Abstract
Vascular endothelial growth factor (VEGF)-neutralizing therapy with bevacizumab has become increasingly important for treating colorectal cancer. It was demonstrated that second-line chemotherapy together with bevacizumab after disease progression (PD) on first-line therapy including bevacizumab showed clinical benefits in metastatic colorectal and breast cancers (ML18147 trial, TANIA trial). One of the rationales for these trials was that the refractoriness to first-line therapy is caused by resistance to not so much bevacizumab as to the chemotherapeutic agents. Nevertheless, resistance to bevacizumab cannot be ruled out because VEGF-independent angiogenesis has been reported to be a mechanism of resistance to anti-VEGF therapy. In this study, we used a xenograft model with the human colon cancer HT-29 cells to investigate the mechanisms underlying the effect of continued administration of bevacizumab plus capecitabine even after resistance to bevacizumab was acquired. The combination of capecitabine plus bevacizumab exhibited significantly stronger antitumor and anti-angiogenic activities than did monotherapy with either agent. Capecitabine treatment significantly increased the intratumoral VEGF level compared with the control group; however, the combination with bevacizumab neutralized the VEGF. Among angiogenic factors other than VEGF, intratumoral galectin-3, which reportedly promotes angiogenesis both dependent on, and independently of VEGF, was significantly decreased in the capecitabine group and the combination group compared with the control group. In an in vitro experiment, 5-fluorouracil (5-FU), an active metabolite of capecitabine, inhibited galectin-3 production by HT-29 cells. These results suggested that capecitabine has a dual mode of action: namely, inhibition of tumor cell growth and inhibition of galectin-3 production by tumor cells. Thus, capecitabine and bevacizumab may work in a mutually complementary manner in tumor angiogenesis inhibition to overcome the resistance caused by angiogenic factors other than VEGF. These results suggest the clinical relevance and the mechanism of action of treatment with bevacizumab in combination therapy beyond PD.
Keywords: angiogenesis, bevacizumab, capecitabine, combination, galectin, resistance, colon, breast, lung
Introduction
Angiogenesis is one of the essential causes of tumor progression (1). The VEGF/VEGF receptor pathway, in particular, contributes to several processes in tumor angiogenesis (2). Indeed, in the clinical setting, anti-angiogenic therapy targeting VEGF has been demonstrated to be effective for several kinds of cancers including colorectal cancer.
Bevacizumab, a humanized anti-VEGF monoclonal antibody, is usually administered in combination with chemotherapeutic agents. Although these combination therapies improve overall survival and progression-free survival in colorectal cancer patients, almost all responders ultimately develop disease progression (PD) (3). Because endothelial cells are genetically stable, it has been considered that refractoriness to combination therapy with chemotherapeutic agents plus bevacizumab is mostly caused by resistance to the chemotherapeutic agents rather than to bevacizumab (4,5). In this context, second-line therapies including bevacizumab (bevacizumab beyond progression therapy; BBP therapy) were intensively studied and were found to demonstrate clinical benefits in patients with metastatic colorectal and breast cancer (6,7). However, VEGF-independent angiogenesis has been reported as a mechanism of resistance to anti-VEGF therapy (8). Therefore, resistance to bevacizumab cannot be excluded as a mechanism of resistance to combination therapy with chemotherapeutic agents plus bevacizumab. We hypothesized that BBP therapy is effective even after a tumor acquires resistance to bevacizumab, and we investigated the effective mechanism of BBP therapy.
To this end, we used the combination of capecitabine plus bevacizumab as BBP therapy because this combination was used for 26% of patients in the metastatic colorectal cancer BBP therapy trial and 60% of patients in the breast cancer BBP therapy trial (6,7). Capecitabine is a prodrug of 5-FU and is designed to generate high levels of 5-FU in tumor cells. It has been reported that capecitabine and 5-FU inhibited angiogenesis in colon and gastric cancer models by suppressing the secretion of angiogenic factors from tumor cells (9,10).
In this study, we used a xenograft model of a human colon cancer cell line with acquired resistance to bevacizumab (hereafter, bevacizumab PD model) to investigate the antitumor effect of the combination of capecitabine plus bevacizumab and its mechanism of action in terms of angiogenic factors produced by stromal cells and tumor cells.
Materials and methods
Test agents
Bevacizumab and capecitabine were provided by F. Hoffmann-La Roche (Basel, Switzerland) as a liquid and fine powder, respectively. Human immunoglobulin G (HuIgG) was purchased from MP Biomedicals (Solon, OH, USA). Capecitabine was dissolved in 40 mM citrate buffer (pH 6.0) containing 5% gum arabic (capecitabine vehicle).
Animals
Five-week-old male BALB/c-nu (CAnN.Cg-Foxn1 <nu>/CrlCrlj) mice were obtained from Charles River Laboratories Japan (Yokohama, Japan). All animals were allowed to acclimatize and recover from shipping-related stress for at least 4 days prior to the study. The health of the mice was monitored by daily observation. The animals were allowed free access to chlorinated water and irradiated food, and the animals were kept under a controlled light-dark cycle (12 h–12 h). All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee at Chugai Pharmaceutical Co., Ltd.
Cell lines and culture conditions
Five human colorectal cancer cell lines (COLO 205, HCT-8, HCT-116, LS411N, and HT-29), and two mouse cancer cell lines (B16-F1 and LLC1) were used in this study. All cancer cell lines were purchased from American Type Culture Collection (Manassas, VA, USA). COLO 205 and LS411N were maintained in RPMI-1640 supplemented with 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 4.5 g/l glucose, 1.5 g/l sodium bicarbonate, and 10% FBS at 37°C under 5% CO2. HCT-116 and HT-29 were maintained in McCoy's 5A supplemented with 10% FBS at 37°C under 5% CO2. HCT-8 was maintained in RPMI-1640 supplemented with 1 mM sodium pyruvate and 10% horse serum at 37°C under 5% CO2. B16-F1 and LLC1 were maintained in D-MEM supplemented with 10% FBS at 37°C under 5% CO2.
In vivo tumor growth inhibition by bevacizumab
Each mouse was inoculated subcutaneously into the right flank with 5×106 cells/mouse of human colorectal cancer cell line (either COLO 205, HCT-8, HCT 116, LS411N or HT-29). Several weeks after tumor inoculation, mice were randomly allocated to control and treatment groups. Bevacizumab (5 mg/kg) was administered intraperitoneally (i.p.) once a week for 3 weeks. To evaluate the antitumor activity of the test agents, tumor volume (TV) was measured twice a week. The tumor volume was estimated from the equation TV = ab2/2, where a and b are tumor length and width, respectively. Tumor volume ratios were calculated by dividing mean tumor volume on day 8 by mean tumor volume on day 1 and mean tumor volume on day 22 by mean tumor volume on day 15 in both the bevacizumab and control groups.
Combination of bevacizumab plus chemotherapy in the bevacizumab PD model
Mice inoculated with HT-29 cells that had been treated with bevacizumab (5 mg/kg) on days 1 and 8 (1st treatment) were randomly allocated to control IgG plus capecitabine vehicle (control), bevacizumab, capecitabine, and bevacizumab plus capecitabine groups on day 29. Bevacizumab (5 mg/kg) was administered i.p. Once a week and capecitabine (359 mg/kg) was administered orally (p.o.) every day for 3 weeks (2nd treatment). To evaluate the antitumor activity and the tolerability of the test agents, TV and body weight was measured twice a week.
Quantification of microvessel density in tumor tissues
HT-29 tumor tissues were resected from the bevacizumab PD model on day 50, and microvessel density (MVD) was evaluated immunohistochemically by using a monoclonal anti-mouse CD31 antibody (rat anti-mouse CD31 monoclonal antibody, clone MEC 13.3; BD Biosciences, Franklin Lakes, NJ, USA). Immunohistochemical staining was performed as described previously (11) using 5-µm-thick sections from freshly frozen tissues. MVD (%) was calculated from the ratio of the CD31-positive staining area to the total observation area. Fields excluding necrotic areas were analyzed. Positive staining areas were calculated by using imaging analysis software (Definiens Tissue Studio; Definiens, Munich, Germany).
Flow cytometric analysis of tumor-infiltrating cells
HT-29 tumors were collected from xenografted mice on day 26 after inoculation. Single cell suspensions were prepared by mincing the tumors, followed by treating with a Tumor Dissociation kit for human tumor tissue (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) in a gentleMACS Dissociator (Miltenyi Biotec). The cells were stained with antibodies to mouse CD11b (PerCP/Cy5.5), Gr1 (APC), and CD45 (FITC) (BioLegend, San Diego, CA, USA) at 1 µg/ml and analyzed by using FACSAria (Becton-Dickinson) and FlowJo software (Tree Star, Ashland, OR, USA).
Measurement of angiogenesis-related proteins
HT-29 tumor tissues collected on day 50 from the bevacizumab PD model were homogenized with cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA) with protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) and phosphatase inhibitor cocktail (Nacalai Tesque, Kyoto, Japan). The supernatant after centrifugation (14,000 × g, 5 min, 4°C) was used for the assays. The protein concentration of the supernatant was quantified using a Direct Detect spectrometer (Merck Millipore, Frankfurter, Germany). The relative expression profiles of human and murine angiogenesis-related proteins were analyzed by membrane-based antibody array (Proteome Profiler Angiogenesis Array kit; R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. The human angiogenesis array simultaneously detect the relative levels of 55 angiogenesis-related proteins (Activin A, ADAMTS-1, Angiogenin, Angiopoietin-1, Angiopoietin-2, Angiostatin/Plasminogen, Amphiregulin, Artemin, Tissue Factor/Factor III, CXCL16, DPPIV/CD26, EGF, EG-VEGF, Endoglin/CD105, Endostatin/Collagen XVIII, Endothelin-1, FGF acidic, FGF basic, FGF-4, FGF-7/KGF, GDNF, GM-CSF, HB-EGF, HGF, IGFBP-1, IGFBP-2, IGFBP-3, IL-1β, CXCL8/IL-8, TGF-β1, Leptin, CCL2/MCP-1, CCL3/MIP-1α, MMP-8, MMP-9, NRG1-β1, Pentraxin 3, PD-ECGF, PDGF-AA, PDGF-AB/PDGF-BB, Persephin, CXCL4/PF4, PlGF, Prolactin, Serpin B5/Maspin, Serpin E1/PAI-1, Serpin F1/PEDF, TIMP-1, TIMP-4, Thrombospondin-1, Thrombospondin-2, uPA, Vasohibin, VEGF, VEGF-C). The murine angiogenesis array simultaneously detects the relative levels of 53 angiogenesis-related proteins (ADAMTS1, Amphiregulin, Angiogenin, Angiopoietin-1, Angiopoietin-3, CCL2/MCP-1, CCL3/MIP-1α, CXCL1, CXCL10, SDF-1, CXCL16, CXCL4, IGFBP-10, DLL4, DPPIV, EGF, Endoglin, Endostatin, FGF acidic, FGF basic, FGF-7/KGF, Fractalkine, GM-CSF, HB-EGF, HGF, IGFBP-1, IGFBP-2, IGFBP-3, IL-1α, IL-1β, IL-10, Leptin, MMP-3, MMP-8, MMP-9, IGFBP-9, Osteopontin, PD-ECGF, PDGF-AA, PDGF-AB/BB, Pentraxin-3, PlGF-2, Prolactin, Proliferin, Serpin E1/PAI-1, Serpin F1/PEDF, Thrombospondin-2, TIMP-1, TIMP-4, Coagulation Factor III, VEGF and VEGF-B). The array was hybridized with 280 µg of total protein. The concentrations of human VEGF, galectin-1, and galectin-3 were measured by a Quantikine ELISA kit (R&D Systems).
In vitro cell treatment with 5-FU
HT-29 cells were seeded on 24-well plates at 4×104 cells/well and were incubated overnight at 37°C under 5% CO2. The cells were then treated with 5-fluorouracil (5-FU) at 0.4, 2, or 20 µM for 24 h.
Statistical analysis
The Wilcoxon test and Dunnett's test were used, with P<0.05 considered statistically significant. Statistical analyses were carried out using the SAS preclinical package (SAS Institute, Cary, NC, USA).
Results
Establishing the bevacizumab PD model
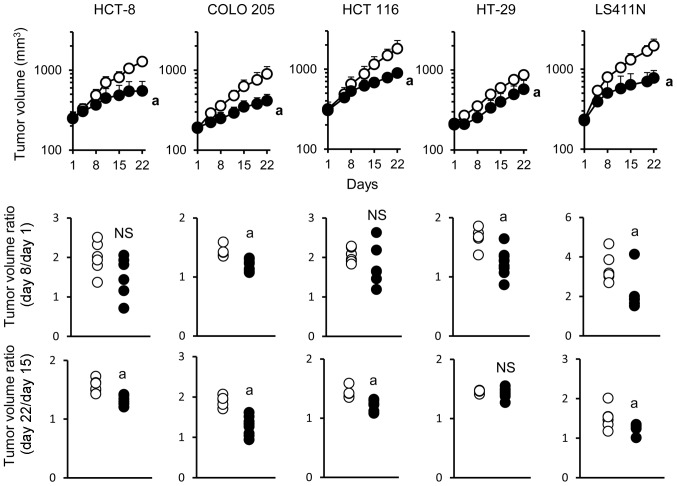
The antitumor activity of bevacizumab monotherapy was examined in five human colorectal cancer xenograft models in which tumors produced VEGF (Table I). Bevacizumab exhibited significant antitumor activity in all of the five models when evaluated on day 22. To analyze in detail the mode of antitumor activity of bevacizumab during the treatment, tumor volume ratio was calculated separately in the early phase (days 1–8) and in the late phase (days 15–22). Acquisition of resistance to bevacizumab was evaluated by comparing the differences in tumor volume ratio in the early and late phases of treatment. In the COLO 205 and LS411N models, bevacizumab exhibited a significant antitumor activity in both the early and late phase. In the HCT-8 and HCT 116 models, bevacizumab exhibited a significant antitumor activity only in the late phase. Of note, in the HT-29 model, bevacizumab exhibited significant antitumor activity in the early phase but not in the late phase (Fig. 1) indicating that the HT-29 model became non-sensitive to bevacizumab during treatment.
Table I.
VEGF expression levels in tumor tissue of colorectal cancer xenograft models.
| Tumor type | VEGF (pg per mg protein) |
|---|---|
| HCT-8 | 1,669 |
| COLO 205 | 1,778 |
| HCT-116 | 2,539 |
| HT-29 | 3,132 |
| LS411N | 3,540 |
Figure 1.
Bevacizumab shows antitumor activity in the early phase but not in the late phase in HT-29 xenograft model. Mice were randomized into 2 groups (n=5–10/group). Bevacizumab and control human IgG were intraperitoneally administered once a week. Data points show mean ± SD of tumor volume. Control, open circles; bevacizumab, closed circles. aP<0.05 versus control IgG group by Wilcoxon test.
Effect of combination therapy with bevacizumab plus capecitabine on tumor growth and MVD in the bevacizumab PD model
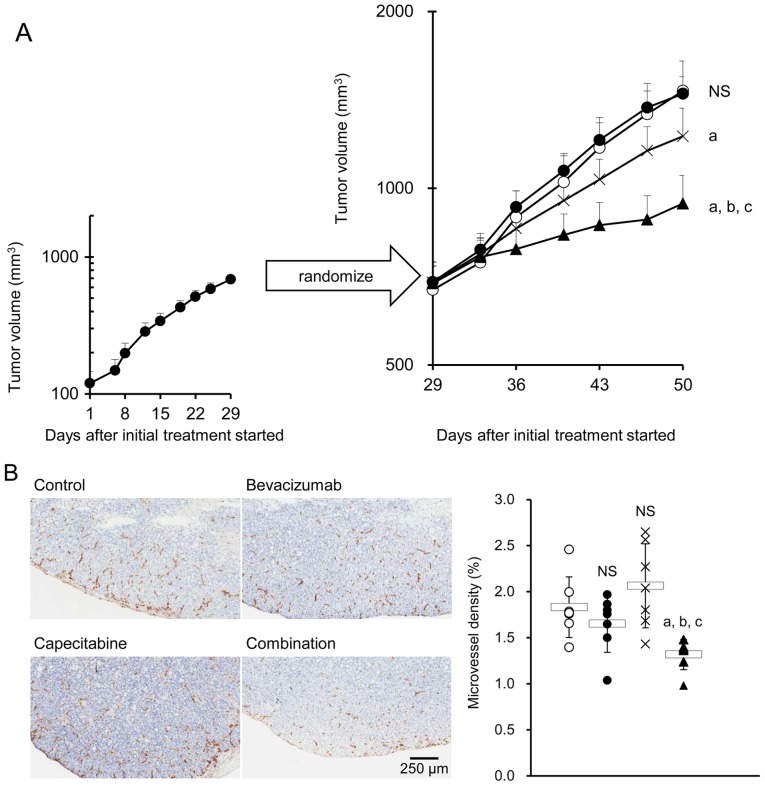
The HT-29 xenografted mice treated with bevacizumab (the bevacizumab PD model mice) were then treated with either control IgG plus capecitabine vehicle, bevacizumab, capecitabine, or bevacizumab plus capecitabine. As was expected, bevacizumab alone did not exhibit any significant antitumor effect, indicating that the HT-29 tumors pretreated with bevacizumab were refractory to bevacizumab. Capecitabine alone exhibited a significant antitumor effect versus control. The combination of bevacizumab plus capecitabine exhibited a stronger antitumor effect than capecitabine alone (Fig. 2A). No difference in MVD was observed in tumor tissues from the control, bevacizumab, and capecitabine groups. However, a significant decrease in MVD was observed in the combination group compared with the groups treated with each drug alone (Fig. 2B).
Figure 2.
Combination therapy with bevacizumab plus capecitabine inhibits tumor growth and tumor angiogenesis in the HT-29 xenograft bevacizumab PD model. Antitumor activity (A) and anti-angiogenic activity (B) of combination therapy with capecitabine plus bevacizumab in the HT-29 xenograft model unresponsive to bevacizumab. Mice that had been treated with bevacizumab on days 1 and 8 were randomly allocated to control, bevacizumab, capecitabine, and bevacizumab plus capecitabine groups on day 29 (n=7/group). CD31 immunostaining in tumor tissue at day 50 (n=7/group). Data points show mean ± SD of tumor volume. Control, open circles; bevacizumab, closed circles; capecitabine, cross marks; combination group, closed triangles. The box-and-whisker plots show mean ± SD. aP<0.05 versus control group; bP<0.05 versus capecitabine group; cP<0.05 versus bevacizumab group by Wilcoxon test.
Intratumoral stromal cell-derived angiogenic factors
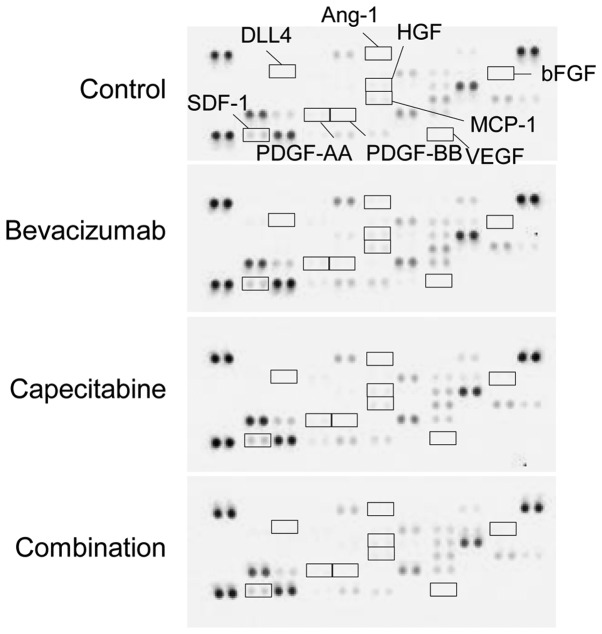
To investigate whether stromal cell-derived angiogenic factors are involved in sensitivity to combination therapy with bevacizumab, we examined the change in expression of murine angiogenesis-related factors in tumors from each treatment group. VEGF, bFGF, HGF, PDGF-AA, PDGF-BB, angiopoietin-1 (Ang-1), and δ-like ligand 4 (DLL4) were not detected. Although SDF-1 and MCP-1 were detected, none of the treatment groups showed any difference in these angiogenic factors (Fig. 3).
Figure 3.
Combination therapy did not change the expression of stromal cell-derived angiogenic factors in tumor tissues. Changes in stromal cell-derived angiogenic factors. Membranes-based antibody arrays were reacted with homogenized tumor tissue harvested from the control, bevacizumab, capecitabine, and combination group on day 50.
Tumor cell-derived angiogenic factors
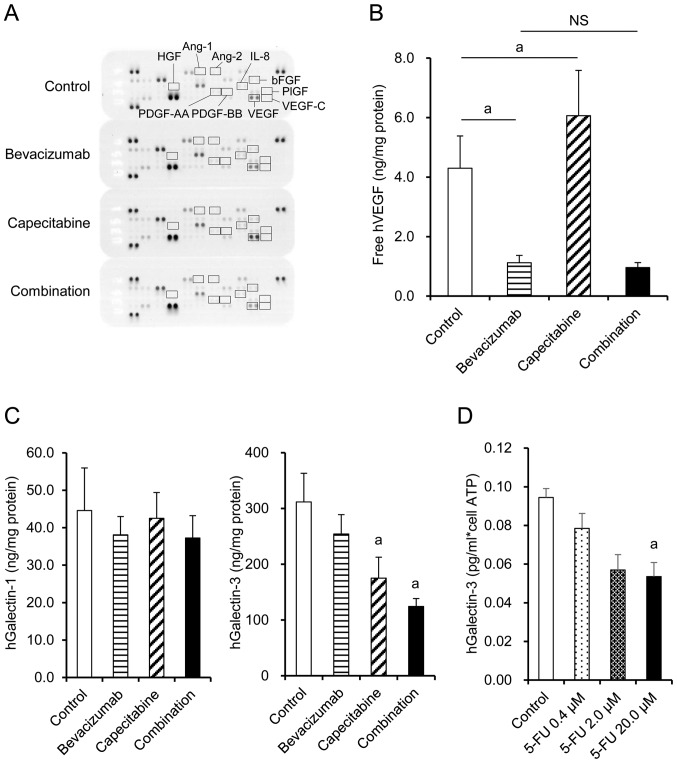
Expression of human angiogenesis-related factors in tumors from each treatment group was examined by Proteome Profiler Angiogenesis Array. VEGF, bFGF, and IL-8 were detected, whereas PDGF-AA, PDGF-BB, VEGF-C, HGF, PlGF, angiopoietin-1 (Ang-1), and angiopoietin-2 (Ang-2) were not detected regardless of the treatment (Fig. 4A). bFGF and IL-8 were similar in the capecitabine group, bevacizumab group, and combination group (Fig. 4A). VEGF level was significantly increased by capecitabine treatment compared with the control group, while VEGF was unsurprisingly neutralized by the bevacizumab-containing treatments when measured by ELISA (Fig. 4B). Galectin-3 levels were significantly decreased in the capecitabine and combination groups compared with the control group, whereas no significant difference was observed in galectin-1 levels (Fig. 4C). When the HT-29 cells were treated in vitro with 5-FU, an active metabolite of capecitabine, production of galectin-3 per cell was inhibited in a dose-dependent manner (Fig. 4D). These data suggested that capecitabine inhibited galectin-3 production by HT-29 cells via a mechanism independent of cell growth inhibition.
Figure 4.
Combination therapy suppresses tumor cell-derived VEGF and galectin-3 in tumor tissues. (A) Membranes-based antibody arrays were reacted with tumor tissue homogenates from the control group, bevacizumab group, capecitabine group, and combination group on day 50. (B) Levels of free VEGF in the tumor tissues (n=7). (C) Levels of galectin-1 and galectin-3 in the tumor tissues (n=7). The control group (open), bevacizumab group (horizontal striped), capecitabine group (hatched), and combination group (closed). Columns show mean ± SD. aP<0.05 versus control by Wilcoxon test. (D) Downregulation of galectin-3 expression by 5-FU in in vitro culture. Culture supernatant was collected and subjected to ELISA (n=3). Columns show mean ± SD. aP<0.05 versus control by Dunnett's test.
Discussion
In colorectal cancer, it was unclear whether maintenance with a combination of bevacizumab plus a chemotherapeutic agent would benefit patients who had acquired resistance to bevacizumab (12). In this study, it was found that the combination of bevacizumab plus capecitabine exhibited a strong antitumor effect on xenografted human colorectal cancer that had acquired resistance to bevacizumab in vivo. The mechanism of action of the combination of bevacizumab plus capecitabine was analyzed in terms of tumor angiogenesis.
We previously reported that the molecular targeted drugs trastuzumab and erlotinib exhibited antitumor effects when administered with chemotherapeutic agents after the tumor had acquired resistance to monotherapy with each if the tumor kept expressing the target molecules (13,14). If a tumor continues to express VEGF, combining bevacizumab with second-line chemotherapy after resistance has been acquired, may be an appropriate therapy. In order to establish a colorectal cancer xenograft model that develops resistance to bevacizumab, five human colorectal cancer xenografts which produced VEGF (Table I) were treated with bevacizumab. Bevacizumab was given as the sole regimen to avoid development of resistance to chemotherapeutic agents. As a result, a bevacizumab PD model was established using the HT-29 xenograft model and was used in the following experiments.
We investigated the antitumor effect of the combination of bevacizumab plus capecitabine by using the HT-29 PD model. Of note, the combination of bevacizumab plus capecitabine exhibited a significantly stronger antitumor effect than capecitabine alone (Fig. 2A). These data suggested that it is important to continue to inhibit VEGF by bevacizumab in combination with an appropriate chemotherapeutic agent even after the tumor has acquired resistance to bevacizumab per se. Indeed, the combination group showed a significant reduction in MVD, while the groups receiving bevacizumab or capecitabine alone did not at this stage (Fig. 2B). These results suggested that a synergistic anti-angiogenic effect was one of the mechanisms underlying the stronger antitumor effect of the combination treatment.
To examine the mechanism through which the combination treatment exerted an anti-angiogenic effect in the bevacizumab PD model, we analyzed the effect of capecitabine on the production of several angiogenic factors by both tumor cells and stromal cells. Since it has been reported that the infiltration of CD11b+/Gr1+ cells into tumors directly or indirectly produces other angiogenic factors causing resistance to anti-VEGF treatment (15), we analyzed stromal cell-derived angiogenic factors and the number of CD11b+/Gr1+ cells in the tumor in the HT-29 PD model. However, the number of CD11b+/Gr1+ cells was as few as in the anti-VEGF antibody sensitive tumor B16-F1 (data not shown). It has also been reported that bFGF, Ang-1, DLL4, HGF, SDF-1, MCP-1, PDGF-AA, and PDGF-BB from stromal cells are implicated in the development of resistance to VEGF inhibition (16–19). MCP-1 and SDF-1 were detected in the HT-29 PD model, but the levels were similar in the capecitabine, bevacizumab, and combination groups (Fig. 3). There were no obvious differences in any of the other murine angiogenic factors detected in the capecitabine, bevacizumab, or combination groups (Fig. 3). Therefore, the mechanism of action of the combination of bevacizumab plus capecitabine in this model could not be explained by the stromal cell-derived angiogenic factors tested.
Next, we investigated whether tumor cell-derived angiogenic factors were involved in the anti-angiogenic effect of the combination therapy. We examined bFGF, IL-8, PDGF-AA, PDGF-BB, VEGF-C, Ang-1, Ang-2, HGF, and PlGF, which are reported to be angiogenic factors for tumors (20–22). PDGF-AA, PDGF-BB, VEGF-C, Ang-1, Ang-2, HGF, and PlGF were not detected in the HT-29 PD model. bFGF and IL-8 were detected but their levels were similar in the capecitabine, bevacizumab and combination groups (Fig. 4A). There were no obvious differences in any of the other human angiogenic factors detected in the capecitabine, bevacizumab, or combination groups (Fig. 4A). Therefore, the anti-angiogenic effect of the combination therapy in this model could not be explained by the factors tested.
Recently, galectins, members of a family of carbohydrate-binding proteins with multiple functions, were reported to be potent prognostic marker in colorectal cancer and to promote angiogenesis (23,24). Galectin-1 maintains angiogenesis in anti-VEGF-refractory tumors (25), and galectin-3 has been shown as an important mediator of VEGF- as well as FGF-mediated angiogenic response (26–28). In light of these findings, we investigated the intratumoral levels of human galectin-1, galectin-3, and VEGF in each treatment group. No significant change was observed in galectin-1 level. However, galectin-3 level was significantly decreased in the capecitabine and combination groups compared with level in the control group (Fig. 4C). In addition, 5-FU, an active metabolite of capecitabine, directly inhibited galectin-3 production by HT-29 cells in vitro (Fig. 4D). Since VEGF- and bFGF-mediated angiogenesis has been reported to be greatly reduced by galectin-3 inhibitors such as dominant negative galectin-3 or in galectin-3 knockdown cells in vitro and also in Gal3−/− mice (26), the decrease in intratumor galectin-3 level by capecitabine in our model was suggested to contribute, at least in part, to synergistic inhibition of angiogenesis in combination with anti-VEGF antibody. Despite the reduction of galectin-3, no difference in MVD was observed between tumor tissues in the control and capecitabine groups. It has been reported that conventional 5-FU treatment may promote tumor angiogenesis by increasing the production of VEGF (9,10). In contrast with galectin-3 levels, VEGF levels indeed increased in the capecitabine treatment group in our model (Fig. 4B). These bilateral effects of capecitabine on the production of angiogenic factors may be a reason for the apparent absence of anti-angiogenic effect by capecitabine alone. However, by neutralization of VEGF through concurrent administration of bevacizumab, a synergistic anti-angiogenic effect emerged (Fig. 4B). These data raise the possibility that the inhibition of galectin-3 production by capecitabine and the neutralization of VEGF by bevacizumab were one of the mechanisms underlying the effect of combination treatment.
Regarding the development of resistance to bevacizumab in the HT-29 model, we investigated whether galectin-3 plays a role in acquisition of resistance. However, galectin-3 levels in the tumors did not change before and after developing PD (data not shown). Galectin-3 interacts with Mgat5-modified N-glycans on cytokine receptors such as EGFR, IGFR, PDGFR, and bFGFR, and activates downstream signal transduction pathways (29). Therefore, alteration in the expression of any of these receptors may cause galectin-3-induced resistance to bevacizumab. Thus, further investigations are required to clarify the mechanisms of resistance to bevacizumab in this model.
In this study, we indicated that combination therapy with bevacizumab plus capecitabine could exert strong antitumor activity, even after resistance to bevacizumab was acquired, by recovering the anti-angiogenic effect, thus suggesting the clinical relevance for treatment with bevacizumab in combination therapy beyond PD.
Acknowledgments
The authors thank Masako Miyazaki and Hiromi Sawamura from Chugai Pharmaceutical Co., Ltd, for technical assistance in the experiments, and also thank Naoki Harada, Kazushige Mori, and Kaori Fujimoto-Ouchi for support and special advice in this study.
References
- 1.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 2.Ellis LM, Hicklin DJ. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz HI, Tebbutt NC, Kabbinavar F, Giantonio BJ, Guan ZZ, Mitchell L, Waterkamp D, Tabernero J. Efficacy and safety of bevacizumab in metastatic colorectal cancer: Pooled analysis from seven randomized controlled trials. Oncologist. 2013;18:1004–1012. doi: 10.1634/theoncologist.2013-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klement G, Huang P, Mayer B, Green SK, Man S, Bohlen P, Hicklin D, Kerbel RS. Differences in therapeutic indexes of combination metronomic chemotherapy and an anti-VEGFR-2 antibody in multidrug-resistant human breast cancer xenografts. Clin Cancer Res. 2002;8:221–232. [PubMed] [Google Scholar]
- 6.Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C, et al. ML18147 Study Investigators Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013;14:29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 7.von Minckwitz G, Puglisi F, Cortes J, Vrdoljak E, Marschner N, Zielinski C, Villanueva C, Romieu G, Lang I, Ciruelos E, et al. Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): An open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1269–1278. doi: 10.1016/S1470-2045(14)70439-5. [DOI] [PubMed] [Google Scholar]
- 8.Jubb AM, Oates AJ, Holden S, Koeppen H. Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer. 2006;6:626–635. doi: 10.1038/nrc1946. [DOI] [PubMed] [Google Scholar]
- 9.Shi H, Jiang J, Ji J, Shi M, Cai Q, Chen X, Yu Y, Liu B, Zhu Z, Zhang J. Anti-angiogenesis participates in antitumor effects of metronomic capecitabine on colon cancer. Cancer Lett. 2014;349:128–135. doi: 10.1016/j.canlet.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Yuan F, Shi H, Ji J, Cai Q, Chen X, Yu Y, Liu B, Zhu Z, Zhang J. Capecitabine metronomic chemotherapy inhibits the proliferation of gastric cancer cells through anti-angiogenesis. Oncol Rep. 2015;33:1753–1762. doi: 10.3892/or.2015.3765. [DOI] [PubMed] [Google Scholar]
- 11.Yanagisawa M, Yorozu K, Kurasawa M, Nakano K, Furugaki K, Yamashita Y, Mori K, Fujimoto-Ouchi K. Bevacizumab improves the delivery and efficacy of paclitaxel. Anticancer Drugs. 2010;21:687–694. doi: 10.1097/CAD.0b013e32833b7598. [DOI] [PubMed] [Google Scholar]
- 12.Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, Adinin R, Overman MJ, Valero V, Wen S, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: Efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimoto-Ouchi K, Sekiguchi F, Yamamoto K, Shirane M, Yamashita Y, Mori K. Preclinical study of prolonged administration of trastuzumab as combination therapy after disease progression during trastuzumab monotherapy. Cancer Chemother Pharmacol. 2010;66:269–276. doi: 10.1007/s00280-009-1160-0. [DOI] [PubMed] [Google Scholar]
- 14.Iwai T, Moriya Y, Shirane M, Fujimoto-Ouchi K, Mori K. Continuous inhibition of epidermal growth factor receptor phosphorylation by erlotinib enhances antitumor activity of chemotherapy in erlotinib-resistant tumor xenografts. Oncol Rep. 2012;27:923–928. doi: 10.3892/or.2011.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maniati E, Hagemann T. IL-17 mediates resistance to anti-VEGF therapy. Nat Med. 2013;19:1092–1094. doi: 10.1038/nm.3333. [DOI] [PubMed] [Google Scholar]
- 16.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 18.Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita-Kashima Y, Fujimoto-Ouchi K, Yorozu K, Kurasawa M, Yanagisawa M, Yasuno H, Mori K. Biomarkers for antitumor activity of bevacizumab in gastric cancer models. BMC Cancer. 2012;12:37. doi: 10.1186/1471-2407-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Xie K, Ding G, Li J, Chen K, Li H, Qian J, Jiang C, Fang J. Tumor resistance to anti-VEGF therapy through up-regulation of VEGF-C expression. Cancer Lett. 2014;346:45–52. doi: 10.1016/j.canlet.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Griffioen AW, Thijssen VL. Galectins in tumor angiogenesis. Ann Transl Med. 2014;2:90. doi: 10.3978/j.issn.2305-5839.2014.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endo K, Kohnoe S, Tsujita E, Watanabe A, Nakashima H, Baba H, Maehara Y. Galectin-3 expression is a potent prognostic marker in colorectal cancer. Anticancer Res. 2005;25:3117–3121. [PubMed] [Google Scholar]
- 25.Croci DO, Cerliani JP, Dalotto-Moreno T, Méndez-Huergo SP, Mascanfroni ID, Dergan-Dylon S, Toscano MA, Caramelo JJ, García-Vallejo JJ, Ouyang J, et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–758. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 26.Markowska AI, Liu FT, Panjwani N. Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. J Exp Med. 2010;207:1981–1993. doi: 10.1084/jem.20090121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markowska AI, Jefferies KC, Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem. 2011;286:29913–29921. doi: 10.1074/jbc.M111.226423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Haene N, Sauvage S, Maris C, Adanja I, Le Mercier M, Decaestecker C, Baum L, Salmon I. VEGFR1 and VEGFR2 involvement in extracellular galectin-1- and galectin-3-induced angiogenesis. PLoS One. 2013;8:e67029. doi: 10.1371/journal.pone.0067029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]