Abstract
Diabetes is a global endemic with rapidly increasing prevalence in both developing and developed countries. The American Diabetes Association has recommended glycated hemoglobin (HbA1c) as a possible substitute to fasting blood glucose for diagnosis of diabetes. HbA1c is an important indicator of long-term glycemic control with the ability to reflect the cumulative glycemic history of the preceding two to three months. HbA1c not only provides a reliable measure of chronic hyperglycemia but also correlates well with the risk of long-term diabetes complications. Elevated HbA1c has also been regarded as an independent risk factor for coronary heart disease and stroke in subjects with or without diabetes. The valuable information provided by a single HbA1c test has rendered it as a reliable biomarker for the diagnosis and prognosis of diabetes. This review highlights the role of HbA1c in diagnosis and prognosis of diabetes patients.
KEYWORDS: diabetes, HbA1c, diagnosis, prognosis, blood test
Introduction
Analysis of glycated hemoglobin (HbA1c) in blood provides evidence about an individual’s average blood glucose levels during the previous two to three months, which is the predicted half-life of red blood cells (RBCs).1 The HbA1c is now recommended as a standard of care (SOC) for testing and monitoring diabetes, specifically the type 2 diabetes.2 Historically, HbA1c was first isolated by Huisman et al.3 in 1958 and characterized by Bookchin and Gallop4 in 1968, as a glycoprotein. The elevated levels of HbA1c in diabetic patients were reported by Rahbar et al.5 in 1969. Bunn et al.6 identified the pathway leading to the formation of HbA1c in 1975. Using the HbA1c as a biomarker for monitoring the levels of glucose among diabetic patients was first proposed by Koenig et al.7 in 1976.
Proteins are frequently glycated during various enzymatic reactions when the conditions are physiologically favorable. However, in the case of hemoglobin, the glycation occurs by the nonenzymatic reaction between the glucose and the N-terminal end of the β-chain, which forms a Schiff base.8,9 During the rearrangement, the Schiff base is converted into Amadori products, of which the best known is HbA1c (Fig. 1). In the primary step of glycated hemoglobin formation, hemoglobin and the blood glucose interact to form aldimine in a reversible reaction. In the secondary step, which is irreversible, aldimine is gradually converted into the stable ketoamine form.10 The major sites of hemoglobin glycosylation, in the order of prevalence, are β-Val-1, β-Lys-66, and α-Lys-61. Normal adult hemoglobin consists predominantly of HbA (α2β2), HbA2 (α2δ2), and HbF (α2γ2) in the composition of 97%, 2.5%, and 0.5%, respectively. About 6% of total HbA is termed HbA1, which in turn is made up of HbA1a1, HbA1a2, HbA1b, and HbA1c fractions, defined by their electrophoretic and chromatographic properties. HbA1c is the most abundant of these fractions and in health comprises approximately 5% of the total HbA fraction. As mentioned above, glucose in the open chain format binds to the N-terminal to form an aldimine before undergoing an Amadori rearrangement to form a more stable ketoamine. This is a nonenzymatic process that occurs continuously in vivo. The formation of the glycated hemoglobin is a normal part of the physiologic function cycle. However, as the average plasma glucose increases, so does the amount of glycated hemoglobin in the plasma. This specific characteristic of the hemoglobin biomarker is utilized for estimating the average blood glucose levels over the previous two to three months.11 In this review, we have described the current trends in diabetes prevalence, diagnostic and prognostic potential of HbA1c, analytical aspects in HbA1c assays, and physiological changes due to hemoglobin glycation.
Figure 1.
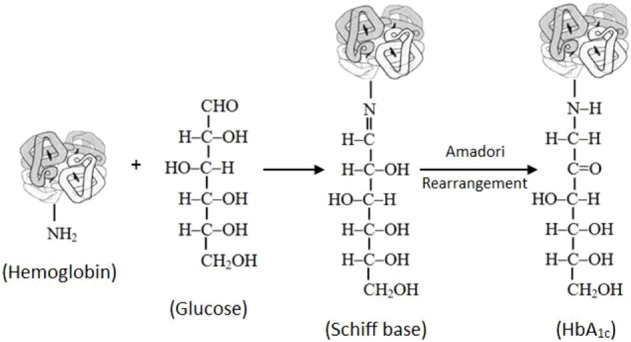
Formation of glycated hemoglobin (HbA1c) from the binding of glucose to hemoglobin.
Diabetes – a Silent Killer
According to the 2014 release of the American Diabetes Association (ADA), as of 2012, 29.1 million Americans, or 9.3% of the total US population, had diabetes.12 Type 1 diabetes is prevalent among approximately 1.25 million American children and adults. A large percentage of Americans (about 28%) were undiagnosed diabetes cases from among the 29.1 million cases (21.0 million diagnosed and 8.1 million undiagnosed). The Americans aged 65 and older (senior citizens) are at a much higher risk (25.9% or 11.8 million, diagnosed and undiagnosed combined). Even though the incidence of new diabetes cases is astounding, the trajectory appeared to have slowed momentarily, with 1.7 million new diagnoses per year as reported in 2012 as compared to 1.9 million in 2010, reflecting fewer cases diagnosed in 2012 than in 2010. This raises the question – is this really a downward trend or have many diabetes cases gone unreported and undiagnosed due to various confounding factors? The prediabetes cases have been on an upward swing with 86 million Americans, aged 20 years or older, having been reported as being prone to diabetes (pre-diabetes) as of 2012, which is higher than the 2010 estimates (79 million). Based on race and ethnicity, diabetes affects 7.6% of non-Hispanic whites, 9.0% of Asian Americans, 12.8% of Hispanics, 13.2% of non-Hispanic blacks, and 15.9% of American Indians/Alaskan Natives, among the US population. Diabetes is the seventh leading cause of death in the US. According to the ADA, 69,071 death certificates listed diabetes as the underlying cause of death in 2010. A total of 234,051 death certificates listed diabetes as an underlying or contributing cause of death. According to the latest statistics available, the total costs of diagnosed diabetes in the US as of 2012 was $245 billion, of which, $176 billion was spent toward direct medical costs and $69 billion costs were associated with reduced productivity. So, it is easy to see how detrimental diabetes is to the overall health of the population and the economy of the United States.
Diabetes – a Global Epidemic
The worldwide picture of diabetes is not much better either, with 387 million people with confirmed diabetes according to the latest census.13 According to the 2014 estimate, the prevalence of diabetes in the world was 9%, among adults aged 18 years or older. It is projected that by the year 2035, those affected by diabetes will be around 592 million. The population with type 2 diabetes continues to increase worldwide. Among the total diabetes patients, 77% live in low- and middle-income countries and 40–49-year olds have the largest number of people of any group. It is estimated that as many as 179 million people remain undiagnosed, for various reasons, but may be affected by diabetes. Every seven seconds, dia betes causes the death of an individual worldwide, and in 2014 alone, 4.9 million deaths were attributed to diabetes with 80% of deaths related to diabetes reported from low- and middle-income countries. In 2014, the overall health expenditure, as a result of diabetes, was estimated as $612 billion, which is approximately 11% of the total spending on adults. In 2013, type 1 diabetes was reported in more than 79,000 children. Gestational diabetes was responsible for more than 21 million live births, affecting both the mother and the newborn, in one way or the other, in 2013.13
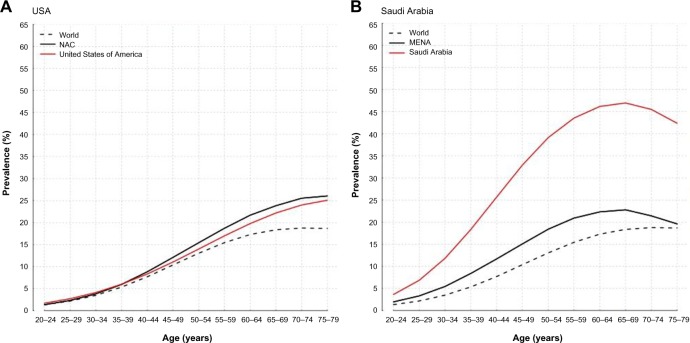
The North America and Caribbean region spends the most amount of money on diabetes health care than any other region of the world and still has more than 39 million diabetes patients, and this number is projected to increase to 50 million by 2035, as shown in Figure 2. The prediction for the South and Central America region does not look good with 2035 projections predicting the diabetes to increase by 60%. In the Middle East and North Africa (MENA) region, 10% of the population (more than 37 million cases) has diabetes, which is predicted to increase to 68 million by 2035, with many prediabetes and undiagnosed cases. Saudi Arabia, representing the MENA region, has reported 3.8 million confirmed cases of diabetes as of 2014, with still many unreported and/or undiagnosed cases. According to the 2014 data, more than 20% (3,806.4 million diabetics out of the total population of 18,546 million) of the Saudi Arabian adult population (aged 20 years and older) has diabetes. The total number of deaths due to diabetes in Saudi Arabia was reported as 25,527 in 2014 and the cost per person with diabetes is estimated to be $1,067.30. And, the total number of undiagnosed cases of diabetes among adults is apprized as more than 1.5 million. The projected trajectory for diabetes in Saudi Arabia is alarming, particularly among the 40–49 age group, as reflected in Figure 2.13 The increase in the incidence of diabetes in Saudi Arabia has been attributed to significant changes in cultural and socioeconomic factors, such as increase in affluence, which unmasks an increase in the genetic or ethnic propensity for diabetes, in addition to physical inactivity and changes in dietary habits with the substitution of animal products and refined foods.14–16
Figure 2.
Prevalence of diabetes in (A) USA and (b) Saudi Arabia versus the entire world.
Source: https://www.idf.org/membership/mena/saudi-arabia.
Abbreviations: NAC, North America and Caribbean; MENA, Middle East and North Africa.
Southeast Asia region remains the biggest challenge with approximately half of the diabetic population, which may still be undiagnosed. Among the Western Pacific region (China, Australia, New Zealand, Malaysia, Mongolia, Philippines, etc.), an estimated 138 million adult individuals have diabetes, which is the highest among any region in the world. In China, diabetes has acquired epidemic proportions and continues to develop at an unprecedented rate. China has overtaken the United States in the prevalence of diagnosed cases of diabetes, with 11.6% of Chinese adults affected by confirmed cases of diabetes.13 The Chinese diabetes population (approximately 114 million) alone is one-third of the entire diabetes population in the world, and the growing number of cases will continue to put enormous strain on China’s health-care system and the overall economy. Representing the South Asia region of the world, India is home to approximately 67 million (66,847.9 million) cases of diabetes, which is about 8.6% of the total adult population (20–79 years) and continues to grow at an alarming rate (2010 estimates: 50.8 million).13 Obesity, associated with diabetes, has reached epidemic proportions among middle-class children and adolescents due to their exposure to fast food diets and lack of exercise and physical activity. In Russia, 6.2% of the entire adult population (20–79 years old) is suffering from diabetes with more than 6.7 million cases of diabetes.13 It is estimated that there may be as many as 2.3 million cases of undiagnosed diabetes among the adults. Regionally, Africa region remains at the forefront with majority of the deaths occurring as a result of diabetes and its complication are confined in people younger than 60 years old. Presently, there are approximately 2 million cases of diabetes in South Africa.13 The total number of people with diabetes may be even higher due to many undiagnosed and unreported cases, which is quite common in many developing countries. As of 2014, more than 11.6 million (8.7% adults) Brazilians had diabetes, which continues to grow with approximately 33 million reporting high blood pressure. More than 80,000 deaths per year are attributed to diabetes in Brazil. The prevalenceof type 1 diabetes is the highest in the Europe (EUR) region. The EUR region has a total of 52 million diabetes patients, and this number is expected to increase to 69 million by 2035. Representing the EUR region, Germany had over 7.2 million cases of diabetes as of 2014.13
Diagnostic Potentials of HbA1c
The ADA has recently recommended HbA1c with a cut-point ≥6.5% for diagnosing diabetes as an alternative to fasting plasma glucose (FPG ≥7.0 mmol/L)-based criteria.17 The levels of HbA1c are strongly correlated with FPG (Fig. 3).18 FPG, 2-hour post-glucose load plasma glucose, and oral glucose tolerance tests are recommended for the diagnosis of diabetes only if HbA1c testing is not possible due to unavailability of the assay, patient factors that preclude its interpretation, and during pregnancy.19 HbA1c provides a reliable measure of chronic glycemia and correlates well with the risk of long-term diabetes complications, so that it is currently considered the test of choice for monitoring and chronic management of diabetes. However, the cut-point of HbA1c from the diagnostic point of view is still controversial. Among diabetics, the blood glucose levels increase in the blood and the glucose attaches to the hemoglobin molecule in a concentration-dependent manner. The glucose-bound (glycated) hemoglobin or HbA1c provides the average glucose levels in an individual’s blood as it becomes glycated with the hemoglobin. It is important to note that the HbA1c levels are directly proportional to the blood glucose levels. A simple blood glucose test such as a fasting glucose test (FGT) is a measure of glucose concentration present in an individual’s blood at a given point of time.20 The blood used for the FGT may be obtained through a needlestick of a finger or directly from the arm. A new techno logy, continuous glucose monitoring, has arrived in the market, which allows for non-prick readings.21–23 A small chip is implanted under the skin, which provides continuous glucose monitoring readings to the sensor kept outside, and if the glucose levels are higher or lower, it sends a special signal to the sensor, thus alerting the patient and/or the health-care provider for intervention.22,23 The FGT is an excellent test for “in the moment” glucose levels, but it does not provide detailed information about the time course trend of the glucose levels. The HbA1c test, however, is a marker of the average glucose levels spread over a two- to three-month period. Contrary to popular belief, along with the type 2 diabetes, the HbA1c is also used to diagnose, manage, and monitor the type 1 diabetes as well.24 In a series of 12,785 male diabetic patients, Khan et al.11 have shown that the HbA1c cut-point of 6.5% was associated with 3.78% false-negative predictions (Fig. 4), while majority of the false-negative patients had borderline FPG (7.0–8.0 mmol/L) and HbA1c (6.0%–6.5%), and therefore belonged to at-risk category on the basis of HbA1c alone criteria. These findings suggest that the status of individuals with HbA1c between 6.0% and 6.5% should be verified by combined FPG and HbA1c criteria.11 Recently, Khan et al.25 have provided regression equations for interconversions between the levels of FGT and HbA1c for predicting their expected values in diabetic patients.
Figure 3.
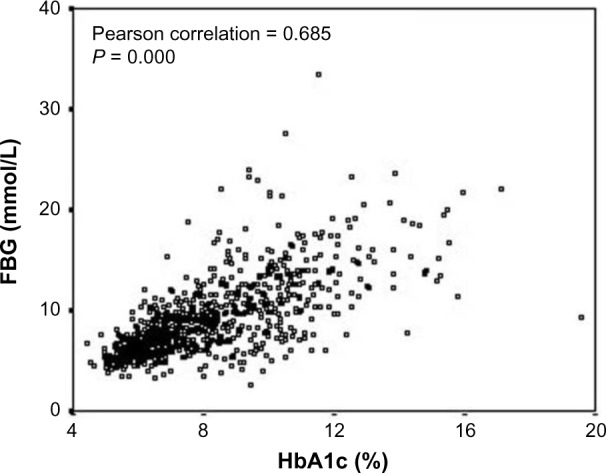
Correlation between HbA1c and FBG in type 2 diabetic patients. Clinical and Experimental Medicine, Association between glycaemic control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia. Volume 7, 2007, 24–29, Khan HA, Sobki SH, Khan SA. (Copyright © 2007, Springer-Verlag Italia) Reused with permission of Springer.
Figure 4.
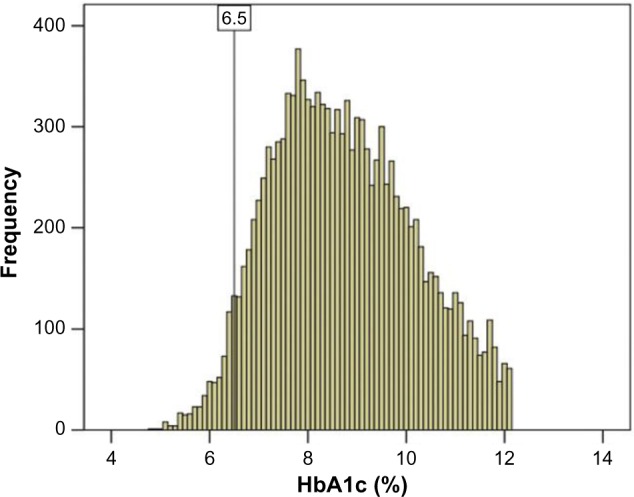
Diagnostic potential of HbA1c.
Notes: The histogram is showing the frequency of patients versus HbA1c. The vertical reference line shows the cutoff value of 6.5% HbA1c. Reprinted from Khan et al.11 with permission from Taylor and Francis, www.tandfonline.com.
Not requiring fasting and also not being bound by the time of the day on the part of the patient, the HbA1c is a very convenient test to administer and evaluate.26 Diabetes, the silent killer, can be detected earlier, and an appropriate treatment regimen can be implemented sooner than later among people. The blood glucose data available from HbA1c are used in prescribing and monitoring the medicines for diabetes and prediabetes, along with exercise and diet. The accuracy of this test has continued to evolve over the last several years and is becoming the go-to option for SOC for detecting blood glucose values among patients in clinics. According to the National Glycohemoglobin Standardization Program (NGSP), which developed the A1C tests, the accuracy has continued to evolve and got more precise over time.27 The HbA1c is recommended to be performed at least twice a year in diabetes patients with stable blood glucose levels.28 Still, there is no substitute for the daily (several times a day) monitoring of the blood glucose, particularly those on insulin regimen as the readings dictate the amount of insulin that a patient must take before each meal. The estimated average glucose can also be calculated from the actual HbA1c levels to help individuals with diabetes to correlate these levels with the daily monitoring of glucose levels.29,30
The HbA1c levels differ for different diabetes patients, depending on their history of diabetes and whether they are on tablets or long-term and/or short-term insulin dosage.29 Type 2 diabetes mellitus (DM) manifests itself in terms of hyperglycemia due to compromised insulin production (no production or nonavailability).31 The significance of the HbA1c test lies in the diagnosis and the prognosis of the diabetes patients, which lends it to a detailed understanding of insulin and insulin resistance. There is a direct correlation between HbA1c and insulin resistance, where HbA1c has been shown to be more strongly associated with the insulin sensitivity in healthy subjects with normal glucose tolerance.32 The HbA1c test has revealed mini mal overlap in values between normal glucose tolerance in subjects with type 2 diabetes while comparing the glycemic spectrum for insulin resistance. As a result, HbA1c is a reliable biomarker and an excellent indicator of insulin resistance for testing individuals for diabetes and prediabetes.33
Kwon et al.34 evaluated the clinical usefulness of HbA1c in diagnosing gestational diabetes mellitus (GDM) and predicting the risk of future type 2 DM development among GDM patients. HbA1c showed high sensitivity with relatively low specificity for diagnosis of GDM in pregnant women but was a potential predictor of postpartum DM. The prognostic value of HbA1c for postpartum DM was evaluated by receiver-operating characteristic curve analysis, with a sensitivity of 78.6% and a specificity of 72.5% at a cut-off value of 5.55%.34 A retrospective cohort study on women who delivered and had an early screening HbA1c test performed at ≤20 weeks of gestation showed that nearly one-third of those patients in the HbA1c 5.7%–6.4% group (27.3%) experienced the development of GDM compared with only 8.7% in the HbA1c <5.7% group.35 Thus, women with HbA1c 5.7%–6.4% have a significantly higher risk of progression to GDM compared with women with normal HgbA1c values and should be considered for closer GDM surveillance and possible intervention. Although paired values of blood glucose and serum fructosamine were also reported for the screening of GDM, there were significant fluctuations during several antenatal visits.36,37
Prognostic Potentials of HbA1c
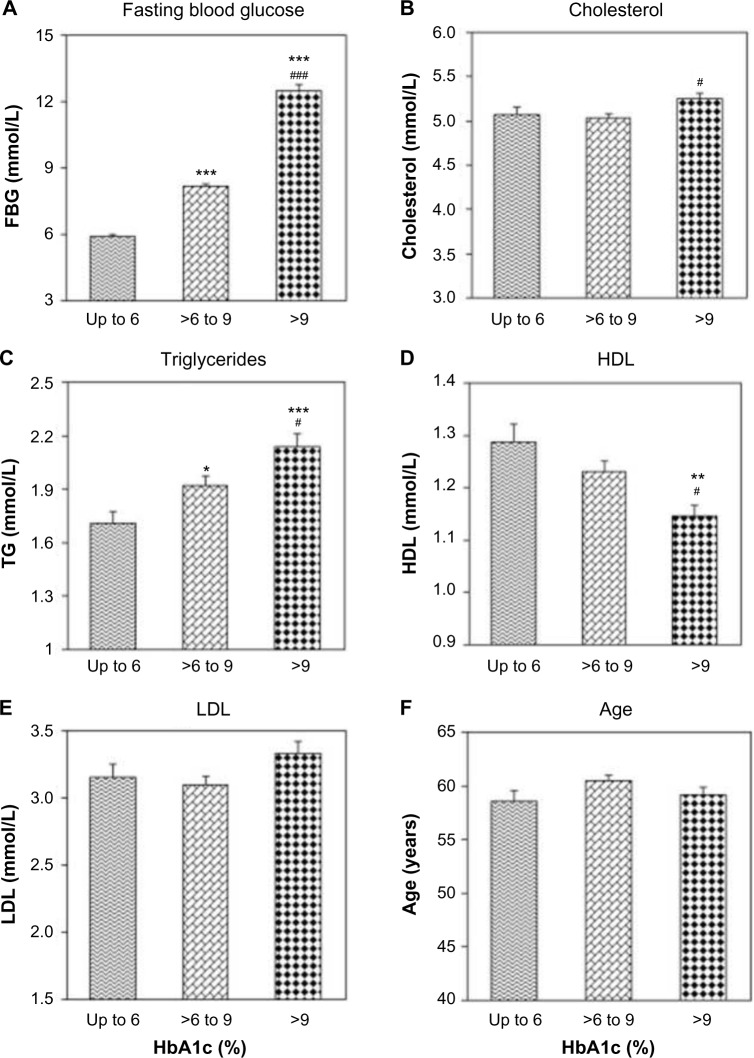
HbA1c is not only a useful biomarker of long-term glycemic control but also a good predictor of lipid profile; thus, monitoring of glycemic control using HbA1c could have additional benefits of identifying diabetes patients who are at a greater risk of cardiovascular complications.18 Thus, a single HbA1c test provides valuable information that can be used for the management of chronic diseases. In a series of 1,011 type 2 diabetic patients, HbA1c exhibited direct correlations with cholesterol, triglycerides, and low density lipoprotein cholesterol and inverse correlation with high-density lipoprotein cholesterol. There was a linear relationship between HbA1c and dyslipidemia as the levels of serum cholesterol and triglycerides were significantly higher and that of high-density lipo-protein cholesterol were significantly lower in patients with worse glycemic control as compared to patients with good glycemic control (Fig. 5).18
Figure 5.
Prognostic potential of HbA1c. Impact of HbA1c on various parameters. The patients were categorized into three groups according to HbA1c levels: group 1 (HbA1c ≤6%), group 2 (HbA1c >6%–9%), and group 3 (HbA1c >9%).
Notes: *P < 0.05, **P < 0.01, and ***P < 0.001 group 1 versus group 2; #P < 0.05 and ###P < 0.001 group 2 versus group 3. Clinical and Experimental Medicine, Association between glycaemic control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia. Volume 7, 2007, 24–29, Khan HA, Sobki SH, Khan SA. (Copyright © 2007, Springer-Verlag Italia) Reused with permission of Springer.
Elevated level of HbA1c has been identified as a significant risk factor for cardiovascular diseases and stroke in subjects who may have diabetes.38 A community-based population study on 11,092 nondiabetic patients found that elevated HbA1c level was strongly associated with the risk of cardiovascular disease and mortality.39 High levels of HbA1c were associated with an increased risk of recurrence of atrial tachyarrhythmia in patients with type 2 DM and paroxysmal atrial fibrillation undergoing catheter ablation.40 Even an increase of 1% in HbA1c concentration was associated with about 30% increase in all-cause mortality and 40% increase in cardiovascular or ischemic heart disease mortality, among individuals with diabetes.41 Whereas reducing the HbA1c level by 0.2% could lower the mortality by 10%.41 Vaag42 has suggested that improving glycemic control in patients with type 2 diabetes may be more important than treating dyslipidemia for the prevention of both microvascular and macrovascular complications.
Cicek et al.43 determined the effect of HbA1c on the outcomes of primary percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction (STEMI). They observed that in-hospital mortality and major adverse cardiac events were significantly higher in patients with HbA1c ≥6.5% (11%) compared with the group of patients with HbA1c between 5.7% and 6.4% (2.8%) or HbA1c ≤5.6% (0.9%). Out of the total 374 patients, 196 (63.6%) patients without a history of DM had elevated HbA1c ≥5.7%, with 31 (10.1%) of them having HbA1c ≥6.5%.43 On the basis of 12-month follow-up of 1,433 patients with stable angina who underwent coronary angiography, it was concluded that high level of baseline HbA1c appeared to be an independent predictor for the severity of coronary artery disease and poor outcome in patients with stable coronary artery disease.44 As the levels of HbA1c increased, patients were more likely to have prior cardiovascular disease and a more unfavorable baseline cardiovascular risk profile in a cohort of AMI patients.45 Although the admission glucose levels may represent a marker for increased risk in the acute and subacute setting after AMI, HbA1c, being a surrogate for more chronic dysglycemia, is clearly a more useful marker of patients with greater long-term risk of death.45
However, in an observational multicenter study on 608 patients with STEMI who underwent primary PCI, the admission level of HbA1c was not found to be an independent prognostic marker for short-term outcomes in STEMI patients treated with primary PCI.46 A prospective cohort of 2,519 nondiabetic patients undergoing elective coronary angiography for suspected stable angina pectoris did not show any association between HbA1c levels and prognosis, questioning an independent role of glycemia in the pathogenesis of atherosclerotic complications in nondiabetic patients.47 Recently, Wang et al.48 have shown that HbA1c level is not a significant and independent marker for the severity of angiography (stenosis) in ACS patients.
Kompoti et al.49 investigated the clinical significance of HbA1c levels on admission in the intensive care unit as a prognostic marker for morbidity and mortality in critically ill patients. The findings showed that HbA1c is a useful tool for the diagnosis of a previously undiagnosed DM in critically ill patients, and HbA1c at admission is significantly associated with intensive care unit mortality. Pimentel et al.50 have shown that HbA1c ≥6.5% is not enough to be used alone in the diagnosis of post-transplantation DM in renal transplant patients. However, the combined use of HbA1c cut-off points of ≤5.8% and ≥6.2% would reduce the number of oral glucose tolerance tests by 85% and the use of an algorithm with HbA1c in combination with FPG proved to be the most efficient strategy to diagnose or rule out post-transplantation DM.50 Poor glycemic control (HbA1c ≥8%) has been associated with decreased survival in the general population of diabetic patients on maintenance hemodialysis, suggesting that moderate hyperglycemia increases the risk for all-cause mortality of diabetic maintenance hemodialysis patients in Han Chinese population.51 Helminen et al.52 assessed the utility of HbA1c levels in predicting the clinical disease in genetically predisposed children with multiple autoantibodies. They observed that a 10% increase in HbA1c levels in samples obtained 3–12 months apart predicted the diagnosis of clinical disease, suggesting the usefulness of HbA1c as a marker for predicting the time to diagnosis of type 1 diabetes in children with multiple autoantibodies.
HbA1c Test Units
As is usually the case with most of the units, the United States and European Union and other countries do not agree with the units of HbA1c measurements.53 In the US, the HbA1c levels are expressed in terms of percentage of the Diabetes Control and Complications Trial units.54,55 The United Kingdom, New Zealand, and Australia, along with many other European and Asian countries, however, express the HbA1c levels as millimoles per mole, keeping in reference with the recommendations of the International Federation of Clinical Chemistry (IFCC).56,57 The International HbA1c Consensus Committee has recommended that the HbA1c levels must be reported in terms of System International (SI) units (millimoles per mole, with no decimal places), which relate better scientifically to a valid measure of HbA1c. The NGSP still recommends using the units in terms of the percentage with one decimal place, for example, an HbA1c level below 5.7% is considered as normal. The SI units allow for avoiding any confusion between the reported HbA1c levels and the traditional fasting glucose levels expressed as millimoles per liter. All of these units can be easily converted using one of the online calculators and the values are interchangeable including those expressed as mg/dL and also allow for calculating the estimated average glucose results.55 It is important to note that the HbA1c levels, expressed in millimoles per mole, must not be confused with blood glucose levels, which are expressed in millimoles per liter, and provide an average long-term trend. The following equation will help to obtain the SI units from the HbA1c expressed in terms of the percentage: HbA1c SI unit (mmol/mol) (HbA1c NGSP unit in % ×10.93) − 23.50. For example, if the HbA1c is 5.7% (Diabetes Control and Complications Trial), then the HbA1c SI unit (mmol/mol) (IFCC) can be calculated as HbA1c SI unit (mmol/mol) (5.7 × 10.93) − 23.50 = 38.8 mmol/mol (IFCC).56,57 The values, based upon different units, are illustrated in Table 1.
Table 1.
HbA1c as an indicator of diabetes control.
| BLOOD GLUCOSE | STATUS | HbA1c | ||
|---|---|---|---|---|
| mmol/L | mg/dL | % | mmol/mol | |
| 5.4 | 97 | Normal | 5 | 31 |
| 7.0 | 126 | 6 | 42 | |
| 8.6 | 155 | Pre-Diabetes | 7 | 53 |
| 10.2 | 184 | Diabetes | 8 | 64 |
| 11.8 | 212 | Diabetes | 9 | 75 |
| 13.4 | 241 | 10 | 86 | |
| 14.9 | 268 | Diabetes | 11 | 97 |
| 16.5 | 297 | 12 | 108 | |
HbA1c Range
Nondiabetes usually falls within the 4.0%–5.6% HbA1c range. The prediabetes usually has the HbA1c levels as 5.7%–6.4%, while those with 6.4% or higher HbA1c levels have diabetes.12,28 Since diabetes is associated with several comorbidities, the recommendations for individuals with diabetes include a healthy lifestyle (diet and exercise) and maintaining the HbA1c levels below 7.0%. Diabetes-related complications are directly proportional to the levels of HbA1c – the increase in the HbA1c levels also increases the risk of such complications. Using HbA1c as a SOC test also provides some complications for the health-care providers and the patients alike. For example, in anemic (low hemoglobin) patients or those with shorter RBC lifespan (glucose-6-phosphate dehydrogenase deficiency, sickle-cell disease, etc.), the HbA1c levels may be compromised indicating a false “good” result.58 The excessive use of vitamin C, B, and E supplements and increased levels of cholesterol, liver, and kidney diseases can also present abnormally high levels of HbA1c.59,60 Dyslipidemia, which is an imbalance of lipids and fats circulating in the blood stream, is another debilitating disease associated with diabetes.61,62 However, maintaining healthy glucose levels for type 2 diabetics is of paramount importance and may help in preventing micro- and macrovascular complications.63 The HbA1c is also used routinely for testing gestational diabetes among pregnant women.64 Other researchers have utilized the serum fructosamine and blood glucose for the screening of GDM.36,37 Both these tests allow the health-care providers to establish whether the pregnant women, with associated risk facts, had developed diabetes before the pregnancy, which may have gone undiagnosed. If the HbA1c levels are not monitored closely to establish acceptable glycemic control, the higher levels of HbA1c may cause the long-axis cardiac dysfunction in the developing fetus.65,66 There is a direct correlation between reduced HbA1c levels and reduced percentage of mortality. Maintaining healthy levels of the HbA1c significantly ameliorates the risk of cardiovascular diseases among individuals with diabetes.67
Methods for HbA1c Analysis
The HbA1c analysis methods can be divided into two categories: methods based on the charge differences and methods based on the structural differences. Ion-exchange chromatography and capillary electrophoresis belong to the first category, while immunoassay, enzymatic assay, and affinity chromatography belong to the second category. Thus, the routine determination of HbA1c can be achieved by methods based on different principles such as immunoturbidimetry, boronate affinity chromatography, ion-exchange high-performance liquid chromatography (HPLC), and enzymatic assay.68–70 Özçelik et al.71 measured HbA1c in blood from 120 patients with prediabetes and diabetes using three different methods including turbidimetric inhibition immunoassay (TINIA), particle-enhanced immunoturbidimetric assay (PEITT), and HPLC. Although the average HbA1c measured by HPLC (7.52% ± 1.40%) was significantly higher than the other methods, including TINIA (7.12% ± 1.66%) and PEITT (7.26% ± 1.39%), there was good concordance between results of PEITT and HPLC methods (r = 0.9401). The measured total time spent on 120 samples was 45 minutes for TINIA, 39 minutes for PEITT, and 384 minutes for HPLC.71
Recently, capillary 2-FP analyzer has been found to be suitable for HbA1c measurement, and sometimes, it showed some advantages with respect to the HPLC analyzers tested, especially when Hb variants are present.72
Since the HbA1c test is now recommended for diagnosing diabetes and minimal variation of the concentration affects the clinical therapy, it is very important that the results are reliable and interference free. One must become more stringent that any unacceptable results are detected, not reported and each method is evaluated for Hb variant interference.73 There are at least 30 different laboratory methods commercially available to measure the proportion of HbA1c in blood.74 Studies have also reported significant bias among analytical methods to measure HbA1c levels.75 Therefore, standardization and comparability of HbA1c results with different methods appear to be an important issue. In 1995, the IFCC established a Working Group (IFCC WG-HbA1c) to achieve international standardization of HbA1c measurement.76 A reference measurement procedure for HbA1c was developed based on the proteolytic digestion of red cell hemoglobins followed by quantitative peptide mapping by HPLC-mass spectrometry or HPLC-capillary electrophoresis.77 The reliability of HbA1c measurement depends on bias (related to proper calibration) and precision (related to the reproducibility of the method). Quality goals can be derived from biological variation, clinical needs, or the state of the art. For HbA1c, a generally accepted rule of thumb is that clinicians interpret a difference of 5 mmol/mol (0.5%) between successive patient samples as a significant change in glycemic control.78
Accessibility to HbA1c Testing for Diagnosis
Although most laboratories in tertiary care hospitals are well equipped with modern instrumentation including HPLC, many of the primary care centers in low- and middle-income countries do not have access to HPLC, some are still struggling with outdated methods or doubtful point-of-care devices that may not be reliable to monitor diabetes. For accurate results, small clinics and health centers have to be dependent on accredited clinical laboratories for HbA1c analysis. However, this strategy becomes more expensive due to the additional cost of sample transportation. Recently, Fokkema et al.79 evaluated the feasibility of HbA1c measurements from dried blood spots collected on filter paper and compared the HbA1c from filter paper (capillary blood) with HbA1c measured in venous blood. HbA1c on filter paper was highly correlated with routine HbA1c (r = 0.987) while the evaluation of samples collected at home showed comparable HbA1c values by filter paper and routine sampling methods. Most of the participants (83%) said that they would like the filter method to be brought into practice, suggesting that HbA1c sampling on filter paper is an acceptable sampling alternative for analysis of HbA1c.79 It is anticipated that a finger prick sample collection on a filter paper would be more convenient for remote and rural health-care centers to send the samples of HbA1c analysis to dedicated laboratories. Moreover, the good relationship and concordance between the immunoturbidimetric and HPLC methods may support the reliability of properly standardized immunoturbidimetric methods for preliminary screening of diabetes in remote areas.71,80–82
Physiological Changes due to Hemoglobin Glycosylation
An increase in HbA1c as observed in conditions of poor diabetic control has been associated with increased blood viscosity.83 Glycosylation of hemoglobin and increased glucose levels tends to affect RBC properties, lowering the RBC flexibility and increasing their aggregation tendency, leading to increased blood viscosity.84 Glycosylation of hemoglobin may also affect membrane lipid protein interactions in RBCs, altering their internal viscosity, modifying viscoelastic properties of erythrocyte membranes, and impairing RBC deformability.85
There is also evidence that glycosylation of hemoglobin impairs nitric oxide (NO)-related relaxation of human mesenteric vessels.86 Hemoglobin glycosylation is also reported to alter NO binding with thiols resulting in lowered NO bioavailability and impaired vasodilatation in rabbit aortic rings.87 Another mechanism by which glycosylation of hemoglobin is proposed to be vasoactive is via the formation of reactive oxygen species.88 Glycosylation of hemoglobin also lowers oxygen-carrying capacity, thereby promoting hypoxia and its related systemic vascular vasodilatory adaptations and responses.89
Glycosylation of hemoglobin appeared to lead to blood pressure reduction in type 2 diabetic patients untreated for hypertension.90 Since 8%–10% HbA1c is considered to be a threshold beyond which the effects of hemoglobin glycosylation become significant, these investigators determined mean arterial blood pressure for patients not treated for hypertension below and above 9% HbA1c and found significant reduction in mean arterial blood pressure below the threshold (86.2 ± 3.9 mmHg) as compared to above the threshold (93.1 ± 12.5 mmHg).90
Conclusion
The HbA1c is an accurate and easy-to-administer test with on-the-spot results availability and can be an effective tool in establishing the diagnosis of diabetes, especially in low- and middle-income countries and hard-to-reach populations. Even though HbA1c has been endorsed for diagnosis of diabetes, in most of the countries worldwide, some testing strategies and cutoff ranges are still being debated. However, combination of FGT and HbA1c significantly enhances the diagnostic accuracy of these individual tests. The prognostic potential of HbA1c lies in its unique ability of assessing retrospective glycemic control as well as predicting the lipid profile in diabetic patients. As the epidemic of diabetes continues to grow worldwide, HbA1c test may continue to be implemented as part of the diagnostic and prognostic tool, leading to better patient care and successful clinical outcomes.
Footnotes
ACADEMIC EDITOR: Karen Pulford, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 694 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: HAK, SIS. Analyzed the data: HAK, SIS, AE. Wrote the first draft of the manuscript: SIS, HAK. Contributed to the writing of the manuscript: HAK, SIS, AE, AM, MKS. Agree with manuscript results and conclusions: HAK, SIS, AE, AM, MKS. Jointly developed the structure and arguments for the paper: HAK, SIS, AE, MKS. Made critical revisions and approved final version: HAK, SIS, AE, AM, MKS. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Khan MI, Weinstock RS. Chapter 16: Carbohydrates. In: McPherson RA, Pincus MR, editors. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia, PA: Saunders Elsevier; 2011. pp. 210–25. [Google Scholar]
- 2.World Health Organization (WHO) Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus Abbreviated Report of a WHO Consultation. Geneva: WHO; 2011. [PubMed] [Google Scholar]
- 3.Huisman TH, Martis EA, Dozy A. Chromatography of hemoglobin types on carboxymethylcellulose. J Lab Clin Med. 1958;52:312–27. [PubMed] [Google Scholar]
- 4.Bookchin RM, Gallop PM. Structure of haemoglobin A1c: nature of the N-terminal beta chain blocking group. Biochem Biophys Res Commun. 1968;32:86–93. doi: 10.1016/0006-291x(68)90430-0. [DOI] [PubMed] [Google Scholar]
- 5.Rahbar S, Blumenfeld O, Ranney HM. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun. 1969;36:838–43. doi: 10.1016/0006-291x(69)90685-8. [DOI] [PubMed] [Google Scholar]
- 6.Bunn HF, Haney DN, Gabbay KH, Gallop PM. Further identification of the nature and linkage of the carbohydrate in haemoglobin A1c. Biochem Biophys Res Commun. 1975;67:103–9. doi: 10.1016/0006-291x(75)90289-2. [DOI] [PubMed] [Google Scholar]
- 7.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N Engl J Med. 1976;295:417–20. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 8.Ohtsubo K, Chen MZ, Olefsky JM, Marth JD. Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat Med. 2011;17:1067–75. doi: 10.1038/nm.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchetti P. Advanced glycation end products (AGEs) and their receptors (RAGEs) in diabetic vascular disease. Medicographia. 2009;31:257–64. [Google Scholar]
- 10.Acharya AS, Roy RP, Dorai B. Aldimine to ketoamine isomerization (Amadori rearrangement) potential at the individual nonenzymic glycation sites of hemoglobin A: preferential inhibition of glycation by nucleophiles at sites of low isomerization potential. J Protein Chem. 1991;10:345–58. doi: 10.1007/BF01025633. [DOI] [PubMed] [Google Scholar]
- 11.Khan HA, Ola MS, Alhomida AS, Sobki SH, Khan SA. Evaluation of HbA1c criteria for diagnosis of diabetes mellitus: a retrospective study of 12785 type 2 Saudi male patients. Endocr Res. 2014;39:62–6. doi: 10.3109/07435800.2013.828740. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association (ADA) Standards of medical care in diabetes. Diabetes Care. 2014;37:S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 13.International Diabetes Federation (IDF) 2015. Available from: www.idf.org.
- 14.Amin TT, Al-Sultan AI, Ali A. Overweight and obesity and their relation to dietary habits and socio-demographic characteristics among male primary schoolchildren in Al-Hassa, Kingdom of Saudi Arabia. Eur J Nutr. 2008;47:310–8. doi: 10.1007/s00394-008-0727-6. [DOI] [PubMed] [Google Scholar]
- 15.Mahfouz AA, Abdelmoneim I, Khan MY, et al. Obesity and related behaviors among adolescent school boys in Abha city, southwestern Saudi Arabia. J Trop Pediatr. 2007;54:120–4. doi: 10.1093/tropej/fmm089. [DOI] [PubMed] [Google Scholar]
- 16.Al-Rubeaan K, Al-Manaa HA, Khoja TA, et al. Epidemiology of abnormal glucose metabolism in a country facing its epidemic: SAUDI-DM study. J Diabetes. 2015;7:622–32. doi: 10.1111/1753-0407.12224. [DOI] [PubMed] [Google Scholar]
- 17.Gillett MJ. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan HA, Sobki SH, Khan SA. Association between glycaemic control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia. Clin Exp Med. 2007;7:24–9. doi: 10.1007/s10238-007-0121-3. [DOI] [PubMed] [Google Scholar]
- 19.Herman WH, Fajans SS. Hemoglobin A1c for the diagnosis of diabetes: practical considerations. Pol Arch Med Wewn. 2010;120:37–40. [PubMed] [Google Scholar]
- 20.Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–9. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 21.Kim HS, Shin JA, Chang JS, Cho JH, Son HY, Yoon KH. Continuous glucose monitoring: current clinical use. Diabetes Metab Res Rev. 2012;28:73–8. doi: 10.1002/dmrr.2346. [DOI] [PubMed] [Google Scholar]
- 22.Pandit K. Continuous glucose monitoring. Ind J Endocrinol Metab. 2012;16(Suppl 2):S263–6. doi: 10.4103/2230-8210.104056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tansey M, Laffel L, Cheng J, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Satisfaction with continuous glucose monitoring in adults and youths with Type 1 diabetes. Diabet Med. 2011;28:1118–22. doi: 10.1111/j.1464-5491.2011.03368.x. [DOI] [PubMed] [Google Scholar]
- 24.Ehehalt S, Gauger N, Blumenstock G, et al. DIARY-Group Baden-Wuerttemberg Hemoglobin A1c is a reliable criterion for diagnosing type 1 diabetes in childhood and adolescence. Pediatr Diabetes. 2010;11:446–9. doi: 10.1111/j.1399-5448.2009.00633.x. [DOI] [PubMed] [Google Scholar]
- 25.Khan HA, Sobki SH, Alhomida AS. Regression analysis for testing association between fasting blood sugar and glycated hemoglobin in diabetic patients. Biomed Res. 2015;26:604–6. [Google Scholar]
- 26.Juarez DT, Demaris KM, Goo R, Mnatzaganian CL, Wong Smith H. Significance of HbA1c and its measurement in the diagnosis of diabetes mellitus: US experience. Diabetes Metab Syndr Obes. 2014;7:487–94. doi: 10.2147/DMSO.S39092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little RR. Glycated hemoglobin standardization-National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med. 2003;41:1191–8. doi: 10.1515/CCLM.2003.183. [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association (ADA) Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62–9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozkaya G, Ozgu E, Karaca B. The association between estimated average glucose levels and fasting plasma glucose levels. Clinics. 2010;65:1077–80. doi: 10.1590/S1807-59322010001100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HY, Lee SY, Suh S, Kim JH, Lee MK, Park HD. The relationship between estimated average glucose and fasting plasma glucose. Clin Chem Lab Med. 2013;51:2195–200. doi: 10.1515/cclm-2013-0045. [DOI] [PubMed] [Google Scholar]
- 31.Buse JB, Polonsky KS, Burant CF. Chapter 31: Type 2 diabetes mellitus. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams Textbook of Endocrinology. 12th ed. Philadelphia, PA: Saunders Elsevier; 2011. pp. 1386–450. [Google Scholar]
- 32.Lin JD, Chang JB, Wu CZ, et al. Identification of insulin resistance in subjects with normal glucose tolerance. Ann Acad Med Singapore. 2014;43:113–9. [PubMed] [Google Scholar]
- 33.Borai A, Livingstone C, Abdelaal F, Bawazeer A, Keti V, Ferns G. The relationship between glycosylated haemoglobin (HbA1c) and measures of insulin resistance across a range of glucose tolerance. Scand J Clin Lab Invest. 2011;71:168–72. doi: 10.3109/00365513.2010.547947. [DOI] [PubMed] [Google Scholar]
- 34.Kwon SS, Kwon JY, Park YW, Kim YH, Lim JB. HbA1c for diagnosis and prognosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2015;110:38–43. doi: 10.1016/j.diabres.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Fong A, Serra AE, Gabby L, Wing DA, Berkowitz KM. Use of hemoglobin A1c as an early predictor of gestational diabetes mellitus. Am J Obstet Gynecol. 2014;211:641.e1–7. doi: 10.1016/j.ajog.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Khan HA, Sobki SH, Alhomida AS, Khan SA. Paired values of serum fructosamine and blood glucose for the screening of gestational diabetes mellitus: a retrospective study of 165 Saudi women. Ind J Clin Biochem. 2007;22:65–70. doi: 10.1007/BF02912884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan HA, Sobki SH, Alhomida AS. Fluctuations in fasting blood glucose and serum fructosamine in pregnant women monitored on successive antenatal visits. Clin Exp Med. 2006;6:134–7. doi: 10.1007/s10238-006-0109-4. [DOI] [PubMed] [Google Scholar]
- 38.Martin-Timon I, Sevillano-Collantes C, Segura-Galindo A, del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5:444–70. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–11. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu ZH, Liu N, Bai R, et al. HbA1c levels as predictors of ablation outcome in type 2 diabetes mellitus and paroxysmal atrial fibrillation. Herz. 2015;40:130–6. doi: 10.1007/s00059-014-4154-6. [DOI] [PubMed] [Google Scholar]
- 41.Khaw KT, Wareham N, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Prospective Investigation of Cancer and Nutrition (EPIC-Norfolk) BMJ. 2001;322:15–8. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaag AA. Glycemic control and prevention of microvascular and macrovascular disease in the Steno 2 study. Endocr Pract. 2006;12:89–92. doi: 10.4158/EP.12.S1.89. [DOI] [PubMed] [Google Scholar]
- 43.Cicek G, Uyarel H, Ergelen M, et al. Hemoglobin A1c as a prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis. 2011;22:131–7. doi: 10.1097/MCA.0b013e328342c760. [DOI] [PubMed] [Google Scholar]
- 44.Hong LF, Li XL, Guo YL, et al. Glycosylated hemoglobin A1c as a marker predicting the severity of coronary artery disease and early outcome in patients with stable angina. Lipids Health Dis. 2014;13:89. doi: 10.1186/1476-511X-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmer JR, Hoekstra M, Nijsten MW, et al. Prognostic value of admission glycosylated hemoglobin and glucose in nondiabetic patients with ST-segment-elevation myocardial infarction treated with percutaneous coronary intervention. Circulation. 2011;124:704–11. doi: 10.1161/CIRCULATIONAHA.110.985911. [DOI] [PubMed] [Google Scholar]
- 46.Tian L, Zhu J, Liu L, Liang Y, Li J, Yang Y. Hemoglobin A1c and short-term outcomes in patients with acute myocardial infarction undergoing primary angioplasty: an observational multicenter study. Coron Artery Dis. 2013;24:16–22. doi: 10.1097/MCA.0b013e32835b3971. [DOI] [PubMed] [Google Scholar]
- 47.Rebnord EW, Pedersen ER, Strand E, et al. Glycated hemoglobin and long-term prognosis in patients with suspected stable angina pectoris without diabetes mellitus: a prospective cohort study. Atherosclerosis. 2015;240:115–20. doi: 10.1016/j.atherosclerosis.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Han Z, Hao G, Li Y, Dong X, Wang C. Hemoglobin A1c level is not related to the severity of atherosclerosis in patients with acute coronary syndrome. Dis Markers. 2015;2015:1–5. doi: 10.1155/2015/192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kompoti M, Michalia M, Salma V, Diogou E, Lakoumenta A, Clouva-Molyvdas PM. Glycated hemoglobin at admission in the intensive care unit: clinical implications and prognostic relevance. J Crit Care. 2015;30:150–5. doi: 10.1016/j.jcrc.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Pimentel AL, Carvalho LS, Marques SS, et al. Role of glycated hemoglobin in the screening and diagnosis of post-transplantation diabetes mellitus after renal transplantation: a diagnostic accuracy study. Clin Chim Acta. 2015;445:48–53. doi: 10.1016/j.cca.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Li X, Xu X, Liu J, et al. HbA1c and survival in maintenance hemodialysis patients with diabetes in Han Chinese population. Int Urol Nephrol. 2014;46:2207–14. doi: 10.1007/s11255-014-0764-4. [DOI] [PubMed] [Google Scholar]
- 52.Helminen O, Aspholm S, Pokka T, et al. HbA1c predicts time to diagnosis of type 1 diabetes in children at risk. Diabetes. 2015;64:1719–27. doi: 10.2337/db14-0497. [DOI] [PubMed] [Google Scholar]
- 53.Karami A, Baradaran A. Comparative evaluation of three different methods for HbA1c measurement with High-performance liquid chromatography in diabetic patients. Adv Biomed Res. 2014;3:94. doi: 10.4103/2277-9175.129364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Little RR, Sacks DB. HbA1c: how do we measure it and what does it mean? Curr Opin Endocrinol Diabetes Obes. 2009;16:113–8. doi: 10.1097/MED.0b013e328327728d. [DOI] [PubMed] [Google Scholar]
- 55.Loh TP, Sethi SK, Wong MS, Tai ES, Kao SL. Relationship between measured average glucose by continuous glucose monitor and HbA1c measured by three different routine laboratory methods. Clin Biochem. 2015;48:514–8. doi: 10.1016/j.clinbiochem.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 56.Hanas R, John G, Behalf of the International HbA1c Consensus Committee 2010 consensus statement on the worldwide standardization of the hemoglobin A1c measurement. Clin Chem. 2010;56:1362–4. doi: 10.1373/clinchem.2010.150540. [DOI] [PubMed] [Google Scholar]
- 57.Consensus Committee Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care. 2007;30:2399–400. doi: 10.2337/dc07-9925. [DOI] [PubMed] [Google Scholar]
- 58.Forbes JM, Soldatos G, Thomas MC. Below the radar: advanced glycation end products that detour “around the side”: is HbA1c not an accurate enough predictor of long term progression and glycaemic control in diabetes? Clin Biochem Rev. 2005;26:123–34. [PMC free article] [PubMed] [Google Scholar]
- 59.Luk AO, Ma RC, Lau ES, et al. Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: prospective analysis of the Hong Kong Diabetes Registry. Diabetes Metab Res Rev. 2013;29:384–90. doi: 10.1002/dmrr.2404. [DOI] [PubMed] [Google Scholar]
- 60.Xu R, Zhang S, Tao A, Chen G, Zhang M. Influence of vitamin E supplementation on glycaemic control: a meta-analysis of randomised controlled trials. PLoS One. 2014;9:e95008. doi: 10.1371/journal.pone.0095008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:150–9. doi: 10.1038/ncpendmet1066. [DOI] [PubMed] [Google Scholar]
- 62.Khan HA. Clinical significance of HbA1c as a marker of circulating lipids in male and female type 2 diabetic patients. Acta Diabetol. 2007;44:193–200. doi: 10.1007/s00592-007-0003-x. [DOI] [PubMed] [Google Scholar]
- 63.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82. [Google Scholar]
- 64.Capula C, Mazza T, Vero R, Costante G. HbA1c levels in patients with gestational diabetes mellitus: relationship with pre-pregnancy BMI and pregnancy outcome. J Endocrinol Invest. 2013;36:1038–45. doi: 10.3275/9037. [DOI] [PubMed] [Google Scholar]
- 65.Hornberger LK. Maternal diabetes and the fetal heart. Heart. 2006;92:1019–21. doi: 10.1136/hrt.2005.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rizzo G, Pietropolli A, Capponi A, Cacciatore C, Arduini D, Romanini C. Analysis of factors influencing ventricular filling patterns in fetuses of type I diabetic mothers. J Perinat Med. 1994;22:149–57. doi: 10.1515/jpme.1994.22.2.149. [DOI] [PubMed] [Google Scholar]
- 67.Pradhan AD, Rifai N, Buring JE, Ridker PM. HbA1c predicts diabetes but not cardiovascular disease in non-diabetic women. Am J Med. 2007;120:720–7. doi: 10.1016/j.amjmed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weykamp C, John WG, Mosca A. A review of the challenge in measuring hemoglobin A1c. J Diabetes Sci Technol. 2009;3:439–45. doi: 10.1177/193229680900300306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee J, Kim M, Chae H, et al. Evaluation of enzymatic BM Test HbA1c on the JCA-BM6010/C and comparison with Bio-Rad Variant II Turbo, Tosoh HPLC 723 G8, and AutoLab immunoturbidimetry assay. Clin Chem Lab Med. 2013;30:1–8. doi: 10.1515/cclm-2013-0238. [DOI] [PubMed] [Google Scholar]
- 70.Ucar F, Erden G, Ginis Z, et al. Estimation of biological variation and reference change value of glycated hemoglobin (HbA1c) when two analytical methods are used. Clin Biochem. 2013;46:1548–53. doi: 10.1016/j.clinbiochem.2013.05.055. [DOI] [PubMed] [Google Scholar]
- 71.Özçelik F, Yiğiner O, Serdar MA, et al. Comparison of three methods for measurement of HbA1c. Turk J Biochem. 2010;35:344–9. [Google Scholar]
- 72.Dessi M, Pieri M, Pignalosa S, Martino FG, Zenobi R. Performances of capillary electrophoresis and HPLC methods in HbA1c determination: diagnostic accuracy in HbS and HbD-Iran variants’ presence. J Clin Lab Anal. 2015;29:57–60. doi: 10.1002/jcla.21728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin CN, Emery TJ, Little RR, et al. Effects of hemoglobin C, D, E, and S traits on measurements of HbA1c by six methods. Clin Chim Acta. 2012;413:819–21. doi: 10.1016/j.cca.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.International Federation of Clinical Chemistry and Laboratory Medicine, IFCC Scientific Division. Mosca A, et al. Global standardization of glycated hemoglobin measurement: the position of the IFCC Working Group. Clin Chem Lab Med. 2007;45:1077–80. doi: 10.1515/CCLM.2007.246. [DOI] [PubMed] [Google Scholar]
- 75.Marinova M, Altinier S, Caldini A, et al. Multicenter evaluation of haemoglobin A1c assay on capillary electrophoresis. Clin Chim Acta. 2013;424:207–11. doi: 10.1016/j.cca.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 76.Hoelzel W, Miedema K. Development of a reference system for the international standardisation of HbA1c/glycohemoglobin determinations. J Int Fed Clin Chem. 1996;9:62–7. [PubMed] [Google Scholar]
- 77.Kobold U, Jeppsson JO, Dülffer T, Finke A, Hoelzel W, Miedema K. Candidate reference method for HbA1c based on peptide mapping. Clin Chem. 1997;43:1944–51. [PubMed] [Google Scholar]
- 78.Little RR, Rohlfing CL, Sacks DB, National Glycohemoglobin Standardization Program (NGSP) Steering Committee Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57:205–14. doi: 10.1373/clinchem.2010.148841. [DOI] [PubMed] [Google Scholar]
- 79.Fokkema MR, Bakker AJ, de Boer F, Kooistra J, de Vries S, Wolthuis A. HbA1c measurements from dried blood spots: validation and patient satisfaction. Clin Chem Lab Med. 2009;47:1259–64. doi: 10.1515/CCLM.2009.274. [DOI] [PubMed] [Google Scholar]
- 80.Metus P, Ruzzante N, Bonvicini P, Meneghetti M, Zaninotto M, Plebani M. Immunoturbidimetric assay of glycated hemoglobin. J Clin Lab Anal. 1999;13:5–8. doi: 10.1002/(SICI)1098-2825(1999)13:1<5::AID-JCLA2>3.0.CO;2-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hamwi A, Schweiger CR, Veitl M, Schmid R. Quantitative measurement of HbA1c by an immunoturbidimetric assay compared to a standard HPLC method. Am J Clin Pathol. 1995;104:89–95. doi: 10.1093/ajcp/104.1.89. [DOI] [PubMed] [Google Scholar]
- 82.Groche D, Hoeno W, Hoss G, Vogt B, Herrmann Z, Witzigmann A. Standardization of two immunological HbA1c routine assays according to the new IFCC reference method. Clin Lab. 2003;49:657–61. [PubMed] [Google Scholar]
- 83.Leiper JM, Lowe GD, Anderson J, et al. Effects of diabetic control and biosynthetic human insulin on blood rheology in established diabetics. Diabetes Res. 1984;1:27–30. [PubMed] [Google Scholar]
- 84.Buhler I, Walter R, Reinhart WH. Influence of D- and L-glucose on erythrocytes and blood viscosity. Eur J Clin Invest. 2001;31:79–85. doi: 10.1046/j.1365-2362.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- 85.Watala C, Witas H, Olszowska L, Piasecki W. The association between erythrocyte internal viscosity, protein non-enzymatic glycosylation and erythrocyte membrane dynamic properties in juvenile diabetes mellitus. Int J Exp Pathol. 1992;73:655–63. [PMC free article] [PubMed] [Google Scholar]
- 86.Vallejo S, Angulo J, Peiro C, et al. Highly glycated oxyhaemoglobin impairs nitric oxide relaxations in human mesenteric microvessels. Diabetologia. 2000;43:83–90. doi: 10.1007/s001250050011. [DOI] [PubMed] [Google Scholar]
- 87.James PE, Lang D, Tufnell-Barret T, Milsom AB, Frenneaux MP. Vasorelaxation by red blood cells and impairment in diabetes: reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ Res. 2004;94:976–83. doi: 10.1161/01.RES.0000122044.21787.01. [DOI] [PubMed] [Google Scholar]
- 88.Angulo J, Sanchez-Ferrer CF, Peiro C, et al. Impairment of endothelium dependent relaxation by increasing percentages of glycosylated human hemoglobin. Possible mechanisms involved. Hypertension. 1996;28:583–92. doi: 10.1161/01.hyp.28.4.583. [DOI] [PubMed] [Google Scholar]
- 89.Paffett ML, Walker BR. Vascular adaptations to hypoxia: molecular and cellular mechanisms regulating vascular tone. Essays Biochem. 2007;43:105–20. doi: 10.1042/BSE0430105. [DOI] [PubMed] [Google Scholar]
- 90.Cabrales P, Salazar Vázquez MA, Salazar Vázquez B, Rodríguez-Morán M, Intaglietta M, Guerrero-Romeros F. Blood pressure reduction due to hemoglobin glycosylation in type 2 diabetic patients. Vasc Health Risk Manag. 2008;4:917–22. doi: 10.2147/vhrm.s3077. [DOI] [PMC free article] [PubMed] [Google Scholar]