Abstract
Cardiovascular disease is the leading cause of death in the developed world, and as such there is a pressing need for treatment options. Cardiac tissue engineering emerged from the need to develop alternate sources and methods of replacing tissue damaged by cardiovascular diseases, as the ultimate treatment option for many who suffer from end-stage heart failure is a heart transplant. In this review we focus on biomaterial approaches to augment injured or impaired myocardium with specific emphasis on: the design criteria for these biomaterials; the types of scaffolds—composed of natural or synthetic biomaterials, or decellularized extracellular matrix—that have been used to develop cardiac patches and tissue models; methods to vascularize scaffolds and engineered tissue, and finally injectable biomaterials (hydrogels)designed for endogenous repair, exogenous repair or as bulking agents to maintain ventricular geometry post-infarct. The challenges facing the field and obstacles that must be overcome to develop truly clinically viable cardiac therapies are also discussed.
1. Motivation for cardiac tissue engineering
Cardiovascular disease (CVD) is currently the leading cause of death in the world and it is projected that it will remain as such throughout the next decade(Mathers and Loncar, 2006; WHO, 2011b). In 2008 alone, CVD accounted for 1 in 3 deaths in the US (811,940 of 2,471,984) and of those, half (approximately 1 in 6 American deaths) were attributable to coronary heart disease(Roger et al., 2012). Furthermore it is estimated that each year 785,000 Americans have a new coronary attack, about 470,000 have a recurrent attack and a further 195,000 suffer their first silent myocardial infarction(Roger et al., 2012). While the disorders categorized as CVDs have such divergent causes as atherosclerosis, rheumatic fever, congenital malformations and thrombosis, they all converge to cause damage to the heart muscle. Unfortunately, the damage is irreversible because the heart muscles cells, cardiomyocytes, are thought to be terminally differentiated and non-proliferative (Sutton and Sharpe, 2000; Laflamme and Murry, 2005), which necessarily limits the regenerative potential of the heart.
Among CVDs, ischemic heart disease is the most prevalent (WHO, 2011a; Roger et al., 2012). It has therefore been the subject of intensive research. The partial or complete blockage of a coronary artery reduces/prevents blood supply to the downstream heart muscle and the affected tissue becomes severely nutrient and oxygen deprived, inducing cardiomyocyte death: an event known as a myocardial infarction (MI). An inflammatory response is activated in consequence wherein macrophages infiltrate the area to remove the dead or dying cardiomyocytes, and are followed in by fibroblasts and endothelial cells, which attempt to repair the damaged tissue by replacing it with tough, rigid, collagenous fibrotic scar tissue(Sutton and Sharpe, 2000; Laflamme and Murry, 2005). However, the “repaired” area is unable to contract and function normally and this mechanical mismatch exerts a strain on the surrounding healthy portions of the heart. With time, cardiac output is reduced and if left untreated a myriad of health complications manifest, ultimately leading to heart failure and premature death. Even with treatment of the underlying cause of heart disease, approximately 50% of patients will experience heart failure within five years of an acute MI(Pantilat and Steimle, 2004). At present, the gold standard treatment for those who have reached end-stage heart failure is a heart transplant; but the insufficiency of donors combined with the need for patient-donor matched organs severely limits the number of patients that can be treated(Zammaretti and Jaconi, 2004; Menasche, 2008). In 2010, there were 2,333 heart transplants performed in the US(Roger et al., 2012). In 2011, greater than 3,000 people were on the waiting list for a heart transplant and thus living with end-stage heart failure(Roger et al., 2012).
Over the last few years left ventricular assist devices (LVADs) have shown great improvement in safety and efficacy. As such, LVADs have been increasingly used as a bridge to transplantation, giving those awaiting a transplant a credible life-saving therapy option by supporting the failing heart, with the consequence of reducing transplant waitlist deaths(Wilson et al., 2009; Kirklin et al., 2013). Additionally, after months to a year on an LVAD, many patients experience improvement in the global contractile properties of the heart (e. g. ejection fraction) allowing their removal from the device. More recently, some VADs have been approved as a destination therapy, providing individuals ineligible for a heart transplant with a long-term therapy option(Wilson et al., 2009; Kirklin et al., 2013). However, there remains a subset of patients that do not experience a significant and/or long-lasting improvement on an LVAD. Moreover, there is a great need to develop therapies that prevent end-stage heart failure. Consequently, the field of cardiac tissue engineering emerged as a means of developing alternative sources of cardiac tissue and methods for replacing tissue damaged by CVD.
Biomaterials have featured prominently in cardiac regenerative therapy and can be divided among two main strategies for inducing functional repair to the heart muscle post-injury: (i) the production of functional cardiac patches in vitro that can be implanted onto the damaged area and thereby directly replace the non-viable portion of a damaged heart, and (ii) injectable biomaterials (hydrogels) to help prevent cardiac remodeling, deliver cells to replace the damaged tissue, and recruit endogenous cell types in an attempt to restore functionality(Menasche, 2008). A complete list of the biomaterials and various strategies discussed within this review is found in Table 1. While the delivery of cells to the injury site is a major aspect of cardiac regeneration therapy and the cell types used are many and varied, the topic has been reviewed in detail elsewhere(Shiba et al., 2009; Hilfiker et al., 2011; Martinez and Kofidis, 2011; Liau et al., 2012) and will not be addressed in the current review.
Table 1.
Biomaterial strategies in cardiac tissue repair
| Scaffold | Material | Design | Use | Reference |
|---|---|---|---|---|
| Natural | Alginate | Prevascularized Scaffold (in the peritoneaum) | Full LV wall tissue replacement | (Amir et al., 2009; Sapir et al., 2011) |
| Alginate | Proangiogenic prevascularized (in the omentum) bioactive scaffold | Tissue engineered patch | (Dvir et al., 2009) | |
| Cell sheets | In vitro prevascularized layered cell sheets | Tissue engineered patch | (Shimizu et al., 2002; Masuda et al., 2008; Sekine et al., 2013) | |
| Collagen | Proangiogenic bioactive scaffold | Tissue engineered patch | (Chiu and Radisic, 2010) | |
| Collagen/Matrigel | Cultured cardiomyocytes in a scaffold | Tissue engineered patch | (Zimmermann et al., 2002; Zimmermann et al., 2006; Yildirim et al., 2007) | |
| Decellularized ECM | Decellularized hearts as a scaffold | Whole organ replacement & tissue patches | (Ott et al., 2008; Sanchez et al., 2012) | |
| Decellularized ECM | Decellularized hearts as a scaffold | Whole organ replacement & tissue patches | (Akhyari et al., 2011; Aubin et al., 2013) | |
| Decellularized ECM | Decellularized hearts in thin sheets as a scaffold | Tissue engineered patch | (Godier-Furnemont et al., 2011) | |
| Decellularized ECM | Scaffold for SC differentiation, whole heart regeneration | Whole organ replacement | (Ng et al., 2011) | |
| Matrigel | Prevascularized (biochamber in hind limb) scaffold | Tissue engineered patch | (Morritt et al., 2007) | |
| Synthetic | (ε-caprolactone-L-lactide) reinforced with a knitted poly-L-lactide fabric | vSMCs seeded in a scaffold | Full LV wall tissue replacement | (Matsubayashi et al., 2003) |
| Poly (2-hydroxyethyl methacrylate-co-methacrylic acid) | Engineered (spatially controlled) scaffold | Tissue engineered patch | (Madden et al., 2010) | |
| Polycaprolactone | Engineered (specifically controlled) scaffold | Tissue engineered patch | (Yeong et al., 2010) | |
| Poly-L-lactic acid &polylactic glycolic acid | Multi-cell seeding to promote vascularization, scaffold | Tissue patch | (Lesman et al., 2010) |
| Hydrogel | Material | Design | Use | Reference |
|---|---|---|---|---|
| Natural | Alginate | Chemically cross-linked hydrogel | Bulking material | (Leor et al., 2009; Lee and Mooney, 2012) |
| Chitosan-collagen | Thermogelling bioactive hydrogel | Endogenous repair | (Reis et al., 2012) | |
| Chitosan-glycerol phosphate | Thermoresponsive chemically cross-linked hydrogel | Cell transplantation | (Lu et al., 2009) | |
| Decellularized ECM | Thermogelling hydrogel | Endogenous repair | (Singelyn et al., 2012) | |
| Fibrin | Thermogelling hydrogel | Cell transplantation | (Christman et al., 2004a) | |
| Semi-synthetic | PEG-fibrinogen | Photocrosslinkable (UV light polymerizable) hydrogel | Endogenous repair, cell transplantation, & bulking material | (Habib et al., 2011; Rufaihah et al., 2013) |
| Synthetic | Methacrylatedhyaluronic acid | Chemically cross-linked hydrogel | Bulking material | (Ifkovits et al., 2010) |
| NIPAAm-co-AAc-co-HEMAPTMC | Thermoresponsive hydrogel | Bulking material | (Fujimoto et al., 2009) | |
| PEG | Chemically cross-linked bioactive hydrogel | Endogenous repair | (Kraehenbuehl et al., 2009) | |
| PEG | Chemically cross-linked hydrogel | Bulking material | (Rane et al., 2011) | |
| Polyester | Thermoresponsive bioactive hydrogel | Endogenous repair & bulking material | (Wu et al., 2011) | |
| α-cyclo-dextrin/MPEG-PCL-MPEG | Chemically cross-linked hydrogel | Cell transplantation | (Wang et al., 2009) |
2. Design criteria for biomaterials in cardiac tissue engineering
The complexity of the native heart environment and pathophysiology of post-MI remodeling challenges the development of strategies for the treatment and design of biomaterials. An important aspect of biomaterial design is the consideration of the objective of the biomaterial application. Design criteria will be different whether the biomaterial is a vehicle for cell delivery, a functionalized material aimed at artificially retaining normal ventricular geometry, or a scaffold to generate tissue patches. However, there are some common design criteria that must first be addressed: (i) biocompatibility, (ii) biodegradability, (iii) mechanical support, (iv) injectability (in the case of hydrogels), (v) clinically relevant thickness (in the case cardiac patches), and finally (vi) envisioned application time post-infarct(Leor et al., 2006; Chen et al., 2008; Vunjak-Novakovic et al., 2010; Bouten et al., 2011).
2.1. Biocompatibility
Biocompatibility today is generally defined as the “ability of a material to perform with an appropriate host response in a specific application”(Williams and European Society for Biomaterials., 1987). In the context of cardiac tissue engineering this encompasses the need for the material to function without initiating a significant foreign body response in vivo, while retaining the ability to support both cardiomyocyte survival in vitro and in vivo readily without cytotoxicity, as well as the contractile function of the myocardium(Leor et al., 2006; Chen et al., 2008; Vunjak-Novakovic et al., 2010). This does not preclude the activation of the host inflammatory and immune response but rather focuses on mitigating and controlling the type of response in order to prevent further injury to the heart and not impede its function. Specifically, a biocompatible material should be resistant to blood clotting and bacterial colonization, and if immunogenic should not recruit cell types that can exacerbate the remodeling process. For example, activation of the host immune response such that there is preferential recruitment of reparative M2 macrophages over cytotoxic M1 macrophages is generally considered to be a beneficial trait for biomaterials.
2.2. Biodegradability
Biodegradability refers to the mechanism through which an implanted material breaks down and the inherent life-span of the material. A thorough discussion of the various mechanisms and subsequent definitions are covered in detail elsewhere(Treiser et al., 2013). However, in brief, there are three modes to consider: (i) bioerosion is degradation of a material through hydrolytic mechanisms (covers both surface and bulk erosion); (ii) bioresorption is degradation through cellular activity; and (iii) biodegradation is degradation through enzymatic activity. In the context of cardiac tissue engineering, a biomaterial is considered to be biodegradable if degradation occurs through disintegration, a hydrolytic mechanism or by enzymatic activity that the biomaterial will encounter in vivo; and that the degradation products similarly conform to the requirements of both biocompatibility and biodegradability(Leor et al., 2006; Chen et al., 2008; Vunjak-Novakovic et al., 2010; Bouten et al., 2011). While biocompatibility and biodegradability are distinct concepts, they are often considered in tandem during biomaterial design as there is little use in designing a biocompatible material that degrades into toxic components.
Many physiological extracellular matrix (ECM)-based biomaterials readily fit these dual criteria (e. g. fibronectin, and collagen) as they inherently contain the correct molecular composition required for cell attachment and survival(Chen et al., 2008), and they are readily degraded in vitro and in vivo within days to weeks by enzymes secreted by cells into biocompatible and biodegradable degradation products. Notably, cells can turnover these ECM biomaterials and replace them with their own ECM components, thereby remodeling their environment as necessary(Li and Guan, 2011). However, sourcing these materials can be complicated by the fact that they may retain many of their surface antigens and may elicit an immune response if used in xeno-transplantation. Despite this limitation there are sources of the ECM-biomaterials that have been approved for human use, including fibrin that can be isolated from a patient’s own blood(Odedra et al., 2011).
Conversely, synthetic materials have been developed such as polyethylene glycol (PEG), poly (glycerol-sebacate) (PGS), and poly (tetrafluoroethylene) (PTFE) with defined chemical compositions and designed to have no foreign body response. Modification of the chemical composition can permit the selection of degradation rates in the range of a few weeks to years. However there is the concern as to whether the degradation products are truly being removed from the body or rather accumulate, the long-term effects of which are unknown(Chen et al., 2008; Li and Guan, 2011). An additional limitation is that these synthetic biomaterials often do not support cell adhesion and survival, and therefore need to be modified with appropriate bioactive molecules(Vunjak-Novakovic et al., 2010).
Another important consideration in terms of biodegradability is the issue of how quickly the material should be removed in order to properly execute its desired function when applied for cardiac ventricular repair. Biomaterials designed for cell delivery, recruitment, and survival (anti-apoptotic, pro-angiogenic) should survive in vivo at least one week, based on the fact that most cell death occurs within the first few days post-MI; and should be fully degraded in 6–8 weeks (in animal models)(Patten et al., 1998; Krzeminski et al., 2008; Li and Guan, 2011) recognizing that pathological remodeling is complete by approximately 6 weeks after MI. Thus, the biomaterial should remain long enough to have the desired effect but no longer than necessary as it may become a hindrance to repair. For example, improved cardiac function post treatment was demonstrated with fibrin glue used to transplant skeletal myoblasts into ischemic myocardium wherein it degraded in 7–10 days(Christman et al., 2004b). Scaffold degradation is likely to require a similar timeframe. Most scaffolds are designed to be quickly replaced by new ECM secreted from the seeded cells with the engineered tissues that are implanted containing very little of the original scaffold material. For scaffolds that are present at the time of implantation, in vivo degradation should not exceed weeks to months and should be quickly replaced by functional tissues. Zimmermann et al. have spent considerable time developing functional cardiac tissue constructs in vitro demonstrating that their collagen-based scaffold can be remodeled and replaced by maturing cardiomyocytes resulting in a scaffold-free transplantable engineered heart tissue construct(Eschenhagen et al., 1997; Zimmermann et al., 2002). In the case of biomaterials designed to provide support to the failing ventricle, they should have relatively slow degradation rates on the order of months to years and while controversy persists as to the timeframe required, it may be desirable for such materials to remain for the very long-term (see Section 4.2 for examples and further discussion)(Nelson et al., 2011). It is important to note however that there is still much debate as to the mechanical properties and degradation requirements that are necessary for certain outcomes due to the large disparity between studies(Nelson et al., 2011). These topics therefore continue to be active areas of investigation.
2.3. Mechanical Support
The third criteria of mechanical support requires some forethought as to the envisioned application with consideration of whether the biomaterial can withstand the mechanical demands that will be placed upon it after ventricular application and also whether it is likely to interfere with the normal mechanical functioning of the surrounding tissue. The in vivo model system for the biomaterial should be considered with respect to species-specific mechanical demands. This is because the mechanical forces placed upon a biomaterial by the human heart will vastly differ from the forces exerted by a small rodent heart in an animal application. Specifically, the human myocardium ranges in stiffness from 20kPa (end of diastole) to 500kPa (end of systole), whereas rat myocardium ranges from 0. 1 to 140kPa(Chen et al., 2008; Vunjak-Novakovic et al., 2010; Bouten et al., 2011; Venugopal et al., 2011). A material envisioned to artificially thicken the ventricle wall and maintain ventricular geometry during remodeling should have a stiffness in the high end of the range characteristic for the native ventricle; whereas a material designed to be injected, to act as a temporary matrix for transplanted cells and/or to recruit endogenous cells can have a low-end stiffness so long as it is sufficiently stiff to withstand the contraction/dilation of the heart. Biomaterials intended for in vitro applications can have a very low stiffness, as long as the cells seeded into it (hydrogels or scaffold) are able to remodel it into a material that as a final product is mechanically similar to the native myocardium. This is especially evident in the case of cardiac tissue grafts for repair of full thickness defects, wherein mismatching the mechanical properties can result in the grave consequences of inducing undue strain on the injured heart if the graft is too stiff or graft failure due to the stresses experienced in vivo if the graft is insufficiently stiff(Ozawa et al., 2002). Furthermore, issues such as burst pressure and suture retention must be considered as the graft has no time to integrate with the host tissue since it experiences full cardiac load immediately upon implantation. Results from tissue engineering of arterial grafts provide useful benchmarks for these properties that are achievable using current tissue engineering methods e.g. burst pressure of over 3000mmHg and suture retention strength of over 160g (L’Heureux et al., 2006; Dahl et al., 2011). Motivated by these challenges, Lang et al have been developing a surgical glue to improve cardiac graft and achieved a success in repairing full thickness defect (Lang et al., 2014).
In general, naturally-derived biomaterials have weak mechanical properties, with moduli in the tens of Pa to tens of kPa range(Chen et al., 2008). Moreover, there is a batch-to-batch and source-to-source variability in the physical properties of these biomaterials. As a consequence, biomaterials made purely of naturally-derived components are limited as to their mechanical support applications. Synthetic materials, on the other hand, are more consistent in their composition between batches and have mechanical properties such as stiffness, elasticity, and porosity that can be precisely controlled(Chen et al., 2008).
2.4. Injectability
A hydrogel that can pass through a fine gauge needle (~27G) is described as injectable as it is possible to safely administer it into the heart in a minimally invasive manner. Injectability can be achieved by two approaches wherein gelation of the hydrogel (by temperature, chemical, light-induced cross linking (Yeo et al., 2007; Habib et al., 2011), etc.) is: (i) initiated but not completed prior to the hydrogel passing through the needle, and (ii) initiated after delivery to the desired site. Importantly, the polymerization time should be in the order of minutes to tens of minutes to ensure the hydrogel is delivered and successfully localized at the site of injection and not completely washed out(Vunjak-Novakovic et al., 2010). This is because for polymerization that requires tens of minutes to hours to complete there is enough time for the biomaterial to be subjected to the contraction of the heart and to be carried away in the blood stream, rather than gel properly in the ventricle wall.
2.5. Clinically Relevant Thickness
Biomaterials used for tissue engineering strategies where cells are cultured with the biomaterial in vitro then implanted in vivo have their own distinct requirement in that they must be capable of supporting the cultivation of tissues of clinically relevant thickness: up to ~10 mm for full thickness cardiac grafts, whereas tissue patches can be thinner(Chiu and Radisic, 2010). The limits of oxygen diffusion within a metabolically active tissue of high cell density (e.g. 108cells/cm3) restricts tissue thickness to approximately 200μm, thus scaffolds often require that a primitive vascular network or a channel array for culture medium perfusion be incorporated into the design to allow sufficient nutrient exchange to the centre of the tissues during in vitro cultivation(Radisic et al., 2006). This is a major issue in producing in vitro cardiac patches that will be discussed in greater detail in Section 3. 3.
2.6. Application Time
An additional consideration that can influence biomaterial design for cardiac regeneration therapy is the time post-infarction at which the biomaterial is to be applied since new and old infarcts present their own unique challenges. The rapid cell death that results from nutrient and oxygen deprivation downstream of a coronary artery blockage suggests that a cell injection-based strategy should be most effective if applied shortly after an MI. The application of a biomaterial modified with both cells and growth factors within hours or days after an MI may promote directed wound repair such that the scar tissue formed would be minimized, the contractile function maintained in the border zone, the ischemic area reduced, and consequently pathological remodeling attenuated. Notably, incorrectly timed administration of the therapy could potentially exacerbate the problem. While early delivery of cells might conceptually be more effective by initiating early re-vascularization and contributing to the protection of the spared myocardium, it may at the same time expose the delivered cells to a very hostile environment because of the significant immune response, the presence of cell death-associated cytokines and the by-products of dead and dying cells in the infarcted area soon after insult, which can compromise the viability of the cells. A scaffold-based contractile tissue engineering strategy while applicable in the acute phase may have a more significant effect if implanted after scar formation. Similarly, larger areas of damaged cardiac muscle evident in chronic cases might benefit from a scaffold-based regenerative therapy approach. Therefore, choosing the right time point post-MI for an intervention is a challenge and no clear consensus has been reached.
3. Scaffolds for engineered cardiac patches and tissue models
Engineered tissue replacements should match the morphology and function of the native myocardium. They should be mechanically stable with a Young’s modulus of 22–50kPa(Bhana et al., 2010), have mm scale thicknesses, and be capable of electrical impulse propagation (~25cm/s)(Chiu et al., 2011; Chiu et al., 2012b). In order to support proper cardiac function, cardiomyocytes within the tissue construct should be elongated and aligned at high cell density of ~108cells/cm3, and organized within a vascular network with intercapillary distance of ~20μm(Chiu and Radisic, 2010; Chiu et al., 2011; Chiu et al., 2012b). Significant work has been done on culture methods to improve in vitro tissue functionality by using topographical and electromechanical cues (reviewed in(Maidhof et al., 2011; Tandon et al., 2011; Riehl et al., 2012)); however this section focuses on the most typical synthetic and natural biomaterials used. Here, we first review common natural and synthetic biomaterials used in preparation of scaffolds for cardiac tissue engineering, then we specifically focus on scaffolds made of decellularized heart tissue, as well as scaffold approaches used to achieve rapid vascularization.
3.1. Natural and synthetic biomaterials used in scaffolds for cardiac tissue engineering
Natural materials that are most commonly used as scaffolds for cardiac tissue engineering include alginate and collagen due to their availability and biocompatibility. In terms of synthetic materials, FDA approved polyesters such as polycaprolactone, poly –L-lactic and poly (lacticco-glycolic) acids are commonly used as they satisfy a number of design criteria outlined above. In addition, due to the need for cyclic strain during relaxation/contraction cycle of the cardiac muscle, elastomers such as poly (glycerol-sebacate) are also commonly used. We outline below representative studies that achieved formation of a contractile cardiac patch using these materials.
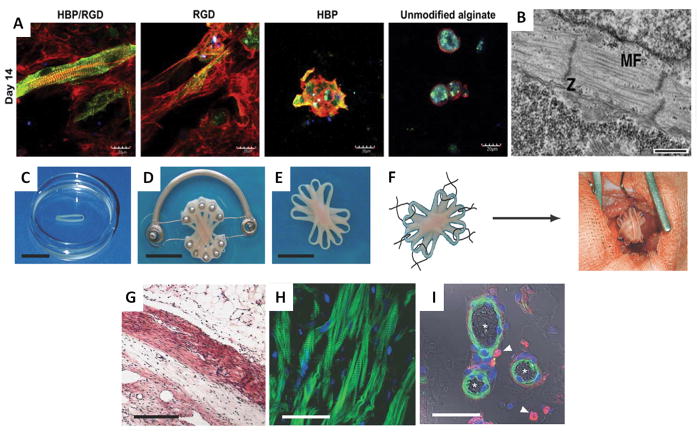
In terms of natural biomaterial-based scaffolds, a porous alginate scaffold was used to culture fetal rat cardiomyocytes in vitro for 4 days, then implanted into the rat peritoneal cavity for 1 week to vascularize the cardiac patch (Amir et al., 2009). The patch was then transplanted into the heart for cardiac repair. In a separate study, the attachment of adhesion peptide G4RGDY and heparin-binding peptide G4SPPRRARVTY to alginate scaffolds improved the formation of a functional cardiac tissue compared to unmodified scaffolds(Sapir et al., 2011). The scaffolds with both peptides showed an isotropic myofiber arrangement of cultivated cardiac cells, with an increase in Connexin-43 expression (Figure 1 A). Similarly, collagen scaffolds were shown to support differentiation of mouse embryoid bodies into beating cardiomyocytes (Figure 1 B)(Dawson et al., 2011).
Figure 1. Natural and synthetic scaffolds for cardiac tissue engineering.
(A) Cardiac cell organization in peptide-attached alginate scaffolds. Cells were grown on scaffolds with both G4RGDY (RGD) and G4SPPRRARVTY (HBP) peptides; scaffolds with a single peptide attached, or unmodified alginate scaffolds. On day 14 post-seeding, cells were double stained with antibodies against α-sarcomeric actinin (green) for cardiomyocytes and F-actin (red) for both myocytes and non-myocytes (nuclei, blue; scale bar = 20 μm)(Sapir et al., 2011).
(B) Isotonic myofiber arrangement of cardiomyocytes in EHT. Collagen scaffold with RGD were seeded with 5-day embryoid body-derived cardiomyocytes. On day 10 post-seeding, the ultrastructure of the EHT was assessed by transmission electron microscopy. Sarcomeric components, including organized bundles of myofibrils (MF) and Z bands (Z), are visible (scale bar = 500 nm)(Dawson et al., 2011).
(C–F) Multi-loop EHT construct as cardiac patch. Individual loop-shaped EHTs were stacked onto a custom device designed to promote the fusion of the individual loops into a single multi-loop EHT and to permit their contraction under auxotonic load (scale bar = 10 mm) (C–E). The resulting synchronously contracting multi-loop EHT was engrafted onto the recipient heart using six single-knot sutures (F) (Zimmermann et al., 2006).
(G–I) Four weeks after engraftment, EHTs form thick, organized, vascularized muscle. H&E staining of paraffin section through infarcted area shows engrafted EHTs form compact and oriented heart muscle (scale bar = 500 μm) (G). Sarcomeric organization of engrafted EHTs is visible by laser scanning microscopy (actin, green; nuclei, blue) (scale bar = 50 μm) (H). Newly formed vessels containing smooth muscle cells, with macrophages in close proximity are visible by confocal microscopy (actin, green; nuclei, blue; ED2, red and arrows) and erythrocytes in the vessels are visible by differential interference contrast imaging (asterisks; scale bar = 50 μm) (I)(Zimmermann et al., 2006).
Similarly, as examples of the success obtained with synthetic biomaterial-based scaffolds, a biodegradable porous scaffold composed of 50% poly-L-lactic acid and 50% polylactic glycolic acid was seeded with human embryonic stem cell (hESC)-derived cardiomyocytes, endothelial cells and embryonic fibroblasts(Lesman et al., 2010). The cardiac constructs were cultivated for 2 weeks and then grafted onto the rat left ventricle for 2 additional weeks, consequently showing formation of both donor and host vasculature within the tissue constructs and functional integration of donor vessels with the host coronary vasculature.
Building upon the idea of using a hydrogel as a substrate for tissue engineering,, Drs. Zimmermann and Eschenhagen used a multi-component natural biomaterial-based scaffolds to produce a more robust and highly functional engineered heart tissue (EHT) construct that has become a benchmark against which other cardiac tissue engineering strategies are compared. By mixing cells with collagen I and Matrigel, seeding the mixture into various molds, combining the resultant scaffolds, then using mechanical stretching platforms to remodel and mature the construct, they succeeded in creating an in vitro cardiac tissue model as well as an organized functional cardiac construct for transplantation in in vivo models (Figure 1 C–F)(Eschenhagen et al., 1997; Zimmermann et al., 2002). The resultant EHTs were transplanted onto the infarcted myocardium of rats with 14 day old infarcts, and after four weeks, the EHT formed thick muscle layers, showed un-delayed electrical coupling with the host myocardium, and displayed no signs of arrhythmia induction. Morphologically the EHT reduced dilation, increased LV wall thickness, and improved fractional area shortening compared to sham operated and non-contractile construct controls (Figure 1G–I)(Zimmermann et al., 2006). This landmark paper clearly demonstrated the power of a tissue engineering approach to prevent pathological remodeling post-MI. By further developing their system, they have also successfully produced pouch-like EHTs that can be slipped over adult rat hearts to completely cover both the left and right ventricles. Analysis 14 days after implantation showed stable coverage as well as indication of integration with host tissue through vascularization(Yildirim et al., 2007).
To investigate the feasibility of truly remuscularizing fully formed scar tissue, Ozawa et al used a modified endoventricular circular patch plasty (EVCPP) on the infarcted area of four week old infarcts in rats in order to replace scar tissue. Having seeded and cultivated vascular smooth muscle cells on a synthetic PCLA sponge for two weeks in vitro, their surgical technique involved fully removing the infarcted tissue and grafting in its place their tissue construct(Matsubayashi et al., 2003). After eight weeks in vivo they recorded improved cardiac function and morphometric measurements, demonstrating the utility of the approach. However, the extreme invasiveness of the surgery combined with the risk of graft failure makes this a much riskier procedure than the conventional strategy of leaving the scar intact and implanting the tissue patch on top.
As far as the design of scaffolds for cardiac tissue engineering has advanced, active research continues to identify the most effective scaffold material and design in order to best mimic the native adult cardiac tissue for in vitro model systems and to induce a functional improvement substantial enough to alleviate some if not all of the lasting effects of an MI. Currently, the complexity of the materials used in scaffolds is increasing as more information becomes available and new scaffold designs are continually being produced. Moreover, there have been instances of success using disparate scaffold types, which all seems to converge on the idea that there is not a single right answer in terms of material or design. However, the development process will be greatly assisted by standardization in experimental protocols and end-points to facilitate comparisons among groups.
3.2. Scaffolds obtained by decellularization of native hearts
Given the challenge of replicating the complexity of the heart ECM and architecture, many researchers looked to decellularized heart as an ideal scaffold for cardiac tissue engineering because the chamber geometry, vascular architecture, heart ECM, and mechanical properties could be preserved, and the seeded cells would therefore be exposed to the same microenvironment as the native heart. Moreover, the generation of an autologous bio-artificial heart using decellularized whole heart as a scaffold would overcome immune-rejection of allograft heart transplantation. Initial experimentation centered on the feasibility of the method in terms of retention of the architecture and ECM components, as well as in supporting seeded cells in various animal models. This was first demonstrated by Ott et al using decellularized rat hearts reseeded with neonatal rat cardiac cells. The pioneering study successfully produced beating hearts in vitro thus the next step in assessing feasibility for human use was to see if the technique could be replicated using human myocardium (Figure 2 A–D)(Ott et al., 2008). Human hearts were decellularized by coronary perfusion with sodium dodecylsulfate for 4–5 days, and were demonstrated to maintain three-dimensional structure, chamber geometry, valve competency, fiber orientation, extracellular matrix components and microvascular structure after decellularization(Sanchez et al., 2012). The decellularized human heart supported the attachment, alignment and survival of human mesenchymal bone marrow derived stem cells cultivated on the left ventricle in vitro(Sanchez et al., 2012). Adult porcine hearts were also decellularized using pulsatile retrograde aortic perfusion, and shown to retain mechanical integrity and ECM components such as collagen, elastin and glycosaminoglycans(Wainwright et al., 2010). The decellularized porcine heart supported the organization of sarcomeres in the cultivated chicken cardiomyocytes in vitro. In a separate study, porcine hearts were also decellularized using the modified Langendorff perfusion decellularization model with an ionic detergent-based protocol(Weymann et al., 2011).
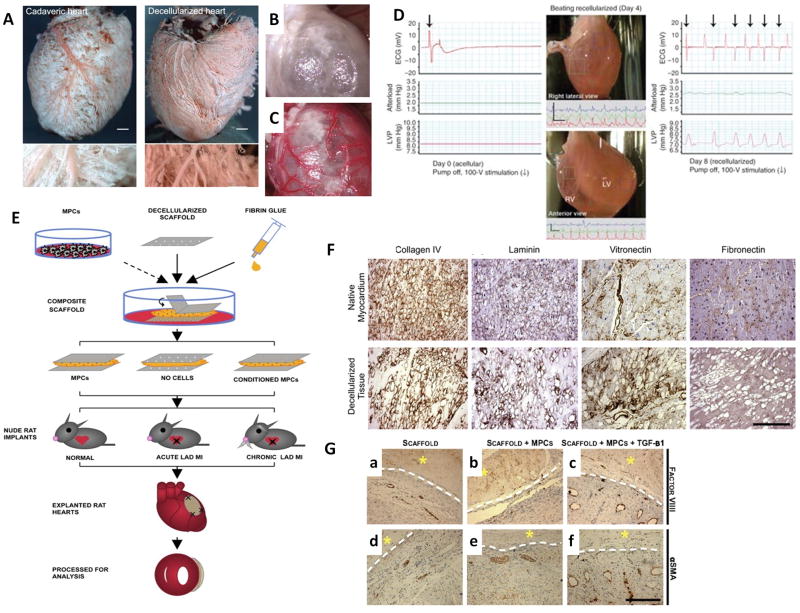
Figure 2. Scaffolds made from decellularized extracellular matrix.
(A–C) Decellularized whole adult rat hearts have patent vasculature and can be perfused. Coronary corrosion casts of cadaveric and decellularized rat hearts demonstrate vessel patency at both the macroscopic (upper row; scale bar = 1000 μm) and microscopic (lower row; scale bar = 250 μm) level (A). Functional perfusion was demonstrated by heterotypic transplantation of the decellularized heart with visualization before (B) and after (C) the host aorta was unclamped(Ott et al., 2008).
(D) Recellularized heart constructs perform stroke work. Decellularized rat hearts were mounted into a working-heart bioreactor, seeded with neonatal rat cardiac cells, and function was assessed by real-time ECG, aortic pressure (after load) and left ventricular pressure (LVP) on day 0, 4 and 8. Decellularized heart construct on day 0 (left). Right lateral (top panel) and anterior view (bottom panel) of regions of movement and corresponding tracings in paced recellularized heart constructs on day 4 (centre). On day 8, electrical stimulation induced an increase in LVP, which was followed by repolarization (right) (Ott et al., 2008).
(E) Composite scaffolds for cell delivery were assembled from thin sheets of decellularized human myocardium, fibrin hydrogel and immune selected MPCs (cultured ±TGF-β conditioning), and implanted into nude rat left anterior descending artery (LAD) ligation models of acute and chronic myocardial infarction. After 4 weeks, the hearts were explanted and function was evaluated by echocardiography (Godier-Furnemont et al., 2011).
(F) Decellularized matrix maintains nearly the same extracellular matrix composition as native tissue, excepting fibronectin content (scale bar = 250μm)(Godier-Furnemont et al., 2011).
(G) Composite scaffolds with MPCs promote angiogenesis and arteriogenesis. Four weeks post-implantation in nude rat models of acute cardiac infarction, the composite constructs with MPCs, with or without TGF-β preconditioning (MPC/scaffold group and TGF-β/MPC/scaffold group, respectively), had increased size and frequency of factor VIII- and SMA-positive blood vessels, relative to the scaffold only group (asterisk, patch; interface, dotted line; scale bar = 250μm)(Godier-Furnemont et al., 2011).
In an attempt to improve upon the decellularization process, Akhyari et al developed a software-controlled automatic coronary perfusion method to decellularize whole rat hearts while keeping the preset perfusion pressure constant(Akhyari et al., 2011). The goal of an automatic protocol was to achieve reproducible scaffolds with the same extracellular matrix components, which may be important for the fate of cardiomyocytes and cardiac progenitors. For example, elastin and collagen IV were found removed by previous protocols. In addition, varying degrees of acellularity were achieved as indicated by the remaining DNA content within the decellularized matrices. Thus, the standardization of an automatic protocol may be necessary to fully exploit the potential of decellularized whole hearts as scaffolds for cardiac tissue engineering.
As an alternative to the whole organ strategy, Godier-Furnemont et al used thin sections of completely decellularized human myocardium as a delivery vehicle for reparative cells(Godier-Furnemont et al., 2011). The cell-matrix composite scaffolds were assembled from thin decellularized sheets of human myocardium and fibrin hydrogel (Figure 2 E–F). Mesenchymal progenitor cells (MPCs) were immunoselected from bone marrow using STRO-1 and STRO-3, which were then cultured in vitro with TGF-β conditioning, suspended in fibrin, and implanted into a nude rat model of cardiac infarction. By adapting this method, great enhancement in vascular network formation was shown in the infarct bed when implanted with this composite scaffold containing TGF-β preconditioned MPCs. This may relate to the increase in the secretion of paracrine factors, such as SDF-1. In this context, paracrine factors are defined as growth factors secreted by the injected cells that positively influence other cells in the native cardiac environment(Gnecchi et al., 2008). Enhanced migration of MPCs into ischemic myocardium was also observed. Furthermore, at 4 weeks post-implantation, TGF-β and MPCs markedly enhanced angiogenesis and arteriogenesis in the infarct bed, as evidenced by the increased size and frequency of blood vessels (Figure 2 G)(Godier-Furnemont et al., 2011).
Recently, Ng et al studied the differentiation potential of hESCs and hESC-derived human mesendodermal cells (hMECs) in decellularized hearts(Ng et al., 2011). These cells showed upregulated expression of cardiac markers after 2 weeks of static culture, demonstrating that the properties of the decellularized heart could direct differentiation of stem cells and progenitors into cardiac cells. Moreover, there was higher expression of myosin light chain (Myl2 and Myl7) in differentiated hMECs and higher expression of myosin heavy chain (Myh6) in differentiated hESCs, showing the presence of different cardiomyocyte subtypes. When subcutaneously implanted in SCID mice, cells that expressed cardiac markers remained in the cardiac tissue but the tissue showed no contractility. While various successful methods for decellularization have been developed, and the feasibility of using decellularized whole hearts and ECM to support cells has been demonstrated, the reality of creating whole hearts for transplantation and of clinical application of decellularized ECM-based scaffolds will require much more research. For example, further investigations into how pluripotent cells or lineage restricted progenitors repopulate the decellularized heart and differentiate in a site-specific manner into different populations of the native heart would be essential.
3.3. Vascularization of scaffolds and engineered cardiac tissues
A major limitation to producing in vitro cardiac patches of clinically relevant thickness is oxygen and nutrient diffusion. The vascularization of the tissue is therefore an essential point of consideration for cardiac tissue engineering. To address this issue, recent research has been focused on making pro-angiogenic scaffolds by (i) controlling the structure of the scaffolds (porosity or introducing topographical cues), (ii) incorporating angiogenic molecules into the scaffolds (biochemical cues), or (iii) incorporating pre-existing vasculature into the engineered tissue constructs.
A variety of work has been published on methods used to modify scaffold structure, with micro-fabrication techniques frequently employed to further control the properties of the scaffolds. A porous synthetic polycaprolactonescaffold was designed with an automated algorithm and fabricated using selective laser sintering(Yeong et al., 2010). The compressive stiffness of the scaffold could be predicted based on the porosity in order to design a scaffold with appropriate mechanical properties for cardiac tissue engineering. The scaffold supported the in vitro growth of C2C12 myoblast cells for 21 days. Multi-layered poly (glycerol-sebacate) (PGS) scaffolds were also fabricated by laser micro-ablation of PGS membranes to create sheets with accordion-like honeycomb shaped pores and a subsequent oxygen plasma treatment of multiple stacked sheets(Park et al., 2011). Cardiac cells that were cultured on these scaffolds responded to electrical field stimulation after 7 days. Similarly, micro-molding and micro-ablation technologies were combined to fabricate biodegradable PGS scaffolds with well defined surface patterns and pores that ultimately guided the orientation of muscle cells(Guillemette et al., 2010). The stiffness of PGS elastomer scaffold could be tailored by controlling the extent of polymer cross-links(Marsano et al., 2010). It was found that the contractility of engineered cardiac tissues using PGS scaffolds had a positive correlation with low compressive stiffness.
In the context of vascularization, Madden et al introduced topographical cues into their scaffold by using micro-templating to make bimodal scaffolds with parallel channels for the organization of cardiomyocytes and interconnected pores to enhance angiogenesis(Madden et al., 2010). These scaffolds supported the survival and proliferation of hESC-derived cardiomyocytes in vitro. When cell-free scaffolds were implanted in the nude rat myocardium, maximal vascularization was achieved with scaffolds with 30–40μm pore diameter.
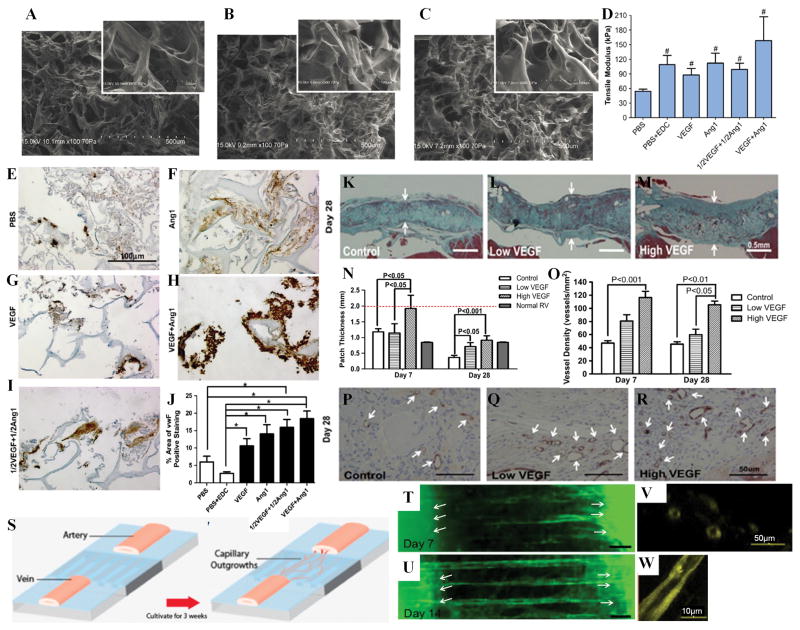
As the alternative strategy, angiogenic molecules such as vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), and basic fibroblast growth factor (bFGF) have been incorporated into scaffolds to improve angiogenesis and in turn cardiac repair. VEGF and Ang-1 were covalently immobilized onto porous collagen scaffolds using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) chemistry (Figure 3 A–C). These modified scaffolds increased the proliferation of seeded H5V endothelial cells(Chiu and Radisic, 2010) and primary rat aortic endothelial cells(Chiu et al., 2011) as compared to cells grown on unmodified scaffolds with or without supplementation of soluble growth factors. These cells also formed capillary-like tubes on modified scaffolds. Scaffolds with co-immobilized VEGF and Ang-1 enhanced angiogenesis in the chicken chorioallantoic membrane assay compared to scaffolds with immobilized VEGF or Ang-1 alone(Chiu and Radisic, 2010), due to the synergistic effects of combined growth factors (Figure 3E–J). When implanted in the rat heart, scaffolds with immobilized VEGF increased angiogenesis as compared to unmodified scaffolds, in turn improving the stability of the cardiac repair (Figure 3 K–R)(Miyagi et al., 2011). These studies demonstrate that covalent immobilization of angiogenic growth factors onto scaffolds can localize and sustain the activity of the biomolecules by preventing their washout from the site of interest and their internalization by the cells, and ultimately improve the efficacy of the bioactive molecules that do not require internalization for their activity.
Figure 3. Scaffolds for engineered cardiac patches and tissue models.
(A–C) SEM images of collagen sponges for (A) PBS only treated scaffold (PBS), (B) scaffold with lower dosed of co-immobilized VEGF and Ang1 (1/2VEGF+1/2Ang), (C) scaffold with higher dose of co-immobilized VEGF and Ang1 (VEGF+Ang1). SEM images suggested there were no significant differences in the pore structure and porosity of growth factor immobilized and control scaffolds(Chiu and Radisic, 2010).
(D) Tensile modulus of scaffolds treated as listed. The tensile moduli of scaffolds with immobilized growth factors were significantly higher than that of PBS control sponges (P=0. 0353)(Chiu and Radisic, 2010).
(E–I) Representative images of Factor VIII staining in Chicken CAM assay (brown represents positive staining; blue represents counter stain)(Chiu and Radisic, 2010).
(J) Scaffolds with co-immobilized VEGF and Ang-1 enhanced angiogenesis in the chicken chorioallantoic membrane assay compared to scaffolds with immobilized VEGF or Ang-1 alone(Miyagi et al., 2011).
(K–M) Representative images of Masson’s trichrome staining at 28 days after patch implantation. Arrows indicate thickness of the patch. At 28 days, wall thicknesses in both VEGF-treated patches were significantly greater than those in the control patches (p<0. 05 for Low VEGF vs. control; p < 0. 001 for High VEGF vs. control)(Miyagi et al., 2011).
(N) Patch thickness at 28 days after implantation (Normal RV = normal right ventricular tissue; red dotted line indicates patch thickness at the time of implantation). Scaffolds with immobilized VEGF increased angiogenesis as compared to unmodified scaffolds(Miyagi et al., 2011).
(O) Blood vessel density within the patches at 28 days after implantation. Scaffolds with immobilized VEGF increased angiogenesis as compared to unmodified scaffolds(Miyagi et al., 2011).
(P–R) Representative images of CD31 expression (arrows identify CD31-positive vascular structures) at 28 days after patch implantation. Blood vessel density within the patch was significantly increased in the High VEGF group at 28 days (p < 0. 01 vs. control; p < 0. 05 vs. Low VEGF) after patch implantation(Miyagi et al., 2011).
(S–W) The engineering of a vascular bed by induction and organization of capillary outgrowths from parent vascular explants. (S) The mouse or human artery and vein explants were placed at the two ends of a polydimethylsiloxane (PDMS) substrate with microgrooves of 25μm, 50μm or 100μm in width and coated with Tβ4-encapsulated collagen-chitosan hydrogel. The samples were cultivated for 3 weeks to achieve capillary outgrowths connected between the parent explants, or for 2 weeks with hepatocyte growth factor (HGF) or VEGF-supplementation in the culture medium. (T–U) The oriented capillary outgrowths connected from the arterial explant to the venous explant at Day 14 with HGF supplementation. Fluorescent microscopy images showing capillary outgrowths extending between mouse artery to vein at (T) Day 7 and (U) Day 14 of in vitro cultivation on PDMS substrate with 100μm grooves and 1500ng encapsulated thymosin β4. Arrows indicate locations of artery and vein explants. Scale bars, 100μm. Confocal microscopy images of capillary outgrowths indicate (V) cross-sectional and (W) longitudinal lumens(Chiu et al., 2012a).
Expanding upon these findings, Chiu et al engineered an organized capillary network anchored by an artery and a vein by mimicking the process of in vivo angiogenesis, normally involving endothelial cell proliferation and sprouting followed by the connection of extended cellular processes and lumen propagation via vacuole fusion(Chiu et al., 2012a). In this approach, an artery and a vein were placed on opposite ends of a micro-patterned substrate coated with a hydrogel containing thymosin β4 (Tβ4, a pro-angiogenic peptide), thus combining the effects of topographical and biochemical cues. Capillary outgrowths were induced from the parent vascular explants by the sustained presence of Tβ4 in the collagen-chitosan hydrogel coating and then organized by the presence of microgrooves such that the capillary outgrowths connected from the artery to the vein after 3 weeks of culture (Figure 3S). Cardiomyocytes could be easily seeded in the parenchymal space, and the resulting engineered cardiac tissues exhibited improved functional properties compared to tissues generated from cardiomyocytes alone without prevascularization (Figure 3T–W). In this case, the engineered vascular bed was not perfused and the cardiomyocyte morphology and function likely improved due to enhanced cell organization and interaction of the vasculature with the cardiomyocytes. The vascular bed could be perfused to further improve viability and function of resulting engineered cardiac tissues. For example, Kofidis et al used pulsatile perfusion to improve cardiomyocyte viability in the tissue engineered constructs(Kofidis et al., 2003). In this study, rat cardiomyocytes were seeded in a fibrin glue scaffold into a circular chamber with an inlet and an outlet, and a rat aorta mounted between the inlet and outlet lines. The cells were cultured for 2 weeks under pulsatile perfusion of 100mL/h and 120 pulsations/min which resulted in significantly higher overall viability. There was also higher cell density near the core vessel. In future studies, the pre-existing vascular structure may be provided by a decellularized donor graft such as an allogenic or xenogenic small caliber artery, making this approach more clinically transferable.
In a novel approach to cardiac patch vascularization, prior to implantation on the heart, the cardiac patches were first implanted into the omentum to induce vascularization. Dvir et al incorporated pro-survival and angiogenic factors, insulin-like growth factor-1 (IGF-1), stromal-cell derived factor (SDF-1) and VEGF, into macroporous alginate scaffolds(Dvir et al., 2009). The alginate scaffolds included alginate-sulfate, which allowed high affinity binding of the factors to the matrix. These scaffolds were then seeded with neonatal rat cardiac cells, cultured for 48 hours and then implanted onto rat omentum for 7 days to induce patch vascularization. There was a clear infiltration of host blood vessels into the cardiac patches with biomolecules, while patches without the factors remained acellular at the centre. When the vascularized cardiac patch was subsequently grafted onto the infarcted rat heart tissue, it fully integrated into the host myocardium after 4 weeks and improved cardiac function.
In another in vivo vascularization approach, Morritt et al engineered thick vascularized cardiac tissues by culturing neonatal rat cardiomyocytes around an arteriovenous blood vessel loop that was surgically created in the groin region of male rats and placed inside a patented tissue engineering chamber(Morritt et al., 2007). At 4 and 10 weeks, the cardiac constructs that grew in the chambers showed spontaneous contractions, extensive vascularization, and positive staining for markers indicating the presence of differentiated cardiomyocytes, including α-sarcomeric actin, troponin and desmin. Importantly, the resulting cardiac tissues reached a physiologically relevant thickness. In a later study, it was found that the viability of the cultivated cells was dependent on the rate of neovascularization within the tissue engineering chamber(Jiang et al., 2008). It was further demonstrates that the redox signaling involving a Nox2-containing nicotinamide adenine dinucleotide phosphate (NADPH) oxidase was important for the neovascularization process, as local treatment with the NADPH oxidase inhibitors apocynin or gp91ds-tat peptide significantly reduced vessel growth. Although this approach of using an arteriovenous loop-containing chamber to engineer vascularized cardiac constructs is quite invasive and therefore poses limitations for a clinical translation, it is one of the very few methods of in vitro tissue engineering of cardiac constructs with a vessel available for surgical anastomosis.
Expanding on their work on cell sheet engineering using temperature-responsive culture surfaces, Shimizu and Okano produced functional cardiac tissues with perfusable vasculature in vitro that could be transplanted and directly anastomozed in vivo(Shimizu et al., 2002; Masuda et al., 2008; Sekine et al., 2013). A resected section of femoral tissue containing a connectable artery and vein was perfused using a specially designed bioreactor in vitro. Using previously described cardiac cell sheets made in coculture with endothelial cells, they were able to create thick cardiac tissues by layering multiple cell sheets on top of the resected vascular tissue and culturing the construct in the bioreactor(Shimizu et al., 2002; Sekine et al., 2013). It was found that endothelial cells can connect to capillaries in the vascular bed and form tubular lumens, creating in vitro perfusable blood vessels in the cardiac cell sheets, and that even thicker tissue could be created by further addition of triple layer cardiac sheets(Sekine et al., 2013). Finally, it was shown that these tissues beat spontaneously and could be successfully transplanted with direct vessel anastomosis.
In another study, rat hearts were decellularized and processed into coronary artery tissue flaps that had patent vessel systems connected to the ascending aorta(Aubin et al., 2013). The de-endothelialized vessels were properly sealed as demonstrated by protein diffusivity analysis and blood perfusion of the arteries. The coronary artery system could be selectively seeded by retrograde aortic perfusion, after which the tissue flaps could be seeded with cardiac cells, thus creating a controlled co-culture. This represents a biologically derived in vitro cardiac model with preserved vascular architecture and cardiac ECM, and therefore provides a platform for studying re-endothelialization and the endothelial function of different donor cell types as well as their interaction with co-cultured cardiac cells. Hence, this model can be used to improve engineered cardiac construct vascularization methods and for in vitro drug testing and stem cell differentiation studies as the recellularization of the flaps can create realistic cardiac tissues.
It is therefore obvious that there is no consensus as to the most effective method for the vascularization of cardiac tissue constructs and researchers are approaching this question from all sides in an attempt to overcome this limitation in order to produce constructs of clinically relevant thickness. There is much to be learned from this body of work and while moving away from the need to include pre-existing vasculature and/or invasive surgical techniques would likely be a necessity for clinical application, to date these are among the most successful methods for vascularization and require due consideration.
4. Injectable biomaterials
The class of hydrogels designed for direct injection into the heart can be classified into three broad groups depending on the desired mode of action. The first includes hydrogels designed to prevent adverse remodeling and recruitment of endogenous cells for repair; the second designed to act as a temporary matrix for cell transplantation and exogenous repair; and the third to act as a bulking material to support the failing left ventricle (LV) and thereby maintain or restore normal heart geometry and promote functional improvement. It must be noted that there is significant overlap between the strategies, and in many cases hydrogels (for cell transplantation) are intended to promote endogenous repair as clinical feasibility of such a treatment is likely much closer to realization than transplantation of exogenous cells.
4.1. Hydrogels for endogenous repair and cell transplantation
Various groups have developed both synthetic and natural biomaterial-based hydrogels that have been demonstrated to induce functional improvements with and/or without cell transplantation. Reis et al have developed a natural, thermo-gelling mix of chitosan and collagen, improved with covalent immobilization of the peptide QHREDGS for cardiac applications(Reis et al., 2012). At physiological temperature and pH the formulation gels without addition of exogenous cross-linkers in ~30 minutes, and provided the appropriate physical properties to support neonatal rat cardiomyocyte survival both in vitro and in vivo(Reis et al., 2012). Covalent immobilization of QHREDGS peptide did not significantly change the gel physical properties, however in vitro culture studies showed improved cell viability and cell/gel construct functional properties. In a sub-cutaneous injection model QHREDGS-gel attracted more reparative myofibroblasts and improved the presence of cardiomyocytes over one week(Reis et al., 2012).
A synthetic peptide modified poly (ethylene glycol) (PEG)-based hydrogel was designed to be susceptible to cell mediated proteolytic degradation by matrix metalloproteinase (MMPs) at the sites of incorporated peptide, therefore providing a biodegradable and remodellable hydrogel matrix for cell and molecule delivery(Kraehenbuehl et al., 2009). The developed hydrogel gelled in ~30 minutes at 37°C. It was capable of physically retaining Tβ4 within the matrix, and controlled release of 90–100% of the incorporated amount in 160–200 hours in vitro was demonstrated. Injection of the developed hydrogel in a rat MI model showed controlled release of Tβ4, degradation to about 25% of the injected amount by day 28, and was undetectable at 6 weeks(Kraehenbuehl et al., 2011). Gel injection alone was able to enhance cardiac function by significantly reducing infarct size as compared to PBS injected control hearts, however addition of Tβ4 with hESC-derived vascular cells further improved host cardiomyocyte functionality in the infarct zone (Figure 4 A). At 6 weeks after combined treatment a modest improvement in cardiac function was observed with an increase in ejection fraction of ~12% compared to PBS injection control. Furthermore, the Tβ4-hESC-gel treatment group showed more vasculature compared to control, and transplanted vascular cells were found to form de novo vascular structures. They hypothesized that the gel provided temporary support and pro-survival factors as it substituted for the degrading endogenous matrix, and hESC-derived vascular cells contributed to formation of capillary-like vessels, stabilization of host vessels, secretion of paracrine factors, or induction of paracrine factor secretion from native rat cells(Kraehenbuehl et al., 2011).
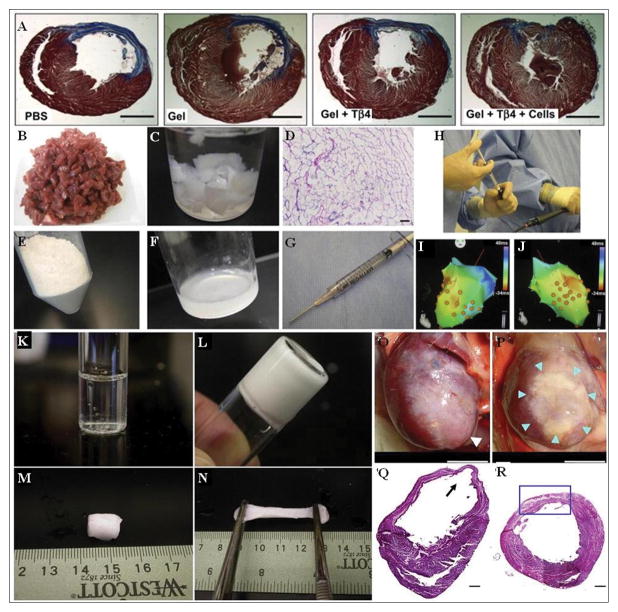
Figure 4. Various injectable hydrogels, injection strategies, and outcomes.
(A) Representative trichrome stains of transverse heart sections shows the effects of different delivery strategies (of modified PEG hydrogel with/without Tβ4 and cells) on cardiac structure 6 weeks after injection. Collagen in the infarct areas is shown in blue, whereas myocytes are in red. Scale bar corresponds to 2. 5 mm(Kraehenbuehl et al., 2011).
(B–J) Fabrication of the myocardial matrix ECM hydrogel is done by (B) slicing porcine ventricular myocardium and then (C) decellularizing using sodium dodecyl sulfate. (D) Hematoxylin and eosin staining of a histological section reveals cellular removal. The decellularized extracellular matrix is then milled into a fine powder (E) and then solubilized through enzymatic digestion (F), which allows for injection via syringe and a 27-gauge needle (G) through percutaneous transendocardial delivery. (H) Image of myocardial matrix being injected through a MyoStar 27-gauge catheter, using a 1-ml Luer lock syringe, attached to the catheter. (I&J) NOGA maps for healthy animal representing final injection locations, indicated by orange dots(Singelyn et al., 2012).
(K–R) Gelation properties of the (NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) hydrogel shows the cooled hydrogel solution (K) transitions to gel after incubation in 37 °C water bath for 30 s (L). Extracted hydrogel formed after 10 min (M) is very pliable and can be stretched to many times its original size (N). Representative images at 8 weeks following the injection procedure of the anterior view of PBS injected (O), and poly (NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) injected hearts (P). White arrow shows an aneurysm formation in the apex area (O), blue arrows indicate the injected hydrogel area (P). The composite histological sections of PBS injected (Q) and hydrogel injected (R) myocardial walls 8 weeks after injection stained with H&E. Black arrow shows an aneurysm formation (Q). Blue box indicates infarct site and injection area of hydrogel (R). Scale bar: 5 mm in (O,P), 500 μm in (Q,R)(Fujimoto et al., 2009).
A synthetic injectable α-cyclodextrin/poly (ethylene glycol)–b-polycaprolactone-(dodecanedioic acid)-polycaprolactone–poly (ethylene glycol) (MPEG–PCL–MPEG) hydrogel was developed by Wang et al to improve cell transplantation therapies into the infarcted MI(Wang et al., 2009). Biocompatibility was shown in vitro by culturing bone marrow-derived stem cells (BMSCs), and co-injection of α-cyclodextrin solution with BMSCs and MPEG–PCL–MPEG solution in the infarct area one week post-MI in a rabbit model showed immediate gelation and localization. Increased LV ejection fraction (~77% increase) and attenuated left ventricular dilatation with hydrogel-BMSC co-injection compared to PBS and BMSC only controls was shown through functional echocardiography. Hearts were excised 4 weeks post treatment and histological analysis revealed significantly increased BMSC retention and vessel density around the infarct compared to injecting BMSCs alone, and no presence of the hydrogel indicating it had been absorbed or degraded(Wang et al., 2009).
Fibrin was one of the earliest biomaterials studied for use in the heart and for cell transplantation. It exhibits several advantages: it is naturally occurring and could be patient-specific; it is biocompatible, biodegradable, and pro-angiogenic. Studies have shown improved cardiac function, increased vascularization, decreased scar area and improved ventricle geometry, when fibrin was injected into the scar zone in animal models of MI, as well as increased retention of various cell types when used as a cell-delivery vehicle(Christman et al., 2004a; Christman et al., 2004b; Huang et al., 2005; Yu et al., 2009). While fibrin-based biomaterials show promise and have the additional benefit of fibrin being FDA approved for human use, these biomaterials may only provide short-term cardiac functional benefits that decrease with time(Yu et al., 2009; Nelson et al., 2011; Segers and Lee, 2011).
Similarly, fibrinogen, the precursor to fibrin, has also been used in biomaterial strategies. Rufaihah et al have demonstrated the applicability of a photo cross linkable semi-synthetic PEG-fibrinogen hydrogel loaded with VEGF in rat models of MI. They showed that a PEG-fibrinogen hydrogel was able to store and release VEGF in a sustained and controlled fashion, as well as significantly improve arteriogenesis and cardiac performance after infarct(Rufaihah et al., 2013). With tunable mechanical properties they have also shown the ability of the PEG-fibrinogen hydrogel on its own to mechanically support the failing LV and to improve cell transplantation, giving the developed hydrogel multiple courses of action as well as promise as a clinically relevant biomaterial(Habib et al., 2011; Rufaihah et al., 2013).
The natural biomaterial chitosan has also been used in the context of cell delivery. Chitosan that was made temperature-responsive by conjugation with glycerol phosphate to improve transplantation of ESCs, was shown previously to be biocompatible in vitro(Lu et al., 2009). In a rat LV MI model co-injection of ESCs with the temperature responsive hydrogel, designed to gel in ~15 minutes at physiological temperatures, improved retention 24h and 4 weeks after injection compared to injection of cells with phosphate buffered saline (PBS). In vivo hydrogel degradation was assessed using histological staining of excised heart sections, showing significant presence of chitosan at 24h after injection, sparse positive chitosan staining at 4 weeks, and no trace of the hydrogel at 6 weeks post injection. Scar thickness was ~54% greater and scar fraction was reduced ~60% in the co-injected vs. PBS (no cells) injected control groups, and correspondingly ejection fraction and fractional shortening were increased by ~20% and 40% respectively(Lu et al., 2009).
In the next step towards clinical application, it has been demonstrated that natural injectable ECM-derived hydrogels can be used in not only small animals but in a large animal (porcine) model for cardiac repair(Singelyn et al., 2012). A hydrogel was prepared by isolating ventricular tissue from Yorkshire farm pigs, rinsing in PBS, then decellularizing the tissue with sodium dodecyl sulfate until the ECM turned white (Figure 4 A–B). Following complete decellularization, aliquots of the ECM were rinsed with deionized water overnight, lyophilized, and milled into a fine powder (Figure 4 D–E). The ECM powder was solubilized by enzymatic digestion using pepsin and HCl for at least 54 hours and adjusted to pH 7. 4 prior to use in two Yucatan mini pigs (Figure 4 F–G). The biotin-labeled myocardial matrix was delivered using the unipolar electromechanical map (NOGA)-guided MyoStar catheter into both an infarcted porcine myocardium after 2 weeks following an MI and in a healthy myocardium as a control (Figure 4 H–J). Histology was then utilized to assess the retention and biodistribution of the myocardial matrix upon injection. These studies demonstrated that myocardial matrix was successfully injected via this percutaneous, transendocardial approach in both healthy and infarcted porcine myocardium without clogging the catheter, which suggests the potential of this technology for treating patients with MI(Singelyn et al., 2012).
4.2. Bulking materials for maintenance of ventricle geometry
As an alternative to inducing endogenous repair or cell delivery, some researchers have sought to induce functional improvements post-MI by using injectable biomaterials as a means of supporting the damaged LV wall. A theoretical modeling study by Wall et al suggested that injection of a bulking material (hydrogel) into the ventricle wall after MI would change the geometry and dilation mechanics of the failing LV by reducing local wall stresses(Wall et al., 2006; Li and Guan, 2011). Furthermore, the mechanical support provided by the hydrogels improved the ejection fraction and the stroke volume/end-diastolic volume relationship in the LV(Wall et al., 2006; Li and Guan, 2011). These findings have led Fujimoto et alto develop a N-isopropylacrylamide (NIPAAm), acrylic acid (AAc), and hydroxyethyl methacrylate-poly (trimethylenecarbonate) (HEMAPTMC) based synthetic hydrogel to prevent progressive adverse remodeling post-MI(Fujimoto et al., 2009). Their optimal ratio of 86/4/10 poly (NIPAAm-co-AAc-co-HEMAPTMC) monomers formed a hydrogel at 37°C (Figure 4 K–N), and showed in vitro biocompatibility and biodegradability over a 5 month period with no cytotoxic degradation products observed(Fujimoto et al., 2009). A rat MI model was used to assess the effect of the hydrogel against a PBS injection control, with treatment done two weeks after MI (Figure 4 O–P). At 6 weeks post treatment hearts injected with hydrogel showed significantly preserved LV cavity area and contractility compared to PBS injection, and sustained hydrogel presence in the LV. Fractional area change (measured by echocardiography) was shown to be at the same level 8-weeks post injection as that measured just prior to injection, and ~55% better than PBS injected control hearts. Histology of excised heart sections also showed the hydrogel promoted tissue in-growth, maintained an ~100% thicker LV wall (Figure 4 Q–R), and showed higher capillary densities in comparison to the control group(Fujimoto et al., 2009).
Similarly, Wu et al developed a temperature-sensitive, aliphatic polyester hydrogel (HG) conjugated with VEGF and looked at its effects on cardiac recovery after MI(Wu et al., 2011). In vitro studies showed gelling in 0 minutes, confirmed biocompatibility, stability of the HG for at least 5 weeks, and complete degradation in 6 months. Rats injected with either hydrogel or PBS in the MI zone one week post-infarct showed preserved ventricular volumes, preload recruitable stroke work, and end-systolic elastance 5 weeks after treatment with HG-VEGF, and furthermore the VEGF conjugated hydrogel led to improved blood vessel density in the infarct area. In comparing function at 5 weeks post treatment between the two groups ejection fraction was ~60% higher, fractional shortening ~37% better, and while the infarct thinned and dilated after PBS injection it was ~30% smaller and 50% thicker in hearts treated with HG-VEGF(Wu et al., 2011). It was noted that these effects could be seen with injection of hydrogel (no VEGF), or hydrogel with VEGF mixed in (not conjugated), however the size was not as significant as those observed with their VEGF conjugated hydrogel. This leads to the conclusion that cardiac regeneration was promoted by the sustained, localized, VEGF presence in the HG-VEGF hydrogel.
In a large animal (ovine) model, Ifkovits et al compared two injectable methacrylated hyaluronic acid (MeHA) formulations that exhibited similar biological profiles but had different mechanical properties in order to correlate material properties with therapeutic outcomes(Ifkovits et al., 2010). MeHA (High and Low moduli) hydrogels were injected 30 min after infarction in an ovine MI model and 8 weeks after both treatments showed significant >200% increase in wall thickness in the apex and >40% basal increase in the infarct regions in comparison to control infarct(Ifkovits et al., 2010). Specifically, higher modulus MeHA treatment group had a significantly ~16% smaller infarct area compared with the control infarct group, and real time 3D echocardiography suggested that MeHA High treatment tended to have a better cardiac output and higher ejection fraction (however not significant) than the low-modulus (MeHA Low) and control infarct groups(Ifkovits et al., 2010). Despite the modest effects demonstrated, this study indicates that the use of hydrogel as bulking materials is not a phenomenon restricted to small animal models.
Importantly, in a recent study by Rane et al it was suggested that passive wall support on its own does not prevent LV remodeling and preservation of cardiac function, and thus calls into question some of the results from the previously mentioned bulking material strategy studies(Rane et al., 2011). Looking to decouple biomaterial effects from mechanical effects of LV bulking materials they injected bio-inert, non-degradable PEG hydrogels into a rat MI model nine days after infarction(Rane et al., 2011). Infarct wall thickness was indeed significantly increased in the PEG versus saline injected control animals, however no difference in cardiac function (ejection fraction, end diastolic and systolic volumes) between the groups was found. Comparison of the cellular response between groups also showed no differences, confirming the decoupling between material bioactivity and mechanical effects, and therefore leading to the conclusion that benefits reported from other studies are likely due to the differences in inflammatory and cellular response to the material and not due to the bulking effect of the material itself(Rane et al., 2011). It therefore remains a matter of debate as to whether using hydrogels as bulking agents is a viable strategy to induce cardiac functional improvements post-MI. It does however illustrate the current limitations in understanding the cause-and-effect relationship between the materials used in cardiac regeneration therapy and the functional outcomes.
4.3. Clinical application
The true implication of any of these injectable biomaterial strategies can only really be assessed in a clinical setting, and currently two groups have developed alginate based hydrogels in Phase II clinical trials (NCT01226563, & NCT01311791). Leor et al developed a calcium cross-linked alginate hydrogel first tested in a swine anterior MI model, and showed improved gross morphometric and cardiac function with intracoronary hydrogel treatment compared to saline(Leor et al., 2009). Dubbed IK-5001, the hydrogel has been approved for Phase II clinical trials and they are currently recruiting patients to test the safety and effectiveness of the device. Intracoronary injection of 4mL of IK-5001 into the blocked artery (after successful stent placement) will be done with the goal of preventing ventricular remodeling and congestive heart failure when administered after a recent acute MI (NCT01226563).
Lee et al have a developed a similar calcium cross-linked alginate hydrogel, however with a short 3–4 minute gelling time and strength of 3–5kPa it is designed for direct injection into the infarcted wall for restoration of LV geometry(Lee et al., 2012). Called Algisyl-LVR™ an initial pilot study in 6 patients suffering from dilated cardiomyopathy demonstrated sustained improvements in LV size and function that were accompanied by statistically significant improvements in clinical status and quality of life 3 months post-injection, with no implant related complications(Lee et al., 2012). The approved Phase II clinical trial will evaluate the concept that direct mid LV intramyocardial injections of the biomaterial into the free wall of the failing LV of patients with dilated cardiomyopathy will reduce LV size, restore LV shape, lower LV wall stress and improve global LV function (NCT01311791). The goal is to show significantly improved peak maximum oxygen uptake 6 months after treatment between Algisyl-LVR™ treated patients versus those receiving medical management.
5. Conclusions
For over a decade, research involving the development of biomaterials in the field of cardiac tissue engineering has focused on improving material properties by mimicking the native cardiac tissue, designing materials with sustained presence of incorporated cells and biomolecules, increasing vascularization within the materials, and evaluating the in vivo lifetime and activity of injected hydrogels or implanted scaffolds. While efforts made by various research groups in developing biomaterials have advanced the field rapidly, it is clear that better standardization of experimental and measurement techniques is necessary in order to properly consolidate findings from individual groups.
Importantly, researchers in the field of cardiac tissue engineering should take on a standardized approach to 1) conduct in vivo animal studies involving the transplantation of biomaterials and 2) measure and compare the functional and morphological properties of the heart after insult and treatment. Currently, a direct comparison between the results reported by different groups cannot be made because of great discrepancies in the timing of biomaterial transplantation and experimental endpoints. Some groups performed injections of hydrogels one hour after infarction and then monitored the animals for six weeks(Kraehenbuehl et al., 2011), while others injected the hydrogels one or two weeks after infarction and then monitored for additional four to eight weeks(Lu et al., 2009);(Fujimoto et al., 2009). Also, different animal models have been used, including rat, rabbit and porcine models(Leor et al., 2009; Wang et al., 2009; Singelyn et al., 2012). In addition, discrepancies are found in the functional and morphological properties that different research groups measured and reported, the methods by which the properties were measured, and the level of cardiac regeneration that was considered sufficient. For example, groups reported values that ranged from 12% to 77% for the difference in ejection fractions between the treatment group and MI only group (Fujimoto et al., 2009; Lu et al., 2009; Wang et al., 2009; Kraehenbuehl et al., 2011; Wu et al., 2011), yet they all used their results as an indication of a significant improvement in cardiac function.
We propose that the timing of biomaterial transplantation and experimental endpoints should be clearly defined for each heart disease model (i.e. acute, chronic MI), and that a set of metrics (i.e. fractional shortening, LV diastolic dimension, LV systolic dimension, end-systolic pressure, LV wall thickness, cellularity, cell types, degree of vascularization) should be required in reports of new biomaterials being evaluated for cardiac repair. This will allow more direct comparisons to be made between biomaterials for cardiac repair instead of focusing on individual approaches, and in turn push the field more rapidly towards finding clinically relevant therapies. Ultimately, there will likely be multiple biomaterial-based methods that are effective for cardiac repair. The standardization of in vivo animal studies will ensure collaboration amongst all researchers in the field in order to come up with the best biomaterials for the treatment of cardiovascular diseases.
References
- Akhyari P, Aubin H, Gwanmesia P, Barth M, Hoffmann S, Huelsmann J, Preuss K, Lichtenberg A. The quest for an optimized protocol for whole-heart decellularization: a comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng Pt C-Meth. 2011;17:915–926. doi: 10.1089/ten.TEC.2011.0210. [DOI] [PubMed] [Google Scholar]
- Amir G, Miller L, Shachar M, Feinberg MS, Holbova R, Cohen S, Leor J. Evaluation of a peritoneal-generated cardiac patch in a rat model of heterotopic heart transplantation. Cell Transplant. 2009;18:275–282. doi: 10.3727/096368909788534898. [DOI] [PubMed] [Google Scholar]
- Aubin H, Kranz A, Hulsmann J, Pinto A, Barth M, Fomin A, Lichtenberg A, Akhyari P. A novel native derived coronary artery tissue-flap model. Tissue Eng Part C Methods. 2013;19:970–980. doi: 10.1089/ten.tec.2012.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105:1148–1160. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- Bouten CV, Dankers PY, Driessen-Mol A, Pedron S, Brizard AM, Baaijens FP. Substrates for cardiovascular tissue engineering. Advanced drug delivery reviews. 2011;63:221–241. doi: 10.1016/j.addr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Chen QZ, Harding SE, Ali NN, Lyon AR, Boccaccini AR. Biomaterials in cardiac tissue engineering: Ten years of research survey. Mat Sci Eng R. 2008;59:1–37. [Google Scholar]
- Chiu LL, Radisic M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31:226–241. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- Chiu LL, Weisel RD, Li RK, Radisic M. Defining conditions for covalent immobilization of angiogenic growth factors onto scaffolds for tissue engineering. J Tissue Eng Regen Med. 2011;5:69–84. doi: 10.1002/term.292. [DOI] [PubMed] [Google Scholar]
- Chiu LL, Montgomery M, Liang Y, Liu H, Radisic M. Perfusable branching microvessel bed for vascularization of engineered tissues. Proc Natl Acad Sci U S A. 2012a;109:E3414–3423. doi: 10.1073/pnas.1210580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu LL, Iyer RK, Reis LA, Nunes SS, Radisic M. Cardiac tissue engineering: current state and perspectives. Frontiers in bioscience. 2012b;17:1533–1550. doi: 10.2741/4002. [DOI] [PubMed] [Google Scholar]
- Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue engineering. 2004a;10:403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004b;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, Carter R, Williams TP, Prichard HL, Dey MS, Begelman KG, Niklason LE. Readily available tissue-engineered vascular grafts. Science translational medicine. 2011;3:68ra69. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- Dawson J, Schussler O, Al-Madhoun A, Menard C, Ruel M, Skerjanc IS. Collagen scaffolds with or without the addition of RGD peptides support cardiomyogenesis after aggregation of mouse embryonic stem cells. In Vitro Cell Dev Biol Anim. 2011;47:653–664. doi: 10.1007/s11626-011-9453-0. [DOI] [PubMed] [Google Scholar]
- Dvir T, Kedem A, Ruvinov E, Levy O, Freeman I, Landa N, Holbova R, Feinberg MS, Dror S, Etzion Y, Leor J, Cohen S. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. P Natl Acad Sci USA. 2009;106:14990–14995. doi: 10.1073/pnas.0812242106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, Dohmen HH, Schafer H, Bishopric N, Wakatsuki T, Elson EL. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1997;11:683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- Fujimoto KL, Ma Z, Nelson DM, Hashizume R, Guan J, Tobita K, Wagner WR. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30:4357–4368. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M, Zhang ZP, Ni AG, Dzau VJ. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godier-Furnemont AF, Martens TP, Koeckert MS, Wan L, Parks J, Arai K, Zhang G, Hudson B, Homma S, Vunjak-Novakovic G. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc Natl Acad Sci U S A. 2011;108:7974–7979. doi: 10.1073/pnas.1104619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette MD, Park H, Hsiao JC, Jain SR, Larson BL, Langer R, Freed LE. Combined technologies for microfabricating elastomeric cardiac tissue engineering scaffolds. Macromol Biosci. 2010;10:1330–1337. doi: 10.1002/mabi.201000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib M, Shapira-Schweitzer K, Caspi O, Gepstein A, Arbel G, Aronson D, Seliktar D, Gepstein L. A combined cell therapy and in-situ tissue-engineering approach for myocardial repair. Biomaterials. 2011;32:7514–7523. doi: 10.1016/j.biomaterials.2011.06.049. [DOI] [PubMed] [Google Scholar]
- Hilfiker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbeck’s archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2011;396:489–497. doi: 10.1007/s00423-011-0762-2. [DOI] [PubMed] [Google Scholar]
- Huang NF, Yu J, Sievers R, Li S, Lee RJ. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue engineering. 2005;11:1860–1866. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, Gorman JH, 3rd, Gorman RC, Burdick JA. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A. 2010;107:11507–11512. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Zhang G, Hashimoto I, Kumar BS, Bortolotto S, Morrison WA, Dusting GJ. Neovascularization in an arterio-venous loop-containing tissue engineering chamber: role of NADPH oxidase. Journal of cellular and molecular medicine. 2008;12:2062–2072. doi: 10.1111/j.1582-4934.2008.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Timothy Baldwin J, Young JB. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Kofidis T, Lenz A, Boublik J, Akhyari P, Wachsmann B, Mueller-Stahl K, Hofmann M, Haverich A. Pulsatile perfusion and cardiomyocyte viability in a solid three-dimensional matrix. Biomaterials. 2003;24:5009–5014. doi: 10.1016/s0142-9612(03)00429-0. [DOI] [PubMed] [Google Scholar]
- Kraehenbuehl TP, Ferreira LS, Zammaretti P, Hubbell JA, Langer R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials. 2009;30:4318–4324. doi: 10.1016/j.biomaterials.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenbuehl TP, Ferreira LS, Hayward AM, Nahrendorf M, van der Vlies AJ, Vasile E, Weissleder R, Langer R, Hubbell JA. Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials. 2011;32:1102–1109. doi: 10.1016/j.biomaterials.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzeminski TF, Nozynski JK, Grzyb J, Porc M. Wide-spread myocardial remodeling after acute myocardial infarction in rat. Features for heart failure progression. Vascular pharmacology. 2008;48:100–108. doi: 10.1016/j.vph.2008.01.002. [DOI] [PubMed] [Google Scholar]
- L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NA, Kyles AE, Gregory CR, Hoyt G, Robbins RC, McAllister TN. Human tissue-engineered blood vessels for adult arterial revascularization. Nature medicine. 2006;12:361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE. Regenerating the heart. Nature biotechnology. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- Lang N, Pereira MJ, Lee Y, Friehs I, Vasilyev NV, Feins EN, Ablasser K, O’Cearbhaill ED, Xu C, Fabozzo A, Padera R, Wasserman S, Freudenthal F, Ferreira LS, Langer R, Karp JM, del Nido PJ. A blood-resistant surgical glue for minimally invasive repair of vessels and heart defects. Science translational medicine. 2014;6:218ra216. doi: 10.1126/scitranslmed.3006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Progress in polymer science. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RJ, Hinson A, Helgerson S, Bauernschmitt R, Sabbah HN. Polymer-based restoration of left ventricular mechanics. Cell Transplant. 2012 doi: 10.3727/096368911X637461. [DOI] [PubMed] [Google Scholar]
- Leor J, Landa N, Cohen S. Renovation of the injured heart with myocardial tissue engineering. Expert review of cardiovascular therapy. 2006;4:239–252. doi: 10.1586/14779072.4.2.239. [DOI] [PubMed] [Google Scholar]
- Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, Petneházy O, Landa N, Feinberg MS, Konen E, Goitein O, Tsur-Gang O, Shaul M, Klapper L, Cohen S. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J Am Coll Cardiol. 2009;54:1014–1023. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, Gepstein L. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Pt A. 2010;16:115–125. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- Li Z, Guan J. Hydrogels for Cardiac Tissue Engineering. Polymers. 2011;3:740–761. [Google Scholar]
- Liau B, Zhang D, Bursac N. Functional cardiac tissue engineering. Regenerative medicine. 2012;7:187–206. doi: 10.2217/rme.11.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WN, Lü S-H, Wang H-B, Li D-X, Duan C-M, Liu Z-Q, He W-J, Hao T, Xie X-H, Wang C-Y, Xu B, Fu Q, Song YC. Functional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogel. Tissue Eng Pt A. 2009;15:1437–1447. doi: 10.1089/ten.tea.2008.0143. [DOI] [PubMed] [Google Scholar]
- Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. P Natl Acad Sci USA. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidhof R, Tandon N, Lee EJ, Luo J, Duan Y, Yeager K, Konofagou E, Vunjak-Novakovic G. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J Tissue Eng Regen Med. 2011 doi: 10.1002/term.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsano A, Maidhof R, Wan LQ, Wang Y, Gao J, Tandon N, Vunjak-Novakovic G. Scaffold stiffness affects the contractile function of three-dimensional engineered cardiac constructs. Biotechnol Prog. 2010;26:1382–1390. doi: 10.1002/btpr.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez EC, Kofidis T. Adult stem cells for cardiac tissue engineering. Journal of molecular and cellular cardiology. 2011;50:312–319. doi: 10.1016/j.yjmcc.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Masuda S, Shimizu T, Yamato M, Okano T. Cell sheet engineering for heart tissue repair. Advanced drug delivery reviews. 2008;60:277–285. doi: 10.1016/j.addr.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi K, Fedak PW, Mickle DA, Weisel RD, Ozawa T, Li RK. Improved left ventricular aneurysm repair with bioengineered vascular smooth muscle grafts. Circulation. 2003;108(Suppl 1):II219–225. doi: 10.1161/01.cir.0000087450.34497.9a. [DOI] [PubMed] [Google Scholar]
- Menasche P. Current status and future prospects for cell transplantation to prevent congestive heart failure. Seminars in thoracic and cardiovascular surgery. 2008;20:131–137. doi: 10.1053/j.semtcvs.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Miyagi Y, Chiu LL, Cimini M, Weisel RD, Radisic M, Li RK. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials. 2011;32:1280–1290. doi: 10.1016/j.biomaterials.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Morritt AN, Bortolotto SK, Dilley RJ, Han X, Kompa AR, McCombe D, Wright CE, Itescu S, Angus JA, Morrison WA. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353–360. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- Nelson DM, Ma Z, Fujimoto KL, Hashizume R, Wagner WR. Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges. Acta Biomater. 2011;7:1–15. doi: 10.1016/j.actbio.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SL, Narayanan K, Gao S, Wan AC. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials. 2011;32:7571–7580. doi: 10.1016/j.biomaterials.2011.06.065. [DOI] [PubMed] [Google Scholar]
- Odedra D, Chiu LLY, Reis LA, Rask F, Chiang K, Radisic M. Cardiac Tissue Engineering. In: Burdick JA, Mauck RL, editors. Biomaterials for Tissue Engineering Applications: A Review of Past & Future Trends. 1. Vienna: Springer Vienna; 2011. p. 562. [Google Scholar]
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nature medicine. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Mickle DA, Weisel RD, Koyama N, Ozawa S, Li RK. Optimal biomaterial for creation of autologous cardiac grafts. Circulation. 2002;106:I176–182. [PubMed] [Google Scholar]
- Pantilat SZ, Steimle AE. Palliative care for patients with heart failure. JAMA : the journal of the American Medical Association. 2004;291:2476–2482. doi: 10.1001/jama.291.20.2476. [DOI] [PubMed] [Google Scholar]
- Park H, Larson BL, Guillemette MD, Jain SR, Hua C, Engelmayr GC, Jr, Freed LE. The significance of pore microarchitecture in a multi-layered elastomeric scaffold for contractile cardiac muscle constructs. Biomaterials. 2011;32:1856–1864. doi: 10.1016/j.biomaterials.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten RD, Aronovitz MJ, Deras-Mejia L, Pandian NG, Hanak GG, Smith JJ, Mendelsohn ME, Konstam MA. Ventricular remodeling in a mouse model of myocardial infarction. The American journal of physiology. 1998;274:H1812–1820. doi: 10.1152/ajpheart.1998.274.5.H1812. [DOI] [PubMed] [Google Scholar]
- Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006;93:332–343. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- Rane AA, Chuang JS, Shah A, Hu DP, Dalton ND, Gu Y, Peterson KL, Omens JH, Christman KL. Increased infarct wall thickness by a bio-inert material is insufficient to prevent negative left ventricular remodeling after myocardial infarction. PloS one. 2011;6:e21571. doi: 10.1371/journal.pone.0021571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LA, Chiu LLY, Liang Y, Hyunh K, Momen A, Radisic M. A peptide-modified chitosan-collagen hydrogel for cardiac cell culture and delivery. Acta Biomater. 2012;8:1022–1036. doi: 10.1016/j.actbio.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Riehl BD, Park JH, Kwon IK, Lim JY. Mechanical Stretching for Tissue Engineering: Two-Dimensional and Three-Dimensional Constructs. Tissue Eng Part B Rev. 2012 doi: 10.1089/ten.teb.2011.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufaihah AJ, Vaibavi SR, Plotkin M, Shen J, Nithya V, Wang J, Seliktar D, Kofidis T. Enhanced infarct stabilization and neovascularization mediated by VEGF-loaded PEGylated fibrinogen hydrogel in a rodent myocardial infarction model. Biomaterials. 2013;34:8195–8202. doi: 10.1016/j.biomaterials.2013.07.031. [DOI] [PubMed] [Google Scholar]
- Sanchez PL, Fernandez-Santos ME, Costanza S, Rodriguez-Abella H, Kren S, Garrido G, Escalante JL, Matesanz R, Taylor D, Francisco FA. Characterization and Biocompatibility of Perfusion-Decellularized Human Heart Matrix: Toward Bioengineering Perfusable Human Heart Grafts. Journal of the American College of Cardiology. 2012;59:E857–E857. [Google Scholar]
- Sapir Y, Kryukov O, Cohen S. Integration of multiple cell-matrix interactions into alginate scaffolds for promoting cardiac tissue regeneration. Biomaterials. 2011;32:1838–1847. doi: 10.1016/j.biomaterials.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Segers VF, Lee RT. Biomaterials to enhance stem cell function in the heart. Circ Res. 2011;109:910–922. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- Sekine H, Shimizu T, Sakaguchi K, Dobashi I, Wada M, Yamato M, Kobayashi E, Umezu M, Okano T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nature communications. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Hauch KD, Laflamme MA. Cardiac applications for human pluripotent stem cells. Current pharmaceutical design. 2009;15:2791–2806. doi: 10.2174/138161209788923804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90:E40–E48. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, Mayle KM, Bartels K, Salvatore M, Kinsey AM, Demaria AN, Dib N, Christman KL. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59:751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- Tandon N, Marsano A, Maidhof R, Wan L, Park H, Vunjak-Novakovic G. Optimization of electrical stimulation parameters for cardiac tissue engineering. J Tissue Eng Regen Med. 2011;5:e115–125. doi: 10.1002/term.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiser M, Abramson S, Langer R, Kohn J. Chapter I.2.6 – Degradable and Resorbable Biomaterials. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science. 3. Academic Press; 2013. pp. 179–195. [Google Scholar]
- Venugopal JR, Prabhakaran MP, Mukherjee S, Ravichandran R, Dan K, Ramakrishna S. Biomaterial strategies for alleviation of myocardial infarction. J Roy Soc Interface. 2011 doi: 10.1098/rsif.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, Radisic M. Challenges in cardiac tissue engineering. Tissue Eng Pt B-Rev. 2010;16:169–187. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, Badylak SF. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Pt C-Meth. 2010;16:525–532. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- Wang T, Jiang X-J, Tang Q-Z, Li X-Y, Lin T, Wu D-Q, Zhang X-Z, Okello E. Bone marrow stem cells implantation with alpha-cyclodextrin/MPEG-PCL-MPEG hydrogel improves cardiac function after myocardial infarction. Acta Biomater. 2009;5:2939–2944. doi: 10.1016/j.actbio.2009.04.040. [DOI] [PubMed] [Google Scholar]
- Weymann A, Loganathan S, Takahashi H, Schies C, Claus B, Hirschberg K, Soos P, Korkmaz S, Schmack B, Karck M, Szabo G. Development and evaluation of a perfusion decellularization porcine heart model--generation of 3-dimensional myocardial neoscaffolds. Circ J. 2011;75:852–860. doi: 10.1253/circj.cj-10-0717. [DOI] [PubMed] [Google Scholar]
- WHO. The top 10 causes of death. World Health Organization, Fact Sheet #310 2011a [Google Scholar]
- Alwan A, editor. WHO. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: World Health Organization; 2011b. p. 176. [Google Scholar]
- Williams DF European Society for Biomaterials. Definitions in biomaterials : proceedings of a consensus conference of the European Society for Biomaterials; Chester, England. March 3–5, 1986; Amsterdam ; New York: Elsevier; 1987. [Google Scholar]
- Wilson SR, Mudge GH, Jr, Stewart GC, Givertz MM. Evaluation for a ventricular assist device: selecting the appropriate candidate. Circulation. 2009;119:2225–2232. doi: 10.1161/CIRCULATIONAHA.109.850610. [DOI] [PubMed] [Google Scholar]
- Wu J, Zeng F, Huang X-P, Chung JC-Y, Konecny F, Weisel RD, Li R-K. Infarct stabilization and cardiac repair with a VEGF-conjugated, injectable hydrogel. Biomaterials. 2011;32:579–586. doi: 10.1016/j.biomaterials.2010.08.098. [DOI] [PubMed] [Google Scholar]
- Yeo Y, Geng WL, Ito T, Kohane DS, Burdick JA, Radisic M. Photo cross linkable hydrogel for myocyte cell culture and injection. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2007;81B:312–322. doi: 10.1002/jbm.b.30667. [DOI] [PubMed] [Google Scholar]
- Yeong WY, Sudarmadji N, Yu HY, Chua CK, Leong KF, Venkatraman SS, Boey YC, Tan LP. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 2010;6:2028–2034. doi: 10.1016/j.actbio.2009.12.033. [DOI] [PubMed] [Google Scholar]
- Yildirim Y, Naito H, Didie M, Karikkineth BC, Biermann D, Eschenhagen T, Zimmermann WH. Development of a biological ventricular assist device: preliminary data from a small animal model. Circulation. 2007;116:I16–23. doi: 10.1161/CIRCULATIONAHA.106.679688. [DOI] [PubMed] [Google Scholar]
- Yu J, Christman KL, Chin E, Sievers RE, Saeed M, Lee RJ. Restoration of left ventricular geometry and improvement of left ventricular function in a rodent model of chronic ischemic cardiomyopathy. The Journal of thoracic and cardiovascular surgery. 2009;137:180–187. doi: 10.1016/j.jtcvs.2008.08.036. [DOI] [PubMed] [Google Scholar]
- Zammaretti P, Jaconi M. Cardiac tissue engineering: regeneration of the wounded heart. Current opinion in biotechnology. 2004;15:430–434. doi: 10.1016/j.copbio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nature medicine. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]