Abstract
BACKGROUND
Regorafenib is an orally administered multikinase inhibitor that has been approved for patients with chemotherapy-refractory metastatic colorectal cancer (mCRC). Even though regorafenib significantly improved survival in two international phase 3 trials (CORRECT and CONCUR), a high rate of treatment-related toxic effects and dose modifications were observed with a modest benefit. The aim of this study was to provide information concerning the efficacy, safety, and cost of regorafenib in patients with mCRC in clinical practice.
MATERIAL AND METHODS
We retrospectively reviewed patients treated with regorafenib monotherapy for unresectable mCRC in five Franche-Comté cancer hospitals (France). The primary end point was overall survival. Secondary end points were safety and descriptive cost analyses of patients treated with regorafenib in clinical practice. Another aim of this study was to assess the impact of regorafenib prescription on the risk of hospitalization in real-life practice.
RESULTS
From January 2014 to August 2014, 29 consecutive patients were enrolled. Patients were heavily pretreated and were refractory to standard chemotherapies. The primary tumor sites were the colon and the rectum for 55% and 45% of patients, respectively. Fifteen patients (51%) harbored an RAS mutation. Eastern Cooperative Oncology Group – Performance Status (PS) was 0–1 for 86% of patients and 2 for 14% of patients. Nineteen patients (66%) initially received reduced doses of 120 or 80 mg/day. The median duration of treatment was 2.5 months (range, 0.13–11.4 months). Treatment-related adverse events occurred in 86% of patients. The most frequent adverse events of any grade were fatigue (35%), diarrhea (20%), and hand–foot skin reaction (20%). Grade 3 or 4 treatment-related adverse events occurred in 10 patients (35%). Three patients (10%) were admitted to hospital due to drug-related severe adverse events. The mean cost of patient management with regorafenib for the duration of treatment was 9908 ± 8191€, and median cost was 7917€ (Interquartile range (IQR) 4469-13,042). The median overall survival was six months (95% confidence interval, five to eight months).
CONCLUSIONS
The safety and efficacy of regorafenib in heavily pretreated mCRC patients was comparable, in our study, to prospective and retrospective trials. Toxic effects were mostly manageable in an outpatient setting. Regorafenib itself represented the most important (93%) part of supported costs. Even though most side effects were manageable in an outpatient setting, severe adverse events occurred from hospitalization in 10% of patients. These data should be confirmed in a larger real-life-based cohort. Identification of predictive biomarkers is needed for mCRC patient selection for regorafenib treatment.
Keywords: metastatic colorectal cancer, regorafenib, cost, efficacy, safety
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world and the fourth leading cause of cancer death.1 In France, 42,000 patients are newly diagnosed with CRC each year.2 Metastasis often occurs in patients with CRC, and the liver is the most frequent site: 10%–25% of patients have hepatic metastases at diagnosis, and 20% of patients initially diagnosed with a localized CRC will subsequently develop liver lesions.3–5 For metastatic or unresectable CRC, standard first- and second-line treatments involve a combination of cytotoxic chemotherapies (eg, 5-fluorouracil, oxaliplatin, and irinotecan)6,7 and molecular targeted agents (eg, bevacizumab, aflibercept, cetuximab, and panitumumab),8–11 which can help to improve survival. These combinations have improved the progression-free survival and overall survival (OS), with a steady increase in median survival in the last two decades reaching to approximately 30 months in more recent trials.12,13 However, many patients see their disease progress after the guideline-recommended standard regimens, while maintaining a good performance status. Regorafenib, an oral multikinase inhibitor, which targets angiogenic, stromal, and oncogenic receptor tyrosine kinases, is approved for the treatment of these metastatic colorectal cancer (mCRC) patients previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, anti-vascular endothelial growth factor (VEGF) therapy, and an anti-epidermal growth factor receptor (EGFR) therapy in KRAS wild-type patients.14
The CORRECT randomized clinical trial, a multinational, multicenter, randomized, placebo-controlled, phase 3 trial, improved the median OS in the regorafenib group, 6.4 months, versus 5.0 months (HR, 0.77; 95% confidence interval [CI], 0.64–0.94, one-sided P = 0.0052).15 This impact of regorafenib for OS improvement was also demonstrated in a second multinational multicenter randomized phase 3 trial, named CONCUR, conducted in 204 Asian patients. A significant prolonged OS value was observed with regorafenib treatment, in comparison to the placebo (HR, 0.55; 95% CI, 0.44–0.77, one-sided P < 0.001), with a median OS of 8.8 months in the regorafenib-treated group versus 6.3 months in the placebo group.16 However, grade 3 or 4 treatment-related adverse events occurred in 54% of the patients assigned to regorafenib treatment in the CONCUR and CORRECT trials. It is important to assess the potential clinical benefit of regorafenib while taking into consideration the clinical impact of its toxicities. Particularly, the impact of such toxicities on the hospitalization rate should be reported in order to provide more evidence that regorafenib is a treatment option compatible with home maintenance of these patients with advanced disease. Therefore, the aim of this study was to provide real-life information on regorafenib efficacy, safety, and cost in a cohort of mCRC patients treated within the regional institute of Franche-Comté.
Methods
We retrospectively reviewed the patients treated with regorafenib monotherapy for unresectable mCRC in the five Franche-Comté cancer centers (France) from January 2014 to August 2014. They received regorafenib monotherapy as salvage treatment. Eligible patients had histologically confirmed adenocarcinoma of the colon or rectum, with measurable or nonmeasurable metastatic disease. Patients were selected using the BPC® software database (IRFC, Federative Regional Cancer Institute of Franche-Comté, France), a computerized physician order entry system. This software is able to track injectable and oral chemotherapy and targeted therapy prescriptions, based on the tumor type. Baseline demographics, clinical history, laboratory findings, treatments, and economic data were retrospectively collected according to the medical records and BPC® software. This work was approved by the regional oncology network Oncolie in the IRFC. All patients provided written informed consent with authorization to collect clinical data retrospectively for research in IRFC.
Assessments
Efficacy
OS was defined as the time from initiation of regorafenib therapy to death from any cause or to last follow-up for survivors. Patients alive on August 31, 2015, were censored.
Imaging assessments of treatment efficacy were performed according to the metastatic sites by bone scan, computerized tomography, or magnetic resonance imaging. We assessed tumor response and progression for metastatic disease according to Response Evaluation Criteria in Solid Tumor (RECIST), version 1.1.17 Progression-free survival was not evaluated due to heterogeneous radiologic assessment in clinical practice.
Drug exposition and safety
Patients received regorafenib 160 mg orally once daily (or reduced doses of 120 or 80 mg/day) in accordance with the treating oncologist’s evaluation and prescribing recommendation, until disease progression, unacceptable toxic effects, or death. Patients had safety assessments by the oncologist at each visit, every month, including adverse events, laboratory changes, and vital signs (blood pressure). Adverse events were assessed and graded according to the National Cancer Institute Common Toxicity Criteria 4.01.
Economic evaluation
The analysis was performed from a health-care payer perspective. Only direct medical cost was computed from the start of treatment until the progression or death of patient. They included: medication (regorafenib, for adverse events), hospitalization (serious adverse event management, follow-up), inpatient and outpatient consultations, and transportation. Neither minor costs and cost considered to be independent of the treatment arm were taken into account nor were indirect medical and intangible costs. Costs are expressed in Euros (€) (reference year 2016, and 1€ = 1.12 USD). Each cost was calculated using the official tariff (for example, for each hospitalization, the national health insurance provider’s tariffs for diagnosis-related group medical consultation = 28€). In France, a pill of regorafenib 40 mg costs around 31€, so a box for monthly treatment with 84 pills of regorafenib at 40 mg costs around 2600€ VAT.
Statistical analysis
SAS 9.4® software (SAS Institute Inc.) was used for data analysis. Continuous variables are described by mean ± standard deviation (SD) and median (ranges). Qualitative variables are described by the size and percent rate. Median OS with its 95% CI and OS were calculated using the Kaplan–Meier method.
Results
Patient characteristics
Between January 2014 and August 2014, 29 patients were enrolled from the five Franche-Comté cancer centers (France). Patients received at least one-week regorafenib, and previous lines included standard chemotherapy (5-fluoropyrimidine, oxaliplatin, and irinotecan) with or without targeted therapy (anti-VEGF or anti-EGFR). Baseline patient characteristics are summarized in Table 1. The primary tumor site was the colon and the rectum in 55% and 45% of patients, respectively. Half of the patients had synchronous metastatic disease. Fifteen patients (51%) harbored a KRAS mutation. Bevacizumab, an anti-VEGF treatment, was previously administered in 26 patients (90%). All KRAS wild-type patients (n = 14, 49%) received anti-EGFR targeted therapy associated with systemic chemotherapies. The median patient age at initiation of regorafenib was 68 years (range, 40–83 years). Eastern Cooperative Oncology Group – Performance Status (ECOG-PS) was 0–1 for 86% of patients, and PS was 2 for 14% of patients. The most frequent metastatic sites were the liver (65%) and the lungs (27%). Patients were heavily pretreated and were refractory to standard chemotherapies. The median number of previous palliative systemic chemotherapies was 3 (Table 1).
Table 1.
Baseline patient characteristics.
| NUMBER OF PATIENTS | n (%) 29 (100) |
|---|---|
| Characteristic at diagnosis | |
| Primary site of disease | |
| Colon | 16 (55) |
| Rectum | 13 (45) |
| Disease status | |
| Synchronous | 15 (51) |
| Metachronous | 14 (49) |
| KRAS mutation | |
| Yes | 15 (51) |
| No | 14 (49) |
| Characteristic at baseline regorafenib | |
| Age, year, median (range) | 68 (40–83) |
| ECOG performance status, | n (%) |
| 0 | 7 (24) |
| 1 | 18 (62) |
| 2 | 4 (14) |
| Site of metastasis | |
| Liver | 21 (72) |
| Lung | 19(65) |
| Lymph Nodes | 8 (27) |
| Peritoneal | 6 (21) |
| Other | 8 (27) |
| No of previous palliative systemic anticancer therapies | |
| <3 | 6 (21) |
| 3 | 12 (41) |
| 4 | 8 (28) |
| 5 | 3 (10) |
| Previous Bevacizumab treatment | |
| Yes | 26 (90) |
| No | 3 (10) |
| Previous anti-EGFR treatment | |
| Yes | 14 (51) |
| No | 15 (49) |
Abbreviations: No, number; ECOG, Eastern Cooperative Oncology Group; EGFR, epithelial growth factor receptor.
Treatment exposition
Ten patients (34%) received once daily oral doses of regorafenib at the initial dose recommendation of 160 mg. Seventeen patients (59%) received an initially reduced dose of 120 mg/day. Table 2 lists the number of patients receiving regorafenib at each dose level initiation and the treatment outcomes. Among the 17 patients who received 120 mg/day at initiation, regorafenib was increased to 160 mg/day in 6 patients (35%), and maintained at 120 mg/day in 6 patients (35%). Dose modifications were performed in overall 15 patients (51%), including a dose reduction in 9 patients (31%). The median duration of treatment was 2.5 months (range, 0.13–11.4 months). Reasons for discontinuation of regorafenib were disease progression (n = 23; 79%) and treatment-related adverse events (n = 6; 21%).
Table 2.
Dose levels, treatment duration, and outcomes.
| INITIAL DOSE OF REGORAFENIB, MG/DAY | n (%) |
|---|---|
| 160 | 10 (34) |
| 120 | 17 (59) |
| 80 | 2 (7) |
| Dose modifications, n (%) | 15 (51) |
| Dose reductions, n (%) | 9 (31) |
| Median duration of treatment, months (range) | 2.5 (0,13–11,4) |
| Reasons for stop, n (%) | |
| Progression | 23 (79) |
| Toxicity | 6 (21) |
| Best response n* (%) | |
| Stable disease | 7 (24) |
| Progression disease | 20 (69) |
Note:
Missing data.
Efficacy
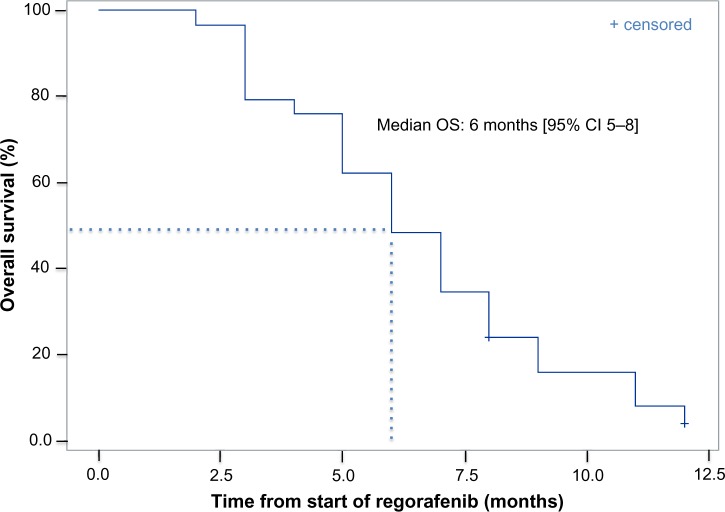
Twenty-seven patients were evaluable for response (Table 2). Best responses included stable disease in seven patients (24%). Twenty patients (69%) had progressive disease as best response. No patient achieved partial or complete responses. All 29 patients were included in the survival analysis on an intent-to-treat basis. OS Kaplan–Meier estimates analysis is described in Figure 1. The median OS was six months (95% CI, five to eight months).
Figure 1.
OS Kaplan–Meier estimates analysis.
Safety
Table 3 shows treatment-related adverse events that occurred in 86% of patients. Most adverse events occurred early in the course of treatment (during cycles 1–2, data not shown). The most frequent adverse events of any grade were fatigue (35%), diarrhea (20%), and hand–foot skin reaction (20%). Grade 3 or 4 treatment-related adverse events occurred in 10 patients (35%). The most frequent laboratory abnormalities were thrombocytopenia (17%), neutropenia (10%), and anemia (3.5%). Three patients (10%) were admitted to hospital due to drug-related severe adverse events. One of them presented Stevens–Johnson syndrome/toxic epidermal necrolysis, one week after regorafenib introduction at 120 mg/day, and the patient was hospitalized seven days. Another patient presented a heart failure five days after introduction of regorafenib at 160 mg/day and was hospitalized for eight days. Finally, the last patient presented bleeding in the neck region (patient previously treated by surgery and radiotherapy for head and neck cancer). He started 160 mg/day of regorafenib four months before, and he had to be hospitalized for several days. There was no treatment-related death.
Table 3.
Treatment-related adverse events.
| TOXICITY TYPE | ANY GRADE (G) | GRADE ≤2 | GRADE 3 | GRADE 4 |
|---|---|---|---|---|
| Any event n(%) | 25 (86) | 13 (45) | 7 (24) | 3* (10) |
| Clinical adverse events | ||||
| Fatigue | 10 (35) | 9 (31) | 1 (3,5) | |
| Diarrhea | 6 (20) | 6 (20) | ||
| Hand foot syndrome | 6 (20) | 4 (14) | 2 (7) | |
| Rash | 5 (17) | 3 (10) | 1 (3,5) | 1*(3,5) |
| Anorexia | 4 (14) | 4 (14) | ||
| Oral mucositis | 4 (14) | 4 (14) | ||
| Muscle pain | 3 (10) | 3 (10) | ||
| Abdominal pain | 3 (10) | 3 (10) | ||
| Voice changes | 3 (10) | 3 (10) | ||
| Bleed | 2 (7,0) | 1 (3,5) | 1*(3,5) | |
| Nausea/Vomiting | 1 (3,5) | 1 (3,5) | ||
| Hypertension | 1 (3,5) | 1 (3,5) | ||
| Heart failure | 1 (3,5) | 1*(3,5) | ||
| Laboratory abnormalities | ||||
| Thrombocytopenia | 5 (17) | 3 (10) | 2 (7,0) | |
| Neutropenia | 3 (10) | 2 (7,0) | 1 (3,5) | |
| Anemia | 1 (3,5) | 1 (3,5) |
Note:
Hospitalization due to severe adverse events.
Economic evaluation
The overall mean and median cost for treating one patient with regorafenib was 9908 ± 8191€, and 7917€ (IQR 4469–13,042), respectively, for the duration of treatment (median time of 2.5 months). The price of the drug represented the most important (93%) part of cost-related treatment (Fig. 2). Three patients required a supplement for a hospital stay due to severe adverse events, amounting to a mean cost of 256 ± 859€ by patients. Costs for hospitalizations, blood tests (177€ [IQR 133–266]), medical transportation (193€ [IQR 157–382]), and medical consultations (112€ [IQR 84–140]) represented 7% of the overall cost.
Figure 2.
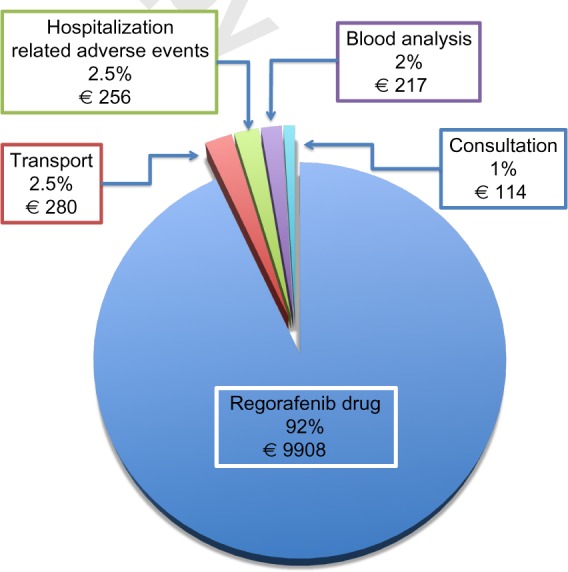
Descriptive cost analysis of patient treated with regorafenib for mCRC in France (data presented in percent and mean cost in euro).
Discussion
Regorafenib offers a new potential line of therapy for patients with mCRC that progresses after all approved standard therapies. However, use of regorafenib requires careful monitoring, for the management of schedule dosing and toxicities. This study investigated treatment efficacy, safety, and cost of regorafenib in clinical practice. Our patients had been heavily pretreated and had failed to standard therapies containing 5-FU, oxaliplatin, and irinotecan. They received either anti-VEGF or anti-EGFR agents. However, a portion of these patients still maintained good performance status and had few additional treatment options. In this setting, regorafenib is a standard-care option for treatment-refractory mCRC. However, clinical benefit appears modest, with median OS increased by six weeks compared to placebo (CORRECT, CONCUR). Therefore, though regorafenib has shown a statistically significant clinical benefit, the safety, cost, and effectiveness need to be evaluated in clinical practice. In our study, the median OS was six months, in concordance with CORRECT trial. In CONCUR trial (Asian population), the median OS was higher (8.8 months), possibly due to a better OS in the subgroup of patients (40%) who did not receive prior anti-VEGF or anti-EGFR target therapy. In fact, OS was higher (median OS 9.7 months) in patients who did not receive any prior targeted therapy than in patients who received at least one prior targeted drug (median OS 7.4 months). Progression-free survival was not assessed in our study because, in clinical practice, we identified an important heterogeneity in the timing of radiologic assessment, leading to potential bias. No objective response was observed in our cohort, in concordance with the pivotal CORRECT trial (1%). The best response was stable disease and concerned a limited number of patients (26%). The apparent lower disease control rate compared to phase 3 trials might be explained by the fact that patients were heavily pretreated, and radiological assessment was usually performed ≥2 months from treatment initiation, compared to 6 weeks in CORRECT and CONCUR trials.
The safety profile of regorafenib is consistent with an important occurrence of adverse events. In the CORRECT and CONCUR trial, grade 3 or 4 treatment-related adverse events occurred in 54% of patients assigned to the regorafenib group and the dose was reduced in 38% of patients. In these pivotal trials, the most frequent adverse events of grade 3 or higher related to regorafenib were hand–foot skin reaction (12%–32%), fatigue (3%–11%), diarrhea (1%–8%), and hypertension (7%–12%; Table 4). In our study, we reported 50% fewer grade 3 or 4 treatment-related adverse events (24%) than in the phase 3 trials. Even though this could be secondary to our clinical practice with two-thirds of the patients starting at lower than 160 mg/day dose and only 31% needs dose reduction compared to about 70% in the CORRECT and CONCUR trials, we cannot exclude missing toxicity data, and no dose recommendation can be drawn from this study. However, the starting dose is relevant and is currently being studied in the phase II Regorafenib Dose Optimization Study (ReDOS, NCT02368886) in the United States.
Table 4.
Review of clinical data for regorafenib monotherapy in mCRC.
| ADVERSE EVENTS > GRADE 3 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAME | STUDY | COUNTRY | NUMBER OF pts TREATED WITH REGORAFENIB | ≥4 LINES (%) | INITIATION DOSE REDUCTION (% pts) | DOSE ADAPTATION (% pts) | MEDIAN DURATION OF TREATMENT (mo) | MEDIAN OS (mo) | MEDIAN PFS (mo) | ADVERSE EVENTS (%) | AE (%) | HAND-FOOT SYNDROME % | HYPERTENSION % | FATIGUE % |
| Strumberg et al.27 | Phase I | Germany | 38 | 50.0 | 0 | 66.0 | 1.8 | NA | 3.5 | 84 | 58 | 32 | 11 | 11 |
| Grothey et al.15 | Phase III (CORRECT) | World | 500 | 49.0 | 0 | 76.0 | 1.7 | 6.4 | 1.9 | 93.0 | 54 | 17 | 7 | 10 |
| Li et al.16 | Phase III (CONCUR) | Asia | 136 | 38.0 | 0 | 75.0 | 2.4 | 8.8 | 3.2 | 97 | 54 | 16 | 11 | 3 |
| Van Cutsem et al.9 | Cohort study Phase IIIb (CONSIGN) | 25 countries | 2864 | 46 | 0 | 87 | 2.5 | NR | 2.7 | 91 | 57 | 14 | 15 | 13 |
| de la Fouchardiere et al.28 | Retrospective Cohort study (REBECCA) | France | 654 | NR | 20 | 50 | 2.2 | 5.6 | 2.7 | 80 | 43.7 | 9 | 4.6 | 14.5 |
| Komatsu et al.29 | Post marketing Surveillance | Japan | 796 | 24 | 34 | NR | 1.7 | 7 | NR | 89 | 51 | 18 | 14 | 2 |
| Tanaka et al.30 | Retrospective | Japan | 16 | NR | NR | 87.5 | 1.6* | 6.6 | 2.3 | NR | ND | 44 | 6 | 13 |
| Hirano et al.31 | Retrospective | Japan | 32 | 22 | 22 | 91.0 | 2.7* | 7.7 | 2.7 | NR | 50 | 22 | 9 | 13 |
| Kim et al.32 | Retrospective | Korean | 32 | NR | 0 | 50 | NR | NR | 4.2 | 90 | 37 | 0 | NR | 3 |
| Calcagno et al | Retrospective | France | 29 | 26 | 66 | 51 | 2.5 | 6 | NR | 86 | 24 | 7 | 3.5 | 3.5 |
Abbreviation: NR, not reported.
Since most severe toxicities occur early after regorafenib exposure, close monitoring should be required from the beginning of the treatment to adapt regorafenib dose and manage most common and severe adverse events.18,19
Our study has several limitations related to its retrospective nature. Quality of life related to health evaluation was not available, and toxicity data recovery may not be exhaustive. At the moment, a prospective observational cohort study (CORRELATE) is being conducted in routine clinical practice settings to evaluate the safety and effectiveness of regorafenib in patients with mCRC. Another aim of the CORRELATE study is to depict health-care resources and health-related quality of life (HRQoL), associated with the management of adverse events due to regorafenib in the real-world setting.20 The results of our analysis suggest that regorafenib monotherapy in our clinical practice presented similar efficacy and safety to those reported in the prospective CORRECT study and retrospective trials (Table 4). The hospitalization rate induced by regorafenib prescription has not been reported so far. The 10% of hospitalizations required to manage severe adverse events is to be taken into account, and supportive care to carefully monitor patients’ tolerance might be considered.
In our study, the price of the drug itself accounts for most of the cost estimate of regorafenib outpatient management (93%). Other health-care costs for management of patients treated by regorafenib, such as medical transportation, blood tests, medical examinations, or adverse event management, are small (7%). Health-care costs have been dramatically increased by the number of new targets for cancer therapies. Standard combination of chemotherapies in mCRC is associated with good clinical effectiveness at a favorable cost.21 We performed a descriptive cost analysis, but a full economic evaluation with cost-effectiveness analysis and cost–utility analysis is required. Economic evaluation is the comparative analysis of alternative courses of action in terms of both their costs and consequences. Recently, Goldstein et al.22 developed a Markov model to compare the cost-effectiveness of regorafenib compared with placebo in third-line treatment of mCRC. The use of regorafenib provides, in the USA, an additional 0.04 QALYs (or 0.13 life years) at a cost of $40,000 for an incremental cost-effectiveness ratio (ICER) >$550,000 per QALY. They concluded that regorafenib provides minimal incremental benefit at high incremental cost per QALY, and the cost-effectiveness of regorafenib could be improved by reduced pricing. In comparison, the treatment with bevacizumab, cetuximab, and panitumumab is not mainly considered to be cost-effective, with an ICER >$100,000.21,23,24 These data provide a reference point of value of regimens for mCRC, but the comparison of cost data remains difficult because of the different health systems and variations among countries (geographic transferability of economic evaluation).25
However, given that regorafenib is associated with significant adverse events with a modest incremental benefit at significant cost, the value of this treatment remains debatable. Hence, regorafenib should be prescribed with caution and patients should be carefully selected by physicians before starting. About one-fourth of patients seem to obtain clinical benefit from regorafenib treatment. New strategies are needed in patient selection for regorafenib treatment in heavily pretreated mCRC patients. Maybe the identification of predictive biomarkers can help to better tailor this therapy to the targeted population. Unfortunately, recent data reported on the analysis of circulating DNA and protein biomarkers did not identify any prognostic or predictive biomarkers that can be used in current clinical practice to predict the clinical activity of regorafenib.26
Conclusion
The safety and efficacy of regorafenib in heavily pretreated mCRC patients was comparable in our study to the pivotal CORRECT trial and retrospective trials. Toxic effects were mostly manageable in an outpatient setting, even though 10% of patients required hospitalization to manage serious adverse events. Given that regorafenib is an outpatient treatment and the drug itself represents the most important (93%) part of supported cost, a decrease in the drug’s price could improve the cost-effectiveness of regorafenib.
Footnotes
ACADEMIC EDITOR: William Chi-shing Cho, Editor in Chief
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,098 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: VN, CB, FC. Analyzed the data: VN, FC, SL. Wrote the first draft of the manuscript: FC. Contributed to the writing of the manuscript: FC, VN, SK, CB. Agree with manuscript results and conclusions: FC, VN, SK, CB, ZL, MJ, TN. Jointly developed the structure and arguments for the paper: FC, VN, SK, CB, ZL, MJ, TN. Made critical revisions and approved final version: FC, VN, SK. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Ferlay JSI, Ervik M, Dikshit R, et al. Cancer Incidence and Mortality Worldwide: IARC CancerBase GLOBOCAN 2012 v10. Lyon: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.Cancer. IINd Incidence nationale du cancer colorectal. 2015. Available at: http://lesdonnees.e-cancer.fr/les-fiches-de-synthese/1-types-cancer/11-cancer-colorectal/43-epidemiologie-du-cancer-colorectal-en-france-metropolitaine-incidence.html.
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93(4):465–74. doi: 10.1002/bjs.5278. [DOI] [PubMed] [Google Scholar]
- 5.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244(2):254–9. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andre T, Louvet C, Maindrault-Goebel F, et al. CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluo-rouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR. Eur J Cancer. 1999;35(9):1343–7. doi: 10.1016/s0959-8049(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 7.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Ciardiello F, Seitz JF, et al. Results from the large, open-label phase 3b CONSIGN study of regorafenib in patients with previously treated metastatic colorectal cancer (mCRC) Ann Oncol. 2015;26(suppl 9):ix42–70. [Google Scholar]
- 10.Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28(31):4706–13. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 11.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–9. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 12.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–75. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 13.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609–18. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 14.(regorafenib) S. [package insert] Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015. [Google Scholar]
- 15.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–29. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Grothey A, George S, van Cutsem E, Blay JY, Sobrero A, Demetri GD. Optimizing treatment outcomes with regorafenib: personalized dosing and other strategies to support patient care. Oncologist. 2014;19(6):669–80. doi: 10.1634/theoncologist.2013-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sastre J, Argiles G, Benavides M, et al. Clinical management of regorafenib in the treatment of patients with advanced colorectal cancer. Clin Transl Oncol. 2014;16(11):942–53. doi: 10.1007/s12094-014-1212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safety and Effectiveness of Regorafenib (CORRELATE) Available at: https://clinicaltrials.gov/ct2/show/NCT02042144.
- 21.Goldstein DA, Chen Q, Ayer T, et al. First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis. J Clin Oncol. 2015;33(10):1112–8. doi: 10.1200/JCO.2014.58.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein DA, Ahmad BB, Chen Q, et al. Cost-effectiveness analysis of regorafenib for metastatic colorectal cancer. J Clin Oncol. 2015;33(32):3727–32. doi: 10.1200/JCO.2015.61.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange A, Prenzler A, Frank M, Kirstein M, Vogel A, von der Schulenburg JM. A systematic review of cost-effectiveness of monoclonal antibodies for metastatic colorectal cancer. Eur J Cancer. 2014;50(1):40–9. doi: 10.1016/j.ejca.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Wade R, Duarte A, Simmonds M, et al. The clinical and cost effectiveness of aflibercept in combination with irinotecan and fluorouracil-based therapy (FOLFIRI) for the treatment of metastatic colorectal cancer which has progressed following prior oxaliplatin-based chemotherapy: a critique of the evidence. Pharmacoeconomics. 2015;33(5):457–66. doi: 10.1007/s40273-015-0257-z. [DOI] [PubMed] [Google Scholar]
- 25.Goeree R, Burke N, O’Reilly D, Manca A, Blackhouse G, Tarride JE. Transferability of economic evaluations: approaches and factors to consider when using results from one geographic area for another. Curr Med Res Opin. 2007;23(4):671–82. doi: 10.1185/030079906x167327. [DOI] [PubMed] [Google Scholar]
- 26.Tabernero J, Lenz HJ, Siena S, et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015;16(8):937–48. doi: 10.1016/S1470-2045(15)00138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strumberg D, Scheulen ME, Schultheis B, et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br J Cancer. 2012;106(11):1722–7. doi: 10.1038/bjc.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de la Fouchardière C, Paule B, Burtin P, et al. Survival benefit, safety, and prognostic factors for outcome with Regorafenib (REG) in patients (pts) with pretreated metastatic colorectal cancer (mCRC). Main analyses of the REBECCA study. Eur J Cancer. 2015;51(suppl 3) Abstract 2095. [Google Scholar]
- 29.Komatsu YM, Yamaguchi K, Uetake H, et al. Safety and efficacy of regorafenibin japanese patients with metastatic colorectal cancer (mCRC) in clinical practice: interim results from post-marketing surveillance (PMS) J Clin Oncol. 2016;34(suppl 4S) Abstract 680. [Google Scholar]
- 30.Tanaka A, Sadahiro S, Suzuki T, Okada K, Saito G. Tolerability and efficacy of regorafenib in patients with unresectable metastatic colorectal cancer. Gan To Kagaku Ryoho. 2014;41(10):1231–6. [PubMed] [Google Scholar]
- 31.Hirano G, Makiyama A, Makiyama C, et al. Reduced dose of salvage-line regorafenib monotherapy for metastatic colorectal cancer in Japan. Anticancer Res. 2015;35(1):371–7. [PubMed] [Google Scholar]
- 32.Kim ST, Kim TW, Kim KP, et al. Regorafenib as salvage treatment in Korean patients with refractory metastatic colorectal cancer. Cancer Res Treat. 2015;47(4):790–5. doi: 10.4143/crt.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]