Abstract
Thrombo-occlusive disease is a leading cause of morbidity and mortality. In this chapter, the use of ultrasound to accelerate clot breakdown alone or in combination with thrombolytic drugs will be reported. Primary thrombus formation during cardiovascular disease and standard treatment methods will be discussed. Mechanisms for ultrasound enhancement of thrombolysis, including thermal heating, radiation force, and cavitation, will be reviewed. Finally, in-vitro, in-vivo and clinical evidence of enhanced thrombolytic efficacy with ultrasound will be presented and discussed.
Keywords: Ultrasound, Thrombus, Cardiovascular disease
19.1 Clinical Challenges of Thrombo-Occlusive Disease
Cardiovascular disease is the number one cause of death worldwide (17.3 million deaths in 2008) (World Health Organization 2013), with a 15% projected mortality rate increase between 2010 and 2020 (Mathers and Loncar 2006). In the United States of America, more than one third of adults (~83.6 million) have one or more forms of cardiovascular disease (Go et al. 2013). Cardiovascular disease is characterized by occlusion of blood flow through the vasculature, in part due to the presence of blood clots, or thrombi.
19.1.1 Thrombus Formation During Cardiovascular Disease
The primary function of thrombus formation is to prevent bleeding from injured blood vessels (Gregg 2003). Platelets adhere to the injury site to initiate the formation of a fibrin mesh (Furie and Furie 2008), a protein network that stabilizes the clot architecture. Under normal circumstances, fibrin strands are cleaved by plasmin as the injured vessel is repaired. In pathologic cases, however, excessive blood clotting occurs in response to Virchow’s triad: hypercoagulability, hemodynamic changes (stasis or turbulence) or endothelial injury (Watson et al. 2009). Atherosclerosis, a disease known to induce Virchow’s triad, is the build-up of lipids, cholesterol, and other substances in arterial tissue (Saric and Kronzon 2012). Lipid-rich plaques are vulnerable and their rupture can cause the formation of platelet- and fibrin-rich thrombi. Thrombi may also form in stagnated blood, such as in the heart during atrial fibrillation (Watson et al. 2009).
Thrombus composition varies based on conditions during formation (Liebeskind et al. 2011). “White” thrombi are formed due to platelet activity in the arterial system, whereas “red” thrombi originate from trapped erythrocytes within a fibrin mesh in the low-pressure venous system (Tan and Lip 2003). Thrombi retrieved from patients exhibit an underlying commonality of histological components and morphology, regardless of origin (Marder et al. 2006). Thromboembolism occurs when a thrombus breaks free and blocks blood flow in the distal vasculature. Hypoxic conditions in tissues distal to the occlusive thrombus cause infarction and tissue death. Depending on the anatomical location of the obstruction, tissue death can occur in the brain (ischemic stroke), extremities and lungs (deep vein thrombosis and pulmonary thromboembolism, respectively), or heart (myocardial infarction).
19.1.1.1 Stroke
Stroke, or brain impairment due to loss of oxygen, is the fourth leading cause of death in the United States (Go et al. 2013), and a leading cause of death worldwide (Mathers et al. 2008). Stroke is classified as either hemorrhagic (13% of cases) or ischemic (87% of cases) (Go et al. 2013). Hemorrhagic stroke has a 40–50% 30-day mortality rate and a 6-month functional independence rate of only 20% (Adeoye et al. 2010). Hemorrhagic stroke results from a weakened vessel that ruptures and bleeds, compressing the surrounding brain tissue. Bleeding can occur in different brain compartments: intracerebral (10% of all stroke cases) or subarachnoid (3% of all stroke cases) (Go et al. 2013). Bleeding extends into the ventricles for 45% of intracerebral cases and 25% of subarachnoid cases (Mohr et al. 1983; Brott et al. 1986). In contrast, ischemic stroke is the result of a thrombus obstructing blood flow to the brain, with a 30-day mortality rate of 8–13% (Rosamond et al. 2007). Thrombi responsible for ischemic stroke are primarily cardioembolic in origin, but can also be atherothromboembolic (Marder et al. 2006). Clinical outcomes are best in patients with distal middle cerebral artery (MCA) occlusions and worst in internal carotid artery occlusions, potentially due to larger thrombus burden and impaired collateral blood flow in the latter (Puetz et al. 2008).
19.1.1.2 Deep Vein Thrombosis and Pulmonary Embolism
Deep vein thrombosis (DVT) is the formation of thrombi in a deep vein, primarily in the calf of the leg (Kearon 2003). Thrombi form due to stagnation of blood in the deep veins (Markel 2005), resulting in “red” thrombi (Hirsh and Hoak 1996). If left untreated, post-thrombotic syndrome, a cause of increased morbidity and disability, can progress due to incompetent valves in deep and superficial veins (Markel 2005). Approximately 20% of venous thrombi occur in the tibial and popliteal veins (Kakkar et al. 1969). Dislodged thrombi, or emboli, travel to the lung in 26–67% of DVT cases (Markel 2005), a condition called pulmonary embolism (PE). The increased workload by the blockage enlarges the heart, potentially resulting in right ventricular dysfunction (Kearon 2003). PE has a 25% morality rate, with a 30-day survival rate of 59.1% (Go et al. 2013). Approximately 10% of PE cases cause death within 1 h of onset (Bell and Simon 1982).
19.1.1.3 Myocardial Infarction
One cause of myocardial infarction (MI), more commonly known as a “heart attack,” is embolic atherosclerotic debris (White and Chew 2008) within the coronary arteries (Saric and Kronzon 2012). There are 600,000 annual incidents of MI, with a mortality rate of 15% (Go et al. 2013). Thrombus formation in the atherosclerotic build-up is composed of a platelet-rich core surrounded by an erythrocyte-rich fibrin mesh. Early platelet thrombus is unstable and fragile (Falk 1991), and may embolize unless stabilized by fibrin (Silvain et al. 2011). Transmural MI results in necrosis in all three muscle layers of the heart (endocardium, myocardium, and epicardium), and is associated with total occlusion of the coronary artery (Turgut and Bates 2000). Non-transmural MI is characterized by ischemic necrosis that does not extend across the full thickness of the myocardial wall, and is limited to the myocardium and the endocardium or the endocardium (Fardanesh and Kian 2014). Vasospasm from endothelial dysfunction, concurrent with chronic atherosclerosis, can also reduce flow and increase ischemia (Fuster 1994). Dissection of the coronary artery is an additional cause of infarction in the absence of occlusive atherosclerosis or vasospasm (Casscells 2003).
19.1.2 Frontline Therapy: Thrombolytic Drugs
Recanalization of the occluded vessels is correlated with functional clinical outcome for DVT (Hirsh and Hoak 1996), MI (Pasceri et al. 1996) and ischemic stroke (Rha and Saver 2007). Regardless of the vascular origin of the thrombus, recanalization can be accelerated with thrombolytic drugs or mechanical intervention. An excellent review of current thrombolytics is provided by Weisel and Litvinov (2008). Most thrombolytic drugs cleave plasminogen bonds to create plasmin. Plasmin, which can also be used as a direct thrombolytic agent (Novokhatny et al. 2004), lyses fibrin to initiate thrombolysis. Early thrombolytic drugs, such as streptokinase (SK) (Baruah et al. 2006) and recombinant urokinase (UK) (Reuning et al. 2003) are administered for treatment of PE and MI. However, both UK and SK lack specificity to fibrin, and their bacterial origin contributes to adverse effects (Perler 2005). These limitations prompted the advent of the fibrin-specific thrombolytic recombinant tissue type plasminogen activator (rt-PA) (Higgins and Bennett 1990; Baruah et al. 2006; Watson et al. 2009), and its derivatives (Baruah et al. 2006; Labreuche et al. 2010; Saric and Kronzon 2012; Jauch et al. 2013). The rt-PA drug is currently the primary thrombolytic in use (Turi et al. 1993; Watson et al. 2009; Jauch et al. 2013).
Strict exclusion criteria (Turi et al. 1993) limit the administration of rt-PA to only 1.5% of total stroke cases (Go et al. 2013). Mechanical thrombolysis with catheter-based intervention has gained some clinical traction in removing or disrupting thrombi (Gralla et al. 2011), either as a stand-alone or adjuvant therapy for ischemic stroke (Jauch et al. 2013) and PE (Jaff et al. 2011). These mechanical devices employ aspiration (The Penumbra Pivotal Stroke Trial Investigators 2009), microcatheters (Smith et al. 2008) or stents (Castano et al. 2010) to remove thrombi. However, endovascular recanalization techniques are reserved for dedicated stroke centers, limiting their widespread use (Gralla et al. 2011). Furthermore, recent studies indicate mechanical intervention has limited clinical improvement compared to rt-PA alone (Ciccone et al. 2013), questioning the value of such an invasive procedure. To improve therapeutic outcome, there is an unmet need for techniques that would either locally increase the efficacy of thrombolytic drugs, or allow a safe and minimally invasive mechanical disruption of the thrombus without drugs. Sonothrombolysis has the potential for both.
19.2 Mechanisms of Thrombolytic Enhancement
The interaction of acoustic waves with tissue is well documented (Nyborg and Miller 1982) and can generally be categorized as thermal, primary mechanical effects (e.g., radiation force), or secondary mechanical effects (acoustic cavitation). The contributions of each of these interactions to thrombolytic enhancement are reviewed in the following sections.
19.2.1 Thermal Effects of Ultrasound
Heating of biological tissues exposed to ultrasound is generated by absorption of the incident wave. In-vitro studies have shown rt-PA clot lysis to be well described by a Arrhenius temperature dependence (Shaw et al. 2007). Increasing the ambient temperature increases enzymatic activity and expedites thrombolysis. However, several studies have shown negligible thermal effects in bench-top (Francis et al. 1992; Shaw et al. 2006; Damianou et al. 2014) or clinical studies (Barlinn and Alexandrov 2013). Numerical computations confirm a negligible temperature rise in clots due to 0.12–3.5 MHz insonations during thrombolysis (Nahirnyak et al. 2007; Bouchoux et al. 2014). In contrast, Sakharov et al. (2000) attributed increased thrombolytic efficacy from 1-MHz insonation in-vitro due to the combined effect of heating and acoustic streaming. However, the in-vitro model employed by Sakharov et al. lacked flow to accelerate the diffusion of heat around the clot.
19.2.2 Primary Mechanical Effects
Ultrasound energy imparts momentum to tissue through absorption and scattering mechanisms. The resultant force, known as the acoustic radiation force, is well described by Nyborg (1953). Radiation force can initiate fluid motion, or acoustic streaming (Lighthill 1978). Fluid mixing may help a thrombolytic drug penetrate a clot (Francis et al. 1995). Traveling wave insonation generates greater acoustic streaming than standing wave insonation (Devcic-Kuhar et al. 2002). Pfaffenberger et al. (2003) found increased thrombolytic efficacy employing 2-MHz traveling waves instead of standing waves in an in-vitro clot model. Furthermore, the thrombolytic efficacy was optimized at a 1-Hz pulse repetition frequency of the traveling wave. These acoustic streaming effects are similar to mild stirring of the medium surrounding the clot (Sakharov et al. 2000).
Displacement of clot surfaces has also been observed due to acoustic radiation force (Wright et al. 2012b). Frenkel et al. (2006), using both an in-vitro clot model and a numerical model to calculate clot displacement, found a correlation between thrombolytic efficacy and acoustic radiation force at 1 MHz. Bader et al. (2015) found a correlation between the root-mean-square velocity of displaced in-vitro human whole blood clots and the lytic rate with sub-megahertz insonation. Radiation force is known to act on bubbles (Leighton 1994), and several groups have shown microbubbles forced into clots in-vitro (Caskey et al. 2009; Acconcia et al. 2013; Everbach and Guarini 2013) (Fig. 19.1). The presence of micro-bubbles could modify both the attenuation and sound speed of the thrombus (Commander and Prosperetti 1989), thereby increasing the acoustic radiation force on the clot (Nyborg 1965).
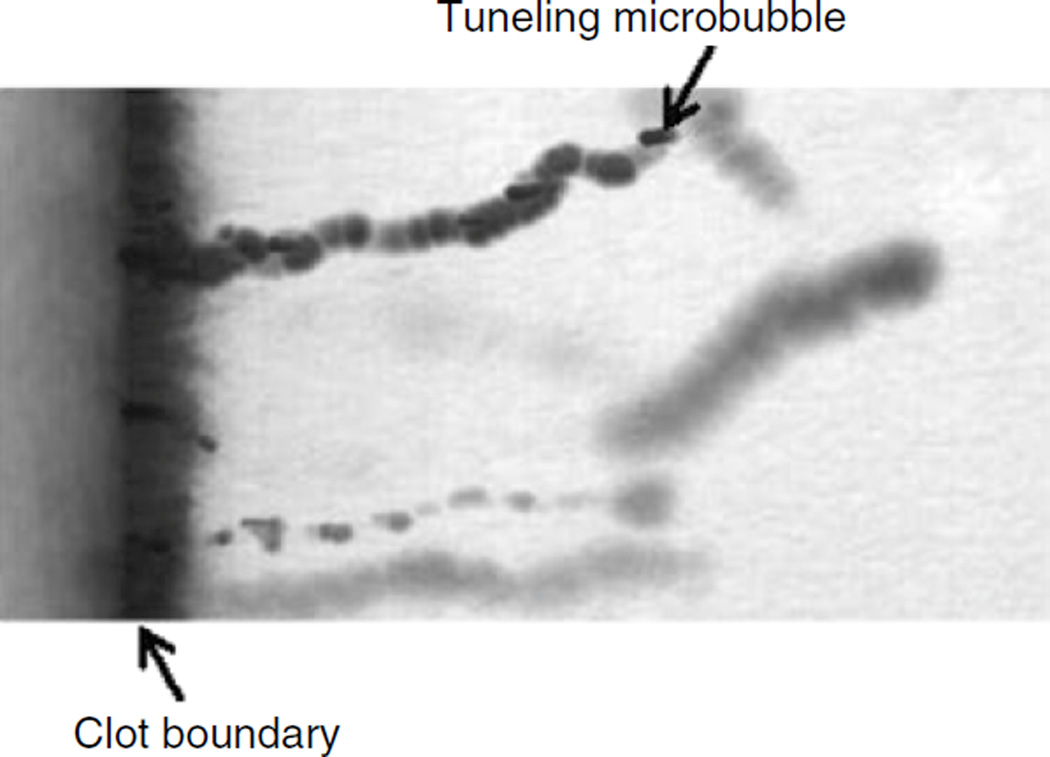
Fig. 19.1.
Microbubble tunneling though a fibrin clot (fluid-clot boundary at left) caused by acoustic radiation force (1 MHz, 0.4 MPa). The blurred, dark line is a motion artifact from deflection of the clot boundary due to radiation force (Acconcia et al. 2013)
19.2.3 Secondary Mechanical Effects (Acoustic Cavitation)
19.2.3.1 Classification of Cavitation
Acoustic cavitation has been a topic of study since the early twentieth century (Rayleigh 1917) and has been extensively reviewed elsewhere (Flynn 1964; Apfel 1981; Leighton 1994; Lauterborn and Kurz 2010). Acoustic cavitation refers to both the formation and oscillation of bubbles due to an acoustic pressure. Cavitation activity can generally be categorized as inertial or stable.
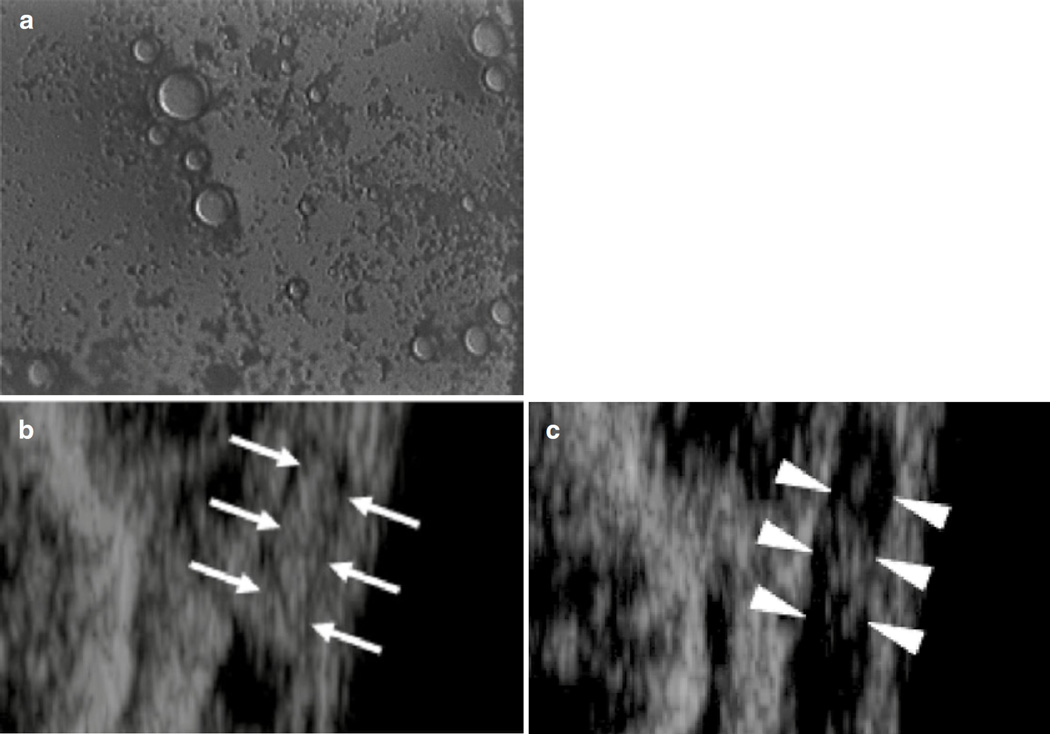
Inertial cavitation encompasses bubble motion dominated by the inertia of the surrounding fluid. Large expansions of the bubbles occur due to relatively large tension generated in the liquid by the acoustic excitation (Holland and Apfel 1989). During the final stages of bubble collapse, the converging liquid compresses and heats the contents of the bubble, creating a high energy density (Young 2005). The sudden halt of the converging liquid generates shock waves (Holzfuss et al. 1998), light emissions (Gaitan et al. 1992), and free radicals (Riesz and Kondo 1992). If the collapse occurs along a surface, a liquid jet with speeds in excess of 1 km/s may form (Brujan et al. 2001). These jets are associated with mechanical damage or deformation of clots (Weiss et al. 2013) (Fig. 19.2a).
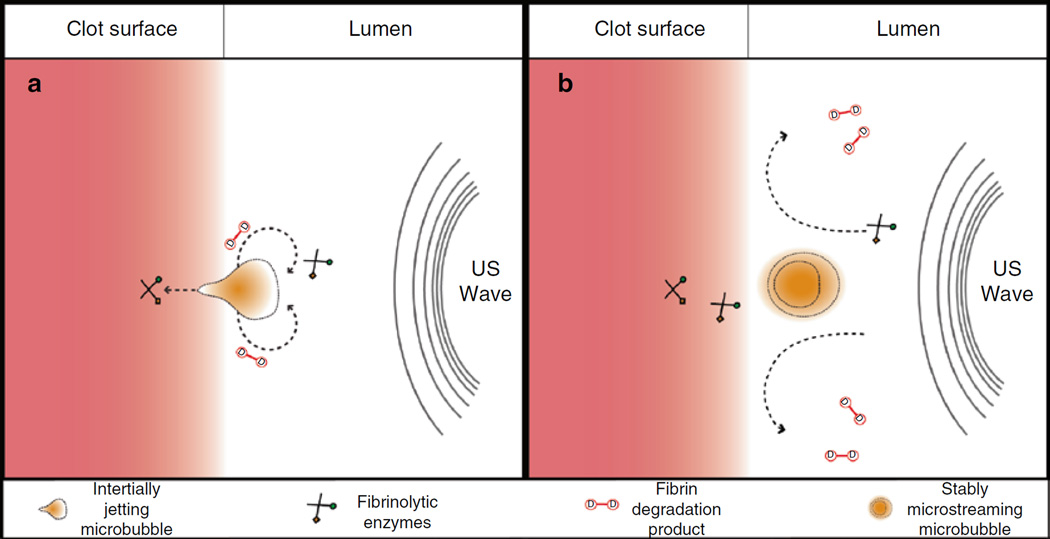
Fig. 19.2.
Interaction of cavitating microbubbles with thrombi, (a) Illustration of inertial cavitation. The asymmetric boundary condition causes a liquid jet to form during the final stages of collapse during inertial cavitation (Brujan et al. 2001). The jet will impinge on the thrombus, resulting in direct mechanical damage and erosion of clot surface, (b) Illustration of stable cavitation. Sustained bubble motion promotes strong fluid mixing through microstreaming (Elder 1959). Local pressure gradients around the microbubble cause enhanced mixing of the fibrinolytic enzymes, and removal of fibrin degradation products from the clot (Datta et al. 2008) (Figure Adapted from Sutton et al. (2013a))
Chen et al. (2014) used high-speed imaging to observe the formation of a defect in a thrombus surface at the location of a microbubble jet after ultrasound exposure (Fig. 19.3). Inertial cavitation can be detected acoustically as broadband emissions (Everbach and Francis 2000; Datta et al. 2008). Chuang et al. (2010) found a positive correlation between the dose of broadband acoustic emissions and the thrombolytic efficacy in-vitro. Similarly, Leeman et al. (2012) observed significant fibrinolysis in-vitro only when insonation of microbubbles produced broadband acoustic emissions. Broadband acoustic emissions have also been detected in-vivo during thrombolysis in porcine (Shi et al. 2011; Maxwell et al. 2011) and rabbit (Wright et al. 2012a) models. Xie et al. (2009) found significant thrombolytic enhancement in a canine model only when microbubbles nucleated inertial cavitation. However, Wu et al. (2014) found that thrombolytic efficacy did not correlate with the amplitude of in-vitro broadband acoustic emissions. They concluded instead that strong inertial cavitation shielded the acoustic energy from the thrombus. Although strong inertial activity appears to enhance thrombolysis in some cases (Chen et al. 2009; Maxwell et al. 2009), sustained bubble activity is difficult (Prokop et al. 2007), potentially due to the destruction of cavitation nuclei (Datta et al. 2006).
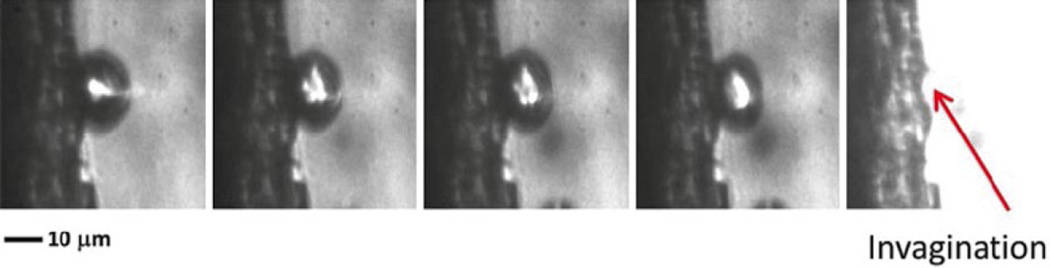
Fig. 19.3.
Interaction of inertially cavitating microbubble with thrombus (1 MHz, 1.5 MPa) recorded at 5 Mfps (200 ns interframe time). As the microbubble disappears at the completion of the inertial collapse, there is a residual “pit” at the thrombus site (Chen et al. 2014)
Stable cavitation is a sustained bubble motion (Datta et al. 2006) where the converging liquid inertia is offset by the restoring force of the gaseous contents of the bubble (Flynn 1964). The acoustic pressure amplitude required to initiate stable oscillations is generally lower than that required for inertial cavitation (Bader and Holland 2012). The highly nonlinear nature of these oscillations generates microstreaming of the surrounding fluid (Elder 1959), promotes strong fluid mixing (Collis et al. 2010) and attracts particulates via secondary radiation force (Nyborg and Miller 1982). Such strong fluid mixing hastens enzymatic fibrinolysis, enhancing the penetration of both rt-PA and plasminogen into clots (Datta et al. 2008; Sutton et al. 2013a) (Fig. 19.2b).
In contrast to the broadband emissions from inertial cavitation, stable cavitation generates harmonic, ultraharmonic, and subharmonic emission lines in the acoustic spectra. Depending on the relative size of the bubble compared to the resonant size (Bader and Holland 2012), harmonic (integer multiples of the fundamental) (Choi and Coussios 2012), subharmonic (rational fractions of the fundamental less than one) (Prokop et al. 2007), or ultraharmonic (rational fractions of the fundamental greater than one) (Datta et al. 2006) emissions can be used to detect stable cavitation acoustically. Datta et al. (2008) found a correlation between thrombolytic enhancement and ultraharmonic emissions in-vitro. Subsequent studies from the same group (Hitchcock et al. 2011) found increased thrombolytic efficacy using an intermittent insonation scheme that optimized ultraharmonic emissions from stable cavitation.
Bader et al. (2015)) used an in-vitro model to monitor the instantaneous reduction in clot width and ultraharmonic emissions from stable cavitation nucleated from Definity® microbubbles. The energy of ultraharmonic emissions was found to correlate significantly with the instantaneous reduction in clot width (i.e., thrombolytic efficacy). Prokop et al. (2007) suggested a correlation between increased thrombolytic efficacy in-vitro and the presence of subharmonic emissions from stable cavitation. The correlation between subharmonic or ultraharmonic emissions established by Prokop et al. (2007), Datta et al. (2008), and Bader et al. (2015)) illustrate the importance of stable cavitation in assisting enzymatic fibrinolysis.
To gauge both types of cavitation activity incited, Apfel (1981) recommends the use of three golden rules: Know thy sound field, Know thy liquid, and Know when something happens. These rules can help optimize sonothrombolysis treatments employing acoustic cavitation.
19.2.3.2 Know Thy Sound Field
Consistent insonification is necessary for safe and efficient sonothrombolysis. Several ultrasound exposure approaches have been proposed, as summarized in Fig. 19.4.
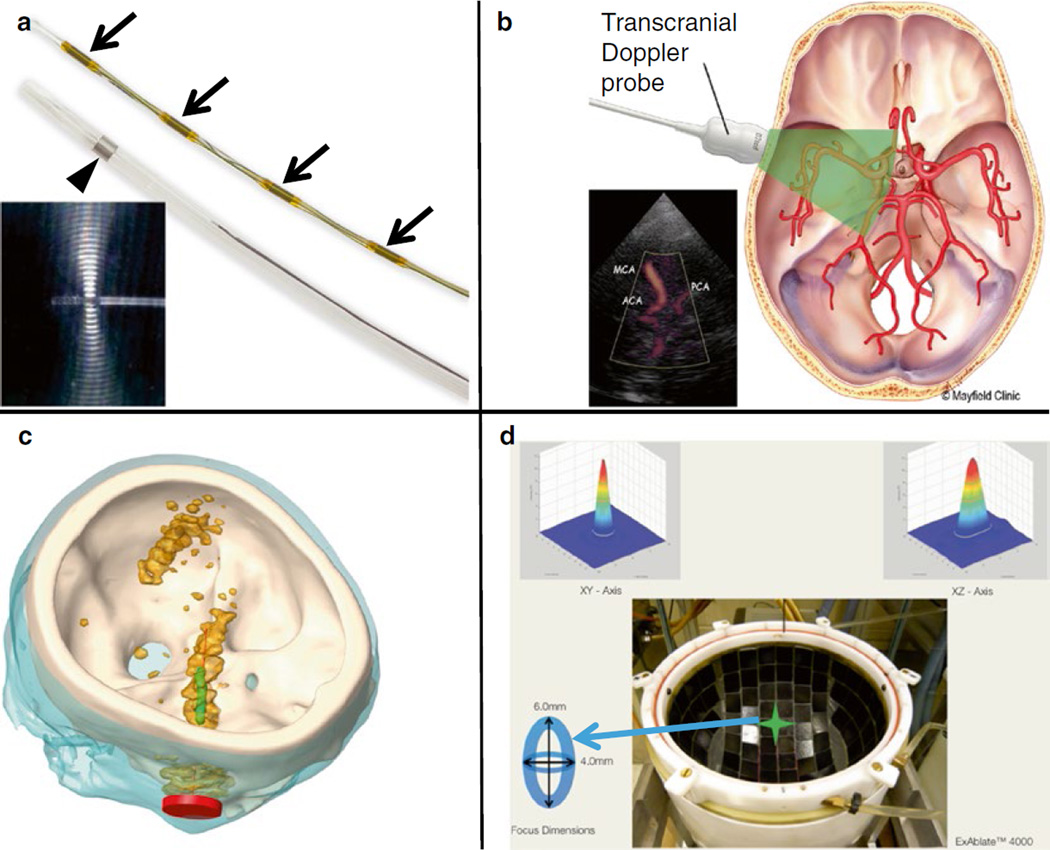
Fig. 19.4.
Ultrasound insonation schemes for sonothrombolysis. (a) Ultrasound-assisted thrombolysis catheter composed of a 5.2-Fr multi-sidehole drug infuser (arrowhead). Ultrasound elements along central core (small arrows) shown separately. During treatment, the central core is placed inside the infusion catheter (Engelberger and Kucher 2014). The omnidirectional ultrasound field promotes cavitation and acoustic streaming, driving thrombolytics into the clot. Images provided by EKOS Corporation (Bothell, WA, USA), (b) Transcranial Doppler. Schematic representation of the penetration of 2-MHz TCCS through the skull. Images reprinted with permission from Mayfield Clinic (Cincinnati, OH, USA). (c) Unfocused, Sub-megahertz Ultrasound. Simulated acoustic fields generated by an unfocused, transcranial sub-megahertz (120 kHz) ultrasound system in a human skull (white). The Ml segment of the MCA is shown in green and the transducer in red. The orange contour indicates the regions where the acoustic pressure is larger than half the maximum pressure in the Ml region of interest (Bouchoux et al. 2014). (d) MR-Guided Focused Ultrasound. Top view of ExAblate™ 4000 HIFU hemispherical 1000-element system for MR-guided transcranial focused ultrasound exposure. A sharp 4 mm×6 mm focus is created along the lateral and elevational orientations, respectively (Hölscher et al. 2013)
Ultrasound Catheter
Ultrasound-emitting catheters have been applied to a wide variety of pathologies (Doomernik et al. 2011). The catheter is inserted into the thrombus allowing combined exposure to ultrasound and local drug infusion. A relatively uniform ultrasound field covering large clots can be obtained using a series of miniaturized unfocused transducers aligned in the lumen of the catheter (Soltani et al. 2007). The acoustic pressure decreases rapidly with distance from the catheter because the transducers are small and unfocused. Nevertheless, effective ultrasound-catheter sonothrombolysis was achieved in large thrombi caused by intracerebral hemorrhage (Newell et al. 2011).
Transcranial Doppler
Extracorporeal insonation of the thrombus, combined with an intravenous injection of a thrombolytic drug and/or microbubbles, is an appealing minimally invasive approach for the treatment of ischemic stroke. The cranial bone attenuates, reflects and distorts the ultrasound waves (Fry et al. 1970). Hence, bone constitutes a major obstacle for transcranial sonothrombolysis. The temporal bone acoustic window is routinely used for transcranial Doppler (TCD) examination of cerebral blood flow (Aaslid et al. 1982). TCD, or transcranial color-coded sonography (TCCS), exposure through the temporal bone accelerates rt-PA thrombolysis (Cintas et al. 2002; Alexandrov et al. 2004; Eggers et al. 2008). TCD and TCCS sonothrombolysis use similar frequencies (around 2 MHz) and similar pulsed-ultrasound waveforms, and comparable results were obtained in clinical trials (Saqqur et al. 2013).
TCD is operator dependent, and the lack of trained operators is an obstacle for widespread adoption (Barlinn et al. 2012). To overcome this limitation, a sonothrombolysis device has been designed in order to be simpler to put into place and is less operator-dependent than TCD (Barlinn et al. 2013). A frame is fixed to the patient’s head using simple landmarks. Eighteen transducers are mounted on the head frame in order to insonify the most frequent locations of thrombi during ischemic stroke through the temporal and suboccipital acoustic windows. The acoustic parameters used by this device are based on those used in FDA-approved TCD devices (2 MHz, 100 kPa derated peak rarefactional pressure). The safety and relevance of this device has been demonstrated in phase I and II clinical trials (Barreto et al. 2013).
Ultrasound waves at 2 MHz are transmitted poorly through the bone (Ammi et al. 2008), which prevents TCD examination in 18% of patients (Wijnhoud et al. 2008). Barlinn et al. (2012) estimated the in-situ acoustic pressure for 20 subjects treated with TCD in the TUCSON trial using a simple attenuation model based on CT data. The calculated in-situ acoustic pressures were higher for patients functionally independent at 3 months. Hence, some patients may receive an insufficient acoustic pressure for thrombolysis enhancement with TCD insonification.
Sub-megahertz Ultrasound
Efficient acoustic transmission through the skull can be achieved in the sub-megahertz frequency range. Ammi et al. (2008) measured a pressure reduction resulting from bone between 77.1 and 96.6% at 2 MHz in 5 human skull specimens. A pressure reduction between 22.5 and 45.5% was observed at 120 kHz. Therefore, sub-megahertz sonothrombolysis may allow a more consistent insonation than 2-MHz TCD. Sub-megahertz (300 kHz) insonation was used in the TRUMBI clinical trial (Daffertshofer et al. 2005). However, a significantly higher rate of symptomatic hemorrhage occurred in the ultrasound group. Baron et al. (2009) numerically simulated TRUMBI trial insonation parameters and concluded that standing waves, due to acoustic reflections in the cranial cavity, could have caused unexpected local pressure maxima. Reflection from contra-lateral bone and associated constructive interference are less likely for 2-MHz TCD because the wave is significantly attenuated while propagating in the brain tissue. The TRUMBI trial and subsequent studies revealed the necessity of producing a well-controlled transcranial ultrasound field for safe and efficient sonothrombolysis.
Taking into account these findings, revised sub-megahertz transcranial sonothrombolysis devices were designed. A dual-frequency array capable of performing 2.5-MHz TCCS and emitting a 500-kHz sonothrombolysis beam was developed for transcranial insonation (Azuma et al. 2010). The transcranial ultrasound field produced by this device was evaluated in-vitro and the safety of this approach was demonstrated in a healthy primate model (Shimizu et al. 2012). Bouchoux et al. (2014) simulated 120 and 500-kHz transcranial ultrasound fields from the head CT scans of 20 ischemic stroke patients using a validated acoustic propagation numerical model (Bouchoux et al. 2012). Consistent and homogeneous insonation of the Ml segment of the MCA was achieved at both 120 and 500 kHz. A positioning strategy based on external head landmarks, which did not require the knowledge of the position of the thrombus, was proposed by Bouchoux et al. and was found to perform similarly to an optimized transducer positioning technique based on CT data analysis. Local acoustic pressure maxima, due to reflection from the contralateral bone, were well controlled and similar to the amplitude in the targeted Ml segment of the MCA.
Transcranial exposure with sub-megahertz ultrasound may allow simple and consistent insonation of the thrombus in ischemic patients, without excluding subjects with an insufficient temporal bone window for TCD or TCCS. Sub-megahertz sonothrombolysis devices should be developed and evaluated carefully. The transcranial ultrasound field can be evaluated by simulation or in-vitro measurements in several skulls. Moreover, sub-megahertz sonothrombolysis devices should be evaluated in a large animal model accounting for constructive interference due to standing waves. Random frequency modulation of the wave can also be employed to suppress standing waves (Tang and Clement 2010; Furuhata and Saito 2013).
Focused Ultrasound
Thrombolysis with focused ultrasound is also a promising approach. A highly focused ultrasound beam allows accurate spatial targeting of the clot. High-pressure amplitudes can be applied locally with a low risk of side effects outside the focal zone. Therefore, thrombolysis based on erosion of the clot by inertial cavitation may be possible with focused ultrasound without a thrombolytic drug. Maxwell et al. (2011) treated acute thrombi in juvenile pig femoral arteries using a 1-MHz transducer with a 1.9×13.5 mm focus (−6 dB). Similarly, a 1.5-MHz transducer (0.9×7.1 mm focal zone) was used to lyse clots in rabbit femoral arteries (Wright et al. 2012a). These studies demonstrated the feasibility of high intensity focused sonothrombolysis using accurate clot targeting. However, the focal zone is usually larger than the targeted vessels. Therefore careful studies on the side effects on tissue surrounding the clot are needed. The treatment of ischemic stroke with a MR-guided transcranial focused ultrasound device (ExAblate™ 4000, InSightec Inc., Tirat Carmel, Israel) was also proposed (Hölscher et al. 2013). This device is a hemispheric 1000-element 220-kHz phased array that can produce an electronically-steered 4×6 mm transcranial focal spot. Nevertheless, the work reported by Hölscher et al. (2013) is in a very early stage and more studies are needed to assess the efficacy and safety of this approach. Safe and efficient intracerebral hemorrhage (ICH) clot liquefaction with a similar MR-guided transcranial ultrasound device was demonstrated in an in-vivo porcine model and in a human cadaver model (Monteith et al. 2013). Though large aperture, focused ultrasound for transcranial insonation appears promising, numerical studies by Pajek and Hynynen (2012) indicate that these devices currently appear to be limited to treatment near the center of the brain (basilar artery and the proximal end of the Ml segment of the middle cerebral artery).
19.2.3.3 Know Thy Liquid (i.e., Know Thy Cavitation Nuclei)
The type and threshold for cavitation activity incited depends highly on the nuclei that are present.
Endogenous Nuclei
Thresholds for cavitation activity depend on the purity of the medium (Roy et al. 1985). However, cavitation thresholds in even the purest sources of water (Herbert et al. 2006; Bader et al. 2012; Maxwell et al. 2013) are much less than theoretically predicted (Church 2002). This discrepancy is generally attributed to nanometer-sized nuclei (Bader et al. 2012; Maxwell et al. 2013) that are difficult to extract from the medium (Flynn 1964). Such nuclei are however not likely to be found in-vivo due to the body’s natural filtration system. Instead, Yount (1979) proposed nuclei composed of an elastic organic skin of amphiphilic molecules which would stabilize bubbles against dissolution. The cavitation thresholds of endogenous nuclei in tissue are summarized by Church (2015). Maxwell et al. (2013) measured the thresholds for a wide range of ex-vivo tissues with short 1-MHz pulses, including clots, using a regime of therapeutic ultrasound termed histotripsy (Xu et al. 2004). The cavitation threshold of clots was similar to that of pure water, suggesting similar nanometer-sized nuclei to that observed in water.
Cavitation from endogenous nuclei has been observed during sonothrombolysis. Maxwell et al. (2011) observed microbubble clouds as regions of hyperechogenicity using 10-MHz diagnostic ultrasound imaging while insonating thrombi in a porcine femoral vein artery at 1 MHz. Additional studies have observed acoustic emissions from inertial (Everbach and Francis 2000; Wright et al. 2012a) or stable cavitation (Datta et al. 2006) during ultrasound-enhanced thrombolysis.
Exogenous Nuclei
Introducing exogenous nuclei allows control of the type and location of cavitation activity. Hydrophobic particulates (Soltani 2013), perfluorocarbon droplets (Pajek et al. 2014) and focused laser pulses (Cui et al. 2013) have been used to nucleate cavitation effectively during sonothrombolysis. However, ultrasound contrast agents (UCAs), or stabilized microbubbles, are also used to nucleate cavitation. Excellent reviews of the principles of UCAs are provided by Stride and Saffari (2003) and Cosgrove (2006). The specific use of UCAs for sonothrombolysis is reviewed by de Saint et al. (2014). Briefly, most commercially available UCAs are composed of a high-molecular-weight inert gas core surrounded by a lipid shell (Faez et al. 2013). The use of a gas with low solubility in the blood stream and pegylation of the lipid shell increases the circulation lifetime (Sarkar et al. 2009). The large compressibility of the gas compared to the surrounding tissue results in a high backscatter of incident acoustic pulse. UCAs are excellent blood pooling agents for left ventricular opacification (Radhakrishnan et al. 2013), detection of focal lesions in the liver (Claudon et al. 2013) and evaluation of brain perfusion (Claudon et al. 2008). UCAs range in size from 1 to 10 um in diameter (Bouakaz and de Jong 2007), which prevents nitration of UCAs by the lungs (Hogg 1987). In addition, UCAs of this size are resonant at diagnostic imaging frequencies (1–10 MHz). Current FDA-approved use of UCAs is restricted to left ventricular opacification, although there is a growing interest in using UCAs for therapeutic applications as well (Cosgrove and Harvey 2009; Stride et al. 2009).
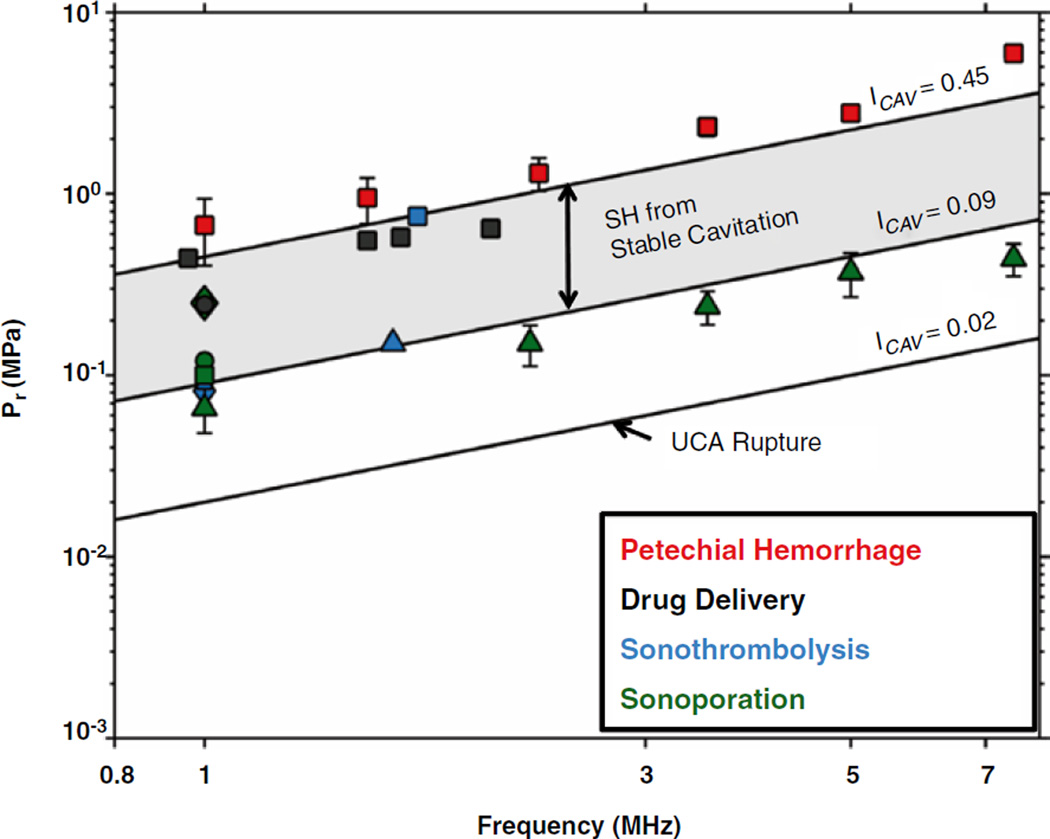
To gauge the potential for generating therapeutic cavitation activity from UCAs, Bader and Holland (2012) investigated the nucleation and detection of stable cavitation numerically. The cavitation index (ICAV) was devised to gauge the likelihood of stable cavitation activity from UCAs in terms of the peak rarefactional pressure in MPa (Pr) and center frequency in MHz (f):
Bader and Holland found there was an increased likelihood of nucleating cavitation activity by rupturing the UCA shell when ICAV > 0.02 , and an increased likelihood of detecting subharmonic emissions from stable cavitation when ICAV > 0.09. Subharmonic emissions are more likely from inertial cavitation when ICAV > 0.45. The parameter space predicted by the cavitation index necessary to promote beneficial bioeffects from stable cavitation is in agreement with reported literature values, including sonothrombolysis studies (Fig. 19.5).
Fig. 19.5.
Comparison of cavitation index to selected bioeffects (Bader and Holland 2012). The line labeled ICAV=0.45 indicates the peak rarefractional pressure (Pr) required to initiate shell rupture of ultrasound contrast agents. The lines labeled ICAV=0.09 and ICAV=0.45 bordering the shaded region demarcate the parameter space over which subharmonic (SH) emissions from stable cavitation are likely. The cavitation index is well suited for predicting beneficial bioeffects associated with stable cavitation, such as drug delivery (Hitchcock et al. 2010; McDannold et al. 2008), sonoporation (Greenleaf et al. 1998; Miller and Dou 2004; Rahim et al. 2006; Juffermans et al. 2009) and sonothrombolysis (Porter et al. 2001; Prokop et al. 2007; Petit et al. 2012a). Beyond ICAV=0.45, subharmonic emissions originate from inertial cavitation. Bioeffects associated with inertial cavitation, such as petechial hemorrhage (Miller et al. 2008), occur at a cavitation index above 0.45
19.2.3.4 Know When Something Happens
Apfel’s final golden rule refers to the detection of cavitation activity. While some in-vitro studies used high-speed imaging to monitor cavitation activity during sonothrombolysis (Caskey et al. 2009; Acconcia et al. 2013; Chen et al. 2014), such observations are not possible in-vivo. Devising methods to monitor cavitation is important to translate these treatments through the regulatory process and into the clinic (Harris 2009). The most common method of monitoring cavitation activity in sonothrombolysis studies is passive cavitation detection (Everbach and Francis 2000; Datta et al. 2006; Prokop et al. 2007), whereby cavitation acoustic emissions are passively received by a transducer. The frequency spectrum of the signal is inspected for the presence of either broadband emissions to indicate the presence of inertial cavitation, harmonic, subharmonic, or ultraharmonic emissions to indicate the presence of stable cavitation. The strength of these characteristic cavitation emissions can be correlated with thrombolytic efficacy in order to gain insight into the type of cavitation activity required to promote maximal thrombolytic efficacy. Chuang et al. (2010) found a positive correlation between the thrombolytic efficacy and the strength of broadband acoustic emissions in an in-vitro human clot model. In contrast, Datta et al. (2008) and Bader et al. (2015) found a positive correlation between thrombolytic efficacy and the strength of ultraharmonic emissions in in-vitro models.
Passive cavitation detection with single-element transducers provides limited information on the spatial distribution of cavitation activity. A new method of cavitation detection, passive cavitation imaging (PCI) (Salgaonkar et al. 2009; Haworth et al. 2012; Jensen et al. 2012), beam-forms received cavitation signals from multiple-element arrays. The resulting passive cavitation images resolve the location and type of cavitation activity. Vignon et al. (2013) demonstrated the feasibility of PCI to detect and map cavitation activity in-vivo for thrombolysis studies. Shi et al. (2011) implemented the technique to monitor cavitation nucleated from Doppler exposure of Definity® in an in-vivo porcine model. Cavitation emissions mapped within the porcine skull were a mixture of both stable and inertial cavitation at the lower Doppler amplitudes (0.46 mechanical index). Only inertial cavitation was observed at the higher Doppler amplitudes (1.7 mechanical index).
19.3 Experimental Evidence for Ultrasound-Enhanced Efficacy
19.3.1 Assessment of Sonothrombolysis in the Laboratory
Since the first demonstration of ultrasound-enhanced thrombolysis by Sobbe et al. in 1974, nearly 500 studies have been published on sonothrombolysis (Scopus search engine, Accessed 9 July 2014). These studies have included "bench top" experiments, designed to elucidate and maximize the mechanisms of ultrasound that enhance thrombolysis. The conclusions of these studies depend on the type of study (i.e., in-vitro, ex-vivo, or in-vivo), modeling of biological parameters (i.e., clot manufacturing process, thrombolytic metric, etc.), and the ultrasound exposure conditions.
19.3.1.1 In-Vitro, Ex-Vivo, and In-Vivo Studies
In-vitro studies minimize the complexity associated with biological systems to assess the interaction of ultrasound with clots in a controlled setting. Clot models have been developed through strict protocols in order to produce consistent, clinically-relevant clots (Holland et al. 2008). Coagulation is initiated by incubating fresh or recalcified blood at physiologic temperature (37 °C) (Roessler et al. 2011). Coagulation can also be initiated by the addition of thrombin to plasma (Lauer et al. 1992; Suchkova et al. 1998). Fibrin clots, which are optically translucent, are also used for optical studies (Acconcia et al. 2013). The degree of clot retraction can be modified by altering the properties of the surface in contact with incubated blood (Sutton et al. 2013b), or by storing the clot at low temperatures (<4 °C) for several days post-incubation (Shaw et al. 2006). Thrombolytic metrics have included mass loss (Datta et al. 2006), dimensional reduction on an image (Cheng et al. 2005; Kim et al. 2012; Petit et al. 2012a), or the presence of fibrin degradation products (Francis et al. 1992; Kimura et al. 1994; Pfaffenberger et al. 2003; Alonso et al. 2009). Interested readers are invited to review an exhaustive list of recent in-vitro studies compiled by Petit et al. (2012b).
Ex-vivo studies incorporate excised living tissue as a first step towards understanding the interaction of vascular tissue exposed to thrombolytics and ultrasound. Excised arteries have been employed to determine the effect on the endothelium during sonothrombolysis (Rosenschein et al. 2000; Hitchcock et al. 2011). Clot models are similar to those used in-vitro, and mass loss is used as the thrombolytic metric.
In-vivo animal models allow the full biological response of a living organism to ultrasound and thrombolytics to be assessed, including the efficacy of the treatment, the ability to track and monitor treatment progress, physiologic alterations, and the potential for collateral damage. These studies are required prior to U.S. Food and Drug Administration approval for new clinical devices (Harris 2009). A variety of in-vivo thrombosis models have been developed and are summarized by Hossmann (1998), Verbeuren (2006), and Mousa (2010). Small animal models, such as rodent (Daffertshofer et al. 2004) and rabbit (Hölscher et al. 2012), are used in sonothrombolysis due to their low cost, ease of handling, and accumulation of data from previous studies. Large animal models, such as swine (Culp 2004) and primates (Shimizu et al. 2012), are needed to model the propagation of ultrasound through the skull and brain architecture. Thrombus formation can be initiated by incubation of autologous blood within a stenotic artery (Culp 2004; Damianou et al. 2014). High-intensity laser pulses can also be used to induce clot formation (Yamashita et al. 2009; Chen et al. 2013). Flow velocity (Maxwell et al. 2011), degree of thrombus size burden (Stone et al. 2007), or the presence of fibrin degradation products (Xie et al. 2005; Yamashita et al. 2009) can be used to assess thrombolytic efficacy. Potential side effects are evaluated using imaging techniques, post-mortem histology and immunohistochemistry techniques.
19.3.1.2 Ultrasound Exposure Conditions
Insonation of thrombi to initiate lysis have been employed with and without thrombolytic drugs. UCAs have also been used to nucleate cavitation activity in both cases. The degree of thrombolysis is dependent on the ultrasound exposure conditions (Holland et al. 2008; Petit et al. 2012a).
Ultrasound Therapy Without a Thrombolytic
In the absence of a thrombolytic drug, lysis is initiated by the mechanical interaction of ultrasound energy with the thrombus. Maxwell et al. employed 1-MHz histotripsy pulses (Xu et al. 2004) to lyse clots in-vitro completely within 5 min (Maxwell et al. 2009). This technique reduced the thrombus size in 10 of 12 cases in an in-vivo swine model (Maxwell et al. 2011). Westermark et al. (1999) determined pulsed ultrasound increased the thrombolytic efficacy compared to continuous wave ultrasound in-vitro. Similarly, Rosenschein et al. (2000) and Wright et al. (2012a) found efficient thrombolysis with pulsed ultrasound both in-vitro and in-vivo. Moderate collateral damage was observed in-vivo in the form of coagulative necrosis (Rosenschein et al. 2000), hemorrhage (Wright et al. 2012a; Burgess et al. 2012), or denudation of the endothelium (Maxwell et al. 2011). These deleterious effects could be explained by the relatively small size of the vessel relative to the focal zone of the transducer (Maxwell et al. 2011), or by standing waves (Burgess et al. 2012).
Ultrasound Therapy with Microbubbles, but Without a Thrombolytic
Sonothrombolysis was investigated in several in-vitro (Borrelli et al. 2012) and in-vivo (Birnbaum et al. 1998; Culp et al. 2003; Xie et al. 2005) studies by insonation of microbubbles alone to initiate thrombolysis. These studies confirm thrombolytic efficacy using ultrasound and microbubbles alone, emphasizing the importance of cavitation activity. Several groups have developed thrombus-specific microbubbles with the ability to target clots for both imaging (Unger et al. 1998; Chen et al. 2009) and therapy (Alonso et al. 2009; Hagisawa et al. 2013) (Fig. 19.6).
Fig. 19.6.
(a) Photograph of thrombus-targeted micro-bubble bound to human whole blood clot in-vitro. Non-targeted microbubbles did not bind to the clot (Unger et al. 1998). (b) Thrombus-targeted bubble liposomes (white arrows’) accumulate on thrombus within an occluded iliac artery in a rabbit model of thromboembolism. (c) After insonation of the thrombus-targeted bubble liposomes, flow was restored and the hyperechogenic area within the iliac artery was reduced (white arrowheads’) (Hagisawa et al. 2013)
Culp (2004) investigated thrombolytic efficacy of a clot-targeted microbubble formulation in a porcine model. In a follow-up study (Culp et al. 2011), a rabbit carotid stroke model insonated with pulsed, 1-MHz ultrasound and microbubbles alone had a significantly smaller infarct volume than with rt-PA alone, or rt-PA with ultrasound exposure. However, there was no significant difference in the infarct volume if targeted or non-targeted microbubbles were used. Several additional studies demonstrated enhanced thrombolytic efficacy in-vitro (Unger et al. 1998; Chen et al. 2009) and in-vivo (Alonso et al. 2009; Hagisawa et al. 2013) using targeted microbubbles. It should be noted that the treatment parameters employed in several other studies did not produce appreciable thrombolytic efficacy with ultrasound alone (Frenkel et al. 2006; Datta et al. 2006; Prokop et al. 2007; Shaw et al. 2008; Petit et al. 2012a) or with microbubbles (Datta et al. 2008; Petit et al. 2012a; Bader et al. 2015).
Ultrasound Therapy with a Thrombolytic
Although lysis with ultrasound alone is a promising means to overcome strict contraindication criteria of thrombolytic drugs (Turi et al. 1993), drug-mediated thrombolysis remains the gold-standard in clinical practice. In this treatment regime, ultrasound is considered an adjuvant therapy, without the intent of completely replacing thrombolytic drugs. Early in-vitro studies by Lauer et al. (1992), and later by Francis and his colleagues (Francis et al. 1992; Blinc et al. 1993; Francis et al. 1995), found enhancement of rt-PA with ultrasound exposure. Meunier et al. (2007) found an increasing thrombolytic efficacy over rt-PA alone as a function of duty cycle over the range 10–80% at 120 kHz. However, subsequent studies by the same group found no distinct trend with duty cycle (Holland et al. 2008). Datta et al. (2006) demonstrated thrombolytic enhancement only when cavitation emissions were detected.
Ultrasound Therapy with Microbubbles and a Thrombolytic
Introducing ultrasound contrast agents, or stabilized microbubbles, can reduce the threshold for cavitation activity. De Saint et al. (2014) provides an extensive review of the use of microbubbles in sonothrombolysis. Tachibana and Tachibana (1995) pioneered the use of microbubbles in sonothrombolysis, insonating Albunex® with urokinase at 170 kHz to increase thrombolytic efficacy over urokinase alone, or urokinase with ultrasound exposure. Subsequent studies confirmed the thrombolytic enhancement in-vitro, with sub-megahertz ultrasound exposure (Datta et al. 2008; Hitchcock et al. 2011; Bader et al. 2015) as well as with diagnostic imaging frequencies (Kondo et al. 1999; Cintas et al. 2004; Xie et al. 2011). Nedelmann et al. (2010) found an increase in thrombolytic efficacy in-vivo with transcranial color-coded duplex Doppler, SonoVue®, and rt-PA when compared with rt-PA alone. Edema and lesion volumes were significantly smaller when microbubbles and rt-PA were exposed to ultrasound than exposed to rt-PA alone. However, Brown et al. (2011) found the infarct volume in a rabbit carotid artery model was not significantly smaller when Definity® and rt-PA were insonified compared to rt-PA alone. Hitchcock et al. (2011) developed a novel ex-vivo porcine carotid model and measured significant thrombolytic efficacy when Definity® and rt-PA were exposed to sub-megahertz ultrasound over rt-PA alone. Sutton et al. (2013b) extended this model, and showed that increased thrombolysis occurred only in unretracted clots. Xie et al. (2013) noted that epicardial recanalization was greatest in a porcine atherosclerotic model when Doppler pulses induced cavitation activity from Definity® in the presence of rt-PA.
Drug-loaded microbubbles also show potential as a “theragnostic” approach for enhancing thrombolytic efficacy. Echogenic liposomes (ELIP), liposomes containing air-filled micro-bubbles, can be used as a vector for therapeutic drugs. Incorporation of rt-PA into ELIP, or t-ELIP, allows acoustic activation (Smith et al. 2010) for localized drug delivery. In-vitro studies have demonstrated enhanced thrombolytic efficacy of t-ELIP and ultrasound over rt-PA alone (Shaw et al. 2009), or over rt-PA, ultrasound and Optison™ combined (Tiukinhoy-Laing et al. 2007). Hua et al. (2014) treated thrombi in a rabbit femoral artery using targeted microbubbles loaded with rt-PA and 2-MHz ultrasound. The recanalization rates obtained with rt-PA targeted microbubbles were similar to those with a combination of untargeted microbubbles and free rt-PA.
19.3.1.3 Conclusions of Bench Top Studies
Ultrasound is well established to enhance thrombolysis in-vitro (Datta et al. 2006; Prokop et al. 2007), ex-vivo (Hitchcock et al. 2011), and in-vivo (Culp 2004; Xie et al. 2013). Mechanical effects, particularly acoustic cavitation (Petit et al. 2012a; Wu et al. 2014; Bader et al. 2015), are clearly responsible for enhanced thrombolysis. These bench top experiments provide proof of concept for a particular insonation scheme, but the results are restricted to the particular clot model employed (Xie et al. 2011). Given the variability in thrombi composition in-vivo (Liebeskind et al. 2011), future studies should focus on both the insonation regime and subtype of clot in order to employ the optimal treatment regime. For example, thrombolytic enhancement was not possible for low-amplitude (<0.25 MPa peak negative pressure), sub-megahertz insonation of retracted clots treated with Definity® and rt-PA (Sutton et al. 2013b). Other insonation schemes need to be developed for these stiff, retracted thrombi. Studies are also needed to determine the thrombus type, for example using elastography techniques (Viola et al. 2004) to determine the optimal ultrasound approach. Finally, methods to monitor and quantify the dose of cavitation energy, possibly employing PCI (Haworth et al. 2012), need to be further developed in order to monitor treatment progress.
19.3.2 Clinical Trials
19.3.2.1 Catheter-Directed Thrombolysis
Significant progress has been made over the past decade to translate ultrasound from the bench to the bedside. Catheter-directed ultrasound-accelerated thrombolysis has been successfully employed to treat peripheral arterial occlusions (Greenberg et al. 1999), stroke (The IMS II Trial Investigators 2007), deep venous thrombosis (Raabe 2006), and pulmonary embolism (PE) (Engelberger and Kucher 2014). Catheter-based ultrasound initiated complete or partial recanalization in 87.9% of cases in a review of 340 clinical cases (Doomernik et al. 2011). Overall rates of non-response (8.2%), complications (7.1%), bleeding (4.1%) and re-occlusion (2.1%) were low compared to conventional thrombolytic treatment. No distal embolization was reported during treatment. Engelberger and Kucher (2014) compiled the results from clinical studies of PE treatment with ultrasound catheter-directed thrombolysis and found similar clinical outcomes compared to administration of the thrombolytic tenecteplase, a genetically modified version of rt-PA (Baruah et al. 2006). In the first randomized trial employing an ultrasound catheter device, 59 patients with acute PE were treated with either the EKOS Endovascular System and rt-PA, or anticoagulation therapy alone (Kucher et al. 2013). The authors concluded ultrasound-assisted thrombolysis was the superior treatment versus anticoagulation at 24 h, without an increased bleeding risk.
19.3.2.2 Transcranial Insonation
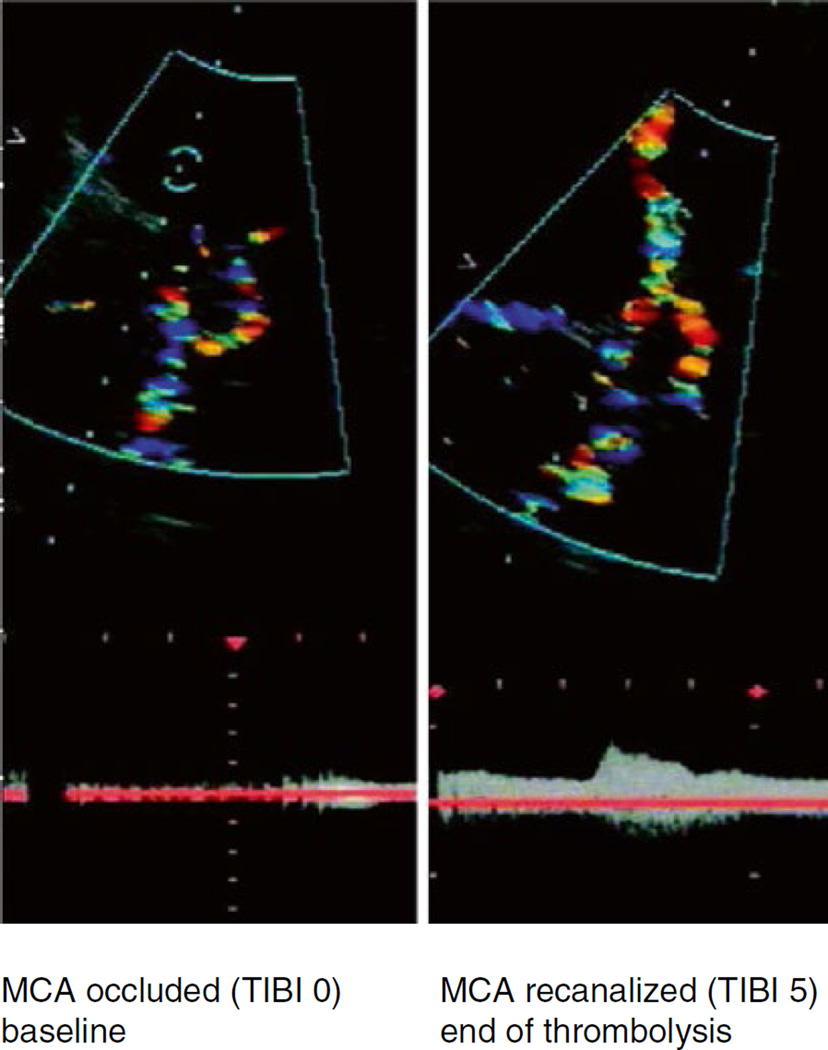
The therapeutic effect of transcranial insonation during intravenous rt-PA treatment for acute stroke has been investigated independently by three stroke therapy centers: University of Texas-Houston Medical School (Alexandrov et al. 2000), University of Lübeck, Germany (Eggers et al. 2003), and University Hospital Ostrava, Czech Republic (Skoloudik et al. 2008). Based on these findings, randomized studies employing either TCD in the CLOTBUST trial (Alexandrov et al. 2004), or TCCS in the Eggers study (Eggers et al. 2008) were conducted (Fig. 19.7). Both studies found significant improved recanalization for the sonothrombolysis groups, with an improvement in neurological deficits after 4 days and functional independence after 3 months. The CLOTBUST trial however found no difference in the rate of symptomatic intracranial hemorrhage between the control and sonothrombolysis groups. The Eggers study found no significant correlation between exposure to TCCS and symptomatic cerebral bleeding. Preliminary studies found further thrombolytic enhancement when employing TCD in combination with Levovist® (Molina et al. 2006) or TCCS with SonoVue® (Perren et al. 2007), and there was no report of an increase in intracranial bleeding. A randomized trial employing MRX-801 (ImaRx Therapeutics, Inc., Redmond, WA, USA), a microbubble developed purposely for enhancing sonothrombolysis, exposed to TCD was discontinued by the sponsor after microbubble dose-escalation resulted in increased bleeding (Molina et al. 2009). The combination of TCD, MRX-801, and rt-PA demonstrated increased early recanalization and clinical recovery rates compared to rt-PA alone.
Fig. 19.7.
Transcranial color-coded sonography in an ischemic stroke patient with an MCA occlusion. Proximal middle cerebral artery stem occlusion (left) with complete recanalization 1 h after intravenous rt-PA plus TCCS exposure (right) (Eggers et al. 2003)
Performing meta-analysis of the results from ten clinical studies of both randomized control trials and case control studies, Saqqur et al. (2013) found TCD and TCCS sonothrombolysis was safe, effective and had more than a twofold likelihood of favorable long-term outcome. Furthermore, subgroup analysis of the use of ultrasound and microbubbles was both safe and more effective than thrombolytic therapy alone, when symptomatic intracranial hemorrhage was considered as the primary safety outcome. In contrast, a meta-analysis by Ricci et al. (2012) of seven randomized control trials found an increased risk of hemorrhage in connection with microbubbles and ultrasound, when both symptomatic and asymptomatic cerebral bleeding were considered. The success of using TCD and TSSC during treatment of ischemic stroke has prompted a host of ongoing trials (Dwedar et al. 2014; Haukeland University Hospital 2014; la Santa Creu i Sant Pau 2014), including successful completion of phase II trials employing a hands-free therapeutic device (Barreto et al. 2013).
19.3.2.3 Conclusions of Clinical Studies
The success of these clinical trials motivates the viability of ultrasound as a therapy for thrombo-occlusive disease. The prospect of co-administration of thrombolytic drugs with microbubbles is especially encouraging. Cavitation detection schemes should be implemented in future studies to inform what specific type of bubble activity promotes thrombolysis. Future TCD studies employing user-free devices may help decrease the patient-to-patient variability (Barlinn et al. 2013). The means to obtain reproducible and predictable transcranial sub-megahertz ultrasound fields have been devised (Bouchoux et al. 2014), and the approach appears safe in large primate models (Shimizu et al. 2012).
19.4 Conclusions and Future Directions
Thrombosis is a major contributor to mortality and morbidity in cardiovascular disease. Although administration of thrombolytic drugs is the current standard clinical treatment, adjuvant therapies are under development. Ultrasound has been demonstrated as an effective adjuvant for thrombolytic drugs in-vitro, in-vivo, and in clinical trials. Of particular interest are insonation schemes that obviate the need for thrombolytic drugs. A considerable degree of variability in clot models exists amongst bench top experiments (Petit et al. 2012b). Thrombus architecture and lytic susceptibility varies depending on the origin of the thrombus (Silvain et al. 2011). Ultrasound-enhanced thrombolysis studies at present have only rarely considered the effect of thrombus composition (Sutton et al. 2013b). Thus a single insonation parameter set may not be effective for all thrombus subtypes. Future studies should focus on the effect of ultrasound on a particular subtype of thrombus to determine what treatment type is optimal for each thrombus type.
Radiation force and acoustic streaming are proposed mechanisms for ultrasound enhancement. In addition, mechanisms related to cavitation are strongly correlated with the enhancement of thrombolytic efficacy. Therefore, potent sonothrombolysis may be achieved by promoting cavitation. In particular, the use of microbubbles to nucleate cavitation both expedites the lytic rate and improves the functional outcome, both in-vivo and in clinical studies. Although these findings are most promising, UCAs are currently only FDA-approved for diagnostic purposes. Furthermore, specific devices designed to produce and monitor safe and efficient cavitation activity are needed. Thus, substantial work is still underway to identify standards to monitor and control therapies employing both spontaneous and UCA nucleated microbubble activity.
In summary, ultrasound shows great promise in the treatment of acute and chronic thrombotic disease. While further bench top measurements are needed to elucidate the mechanisms and assess potential risks, clinical trials are also required for optimal and safe clinical implementation.
References
- Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Acconcia C, Leung BYC, Hynynen K, Goertz DE. Interactions between ultrasound stimulated microbubbles and fibrin clots. Appl Phys Lett. 2013;103:053701. [Google Scholar]
- Adeoye O, Clark JF, Khatri P, Wagner KR, Zuccarello M, Pyne-Geithman GJ. Do current animal models of intracerebral hemorrhage mirror the human pathology? Transl Stroke Res. 2010;2:17–25. doi: 10.1007/s12975-010-0037-1. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Demchuk AM, Felberg RA, Christou I, Barber PA, Burgin WS, Malkoff M, Wojner AW, Grotta JC. High rate of complete recanalization and dramatic clinical recovery during tPA infusion when continuously monitored with 2-MHz transcranial Doppler monitoring. Stroke. 2000;31:610–614. doi: 10.1161/01.str.31.3.610. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moyé LA, Hill MD, Wojner AW Clotbust Investigators. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- Alonso A, Dempfle C-E, Martina Delia A, Stroick M, Fatar M, Zohsel K, Allémann E, Hennerici MG, Meairs S. In vivo lysis of human thrombus with intravenous abciximab immunobubbles and ultrasound. Thromb Res. 2009;124:70–74. doi: 10.1016/j.thromres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Ammi AY, Mast TD, Huang IH, Abruzzo TA, Coussios CC, Shaw GJ, Holland CK. Characterization of ultrasound propagation through ex-vivo human temporal bone. Ultrasound Med Biol. 2008;34:1578–1589. doi: 10.1016/j.ultrasmedbio.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel RE. Acoustic cavitation. In: Edmonds PD, editor. Methods in experimental physics. New York: Academic; 1981. pp. 355–411. [Google Scholar]
- Azuma T, Ogihara M, Kubota J, Sasaki A, Umemura S, Furuhata H. Dual-frequency ultrasound imaging and therapeutic bilaminar array using frequency selective isolation layer. IEEE Trans Ultrason Ferrelectr Freq Control. 2010;57:1211–1224. doi: 10.1109/TUFFC.2010.1534. [DOI] [PubMed] [Google Scholar]
- Bader KB, Holland CK. Gauging the likelihood of stable cavitation from ultrasound contrast agents. Phys Med Biol. 2012;58:127–144. doi: 10.1088/0031-9155/58/1/127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader KB, Raymond JL, Mobley J, Church CC, Felipe Gaitan D. The effect of static pressure on the inertial cavitation threshold. J Acoust Soc Am. 2012;132:728. doi: 10.1121/1.4733539. [DOI] [PubMed] [Google Scholar]
- Bader KB, Gruber MJ, Holland CK. Shaken and stirred: mechanisms enhancing thrombolysis with submegahertz ultrasound. Ultrasound Med Biol. 2015;41:187–196. doi: 10.1016/j.ultrasmedbio.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlinn K, Alexandrov AV. Sonothrombolysis in ischemic stroke. Curr Treat Options Neurol. 2013;15:91–103. doi: 10.1007/s11940-012-0214-5. [DOI] [PubMed] [Google Scholar]
- Barlinn K, Tsivgoulis G, Molina CA, Alexandrov DA, Schafer ME, Alleman J, Alexandrov AV TUCSON Investigators. Exploratory analysis of estimated acoustic peak rarefaction pressure, recanalization, and outcome in the transcranial ultrasound in clinical sonothrombolysis trial. J Clin Ultrasound. 2012;41:354–360. doi: 10.1002/jcu.21978. [DOI] [PubMed] [Google Scholar]
- Barlinn K, Barreto AD, Sisson A, Liebeskind DS, Schafer ME, Alleman J, Zhao L, Shen L, Cava LF, Rahbar MH, Grotta JC, Alexandrov AV. CLOTBUST-hands free: initial safety testing of a novel operator-independent ultrasound device in stroke-free volunteers. Stroke. 2013;44:1641–1646. doi: 10.1161/STROKEAHA.113.001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C, Aubry J-F, Tanter M, Meairs S, Fink M. Simulation of intracranial acoustic fields in clincial trials of sonothrombolysis. Ultrasound Med Biol. 2009;35:1148–1158. doi: 10.1016/j.ultrasmedbio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Barreto AD, Alexandrov AV, Shen L, Sisson A, Bursaw AW, Sahota P, Peng H, Ardjomand-Hessabi M, Pandurengan R, Rahbar MH, Barlinn K, Indupuru H, Gonzales NR, Savitz SI, Grotta JC. CLOTBUST-Hands Free: pilot safety study of a novel operator-independent ultrasound device in patients with acute ischemic stroke. Stroke. 2013;44:3376–3381. doi: 10.1161/STROKEAHA.113.002713. [DOI] [PubMed] [Google Scholar]
- Baruah DB, Dash RN, Chaudhari MR, Kadam SS. Plasminogen activators: a comparison. Vase Pharmacol. 2006;44:1–9. doi: 10.1016/j.vph.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Bell WR, Simon TL. Current status of pulmonary thromboembolic disease: pathophysiology, diagnosis, prevention, and treatment. Am Heart J. 1982;103:239–262. doi: 10.1016/0002-8703(82)90498-7. [DOI] [PubMed] [Google Scholar]
- Birnbaum Y, Luo H, Nagai T, Fishbein MC, Peterson TM, Li S, Kricsfeld D, Porter TR, Siegel RJ. Noninvasive in vivo clot dissolution without a throm-bolytic drug: recanalization of thrombosed iliofemoral arteries by transcutaneous ultrasound combined with intravenous infusion of microbubbles. Circulation. 1998;97:130–134. doi: 10.1161/01.cir.97.2.130. [DOI] [PubMed] [Google Scholar]
- Blinc A, Francis CW, Trudnowski JL, Carstensen EL. Characterization of ultrasound-potentiated fibrinolysis in vitro. Blood. 1993;81:2636–2643. [PubMed] [Google Scholar]
- Borrelli MJ, O'Brien WD, Hamilton E, Oelze ML, Wu J, Bernock LJ, Tung S, Rokadia H, Culp WC. Influences of microbubble diameter and ultrasonic parameters on in vitro sonothrombolysis efficacy. J Vase Interv Radiol. 2012;23:1677–1684. doi: 10.1016/j.jvir.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouakaz A, de Jong N. WFUMB safety symposium on echo-contrast agents: nature and types of ultrasound contrast agents. Ultrasound Med Biol. 2007;33:187–196. doi: 10.1016/j.ultrasmedbio.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Bouchoux G, Bader KB, Korfhagen JJ, Raymond JL, Shivashankar R, Abruzzo TA, Holland CK. Experimental validation of a finite-difference model for the prediction of transcranial ultrasound fields based on CT images. Phys Med Biol. 2012;57:8005–8022. doi: 10.1088/0031-9155/57/23/8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchoux G, Shivashankar R, Abruzzo TA, Holland CK. In silico study of low-frequency transcranial ultrasound fields in acute ischemic stroke patients. Ultrasound Med Biol. 2014;40:1154–1166. doi: 10.1016/j.ultrasmedbio.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brott T, Thalinger K, Hertzberg V. Hypertension as a risk factor for spontaneous intracerebral hemorrhage. Stroke. 1986;17:1078–1083. doi: 10.1161/01.str.17.6.1078. [DOI] [PubMed] [Google Scholar]
- Brown AT, Flores R, Hamilton E, Roberson PK, Borrelli MJ, Culp WC. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Invest Radiol. 2011;46:202–207. doi: 10.1097/RLI.0b013e318200757a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brujan E, Nahen K, Schmidt P, Vogel A. Dynamics of laser-induced cavitation bubbles near an elastic boundary. J Fluid Mech. 2001;433:251–281. [Google Scholar]
- Burgess A, Huang Y, Waspe AC, Ganguly M, Goertz DE, Hynynen K. High-Intensity Focused Ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke. PLoS One. 2012;7:e42311. doi: 10.1371/journal.pone.0042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey CF, Qin S, Dayton PA, Ferrara KW. Microbubble tunneling in gel phantoms. J Acoust Soc Am. 2009;125:EL183. doi: 10.1121/1.3097679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casscells W. Vulnerable atherosclerotic plaque: a multifocal disease. Circulation. 2003;107:2072–2075. doi: 10.1161/01.CIR.0000069329.70061.68. [DOI] [PubMed] [Google Scholar]
- Castano C, Dorado L, Guerrero C, Millan M, Gomis M, Perez de la Ossa N, Castellanos M, Garcia MR, Domenech S, Davalos A. Mechanical throm-bectomy with the solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke. 2010;41:1836–1840. doi: 10.1161/STROKEAHA.110.584904. [DOI] [PubMed] [Google Scholar]
- Chen SC, Ruan JL, Cheng PW, Chuang YH, Li PC. In vitro evaluation of ultrasound-assisted thrombolysis using a targeted ultrasound contrast agent. Ultrason Imaging. 2009;31:235–246. doi: 10.1177/016173460903100402. [DOI] [PubMed] [Google Scholar]
- Chen R, Paeng D-G, Lam KH, Zhou Q, Shung KK, Matsuoka N, Humayun MS. In vivo sonothrombolysis of ear marginal vein of rabbits monitored with high-frequency ultrasound needle transducer. J Med Biol Eng. 2013;33:103–110. [PMC free article] [PubMed] [Google Scholar]
- Chen X, Leeman JE, Wang J, Pacella JJ, Villanueva FS. New insights into mechanisms of sonothrombolysis using ultra-high-speed imaging. Ultrasound Med Biol. 2014;40:258–262. doi: 10.1016/j.ultrasmedbio.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Cheng JY, Shaw GJ, Holland CK. In vitro microscopic imaging of enhanced thrombolysis with 120-kHz ultrasound in a human clot model. Acoust Res Lett Online. 2005;6:25–29. doi: 10.1121/1.1815039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JJ, Coussios CC. Spatiotemporal evolution of cavitation dynamics exhibited by flowing micro-bubbles during ultrasound exposure. J Acoust Soc Am. 2012;132:3538. doi: 10.1121/1.4756926. [DOI] [PubMed] [Google Scholar]
- Chuang YH, Cheng PW, Chen SC, Ruan JL, Li PC. Effects of ultrasound-induced inertial cavitation on enzymatic thrombolysis. Ultrason Imaging. 2010;32:81–90. doi: 10.1177/016173461003200202. [DOI] [PubMed] [Google Scholar]
- Church CC. Spontaneous homogeneous nucle-ation, inertial cavitation and the safety of diagnostic ultrasound. Ultrasound Med Biol. 2002;28:1349–1364. doi: 10.1016/s0301-5629(02)00579-3. [DOI] [PubMed] [Google Scholar]
- Church CC, Labuda C, Nightingale K. A theoretical study of inertial cavitation from acoustic radiation force impulse (ARFI) imaging and implications for the mechanical index. Ultrasound Med Biol. 2015;41:472–485. doi: 10.1016/j.ultrasmedbio.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R Boccardi and SYNTHESIS Expansion Investigators. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintas P, Le Traon AP, Larrue V. High rate of recanalization of middle cerebral artery occlusion during 2-MHz transcranial color-coded doppler continuous monitoring without thrombolytic drug. Stroke. 2002;33:626–628. doi: 10.1161/hs0202.103073. [DOI] [PubMed] [Google Scholar]
- Cintas P, Nguyen F, Boneu B, Larrue V. Enhancement of enzymatic fibrinolysis with 2-MHz ultrasound and microbubbles. J Thromb Haemost. 2004;2:1163–1166. doi: 10.1111/j.1538-7836.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D'Onofrio M, Evans DH, Filice C, Greiner L, Jager K, Nd J, Leen E, Lencioni R, Lindsell D, Martegani A, Meairs S, Nolsoe C, Piscaglia F, Ricci P, Seidel G, Skjoldbye B, Solbiati L, Thorelius L, Tranquart F, Weskott HP, Whittingham T. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) – update 2008. Ultraschall Med. 2008;29:28–24. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- Claudon M, Dietrich CF, Choi BI, Cosgrove D, Kudo M, Nolsoe C, Piscaglia F, Wilson SR, Barr RG, Chammas MC, Chaubal NG, Chen MH, Clevert DA, Correas JM, Ding H, Forsberg F, Fowlkes JB, Gibson RN, Goldberg BB, Lassau N, Leen EL, Mattrey RF, Moriyasu F, Solbiati L, Weskott HP, Xu HX World Federation for Ultrasound in Medicine, European Federation of Societies for Ultrasound. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver-update 2012. Ultrasound Med Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Collis J, Manasseh R, Liovic P, Tho P, Ooi A, Petkovic-Duran K, Zhu Y. Cavitation microstreaming and stress fields created by microbubbles. Ultrasonics. 2010;50:273–279. doi: 10.1016/j.ultras.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Commander KW, Prosperetti A. Linear pressure waves in bubbly liquids: comparison between theory and experiments. J Acoust Soc Am. 1989;85:1–15. [Google Scholar]
- Cosgrove D. Ultrasound contrast agents: an overview. Eur J Radiol. 2006;60:324–330. doi: 10.1016/j.ejrad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Harvey C. Clinical uses of micro-bubbles in diagnosis and treatment. Med Biol Eng Comput. 2009;47:813–826. doi: 10.1007/s11517-009-0434-3. [DOI] [PubMed] [Google Scholar]
- Cui H, Zhang T, Yang X. Laser-enhanced cavitation during high intensity focused ultrasound. 2013;102:133702. doi: 10.1063/1.4800780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp WC. Intracranial clot lysis with intravenous microbubbles and transcranial ultrasound in swine. Stroke. 2004;35:2407–2411. doi: 10.1161/01.STR.0000140890.86779.79. [DOI] [PubMed] [Google Scholar]
- Culp WC, Porter TR, McCowan TC, Roberson PK, James CA, Matchett WJ, Moursi M. Microbubble-augmented ultrasound declotting of thrombosed arte-riovenous dialysis grafts in dogs. J Vase Interv Radiol. 2003;14:343–347. doi: 10.1097/01.rvi.0000058409.01661.b4. [DOI] [PubMed] [Google Scholar]
- Culp WC, Flores R, Brown AT, Lowery JD, Roberson PK, Hennings LJ, Woods SD, Hatton JH, Culp BC, Skinner RD, Borrelli MJ. Successful micro-bubble sonothrombolysis without tissue-type plas-minogen activator in a rabbit model of acute ischemic stroke. Stroke. 2011;42:2280–2285. doi: 10.1161/STROKEAHA.110.607150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffertshofer M, Huang Z, Fatar M, Popolo M, Schroeck H, Kuschinsky W, Moskowitz MA, Hennerici MG. Efficacy of sonothrombolysis in a rat model of embolic ischemic stroke. Neurosurgery. 2004;361:115–119. doi: 10.1016/j.neulet.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, Sedlaczek O, Koroshetz WJ, Hennerici MG. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36:1441–1446. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- Damianou C, Hadjisavvas V, Mylonas N, Couppis A, Ioannides K. MRI-guided sonothrombolysis of rabbit carotid artery. J Stroke Cerebrovasc. 2014;23:el13–el21. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Datta S, Coussios C-C, McAdory LE, Tan J, Porter T, De Courten-Myers G, Holland CK. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol. 2006;32:1257–1267. doi: 10.1016/j.ultrasmedbio.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Coussios C-C, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity® as a cavitation nucle-ation agent. Ultrasound Med Biol. 2008;34:1421–1433. doi: 10.1016/j.ultrasmedbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint VM, Crake C, Coussios CC, Stride E. Properties, characteristics and applications of micro-bubbles for sonothrombolysis. Expert Opin Drug Deliv. 2014;11:187–209. doi: 10.1517/17425247.2014.868434. [DOI] [PubMed] [Google Scholar]
- Devcic-Kuhar B, Pfaffenberger S, Grschl M, Kollmann C, Benes E, Gottsauner-Wolf M. In vitro thrombolysis enhanced by standing and travelling ultrasound wave fields. Ultrasound Med Biol. 2002;28:1181–1187. doi: 10.1016/s0301-5629(02)00563-x. [DOI] [PubMed] [Google Scholar]
- Doomernik DE, Schrijver AM, Zeebregts CJ, de Vries JP, Reijnen MM. Advancements in catheter-directed ultrasound-accelerated thrombolysis. J Endovasc Ther. 2011;18:418–434. doi: 10.1583/10-3362.1. [DOI] [PubMed] [Google Scholar]
- Dwedar AZ, Ashour S, Haroun M, El Nasser AA, Moustafa RR, Ibrahim MH, Elsadek A. Sonothrombolysis in acute middle cerebral artery stroke. Neurol India. 2014;62:62–65. doi: 10.4103/0028-3886.128308. [DOI] [PubMed] [Google Scholar]
- Eggers J, Koch B, Meyer K, Konig I, Seidel G. Effect of ultrasound on thrombolysis of middle cerebral artery occlusion. Ann Neurol. 2003;53:797–800. doi: 10.1002/ana.10590. [DOI] [PubMed] [Google Scholar]
- Eggers J, Konig IR, Koch B, Handler G, Seidel G. Sonothrombolysis with transcranial color-coded sonog-raphy and recombinant tissue-type plasminogen activator in acute middle cerebral artery main stem occlusion: results from a randomized study. Stroke. 2008;39:1470–1475. doi: 10.1161/STROKEAHA.107.503870. [DOI] [PubMed] [Google Scholar]
- Elder SA. Cavitation microstreaming. J Acoust Soc Am. 1959;31:54–64. [Google Scholar]
- Engelberger RP, Kucher N. Ultrasound-assisted thrombolysis for acute pulmonary embolism: a systematic review. Eur Heart J. 2014;35:758–764. doi: 10.1093/eurheartj/ehu029. [DOI] [PubMed] [Google Scholar]
- Everbach EC, Francis CW. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound Med Biol. 2000;26:1153–1160. doi: 10.1016/s0301-5629(00)00250-7. [DOI] [PubMed] [Google Scholar]
- Everbach EC, Guarini A. International congress on acoustics montreal. New York, NY: 2013. Jun 2–7, Modeling of microbubbles pushed through clots via acoustic radiation force. [Google Scholar]
- Faez T, Emmer M, Kooiman K, Versluis M, van der Steen A, de Jong N. 20 years of ultrasound contrast agent modeling. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:7–20. doi: 10.1109/TUFFC.2013.2533. [DOI] [PubMed] [Google Scholar]
- Falk E. Coronary thrombosis: pathogenesis and clinical manifestations. Am J Cardiol. 1991;68:B28–B35. doi: 10.1016/0002-9149(91)90382-u. [DOI] [PubMed] [Google Scholar]
- Fardanesh R, Kian L. Nontransmural myocardial infarcion. In: White CS, Haramati LB, Chen JJ-S, Levsky JM, editors. Cardiac imaging. 1st. Oxford: Oxford University Press; 2014. pp. 269–272. [Google Scholar]
- Flynn HG. Physics of acoustic cavitation in liquids. In: Mason WP, editor. Physical acoustics. New York: Academic; 1964. pp. 58–172. [Google Scholar]
- Francis CW, Onundarson PT, Carstensen EL, Blinc A, Meltzer RS, Schwarz K, Marder VJ. Enhancement of fibrinolysis in vitro by ultrasound. J Clin Invest. 1992;90:2063–2068. doi: 10.1172/JCI116088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol. 1995;21:419–124. doi: 10.1016/0301-5629(94)00119-x. [DOI] [PubMed] [Google Scholar]
- Frenkel V, Oberoi J, Stone MJ, Park M, Deng C, Wood BJ, Neeman Z, Home M, 3rd, Li KC. Pulsed high-intensity focused ultrasound enhances thrombolysis in an in vitro model 1. Radiology. 2006;239:86–93. doi: 10.1148/radiol.2391042181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry FJ, Heimburger RF, Gibbons LV, Eggleton RC. Ultrasound for visualization and modification of brain tissue. IEEE Trans Ultrason Ferroelectr Freq Control. 1970;17:165–169. [Google Scholar]
- Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- Furuhata H, Saito O. Comparative study of standing wave reduction methods using random modulation for transcranial ultrasonication. Ultrasound Med Biol. 2013;39:1440–1450. doi: 10.1016/j.ultrasmedbio.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Fuster V. Lewis A. Conner Memorial Lecture. Mechanisms leading to myocardial infarction: insights from studies of vascular biology. Circulation. 1994;90:2126–2146. doi: 10.1161/01.cir.90.4.2126. [DOI] [PubMed] [Google Scholar]
- Gaitan DF, Cram LA, Church CC, Roy RA. Sonoluminescence and bubble dynamics for a single, stable, cavitation bubble. J Acoust Soc Am. 1992;91:3166. [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matcher DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi TA, Turan TN, Virani SS, Wing ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla J, Brekenfeld C, Mordasini P, Schroth G. Mechanical thrombolysis and stenting in acute ischemic stroke. Stroke. 2011;43:280–285. doi: 10.1161/STROKEAHA.111.626903. [DOI] [PubMed] [Google Scholar]
- Greenberg R, Ivancev K, Ouriel K. High frequency ultrasound thrombolysis: phase I results. Am J Cardiol. 1999;84:42. [Google Scholar]
- Greenleaf WJ, Bolander ME, Sarkar G, Goldring MB, Greenleaf JF. Artifical cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound Med Biol. 1998;24:587–595. doi: 10.1016/s0301-5629(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Gregg D. Platelets and cardiovascular disease. Circulation. 2003;108:88e–90e. doi: 10.1161/01.CIR.0000086897.15588.4B. [DOI] [PubMed] [Google Scholar]
- Hagisawa K, Nishioka T, Suzuki R, Maruyama K, Takase B, Ishihara M, Kurita A, Yoshimoto N, Nishida Y, Iida K, Luo H, Siegel RJ. Thrombus-targeted perfluorocarbon-containing liposomal bubbles for enhancement of ultrasonic thrombolysis: in vitro and in vivo study. J Thromb Haemost. 2013;11:1565–1573. doi: 10.1111/jth.12321. [DOI] [PubMed] [Google Scholar]
- Harris GR. FDA regulation of clinical high intensity focused ultrasound (HIFU) devices. 31st annual international conference of the IEEE EMBS. IEEE; Minneapolis. 2009. pp. 145–148. [DOI] [PubMed] [Google Scholar]
- Haukeland University Hospital The Norwegian Sonothrombolysis in Acute Stroke Study. [Accessed 19 May 2014];clinical-trials.gov. http://clinicaltrials.gov/show/NCT01949961.
- Haworth KJ, Mast TD, Radhakrishnan K, Burgess MT, Kopecheck JA, Huang SL, McPherson DD, Holland CK. Passive imaging with pulsed ultrasound insonations. J Acoust Soc Am. 2012;132:544. doi: 10.1121/1.4728230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert E, Balibar S, Caupin F. Cavitation pressure in water. Phys Rev E. 2006;74:041603. doi: 10.1103/PhysRevE.74.041603. [DOI] [PubMed] [Google Scholar]
- Higgins DL, Bennett RM. Tissue plasminogen-activator – the biochemistry and pharmacology of variants produced by mutagenesis. Ann Rev Pharmacol. 1990;30:91–121. doi: 10.1146/annurev.pa.30.040190.000515. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. Circulation. 1996;93:2212–2245. doi: 10.1161/01.cir.93.12.2212. [DOI] [PubMed] [Google Scholar]
- Hitchcock KE, Caudell DN, Sutton JT, Klegerman ME, Vela D, Pyne-Geithman GJ, Abruzzo T, Cyr PE, Geng YJ, McPherson DD, Holland CK. Ultrasound-enhanced delivery of targeted echogenic liposomes in a novel ex vivo mouse aorta model. J Control Release. 2010;144:288–295. doi: 10.1016/j.jconrel.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock KE, Ivancevich NM, Haworth KJ, Caudell Stamper DN, Vela DC, Sutton JT, Pyne-Geithmann GJ, Holland CK. Ultrasound-enhanced rt-PA thrombolysis in an ex vivo porcine carotid artery model. Ultrasound Med Biol. 2011;37:1240–1251. doi: 10.1016/j.ultrasmedbio.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg JC. Neutrophil kinetics and lung injury. Physiol Rev. 1987;67:1249–1295. doi: 10.1152/physrev.1987.67.4.1249. [DOI] [PubMed] [Google Scholar]
- Holland CK, Apfel RE. Improved theory for the prediction of microcavitation thresholds. IEEE Trans Ultras Ferroelectr Freq Control. 1989;36:204–208. doi: 10.1109/58.19152. [DOI] [PubMed] [Google Scholar]
- Holland CK, Vaidya SS, Datta S, Coussios CC, Shaw GJ. Ultrasound-enhanced tissue plasminogen activator thrombolysis in an in vitro porcine clot model. Thromb Res. 2008;121:663–673. doi: 10.1016/j.thromres.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher T, Fisher DJ, Ahadi G, Voie A. Introduction of a rabbit carotid artery model for sonothrombolysis research. Transl Stroke Res. 2012;3:397–407. doi: 10.1007/s12975-012-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher T, Ahadi G, Fisher D, Zadicario E, Voie A. MR-guided focused ultrasound for acute stroke: a rabbit model. Stroke. 2013;44:S58–S60. doi: 10.1161/STROKEAHA.111.000688. [DOI] [PubMed] [Google Scholar]
- Hölscher T, Raman R, Fisher DJ, Ahadi G, Zadicario E, Voie A. Effects of varying duty cycle and pulse width on high-intensity focused ultrasound (HIFU)-induced transcranial thrombolysis. J Therap Ultrasound. 2013;1:18–22. doi: 10.1186/2050-5736-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzfuss J, Rüggeberg M, Billo A. Shock wave emissions of a sonoluminescing bubble. Phys Rev Lett. 1998;81:5434–5437. [Google Scholar]
- Hossmann KA. Experimental models for the investigation of brain ischemia. Cardiovasc Res. 1998;39:106–120. doi: 10.1016/s0008-6363(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Hua X, Zhou L, Liu P, He Y, Tan K, Chen Q, Gao Y, Gao Y. In vivo thrombolysis with targeted microbubbles loading tissue plasminogen activator in a rabbit femoral artery thrombus model. J Thromb Thrombolysis. 2014;38:57–64. doi: 10.1007/s11239-014-1071-8. [DOI] [PubMed] [Google Scholar]
- Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, American Heart Association Council on Peripheral Vascular Disease, American Hear Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Saver JL, Adams HP, Brino A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi A, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Peripheral Vascular Disease, Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- Jensen CR, Ritchie RW, Gyöngy M, Collin JR, Leslie T, Coussios CC. Spatiotemporal monitoring of high-intensity focused ultrasound therapy with passive acoustic mapping. Radiology. 2012;262:252–261. doi: 10.1148/radiol.11110670. [DOI] [PubMed] [Google Scholar]
- Juffermans LJM, van Dijk A, Jongenelen CAM, Drukarch B, Reijerkerk A, de Vries HE, Kamp O, Musters RJ. Ultrasound and microbubble-induced intra- and intercellular bioeffects in primary endothelial cells. Ultrasound Med Biol. 2009;35:1917–1927. doi: 10.1016/j.ultrasmedbio.2009.06.1091. [DOI] [PubMed] [Google Scholar]
- Kakkar VV, Flanc C, Howe CT, Clarke MB. Natural history of postoperative deep-vein thrombosis. Lancet. 1969;2:230–232. doi: 10.1016/s0140-6736(69)90002-6. [DOI] [PubMed] [Google Scholar]
- Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:122–130. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- Kim JS, Leeman JE, Kagemann L, Yu FT, Chen X, Pacella JJ, Schuman JS, Villanueva FS, Kim K. Volumetric quantification of in vitrosonothrombolysis with microbubbles using high-resolution optical coherence tomography. J Biomed Opt. 2012;17:0705021. doi: 10.1117/1.JBO.17.7.070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Iijima S, Kobayashi K, Furuhata H. Evaluation of the thrombolytic effect of tissue-type plas-minogen activator with ultrasonic irradiation: in vitro experiment involving assay of the fibrin degradation products from the clot. Biol Pharm Bull. 1994;17:126–130. doi: 10.1248/bpb.17.126. [DOI] [PubMed] [Google Scholar]
- Kondo I, Mizushige K, Ueda T, Masugata H, Ohmori K, Matsuo H. Histological observations and the process of ultrasound contrast agent enhancement of tissue plasminogen activator thrombolysis with ultrasound exposure. Jap Circ J (English Edition) 1999;63:478–484. doi: 10.1253/jcj.63.478. [DOI] [PubMed] [Google Scholar]
- Kucher N, Boekstegers P, Müller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, Tebbe U, Horstkotte J, Muller R, Blessing E, Greid M, Lange P, Hoffmann RT, Werth S, Barmeyer A, Hartel D, Greunwald H, Empen K, Baumgartner I. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2013;129:479–486. doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- la Santa Creu i Sant Pau de FI de R de L Sonothrombolysis Potentiated by Microbubbles for Acute Ischemic Stroke. [Accessed 19 May 2014];clinicaltrials.gov. http://clinicaltrials.gov/ct2/show/NCT01678495?term=sonothrombolysis&rank=2.
- Labreuche J, Deplanque D, Touboul PJ, Bruckert E, Amarenco P. Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis: systematic review and meta-regression analysis. Atherosclerosis. 2010;212:9–15. doi: 10.1016/j.atherosclerosis.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Lauer CG, Burge R, Tang DB, Bass BG, Gomez ER, Alving BM. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation. 1992;86:1257–1264. doi: 10.1161/01.cir.86.4.1257. [DOI] [PubMed] [Google Scholar]
- Lauterborn W, Kurz T. Physics of bubble oscillations. Rep Prog Phys. 2010;73:106501. [Google Scholar]
- Leeman JE, Kim JS, Yu FTH, Chen X, Kim K, Wang J, Chen X, Villanueva FS, Pacella JJ. Effect of acoustic conditions on microbubble-mediate microvascular sonothrombolysis. Ultrasound Med Biol. 2012;38:1589–1598. doi: 10.1016/j.ultrasmedbio.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Leighton TG. The acoustic bubble. London: Academic; 1994. [Google Scholar]
- Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, Zheng DD, Abolian AM, Kim D, Ali LK, Shah SH, Towfighi A, Ovbiagele B, Kidwell CS, Tateshima S, Jahan R, Duckwiler GR, Vinuela F, Salamon N, Villablanca JP, Vinters HV, Marder VJ, Saver JL. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthill SJ. Acoustic streaming. J Sound Vib. 1978;61:391–418. [Google Scholar]
- Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, Ovbiagele B, Vinuela F, Duckwiler G, Jahan R, Vespa PM, Selco S, Rajajee V, Kim D, Sanossian N, Saver JL. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- Markel A. Origin and natural history of deep vein thrombosis of the legs. Sem Vase Med. 2005;5:65–74. doi: 10.1055/s-2005-871743. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers C, Fat DM, Boerma JT. The global burden of disease: 2004 update. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- Maxwell AD, Cain CA, Duryea AP, Yuan L, Gurm HS, Xu Z. Noninvasive thrombolysis using pulsed ultrasound cavitation therapy – histotripsy. Ultrasound Med Biol. 2009;35:1982–1994. doi: 10.1016/j.ultrasmedbio.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Owens G, Gurm HS, Ives K, Myers DD, Jr, Xu Z. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. J Vase Interv Radiol. 2011;22:369–377. doi: 10.1016/j.jvir.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AD, Cain CA, Hall TL, Fowlkes JB, Xu Z. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med Biol. 2013;39:449–465. doi: 10.1016/j.ultrasmedbio.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med Biol. 2008;34:834–840. doi: 10.1016/j.ultrasmedbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JM, Holland CK, Lindsell CJ, Shaw GJ. Duty cycle dependence of ultrasound enhanced thrombolysis in a human clot model. Ultrasound Med Biol. 2007;33:576–583. doi: 10.1016/j.ultrasmedbio.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Dou C. Membrane damage thresholds for 1- to 10-MHz pulsed ultrasound exposure of phagocytic cells loaded with contrast agent gas bodies in vitro. Ultrasound Med Biol. 2004;30:973–977. doi: 10.1016/j.ultrasmedbio.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Miller DL, Dou C, Wiggins RC. Frequency dependence of kidney injury induced by contrast-aided diagnostic ultrasound in rats. Ultrasound Med Biol. 2008;34:1678–1687. doi: 10.1016/j.ultrasmedbio.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr G, Ferguson G, Khan M, Malloy D, Watts R, Benoit R, Weir B. Intraventricular hemorrhage from ruptured aneurysm: retrospective analysis of 91 cases. J Neurosurg. 1983;58:482–487. doi: 10.3171/jns.1983.58.4.0482. [DOI] [PubMed] [Google Scholar]
- Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, Alvarez-Sabin J. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–429. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- Molina CA, Barreto AD, Tsivgoulis G, Sierzenski P, Malkoff MD, Rubiera M, Gonzales N, Mikulik R, Pate G, Ostrem J, Singleton W, Manvelian G, Unger EC, Grotta JC, Schellinger PD, Alexandrov AV. Transcranial ultrasound in clinical sonothrombolysis (TUCSON) trial. Ann Neurol. 2009;66:28–38. doi: 10.1002/ana.21723. [DOI] [PubMed] [Google Scholar]
- Monteith SJ, Harnof S, Medel R, Popp B, Wintermark M, Lopes MB, Kassell NF, Elias WJ, Snell J, Eames M, Zadicario E, Moldovan K, Sheehan J. Minimally invasive treatment of intracerebral hemorrhage with magnetic resonance-guided focused ultrasound. J Neurosurg. 2013;118:1035–1045. doi: 10.3171/2012.12.JNS121095. [DOI] [PubMed] [Google Scholar]
- Mousa SA. Methods in molecular biology. Totowa: Humana Press; 2010. In vivo models for the evaluation of antithrombotics and thrombolytics; pp. 29–107. [DOI] [PubMed] [Google Scholar]
- Nahirnyak V, Mast TD, Holland CK. Ultrasound-induced thermal elevation in clotted blood and cranial bone. Ultrasound Med Biol. 2007;33:1285–1295. doi: 10.1016/j.ultrasmedbio.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelmann M, Ritschel N, Doenges S, Langheirich AC, Acker T, Reuter P, Yeniguen M, Pukropski J, Kaps M, Mueller C, Bachmann G, Gerriets T. Combined contrast-enhanced ultrasound and rt-PA treatment is safe and improves impaired microcirculation after reperfusion of middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2010;30:1712–1720. doi: 10.1038/jcbfm.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell DW, Shah MM, Wilcox R, Hansmann DR, Melnychuk E, Muschelli J, Hanley DF. Minimally invasive evacuation of spontaneous intracerebral hemorrhage using sonothrombolysis. J Neurosurg. 2011;115:592–601. doi: 10.3171/2011.5.JNS10505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novokhatny VV, Jesmok GJ, Landskroner KA, Vander VJ, Zimmerman TP. Locally delivered plasmin: why should it be superior to plasminogen activators for direct thrombolysis? Trends Pharmacol Sci. 2004;25:72–75. doi: 10.1016/j.tips.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Nyborg WL. Acoustic streaming due to attenuated plane waves. J Acoust Soc Am. 1953;25:68. [Google Scholar]
- Nyborg WL. Acoustic streaming. In: Mason WP, editor. Physical acoustics. Waltham: Academic; 1965. pp. 265–331. [Google Scholar]
- Nyborg WL, Miller DL. Biophysical implications of bubble dynamics. Appl Sci Res. 1982;38:17–24. [Google Scholar]
- Pajek D, Hynynen K. The design of a focused ultrasound transducer array for the treatment of stroke: a simulation study. Phys Med Biol. 2012;57:4951–4968. doi: 10.1088/0031-9155/57/15/4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajek D, Burgess A, Huang Y, Hynynen K. High-intensity focused ultrasound sonothrombolysis: The use of perfluorocarbon droplets to achieve clot lysis at reduced acoustic power. Ultrasound Med Biol. 2014;40:2131–2161. doi: 10.1016/j.ultrasmedbio.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasceri V, Andreotti F, Maseri A. Clinical markers of thrombolytic success. Eur Heart J. 1996;17(Suppl E):35–41. doi: 10.1093/eurheartj/17.suppl_e.35. [DOI] [PubMed] [Google Scholar]
- Perler B. Thrombolytic therapies: the current state of affairs. J Endovasc Ther. 2005;12:224–232. doi: 10.1583/04-1438.1. [DOI] [PubMed] [Google Scholar]
- Perren F, Loulidi J, Poglia D, Landis T, Sztajzel R. Microbubble potentiated transcranial duplex ultrasound enhances IV thrombolysis in acute stroke. J Thromb Thrombolysis. 2007;25:219–223. doi: 10.1007/s11239-007-0044-6. [DOI] [PubMed] [Google Scholar]
- Petit B, Gaud E, Colevret D, Arditi M, Yan F, Tranquart F, Allemann E. In vitro sonothromboly sis of human blood clots with BR38 microbubbles. Ultrasound Med Biol. 2012a;38:1222–1233. doi: 10.1016/j.ultrasmedbio.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Petit B, Yan F, Tranquart F, Allemann E. Microbubbles and ultrasound-mediated thrombolysis: a review of recent in vitro studies. J Drug Deliv Sci Tec. 2012b;22:381–392. [Google Scholar]
- Pfaffenberger S, Devcic-Kuhar B, El-Rabadi K, Groschl M, Speidl WS, Weiss TW, Huber K, Benes E, Maurer G, Wojta J, Gottsauner-Wolf M. 2MHz ultra sound enhances t-PA-mediated thrombolysis: com parison of continuous versus pulsed ultrasound and standing versus travelling acoustic waves. Thromb Haemost. 2003;89:583–589. [PubMed] [Google Scholar]
- Porter TR, Kricsfeld D, Lof J, Everbach EC, Xie F. Effectiveness of transcranial and transthoracic ultrasound and microbubbles in dissolving intravascular thrombi. J Ultrasound Med. 2001;20:1313–1325. doi: 10.7863/jum.2001.20.12.1313. [DOI] [PubMed] [Google Scholar]
- Prokop AF, Soltani A, Roy RA. Cavitational mechanisms in ultrasound-accelerated fibrinolysis. Ultrasound Med Biol. 2007;33:924–933. doi: 10.1016/j.ultrasmedbio.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Puetz V, Dzialowski I, Hill MD, Subramaniam S, Sylaja PN, Krol A, O'Reilly C, Hudon ME, Hu WY, Courts SB, Barber PA, Watson T, Roy J, Demchuk AM Calgary CTA Study Group. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke. 2008;3:230–236. doi: 10.1111/j.1747-4949.2008.00221.x. [DOI] [PubMed] [Google Scholar]
- Raabe RA. Ultrasound facilitated thrombolysis in treating DVT. Endovascular Today. 2006;4:1–14. [Google Scholar]
- Radhakrishnan K, Bader KB, Haworth KJ, Kopechek JA, Raymond JL, Huang SL, McPherson DD, Holland CK. Relationship between cavitation and loss of echogenicity from ultrasound contrast agents. Phys Med Biol. 2013;58:6541–6563. doi: 10.1088/0031-9155/58/18/6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim A, Taylor SL, Bush NL, ter Haar GR, Bamber JC, Porter CD. Physical parameters affecting ultra-sound/microbubble-mediated gene delivery efficiency in vitro. Ultrasound Med Biol. 2006;32:1269–1279. doi: 10.1016/j.ultrasmedbio.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Rayleigh L. On the pressure developed in a liquid during the collapse of a spherical cavity. Philos Mag. 1917;34:94–98. [Google Scholar]
- Reuning U, Magdolen V, Hapke S, Schmitt M. Molecular and functional interdependence of the urokinase-type plasminogen activator system with integrins. Biol Chem. 2003;384:1119–1131. doi: 10.1515/BC.2003.125. [DOI] [PubMed] [Google Scholar]
- Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- Ricci S, Dinia L, Del Sette M, Anzola P, Mazzoli T, Cenciarelli S, Gandolfo C. Sonothrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2012;10:CD008348. doi: 10.1002/14651858.CD008348.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesz P, Kondo I. Free-radical formation induced by ultrasound and its biological implications. Free Radical Bio Med. 1992;13:247–270. doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- Roessler FC, Ohlrich M, Marxsen JH, Stellmacher F, Sprenger A, Dempfle CE, Seidel G. The platelet-rich plasma clot: a standardized in-vitro clot formation protocol for investigations of sonothrombolysis under physiological flows. Blood Coagul Fibrin. 2011;22:407–415. doi: 10.1097/MBC.0b013e3283468a60. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y American Heart Association Statistics Committee and Stroke Statistics Subcommitee. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–el71. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- Rosenschein U, Furman V, Kerner E, Fabian I, Bernheim J, Eshel Y. Ultrasound imaging-guided nonin-vasive ultrasound thrombolysis: preclinical results. Circulation. 2000;102:238–245. doi: 10.1161/01.cir.102.2.238. [DOI] [PubMed] [Google Scholar]
- Roy RA, Atchley AA, Cram LA, Fowlkes JB, Reidy JJ. A precise technique for the measurement of acoustic cavitation thresholds and some preliminary results. J Acoust Soc Am. 1985;78:1799–1805. doi: 10.1121/1.392767. [DOI] [PubMed] [Google Scholar]
- Sakharov DV, Hekkenberg RT, Rijken DC. Acceleration of fibrinolysis by high-frequency ultrasound: the contribution of acoustic streaming and temperature rise. Thromb Res. 2000;100:333–340. doi: 10.1016/s0049-3848(00)00319-4. [DOI] [PubMed] [Google Scholar]
- Salgaonkar VA, Datta S, Holland CK, Mast TD. Passive cavitation imaging with ultrasound arrays. J Acoust Soc Am. 2009;126:3071. doi: 10.1121/1.3238260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saqqur M, Tsivgoulis G, Nicoli F, Skoloudik D, Vk S, Larrue V, Eggers J, Perren F, Charalampidis P, Stories D, Shuaib A, Alexandrov AV. The role of sonolysis and sonothrombolysis in acute ischemic stroke: a systematic review and meta-analysis of randomized controlled trials and case-control studies. J Neuroimaging. 2013;24:209–220. doi: 10.1111/jon.12026. [DOI] [PubMed] [Google Scholar]
- Saric M, Kronzon I. Aortic atherosclerosis and embolic events. Curr Cardiol Rep. 2012;14:342–349. doi: 10.1007/s11886-012-0261-2. [DOI] [PubMed] [Google Scholar]
- Sarkar K, Katiyar A, Jain P. Growth and dissolution of an encapsulated contrast microbubble: effects of encapsulation permeability. Ultrasound Med Biol. 2009;35:1385–1396. doi: 10.1016/j.ultrasmedbio.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GJ, Bavani N, Dhamija A, Lindsell CJ. Effect of mild hypothermia on the thrombolytic efficacy of 120 kHz ultrasound enhanced thrombolysis in an in-vitro human clot model. Thromb Res. 2006;117:603–608. doi: 10.1016/j.thromres.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Shaw GJ, Dhamija A, Bavani N, Wagner KR, Holland CK. Arrhenius temperature dependence of in vitro tissue plasminogen activator thrombolysis. Phys Med Biol. 2007;52:2953–2967. doi: 10.1088/0031-9155/52/11/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GJ, Meunier JM, Lindsell CJ, Holland CK. Tissue plasminogen activator concentration dependence of 120 kHz ultrasound-enhanced thrombolysis. Ultrasound Med Biol. 2008;34:1783–1792. doi: 10.1016/j.ultrasmedbio.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GJ, Meunier JM, Huang SL, Lindsell CJ, McPherson DD, Holland CK. Ultrasound-enhanced thrombolysis with tPA-loadedechogenicliposomes. Thromb Res. 2009;124:306–310. doi: 10.1016/j.thromres.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WT, Porter TR, Vignon F, Powers JE, Shunji G, Jinjin L, Feng X, Drvol L, Lof J, Everbach EC. 2011 IEEE International Ultrasonics Symposium (IUS) Orlando, FL: 2011. Investigation of image-guided sonothrombolysis in a porcine acute ischemic stroke model; pp. 5–8. [Google Scholar]
- Shimizu J, Fukuda T, Abe T, Ogihara M, Kubota J, Sasaki A, Azuma T, Sasaki K, Shimizu K, Oishi T, Umemura S, Furuhata H. Ultrasound safety with midfrequency transcranial sonothrombolysis: preliminary study on normal macca monkey brain. Ultrasound Med Biol. 2012;38:1040–1050. doi: 10.1016/j.ultrasmedbio.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O, Montalescot G, Wisel JW. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 2011;57:1359–1367. doi: 10.1016/j.jacc.2010.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoloudik D, Bar M, Skoda O, Vaclavik D, Hradilek P, Allendoerfer J, Sanak D, Hlustik P, Langova K, Herzig R, Kanovsky P. Safety and efficacy of the sonographic acceleration of the middle cerebral artery recanalization: results of the pilot thrombotripsy study. Ultrasound Med Biol. 2008;34:1775–1782. doi: 10.1016/j.ultrasmedbio.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, Lutsep HL, Rymer MM, Higashida RT, Starkman S, Gobin YP, MERCI Multi Investigators. Frei D, Grobelny T, Hellinger F, Huddle D, Kidwell C, Koroshetz W, Marks M, Nesbit G, Silverman IE. Mechanical thrombectomy for acute ischemic stroke: final results of the multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- Smith DAB, Vaidya SS, Kopechek JA, Huang SL, Klegerman ME, McPherson DD, Holland CK. Ultrasound-triggered release of recombinant tissue-type plasminogen activator from echogenic liposomes. Ultrasound Med Biol. 2010;36:145–157. doi: 10.1016/j.ultrasmedbio.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani A. Application of cavitation promoting surfaces in management of acute ischemic stroke. Ultrasonics. 2013;53:580–587. doi: 10.1016/j.ultras.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani A, Singhal R, Garcia JL, Raju NR. Absence of biological damage from prolonged exposure to intravascular ultrasound: a swine model. Ultrasonics. 2007;46:60–67. doi: 10.1016/j.ultras.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Stone MJ, Frenkel V, Dromi S, Thomas P, Lewis RP, Li KC, Home M, 3rd, Wood BJ. Pulsed-high intensity focused ultrasound enhanced tPA mediated thrombolysis in a novel in vivo clot model, a pilot study. Thromb Res. 2007;121:193–202. doi: 10.1016/j.thromres.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stride E, Saffari N. Microbubble ultrasound contrast agents: a review. Proc Inst Mech Eng H. 2003;217:429–447. doi: 10.1243/09544110360729072. [DOI] [PubMed] [Google Scholar]
- Stride EP, Coussios CC, Wells PNT. Cavitation and contrast: the use of bubbles in ultrasound imaging and therapy. Proc Inst Mech Eng H. 2009;224:171–191. doi: 10.1243/09544119JEIM622. [DOI] [PubMed] [Google Scholar]
- Suchkova V, Siddiqi FN, Carstensen EL, Dalecki D, Child S, Francis CW. Enhancement of fibrinolysis with 40-kHz ultrasound. Circulation. 1998;98:1030–1035. doi: 10.1161/01.cir.98.10.1030. [DOI] [PubMed] [Google Scholar]
- Sutton JT, Haworth KJ, Pyne-Geithman G, Holland CK. Ultrasound-mediated drug delivery for cardiovascular disease. Expert Opin Drug Deliv. 2013a;10:573–592. doi: 10.1517/17425247.2013.772578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton JT, Ivancevich NM, Perrin SR, Jr, Vela DC, Holland DC. Clot retraction affects the extent of ultrasound-enhanced thrombolysis in an ex vivo porcine thrombosis model. Ultrasound Med Biol. 2013b;39:813–824. doi: 10.1016/j.ultrasmedbio.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K, Tachibana S. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation. 1995;92:1148–1150. doi: 10.1161/01.cir.92.5.1148. [DOI] [PubMed] [Google Scholar]
- Tan KT, Lip GYH. Red vs white thrombi: treating the right clot is crucial. Arch Intern Med. 2003;163:2534–2535. doi: 10.1001/archinte.163.20.2534-b. author reply 2535. [DOI] [PubMed] [Google Scholar]
- Tang SC, Clement GT. Standing-wave suppression for transcranial ultrasound by random modulation. IEEE Trans Biomed Eng. 2010;57:203–205. doi: 10.1109/TBME.2009.2028653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II study. Stroke. 2007;38:2127–2135. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- The Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland DC, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res. 2007;119:777–784. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgut T, Bates ER. Acute transmural myocardial infarction. Curr Treat Options Cardiovasc Med. 2000;2:13–18. doi: 10.1007/s11936-000-0024-z. [DOI] [PubMed] [Google Scholar]
- Turi ZG, Goldberg S, Little John JK, Vander Ark C, Shadoff N, Karlsberg R, Williams J, Butman S, Stadius ML, Wise K. Dose-related efficacy and bleeding complications of double-chain tissue plas-minogen activator in acute myocardial infarction. Am J Cardiol. 1993;71:1009–1014. doi: 10.1016/0002-9149(93)90564-s. [DOI] [PubMed] [Google Scholar]
- Unger EC, McCreery TP, Sweitzer RH, et al. In vitro studies of a new thrombus-specific ultrasound contrast agent. Am J Cardiol. 1998;81:58G–61G. doi: 10.1016/s0002-9149(98)00055-1. [DOI] [PubMed] [Google Scholar]
- Verbeuren TJ. Experimental models of thrombosis and atherosclerosis. Therapie. 2006;61:379–387. doi: 10.2515/therapie:2006069. [DOI] [PubMed] [Google Scholar]
- Vignon F, Shi WT, Powers JE, Everbach EC, Liu J, Gao S, Xie F, Porter TR. Microbubble cavitation imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:661–670. doi: 10.1109/TUFFC.2013.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola F, Kramer MD, Lawrence MB, Oberhauser JP, Walker WF. Sonorheometry: a noncontact method for the dynamic assessment of thrombosis. Ann Biomed Eng. 2004;32:696–705. doi: 10.1023/b:abme.0000030235.72255.df. [DOI] [PubMed] [Google Scholar]
- Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009;373:155–166. doi: 10.1016/S0140-6736(09)60040-4. [DOI] [PubMed] [Google Scholar]
- Weisel JW, Litvinov RI. The biochemical and physical process of fibrinolysis and effects of clot structure and stability on the lysis rate. Cardiovasc Hematol Agents Med Chem. 2008;6:161–180. doi: 10.2174/187152508784871963. [DOI] [PubMed] [Google Scholar]
- Weiss HL, Selvaraj P, Okita K, Matsumoto Y, Voie A, Hoelscher T, Szeri AJ. Mechanical clot damage from cavitation during sonothrombolysis. J Acoust Soc Am. 2013;133:3159. doi: 10.1121/1.4795774. [DOI] [PubMed] [Google Scholar]
- Westermark S, Wiksell H, Elmqvist H, Hultenby K, Berglund H. Effect of externally applied focused acoustic energy on clot disruption in vitro. Clin Sci. 1999;97:67–71. [PubMed] [Google Scholar]
- White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–584. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- Wijnhoud AD, Franckena M, van der Lugt A, Koudstaal PJ, Dippel ED. Inadequate acoustical temporal bone window in patients with a transient ischemic attack or minor stroke: role of skull thickness and bone density. Ultrasound Med Biol. 2008;34:923–929. doi: 10.1016/j.ultrasmedbio.2007.11.022. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: 2011. [Google Scholar]
- Wright C, Hynynen K, Goertz D. In vitro and in vivo high-intensity focused ultrasound thrombolysis. Invest Radiol. 2012a;47:217–225. doi: 10.1097/RLI.0b013e31823cc75c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CC, Hynynen K, Goertz DE. Pulsed focused ultrasound-induced displacements in confined in vitro blood clots. IEEE Trans Biomed Eng. 2012b;59:842–851. doi: 10.1109/TBME.2011.2180904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xie F, Kumar T, Liu J, Lof J, Shi W, Everbach EC, Porter ER. Improved sonothrombolysis from a modified diagnostic transducer delivering impulses containing a longer pulse duration. Ultrasound Med Biol. 2014;40:1545–1553. doi: 10.1016/j.ultrasmedbio.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Tsutsui JM, Lof J, Unger EC, Johanning J, Culp WC, Matsunaga T, Porter TR. Effectiveness of lipid microbubbles and ultrasound in declotting thrombosis. Ultrasound Med Biol. 2005;31:979–985. doi: 10.1016/j.ultrasmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Xie F, Lof J, Everbach C, He A, Bennet RM, Matsunaga T, Johanning J, Porter TR. Treatment of acute intravascular thrombi with diagnostic ultrasound and intravenous microbubbles. J Am Coll Cardiol. 2009;2:511–518. doi: 10.1016/j.jcmg.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Xie F, Everbach EC, Gao S, Drvol LK, Shi WT, Vignon F, Powers JE, Lof J, Porter TR. Effects of attenuation and thrombus age on the success of ultrasound and microbubble-mediated thrombus dissolution. Ultrasound Med Biol. 2011;37:280–288. doi: 10.1016/j.ultrasmedbio.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Gao S, Wu J, Lof J, Radio S, Vignon F, Shi W, Powers J, Unger E, Everbach EC, Liu J, Porter TR. Diagnostic ultrasound induced inertial cavitation to non-invasively restore coronary and microvascular flow in acute myocardial infarction. PLoS One. 2013;8:e69780. doi: 10.1371/journal.pone.0069780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Ludomirsky A, Eun LY, Hall TL, Tran BC, Fowlkes JB, Cain CA. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:726–736. doi: 10.1109/tuffc.2004.1308731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Ohtsuka H, Arimura N, Sonoda S, Kato C, Ushimaru K, Hara N, Tachibana K, Sakamoto T. Sonothrombolysis for intraocular fibrin formation in an animal model. Ultrasound Med Biol. 2009;35:1845–1853. doi: 10.1016/j.ultrasmedbio.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Young FR. Sonoluminescence. Boca Raton: CRC Press; 2005. [Google Scholar]
- Yount DE. Skins of varying permeability: a stabilization mechanism for gas cavitation nuclei. J Acoust Soc Am. 1979;65:1429. [Google Scholar]