Abstract
Flavonoids are important plant secondary metabolites, which protect plants from various stresses, including herbivory. Plants differentially respond to insects with different modes of action. High performance liquid chromatography (HPLC) fingerprinting of phenols of groundnut (Arachis hypogaea) plants with differential levels of resistance was carried out in response to Helicoverpa armigera (chewing insect) and Aphis craccivora (sucking pest) infestation. The genotypes used were ICGV 86699, ICGV 86031, ICG 2271 (NCAc 343), ICG 1697 (NCAc 17090), and JL 24. Most of the identified compounds were present in H. armigera- and A. craccivora-infested plants of ICGV 86699. Syringic acid was observed in all the genotypes across the treatments, except in the uninfested control plants of ICG 2271 and aphid-infested plants of ICG 1697. Caffeic acid and umbelliferone were observed only in the H. armigera-infested plants of ICGV 86699. Similarly, dihydroxybenzoic acid and vanillic acid were observed in H. armigera- and aphid-infested plants of ICG 2271 and JL 24, respectively. The peak areas were transformed into the amounts of compounds by using internal standard peak areas and were expressed in nanograms. Quantities of the identified compounds varied across genotypes and treatments. The common compounds observed were chlorogenic, syringic, quercetin, and ferulic acids. These results suggest that depending on the mode of feeding, flavonoids are induced differentially in groundnut plants.
Keywords: flavonoids, HPLC, herbivory, plant defense, induced resistance
Introduction
Plants produce a number of secondary metabolites in response to insect herbivory, pathogens, and other stresses.1,2 Secondary metabolites such as phenols are the most important and abundant group of plant defensive compounds involved in defense against herbivory.1–5 Flavonoids and isoflavonoids directly affect insect behavior, growth, and development by influencing the steroid hormone systems.3,5,6 They are powerful antibiotics and form complexes with various enzymes, thus restricting the availability of dietary proteins to insect pests.7,8 Moreover, phenols are oxidized by plant defensive enzymes into toxic compounds such as quinones, which in turn bind to leaf proteins, inhibiting digestion in herbivores.9,10 They also act as scavengers of a number of highly reactive and unstable reactive oxygen species.11 Flavonoids such as chlorogenic and caffeic acids are highly toxic to insect pests.12,13 Some flavonoids including isorhamnetin-3-sophoroside-7-glucoside and kaempferol-3,7-diglucoside act as feeding deterrents against Mamestra configurata (Walk.).14 In addition, phenols attract the natural enemies of insect pests, thus indirectly defending the plants.15 Alteration in phenols occurs in plants when they encounter various stresses including insect herbivory.2,4,5,7,16
Induction of flavonoids in plants in response to insect pests will serve as important biochemical markers for induced resistance against insect pests and for the selection of resistant lines in breeding programmes. To test the hypothesis that induction of flavonoids in plants depends on the insect-feeding habits and the levels of host plant resistance, groundnut genotypes with differential levels of resistance were infested with Helicoverpa armigera (Hub.) and Aphis craccivora Koch, chewing and sap-sucking pests, respectively.
Materials and Methods
Groundnut (Arachis hypogaea L.) plants and insect infestation
Five groundnut genotypes ICGV 86699, ICGV 86031, ICG 2271 (NCAc 343), ICG 1697 (NCAc 17090) (with moderate levels of resistance to insects), and JL 24 (susceptible check)17 were grown under greenhouse conditions at the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, Andhra Pradesh, India. The greenhouse was cooled by desert coolers to maintain the temperature at 26°C ± 5°C and the relative humidity at 65% ± 5%. The H. armigera larvae were obtained from the stock culture maintained under laboratory conditions (26°C ± 1°C; 11 ± 0.5 hours photoperiod, and 75% ± 5% RH) from the insect-rearing laboratory at ICRISAT. The aphid culture was maintained on groundnut plants under glasshouse conditions. Fifteen pots, with three plants in each pot, were maintained for each genotype. A 20-day-old plant in each pot was enclosed in a plastic cage (11 cm diameter and 26 cm in height). In each genotype, five caged plants were infested with 10 H. armigera neonates and five with 10 apteral adults of A. craccivora. Five plants similarly enclosed in plastic cages were maintained as uninfested control.
HPLC fingerprinting
After six days of insect infestation, leaves were collected from insect-infested and uninfested control plants and extracted in methanol for HPLC fingerprinting. The leaves were extracted thrice with methanol, and the extracts were pooled together. There were three replicates for each treatment/genotype. The HPLC system used was of Waters Series consisting of a Separation module (2695) with Controller (600) and equipped with photodiode array detector (2996). Methanolic extracts were filtered through a polyvinyl difluoride filter (PVDF; Millipore, Millex-GV, filter 0.22 μm diameter) membrane before HPLC analysis. Separation of the compounds was performed on an Atlantis C18 column (4.6 mm × 250 mm) at a flow rate of 1 mL minute−1 for 40 minutes with 20 μL injected volume of the extract. The column was used at ambient temperature. The mobile phase was water (A) and acetonitrile (B) (v/v) containing 1% orthophosphoric acid. The mobile phase was filtered through a 0.45 μm membrane filter and deaerated using a sonicator (D-Compact, 443). The elution profile used was 0–5 minutes, 65% A, 35% B (isocratic); 5–12 minutes, 35%–40% B in A (linear gradient); 12–20 minutes, 40%–45% B in A (linear gradient); 20–30 minutes, 55% A, 45% B (isocratic); 30–35 minutes, 45%–35% B in A (linear gradient); and 35–40 minutes, 65% A, 35% B. All compounds were identified by comparing their HPLC retention times to those of authentic standards. The peak area of each identified compound was transformed into quantities of the compounds and was expressed in nanograms using internal standard peak areas.
Statistical analysis
The quantities of the compounds across treatments and genotypes were subjected to analysis of variance using SPSS (15.1). The mean values were separated by Tukey’s/multiple comparison tests when the treatment effects were statistically significant (P ≤ 0.05).
Results
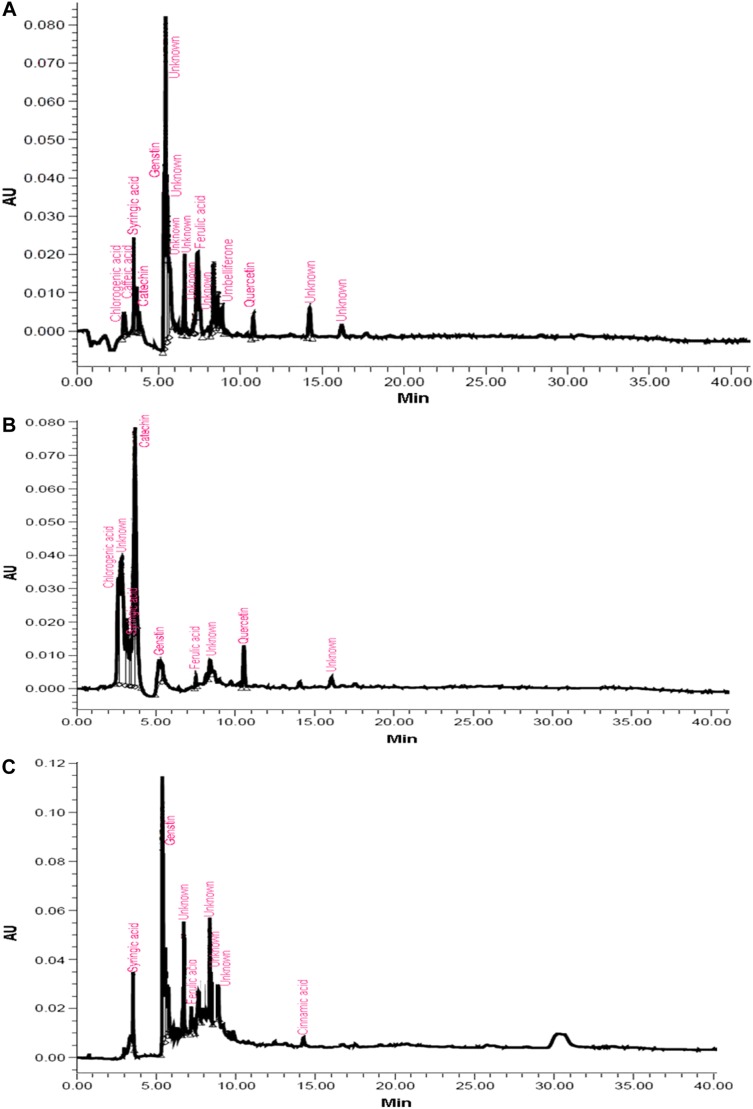
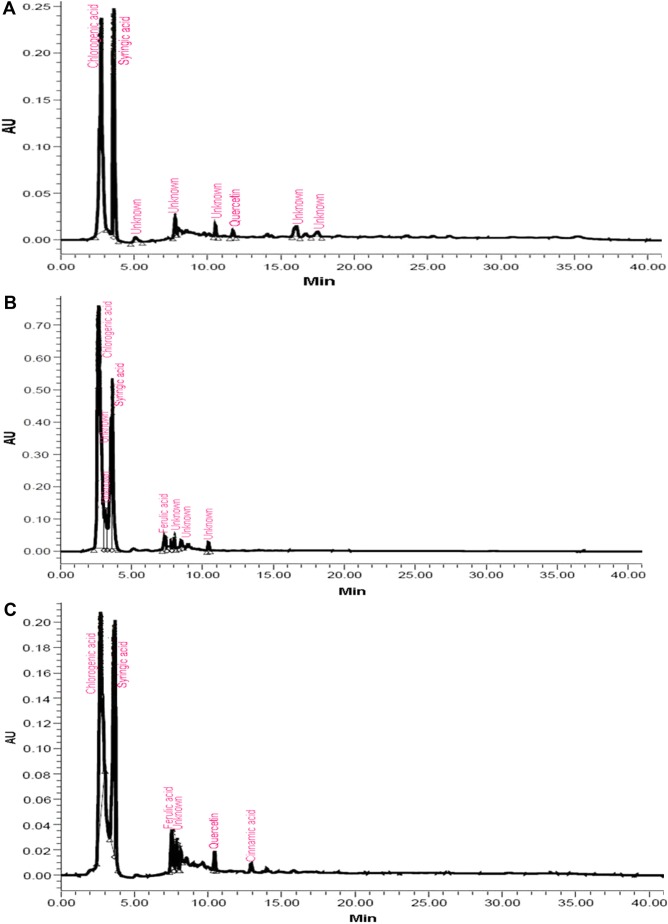
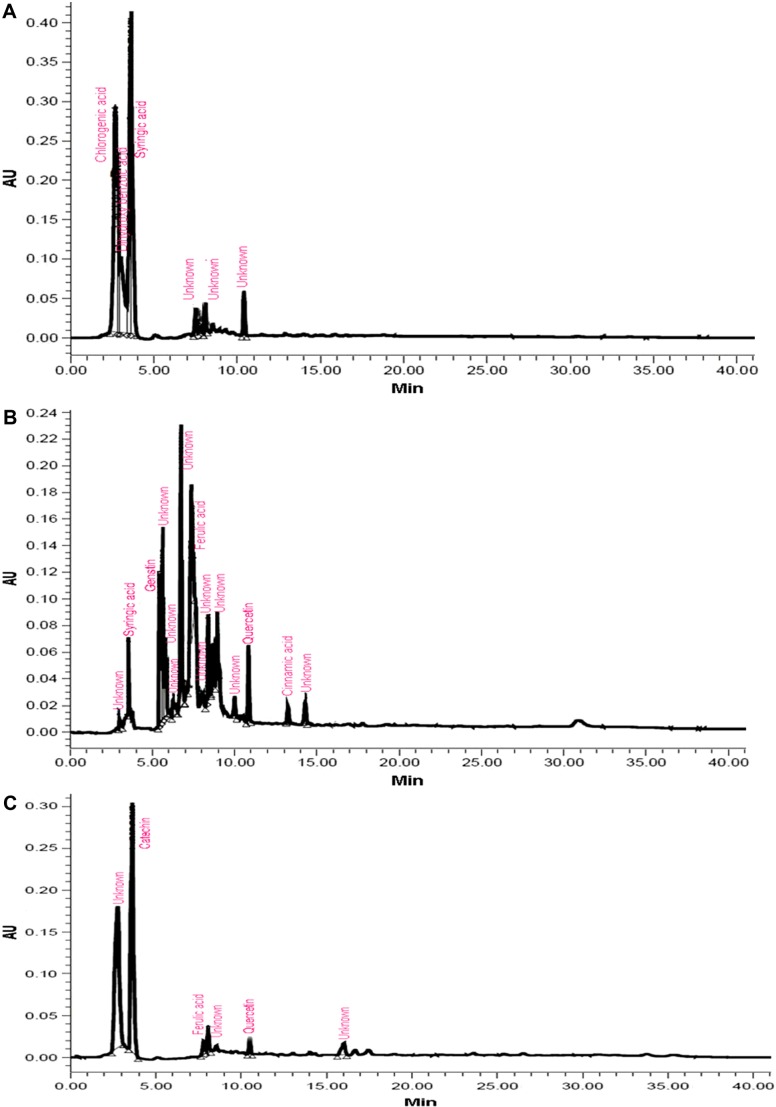
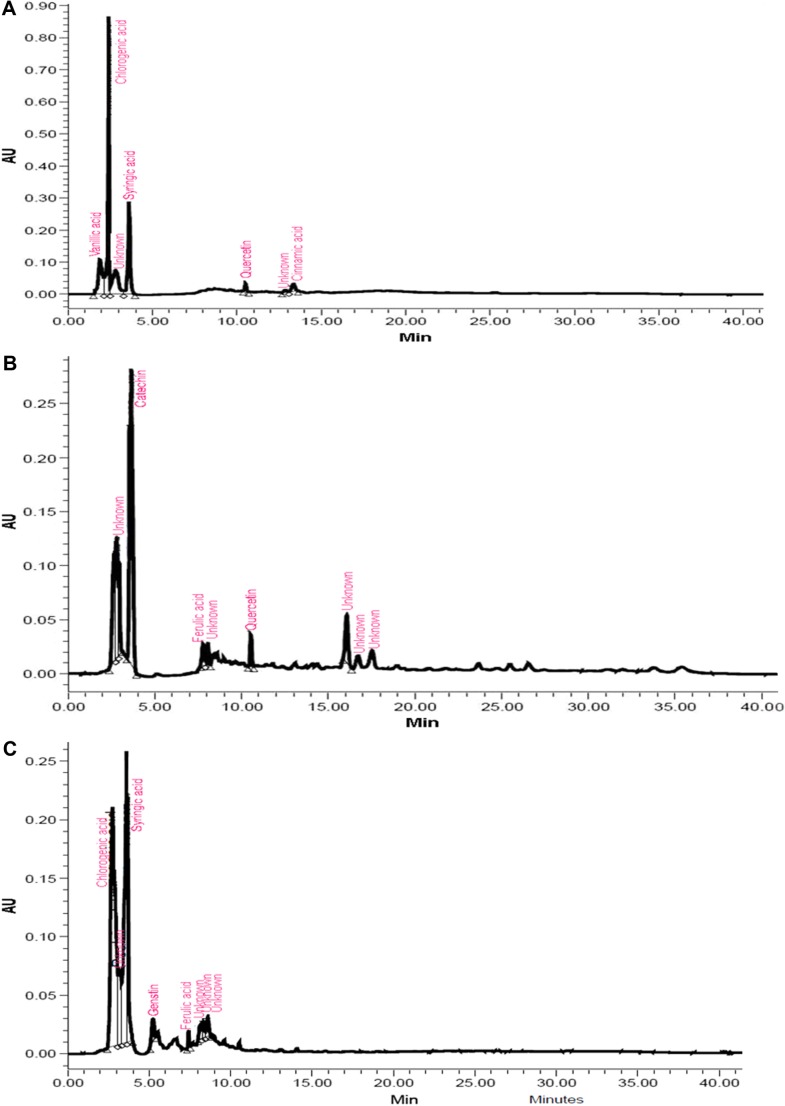
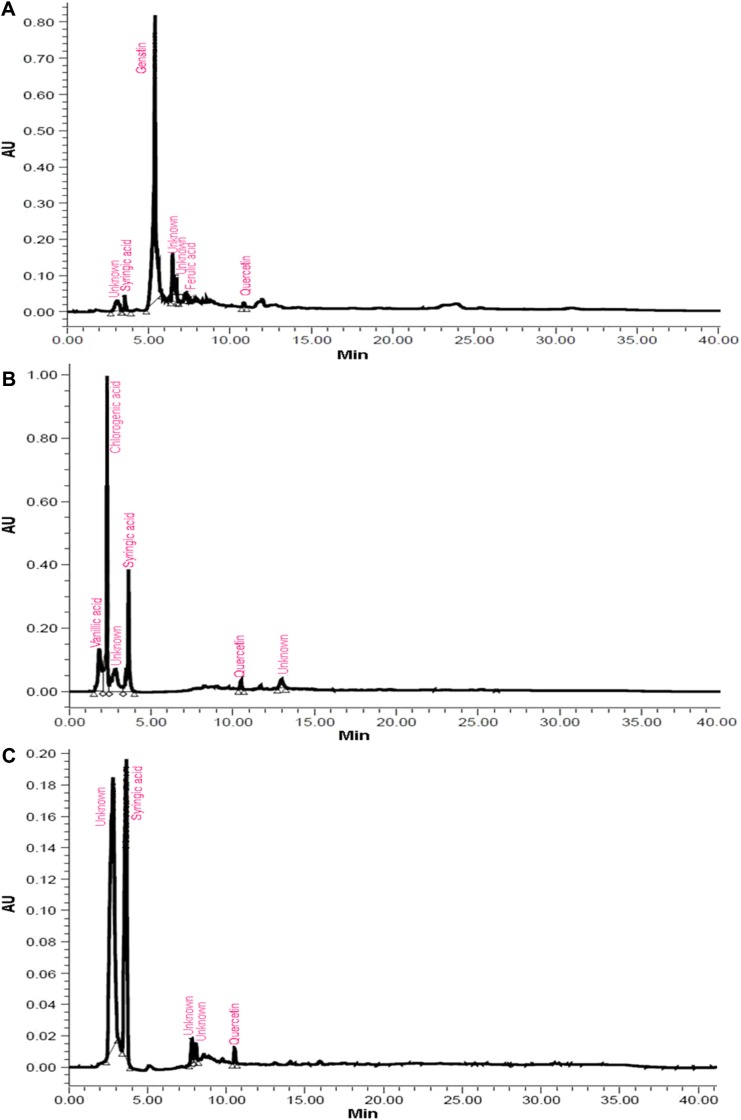
Most of the identified compounds were present in H. armigera- and aphid-infested plants of ICGV 86699. Syringic acid was observed in all the genotypes and treatments, except in the control and aphid-infested plants of ICG 2271 and ICG 1697, respectively. The quantities of identified compounds differed significantly across treatments and the genotypes (Table 1). Concentration of chlorogenic acid was significantly greater in A. craccivora (32,954.21 ng)-infested plants of ICGV 86031 followed by H. armigera-infested plants of ICG 1697 (11,477.63 ng), uninfested control plants of ICG 1697 (9719.77 ng), and H. armigera-infested plants of ICG 2271 (9222.49 ng). Aphid-infested plants of ICGV 86031 had significantly greater syringic acid content (5942.56 ng) than the rest of the genotypes and/or treatments. This was followed by the infested plants of ICG 1697 (4304.26 ng). Catechin content was greater in the aphid-infested plants of ICG 1697 (5813.26 ng) followed by the uninfested control plants of ICG 2271 (5178.97 ng) across all the genotypes and/or treatments. Genistin percentage peak area was also significantly greater in H. armigera-infested plants of JL 24 (64,493.32 ng). The uninfested control plants of ICGV 86699 had greater genistin content (5627.37 ng) than in the control plants of rest of the genotypes. Ferulic acid content was significantly greater in aphid-infested plants of ICG 2271 (1775.45 ng) than the rest of the treatments and/or genotypes. Significantly greater quercetin content was found in aphid-infested plants of ICG 2271 (308.91 ng) than the rest of the treatments and/or genotypes. Cinnamic acid content was high in H. armigera-infested plants of ICG 1697 (308.03 ng). Caffeic acid and umbelliferone were observed only in H. armigera-infested plants of ICGV 86699 (20.91 and 87.80 ng, respectively). Dihydroxybenzoic acid was reported only in H. armigera-infested plants of ICG 2271 (1201.06 ng), while vanillic acid was observed in aphid-infested plants of JL 24 (190.33 ng). The peak heights and areas were greater in the insect-infested plants than in the uninfested control plants (data not shown). The number of peaks also varied across the genotypes and treatments. The H. armigera-infested plants of ICGV 86699 had more number of peaks (16) as compared to A. craccivora-infested (9) and uninfested control plants (8; Fig. 1). H. armigera-infested and A. craccivora-infested plants of ICGV 86031 showed equal number of peaks (eight each). The uninfested control plants had six peaks (Fig. 2). In ICG 2271, more number of peaks were observed in A. craccivora-infested plants (15) as compared to the H. armigera-infested (6) and uninfested control plants (6; Fig. 3). The number of peaks observed in HPLC chromatogram of ICG 1697 was seven in H. armigera-infested, eight in A. craccivora-infested, and eight in uninfested control plants (Fig. 4). The chromatogram of JL 24 had seven, six, and five peaks, respectively, for H. armigera, A. craccivora, and uninfested control plants (Fig. 5). Chlorogenic and syringic acids were the main compounds found in all the genotypes. The H. armigera-infested plants of ICGV 86699 and A. craccivora-infested plants of ICG 2271 had chlorogenic acid, caffeic acid, syringic acid, catechin, genistin, ferulic acid, vanillic acid, umbelliferone, and quercetin as the main identified compounds in the former and syringic acid, genistin, ferulic acid, and cinnamic acid in the latter. Moreover, chlorogenic and syringic acids were found in almost all the chromatograms.
Table 1.
Amounts of the identified compounds in HPLC chromatograms of H. armigera-, aphid-infested, and uninfested control plants of groundnut.
| PEAK (COMPOUND) | AMOUNT OF THE COMPOUNDS (ng) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICGV 86699 | ICGV 86031 | ICG 2271 | ICG 1697 | JL 24 | |||||||||||
| H. armigera | APHID | CONTROL | H. armigera | APHID | CONTROL | H. armigera | APHID | CONTROL | H. armigera | APHID | CONTROL | H. armigera | APHID | CONTROL | |
| Chlorogenic acid | 2339.65fA | 1474.41gB | NA | 7064.34dB | 32954.21aA | 5987.67eC | 9222.49c | NA | NA | 11477.63bA | NA | 9719.77cB | NA | 2282.57f | NA |
| Syringic acid | 101.90fgA | 135.64fA | 93.94fgAB | 2295.81deB | 5942.56aA | 2209.69deB | 3795.67cA | 167.73fB | NA | 4304.26bA | NA | 3864.49cB | 277.23fC | 3723.02cA | 3527.23dB |
| Catechin | 786.89bcCD | 1031.21bC | NA | NA | NA | NA | NA | NA | 5178.97aB | NA | 5813.26aA | NA | NA | NA | NA |
| Genistin | 120.51fF | 983.09eE | 5726.37bB | NA | NA | NA | NA | 2534.14cC | NA | NA | NA | 1843.45dD | 64493.32aA | NA | NA |
| Ferulic acid | 345.43bB | 12.19deDE | 89.24dD | NA | 74.46dD | 372.28bB | NA | 1775.46aA | 179.79cCD | NA | 239.88bcBC | 81.73dD | 62.04dD | NA | NA |
| Quercetin | 27.73cdC | 77.04cC | NA | 52.61cC | NA | 78.10cC | NA | 308.91aA | 122.01bBC | 141.76bB | 173.01bB | NA | 89.73cC | 160.99bB | 56.63cC |
| Cinnamic acid | NA | NA | 8.27cC | NA | NA | 10.29cB | NA | 68.96bB | NA | 308.03aA | NA | NA | NA | NA | NA |
| Caffeic acid | 20.91 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Umbelliferone | 87.80 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Dihydroxybenzoic acid | NA | NA | NA | NA | NA | NA | 1201.06 | NA | NA | NA | NA | NA | NA | NA | NA |
| Vanillic acid | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 190.33 | NA |
Notes: Values within a row with the same superscript upper case letters are not significantly different (P ≤ 0.05) across the treatments within a genotype. Values within a row with the same superscript lowercase letters are not significantly different (P ≤ 0.05) across the genotypes within a treatment.
Abbreviations: H. armigera, plants infested with Helicoverpa armigera; aphid, plants infested with Aphis craccivora.
Figure 1.
HPLC chromatogram of ICGV 86699 plants infested with (A) H. armigera, (B) A. craccivora, and (C) untreated control plants.
Figure 2.
HPLC chromatogram of ICGV 86031 plants infested with (A) H. armigera, (B) A. craccivora, and (C) untreated control plants.
Figure 3.
HPLC chromatogram of ICG 2271 plants infested with (A) H. armigera, (B) A. craccivora, and (C) untreated control plants.
Figure 4.
HPLC chromatogram of ICG 1697 plants infested with (A) H. armigera, (B) A. craccivora, and (C) untreated control plants.
Figure 5.
HPLC chromatogram of JL 24 plants infested with (A) H. armigera, (B) A. craccivora, and (C) untreated control plants.
Discussion
The role of flavonoids in plants against insect herbivory has been well documented.11,18 They act as antifeedants and also affect the insect through antibiosis, thus regulating insect growth and development.12,19 The HPLC fingerprinting showed the presence/absence of peaks in H. armigera and A. craccivora-infested and uninfested groundnut genotypes. More number of peaks were observed in insect-infested plants, especially in the insect-resistant genotypes (ICGV 86699, ICGV 86031, ICG 2271, and ICG 1697) than in the susceptible check, JL 24. The content of the identified compounds differed across the treatments and the genotypes. The chlorogenic acid content in the insect-infested plants was greater than the control plants, except in ICGV 1697. Syringic acid of aphid-infested plants of ICGV 86031 was higher than the rest of the genotypes and/or treatments. Ferulic amounts were greater in the aphid-infested plants of ICG 2271 than the rest of the treatments and across genotypes. Peak areas and heights also differed across treatments and the genotypes. The most common compounds observed in insect-resistant genotypes were chlorogenic, syringic, quercetin, and ferulic acids. The ICGV 86031 plants infested with H. armigera showed larger peaks corresponding to chlorogenic acid and syringic acid. Infestation by A. craccivora also induced the production of several phenolic compounds, including chlorogenic acid, syringic acid, and ferulic acid, in ICGV 86699, ICGV 86031, ICG 2271, and ICG 1697. Caffeic acid and umbelliferone were observed only in H. armigera-infested plants of ICGV 86699. Similarly, dihydroxybenzoic acid and vanillic acid were observed in H. armigera- and aphid-infested plants of ICG 2271 and JL 24, respectively. Differences in induction of phenolics by H. armigera and A. craccivora were possibly due to the differences in the nature of damage by these insects. In addition, differences in the presence of various unknown compounds were observed in the insect-infested plants, although some of them were also expressed constitutively in the uninfested control plants. Many of these compounds are deployed by the plants against insect pests.2,4,9,20 Chewing and sap-sucking insects induce similar defensive responses in groundnut with varying degrees.20 The results showed that depending on the mode of feeding, flavonoids are induced differentially. Chlorogenic acid is considered as an important component of host plant resistance to insects in groundnut.21 The toxicity of chlorogenic acid against insect pests is ascribed to the production of the highly reactive chlorogenoquinone that reacts with nucleophilic –SH and –NH2 groups in proteins, thus reducing their availability to insect pests.7 Furthermore, differences in the number of peaks in control plants in different genotypes showed the variation of constitutive levels of resistance across the genotypes. Sharma and Norris3 observed the negative effect of flavonoids from soybean on Trichoplusia ni (Hub.). Flavonoid production has been found to confer resistance in Arabidopsis thaliana (L.) against Spodoptera frugiperda (J.E. Smith).22 Rutin (quercetin 3-O-glucosyl rhamnoside) and genistin negatively affected the behavior and physiology of Helicoverpa zea (Boddie) and T. ni in soybean.23,24 Flavonoids drastically affect the insect growth and development when incorporated in artificial diets, for example, incorporation of rutin in artificial diet resulted in poor growth and development of a number of insect pests.21,25 H. armigera larvae fed on flavonoid-containing diet exhibited reduced larval survival and weights, which has been attributed to the alteration in insect digestive and detoxifying enzymes.12 The flavonoids quercetin dehydrate, rutin hydrate, and naringin at 1000 ppm showed mortalities of 85%, 93%, and 86%, respectively, in Eriosoma lanigerum (Haus.) in a twig dip assay.25 In addition, flavonoids scavenge the free radicals including reactive oxygen species and reduce their formation by chelating metals.11 However, some flavonoids have been found to act as feeding stimulants.26
Conclusion
Differential induction of plant secondary metabolites was observed in groundnut plants due to feeding by the chewing (H. armigera) and sucking (A. craccivora) type of insect pests, and the response varied between the insect-resistant and -susceptible genotypes. Some compounds such as caffeic acid and umbelliferone were found only in H. armigera-infested plants of ICGV 86699, while dihydroxybenzoic acid was observed in H. armigera-infested plants of the insect-resistant genotype, ICG 2271, and vanillic acid in aphid-infested plants of the susceptible genotype, JL 24.
Acknowledgments
We are thankful to the Entomology staff, ICRISAT, for assistance in carrying out the experiments.
Footnotes
ACADEMIC EDITOR: Paul-André Calatayud, Editor in Chief
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,956 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: ARW and HCS. Analyzed the data: ARW and SPS. Wrote the first draft of the manuscript: ARW. Contributed to the writing of the manuscript: ARW and SPS. Agreed with manuscript results and conclusions: ARW, SPS, and HCS. Jointly developed the structure and arguments for the paper: ARW, SPS, and HCS. Made critical revisions and approved the final version: HCS. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Sharma HC, Sujana G, Rao DM. Morphological and chemical components of resistance to pod borer, Helicoverpa armigera in wild relatives of pigeon pea. Arthropod Plant Interact. 2009;3:151–161. [Google Scholar]
- 2.Usha Rani P, Jyothsna Y. Biochemical and enzymatic changes in rice as a mechanism of defense. Acta Physiol Plant. 2010;32:695–701. [Google Scholar]
- 3.Sharma HC, Norris DM. Chemical basis of resistance in soya bean to cabbage looper Trichoplusia ni. J Agric Food Chem. 1991;55:353–364. [Google Scholar]
- 4.Stevenson PC, Blaney WL, Simmonds MSJ, Wightman JA. The identification and characterization of resistance in wild species of Arachis to Spodoptera litura (Lepidoptera: Noctuidae) Bull Entomol Res. 1993;83:421–429. [Google Scholar]
- 5.Simmonds MSJ. Flavonoid-insect interactions: recent advances in our knowledge. Phytochemistry. 2003;64:21–30. doi: 10.1016/s0031-9422(03)00293-0. [DOI] [PubMed] [Google Scholar]
- 6.Oberdorster E, Clay MA, Cottam DM, Wilmot FA, Mclachlan JA, Milner MJ. Common phytochemicals are ecdysteroid agonists and antagonists: a possible evolutionary link between vertebrate and invertebrate steroid hormones. J Steroid Biochem Mol Biol. 2001;77:229–238. doi: 10.1016/s0960-0760(01)00067-x. [DOI] [PubMed] [Google Scholar]
- 7.Felton GW, Donato KK, Broadway RM, Duffey SS. Impact of oxidized plant phenolics on the nutritional quality of dietary protein to a noctuid herbivore Spodoptera exigua. J Insect Physiol. 1992;38:277–285. [Google Scholar]
- 8.Renwick JAA, Zhang W, Haribal M, Attygalle AB, Lopez KD. Dual chemical barriers protect a plant against different larval stages of an insect. J Chem Ecol. 2001;27:1575–1583. doi: 10.1023/a:1010402107427. [DOI] [PubMed] [Google Scholar]
- 9.Ramiro DA, Guerreiro-Filho O, Mazzafera P. Phenol contents, oxidase activities, and the resistance of coffee to the leaf miner Leucoptera coffeella. J Chem Ecol. 2006;32:1977–1988. doi: 10.1007/s10886-006-9122-z. [DOI] [PubMed] [Google Scholar]
- 10.Bhonwong A, Stout MJ, Attajarusit J, Tantasawat P. Defensive role of tomato polyphenol oxidase against cotton bollworm (Helicoverpa armigera) and Beet armyworm (Spodoptera exigua) J Chem Ecol. 2009;35:28–38. doi: 10.1007/s10886-008-9571-7. [DOI] [PubMed] [Google Scholar]
- 11.Treutter D. Significance of flavonoids in plant resistance: a review. Environ Chem Lett. 2006;4:147–157. [Google Scholar]
- 12.War AR, Paulraj MG, Hussain B, Buhroo AA, Ignacimuthu S, Sharma HC. Effect of plant secondary metabolites on legume pod borer Helicoverpa armigera. J Pest Sci. 2013;86:399–408. [Google Scholar]
- 13.Summers CB, Felton GW. Prooxidant effects of phenolic acids on the generalist herbivore Helicoverpa zea (Lepidoptera: Noctuidae): potential mode of action for phenolic compounds in plant anti-herbivore chemistry. Insect Biochem Mol Biol. 1994;24:943–953. [Google Scholar]
- 14.Onyilagha JC, Lazorko J, Gruber MY, Soroka JJ, Erlandson MA. Effect of flavonoids on feeding preference and development of the crucifer pest Mamestra configurata Walker. J Chem Ecol. 2004;30:109–124. doi: 10.1023/b:joec.0000013185.62475.65. [DOI] [PubMed] [Google Scholar]
- 15.Heil M. Indirect defense via tritophic interactions. New Phytologist. 2008;178:41–61. doi: 10.1111/j.1469-8137.2007.02330.x. [DOI] [PubMed] [Google Scholar]
- 16.War AR, Munghate RS, Sharma HC. Expression of different mechanisms of resistance to insects in groundnut under field conditions. Phytoparasitica. 2015;43(5):669–677. [Google Scholar]
- 17.Sharma HC, Pampathy G, Dwivedi SL, Reddy LJ. Mechanism and diversity of resistance to insect pests in wild relatives of groundnut. J Econ Entomol. 2003;96(6):1886–1897. doi: 10.1093/jee/96.6.1886. [DOI] [PubMed] [Google Scholar]
- 18.Erb M, Lenk C, Degenhardt J, Turlings TCJ. The underestimated role of roots in defense against leaf attackers. Trends Plant Sci. 2009;14:653–659. doi: 10.1016/j.tplants.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto M, Kumeda S, Komai K. Insect antifeedant flavonoids from Gnaphalium affine D. Don. J Agric Food Chem. 2000;48:1888–1891. doi: 10.1021/jf990282q. [DOI] [PubMed] [Google Scholar]
- 20.War AR, Paulraj MG, Ignacimuthu S, Sharma HC. Defensive responses in groundnut against chewing and sap sucking insects. J Plant Growth Regul. 2013;32:259–272. [Google Scholar]
- 21.Mallikarjuna N, Kranthi KR, Jadhav DR, Kranthi S, Chandra S. Influence of foliar chemical compounds on the development of Spodoptera litura (Fab.) in interspecific derivatives of groundnut. J Appl Entomol. 2004;128:321–328. [Google Scholar]
- 22.Johnson ET, Dowd PF. Differentially enhanced insect resistance, at a cost, in Arabidopsis thaliana constitutively expressing a transcription factor of defensive metabolites. J Agric Food Chem. 2004;52:5135–5138. doi: 10.1021/jf0308049. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann-Campo CB. Role of Flavonoids in the Natural Resistance of Soyabean to Heliothis virescens (F.) and Trichoplusia ni (Hubner) [PhD Thesis] Reading, Berkshire: The University of Reading; 1995. p. 165. [Google Scholar]
- 24.Hoffmann-Campo C, Harborne J, McCaffery A. Pre-ingestive and post-ingestive effects of soya bean extracts and rutin on Trichoplusia ni growth. Entomol Exp Appl. 2001;98:181–194. [Google Scholar]
- 25.Atteyat M, Abu-Romann S, Abu-Darwish M, Ghabeish I. Impact of flavonoids against woolly apple aphid, Eriosoma lanigerum (Hausmann) and its sole parasitoid, Aphelinus mali (Hald.) J Agric Sci. 2012;4:227–236. [Google Scholar]
- 26.van Loon JJA, Wang CZ, Nielsen JK, Gols R, Qiu YT. Flavonoids from cabbage are feeding stimulants for diamondback moth larvae additional to glucosinolates: chemoreception and behavior. Entomol Exp Appl. 2002;104:27–34. [Google Scholar]