Abstract
Background
This investigation aimed to evaluate changes in apparent diffusion coefficient (ADC) and fractional anisotropy (FA) of white matter injury (WMI) in preterm neonates with hypoxic-ischemic encephalopathy (HIE) using diffusion tension imaging (DTI).
Material/Methods
Thirty-eight neonates less than 37 weeks of gestation with leukoencephalopathy (as observation group) and 38 full-term infants with no leukoencephalopathy (as control group) were selected from the Neonatal Care Center in Taian Central Hospital from January 2012 to December 2013. A DTI scan was obtained within 1 week after birth.
Results
In the observation group, on both sides the ADC values in regions of interest (ROI) of white matter, lesions were greater and FA values were lower than in the control group. ADC and FA values in genu and splenum of corpus callosum were statistically different between the mild and severe injury groups (p<0.05).
Conclusions
This study demonstrates that DTI provides sensitive detection and early diagnosis of WMI in brains of premature infants with HIE.
MeSH Keywords: Brain Injuries, Leukoencephalopathies, Magnetic Resonance Imaging
Background
Hypoxic ischemia in preterm neonates can easily damage the white matter, especially around the cerebral ventricle. Since the blood volume of vessels flowing from the brain surface toward the ventricle is only a quarter of that flowing towards grey matter, hypoxic ischemia can easily cause damage and malacia. Moreover, oligodendrocytes of neonates born at 34 weeks of gestation are in the period of high differentiation and are very susceptible to damage [1]. There are 2 important causes of neonatal brain paralysis and mental retardation: corticospinal tract traveling through the regions around the ventricle, and easy damage of motor function [2–4]. According to the literature, every year 1.2 million neonates are born with hypoxic-ischemic brain damage and 1 million of them end up with permanent dysfunction of the nervous system [5]. Therefore, early diagnosis and treatment is of great significance in preventing irreversible damage to brain tissues.
For neonates with damaged white matter of the brain, diffusion tensor imaging (DTI) is currently the only non-invasive technology available to study the cerebral white matter fibers. It can directly observe abnormalities of white matter fiber bundles caused by hypoxic ischemia, such as rarefaction, breakage, and damage, and can reflect the running, circumventing, and crossing of cerebral white matter fiber bundles [6]. With DTI, fractional anisotropy of brain-water diffusion can be quantified and maturation of the myelin sheath of brain white matter fiber bundles can be evaluated correctly through changes in apparent diffusion coefficient (ADC) and FA. In this retrospective study, we explored the role of DTI in early diagnosis of leukoencephalopathy for preterm neonates by evaluating ADC and FA of white matter in nerve fiber bundles of preterm neonates with leukoencephalopathy. In predicting results, ADC has a specificity of 64% and sensitivity of 66% [7]. Therefore, DTI can importantly contribute to early treatment of neonates with damaged brain white matter.
Material and Methods
Patients
Neonates with hypoxic-ischemic brain damage were selected from the Neonatal Care Center of Taian City Central Hospital from January 2012 to December 2013 and were included in the study. Early clinical symptoms of damaged white matter of preterm neonates are non-specific. MRI and DTI scans were taken within 14 days for all patients included in the study. The diagnostic criteria were as described previously by Volpe et al. (2003) [8]: (1) MRI of focal damage in cerebral white matter of preterm neonates in early stage is presented with dotted or linear DWI high signals in centrum semiovale and near lateral ventricle, accompanied with or without short TI and short T2. (2) Diffused damage of cerebral white matter is represented with changes of diffused high signals in DWI, like a sheet of high signals in white matter near the lateral ventricle with no signal changes in regular M. Patients who met any of these criteria were identified to have cerebral white matter injury; 38 neonates (26 boys and 12 girls) were included in the study. All the patients in the disease group were newly diagnosed with cerebral white matter injury.
Nervous system symptoms of neonates within 3 days are: altered mental status (over-excitation, somnolence, and coma), altered muscular tension (increase or decrease), abnormal primitive reflex (decreased or lost sucking reflex and embrace reflex), convulsions when seriously ill, symptoms of brain stem dysfunction (change of respiratory rhythm and slowing or absence of light response of the pupil), and increase in bregma tension. According to these symptoms, 38 preterm neonates with cerebral white matter injury were divided into 3 levels: 18 were in the mild group, 13 in the moderate group, and 7 in the severe group.
The clinical information was retrospectively analyzed from medical records. The control group included subjects who matched the disease group in terms of age, sex, and gestation period. Therefore, 38 full-term (adjusted gestation) neonates without leukoencephalopathy (25 boys and 13 girls) were included in the control group. The inclusion criteria were: (1) full-term neonates based on the adjusted gestation period; (2) no statistically significant difference between control and disease groups in terms of gestation period, birth weight, delivery method, or age in days in scan tests; and (3) neonates without central nervous system diseases, convulsions, perinatal asphyxia, congenital malformation, or hereditary metabolic illness. The patients included in the study had a clear history of anoxia and cyanosis, without obvious abnormal signals in brain MRI. Written informed consent was obtained from parents of all patients, and the study was approved by the Medical Ethics Committee of Taian City Central Hospital.
Scan equipment and methods
Before treatment, neonates in the 2 groups were scanned using MRI and DTI. When undergoing the MRI scan, the patients were in stable condition and in continuous intensive care of a doctor and nurses in the Neonatal Care Center. If the patients were not cooperative, they were injected with 0.5 ml/kg of 10% chloral hydrate and scanned after falling asleep. A cotton ball was stuck into each ear of patients to decrease the influence of noise. All the tests were completed within 2 weeks after birth. The brains of all patients were scanned with a SIEMENS MAGNETOM VERIO 3.0 device.
Regular T1WI, T2WI, and FLAIR were taken once each. The scan indexes of regular T1WI were 6 mm collimation, 2 mm interlayer spacing, 384×192 matrix, TR/TE 360/15 ms, and FOV 240×180 mm. The scan indexes of T2WI were 6 mm collimation, 2 mm interlayer spacing, 480×256 matrix, TR/TE3600/115 ms, and FOV 240×180 mm. The scan indexes of FLAIR were 6 mm collimation, 2 mm interlayer spacing, 320×192 matrix, TR/TE8500/120 ms, and FOV 240×240 mm.
A single-shot SE EPI was taken once with DTI. The scan indexes were 5 mm collimation, 0 mm interlayer spacing, 128×128 matrix, TR/TE 8000/89.2 ms, FOV 240×240 mm, b=1000 s/mm2, and 15 directions of diffusion-sensitive gradient. The scan was performed within 15 min.
The data was entered into a computer in DICOM format and processed with Volume-one 1.72 and diffusion TENSOR Visualizer II (dTV II) in z-axis to obtain an FA map and an ADC map.
Measurement of FA and ADC, range of ROI
FA and ADC were evaluated by 2 neurology radiologists who are associate chief physicians. Any conflicts were resolved through mutual discussions. FA and ADC of 6 regions sensitive to hypoxic ischemia (i.e., regions of interest [ROI]) were measured: corona radiate, superior longitudinal fasciculus, inferior longitudinal fasciculus, genu and splenium of corpus callosum, and posterior limb of internal capsule. Except for genu and splenum of corpus callosum, ADC and FA of both sides of the 4 other ROIs were measured. Referring to the ROI placement method of Shimony et al. [9], ROIs were manually placed in the FA map according to different anatomic locations. To prevent bias in evaluation, MRI radiologists were blinded to the patient groups. The size of ROI was 30±10 mm2. According to the anatomical structure and ADC map, the size of ROI was altered accordingly to cover all anatomical structures of the scanned region and to prevent the mutual influence of volume effects between neighboring regions.
Data recording
The following data were recorded: (1) gestation period, sex, delivery method, weight at birth, and Apgar score; (2) clinical symptoms and graduation of patients; and (3) results of MRI and DTI scans. All results were evaluated by single-blinding. After observing the image film of brain MRIs, 2 radiologists gave the results. DTI data were evaluated by 1 radiologist after testing.
Statistical method
All data were processed with SPSS18.0. The values of FA and ADC obtained from brain scans in different groups are presented as mean ±SD (χ̄±s). After normality testing and homogeneity testing for variance, ADC value and FA value of different regions and different groups were compared using the t-test. One-way ANOVA was used to compare FA and ADC in the same region of 3 groups and SNK was used to compare FA and ADC in pairs. If p<0.05, the differences were considered statistically significant.
Results
The observation and control groups were well balanced in terms of gestation, weight at birth, delivery method, and age in days, but there was a statistically significant difference between the groups in terms of Apgar scores at 1 and 5 min (p<0.05). Table 1 compares the demographic and clinical characteristics at baseline.
Table 1.
Demographic and clinical characteristics of observation and control groups (χ̄±s).
| Basic information | Observation group | Control group | p-Value |
|---|---|---|---|
| Male/femalea | 26/12 | 25/13 | 0.807 |
| Gestation (weeks)b | 35.2±1.5 | 35.1±1.1 | 0.741 |
| Weight (g)b | 2224.6±205.3 | 2316.5±238.4 | 0.076 |
| C-section/natural birth/deliverya | 23/11/4 | 18/14/6 | 0.504 |
| Age in days (days)b | 4.1±2.8 | 4.5±2.4 | 0.506 |
| Apagar score (1 min)b | 7.8±1.8 | 9.1±1.5 | 0.001 |
| Apagar score (5 min)b | 9.2±0.8 | 9.6±0.5 | 0.011 |
with χ2 test
with t-test.
Measurement of FA and ADC in ROI of cerebral white matter
According to the degree of damage from hypoxic ischemia to cerebral white matter, the 38 preterm neonates (gestation <37 weeks) were divided into 3 groups: 18 in the mild damage group, 13 in the moderate damage group, and 7 in the severe damage group. ADC and FA in ROIs of corona radiate, superior longitudinal fasciculus, inferior longitudinal fasciculus, and posterior limb of internal capsule were measured and compared to the control group. Table 2 compares the ADC and FA between the observation and control groups. After statistical analysis, ADC and FA were significantly different between the 2 groups. Compared with the control group, the observation group had higher ADC in different regions (p<0.05) and lower FA in corona radiate, superior longitudinal fasciculus, inferior longitudinal fasciculus, and posterior limb of internal capsule (p<0.05). The relationship between the cerebral white matter injury graduation of hypoxic ischemia in the observation group and ADC and FA in genu and splenum of corpus callosum was analyzed. As Table 3 shows, the ADC values of genu and splenum of corpus callosum in the mild damage group were lower than in the moderate and severe groups. The FA values of genu and splenum of corpus callosum in the mild damage group were higher.
Table 2.
Mean of ADC and FA in ROIs of cerebral white matter between experimental and control groups (N=38).
| ADC | FA | |||
|---|---|---|---|---|
| Observation group | Control group | Observation group | Control group | |
| Corona radiate* | 1.74±0.27 | 1.54±0.23a | 0.29±0.04 | 0.39±0.14e |
| Superior longitudinal fasciculus | 1.63±0.25 | 1.50±0.22b | 0.24±0.05 | 0.33±0.05f |
| Inferior longitudinal fasciculus | 1.72±0.23 | 1.61±0.22c | 0.29±0.03 | 0.32±0.05g |
| Posterior limb of internal capsule | 1.08±0.048 | 1.04±0.03d | 0.51±0.04 | 0.54±0.03h |
ROI – region of interest; ADC – apparent diffusion coefficient; FA – fractional anisotropy.
The measured value of ADC and FA were the mean of both sides;
comparison between experimental and control groups:
t=3.563, p=0.001;
t=2.372, p=0.020;
t=2.093, p=0.039;
t=4.0379, p<0.001;
t=4.0456, p<0.001;
t=7.445, p<0.001;
t=2.371, p=0.020;
t=3.406, p=0.001.
Table 3.
Relationship between the cerebral white matter damage graduation of hypoxic ischemia in observation group and ADC and FA in genu and splenum of corpus callosum.
| Graduation | ADC in genu | ADC in splenum | FA in genu | FA in splenum |
|---|---|---|---|---|
| Mild | 1.30±0.12 | 1.16±0.12 | 0.58±0.07 | 0.68±0.08 |
| Moderate | 1.47±0.18a | 1.36±0.26a | 0.50±0.10a | 0.63±0.12 |
| Severe | 1.55±0.15a | 1.50 ±0.21a | 0.42±0.09a | 0.51±0.08a,b |
| F value | 8.126 | 8.965 | 9.700 | 7.968 |
| p-Value | 0.001 | 0.001 | 0.000 | 0.001 |
ADC – apparent diffusion coefficient; FA – fractional anisotropy.
Compared with mild group p<0.05;
compared with moderate group p<0.05.
FA maps and DTI maps of nerve fiber growth in preterm group (gestation <37 weeks) and full-term group (adjusted gestation) without cerebral white matter injury
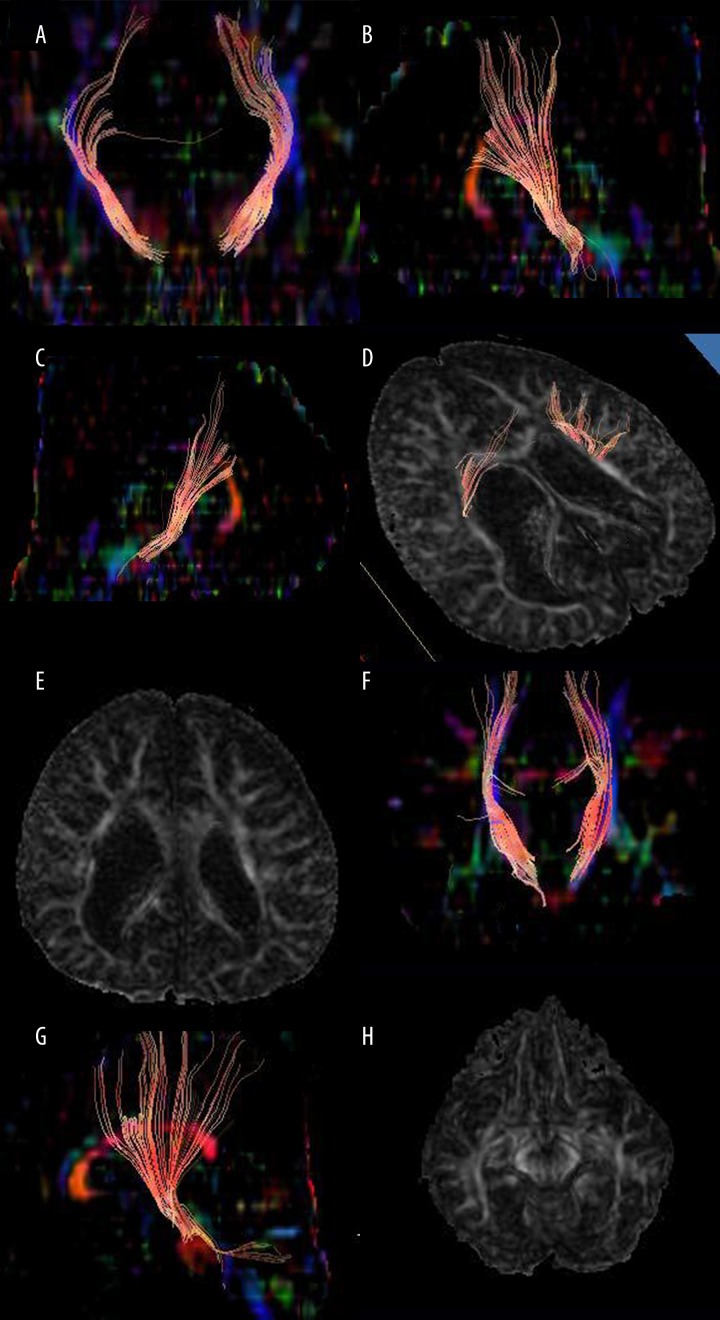
DTI can quantify the microstructure of brain tissues and has an overwhelming advantage in showing the fine structure of brain tissues, especially white matter fibers. It is particularly sensitive to the brain tissue damage caused by ischemia, anoxia, and swelling. Results of DTI can directly describe the spatial distribution of white matter fiber bundles, and track its running, circumventing, and crossing. DTI map and FA map of preterm neonates (Figure 1A–1E) showed rarefaction, breakage, and induced volume change of the lateral ventricle in cerebral white matter and rarefaction and disruption in nerve fibers on both sides. The nerve fibers on the right side were less numerous and had more breaks than those on the left side. The DTI map and FA map of full-term (adjusted gestation) neonates without cerebral white matter injury (Figure 1F–1H) revealed the projection of nerve fibers. After local 3-dimensional reconstruction, rich nerve fibers extending radially were seen. After comparing the tractus corticospinalis of those with nearly the same gestation, full-term neonates with adjusted gestation had thicker and more numerous tractus corticospinalis than preterm neonates.
Figure 1.
Diffusion tensor imaging (DTI) maps of neonates. (A) DTI coronal map of both sides of tractus corticospinalis for preterm neonates (adjusted gestation: 12 months). (B) DTI sagittal map of right tractus corticospinalis for preterm neonates (adjusted gestation: 12 months). (C) DTI sagittal map of left tractus corticospinalis for preterm neonates (adjusted gestation: 12 months). (D) DTI map of both sides of tractus corticospinalis for preterm neonates (adjusted gestation: 12 months). (E) DTI coronal map of both sides of tractus corticospinalis for preterm neonates (adjusted gestation: 12 months). (F) DTI sagittal map of right tractus corticospinalis for preterm neonates (adjusted gestation: 12 months). (G) FA map of patients (male, 10 months) with severe white matter damage. Due to periventricular leukomalacia caused by hypoxic ischemia, lateral ventricle is enlarged, white matter near the ventricle is decreased; and local white matter signals at the right side of lateral ventricle are reduced. (H) Male, adjusted gestation 1 year. The FA map of a healthy brain shows inferior longitudinal fasciculus and tractus corticospinalis (indicated by arrows).
Discussion
1. Changes of DTI indexes in different stages of preterm neonates can reflect the maturation and damage to cerebral white matter. Under pathologic status, differences in water diffusion speed in various tissues can reveal the differences in tissues and structures. Within 1 week of acute damage in preterm neonates, swollen axons, damaged oligodendrocyte precursor cells, and cellular edema in the early stage of neuronal damage have been observed. A week later, after progressing into vasogenic edema, cell membranes ruptured; water molecules flowed from the inside of cells to the outside and free water outside the cells increased. Alexander et al. (2007) concluded that FA is highly sensitive to microstructural changes, so all these may cause FA to decrease more sharply or to fall below the normal level [10]. If running DTI during this time, the increase of free water outside the cells could lead to ADC growth, influencing the structure and running of cerebral white matter fiber bundles and even decreasing FA [11,12]. The present study found an inverse correlation between increasing age and decreasing ADC. The differences between the observation group and the control group in corona radiate, superior longitudinal fasciculus, inferior longitudinal fasciculus, posterior limb of internal capsule, and genu and splenium of corpus callosum were statistically significant (p<0.05). ADC in the observation group was higher than that in the control group. This may have a correlation with increase in free water outside of cells after brain damage, or with confined movement of water molecules caused by gradual decrease of water in brain tissue, compact arrangement of brain white matter, and maturation of myelinization, but the exact cause is still unclear [13,14]. However, ADC in different regions was different. Researchers found that the lowest ADC was in the posterior limb of the internal capsule, because at birth the white matter fibers had already been myelinized, inhibiting the diffusion of water molecules. According to Bednark et al. [15], when neonates had hypoxic ischemia, ADC decreased. However, a week later ADC returned to normal levels in spite of brain damage shown in scan films, which was pseudonormal, but this phenomenon did not occur in FA. Thus, FA can offset the defects of pseudonormal ADC. Compared with full-term (adjusted gestation) neonates, preterm neonates had a lower FA. With increasing gestation age, FA increased [16]. The FA differences between observation and control groups in corona radiate, superior longitudinal fasciculus, inferior longitudinal fasciculus, posterior limb of internal capsule, and genu and splenium of corpus callosum were statistically significant (p<0.05). FA in the control group was higher than that of the observation group. In the present study, FA in genu and splenium of corpus callosum of preterm neonates (gestation <37 weeks) was lower than that of full-term neonates with adjusted gestation, which indicates that myelinization in preterm neonates is slower than in full-term ones.
By measuring FA in cerebral white matter nerve fibers of preterm neonates, Berman et al. [17] found that FA was altered differently in different regions of cerebral white matter. For preterm neonates, damage to the cerebral white matter usually presented with water diffusion disorders. FA in the same region at different times was also different. Provenzale et al. [18] found that for babies who were 3 months post-birth, FA in the posterior limb of the internal capsule was 0.46, lower than that in the present study, which found 0.54. The changes of measured FA corresponded with the trend of FA in different development stages of neonates. The smaller the gestation period in preterm neonates, the sharper was the FA reduction [19].
At present there are a few ongoing studies worldwide on DTI of damages within 24 h. Due to the severe condition of preterm neonates there is limited information on DTI tests. Some researchers studied brain DTI in neonates at between 1 and 6 days [20]. According to Ward et al. [21], reduction of ADC and FA in the first week indicates a severe condition. Normal or increased ADC, coupled with reduced FA, indicates moderate lesions. In conclusion, DTI data may become a biomarker in early target imaging after treatment. ADC and FA provide objective and reliable measures [22].
2. Through DTI scan imaging coupled with clinical graduation of cerebral white matter injury, fine structures of white matter can be directly and clearly detected. It can provide imaging of white matter development in healthy babies, and lays a foundation for white matter disease diagnosis and evaluation of preterm neonatal development.
Damage to corticospinal tract fibers can to some extent predict the influence on motor and sensory pathways of specific regions. The decrease in the number of nerve fibers and the reduction or breakage of projections can lead to abnormal neural connections and central nervous system dysfunction by affecting the neural pathways. Corticospinal tract damage in periventricular leukomalacia mainly influences motor development. For patients with periventricular leukomalacia and periventricular hemorrhagic infarction, the connection was broken between the cortex and the corpus callosum, posterior limb of internal capsule, superior longitudinal fasciculus, and thalamus, which is correlated with the immaturity of the endothelial monolayer and rich mitochondria in germinal matrix capillaries and its high sensitivity to anoxia [23]. Therefore, for these diseases, early diagnosis is very important; the associated causative agent should be removed in a timely manner and symptomatic treatment should be given. Standard early intervention and follow-up visits should be performed systematically.
Due to the small sample size, the conclusions of the present study are limited. In addition, there was a lack of imaging and research data during long-term follow-up. Some researchers have analyzed the data on DTI with FMRIB to improve the image quality [24]. As a next step, further improvements are warranted in several aspects.
Conclusions
In summary, DTI is currently the only way to quantify the maturation and damage of brain development in preterm neonates. In the early stage, it can identify damages that cannot be screened by MRI. Thus, it can provide evidence for developing effective measures of prevention, protection, and rehabilitation for damage to the brain. Coupled with a good intervention treatment system established with clinical development assessment and pathogenic factors, DTI is an important aid to improve the prognosis of nerve development for preterm neonates, owing to its ability to reflect the damage to cells in white matter and nerve fibers at an early stage and at a microscopic level.
Footnotes
Source of support: Departmental sources
References
- 1.Wang XY, Mao J. Pathogenesis of white matter damage in preterm children. Foreign Medical Sciences. 2004;3:132–34. [Google Scholar]
- 2.Chau V, Synnes A, Grunau RE, et al. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology. 2013;81(24):2082–89. doi: 10.1212/01.wnl.0000437298.43688.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins RD, Raju T, Edwards AD, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011;159(5):851–58.e1. doi: 10.1016/j.jpeds.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasiljevic B, Maglajlic-Djukic S, Gojnic M, et al. New insights into the pathogenesis of perinatal hypoxic-ischemic brain injury. Pediatr Int. 2011;53(4):454–62. doi: 10.1111/j.1442-200X.2010.03290.x. [DOI] [PubMed] [Google Scholar]
- 6.Cai Q, Xue XD, Fu JH. Progresses in studies on magnetic resonance imaging of neonatal hypoxic-ischemic encephalopathy. Zhonghua Er Ke Za Zhi. 2010;48(3):227–31. [PubMed] [Google Scholar]
- 7.Thayyil S, Chandrasekaran M, Taylor A, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: A meta-analysis. Pediatrics. 2010;125(2):e382–95. doi: 10.1542/peds.2009-1046. [DOI] [PubMed] [Google Scholar]
- 8.Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112(1 Pt 1):176–80. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 9.Shimony JS, McKinstry RC, Akbudak E, et al. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology. 1999;212(3):770–84. doi: 10.1148/radiology.212.3.r99au51770. [DOI] [PubMed] [Google Scholar]
- 10.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapertica. 2007;4(3):316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotak CH. The role of diffusion tensor imaging in the evaluation of ischemic brain injury – a review. NMR Biomed. 2002;15(7–8):561–69. doi: 10.1002/nbm.786. [DOI] [PubMed] [Google Scholar]
- 12.Neil J, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain – a technical review. NMR Biomed. 2002;15(7–8):543–52. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- 13.Partridge SC, Mukherjee P, Henry RG, et al. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004;22(3):1302–14. doi: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 14.Giorgio A, Watkins KE, Douaud G, et al. Changes in white matter microstructure during adolescence. Neuroimage. 2008;39(1):52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 15.Bednarek N, Mathur A, Inder T, et al. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology. 2012;78(18):1420–27. doi: 10.1212/WNL.0b013e318253d589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobel U, Sedlacik J, Gullmar D, et al. Diffusion tensor imaging: the normal evolution of ADC, RA, FA, and eigenvalues studied in multiple anatomical regions of the brain. Neuroradiology. 2009;51(4):253–63. doi: 10.1007/s00234-008-0488-1. [DOI] [PubMed] [Google Scholar]
- 17.Berman JI, Mukherjee P, Partridge SC, et al. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27(4):862–71. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Provenzale JM, Liang L, DeLong D, White LE. Diffusion tensor imaging assessment of brain white matter maturation during the first postnatal year. Am J Roentgenol. 2007;189(2):476–86. doi: 10.2214/AJR.07.2132. [DOI] [PubMed] [Google Scholar]
- 19.Huppi PS, Murphy B, Maier SE, et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107(3):455–60. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- 20.Brissaud O, Amirault M, Villega F, et al. Efficiency of fractional anisotropy and apparent diffusion coefficient on diffusion tensor imaging in prognosis of neonates with hypoxic-ischemic encephalopathy: A methodologic prospective pilot study. Am J Neuroradiol. 2010;31(2):282–87. doi: 10.3174/ajnr.A1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward P, Counsell S, Allsop J, et al. Reduced fractional anisotropy on diffusion tensor magnetic resonance imaging after hypoxic-ischemic encephalopathy. Pediatrics. 2006;117(4):e619–30. doi: 10.1542/peds.2005-0545. [DOI] [PubMed] [Google Scholar]
- 22.Tusor N, Wusthoff C, Smee N, et al. Prediction of neurodevelopmental outcome after hypoxic-ischemic encephalopathy treated with hypothermia by diffusion tensor imaging analyzed using tract-based spatial statistics. Pediatr Res. 2012;72(1):63–69. doi: 10.1038/pr.2012.40. [DOI] [PubMed] [Google Scholar]
- 23.Huang BY, Castillo M. Hypoxic-ischemic brain injury: Imaging findings from birth to adulthood. Radiographics. 2008;28(2):417–39. doi: 10.1148/rg.282075066. quiz 617. [DOI] [PubMed] [Google Scholar]
- 24.Ball G, Counsell SJ, Anjari M, et al. An optimised tract-based spatial statistics protocol for neonates: Applications to prematurity and chronic lung disease. Neuroimage. 2010;53(1):94–102. doi: 10.1016/j.neuroimage.2010.05.055. [DOI] [PubMed] [Google Scholar]