoral Distribution and Tumor Penetration
There have been several techniques reported for quantifying the intratumoral distribution and penetration of liposomes in relationship to the tumor vasculature and tumor microenvironment [4, 5, 33, 34]. These include using in vitro multicellular spheroid models to track the penetration of the empty liposome carrier or liposomal drug. The liposomes are usually labeled with fluorescent probes or radioactivity for monitoring their distribution by fluorescent microscopy or autoradiography, respectively. In some cases, the drug (i.e. doxorubicin as an example) has endogeneous fluorescent properties that do not require tagging of the fluorescent probe to the liposomes. In vivo methods include intravital microscopy to monitor the tumor distribution of fluorescent liposomes through window chambers placed in the tumors of living animals. Another in vivo method uses immunohistochemistry of tumor tissue collected from animal tumor models following infusion of the liposomal drug. An additional feature of immunohistochemistry is the ability to co-localize the fluorescent liposomes with tumor biomarkers to compare the liposome distribution with changes in these markers following treatment. Currently, whole body clinical scanners as well as pre-clinical small animal imaging systems (single photon emission computed tomography (SPECT), positron emission tomography (PET), computed tomography (CT), magnetic resonance imaging (MRI) and optical imaging) are being integrated into studies of the macro- and micro-distribution of liposomes within tumors. Although the spatial resolution of these scanners is limited to millimeter range, they can provide important information regarding regional distribution within a tumor and overall percent global liposome uptake by the tumor. Additionally, this global and regional information of liposome distribution can be correlated with immunohistochemical or autoradiographical information from the ex-vivo tumor sections. Finally, imaging can also play a role in guidance of the liposomal drug infusion into the tumor interstitium during direct or convection enhanced intratumoral delivery. Examples of the use of imaging for monitoring the macro- and micro-distribution of liposomes in tumor are included in this review.
3. Current Strategies
A variety of strategies to improve the intratumoral distribution of liposomal cancer therapies has been investigated. This review will focus on pharmacologic or external stimuli approaches to modify the accumulation and intratumoral distribution of liposomes following either systemic or loco-regional administration (Table 1).
Table 1.
Strategies to improve intratumoral distribution of liposome therapies.
| Strategy | Method | Route | Key Studies | Cite |
|---|---|---|---|---|
| Pharmacologic | Anti-angiogenesis Inhibitors | IV | Pretreatment with DC-101 monoclonal antibody normalized blood vessel pores resulting in decreased liposomal doxorubicin accumulation. In contrast, conjugation of DC101 on the liposomal surface did not cause vascular normalization as observed with co-administration. DC101-immunoliposomal doxorubicin was more efficacious compared with liposomal doxorubicin. | [38, 39] |
| Pharmacologic | Angiogenic Endothelial Cell Targeting | IV | A specific interaction of RGD- liposomes with tumor blood vessels has been observed. Tumor growth inhibition was increased for liposomal doxorubicin conjugated with RGD or APRPG peptides on their surface compared with non-targeted liposomal doxorubicin. | [40–43] |
| Pharmacologic | Vascular Disrupting Agents | IV | Liposome encapsulation of vascular disrupting agents is one approach to improve vascular disrupting agent release kinetics and toxicity profile. | [45, 47–50] |
| Pharmacologic | Vascular Priming | IV | TNF-α pretreatment increased tumor uptake of 100 nm liposomal doxorubicin, contributing to improved tumor response in a melanoma tumor model. | [51] |
| Pharmacologic | Tumor Penetrating Peptides | IV | Increased tumor penetration of liposomal doxorubicin was observed when tumor penetrating peptides were co-administered or attached to liposomal surface. | [52–55] |
| Pharmacologic | Collagen Depletion | IV | Collagenase pretreatment resulted in higher tumor accumulation and more penetration from blood vessels for TP3 monoclonal antibody and liposomal doxorubicin. | [57, 58] |
| Pharmacologic | Inhibition of Collagen Synthesis | IV | After Lorastan pretreatment, an increase in tumor accumulation of fluorescent polystyrene nanoparticles with more penetration from blood vessels was observed. Liposomal doxorubicin combined with Lorastan pretreatment resulted in better therapy response compared with liposomal doxorubicin alone. | [59] |
| Pharmacologic | Hyaluronan Depletion | IV | Hyaluronidase pretreatment resulted in more tumor penetration of liposomal doxorubicin. | [60] |
| Pharmacologic | Inhibition of Hyaluronan Synthesis | IV | DiD-labeled empty liposomes penetrated further from the vasculature and were distributed more within 4T1 tumors following pretreatment with MU-prodrug liposomes. Liposomal doxorubicin combined with MU-prodrug liposome pretreatment resulted in reduced tumor growth and increased survival in mice bearing 4T1 tumors. | [20] |
| Pharmacologic | Tumor Priming | IV | Chemotherapy pretreatment can prime the tumor microenvironment thereby providing more gaps for liposomal drugs to penetrate through. Cyclophosphamide priming enhanced delivery and efficacy of HER2-targeted liposomal doxorubicin. | [61–63] |
| Pharmacologic | Tumor Associated Macrophages | IV | Recent evidence suggests tumor associated macrophages are capable of assisting in the movement of phagocytosed liposomes and other nanoparticles throughout the tumor. | [65–67] |
| External Stimuli | Hyperthermia | IV | Recent studies focus on improving drug release from the liposome and increasing free drug concentrations within the tumor. Approaches include modification of thermosensitive liposome formulations and hyperthermia protocol design. | [25, 26, 71–78, 148] |
| External Stimuli | Magnetic Field | IV | External permanent magnetic exposure to tumor following SPION magnetoliposomes administration resulted in increased accumulation of magnetoliposomes in the exposed tumor. | [82, 83] |
| External Stimuli | Radiation | IV | Liposomal doxorubicin with radiotherapy can delay tumor growth in preclinical tumor models. Concomitant radiotherapy and liposomal doxorubicin administration was shown to increase the tumor vascular permeability and intratumoral doxorubicin penetration, and delay tumor growth in an osteosarcoma model. | [87, 88] |
| External Stimuli | RF Ablation | IV | Advantages of combining pegylated liposomal doxorubicin with RF ablation include improved liposome uptake, larger RF ablation zone and reduced tumor growth. For further improvements in therapeutic efficacy of this combination therapy, the role of heat shock protein 70 and apoptosis as well as liposome formulation modifications for increased drug delivery are being investigated. Phase III ThermoDox™ study reported thermosensitive liposomes with RF ablation did not improve liver therapy outcomes compared with RF ablation alone. Further preclinical studies comparing non-thermosensitive and thermosensitive liposomes highlighted the importance of the timing of doxorubicin release with RF ablation-induced tumor microenvironment changes. | [24, 91–98, 100, 101] |
| External Stimuli | Ultrasound | IV | HIFU can improve vascular permeability of liposomes in tumors. HIFU studies have been combined with MRI image guidance to quantify intratumoral drug release. Ultrasound microbubble contrast combined with liposomal therapeutics can increase liposome permeability through blood vessels and blood brain barrier as well as improve drug and gene transport through sonoporation of cell membranes. | [27, 103, 105–123] |
| Loco-regional | Direct injection | IT | Liposomes carrying chemotherapeutic agents, radionuclides and genes have been investigated as a way to improve the dispersion and local retention of the encapsulated agent in the tumor. | [17, 18, 28, 124–126] |
| Loco-regional | CED | IT | Liposomal doxorubicin had large area of coverage compared with doxorubicin in a rodent intracranial brain tumor model. In an intracranial SF7796 brain cancer model, improved survival was observed with a single infusion of liposomal irinotecan compared with two intravenous infusions of irinotecan alone. Survival was greatly increased in rats with an aggressive U251 intracranial tumor after receiving a single infusion of rhenium-186 liposomes compared with tumor-bearing rats infused with unlabeled liposomes. |
[130, 132, 135] |
APRPG, alanine-proline-arginine-proline-glycine; CED, convection enhanced delivery; DiD, 1,1-dioctadecyl-3,3,3,3-tetramethylindodicarbocyanine; HER-2, human epidermal growth factor receptor-2; HIFU, high intensity focused ultrasound; IV, intravenous; IT, intratumoral; MU, 4-methylumbelliferone; MRI, magnetic resonance imaging; RF, radiofrequency; RGD, arginine-glycine-aspartic acid; SPION, superparamagnetic iron oxide nanoparticles; TNF-α, tumor necrosis factor-alpha
3.1 Systemic Administration
Following intravenous injection, the intratumoral distribution of liposome drugs at distances away from the tumor vasculature is limited due to high interstitial fluid pressure and dense extracellular matrix [2]. This section will review a number of pharmacologic and external stimuli methods that have been attempted to improve intratumoral distribution following intravenous liposomal administration.
3.1.1 Tumor Physiological Modifying Agents
Researchers have explored combination therapies with different pharmacological agents to influence the tumor microenvironment components with the goal of improving liposome accumulation and distribution within the tumor. In the following section, pharmacological approaches to modify tumor vasculature and tumor penetrability, interstitial fluid pressure, stroma composition and the interaction of non-tumor cells located within the extracellular matrix (tumor-associated macrophages) will be discussed.
3.1.1.1 Abnormal Tumor Vasculature
Targeting abnormal tumor vasculature to improve liposome extravasation and tumor penetrability has been actively studied [2]. Various markers observed in abnormal tumor vasculature have been identified. These include vascular endothelial growth factor (VEGF) and VEGF receptor-2 which target tumor cells as well as αvβ3 integrin (RGD) and galectin-1 receptor (anginex) which target angiogenic endothelial cells [3, 35–38]. The potential of liposomes for targeting these tumor vasculature markers was recognized early and may play a role in tumor vasculature imaging [3, 35–37]. Besides imaging applications, a number of pharmacological approaches have been investigated. Strategies targeting VEGF and VEGF receptor-2 to prevent new vessel growth, leading to tumor cell death and possible tumor vessel normalization have been studied [38, 39]. Chauhan et al found pretreatment of the anti-angiogenic monoclonal antibody DC101 did not improve liposomal doxorubicin therapy because DC101 modified the leaky tumor vessels and made them more like normal tissue vessels with smaller sized pores that the liposomes could not readily pass through [39]. Another approach has been to prepare liposomes targeting pro-angiogenic factors by conjugating the anti-angiogenic inhibitor (anti-VEGFR2 monoclonal antibody DC101) on the liposome surface [38]. Liposomal doxorubicin with DC101 monoclonal antibodies conjugated on their surface was more efficacious compared with liposomal doxorubicin. Interestingly, the researchers also observed a one-third decrease in tumor-associated intact capillaries in DC101-liposomal doxorubicin treated mice compared with the liposomal doxorubicin group. They also noted DC101-liposomal doxorubicin did not cause vessel normalization as noted by Chauhan et al. Liposomes targeting angiogenic tumor endothelial cell markers (αvβ3 integrin (RGD) and galectin-1 receptor (anginex) have been investigated, especially RGD targeting [40]. In this strategy, the targeted liposomes can bind and deliver their payload to angiogenic endothelial cells causing cell death which in turn starves the tumor of oxygen and nutrients. Early studies by Schiffelers et al reported visualization of a specific interaction of the RGD liposomes with tumor blood vessels and an improved tumor growth inhibition for RGD-liposomal doxorubicin over non-RGD liposomal doxorubicin [40, 41]. Similar tumor growth inhibition was noted by Asai for APRPG-liposomal doxorubicin [42, 43].
Vascular disrupting agents (VDA) cause immediate ischemia and nutrient depletion of established angiogenic blood vessels by interfering with microtubule formation, thus resulting in vascular collapse and tumor cell death [44–46]. By shutting down blood flow in the tumor, further intratumoral penetration of liposomal drug is decreased. The liposomal drug trapped within the tumor after vascular disruption could provide focused cell killing, but the extent of cell killing depends on the effectiveness of the entrapped drug within a more hypoxic and acidic pH tumor. A clinical trial update for the two main VDA classes, flavonoids and tubulin binding agents, was recently published [46]. The biggest challenges with the co-administration of VDAs with liposome therapies are controlling the degree of the vascular disruption and optimizing the timing sequence of the administration [46]. One approach to improve the drug release kinetics and toxicity of VDAs has been through liposome encapsulation of the VDA alone or in combination with a cytotoxic agent [45, 47–50]. In a melanoma model, RGD-targeted liposomes loaded with both combretastatin A-4 and doxorubicin had improved tumor growth inhibition over the RGD-liposome monotherapies [50].
The manipulation of abnormal tumor vasculature using agents (tumor necrosis factor-alpha (TNF-α), histamine, bradykinin, interleukin-2) that prime the tumor vasculature have also been studied to enhance liposome drug delivery [51].
3.1.1.2 Tumor Penetrating Peptides
The identification of a series of tumor penetrating peptides (TPP) containing the sequence motif R/KXXR/K at the C terminal has been shown to improve drug penetration in tumors [52, 53]. These peptides contain a tumor-homing sequence which transports the peptide to the tumor vasculature endothelium where it is proteolytically cleaved to expose an activated C-terminal CendR motif. The activated CendR then binds to neuropilin-1 which assists in extravasation, tumor penetration and cell internalization of the CendR peptide and any nanoparticle attached to it. For example, co-administration of the 9 amino acid-based arginine-glycine-aspartic acid (RGD) based TPP (iRGD) with liposomal doxorubicin led to improved tumor penetration of the liposomal doxorubicin [53]. More recently, improved tumor tissue transport and tumor growth inhibition was reported in pre-clinical glioblastoma and prostate tumor models following treatment of liposomal doxorubicin with TPPs conjugated on their surface [54, 55].
3.1.1.3 Interstitial Fluid Pressure
Tumors have an elevated interstitial fluid pressure because permeable blood vessels allow tumor uptake of increased fluid and serum proteins which cannot be easily removed through the defective lymphatic vessels. Also, as tumor cells proliferate, there is increased stromal burden as well as increased contractility of fibroblasts and dense collagen and elastin matrix. This high IFP greatly reduces drug movement from the capillaries into the tumor as well as movement within the tumor microenvironment [23].
Measurement of IFP in tumors can be challenging especially with tumors located deep within the body. Also it has been difficult to determine whether a clear relationship of IFP with a tumor therapy response is present. Pre-clinical measurement of IFP has generally been performed using wick-in-needle method which is invasive and difficult to use before and after treatment.
Recently, Stapleton et al reported a technique which will allow the measurement of spatial IFP [56]. These measurements use a robotic needle placement device guided under microcomputed tomography (CT) (Figure 2). Using liposomes encapsulating iodine contrast, the researchers demonstrated that they could compare IFP measurements within different regions of the tumor with the location of the liposome contrast in microCT images. Moreover, they were able to compare differences in liposome uptake and IFP in an orthotopic versus subcutaneous tumor xenograft model. Interestingly, they noted only a moderate relationship between IFP and liposome uptake. On the contrary, they observed a strong relationship between tumor perfusion and liposome uptake, suggesting tumor perfusion imaging could be used to stratify patients likely to benefit from liposome therapy.
Figure 2.
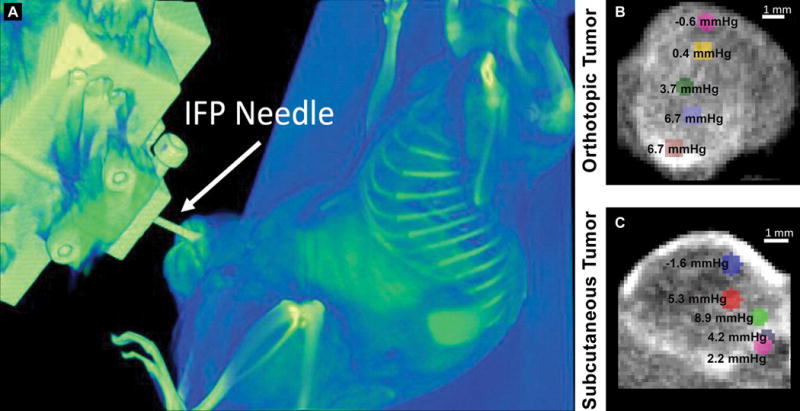
(A) A 3D volume rendering showing the position of the IFP needle penetrating the tumor volume for robotic IFP measurement during microCT scanning of MDA-MB-231 tumor-bearing mouse after injection of liposomes containing iodine contrast. Images showing point-based measurements of IFP overlaid on microCT images of orthotopic (B) or subcutaneous (C) tumors depicting the intratumoral distribution of liposomes containing iodine contrast. IFP measurements were higher in the tumor center than at the tumor periphery. Reproduced with permission from [56].
3.1.1.4 Modulation of Extracellular Matrix
Another target for improving intratumoral distribution and penetration of liposomes has been the depletion of the extracellular matrix constituents of collagen and hyaluronic acid, which pose a barrier for liposome movement as well as contribute to elevated IFP. Collagen depletion by collagenase pretreatment was one of the first tumor matrix modulation approaches. IFP was decreased in an osteosarcoma tumor model by pretreatment with intravenous and intratumoral collagenase-1. Also there was greater tumor penetration of TP3 monoclonal antibody in pretreated collagenase animals and the TP3 antibody was located further from blood vessels [57]. Use of collagenase has not been clinically translated because of the important role of collagen in healthy tissue and concerns for the promotion of metastasis. Rather than using collagenase-1, Zheng et al tested the role of collagen depletion on liposome distribution using collagenase-2 which cleaves collagen type-1 and has little effect on basement membrane type IV collagens [58]. They demonstrated that intravenous or intratumoral treatment with collagenase-2 decreased IFP measured with a fiber optic pressure device placed in the tumor [58]. The IFP was compared with non-invasive power Doppler ultrasound measurements to determine blood perfusion vessel fraction. Using intravenously administered liposomal doxorubicin radiolabeled with technetium-99m (99mTc), the whole body liposome distribution was tracked by gamma camera imaging followed by ex vivo autoradiography and histopathology (Figure 3). Using intravenous collagenase-2 or deactivated collagenase-2, there were no differences in liposome penetration. In contrast, intratumoral collagenase treatment caused more collagen depletion over 24 hours which allowed enough time for the liposomes to accumulate. Moreover, liposomal doxorubicin was located in tumor cell nests in animals pretreated with intratumoral collagenase-2.
Figure 3.
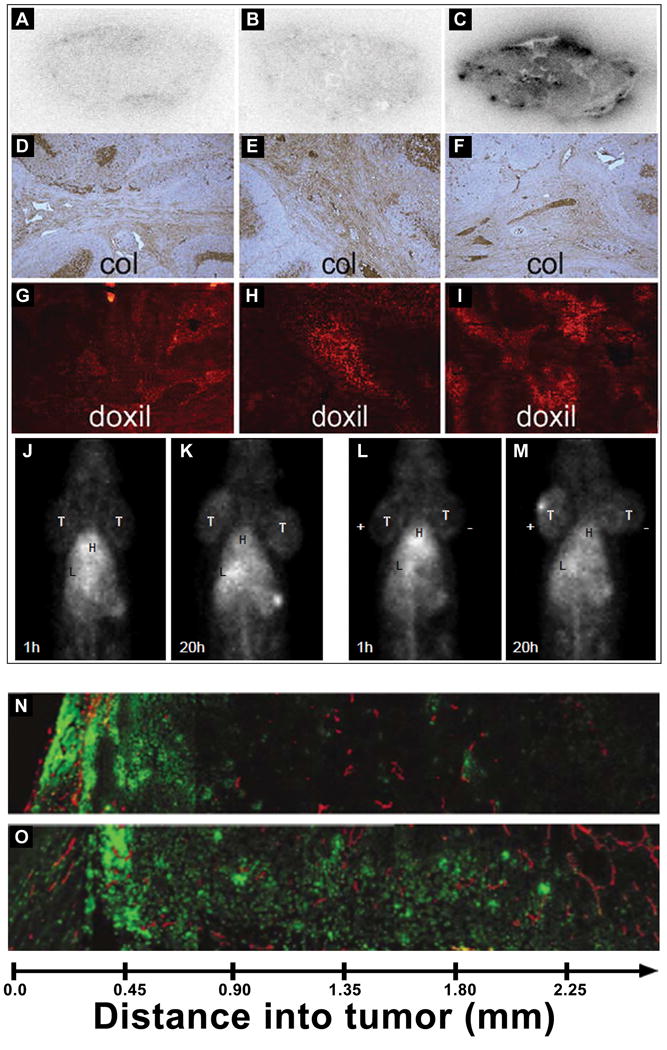
Effect of depletion of extracellular matrix components on liposomal doxorubicin intratumoral distribution and penetration. Autoradiographs of tumors receiving intravenous collagenase-2 (A), intratumoral deactivated collagenase-2 (B) or intratumoral active collagenase-2 (C) and collected 24 hours after intravenous injection of 99mTc-liposomal doxorubicin. Microscopic examination of collagen from tumor sections receiving intravenous collagenase-2 (D), intratumoral deactivated collagenase-2 (E) or intratumoral active collagenase-2 (F) and liposomal doxorubicin distribution in tumor sections from rats pretreated with intravenous collagenase-2 (G), intratumoral deactivated collagenase-2 (H) or intratumoral active collagenase-2 (I). Gamma camera images of rats bearing two head and neck tumors at the nape of the neck pretreated with intravenous collagenase-2 and intravenously injected with 99mTc-liposomal doxorubicin to monitor the tumor accumulation of 99mTc-liposomal doxorubicin at 1 h (J) or 24 h (K) post-injection. Images of tumor-bearing rats pretreated with intratumoral collagenase-2 and intravenously injected with 99mTc-liposomal doxorubicin to monitor tumor accumulation at 1 h (L) or 20 h (M). Reproduced with permission from [58]. Fluorescent microscopic images of doxorubicin distribution collected from tumors excised from mice intravenously injected with liposomal doxorubicin without (N) or with (O) hyaluronidase pretreatment. Reproduced with permission from [60].
Another approach has been to suppress tumor collagen type I synthesis. Lorastan, an active transforming growth factor β1 inhibitor with anti-fibrotic effects, was reported to inhibit collagen type I synthesis in a dose dependent manner by decreasing the levels of collagen type I in several preclinical tumor models [59]. Lorastan pretreatment two weeks before intravenous administration of 100 nm fluorescent polystyrene nanoparticles resulted in a 2-fold increase in the intratumoral nanoparticle content with more nanoparticles located further from the blood vessels. In a separate study, pancreatic tumor volumes in mice pretreated with lorastan followed by intravenous liposomal doxorubicin therapy were significantly smaller than mice administered liposomal doxorubicin monotherapy.
Modulation of tumor hyaluronan levels to improve intratumoral liposome distribution has been studied. Hyaluronan depletion by hyaluonidase has been investigated in a mouse osteosarcoma xenograft model [60]. Tumor uptake of intravenous liposomal doxorubicin administered 1 hour after intratumoral hyaluronidase pretreatment was increased 4-fold in the tumor center compared with liposomal doxorubicin alone (Figure 3 N–O). Recently, a different strategy based on inhibition of hyaluronan synthesis was reported by Kohli et al [20]. They reformulated the hyaluronan synthesis inhibitor, 4-methylumbelliferone (MU), by preparing a phosphorylated prodrug to increase MU solubility and encapsulating the MU-prodrug in neutral liposomes. The MU-prodrug liposomes could deplete tumor hyaluronan. Fluorescent co-localization imaging of ex vivo tumor sections from 4T1 tumor bearing mice administered DiD-labeled empty liposomes with or without pretreatment with the MU-prodrug liposomes showed the DiD-labeled liposomes penetrated further from the vasculature and had increased intratumoral distribution in animals pretreated with MU-prodrug liposomes. Furthermore, hyluronan depleted tumor-bearing mice administered intravenous liposomal doxorubicin therapy had reduced tumor growth and extended overall survival compared with tumor-bearing mice receiving only liposomal doxorubicin monotherapy. From these results it was inferred that the increased intratumoral distribution of liposomes following hyluronan depletion was responsible for the improved liposomal doxorubicin efficacy.
Several drugs have been shown to prime the tumor microenvironment by inducing apoptosis and reducing tumor cell density which in turn causes interstitial space expansion and thereby provides more gaps for liposomes to penetrate through [61]. Examples include paclitaxel and liposomal doxorubicin as well as TNF related apoptosis inducing ligand (Apo2/TRAIL) and liposome gemcitabine [61, 62]. Recently, cyclophosphamide priming was shown to enhance delivery and efficacy of HER2-targeted liposomal doxorubicin leading to a phase I clinical study of this combination therapy [63].
3.1.1.5 Tumor Associated Macrophages
Abundant within the tumor mass, tumor-associated macrophages (TAMs) are a distinct phenotype with expression of scavenger receptor A and mannose receptors on their surface [64]. They play a role in tumor progression by suppressing immunocompetent cells and facilitating tumor growth through pro-angiogenic factors [64]. Intravenously administered liposomes have been observed in the endosomal lysosomal compartment of TAMs near tumor blood vessels [65]. It has been postulated that TAMs may play a role in the movement of phagocytosed nanoparticle drug carriers throughout the tumor [28, 66, 67]. Although many questions remain on the role of TAMs as an approach to improve intratumoral distribution, current evidence suggests future research is warranted.
3.1.2 External Stimuli
Various external stimuli have been employed in an attempt to improve the intratumoral distribution and drug release of liposomes. Although there is evidence that these external stimulation techniques cause increased tumor uptake of liposomes, less information is known about their ability to cause the liposomes to penetrate further and distribute throughout the tumor (Figure 4). This section highlights some of these strategies.
Figure 4.
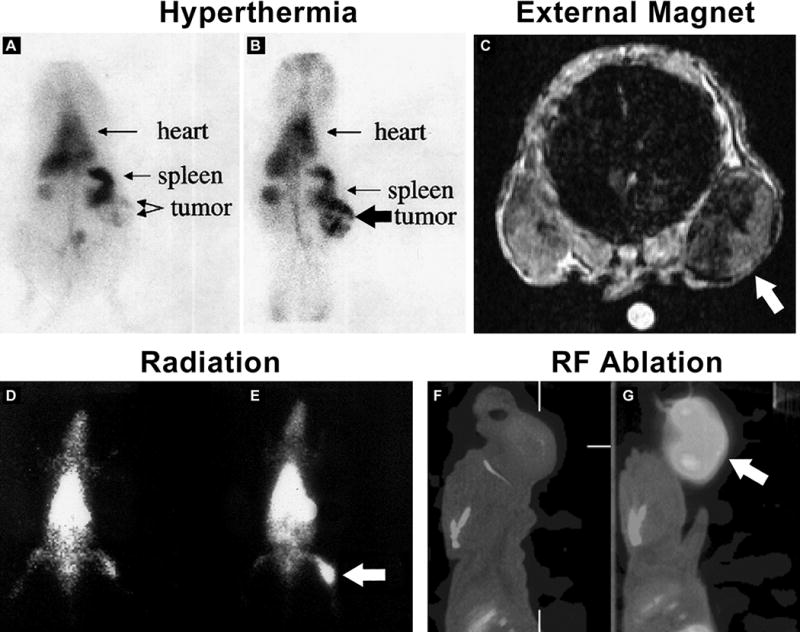
Images of tumor-bearing animals infused with liposomes and treated with an external physical stimulus to increase tumor accumulation. Gamma camera images of sarcoma-bearing cat acquired 3.5 h after 99mTc-PEG-liposomes and before (A) or after (B) ultrasound generated hyperthermia. Reproduced with permission from [69]. Transverse MR image of mouse with subcutaneously implanted tumors in each flank. A magnet was glued to skin over left tumor for 24 h after intravenous injection of pegylated magnetoliposomes. The magnet was removed before imaging. The magnetically targeted tumor (Arrow) appears darker than contralateral control tumor indicating preferential accumulation of magnetoliposomes. Reproduced with permission from [82]. Gamma camera images of rats bearing prostate tumors implanted in the thigh and receiving no (D) or 500 cGy (E) of ionizing radiation directed at the tumor. Images were acquired 20 h after intravenous injection of 99mTc-PEG-liposomes. The ionizing radiation was delivered 24 h before injection of the 99mTc-PEG-liposomes. Reproduced with permission from [69]. Sagittal SPECT/CT images of tumor-bearing rats intravenously injected with 99mTc-liposomal doxorubicin and without (F) or with (G) radiofrequency ablation.
3.1.2.1 Hyperthermia
The idea of using heat to improve chemotherapy drug delivery to superficial solid tumors has been around for many years. Heating in early studies was provided by local hyperthermia, but today many devices use microwave or high intensity focused ultrasound (HIFU). Hyperthermia can affect tumor therapy in several ways [8]. Besides causing direct cell killing from heat ablation, hyperthermia causes the tumor vasculature to become more permeable, thus allowing more drug to pass through into the tumor interstitium. Once within the tumor interstitium, the drug can freely diffuse depending on its tissue binding affinity. Since hyperthermia also can increase cellular membrane fluidity, drug diffusion through the cell membrane can be increased. Early hyperthermia studies with passively targeted conventional and stealth liposomes revealed heating allowed more liposome extravasation, however the temperatures used were not sufficient for drug release within the tumor [25, 26]. These findings led to the design of thermosensitive liposomes which have been formulated to release their contents upon heating by incorporating lipids with phase transition properties in the heating temperature range, lysolipids or thermosensitive polymers [21, 23]. The most studied thermosensitive liposome formulation is ThermoDox™ which uses lysolipids to cause a rapid intravascular release of doxorubicin within the tumor blood vessels. This high local concentration of doxorubicin in the bloodstream increases the intratumoral penetration of the doxorubicin and availability to the tumor cells.
From fluorescent images of FaDu tumor sections, more released doxorubicin penetrated away from the tumor vasculature in thermosensitive liposomal doxorubicin formulation compared with non-thermosensitive liposomal doxorubicin [68]. Thermosensitive 99mTc-pegylated liposomes had increased tumor accumulation in sarcoma-bearing cat following ultrasound heating (Figure 4A–B) [69, 70]. However, thus far, heat triggered drug release by thermosensitive liposomes have not lived up to their promise. Some of the reasons have been premature release of the drug from the liposome at normal body temperatures before reaching the tumor target. Another problem has been low intratumoral penetration of the drug released from the liposome because of redistribution in the circulation following intravascular release [3, 71–73]. Currently, several strategies are being investigated to improve liposome-based hyperthermia [25, 26]. These approaches include utilizing newer pegylated thermosensitive liposome and leucine zipper peptide-lipid hybrid formulations with improved in vivo drug retention and increased doxorubicin bioavailability in tumors [71, 73]. Two-step hyperthermia protocols have been described in which the first temperature step increases extravasation and intratumoral penetration of longer circulating thermosensitive liposome formulations and a second heating step increases intratumoral drug release [74]. Another approach targets more liposomes to angiogenic endothelial cells and tumor cells by modifying the thermosensitive liposomes formulation with cRGD, MUC-1 monoclonal antibody, or cationic lipid [75–78]. In a B16 melanoma model, cationic thermosensitive liposomes had 3 times more doxorubicin located in the tumor, and also caused more tumor vessel damage [76].
3.1.2.2 Magnetic Field
Magnetoliposomes, liposomes containing superparamagnetic iron oxide nanoparticles (SPION), have been studied as MR contrast agents and as magnetic sensitive drug delivery platforms [79–81]. The feasibility of using external permanent magnet exposure to guide magnetoliposomes to liver in situ or prostate tumor xenografts was reported [82, 83]. In the prostate tumor xenograft model, a tumor was placed on both flanks [82]. The mice were infused with pegylated magnetoliposomes and one tumor was exposed to a permanent magnet for varying times. MRI imaging of the accumulation was then conducted. The tumor exposed to the magnet had more magnetoliposome uptake (Figure 4C). From histologic analysis of tumor sections, more iron staining was noted in capillaries and tumor interstitium in the magnetic-stimulated tumor. Interestingly, tumor cells did not contain magnetoliposomes whereas fibroblasts, macrophages and endothelial cells did.
More recent research has focused on using magnetoliposomes composed of thermosensitive lipids and containing SPION and a chemotherapeutic agent [84, 85]. Once the liposomes have accumulated in the target tumor following magnetic stimulation, an alternating current magnetic field operating a radiofrequency levels can cause localized heating of the tumor, thereby triggering drug release. Folate targeting has also been used to localize the magnetoliposomes in closer proximity to tumor cells for better hyperthermia drug release [86]. Other groups are focusing on controlling and maximizing drug leakage by embedding oleic acid coated maghemite within the lipid bilayer. Most of these studies have shown favorable cell killing and intracellular uptake of the chemotherapeutic in vitro. Whether these strategies will be translatable will require testing in pre-clinical animal models.
3.1.2.3 Radiation
Several groups have reported delayed tumor growth in pre-clinical tumor models by combining radiotherapy with intravenous liposomal doxorubicin [87, 88]. Radiation therapy directed at the tumor in prostate tumor-bearing rats has been shown to increase accumulation of 99mTc-pegylated liposomes administered 20 hours after receiving a 500 cGy (5 Gy) radiation dose [69] (Figure 4D–E). This increased accumulation was attributed to the increased vascular permeability caused by the radiotherapy. In an osteosarcoma model, concomitant radiotherapy and liposomal doxorubicin had an increased delay in tumor growth compared with the monotherapies. There was also more tumor penetration of doxorubicin observed in tissue collected from the irradiated mice receiving liposomal doxorubicin [87]. Liposome-based radiochemotherapy has not advanced past the pre-clinical stage.
3.1.2.4 Radiofrequency Ablation
Focal thermal therapy using radiofrequency (RF) to treat tumors is a common minimally invasive therapy for treatment of liver and other tumors [24]. As a minimally invasive therapy, RF ablation can potentially reduce risks associated with major surgery and provide a treatment opportunity for non-surgical patients[24]. It is delivered through a needle probe placed into a solid tumor by image guidance to induce short duration heating of tumor tissue to >50 degrees C to cause tumor coagulation within the ablative zone [24]. RF ablation procedures still have challenges, especially in situations requiring ablation in tumors located near adjacent blood vessels or > 3 cm in diameter. Particularly in large tumors, residual viable tumor cells within the ablation zone and surrounding margin have been observed [89, 90]. Targeting of these residual viable tumor cells using intravenously administered liposomes carrying a variety of chemotherapeutics or therapeutic radionuclides as an adjuvant to RF ablation has been investigated [24, 91–96]. When liposomal doxorubicin is injected intravenously immediately following RF thermal ablation, a greatly increased accumulation of the liposomes in the region of the thermal ablation occurs with some studies reporting uptake that was 5.6-fold greater as compared with un-ablated tumors in a breast tumor model [97]. The significantly increased RF ablation-induced uptake of liposomal doxorubicin has been observed in SPECT/CT images of head and neck tumor-bearing rats administered with 99mTc-liposomal doxorubicin (Figure 4F–G). In this imaging study, a 3-fold increased accumulation of 99mTc-liposomal doxorubicin in tumors treated with RF ablation was measured and intratumoral doxorubicin levels correlated with the quantitatively measured activity in the tumor using SPECT/CT imaging [98]. Since RF ablation has been shown to increase microvasculature perfusion in the peri-ablation rim due to RF-induced sub-lethal hyperthermia, this increased liposomal doxorubicin accumulation is most likely due to the increased liposome permeability through leaky endothelium [99, 100]. The increased intratumoral liposomal doxorubicin leads to early vascular injury and reduced perfusion resulting in a hypoxic environment which potentiates the effects of doxorubicin locally and contributes to increased apoptosis, creating a larger tumor ablation zone. The influence of RF ablation on microvessel morphology in the presence or absence of liposomal doxorubicin was recently reported [100]. Interestingly, RF ablation and liposomal doxorubicin treatment reduced microvessel diameters compared with RF ablation alone with the greatest reduction occurring 12 hours post-treatment. This study highlights the importance of the timing of the liposome administration to ensure an optimal intratumoral liposome accumulation and therapeutic effect.
Thermosensitive liposomal doxorubicin formulations such as ThermoDox™ have been combined with RF ablation and are currently in clinical development for treatment of liver and breast cancer [21]. Although ThermoDox™ was found to be safe in phase I testing, a large phase III efficacy trial of RF ablation/ThermoDox™ for liver cancer therapy recently failed [101, 102]. A recent comparison of non-thermosensitive liposomal doxorubicin or thermosensitive liposomal doxorubicin combined with RF ablation was reported in a medulloblastoma tumor model [101]. A delayed (> 75 days post-therapy) effect on survival was observed with 100% of the non-thermosensitive group and only 70% for the thermosensitive group. This difference was attributed to the ability of the more stable non-thermosensitive liposome formulation to slowly release doxorubicin over a long period of time compared with the rapid release by the thermosensitive liposomes following RF ablation. This study highlights the need to consider the liposome drug release profile in relationship to the changes that may occur in the tumor microenvironment induced by the external stimuli applied.
3.1.2.5 Ultrasound
Another area of significant research is the application of HIFU to improve the drug delivery and triggered drug release from nanoparticles. Several good reviews on the use of HIFU in nanoparticle drug delivery have been published [27, 103–105]. Ultrasound technology can impart both thermal and mechanical effects to improve liposome drug delivery. For mechanical effects, gas bubbles in the mm size range are used to increase the permeability of liposomes through the vessel wall or blood brain barrier following intravenous infusion or through sonoporation of cell membranes, thereby enhancing drug and gene transport into the cell [106–111]. Thermal effects from HIFU have been used for tumor ablation and mild hyperthermia. One approach is to use ultrasound image guidance to localize the tumor for HIFU therapy following intravenous liposome infusion [73, 112–115]. The increased heating in the area causes increased permeability of the liposomes in the tumor. To track the drug delivery in many of the studies, the liposomes have been designed to contain various contrast agents including MRI, fluorescent and radiolabeled agents for non-invasive imaging [73, 114–118]. Other groups have also tracked the accumulation of liposomes after HIFU using radiolabeled liposomes [70, 119]. Several researchers are combining MRI to monitor drug release following heating of the temperature sensitive liposomes [120, 121]. An advantage of MRI is that it can provide chemodosimetry or dose painting feedback to quantify the drug from the signal of MRI contrast within the tumor [103, 122, 123].
3.2 Loco-regional Administration
3.2.1 Intratumoral Administration
Compared with other nanoparticle platforms, liposomes are good candidates for direct interstitial therapy of cancer. Direct intratumoral drug delivery of chemotherapeutic agents, radionuclides, and genes has been investigated [17, 18, 28, 124, 125]. The liposome carrier helps the encapsulated agent disperse through the local tissue while facilitating the retention of the agent in the local region. These advantageous features of liposomes were noted when radiolabeled liposomes were infused directly into a solid tumor as compared to the infusion of the small molecule, N,N-bis(2-mercapto-ethyl-N′,N′-diethyl-ethylenediamine (BMEDA) [126]. The small BMEDA molecules had decreased local spread and greatly decreased local retention which was likely due to a more rapid absorption into blood capillaries.
3.2.2 Convection Enhanced Delivery
Convection enhanced delivery (CED) has been developed as an alternative to intravenous drug delivery and an improvement on direct intratumoral injection. CED for cancer therapy is an approach that uses the direct injection of agents into solid tumors under convective force [127, 128]. CED is performed by using image-guidance to place a catheter directly into solid tumor parenchyma while therapeutic agents are infused through the catheter under constant pressure. The resulting pressure gradient moves therapeutic agents through the interstitial spaces of the targeted tumor by bulk flow. By use of CED, a much higher tumor-to-normal tissue concentration of therapeutic agents is achieved as compared with intravenous administration, which relies solely on the diffusion of drug from the blood into the tumor. Bobo et al originally demonstrated that CED enhanced the volume of distribution of a radiolabeled large molecule in the brain while also achieving concentrations in brain tissue that were orders of magnitude greater than systemic levels [129]. In a direct comparison of liposomal doxorubicin versus doxorubicin alone infused in rodent intracranial brain tumors by CED, Saito et al demonstrated the liposomal formulation provided a more reliable volume of distribution. Furthermore, they determined by fluorescent microscopy that liposomal doxorubicin provided a larger area of coverage than doxorubicin alone (Figure 5A–B) [130]. The advantage of using nanoparticles like liposomes to improve the intratumoral distribution during CED has been termed Enhanced Dispersion and Retention (EDR) [28].
Figure 5.
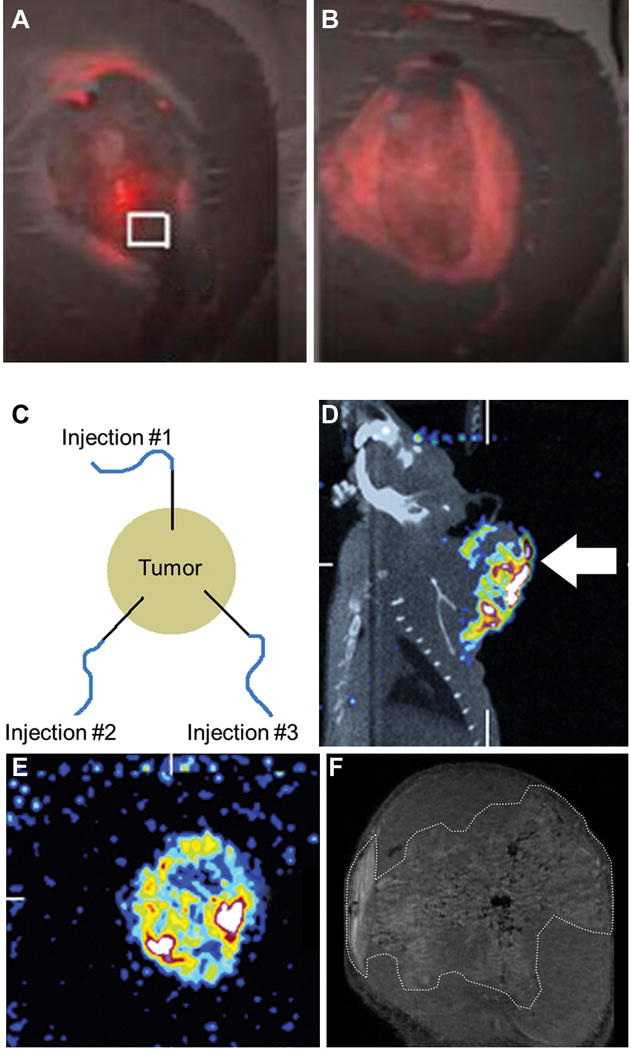
Fluorescent images of brain sections from rats bearing 9L-2 intracranial xenograft tumors following CED infusion of doxorubicin alone (A) or liposomal doxorubicin (B). Fluorescent images were superimposed on hematoxylin and eosin staining. Reproduced with permission from [130]. (C) Schematic diagram depicting the placement of catheters in a head and neck tumor for intratumoral CED infusion. (D) Sagittal SPECT/CT image of rat bearing head and neck tumor acquired 20 h after multiple intratumoral CED infusions of 186Re-liposomes to monitor tumor coverage by the liposomes. (E) Transaxial pinhole SPECT image of head and neck tumor acquired 20 h after multiple intratumoral CED infusions of 186Re-liposomes. (F) T1-weighted 7-Tesla MR image focused on the head and neck tumor after intratumoral CED infusion of gadolinium-liposomes. Intratumoral distribution of the gadolinium-liposomes has been circumscribed with dashed white line. Reproduced with permission from [145].
The majority of current investigations using CED have been in the brain due to the limited ability of intravenously administered drugs to cross the blood-brain barrier [127]. For brain cancer, liposomal agents, solid lipid nanocapsules, metallofullerene nanoparticles, immunotoxins and gene therapies have been tested by CED [127, 131–137]. Liposomal irinotecan has been tested in both intracranial rodent and spontaneous canine brain cancer [133, 138]. The survival benefit of CED intratumoral administration compared with intravenous delivery for nanoliposomal irinotecan in two intracranial glioblastoma rodent models was reported [132]. Impressively, in the intracranial SF7796 model, 50% survival was >60 days following one CED infusion of 0.4 mg irinotecan compared with ~40 days for animals receiving the two intravenous infusions of 0.4 mg irinotecan. Liposomes administered by CED containing rhenium-186 (186Re) have also been tested in intracranial rodent glioblastoma models [135]. In an aggressive U251 tumor, 83% of rats receiving a single mean radiation dose of 1700 Gy survived > 130 days compared with no survival in unlabeled liposome control rats by 35 days post-tumor implantation.
Although CED has significant advantages in cancer drug delivery, in initial clinical research it has faced several challenges related to the 1) inability to verify the distribution and retention of the CED administered drugs, 2) inability to accurately locate the catheter within the tumor, 3) backflow of CED administered drug along the catheter [139], and 4) lack of use of liposome carrier agents to improve the distribution and retention of therapeutic agents within the tumor. In the last 10 years, significant progress has been made in all of these areas including 1) significant improvements in image-guidance technologies and catheter design [140], 2) development of new nanoparticles [28] and 3) development of techniques to improve the intratumoral distribution and retention of CED infused agents based on newly gained scientific understandings of the many factors that influence CED therapy [141].
Another important area where progress has been made is the in vivo imaging technology to monitor the CED infusate distribution. Some of the tracers used in conjunction with the therapeutic agent are radiolabeled albumin, and gadolinium alone or conjugated to albumin or liposomes [134]. In the case of brain cancer, many of the liposome distribution studies have focused on the dispersion in normal brain tissue. Magnetic resonance imaging (MRI) of liposomes has shown that the volume of liposomes infused by CED into the normal brain tissue is highly correlated with the total brain tissue distribution volume. In these studies, the brain distribution volumes are approximately ~3-fold greater than the volume of liposomes infused [142]. During the intratumoral CED infusion of 186Re-liposomes, gamma camera imaging was found to be useful for optimizing the volume and rates of infusion of the 186Re-liposomes [135]. A recent article by Allard et al provides a thorough review of the current state of our knowledge on the delivery parameters and nanoparticle characteristics for successful convection enhanced delivery of nanoparticles to brain [143]. They proposed optimal nanoparticle properties including a particle size of <100 nm, neutral or negative surface charge and a steric coating to reduce binding of the nanoparticles to brain cells.
CED also has potential for use in all types of solid tumors, since all solid tumors tend to have high tumor interstitial pressure, limiting drug diffusion from the blood [2]. However, limited research has been reported in other solid tumors. In French et al, 186Re-liposomes were intratumorally infused by CED into head and neck tumors using three infusion locations to increase coverage of the tumor as diagrammed in Figure 5C [144]. They used SPECT/CT imaging to monitor the retention and distribution of the 186Re-liposomes within the tumor (Figure 5D–E). Compared with the control agents of 186Re-perrhenate and 186Re-BMEDA, the 186Re-liposomes had much better retention of 39.7% within the tumor at 20 hours post-administration whereas the intratumoral retention of 186Re-perrhenate and 186Re-BMEDA was only 0.1% and 0.7%, respectively. The improved tumor retention and higher radiation absorbed dose delivered to the tumor by 186Re-liposomes resulted in an 87% reduction in average tumor volume compared with a 395% increase in tumor volume for 186Re-perrhenate and 186Re-BMEDA. This work clearly demonstrates the importance of increased dispersion and retention within the tumor to achieve effective tumor therapy. This same research group has reported using high resolution MRI to track the dispersion of multifunctional liposomes with the capability to be labeled with 186Re as well as gadolinium for multimodality imaging studies in a head and neck tumor model [145]. Using high resolution MRI may allow non-invasive comparisons of the liposome dispersion following modulation of the tumor interstitium with pharmacologic or external stimuli in the future.
3.2.3 Combination Therapies
Few studies have combined intratumorally administered liposomes with pharmacologic agents or an external stimulus to improve intratumoral liposome distribution. In a recent example, pretreatment of the collagen I synthesis inhibitor, lorastan, did delay the growth of HSTS26T tumors intratumorally injected with oncolytic HSV virus [59]. Presumably the decreased tumor growth was due to the increased movement of virus through the less collagen dense tumor. Adding lorastan to intratumorally administered liposomes therapy may also improve liposome dispersion and lead to better therapy response.
3.3 Conclusion
This article reviewed the current strategies to improve the intratumoral distribution of liposome therapies. These strategies included using combinations of liposome therapies with pharmacological agents to modify the tumor vasculature, extracellular matrix, and interstitial fluid pressure as well as use of tumor penetrating peptides and tumor associated macrophages to improve the intratumoral distribution of therapeutic agents. Tumor priming and agents to modulate the tumor extracellular matrix appear promising. The intratumoral distribution of liposomes will likely benefit from their uptake by tumor associated macrophages. Likewise, more research to determine the benefit of tumor penetrating peptides for improving the homogeneity of tumor coverage of liposome therapies within the tumor will be required, especially when the liposome therapy is administered intratumorally. External stimuli strategies including hyperthermia, magnetic field, radiofrequency ablation, radiotherapy, and ultrasound in combination with liposome therapies were also reviewed. To date, high frequency focused ultrasound and radiofrequency ablation continue to hold the most promise and appear most adaptable to the clinical setting. Improvements in the stimuli devices and consideration of the properties of the liposomes following stimulation that provide intratumoral drug release and bioavailability are important areas for continued investigation. For both combination strategies, additional research to determine the optimal administration protocols will be needed to take full advantage of these new combination therapies. Finally, intratumoral infusion using CED as another promising approach to improve the distribution and retention of liposomes within the tumor was reviewed. More research on the best CED delivery methods including widespread adoption of image-guidance technology will be required.
4. Expert Opinion
Liposome cancer therapies are limited by their inability to distribute throughout the entire tumor mass. In the past 10 years, there has been an increasing understanding that poor tumor penetration and inhomogeneous distribution of therapeutic agents within tumors are major reasons for tumor treatment failure [4]. With this understanding having increased, significant progress has been made to improve intratumoral therapy distributions. In particular, our understanding of the important role of the tumor microenvironment in the distribution of nanoparticulate liposome therapies within the tumor has increased. Moreover, a number of strategies are being tested based on tumor biology including 1) new liposome systems that are responsive to tumor-specific characteristics for better tumor penetration and drug release [21, 22] and 2) liposome therapies in combination with pharmacologic agents or external stimuli which act on the tumor vasculature or microenvironment to facilitate liposome accumulation, penetration and drug release. Thus far, tumor responsive liposome systems have not delivered effective therapies despite a major effort being put forth. To move these new liposome systems towards the clinic, the current designs may need to be simplified and put in perspective of current medical practices. In the near future, combination therapies seem to hold more promise than tumor responsive liposome systems with several combination therapies having reached clinical testing. However, due to the large number of possible combinations between liposome therapies and pharmacologic agents and/or external stimuli, strategic optimization will need to take into account the injection sequence, timing between therapies as well as how the therapy will affect the properties of the liposomes during the combination therapy procedure. Non-invasive imaging with PET, SPECT and MRI are logical tools to study different combined protocols in clinical trials to assess total tumor accumulation and intratumoral liposome and release drug distribution [4, 5, 28, 98, 115, 119, 122, 142]. Continued optimization of liposome formulations capable of intratumoral drug release following external stimulation is needed.
A third approach using intratumoral CED of liposomes to improve intratumoral distribution has been generally overlooked but may be a viable alternative especially for loco-regional tumors. In the past few years, encouraging results have been reported with liposome-based chemotherapy and radionuclide therapy in brain cancer therapy. Yet, further improvements to ensure tumor coverage are needed especially in the areas of catheter design and robotic catheter placement as well as non-invasive image guidance techniques and treatment planning systems. Expansion of intratumoral CED to other solid tumors should be undertaken.
Further research should be conducted on the role of tumor associated macrophages in liposome uptake and mechanism by which TAMs possibly can alter the liposome distribution within the tumor. Recent studies reporting the ability of macrophages laden with small gold nanorods to distribute throughout tumor after intratumoral administration suggests TAMs could be used to improve the tumor coverage of intratumorally administered liposomes [66]. This mechanism has been proposed as a way to distribute liposomes at the invasive margins of brain tumors [28].
Another interesting area for further study would be using tumor penetrating peptide-targeted liposomes to improve their intratumoral distribution. Initial studies using an intravenous route to deliver TPP-conjugated liposomes in pre-clinical models seem promising. Recent studies of a recombinant protein consisting of cell death domain and iRGD peptide administered directly into tumors could disperse widely and provided strong tumor growth inhibition in an orthotopic breast tumor model [146]. It would be interesting to further investigate the spread of intratumorally administered iRGD-liposomes within the tumor [54, 55].
As personalized medicine continues to be adopted, new tools to stratify patients based on their tumor microenvironment characteristics need to be developed. For example, determination of the best non-invasive imaging modality to use and protocol optimization for imaging the tumor microenvironment is actively being investigated and beginning to offer new insights [147]. An important area to address will be the development of methods to distinguish whether changes observed in the images of the tumor microenvironment following a liposome therapy are truly related to a response to the liposome therapy and not simply an artifact caused by perturbation of the microenvironment by the presence of the liposomes. Further, the development of new imaging tools with robotic accessories, providing real time measurement of tumor microenvironment parameters such as interstitial fluid pressure overlaid with spatial information could also aid in patient stratification. Although these tools are still in the pre-clinical stage, it may be possible to adapt them to clinical practice. Imaging with rapid feedback can also play a role during the performance of the clinical therapy to clearly demonstrate the quantity and intratumoral distribution of a liposome therapy providing significant potential to improve cancer therapy.
The clinical success of liposome therapies will require an interdisciplinary team of researchers with expertise in liposome technology, oncology, imaging technology, as well as robotic and computer aided assisted image guidance technologies. More effort to set up close collaborations among scientists and physicians to implement these new strategies in the clinical setting should be a high priority. Through more collaboration among these researchers as a team, it may be possible to improve the intratumoral distribution and drug release of liposome therapies, and cancer therapy in the future.
Article highlights box.
A major challenge for effective liposome cancer therapy is the inability to deliver the therapeutics throughout the entire tumor mass.
Strategies using tumor priming or modulation of the extracellular matrix to improve the intratumoral distribution of liposome therapeutics have potential.
At the present time, radiofrequency ablation and high intensity focused ultrasound appear to be the best external stimuli to combine with liposome therapies for effective tumor coverage.
Improved tumor coverage may be possible using tumor associated macrophages and tumor penetrating peptides to assist in the transport of liposome therapeutics within the tumor.
Liposomes have improved intratumoral distribution and retention as compared to small molecules administered by convection enhanced delivery.
Convection enhanced delivery is a promising strategy to improve the intratumoral distribution of liposome therapeutics especially for brain cancer therapy.
Acknowledgments
The authors would like to thank Jonathan Sumner for preparing the figures.
Research discussed in this manuscript was funded in part by NIH grants 5P30 CA054174-16 and R01 CA131039. B Goins, W Phillips and A Bao are co-founders and shareholders in NanoTX Therapeutics which is developing the rhenium-186 liposomes discussed in this article.
List of Abbreviations
- Apo2/TRAIL
tumor necrosis factor related apoptosis inducing factor
- APRPG
alanine-proline-arginine-proline-glycine
- BMEDA
N,N-bis(2-mercapto-ethy)l-N′,N′-diethyl-ethylenediamine
- CED
convection enhanced delivery
- CT
computed tomography
- 64Cu
copper-64
- DiD
1,1-dioctadecyl-3,3,3,3-tetramethylindodicarbocyanine
- EDR
enhanced dispersion and retention
- EPR
enhanced permeability and retention
- Gy
Gray
- HER-2
human epidermal growth factor receptor-2
- HIFU
high intensity focused ultrasound
- IFP
interstitial fluid pressure
- IT
intratumoral
- IV
intravenous
- MRI
magnetic resonance imaging
- MU
4-methylumbelliferone
- PET
positron emission tomography
- 186Re
rhenium-186
- RES
reticuloendothelial system
- RF
radiofrequency
- RGD
arginine-glycine-aspartic acid
- SPECT
single photon emission computed tomography
- SPION
superparamagnetic iron oxide nanoparticles
- TAM
tumor associated macrophage
- 99mTc
technetium-99m
- TNF-α
tumor necrosis factor-alpha
- TPP
tumor penetrating peptide
- VDA
vascular disruption agent
- VEGF
vascular endothelial growth factor
Footnotes
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Ernsting MJ, Murakami M, Roy A, et al. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release. 2013;172:782–94. doi: 10.1016/j.jconrel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2••.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–64. doi: 10.1038/nrclinonc.2010.139. This review describes the barriers nanomedicines must overcome to be effective cancer therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Lammers T, Kiessling F, Hennink WE, et al. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161:175–87. doi: 10.1016/j.jconrel.2011.09.063. This review describes the shortcomings of current nanomedicines for cancer therapy and possible improvements. [DOI] [PubMed] [Google Scholar]
- 4•.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–92. doi: 10.1038/nrc1893. This paper describes the relationship between poor drug penetration and cancer treatment failure. [DOI] [PubMed] [Google Scholar]
- 5.Waite CL, Roth CM. Nanoscale drug delivery systems for enhanced drug penetration into solid tumors: current progress and opportunities. Crit Rev Biomed Eng. 2012;40:21–41. doi: 10.1615/critrevbiomedeng.v40.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sriraman SK, Aryasomayajula B, Torchilin VP. Barriers to drug delivery in solid tumors. Tissue Barriers. 2014;2:e29528. doi: 10.4161/tisb.29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 8.Pattni BS, Chupin VV, Torchilin VP. New Developments in Liposomal Drug Delivery. Chem Rev. 2015;115:10938–66. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 9.Yuan F, Leunig M, Huang SK, et al. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352–6. [PubMed] [Google Scholar]
- 10.Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Control Release. 2012;164:138–44. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 11.Prabhakar U, Maeda H, Jain RK, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–7. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen AE, Petersen AL, Henriksen JR, et al. Positron Emission Tomography Based Elucidation of the Enhanced Permeability and Retention Effect in Dogs with Cancer Using Copper-64 Liposomes. ACS Nano. 2015;9:6985–95. doi: 10.1021/acsnano.5b01324. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2013;4:81–9. doi: 10.7150/thno.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirtane AR, Kalscheuer SM, Panyam J. Exploiting nanotechnology to overcome tumor drug resistance: Challenges and opportunities. Adv Drug Deliv Rev. 2013;65:1731–47. doi: 10.1016/j.addr.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–13. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCready VR, Cornes P. The potential of intratumoural unsealed radioactive source therapy. Eur J Nucl Med. 2001;28:567–9. doi: 10.1007/s002590000380. [DOI] [PubMed] [Google Scholar]
- 17.Ueno NT, Bartholomeusz C, Xia W, et al. Systemic gene therapy in human xenograft tumor models by liposomal delivery of the E1A gene. Cancer Res. 2002;62:6712–6. [PubMed] [Google Scholar]
- 18.Yoo GH, Hung MC, Lopez-Berestein G, et al. Phase I trial of intratumoral liposome E1A gene therapy in patients with recurrent breast and head and neck cancer. Clin Cancer Res. 2001;7:1237–45. [PubMed] [Google Scholar]
- 19.Holback H, Yeo Y. Intratumoral drug delivery with nanoparticulate carriers. Pharm Res. 2011;28:1819–30. doi: 10.1007/s11095-010-0360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohli AG, Kivimae S, Tiffany MR, et al. Improving the distribution of Doxil(R) in the tumor matrix by depletion of tumor hyaluronan. J Control Release. 2014;191:105–14. doi: 10.1016/j.jconrel.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13:813–27. doi: 10.1038/nrd4333. This paper summarizes the use of nanoparticle -responsive systems to improve tumor drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, Torchilin VP. Stimulus-responsive nanopreparations for tumor targeting. Integr Biol (Camb) 2013;5:96–107. doi: 10.1039/c2ib20135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bibi S, Lattmann E, Mohammed AR, et al. Trigger release liposome systems: local and remote controlled delivery? J Microencapsul. 2012;29:262–76. doi: 10.3109/02652048.2011.646330. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed M, Moussa M, Goldberg SN. Synergy in cancer treatment between liposomal chemotherapeutics and thermal ablation. Chem Phys Lipids. 2012;165:424–37. doi: 10.1016/j.chemphyslip.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kneidl B, Peller M, Winter G, et al. Thermosensitive liposomal drug delivery systems: state of the art review. Int J Nanomedicine. 2014;9:4387–98. doi: 10.2147/IJN.S49297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koning GA, Eggermont AM, Lindner LH, et al. Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharm Res. 2010;27:1750–4. doi: 10.1007/s11095-010-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai CY, Fite BZ, Ferrara KW. Ultrasonic enhancement of drug penetration in solid tumors. Front Oncol. 2013;3:204. doi: 10.3389/fonc.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips WT, Bao A, Brenner AJ, et al. Image-guided interventional therapy for cancer with radiotherapeutic nanoparticles. Adv Drug Deliv Rev. 2014;76:39–59. doi: 10.1016/j.addr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–51. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durymanov MO, Rosenkranz AA, Sobolev AS. Current Approaches for Improving Intratumoral Accumulation and Distribution of Nanomedicines. Theranostics. 2015;5:1007–20. doi: 10.7150/thno.11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacEwan SR, Callahan DJ, Chilkoti A. Stimulus-responsive macromolecules and nanoparticles for cancer drug delivery. Nanomedicine (Lond) 2010;5:793–806. doi: 10.2217/nnm.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miao L, Lin CM, Huang L. Stromal barriers and strategies for the delivery of nanomedicine to desmoplastic tumors. J Control Release. 2015;219:192–204. doi: 10.1016/j.jconrel.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee H, Hoang B, Fonge H, et al. In vivo distribution of polymeric nanoparticles at the whole-body, tumor, and cellular levels. Pharm Res. 2010;27:2343–55. doi: 10.1007/s11095-010-0068-z. [DOI] [PubMed] [Google Scholar]
- 34.Saggar JK, Yu M, Tan Q, et al. The tumor microenvironment and strategies to improve drug distribution. Front Oncol. 2013;3:154. doi: 10.3389/fonc.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandwijk RJ, Mulder WJ, Nicolay K, et al. Anginex-conjugated liposomes for targeting of angiogenic endothelial cells. Bioconjug Chem. 2007;18:785–90. doi: 10.1021/bc060316h. [DOI] [PubMed] [Google Scholar]
- 36.Kuesters GM, Campbell RB. Conjugation of bevacizumab to cationic liposomes enhances their tumor-targeting potential. Nanomedicine (Lond) 2010;5:181–92. doi: 10.2217/nnm.09.105. [DOI] [PubMed] [Google Scholar]
- 37.Mulder WJ, Strijkers GJ, Habets JW, et al. MR molecular imaging and fluorescence microscopy for identification of activated tumor endothelium using a bimodal lipidic nanoparticle. Faseb J. 2005;19:2008–10. doi: 10.1096/fj.05-4145fje. [DOI] [PubMed] [Google Scholar]
- 38.Wicki A, Rochlitz C, Orleth A, et al. Targeting tumor-associated endothelial cells: anti-VEGFR2 immunoliposomes mediate tumor vessel disruption and inhibit tumor growth. Clin Cancer Res. 2012;18:454–64. doi: 10.1158/1078-0432.CCR-11-1102. [DOI] [PubMed] [Google Scholar]
- 39.Chauhan VP, Stylianopoulos T, Martin JD, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7:383–8. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Temming K, Schiffelers RM, Molema G, et al. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist Updat. 2005;8:381–402. doi: 10.1016/j.drup.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Schiffelers RM, Koning GA, ten Hagen TL, et al. Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J Control Release. 2003;91:115–22. doi: 10.1016/s0168-3659(03)00240-2. [DOI] [PubMed] [Google Scholar]
- 42.Asai T. Nanoparticle-mediated delivery of anticancer agents to tumor angiogenic vessels. Biol Pharm Bull. 2012;35:1855–61. doi: 10.1248/bpb.b212013. [DOI] [PubMed] [Google Scholar]
- 43.Asai T, Oku N. Liposomalized oligopeptides in cancer therapy. Methods Enzymol. 2005;391:163–76. doi: 10.1016/S0076-6879(05)91009-4. [DOI] [PubMed] [Google Scholar]
- 44.Al-Abd AM, Aljehani ZK, Gazzaz RW, et al. Pharmacokinetic strategies to improve drug penetration and entrapment within solid tumors. J Control Release. 2015;219:269–77. doi: 10.1016/j.jconrel.2015.08.055. [DOI] [PubMed] [Google Scholar]
- 45.Crielaard BJ, van der Wal S, Le HT, et al. Liposomes as carriers for colchicine-derived prodrugs: vascular disrupting nanomedicines with tailorable drug release kinetics. Eur J Pharm Sci. 2012;45:429–35. doi: 10.1016/j.ejps.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 46.Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat Rev. 2011;37:63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai W, Jin W, Zhang J, et al. Spatiotemporally controlled co-delivery of anti-vasculature agent and cytotoxic drug by octreotide-modified stealth liposomes. Pharm Res. 2012;29:2902–11. doi: 10.1007/s11095-012-0797-2. [DOI] [PubMed] [Google Scholar]
- 48.Fens MH, Hill KJ, Issa J, et al. Liposomal encapsulation enhances the antitumour efficacy of the vascular disrupting agent ZD6126 in murine B16.F10 melanoma. Br J Cancer. 2008;99:1256–64. doi: 10.1038/sj.bjc.6604675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Yang T, Wang X, et al. Materializing sequential killing of tumor vasculature and tumor cells via targeted polymeric micelle system. J Control Release. 2011;149:299–306. doi: 10.1016/j.jconrel.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 50.Zhang YF, Wang JC, Bian DY, et al. Targeted delivery of RGD-modified liposomes encapsulating both combretastatin A-4 and doxorubicin for tumor therapy: in vitro and in vivo studies. Eur J Pharm Biopharm. 2010;74:467–73. doi: 10.1016/j.ejpb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Seynhaeve AL, Hoving S, Schipper D, et al. Tumor necrosis factor alpha mediates homogeneous distribution of liposomes in murine melanoma that contributes to a better tumor response. Cancer Res. 2007;67:9455–62. doi: 10.1158/0008-5472.CAN-07-1599. [DOI] [PubMed] [Google Scholar]
- 52.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J Cell Biol. 2010;188:759–68. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugahara KN, Teesalu T, Karmali PP, et al. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031–5. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan Z, Yang Y, Wei X, et al. Tumor-penetrating peptide mediation: an effective strategy for improving the transport of liposomes in tumor tissue. Mol Pharm. 2014;11:218–25. doi: 10.1021/mp400393a. [DOI] [PubMed] [Google Scholar]
- 55.Yang Y, Yan Z, Wei D, et al. Tumor-penetrating peptide functionalization enhances the anti-glioblastoma effect of doxorubicin liposomes. Nanotechnology. 2013;24:405101. doi: 10.1088/0957-4484/24/40/405101. [DOI] [PubMed] [Google Scholar]
- 56.Stapleton S, Milosevic M, Tannock IF, et al. The intra-tumoral relationship between microcirculation, interstitial fluid pressure and liposome accumulation. J Control Release. 2015;211:163–70. doi: 10.1016/j.jconrel.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Eikenes L, Bruland OS, Brekken C, et al. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res. 2004;64:4768–73. doi: 10.1158/0008-5472.CAN-03-1472. [DOI] [PubMed] [Google Scholar]
- 58.Zheng X, Goins BA, Cameron IL, et al. Ultrasound-guided intratumoral administration of collagenase-2 improved liposome drug accumulation in solid tumor xenografts. Cancer Chemother Pharmacol. 2011;67:173–82. doi: 10.1007/s00280-010-1305-1. [DOI] [PubMed] [Google Scholar]
- 59.Diop-Frimpong B, Chauhan VP, Krane S, et al. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108:2909–14. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eikenes L, Tari M, Tufto I, et al. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. Br J Cancer. 2005;93:81–8. doi: 10.1038/sj.bjc.6602626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu D, Wientjes MG, Lu Z, et al. Tumor priming enhances delivery and efficacy of nanomedicines. J Pharmacol Exp Ther. 2007;322:80–8. doi: 10.1124/jpet.107.121632. [DOI] [PubMed] [Google Scholar]
- 62.Hylander BL, Sen A, Beachy SH, et al. Tumor priming by Apo2L/TRAIL reduces interstitial fluid pressure and enhances efficacy of liposomal gemcitabine in a patient derived xenograft tumor model. J Control Release. 2015;217:160–9. doi: 10.1016/j.jconrel.2015.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geretti E, Leonard SC, Dumont N, et al. Cyclophosphamide-Mediated Tumor Priming for Enhanced Delivery and Antitumor Activity of HER2-Targeted Liposomal Doxorubicin (MM-302) Mol Cancer Ther. 2015;14:2060–71. doi: 10.1158/1535-7163.MCT-15-0314. [DOI] [PubMed] [Google Scholar]
- 64.Vinogradov S, Warren G, Wei X. Macrophages associated with tumors as potential targets and therapeutic intermediates. Nanomedicine (Lond) 2014;9:695–707. doi: 10.2217/nnm.14.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schiffelers RM, Metselaar JM, Fens MH, et al. Liposome-encapsulated prednisolone phosphate inhibits growth of established tumors in mice. Neoplasia. 2005;7:118–27. doi: 10.1593/neo.04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Z, Huang H, Tang S, et al. Small gold nanorods laden macrophages for enhanced tumor coverage in photothermal therapy. Biomaterials. 2016;74:144–54. doi: 10.1016/j.biomaterials.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 67.Madsen SJ, Baek SK, Makkouk AR, et al. Macrophages as cell-based delivery systems for nanoshells in photothermal therapy. Ann Biomed Eng. 2012;40:507–15. doi: 10.1007/s10439-011-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 2001;61:3027–32. [PubMed] [Google Scholar]
- 69.Goins BA, Phillips WT. The use of scintigraphic imaging as a tool in the development of liposome formulations. Prog Lipid Res. 2001;40:95–123. doi: 10.1016/s0163-7827(00)00014-x. [DOI] [PubMed] [Google Scholar]
- 70.Matteucci ML, Anyarambhatla G, Rosner G, et al. Hyperthermia increases accumulation of technetium-99m-labeled liposomes in feline sarcomas. Clin Cancer Res. 2000;6:3748–55. [PubMed] [Google Scholar]
- 71.Al-Ahmady ZS, Scudamore CL, Kostarelos K. Triggered doxorubicin release in solid tumors from thermosensitive liposome-peptide hybrids: Critical parameters and therapeutic efficacy. Int J Cancer. 2015;137:731–43. doi: 10.1002/ijc.29430. [DOI] [PubMed] [Google Scholar]
- 72.Li L, ten Hagen TL, Bolkestein M, et al. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J Control Release. 2013;167:130–7. doi: 10.1016/j.jconrel.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 73.Li L, ten Hagen TL, Hossann M, et al. Mild hyperthermia triggered doxorubicin release from optimized stealth thermosensitive liposomes improves intratumoral drug delivery and efficacy. J Control Release. 2013;168:142–50. doi: 10.1016/j.jconrel.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 74.Li L, ten Hagen TL, Haeri A, et al. A novel two-step mild hyperthermia for advanced liposomal chemotherapy. J Control Release. 2014;174:202–8. doi: 10.1016/j.jconrel.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 75.Al-Ahmady ZS, Chaloin O, Kostarelos K. Monoclonal antibody-targeted, temperature-sensitive liposomes: in vivo tumor chemotherapeutics in combination with mild hyperthermia. J Control Release. 2014;196:332–43. doi: 10.1016/j.jconrel.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 76.Dicheva BM, ten Hagen TL, Li L, et al. Cationic thermosensitive liposomes: a novel dual targeted heat-triggered drug delivery approach for endothelial and tumor cells. Nano Lett. 2013;13:2324–31. doi: 10.1021/nl3014154. [DOI] [PubMed] [Google Scholar]
- 77.Dicheva BM, Ten Hagen TL, Seynhaeve AL, et al. Enhanced Specificity and Drug Delivery in Tumors by cRGD - Anchoring Thermosensitive Liposomes. Pharm Res. 2015;32:3862–76. doi: 10.1007/s11095-015-1746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim MS, Lee DW, Park K, et al. Temperature-triggered tumor-specific delivery of anticancer agents by cRGD-conjugated thermosensitive liposomes. Colloids Surf B Biointerfaces. 2014;116:17–25. doi: 10.1016/j.colsurfb.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 79.Al-Jamal WT, Kostarelos K. Liposome-nanoparticle hybrids for multimodal diagnostic and therapeutic applications. Nanomedicine (Lond) 2007;2:85–98. doi: 10.2217/17435889.2.1.85. [DOI] [PubMed] [Google Scholar]
- 80.Bakandritsos A, Fatourou AG, Fatouros DG. Magnetoliposomes and their potential in the intelligent drug-delivery field. Ther Deliv. 2012;3:1469–82. doi: 10.4155/tde.12.129. [DOI] [PubMed] [Google Scholar]
- 81.Bulte JW, De Cuyper M. Magnetoliposomes as contrast agents. Methods Enzymol. 2003;373:175–98. doi: 10.1016/s0076-6879(03)73012-2. [DOI] [PubMed] [Google Scholar]
- 82.Fortin-Ripoche JP, Martina MS, Gazeau F, et al. Magnetic targeting of magnetoliposomes to solid tumors with MR imaging monitoring in mice: feasibility. Radiology. 2006;239:415–24. doi: 10.1148/radiol.2392042110. [DOI] [PubMed] [Google Scholar]
- 83.Viroonchatapan E, Sato H, Ueno M, et al. Magnetic targeting of thermosensitive magnetoliposomes to mouse livers in an in situ on-line perfusion system. Life Sci. 1996;58:2251–61. doi: 10.1016/0024-3205(96)00220-2. [DOI] [PubMed] [Google Scholar]
- 84.Chen Y, Chen Y, Xiao D, et al. Low-dose chemotherapy of hepatocellular carcinoma through triggered-release from bilayer-decorated magnetoliposomes. Colloids Surf B Biointerfaces. 2014;116:452–8. doi: 10.1016/j.colsurfb.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kulshrestha P, Gogoi M, Bahadur D, et al. In vitro application of paclitaxel loaded magnetoliposomes for combined chemotherapy and hyperthermia. Colloids Surf B Biointerfaces. 2012;96:1–7. doi: 10.1016/j.colsurfb.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 86.Pradhan P, Giri J, Rieken F, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142:108–21. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 87.Davies Cde L, Lundstrom LM, Frengen J, et al. Radiation improves the distribution and uptake of liposomal doxorubicin (caelyx) in human osteosarcoma xenografts. Cancer Res. 2004;64:547–53. doi: 10.1158/0008-5472.can-03-0576. [DOI] [PubMed] [Google Scholar]
- 88.Harrington KJ, Rowlinson-Busza G, Syrigos KN, et al. Pegylated liposome-encapsulated doxorubicin and cisplatin enhance the effect of radiotherapy in a tumor xenograft model. Clin Cancer Res. 2000;6:4939–49. [PubMed] [Google Scholar]
- 89.Sofocleous CT, Garg SK, Cohen P, et al. Ki 67 is an independent predictive biomarker of cancer specific and local recurrence-free survival after lung tumor ablation. Ann Surg Oncol. 2013;20(Suppl 3):S676–83. doi: 10.1245/s10434-013-3140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sofocleous CT, Nascimento RG, Petrovic LM, et al. Histopathologic and immunohistochemical features of tissue adherent to multitined electrodes after RF ablation of liver malignancies can help predict local tumor progression: initial results. Radiology. 2008;249:364–74. doi: 10.1148/radiol.2491071752. [DOI] [PubMed] [Google Scholar]
- 91.Ahmed M, Monsky WE, Girnun G, et al. Radiofrequency thermal ablation sharply increases intratumoral liposomal doxorubicin accumulation and tumor coagulation. Cancer Res. 2003;63:6327–33. [PubMed] [Google Scholar]
- 92.Moussa M, Goldberg SN, Kumar G, et al. Nanodrug-enhanced radiofrequency tumor ablation: effect of micellar or liposomal carrier on drug delivery and treatment efficacy. PLoS One. 2014;9:e102727. doi: 10.1371/journal.pone.0102727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Soundararajan A, Dodd GD, 3rd, Bao A, et al. Chemoradionuclide therapy with 186Re-labeled liposomal doxorubicin in combination with radiofrequency ablation for effective treatment of head and neck cancer in a nude rat tumor xenograft model. Radiology. 2011;261:813–23. doi: 10.1148/radiol.11110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang W, Ahmed M, Elian M, et al. Do liposomal apoptotic enhancers increase tumor coagulation and end-point survival in percutaneous radiofrequency ablation of tumors in a rat tumor model? Radiology. 2010;257:685–96. doi: 10.1148/radiol.10100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang W, Ahmed M, Tasawwar B, et al. Radiofrequency ablation combined with liposomal quercetin to increase tumour destruction by modulation of heat shock protein production in a small animal model. Int J Hyperthermia. 2011;27:527–38. doi: 10.3109/02656736.2011.582474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang W, Ahmed M, Tasawwar B, et al. Combination radiofrequency (RF) ablation and IV liposomal heat shock protein suppression: reduced tumor growth and increased animal endpoint survival in a small animal tumor model. J Control Release. 2012;160:239–44. doi: 10.1016/j.jconrel.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Monsky WL, Kruskal JB, Lukyanov AN, et al. Radio-frequency ablation increases intratumoral liposomal doxorubicin accumulation in a rat breast tumor model. Radiology. 2002;224:823–9. doi: 10.1148/radiol.2243011421. [DOI] [PubMed] [Google Scholar]
- 98.Head HW, Dodd GD, 3rd, Bao A, et al. Combination radiofrequency ablation and intravenous radiolabeled liposomal Doxorubicin: imaging and quantification of increased drug delivery to tumors. Radiology. 2010;255:405–14. doi: 10.1148/radiol.10090714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kruskal JB, Oliver B, Huertas JC, et al. Dynamic intrahepatic flow and cellular alterations during radiofrequency ablation of liver tissue in mice. J Vasc Interv Radiol. 2001;12:1193–201. doi: 10.1016/s1051-0443(07)61679-0. [DOI] [PubMed] [Google Scholar]
- 100.Moussa M, Goldberg SN, Tasawwar B, et al. Adjuvant liposomal doxorubicin markedly affects radiofrequency ablation-induced effects on periablational microvasculature. J Vasc Interv Radiol. 2013;24:1021–33. doi: 10.1016/j.jvir.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Andriyanov AV, Koren E, Barenholz Y, et al. Therapeutic efficacy of combining pegylated liposomal doxorubicin and radiofrequency (RF) ablation: comparison between slow-drug-releasing, non-thermosensitive and fast-drug-releasing, thermosensitive nano-liposomes. PLoS One. 2014;9:e92555. doi: 10.1371/journal.pone.0092555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wood BJ, Poon RT, Locklin JK, et al. Phase I study of heat-deployed liposomal doxorubicin during radiofrequency ablation for hepatic malignancies. J Vasc Interv Radiol. 2012;23:248–55 e7. doi: 10.1016/j.jvir.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grull H, Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J Control Release. 2012;161:317–27. doi: 10.1016/j.jconrel.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 104.Manthe RL, Foy SP, Krishnamurthy N, et al. Tumor ablation and nanotechnology. Mol Pharm. 2010;7:1880–98. doi: 10.1021/mp1001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Phenix CP, Togtema M, Pichardo S, et al. High intensity focused ultrasound technology, its scope and applications in therapy and drug delivery. J Pharm Pharm Sci. 2014;17:136–53. doi: 10.18433/j3zp5f. [DOI] [PubMed] [Google Scholar]
- 106.Manzoor AA, Lindner LH, Landon CD, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72:5566–75. doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suzuki R, Klibanov AL. Co-administration of Microbubbles and Drugs in Ultrasound-Assisted Drug Delivery: Comparison with Drug-Carrying Particles. Adv Exp Med Biol. 2016;880:205–20. doi: 10.1007/978-3-319-22536-4_12. [DOI] [PubMed] [Google Scholar]
- 108.Suzuki R, Maruyama K. Effective in vitro and in vivo gene delivery by the combination of liposomal bubbles (bubble liposomes) and ultrasound exposure. Methods Mol Biol. 2010;605:473–86. doi: 10.1007/978-1-60327-360-2_33. [DOI] [PubMed] [Google Scholar]
- 109.Yang FY, Wong TT, Teng MC, et al. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J Control Release. 2012;160:652–8. doi: 10.1016/j.jconrel.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 110.Yin T, Wang P, Li J, et al. Tumor-penetrating codelivery of siRNA and paclitaxel with ultrasound-responsive nanobubbles hetero-assembled from polymeric micelles and liposomes. Biomaterials. 2014;35:5932–43. doi: 10.1016/j.biomaterials.2014.03.072. [DOI] [PubMed] [Google Scholar]
- 111.Yudina A, de Smet M, Lepetit-Coiffe M, et al. Ultrasound-mediated intracellular drug delivery using microbubbles and temperature-sensitive liposomes. J Control Release. 2011;155:442–8. doi: 10.1016/j.jconrel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 112.Dicheva BM, Seynhaeve AL, Soulie T, et al. Pharmacokinetics Tissue Distribution and Therapeutic Effect of Cationic Thermosensitive Liposomal Doxorubicin Upon Mild Hyperthermia. Pharm Res. 2015 doi: 10.1007/s11095-015-1815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dromi S, Frenkel V, Luk A, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13:2722–7. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kheirolomoom A, Lai CY, Tam SM, et al. Complete regression of local cancer using temperature-sensitive liposomes combined with ultrasound-mediated hyperthermia. J Control Release. 2013;172:266–73. doi: 10.1016/j.jconrel.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koning GA, Krijger GC. Targeted multifunctional lipid-based nanocarriers for image-guided drug delivery. Anticancer Agents Med Chem. 2007;7:425–40. doi: 10.2174/187152007781058613. [DOI] [PubMed] [Google Scholar]
- 116.Fowler RA, Fossheim SL, Mestas JL, et al. Non-invasive magnetic resonance imaging follow-up of sono-sensitive liposome tumor delivery and controlled release after high-intensity focused ultrasound. Ultrasound Med Biol. 2013;39:2342–50. doi: 10.1016/j.ultrasmedbio.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 117.Kheirolomoom A, Kruse DE, Qin S, et al. Enhanced in vivo bioluminescence imaging using liposomal luciferin delivery system. J Control Release. 2010;141:128–36. doi: 10.1016/j.jconrel.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Watson KD, Lai CY, Qin S, et al. Ultrasound increases nanoparticle delivery by reducing intratumoral pressure and increasing transport in epithelial and epithelial-mesenchymal transition tumors. Cancer Res. 2012;72:1485–93. doi: 10.1158/0008-5472.CAN-11-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.de Smet M, Langereis S, van den Bosch S, et al. SPECT/CT imaging of temperature-sensitive liposomes for MR-image guided drug delivery with high intensity focused ultrasound. J Control Release. 2013;169:82–90. doi: 10.1016/j.jconrel.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 120.de Smet M, Hijnen NM, Langereis S, et al. Magnetic resonance guided high-intensity focused ultrasound mediated hyperthermia improves the intratumoral distribution of temperature-sensitive liposomal doxorubicin. Invest Radiol. 2013;48:395–405. doi: 10.1097/RLI.0b013e3182806940. [DOI] [PubMed] [Google Scholar]
- 121.Negussie AH, Yarmolenko PS, Partanen A, et al. Formulation and characterisation of magnetic resonance imageable thermally sensitive liposomes for use with magnetic resonance-guided high intensity focused ultrasound. Int J Hyperthermia. 2011;27:140–55. doi: 10.3109/02656736.2010.528140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ponce AM, Viglianti BL, Yu D, et al. Magnetic resonance imaging of temperature-sensitive liposome release: drug dose painting and antitumor effects. J Natl Cancer Inst. 2007;99:53–63. doi: 10.1093/jnci/djk005. [DOI] [PubMed] [Google Scholar]
- 123.Viglianti BL, Ponce AM, Michelich CR, et al. Chemodosimetry of in vivo tumor liposomal drug concentration using MRI. Magn Reson Med. 2006;56:1011–8. doi: 10.1002/mrm.21032. [DOI] [PubMed] [Google Scholar]
- 124.Harrington KJ, Rowlinson-Busza G, Syrigos KN, et al. Pegylated liposomes have potential as vehicles for intratumoral and subcutaneous drug delivery. Clin Cancer Res. 2000;6:2528–37. [PubMed] [Google Scholar]
- 125.Ren S, Li C, Dai Y, et al. Comparison of pharmacokinetics, tissue distribution and pharmacodynamics of liposomal and free doxorubicin in tumour-bearing mice following intratumoral injection. J Pharm Pharmacol. 2014;66:1231–9. doi: 10.1111/jphp.12257. [DOI] [PubMed] [Google Scholar]
- 126.Bao A, Phillips WT, Goins B, et al. Potential use of drug carried-liposomes for cancer therapy via direct intratumoral injection. Int J Pharm. 2006;316:162–9. doi: 10.1016/j.ijpharm.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 127•.Fiandaca MS, Berger MS, Bankiewicz KS. The use of convection-enhanced delivery with liposomal toxins in neurooncology. Toxins (Basel) 2011;3:369–97. doi: 10.3390/toxins3040369. A good review describing convection enhanced delivery of liposomal agents for brain cancer treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mehta AI, Choi BD, Ajay D, et al. Convection enhanced delivery of macromolecules for brain tumors. Curr Drug Discov Technol. 2012;9:305–10. doi: 10.2174/157016312803305951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bobo RH, Laske DW, Akbasak A, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–80. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saito R, Krauze MT, Noble CO, et al. Tissue affinity of the infusate affects the distribution volume during convection-enhanced delivery into rodent brains: implications for local drug delivery. J Neurosci Methods. 2006;154:225–32. doi: 10.1016/j.jneumeth.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 131.Allard E, Hindre F, Passirani C, et al. 188Re-loaded lipid nanocapsules as a promising radiopharmaceutical carrier for internal radiotherapy of malignant gliomas. Eur J Nucl Med Mol Imaging. 2008;35:1838–46. doi: 10.1007/s00259-008-0735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen PY, Ozawa T, Drummond DC, et al. Comparing routes of delivery for nanoliposomal irinotecan shows superior anti-tumor activity of local administration in treating intracranial glioblastoma xenografts. Neuro Oncol. 2013;15:189–97. doi: 10.1093/neuonc/nos305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging: laboratory investigation. J Neurosurg. 2008;108:989–98. doi: 10.3171/JNS/2008/108/5/0989. [DOI] [PubMed] [Google Scholar]
- 134.Mehta AI, Choi BD, Raghavan R, et al. Imaging of convection enhanced delivery of toxins in humans. Toxins (Basel) 2011;3:201–6. doi: 10.3390/toxins3030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Phillips WT, Goins B, Bao A, et al. Rhenium-186 liposomes as convection-enhanced nanoparticle brachytherapy for treatment of glioblastoma. Neuro Oncol. 2012;14:416–25. doi: 10.1093/neuonc/nos060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rainov NG, Heidecke V. Clinical development of experimental virus-mediated gene therapy for malignant glioma. Anticancer Agents Med Chem. 2011;11:739–47. doi: 10.2174/187152011797378724. [DOI] [PubMed] [Google Scholar]
- 137.Shultz MD, Wilson JD, Fuller CE, et al. Metallofullerene-based nanoplatform for brain tumor brachytherapy and longitudinal imaging in a murine orthotopic xenograft model. Radiology. 2011;261:136–43. doi: 10.1148/radiol.11102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Noble CO, Krauze MT, Drummond DC, et al. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res. 2006;66:2801–6. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- 139.Sampson JH, Archer G, Pedain C, et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg. 2010;113:301–9. doi: 10.3171/2009.11.JNS091052. [DOI] [PubMed] [Google Scholar]
- 140.Vogelbaum MA, Aghi MK. Convection-enhanced delivery for the treatment of glioblastoma. Neuro Oncol. 2015;17(Suppl 2):ii3–ii8. doi: 10.1093/neuonc/nou354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barua NU, Gill SS, Love S. Convection-enhanced drug delivery to the brain: therapeutic potential and neuropathological considerations. Brain Pathol. 2014;24:117–27. doi: 10.1111/bpa.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saito R, Bringas JR, McKnight TR, et al. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64:2572–9. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- 143.Allard E, Passirani C, Benoit JP. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials. 2009;30:2302–18. doi: 10.1016/j.biomaterials.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 144.French JT, Goins B, Saenz M, et al. Interventional therapy of head and neck cancer with lipid nanoparticle-carried rhenium 186 radionuclide. J Vasc Interv Radiol. 2010;21:1271–9. doi: 10.1016/j.jvir.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li S, Goins B, Zhang L, et al. Novel multifunctional theranostic liposome drug delivery system: construction, characterization, and multimodality MR, near-infrared fluorescent, and nuclear imaging. Bioconjug Chem. 2012;23:1322–32. doi: 10.1021/bc300175d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen R, Braun GB, Luo X, et al. Application of a proapoptotic peptide to intratumorally spreading cancer therapy. Cancer Res. 2013;73:1352–61. doi: 10.1158/0008-5472.CAN-12-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.LeBleu VS. Imaging the Tumor Microenvironment. Cancer J. 2015;21:174–8. doi: 10.1097/PPO.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li L, ten Hagen TL, Schipper D, et al. Triggered content release from optimized stealth thermosensitive liposomes using mild hyperthermia. J Control Release. 2010;143:274–9. doi: 10.1016/j.jconrel.2010.01.006. [DOI] [PubMed] [Google Scholar]


