Abstract
Accumulating evidence has shown that T cells are crucial in shaping the tumor microenvironment and regulating tumor development. However, the roles of IL-17A-producing T cells (IL-17A+CD4+ Th17, IL-17A+CD8+ Tc17 and IL-17A+ γδT17 cells) and related cytokines in the progression of lung cancer (LC) remain uncertain. Here, we found that the frequencies of both Th17 and γδT17 cells in the peripheral blood of patients with lung adenocarcinoma (LA) were higher than those in healthy controls (HCs), whereas the frequency of Tc17 cells in the patients with LA was decreased. In addition, the frequencies of circulating Th17 and γδT17 cells, but not Tc17 cells, were positively associated with tumor invasion and metastasis. Furthermore, the major source of IL-17A production was Th17 cells, followed by Tc17 and γδT17 cells, in peripheral blood from patients with LA and HCs; but the percentages of Th17 and γδT17 cells in total intracellular IL-17A+ cells obtained from the patients with LC were higher than those from HCs. Moreover, the protein and corresponding mRNA levels of IL-17A, IL-23, IL-1β, and TGF-β1 were much higher in the patients with LA than those in HCs, and the levels of IL-17A in patients were positively correlated with numbers of both Th17 and γδT17 cells, but not Tc17 cells. Finally, the frequencies of circulating Th17 and γδT17 cells, along with the levels of IL-17A, IL-23, IL-1β, and TGF-β1 were decreased in the patients with LA after tumor resection, whereas the frequency of circulating Tc17 cells was inversely increased in these patients. Our findings indicate that Th17, Tc17, γδT17 cells, and IL-17A-associated cytokines contribute to the development of LA and thus represent promising targets for therapeutic strategies.
Keywords: IL-17A-producing T cells, Th17 cells, Tc17 cells, γδT17 cells, IL-17A, IL-23, lung cancer, lung adenocarcinoma
Introduction
Lung cancer (LC) is currently the leading cause of cancer-related mortality among both males and females worldwide and was responsible for approximately 1.59 million deaths in 2012 (1,2). LC has also been the number one cause of cancer-related deaths among patients with malignant tumors since 2008 in China (3). In addition, the mortality rate of LC in China has increased dramatically during the past three decades due to smoking and air pollution, imposing a huge economic burden on patients, medical professionals, and society (3).
LC is characterized by a series of hallmarks, such as tumor-promoting inflammation, avoidance of immune destruction, genomic instability, and induction of angiogenesis (4). Inflammatory responses contribute to the initiation, progression, and metastases of malignancies as proposed by Virchow in 1863, by promoting proliferative signaling, destabilizing genomic integrity, and inducing the invasion of cancer cells (5,6). Chronic inflammation triggered by bacterial and viral infections, tobacco smoking, and chemicals suppress wound healing and tissue regenerative responses, promoting cancer development and progression, as described as 'wounds that do not heal' (7,8). In addition, inflammation compromises genomic maintenance and repair pathways, inducing genomic instability (6,9). Furthermore, cancer cells modulate inflammation by secreting soluble mediators and interfering with innate and adaptive immune cells, such as, macrophages, dendritic cells and lymphocytes (6). Multiple inflammatory mediators may trigger and maintain tumorigenesis individually or coordinately in the tumor microenvironment (6). The crosstalk between the inflammatory microenvironment and cancer cells controls and shapes tumor growth and metastasis (5–7).
Interleukin (IL)-17A, a proinflammatory cytokine discovered in 1993, induces tissue inflammation mainly by promoting expression of various cytokines, chemokines, antimicrobial peptides, and tissue-remodeling molecules (10–12). IL-17A exerts complicated functions in allergic, autoimmune, and malignant diseases, by targeting mesenchymal and myeloid cells (10,13). Although originally linked to IL-17-producing CD4+ T helper (Th17) cells, a distinct T cell subtype different from Th1 and Th2 cells, IL-17A was subsequently found to be produced by several other immune cells, including IL-17A-producing γδT (γδT17) cells, IL-17A-producing CD8+ T (Tc17) cells, natural killer T cells, and mast cells (10,14,15). IL-17A-producing T cells and associated cytokines, such as IL-17, IL-23, and IL-1β, have been shown to be involved in both inflammation and immune responses in various types of cancers including gastric, breast, prostate and hepatocellular cancer (10,16–19). However, the roles of these T cells and associated cytokines are conflicting in various animal models and patients (10,20–22). IL-17-producing T cells have displayed both antitumor and protumor functions, due to their plasticity and functions in the tumor microenvironments (23). The numbers of circulating Th17 and γδT17 cells were significantly higher in patients with gastric cancer than those in healthy controls (HCs) (16). On the contrary, data have shown that Th17 cells elicit antitumor effects, by promoting cytotoxic activities, enhancing Th1 response, and augmenting the expression of MHC antigens (24–26). In a murine model of LC, enhanced Th17 cells and overexpression of IL-17A stimulated tumor growth in the lungs (27,28). Similarly, an increased number of intratumoral IL-17-positive cells in patients with LC was correlated with poor prognosis (29). However, a higher percentage of Tregs but a lower frequency of Th17 cells was found in malignant pleural effusion, as compared with those in parapneumonic effusion, and a higher ratio of Treg/Th17 cells in malignant pleural effusion was found to indicate a poor prognosis of patients with LC (30). Thus, the roles of IL-17A-producing T cells, especially Tc17 and γδT17 cells, and associated cytokines in the progression of LC remain to be defined.
In the present study, we investigated the frequencies of IL-17-producing T cells (Th17, Tc17 and γδT17 cells) and levels of IL-17A-associated cytokines (IL-17A, IL-23, IL-1β, and TGF-β1) in patients with lung adenocarcinoma (LA) and HCs. We found that the frequencies of both Th17 and γδT17 cells in the peripheral blood of patients with LA were higher than those in HCs and were positively associated with tumor invasion and metastasis, whereas the frequency of Tc17 cells in patients with LA was decreased. Furthermore, the major source of IL-17A production was Th17 cells in peripheral blood from both patients with LA and HCs. In addition, the protein and corresponding mRNA levels of IL-17A, IL-23, IL-1β, and TGF-β1 were much higher in patients with LA than those in HCs, and the levels of IL-17A were positively correlated with numbers of both Th17 and γδT17 cells, but not Tc17 cells. Finally, the frequencies of circulating Th17 and γδT17 cells, along with levels of IL-17A, IL-23, IL-1β, and TGF-β1 were decreased in the patients with LA after tumor resection, whereas the frequency of circulating Tc17 cells was inversely increased in these patients. This study provides further insight into the association between IL-17A-producing T cells and progression of LA, and offers promising targets for therapeutic strategies.
Materials and methods
Subjects
Forty patients, diagnosed with LA and admitted to the Department of Respiratory Medicine at the First Affiliated Hospital of Zhejiang University, and 35 HCs were enrolled in this study from May 2014 to June 2015. All patients were histologically confirmed with LA by two pathologists. The patients were excluded if they had autoimmune diseases, immune compromised diseases, and pulmonary infections, if they were taking any drugs which affect immune responses, or if they had already received anticancer therapies including chemotherapy, radiotherapy, targeted therapy, surgery or immune therapy. The characteristics of the enrolled 40 patients and 35 HCs, are summarized in Table I. All cases with LA were staged according to the 7th edition of the tumor, node, and metastasis (TNM) classification for LC (31). The study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University, and informed consent was obtained from all patients and HCs.
Table I.
Characteristics of the patients with lung adenocarcinoma and healthy controls.
| Characteristics | Patients | Healthy controls |
|---|---|---|
| Gender | ||
| Male | 27 | 24 |
| Female | 13 | 11 |
| Age (years) | ||
| Median | 53 | 54 |
| Range | 36–77 | 32–76 |
| Tumor grade | ||
| Well/moderate | 26 | |
| Poor | 14 | |
| Tumor stage | ||
| T1 | 19 | |
| T2 | 13 | |
| T3 | 0 | |
| T4 | 8 | |
| Node status | ||
| N0 | 21 | |
| N1 | 9 | |
| N2 | 7 | |
| N3 | 3 | |
| Metastasis | ||
| M0 | 34 | |
| M1a/b | 6 | |
| TNM stage | ||
| I | 16 | |
| II | 10 | |
| III | 8 | |
| IV | 6 | |
All patients were staged according to the 7th edition of the tumor, node, and metastasis (TNM) classification for lung cancer.
Sample collection and processing
Peripheral blood samples were collected from the subjects before any regional or systemic anticancer treatments and re-collected from 15 patients after thoracic surgery. The fresh peripheral blood of all individuals was stored in heparin-coated tubes (BD Biosciences, San Jose, CA, USA) and centrifuged at 4,000 rpm for 10 min at 4°C. Then, the cell-free supernatants, allocated into 1.5-ml Eppendorf tubes, were frozen at −80°C for the detection of cytokines (30). The cell pellets were re-suspended in saline for further analyses of flow cytometry and real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) (30).
Flow cytometric analysis
Human peripheral blood mononuclear cells (PBMCs), isolated from cell pellets using Ficoll-Hypaque density gradient centrifugation, were re-suspended in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen Life Technologies, Carlsbad, CA, USA). Next, they were stimulated for 5 h with 50 ng/ml phorbol 12-myristate 13-acetate (PMA; BioVision, Mountain View, CA, USA), 1 µg/ml ionomycin (Enzo Life Sciences, Inc., Farmingdale, NY, USA) and 500 ng/ml monensin (eBioscience, San Diego, CA, USA) in 24-well plates (16). To analyze IL-17A-producing T cells, stimulated PBMCs were stained with phycoerythrin (PE)-conjugated anti-human CD3, fluorescein isothiocyanate (FITC)-conjugated anti-human γδTCR, allophycocyanin (APC)-conjugated anti-human CD8, and Pacific Blue-conjugated anti-human CD4 antibodies at 4°C for 30 min (16). Then, the cells, fixed and permeabilized with IC fixation/permeabilization buffer (eBioscience), were intracellularly stained with PerCP-Cy5.5-conjugated anti-human IL-17 antibody according to the manufacturer's instructions (16). All antibodies used in the flow cytometric analysis were obtained from Biolegend (San Diego, CA, USA) and isotype-matched antibody controls were used in all procedures. Flow cytometric acquisition was performed using a FACSCalibur (BD Biosciences), and data were analyzed using FlowJo software, version 7.6.5 (TreeStar, Inc., San Carlos, CA, USA).
ELISA measurement of serum cytokines
The cell-free supernatants of all individuals were tested using ELISA kits according to the manufacturer's instructions (eBioscience), for cytokines including IL-17A, IL-23, IL-1β, and TGF-β1. All samples were tested in triplicate.
qRT-PCR
RNA samples were prepared from stimulated human PBMCs using TRIzol (Invitrogen Life Technologies) (16). cDNA was synthesized using reverse transcription reagent kits (Takara Biotechnolgy Co., Inc., Dalian, China) and real-time PCR was performed in triplicate using the QuantiFast™ SYBR Green PCR kit (Qiagen, Hilden, Germany) in an ABI 7500 analysis system (Applied Biosystems, Foster City, CA, USA) (16). The following primer pairs were used: IL-1β forward, 5′-CCACAGACCTTCCAGGAGAATG-3′, and reverse, 5′-GTGCAGTTCAGTGATCGTACAGG-3′; IL-17A forward, 5′-CGGACTGTGATGGTCAACCTGA-3′, and reverse, 5′-GCACTTTGCCTCCCAGATCACA-3′; IL-23p19 forward, 5′-GAGCCTTCTCTGCTCCCTGATA-3′, and reverse, 5′-GACTGAGGCTTGGAATCTGCTG-3′; TGF-β1 forward, 5′-CAGAAATACAGCAACAATTCCTGG-3′, and reverse, 5′-TTGCAGTGTGTTATCCGTGCTGTC-3′; GAPDH forward, 5′-GGTCTCCTCTGACTTCAACA-3′, and reverse, 5′-GTGAGGGTCTCTCTCTTCCT-3′. The data were analyzed by ABI 7500 software (Applied Biosystems).
Statistical analysis
Values are presented as means ± SEM. Differences among groups were tested by one-way ANOVA. Differences between two groups were tested using non-paired Student's t-test. For non-parametric data, the Mann-Whitney U test was performed between groups. Correlations between values were determined using Spearman's correlation coefficient. Analysis was performed with SPSS statistical software (version 21.0; SPSS, Inc., Chicago, IL, USA), and P<0.05 was considered statistically significant.
Results
Characteristics of the subjects
Clinical characteristic of the 40 patients with LA and 35 HCs are summarized in Table I. The median age was 53 years (range, 36–77 years) in the patient group including 27 males and 13 females, and 54 years (range, 32–76 years) in the control group including 24 males and 11 females. Baseline characteristics were balanced between the two groups. In patients with LA, there were 26 cases with well/moderate differentiation and 14 cases with poor differentiation; 32 cases in tumor stage T1+T2 and 8 cases in T3+T4; 21 cases of node status N0 and 19 cases of node status N1+N2+N3; 34 cases in metastasis status M0 and 6 cases in M1a/b; 26 cases in stages I+II and 14 cases in TNM stages III+IV.
Frequency of circulating Th17, Tc17, and γδT17 cells in peripheral blood
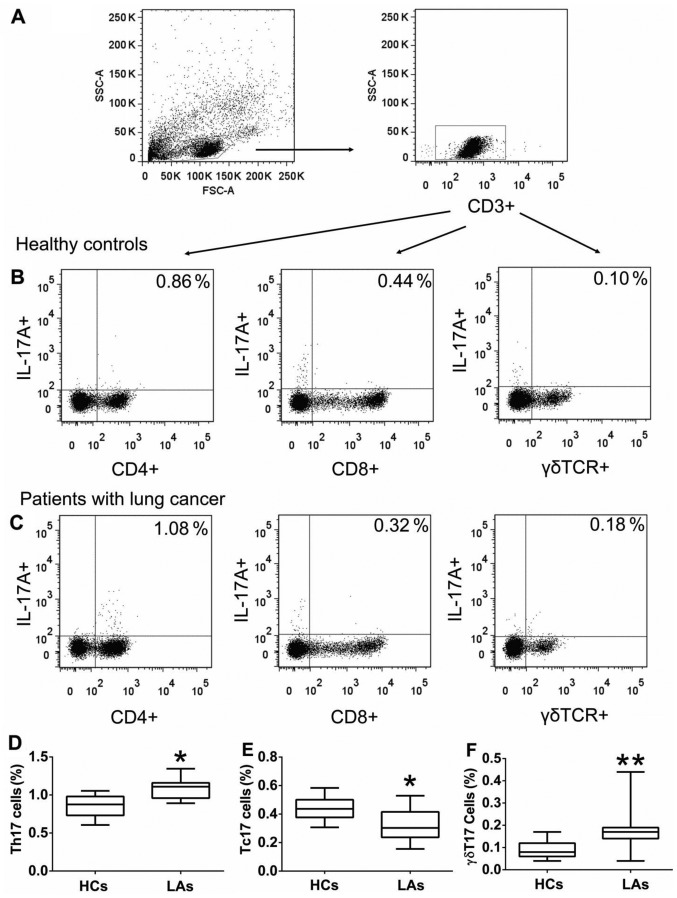
To investigate the roles of IL-17A-producing T cells in the development of LC, we first determined the frequencies of circulating Th17, Tc17, and γδT17 cells in the PBMCs obtained from all individuals. The frequencies of circulating Th17 and γδT17 cells were considerably higher in patients with LA than those in the HCs (Fig. 1), but the frequency of circulating Tc17 cells was markedly lower in patients with LA than that in HCs (Fig. 1). In addition, there were no significant differences in the frequencies of circulating Th17, Tc17, and γδT17 cells regarding tumor differentiation (well-moderate vs. poor); however, there were substantial differences between tumor invasion (T1+T2 vs. T3+T4), distant metastasis (M0 vs. M1a/b), and TNM stage (I+II and III+IV) (Fig. 2). The percentage of Th17 cells in patients with extensive tumor invasion (T3+T4) was higher than that in patients with T1+T2, while the percentage of Tc17 cells was low in patients with T3+T4 and the percentage of γδT17 cells showed no difference in regards to tumor invasion (Fig. 2). The percentage of γδT17 cells in patients with lymphatic metastasis (N1+N2+N3) was significantly higher than that in patients with lymphatic metastasis (N0), while there were no differences in Th17 and Tc17 cells regarding lymphatic metastasis (Fig. 2). The percentage of Th17 and γδT17 cells in patients with distant metastasis (M1) were higher than that in patients without metastasis (M0), while the percentage of Tc17 cells was low in patients with distant metastasis (M1) (Fig. 2). The percentage of γδT17 cells was higher in patients with III+IV stage than that in patients with I+II stage, while the percentage of Tc17 cells was low in patients with III+IV stage and there was no difference in the percentage of Th17 cells regarding TNM staging (Fig. 2). These findings suggest that a high percentage of circulating Th17 and γδT17 cells may contribute to the metastases of LA and indicate poor prognosis.
Figure 1.
The frequency of circulating Th17, Tc17 and γδT17 cells in all individuals. Peripheral blood mononuclear cells (PBMCs) obtained from patients with lung adenocarcinoma (LA) and healthy controls (HCs) were stained with labeled antibodies and analyzed using flow cytometric analysis. (A) PBMCs were gated initially on living lymphocytes and then on CD3+ T cells. (B) Representative plots of IL-17A+CD4+ T (Th17), IL-17A+CD8+ T (Tc17) and, IL-17A+γδ+ T (γδT17) cells in total CD3+ T cells from HCs. (C) Representative plots of Th17, Tc17, and γδT17 cells in total CD3+ T cells from patients with LA. Comparison of (D) Th17, (E) Tc17, and (F) γδT17 cells presented as percentages of total CD3+ T cells, between patients with LA and HCs. The data shown represent the means ± SEM. *P<0.05, **P<0.01 were considered to represent significant differences compared with HCs.
Figure 2.
Analysis of circulating Th17, Tc17 and γδT17 cells in patients with different tumor characteristics. The percentages of (A) Th17, (B) Tc17 and (C) γδT17 cells in patients with lung adenocarcinoma (LA) were compared according to tumor differentiation (good/moderate vs. poor), tumor invasion (T1+T2 vs. T3+T4), lymph node status (N0 vs. N1+N2+N3), metastasis (M0 vs. M1), and TNM stage (I+II and III+IV); The data shown represent the means ± SEM. *P<0.05, **P<0.01 were considered to represent significant differences.
The main T cells secreting IL-17A in peripheral blood
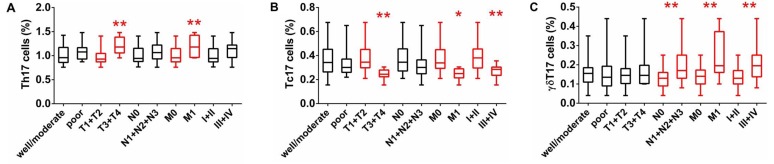
To further explore the main T cells secreting IL-17A in the peripheral blood, we measured the frequencies of circulating Th17, Tc17, and γδT17 cells in gated intracellular IL-17A+ cells obtained from patients with LA and HCs by flow cytometric analysis. The highest population among the intracellular IL-17A+ T cells in both patients with LA and HCs was circulating Th17 cells, followed by Tc17 and γδT17 cells (Fig. 3). Furthermore, the percentages of Th17 and γδT17 cells in the total intracellular IL-17A+ cells obtained from patients with LA were higher than those from the HCs, while the percentage of intracellular IL-17A+ cells represented by circulating Tc17 cells was low in patients with LA compared to that in patients with LCs (Fig. 3). These data indicated that the main source of intracellular IL-17A in the peripheral blood was Th17 cells for both the patients and the HCs, and that circulating IL-17A+ T cells and IL-17A play key roles in the initiation and progression of LC.
Figure 3.
Percentages of Th17, Tc17, and γδT17 cells in IL-17A+ cells. Peripheral blood mononuclear cells (PBMCs) obtained from patients with lung adenocarcinoma (LA) and healthy controls (HCs) were stained with labeled antibodies and analyzed using flow cytometric analysis. (A) PBMCs were gated initially on living lymphocytes and then on intracellular IL-17A. (B) Representative plots of Th17, Tc17, and γδT17 cells in IL-17A+ cells from patients with LA. Comparison of (C) Th17, (D) Tc17, and (E) γδT17 cells presented as percentages of total IL-17A+ cells between patients with LA and HCs. The data shown represent the means ± SEM. *P<0.05, **P<0.01 were considered to represent significant differences.
Analysis of IL-17A and associated cytokines in peripheral blood
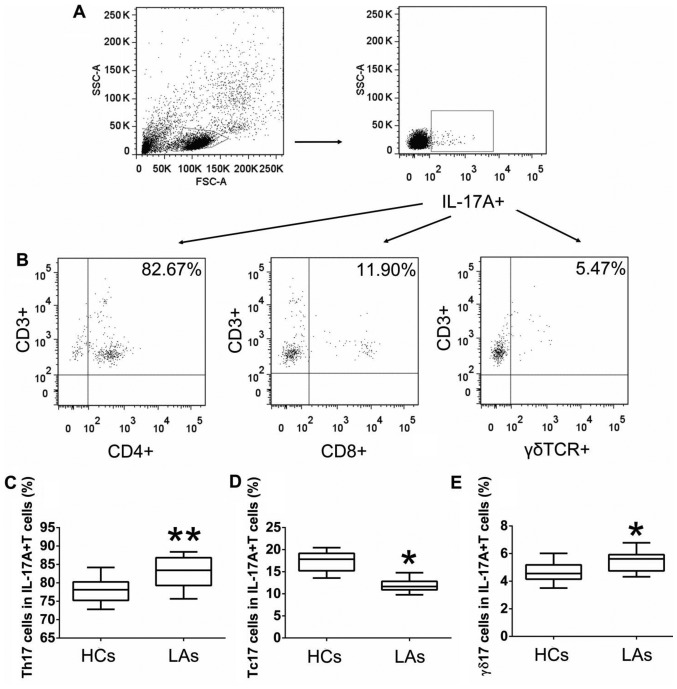
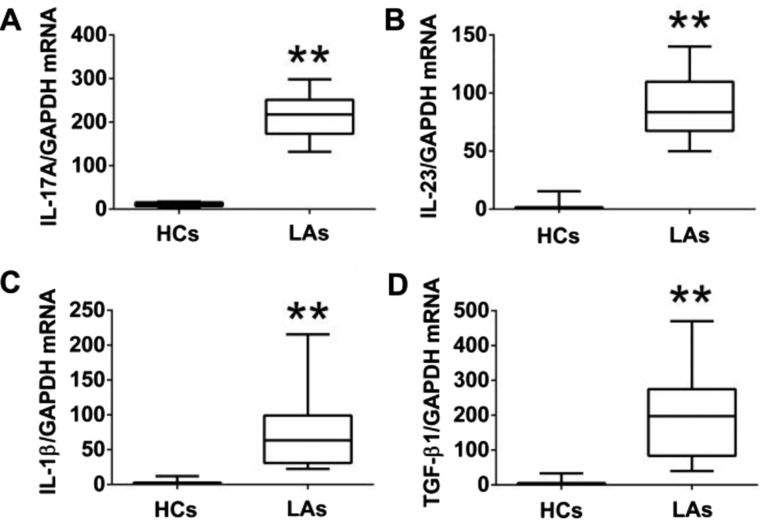
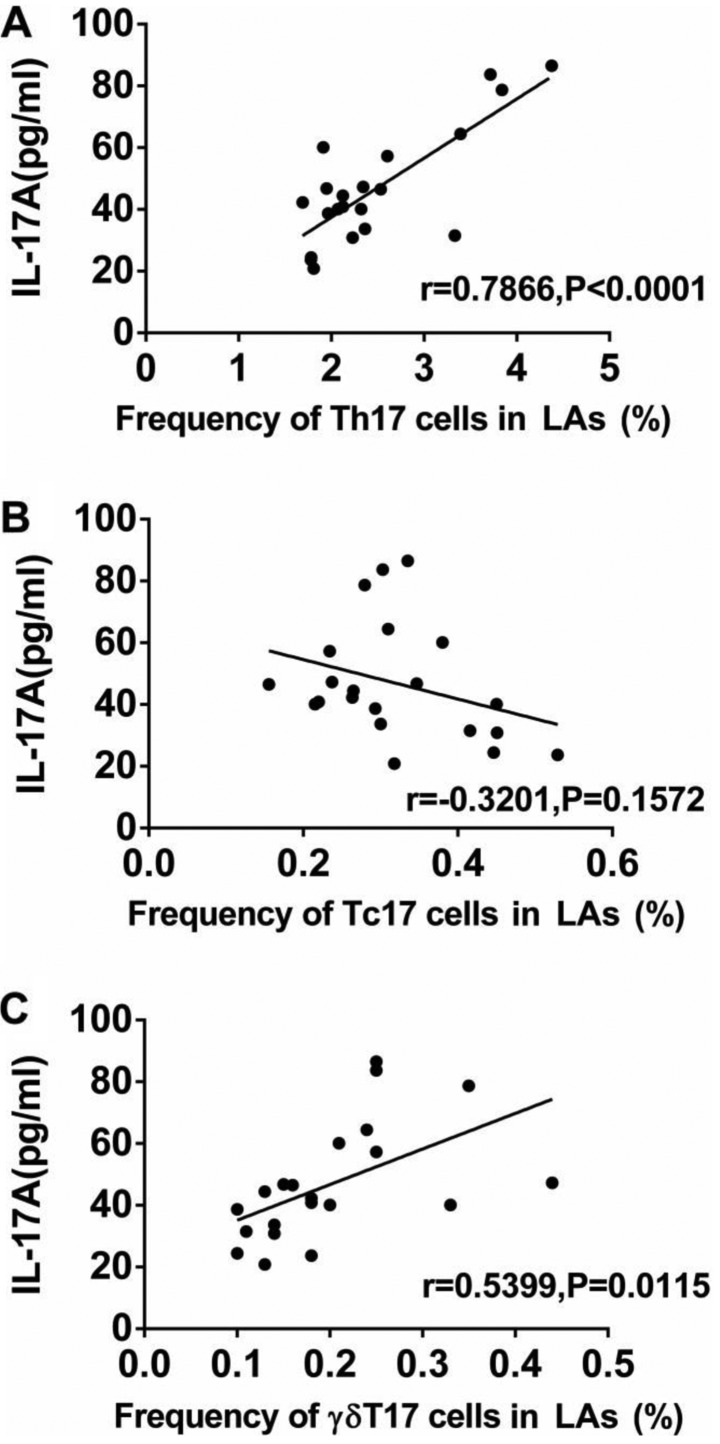
To further explore the functions of IL-17A-producing T cells, the protein levels of IL-17A and associated cytokines (IL-23, IL-1β, and TGF-β1) in serum and their mRNA levels in PBMCs from both the patients and the HCs were measured by ELISA and qRT-PCR. The protein levels of IL-17A, IL-23, IL-1β, and TGF-β1 in the serum of patients with LA were much higher than those in the HCs (Fig. 4). Similarly, the mRNA levels of IL-17A IL-23, IL-1β, and TGF-β1 in PBMCs of patients with LA were markedly higher than those in the HCs (Fig. 5). In addition, we analyzed the association between the levels of IL-17A and the frequencies of Th17, Tc17 and γδT17 cells in patients with LA. The expression of IL-17A in serum from patients was positively associated with the frequencies of Th17 and γδT17 cells, but was not related to the frequency of Tc17 cells (Fig. 6).
Figure 4.
Protein levels of IL-17A and associated cytokines (IL-23, IL-1β and TGF-β1) in serum. Comparison of (A) IL-17A, (B) IL-23, (C) IL-1β, and (D) TGF-β1 concentration between patients with lung adenocarcinoma (LA) and healthy controls (HCs). The data shown represent the means ± SEM. *P<0.05, **P<0.01 were considered to represent significant differences.
Figure 5.
The mRNA levels of IL-17A and associated cytokines (IL-23, IL-1β and TGF-β1) in PBMCs. Comparison of (A) IL-17A, (B) IL-23, (C) IL-1β, and (D) TGF-β1 mRNA levels between patients with lung adenocarcinoma (LA) and healthy controls (HCs). The data shown represent the means ± SEM. **P<0.01 was considered to represent significant differences.
Figure 6.
Association between IL-17A protein levels and frequencies of circulating Th17, Tc17 and γδT17 cells in patients with lung adenocarcinoma (LA). (A) Association between the protein level of IL-17A and the frequencies of circulating (A) Th17, (B) Tc17, and (C) γδT17 cells. P<0.05 was considered to represent significant difference.
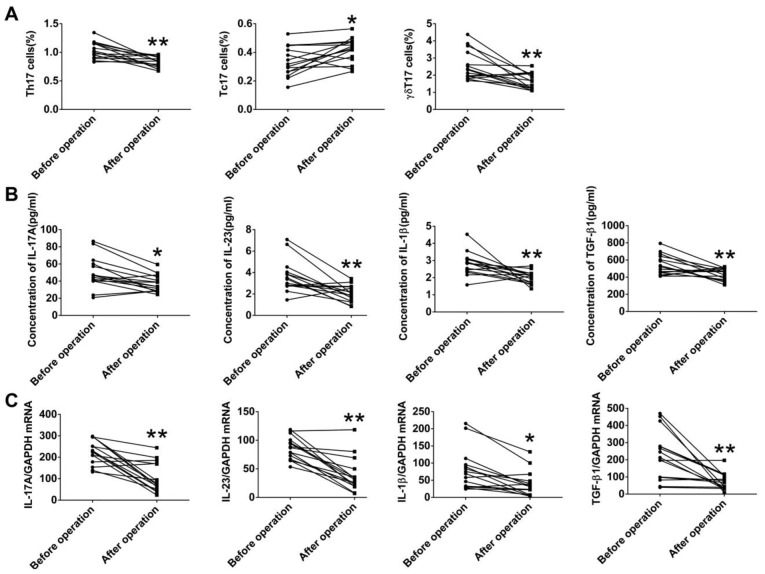
Alterations of IL-17A-producing T cells and IL-17A-associated cytokines in patients with LA after thoracic surgery
To explore the effects of tumor burden on IL-17A-producing T cells, we also measured both the frequencies of circulating Th17, Tc17, and γδT17 cells and levels of IL-17A and associated cytokines in 15 patients with LA, who received surgery, at 1 month after the resection. The frequencies of Th17 and γδT17 cells in the patients with LA were markedly decreased after surgery, while the frequency of Tc17 was significantly increased after surgery (Fig. 7A). Furthermore, both the protein levels of IL-17A IL-23, IL-1β, and TGF-β1 in serum and the corresponding mRNA levels in PBMCs were markedly decreased in the patients with LA after surgery (Fig. 7B and C). These data suggested that tumor resection resulted in the alteration of IL-17A-producing cells, IL-17A-associated cytokines, and the tumor-related microenvironment.
Figure 7.
Alterations in IL-17A-producing T cells and IL-17A-associated cytokines in patients with lung adenocarcinoma (LA) after surgery. (A) Alterations in Th17, Tc17 and γδT17 cells in patients with LA after surgery. (B) Alterations in protein levels of IL-17A and associated cytokines (IL-23, IL-1β and TGF-β1) in patients with LA after thoracic surgery. (C) Alterations of mRNA levels of IL-17A, IL-23, IL-1β and TGF-β1 in patients with LA after surgery. *P<0.05, **P<0.01 were considered to represent significant differences.
Discussion
Evidence has shown the dual roles of T cells and associated cytokines in the initiation, progression, and metastasis of LC. The present study investigated the frequencies of IL-17A-producing cells (Th17, Tc17, and γδT17 cells), and expression levels of IL-17-associated cytokines in patients with LA and HCs. We found that the frequencies of circulating Th17 and γδT17 cells, and the protein and corresponding mRNA levels of IL-17A, IL-23, IL-1β, and TGF-β1 in the peripheral blood of patients with LA were higher than those in the HCs, whereas the frequency of Tc17 cells in patients with LA was decreased. Moreover, the frequencies of circulating Th17 and γδT17 cells, along with the levels of IL-17A, IL-23, IL-1β, and TGF-β1 were decreased in patients with LA after tumor resection. Our findings suggest that Th17, γδT17, Tc17 cells, and IL-17A-associated cytokines contribute to the development of LA and thus represent promising targets for therapeutic strategies.
Th17 cells were originally discovered to promote inflammatory responses in autoimmune diseases and act in host defenses against microbes (32,33). Recently, their functions in the initiation and development of cancers have been extensively studied (34). However, the roles of Th17 cells and its main cytokine, IL-17A, in carcinogenesis are still controversial (35). Accumulation of tumor-infiltrating Th17 cells have been found in a variety of cancers, including gastric, hepatocellular, breast, and LCs, and contributed to poor patient prognosis (28,36–38). Cytokines secreted by cancer cells, such as RANTES, MCP1, IL-6, IL-1β, and CCL 20, contribute to the differentiation and expansion of Th17 cells, in the tumor microenvironment (35,39). Moreover, Th17 cells and IL-17 resulted in the recruitment of Gr-1+CD11b+ myeloid cells, promoting the inflammation and tumor growth in a mouse model with oncogenic K-ras mutation expressed only in the lungs (28). Smoking-mediated Th17 inflammation by induction of osteopontin and IL-17A deficiency attenuated smoking-induced emphysema in mice (40). In contrast, elevated Th17 cells or a high ratio of Th17/Treg, in malignant pleural effusion, partially promoted by chemokines, predicted a good prognosis in patients with LC (30,41). Th17 cells may promote the recruitment of dendritic cells and subsequent activation of CD8+ T cells in tumors, exerting antitumor immunity (26). In IL-17-deficient mice, tumor growth and lung metastasis were augmented, accompanied by decreased natural killer cells and T cells, suggesting the protective roles of endogenous IL-17 and Th17 in tumors (25). Consistent with previous studies, our results demonstrated that the frequency of circulating Th17 cells was increased in the patients with LA, and was associated with tumor invasion and metastasis, indicating the pro-tumoral roles of Th17 cells in the initiation and progression of LA (42). It was also confirmed that Th17 cells were the main source of IL-17A in human peripheral blood in both patients with LA and HCs. However, the mechanisms involved in the accumulation of Th17 cells in the tumor microenvironment are still unknown, and Th17 cells could be induced, expanded, or converted from other T cells.
Tc17 cells, a minor subset of CD8+ T cells characterized by the production of IL-17, play various roles in infection, cancers and autoimmune diseases (43,44). Although most of the knowledge concerning the differentiation and plasticity of Tc17 cells stems from autoimmune diseases, emerging evidence has also shown that Tc17 cells are associated with the development of cancers in both animal models and humans (43). In patients with hepatocellular carcinoma, tumor-activated monocytes triggered the proliferation of Tc17 cells at the edge of invading tumors, by a set of proinflammatory cytokines (45). In patients with uterine cervical cancer, high levels of Tc17 cells were found in both the peripheral blood and in cervical tissues, and were involved in the metastases of pelvic lymph nodes and tumor vasculogenesis (46). Notably, adoptive transfer of tumor-specific Tc17 cells led to the regression of melanoma in mice by the recruitment of neutrophils and induction of chemokines, and elicited antitumor immunity, which was reversed by the blocking of the inducible costimulator pathway (47–49). The numbers of both CD4+ and CD8+ T cells were significantly elevated in lung tumor tissues compared with HCs, and were positively related to the staging of cancers (50). In spite of some functional similarities shared with Th17 cells, it has become gradually clear that Tc17 cells are a distinct subset of T cells regarding differentiation, development, and plasticity. Contrary to Th17 cells, the frequency of circulating Tc17 cells was reduced in the patients with LA, especially those with tumor invasion and distant metastasis. These results are consistent with data from patients with thyroid tumors or gastric carcinoma, suggesting that Tc17 cells may exhibit some antitumor activities in lung carcinoma and Th17 cells may suppress the development of Tc17 in the tumor microenvironment (16,51).
γδT cells, compared with conventional αβT cells, exhibit distinct and versatile functions by recognition of non-peptide antigens, production of proinflammatory cytokines (IL-17, interferon-γ, and TNF-α), and interaction with activation of adaptive immune cells (21,52). γδT17 cells, the primary source of IL-17 in the early stage of some diseases and one of the pivotal players in immune surveillance, display diverse responses to tumors (20,53). In patients with colorectal cancers, γδT17 cells were found to be a major source of IL-17, related to the expansion of myeloid-derived suppressor cells, and positively associated with tumor stages (54). Murine γδT17 cells also mobilized small peripheral macrophages, which expressed proangiogenic and proinflammatory mediators, and promoted ovarian cancer growth in vivo (55). Recently, in a murine model of breast cancer, γδT17 cells resulted in expansion and polarization of specific neutrophils which subsequently inhibited cytotoxic CD8+ lymphocytes, and led to pulmonary and lymph nodal metastases, indicating a cooperative mechanism among γδT17 cells, cytotoxic T cells and neutrophils in the metastatic microenvironment (18). In our study, γδT17 cells were the third source of IL-17A, which were consistent with gastric patients but not with colorectal cancers (16,54). In addition, increased frequency of γδT17 cells was found in patients with LA and was positively related to the metastasis and staging of cancers, and was markedly decreased after the resection of the tumor. The prevalence and variety of γδT17 cells in patients with LA were very similar with those of Th17 cells, suggesting these two IL17-producting T cells may collaboratively promote pulmonary carcinogenesis.
In response to stress, injury, and pathogenic stimuli, IL-17-associated cytokines, including IL-23, IL-1β, and TGF-β1, drive the differentiation of naïve T cells into IL-17-producing T cells (14,43,56). IL-23 further induces the production of IL-17 by Th17 and γδT cells, and promotes tumor growth (22,57). IL-17 targets myeloid and mesenchymal cells, and induces tissue inflammation by promoting the expression of proinflammatory cytokines, chemokines, and antimicrobial peptides (10). In addition, IL-17 resulted in the infiltration of myeloid-derived suppressor cells and angiogenesis in tumors, and contributes to the tumor-promoting microenvironments in mice (58,59). Elevated levels of IL-17 were found in patients with gastric, colorectal and prostatic cancers, and are associated with poor prognosis (60). Recently, increased levels of IL-23, IL-1β, and IL-17A were found in gastric patients and were positively related to tumor invasion and metastasis (24). In experimental silicosis, IL-17A produced by both Th17 and γδT17 cells was required for acute pulmonary inflammation and injury, but not chronic responses and fibrosis (61). Our study showed that both the mRNA and protein levels of IL-17A IL-23, IL-1β, and TGF-β1 in PBMCs of patients with LA were markedly higher than those in the HCs. In addition, the expression of IL-17A in serum was positively associated with the number of Th17 and γδT17 cells, but not Tc17 cells. Results indicated that these inflammatory cytokines contribute to the proliferation of Th17 and γδT17 cells, and the progression of LC in the tumor microenvironment.
We further explored the effects of the resection of lung tumors on the alterations of IL-17-producing T cells and inflammatory cytokines. Notably, after surgery in patients with LA, the frequencies of Th17 and γδT17 cells, and cytokines including IL-17A IL-23, IL-1β, and TGF-β1 were markedly reduced, whereas the frequency of Tc17 cells recovered, suggesting that removal of tumors may restore immune hemostasis and surveillance, and IL-17-producing cells may be critical to tumor progression.
In conclusion, our data demonstrated that the frequencies of circulating Th17 and γδT17 cells, and the protein and corresponding mRNA levels of IL-17A, IL-23, IL-1β, and TGF-β1 in the peripheral blood of patients with LA were higher than those in HCs, whereas the frequency of Tc17 cells in patients with LA was decreased. In addition, Th17 cells were the major source of IL-17A in patients with LA and HCs. Moreover, the frequencies of circulating Th17 and γδT17 cells, along with levels of IL-17A, IL-23, IL-1β, and TGF-β1 were decreased in the patients with LA after tumor resection. Our data suggest that Th17, γδT17 and Tc17 cells, and IL-17A-associated cytokines play pivotal roles in the crosstalk between tumor-related inflammation and immunity, and contribute to the development of LC. In the future, a better understanding of the distribution and cooperation among IL-17-producing T cells would provide us with a rationale for novel anticancer strategies.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (nos. 81472171, and 81300009), the Major Project of the Science Technology Department of Zhejiang Province, China (no. 2012C13022-2), the Zhejiang Provincial Natural Science Foundation of China (no. LY14H010002), the Key Personnel Grant of Zhejiang Medicine and Health Platform (no. 2012RCA025), and the Grant for Returned Overseas Chinese Scholars of the Personnel Department of Zhejiang Province (no. J20120565).
References
- 1.Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res. 2015;4:327–338. doi: 10.3978/j.issn.2218-6751.2015.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.She J, Yang P, Hong Q, Bai C. Lung cancer in China: Challenges and interventions. Chest. 2013;143:1117–1126. doi: 10.1378/chest.11-2948. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 6.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvorak HF. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 9.Pálmai-Pallag T, Bachrati CZ. Inflammation-induced DNA damage and damage-induced inflammation: A vicious cycle. Microbes Infect. 2014;16:822–832. doi: 10.1016/j.micinf.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Xu S, Cao X. Interleukin-17 and its expanding biological functions. Cell Mol Immunol. 2010;7:164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 12.Rouvier E, Luciani MF, Mattéi MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–5456. [PubMed] [Google Scholar]
- 13.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Cua DJ, Tato CM. Innate IL-17-producing cells: The sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 16.Zhong F, Cui D, Tao H, Du H, Xing C. IL-17A-producing T cells and associated cytokines are involved in the progression of gastric cancer. Oncol Rep. 2015;34:2365–2374. doi: 10.3892/or.2015.4246. [DOI] [PubMed] [Google Scholar]
- 17.Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, Shi L, Wu D, Dong C, Liu H. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014;74:1969–1982. doi: 10.1158/0008-5472.CAN-13-2534. [DOI] [PubMed] [Google Scholar]
- 18.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M, Hawinkels LJ, Jonkers J, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Liu S, Ge D, Zhang Q, Xue Y, Xiong Z, Abdel-Mageed AB, Myers L, Hill SM, Rowan BG, et al. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Res. 2012;72:2589–2599. doi: 10.1158/0008-5472.CAN-11-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva-Santos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunol. 2015;15:683–691. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 21.Patil RS, Bhat SA, Dar AA, Chiplunkar SV. The Jekyll and Hyde story of IL17-producing γδT cells. Front Immunol. 2015;6:37. doi: 10.3389/fimmu.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, Karin M. The IL-23 to IL-17 cascade inflammation-related cancers. Clin Exp Rheumatol. 2015;33(Suppl 92):S87–S90. [PubMed] [Google Scholar]
- 23.Shalapour S, Karin M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J Clin Invest. 2015;125:3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemdan NY. Anti-cancer versus cancer-promoting effects of the interleukin-17-producing T helper cells. Immunol Lett. 2013;149:123–133. doi: 10.1016/j.imlet.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu B, Guenther JF, Pociask DA, Wang Y, Kolls JK, You Z, Chandrasekar B, Shan B, Sullivan DE, Morris GF. Promotion of lung tumor growth by interleukin-17. Am J Physiol Lung Cell Mol Physiol. 2014;307:L497–L508. doi: 10.1152/ajplung.00125.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, Moghaddam SJ, Dong C. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci USA. 2014;111:5664–5669. doi: 10.1073/pnas.1319051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69:348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Yang G, Li H, Yao Y, Xu F, Bao Z, Zhou J. Treg/Th17 imbalance in malignant pleural effusion partially predicts poor prognosis. Oncol Rep. 2015;33:478–484. doi: 10.3892/or.2014.3576. [DOI] [PubMed] [Google Scholar]
- 31.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L, International Association for the Study of Lung Cancer International Staging Committee et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 32.Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121:2402–2414. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 34.Ye J, Livergood RS, Peng G. The role and regulation of human Th17 cells in tumor immunity. Am J Pathol. 2013;182:10–20. doi: 10.1016/j.ajpath.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guéry L, Hugues S. Th17 Cell plasticity and functions in cancer Immunity. Biomed Res Int. 2015;2015:314620. doi: 10.1155/2015/314620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Li Q, Chen J, Liu Y, Zhao X, Tan B, Ai J, Zhang Z, Song J, Shan B. Prevalence of Th17 and Treg cells in gastric cancer patients and its correlation with clinical parameters. Oncol Rep. 2013;30:1215–1222. doi: 10.3892/or.2013.2570. [DOI] [PubMed] [Google Scholar]
- 38.Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology. 2013;63:225–233. doi: 10.1111/his.12156. [DOI] [PubMed] [Google Scholar]
- 39.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 40.Shan M, Yuan X, Song LZ, Roberts L, Zarinkamar N, Seryshev A, Zhang Y, Hilsenbeck S, Chang SH, Dong C, et al. Cigarette smoke induction of osteopontin (SPP1) mediates TH17 inflammation in human and experimental emphysema. Sci Transl Med. 2012;4:117ra9. doi: 10.1126/scitranslmed.3003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, Tao XN, Shi HZ. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185:6348–6354. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 42.Li S, Li Y, Qu X, Liu X, Liang J. Detection and significance of TregFoxP3+ and Th17 cells in peripheral blood of non-small cell lung cancer patients. Arch Med Sci. 2014;10:232–239. doi: 10.5114/aoms.2014.42573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang Y, Pan HF, Ye DQ. Tc17 cells in immunity and systemic autoimmunity. Int Rev Immunol. 2015;34:318–331. doi: 10.3109/08830185.2014.954698. [DOI] [PubMed] [Google Scholar]
- 44.Kondo T, Takata H, Matsuki F, Takiguchi M. Cutting edge: Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J Immunol. 2009;182:1794–1798. doi: 10.4049/jimmunol.0801347. [DOI] [PubMed] [Google Scholar]
- 45.Kuang DM, Peng C, Zhao Q, Wu Y, Zhu LY, Wang J, Yin XY, Li L, Zheng L. Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T cells in hepatocellular carcinoma patients. J Immunol. 2010;185:1544–1549. doi: 10.4049/jimmunol.0904094. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Hou F, Liu X, Ma D, Zhang Y, Kong B, Cui B. Tc17 cells in patients with uterine cervical cancer. PLoS One. 2014;9:e86812. doi: 10.1371/journal.pone.0086812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y, Cho HI, Wang D, Kaosaard K, Anasetti C, Celis E, Yu XZ. Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol. 2013;190:1873–1881. doi: 10.4049/jimmunol.1201989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Hernandez ML, Hamada H, Reome JB, Misra SK, Tighe MP, Dutton RW. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J Immunol. 2010;184:4215–4227. doi: 10.4049/jimmunol.0902995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson MH, Kundimi S, Bowers JS, Rogers CE, Huff LW, Schwartz KM, Thyagarajan K, Little EC, Mehrotra S, Cole DJ, et al. The inducible costimulator augments Tc17 cell responses to self and tumor tissue. J Immunol. 2015;194:1737–1747. doi: 10.4049/jimmunol.1401082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banat GA, Tretyn A, Pullamsetti SS, Wilhelm J, Weigert A, Olesch C, Ebel K, Stiewe T, Grimminger F, Seeger W, et al. Immune and inflammatory cell composition of human lung cancer stroma. PLoS One. 2015;10:e0139073. doi: 10.1371/journal.pone.0139073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang G, Ma S, Wei Y, Wu Y, Yu X, Liu H. The prevalence and distribution of Th17 and Tc17 cells in patients with thyroid tumor. Immunol Lett. 2014;162:68–73. doi: 10.1016/j.imlet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Vantourout P, Hayday A. Six-of-the-best: Unique contributions of γδ T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rei M, Pennington DJ, Silva-Santos B. The emerging protumor role of γδ T lymphocytes: Implications for cancer immunotherapy. Cancer Res. 2015;75:798–802. doi: 10.1158/0008-5472.CAN-14-3228. [DOI] [PubMed] [Google Scholar]
- 54.Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W, et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rei M, Gonçalves-Sousa N, Lança T, Thompson RG, Mensurado S, Balkwill FR, Kulbe H, Pennington DJ, Silva-Santos B. Murine CD27− Vγ6+ γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci USA. 2014;111:E3562–E3570. doi: 10.1073/pnas.1403424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bystrom J, Taher TE, Muhyaddin MS, Clanchy FI, Mangat P, Jawad AS, Williams RO, Mageed RA. Harnessing the therapeutic potential of Th17 cells. Mediators Inflamm. 2015;2015:205156. doi: 10.1155/2015/205156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 58.He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184:2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 60.Yang B, Kang H, Fung A, Zhao H, Wang T, Ma D. The role of interleukin 17 in tumour proliferation, angiogenesis, and metastasis. Mediators Inflamm. 2014;2014:623759. doi: 10.1155/2014/623759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lo Re S, Dumoutier L, Couillin I, Van Vyve C, Yakoub Y, Uwambayinema F, Marien B, van den Brûle S, Van Snick J, Uyttenhove C, et al. IL-17A-producing gammadelta T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis. J Immunol. 2010;184:6367–6377. doi: 10.4049/jimmunol.0900459. [DOI] [PubMed] [Google Scholar]