Abstract
Background:
Renal denervation (RDN), treating resistant hypertension, has, in open trial design, been shown to lower blood pressure (BP) dramatically, but this was primarily with respect to office BP.
Method:
We conducted a SHAM-controlled, double-blind, randomized, single-center trial to establish efficacy data based on 24-h ambulatory BP measurements (ABPM). Inclusion criteria were daytime systolic ABPM at least 145 mmHg following 1 month of stable medication and 2 weeks of compliance registration. All RDN procedures were carried out by an experienced operator using the unipolar Medtronic Flex catheter (Medtronic, Santa Rosa, California, USA).
Results:
We randomized 69 patients with treatment-resistant hypertension to RDN (n = 36) or SHAM (n = 33). Groups were well balanced at baseline. Mean baseline daytime systolic ABPM was 159 ± 12 mmHg (RDN) and 159 ± 14 mmHg (SHAM). Groups had similar reductions in daytime systolic ABPM compared with baseline at 3 months [−6.2 ± 18.8 mmHg (RDN) vs. −6.0 ± 13.5 mmHg (SHAM)] and at 6 months [−6.1 ± 18.9 mmHg (RDN) vs. −4.3 ± 15.1 mmHg (SHAM)]. Mean usage of antihypertensive medication (daily defined doses) at 3 months was equal [6.8 ± 2.7 (RDN) vs. 7.0 ± 2.5 (SHAM)].
RDN performed at a single center and by a high-volume operator reduced ABPM to the same level as SHAM treatment and thus confirms the result of the HTN3 trial.
Conclusion:
Further, clinical use of RDN for treatment of resistant hypertension should await positive results from double-blinded, SHAM-controlled trials with multipolar ablation catheters or novel denervation techniques.
Keywords: ambulatory blood pressure measurement, randomized controlled trial, renal denervation, SHAM procedure, treatment-resistant hypertension
INTRODUCTION
Renal denervation (RDN), using catheter-based low-energy radiofrequency ablation in the renal arteries, has developed as a potential treatment modality in patients with treatment-resistant hypertension (TRH). Sympathetic signaling between the central nervous system and the kidneys plays a role in the maintenance of high blood pressure (BP) and should therefore be considered a relevant target for therapy. This was encouraged by open-labeled clinical trials and case series showing sustained BP-lowering effect of RDN treatment without concomitant hazards [1–3]. As hypertension is a major cardiovascular risk factor and the prevalence of TRH among diagnosed hypertensive patients has been estimated to be around 10% [4–7], the interest in RDN therapy has been enormous.
Surprisingly, the first published SHAM-controlled, randomized clinical trial on RDN treatment, the HTN3 trial, came out neutral [8]. The conflicting results with RDN treatment have been debated and may reflect important challenges in patient selection, blood-pressure (BP) monitoring, drug adherence and denervation technique [9–10]. TRH is defined as a sustained BP level above target despite concurrent use of at least three different antihypertensive drugs including a diuretic [11–12]. However, when thoroughly examined, it appears that many patients with TRH are prone to poor medical adherence and have white coat hypertension or secondary forms of hypertension [13]. Careful patient selection and BP evaluation using 24-h ambulatory BP measurements (ABPM) should therefore be mandatory in all patients with suspected TRH. This has not been the case in many previous RDN studies and case series [9].
The ReSET study was conducted as a double-blinded, SHAM-controlled, single-center intervention study to address the effect of RDN on BP measured by ABPM. The study was supported by a grant from the Danish Heart Foundation, but was otherwise unsponsored.
METHODS
Study design
Patients with therapy-resistant essential hypertension, aged between 30 and 70 years, were randomly assigned to undergo catheter-based RDN or a SHAM procedure in a 1 : 1 ratio. Patients and caretaking physicians were blinded concerning the randomization outcome during 6-month follow-up period. After unblinding, the SHAM-treated patients were offered open-labeled RDN treatment.
Study patients
Seven dedicated hypertension outpatient clinics, all located in the same region of Denmark, participated in the trial. Study criteria are presented in Table 1. Patients underwent a thorough run in and baseline examination before final submission for study randomization. During the run in of at least 1 month, medication was kept unaltered. The qualifying ABPM was done after another 14-day period with scheduled intake of antihypertensive medication, during which the patient registered each single pill taken, however, unsupervised. If the qualifying systolic daytime ABPM was at least 145 mmHg, the patient was finally included. Secondary forms of hypertension were excluded by means of computed axial tomography imaging of renal arteries (renal artery stenosis), echocardiography (coarctation of the aorta), hormone analysis (aldosteronism, hyperthyroidism and pheocromocytoma) and physical examination (Cushing disease). Follow-up examination and medical treatment of all study patients were performed in the hypertension outpatient clinic by physicians who were blinded toward the randomization outcome throughout the study. The trial was approved by the local Ethical Committee and conducted in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent before randomization.
TABLE 1.
ReSET study criteria
| Inclusion |
| • Age (30–70) years |
| • One month of stable antihypertensive treatment with at least three antihypertensive agents including a diuretic (or in case of diuretics intolerance a minimum of three nondiuretic antihypertensive drugs) |
| • Daytime ABPM SBP ≥ 145 mmHg (preceded by 14 days of scheduled drug intake showing at least 85% adherence) |
| Exclusion |
| General |
| • Noncompliant personality (abuse and mental illness) |
| • Pregnancy/inadequate contraception in fertile women |
| • Known allergy to iodine-containing radiograph contrast agent |
| Comorbidity |
| • Secondary hypertension |
| • Malignant disease |
| • Congestive heart failure NYHA 3–4 |
| • Chronic renal failure stage 4–5 (eGFR ≤ 30 ml/min per 1.73 m2) |
| • Stable angina pectoris CCS class 2–4 |
| • Unstable angina pectoris |
| • Coronary artery disease with indication for coronary intervention |
| • Recent myocardial infarction or coronary intervention (<6 months) |
| • Permanent atrial fibrillation |
| • Orthostatic syncope (<6 months) |
| • Symptomatic peripheral artery disease |
| Paraclinical |
| • Clinically significant abnormal electrolytes and liver function tests |
| • Hemoglobin <7.0 mmol/l |
| • Abnormal thyroidea function |
| • Macroscopic hematuria |
| • ECG: atrioventricular block grades 2 and 3 |
| Echocardiography |
| • Left ventricular ejection fraction <50% |
| • Significant valvular disease |
| Computed axial tomography angiography and selective angiography of renal arteries |
| • Pronounced calcification in iliaco-aortic or renal arteries |
| • Multiple renal arteries: accessory renal arteries estimated to carry more than 10% of the kidney's blood supply (small polar arteries accepted) and being undersized (see below) for ablation procedure |
| • Renal artery diameter <4 mm |
| • Renal artery length (from ostium to first major sidebranch) <20 mm |
| • Renal artery disease (stenosis, fibromuscular dysplasia, prior intervention and dissection) |
ABPM, 24-h ambulatory blood pressure monitoring; eGFR, estimated glomerular filtration rate.
Study procedure
All invasive procedures were carried out at one single invasive cardiovascular center and performed by one single experienced invasive cardiologist who, in addition, was both proctored and further qualified by 10 pretrial technically successful RDN procedures [14]. Patients were submitted in the morning and prepared for femoral artery catheterization with a 6F diagnostic catheter. Pretreatment included oral acetaminophen and 10 mg oral morphine. Unless previously examined, a coronary angiography was performed, at first, to exclude possible asymptomatic severe proximal coronary stenosis. Thereafter, renal angiography was performed to confirm the findings from the renal computed axial tomography angiography that renal artery anatomy was suitable for RDN therapy (Table 1). At this moment, sedative drugs were administered (fentanyl and midazolam) to obtain a heavy sedation, and the patient was then randomized for a SHAM procedure or a RDN procedure using a computer. If allocated to a SHAM procedure, the diagnostic catheter was kept in situ and dummy radiograph scan performed for another 10–15 min before removing the femoral sheath from the sedated patient. If allocated to RDN treatment, the Simplicity renal denervation catheter (Medtronic) was advanced and four-to-six discrete, low-power radio frequency treatments were systematically applied to cover the entire circumference in a spiral manner along the length of each main renal artery. Each single point of ablation was considered technically successful when 2 min of ramped (5–8 W) and undisrupted energy delivery could be achieved, meaning that impedance and/or temperature levels stayed within the program limits, by which the generator would otherwise switch off. After the procedure, patients were submitted to the ward for routine observation and were discharged in the evening or the next morning. The staff at the ward was blinded concerning the randomization outcome. At discharge, the patients were asked to complete a questionnaire and to state whether he or she believed his or her treatment to be RDN or SHAM or uncertain.
Blood pressure measurement
All ABPM was done using either the SpaceLabs 90207 or 90217 ABPM monitor with BP readings every 20 min. Nighttime and daytime periods were defined according to the European Society of Hypertension recommendation of short fixed interval on the basis of hour-averaged values; 0100–0600 (night) and 0900–2100 (day) [15]. A minimum of 50% successful readings during night-time and daytime were demanded for each ABPM to qualify for analysis. Patients with atrial fibrilation were excluded from the study as ABPM is more reliable during sinus rhythm.
Vasoactive hormones
For vasoactive hormones analysis, all blood samples were centrifuged for 15 min at 3000 rpm at 4 °C. Plasma was separated from blood cells and kept frozen at −20 °C, until assayed. Angiotensin II and arginine–vasopressin (AVP) were extracted from plasma with C18 Sep-Pak (Water Associates, Milford, Massachusetts, USA) and, hereafter, determined by radioimmunoassay [16]. The antibody against angiotensin II was obtained from Department of Clinical Physiology, Glostrup Hospital, Denmark. Minimal detection level was 2 pmol/l. The coefficients of variation were 12% (interassay) and 8% (intra-assay). The antibody against AVP was obtained from Professor Jacques Dürr, Miami, Florida, USA. Minimal detection level was 0.5 pmol/l. The coefficients of variation were 13% (interassay) and 9% (intra-assay).
Aldosterone was determined by a commercial RIA assay (Diagnostic Systems Laboratories Inc., Webster, Texas, USA). Minimal detection level was 22 pmol/l. Variations were 8.2% (interassay) and 3.9% (intra-assay).
Plasma renin concentration was determined by a commercial RIA assay (CIS bio international, Gif-Sur-Yvette Cedex, France). Minimal detection level was 1 pg/ml. Within-run and between-run coefficient of variations were 4.5%, and 14.5%, respectively.
Antihypertensive medication
Changes in antihypertensive medication during follow-up were only allowed if requested by the patient or if potentially harmful changes in BP, clinical appearance or biochemistry markers arose. Any changes in antihypertensive medication were decided by physician in the outpatient clinic, who was blinded concerning the invasive study treatment. The combination of antihypertensive medication was optional, apart from the mandatory use of a diuretic unless not tolerated by the patient.
Efficacy endpoints
The primary efficacy endpoint was defined as the mean change in daytime systolic ABPM from baseline to 3 months in the RDN group as compared with the SHAM group. This analysis was repeated at 1 and 6 months as a secondary endpoint. To address the potential effects on BP by confounding changes in study medication during follow-up, we assumed a mean effect of ±5 mmHg for one discontinued or added antihypertensive defined daily dose (DDD) [17]. This assumption was based on a meta-analysis [18], and for each patient an adjusted mean value of daytime systolic ABPM was derived and analyzed in the same way as the primary efficacy endpoint.
Tertiary efficacy parameters consisted of other ABPM measures (DBP, mean BP, night-time BP and 24-h BP).
Vasoactive hormones were evaluated at baseline and after 1 month of follow-up.
Safety
All adverse events and complications were systematically recorded in the trial CRF during each study visit. Specific interventional related safety data included bleeding or femoral pseudoaneurysm requiring intervention, renal artery dissection, myocardial infarction, stroke and death. Specific follow-up-related safety record concerned BP, renal function, electrolyte disarrangement, stroke, transitory ischemic attack, myocardial infarction and symptomatic hypotension.
Statistical analysis
The ReSET trial was initiated before the HTN3 trial. Therefore, according to ABPM data from the HTN2 trial [2] and according to our own pilot data [14], we hypothesized a between-group difference on the primary endpoint of 10 mmHg (daytime systolic ABPM after 3 months). Expecting a SD of approximate 13 mmHg on ABPM (own data), we calculated a minimum sample size of 28 patients in each group, beta value 0.8 and alpha value 0.05. Analysis was planned according to the intention-to-treat principle (meaning from the time of randomization), and we therefore decided to randomize a total of 70 patients.
Between-group differences and differences from baseline to the follow-up assessment were tested two-sided with the use of unpaired and paired t tests, respectively. Data on vasoactive hormones were analyzed using the Mann–Whitney test (between-group) and the Wilcoxon test (within-group) as they did not show a normal distribution. Fischer's exact test and chi-square test were used for testing binary and multilevel categorical variables, respectively. Level of significance was 0.05. Data are presented as mean values ± SD, unless otherwise shown.
RESULTS
Patient characteristics and medication
A total of 69 patients were randomized to either RDN (n = 36) or SHAM (n = 33) treatment. The last patient planned for randomization suffered a myocardial infarction a few days in advance and had to be excluded. A total of 87 patients were included in the study while meeting the baseline ABPM criteria. However, seventeen (19%) of these were excluded because of unsuitable renal artery anatomy based on computed axial tomography angiography. Baseline characteristics are shown in Table 2. A total of nine patients (14%) did not receive a diuretic at baseline due to intolerance in terms of tiredness (n = 3), dizziness (n = 3), hyponatriamia (n = 1), acute gout (n = 1) and worsening of nocturia (n = 1). More SHAM patients, than RDN patients, were treated with calcium channel blocker at baseline. There was a borderline significant baseline difference in left ventricular mass index assessed by echocardiography. Otherwise, the two groups were very similar. No patients were lost to follow-up, and no patients were unblinded prematurely. Patient blinding index at discharge was 0.83. (index value <0.5 indicates insufficient blinding, and index value of 1.0 indicates perfect blinding). There were only a few missing or unsuccessful ABPM readings during follow-up. Most occurred at 1 month, and patients with missing ABPM data all had a successful ABPM reading at either 3 or 6 months (Table 3). The overall average of successful readings for each ABPM was 86%. Almost half of the patients had changes in antihypertensive medication at 3 and 6 months follow-up. Most medical changes were minor, and differences between groups in antihypertensive treatment remained insignificant, although a tendency toward a DDD reduction in the RDN group and a DDD increment in the SHAM group were observed (P = 0.08, Table 4).
TABLE 2.
Baseline characteristics of ReSET study patients
| Characteristic | RDN group (n = 36) | SHAM group (n = 33) | P |
| Demography | |||
| Age (years) | 54.3 ± 7.8 | 57.1 ± 9.6 | 0.18 |
| Men | 75% | 73% | 0.83 |
| BMI | 28.2 ± 5.0 | 28.8 ± 3.9 | 0.38 |
| Smokers | 19% | 15% | 0.64 |
| White race | 97% | 97% | 0.95 |
| Comorbidity | |||
| eGFR ≥ 60 (ml/min per 1.73 m2) | 92% | 82% | 0.23 |
| eGFR 45–60 (ml/min per 1.73 m2) | 5% | 9% | 0.58 |
| eGFR 30–45 (ml/min per 1.73 m2) | 3% | 9% | 0.27 |
| Creatinine (μM/l) | 81 ± 27 | 87 ± 33 | 0.44 |
| History of coronary artery disease | 6% | 15% | 0.19 |
| Previous stroke | 3% | 0% | 0.34 |
| Duration of hypertension (years) | 10.9 ± 6.5 | 11.4 ± 5.9 | 0.78 |
| Hypertension >10 years | 61% | 73% | 0.31 |
| Type 2 diabetes | 25% | 31% | 0.57 |
| Type 1 diabetes | 3% | 3% | 0.93 |
| Sleep apnea (CPAP treatment) | 8% | 12% | 0.61 |
| U albumin/creatinine >30 mg/g | 36% | 36% | 0.90 |
| Heart rate ABPM (min−1) | 71 ± 10 | 70 ± 11 | 0.94 |
| LVMI (g/m2) | 109 ± 19 | 123 ± 35 | 0.07 |
| Blood pressures | |||
| Office SBP (mmHg) | 160 ± 20 | 166 ± 19 | 0.33 |
| Office DBP (mmHg) | 95 ± 15 | 90 ± 17 | 0.21 |
| ABPM systolic (mmHg) | 152 ± 12 | 153 ± 13 | 0.71 |
| ABPM diastolic (mmHg) | 91 ± 9 | 89 ± 11 | 0.48 |
| Daytime ABPM systolic (mmHg) | 159 ± 12 | 159 ± 14 | 0.97 |
| Daytime ABPM diastolic (mmHg) | 96 ± 9 | 93 ± 12 | 0.27 |
| Night-time ABPM systolic (mmHg) | 136 ± 17 | 141 ± 18 | 0.22 |
| Night-time ABPM diastolic (mmHg) | 79 ± 11 | 80 ± 10 | 0.73 |
| Drug treatment | |||
| Number of antihypertensive drugs | 4.1 ± 1.2 | 4.2 ± 1.1 | 0.89 |
| Number of antihypertensive drugs (DDD) | 6.9 ± 2.9 | 6.8 ± 2.5 | 0.89 |
| ACE inhibitor | 53% | 45% | 0.55 |
| Angiotensin-receptor blocker | 61% | 61% | 0.97 |
| Calcium-channel blocker | 53%a | 85%a | <0.01 |
| Beta-blocker | 81% | 76% | 0.64 |
| Diuretic | 86% | 85% | 0.88 |
| Thiazide diuretic | 50% | 52% | 0.90 |
| Loop diuretic | 28% | 30% | 0.82 |
| Aldosterone inhibitor | 22% | 21% | 0.92 |
| Alpha-adrenergic blocker | 11% | 21% | 0.26 |
| Direct-acting renin inhibitor | 3% | 6% | 0.51 |
| Direct-acting vasodilator | 17% | 6% | 0.17 |
| Centrally acting sympatholytic agent | 17% | 15% | 0.87 |
| Low dose aspirin (%) | 33% | 45% | 0.31 |
| Statin (%) | 36% | 48% | 0.31 |
ABPM, 24-h ambulatory blood pressure monitoring; DDD, daily defined dose; eGFR, estimated glomerular filtration rate; RDN, renal denervation.
All differences in baseline characteristics were insignificant except for aP < 0.01.
TABLE 3.
Changes in 24-h ambulatory blood pressure monitoring parameters from baseline (mmHg), paired data
| Daytime ABPM | Night-time ABPM | ABPM | |||||||||||
| Systolic | P value change | Diastolic | P value change | Systolic | P value change | Diastolic | P value change | Systolic | P value change | Diastolic | P value change | ||
| 1 month | RDN (n 31) | −6.0 ± 11.0 | <0.01 | −4.2 ± 6.6 | <0.01 | −2.8 ± 17.2 | 0.36 | −1.7 ± 10.5 | 0.38 | −4.5 ± 11.2 | <0.05 | −2.6 ± 6.3 | <0.05 |
| SHAM (n 31) | 0.0 ± 15 | 0.99 | 0.2 ± 8.4 | 0.87 | 0.7 ± 15.0 | 0.80 | 0.4 ± 7.7 | 0.79 | 0.6 ± 12.8 | 0.79 | 0.0 ± 6.6 | 0.99 | |
| P value RDN vs. SHAM | 0.08 | <0.05 | 0.39 | 0.39 | 0.10 | 0.12 | |||||||
| 3 months | RDN (n 35) | −6.2 ± 18.8 | 0.06 | −2.4 ± 10.3 | 0.18 | −0.4 ± 20.5 | 0.92 | 0.8 ± 13.4 | 0.72 | −3.9 ± 17.0 | 0.19 | −1.3 ± 9.7 | 0.43 |
| SHAM (n 32) | −6.0 ± 13.5 | <0.02 | −3.2 ± 6.2 | <0.02 | −4.7 ± 16.6 | 0.12 | −2.6 ± 8.9 | 0.12 | −4.4 ± 12.0 | <0.05 | −2.7 ± 5.6 | <0.05 | |
| P value RDN vs. SHAM | 0.95 | 0.71 | 0.35 | 0.23 | 0.88 | 0.47 | |||||||
| 6 months | RDN (n 35) | −6.1 ± 18.9 | 0.07 | −3.2 ± 10.8 | 0.09 | −1.4 ± 18.2 | 0.65 | −0.6 ± 10.1 | 0.74 | −3.7 ± 16.4 | 0.19 | −1.7 ± 8.6 | 0.25 |
| SHAM (n 33) | −4.3 ± 15.1 | 0.12 | −3.6 ± 8.3 | <0.02 | −1.1 ± 14.4 | 0.66 | −0.7 ± 8.8 | 0.67 | −2.6 ± 12.8 | 0.24 | −2.6 ± 7.5 | 0.05 | |
| P value RDN vs. SHAM | 0.66 | 0.87 | 0.95 | 0.97 | 0.76 | 0.64 | |||||||
ABPM, 24-h ambulatory blood pressure monitoring; RDN, renal denervation.
TABLE 4.
Antihypertensive drug consumption at baseline and follow-up. P values for the change from baseline within groups (paired data) are denoted in round brackets
| RDN | SHAM | P value group comparison | ||
| Baseline | Antihypertensive drugs (numbers) | 4.1 ± 1.2 | 4.2 ± 1,1 | 0.96 |
| Antihypertensive drugs (DDD) | 6.9 ± 2.7 | 6.8 ± 2.5 | 0.86 | |
| 1 month | Antihypertensive drugs (numbers) | 4.2 ± 1.2 (0.16) | 4.2 ± 1.1 (1.00) | 0.88 |
| Antihypertensive drugs (DDD) | 7.0 ± 2.8 (0.61) | 6.8 ± 2.5 (0.10) | 0.80 | |
| Patients with changes in drugs | 14% | 12% | ||
| 3 months | Antihypertensive drugs (numbers) | 4.2 ± 1.2 (0.66) | 4.2 ± 1.2 (0.26) | 0.79 |
| Antihypertensive drugs (DDD) | 6.8 ± 2.7 (0.72) | 7.0 ± 2.5 (0.14) | 0.77 | |
| Patients with changes in drugs | 41% | 24% | ||
| 6 months | Antihypertensive drugs (numbers) | 4.1 ± 1.2 (0.99) | 4.2 ± 1.3 (0.45) | 0.39 |
| Antihypertensive drugs (DDD) | 6.5 ± 2.8 (0.19) | 7.1 ± 2.5 (0.16) | 0.72 | |
| Patients with changes in drugs | 46% | 33% |
No significant difference between groups and no significant changes within groups. DDD, daily defined dose; RDN, renal denervation.
Procedure data
Data for the combined procedure of renal angiography and renal denervation showed a mean procedure time of 42 ± 11 min and a mean iodine contrast volume of 85 ± 26 ml. The mean number of successful ablations in each renal artery was 5.4 ± 1.0 (left) and 5.5 ± 0.9 (right).
Blood pressure data
Changes in ABPM at 1, 3 and 6 months are shown in Table 3. Groups showed very similar changes in ABPM at 3 and 6 months. The primary efficacy endpoint came out neutral with a reduction in daytime systolic ABPM of 6.2 mmHg (RDN group) vs. 6.0 mmHg (SHAM group) at 3 months (Fig. 1). When adjusting for changes in antihypertensive medication during follow-up, the RDN group showed a reduction in daytime systolic ABPM of 6.1 ± 19.6 mmHg at 3 months and 6.9 ± 21.6 mmHg at 6 months. This remained insignificant compared with the adjusted reduction in the SHAM group of 4.7 ± 13.1 mmHg at 3 months and 2.6 ± 17.5 mmHg at 6 months (P = 0.73 and 0.35, respectively).
FIGURE 1.
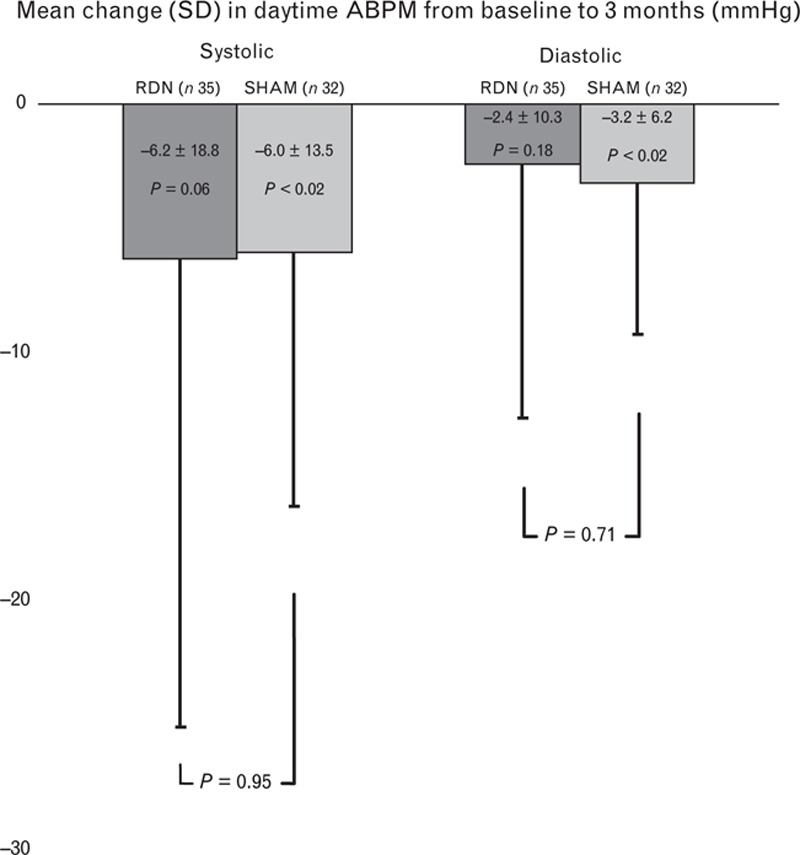
Mean change from baseline to 3 months in daytime SBP and daytime DBP, measured by means of 24-h ambulatory blood pressure measurement. There was no difference in the change in blood pressure between groups.
However, at 1 month, when medical changes were few, we observed a borderline significant difference in daytime systolic ABPM of −6.0 mmHg in favor of RDN (P = 0.08) and a significant difference in daytime diastolic ABPM daytime DBP of −4.4 mmHg (P = 0.02).
Vasoactive hormone data
The effect of RDN on vasoactive hormones was assessed in a subset of patients (28 patients in the RDN group and 30 patients in the RDN group) at baseline and at 1 month. The results are presented in Table 5. Plasma levels of aldosterone were significantly reduced in the RDN group compared with the SHAM group. RDN had no impact on plasma levels of angiotensin II, renin and vasopressin.
TABLE 5.
Plasma levels of vasoactive hormones at baseline and changes from baseline to 1 month follow-up. P values for a difference between groups are shown
| Baseline | Changes from baseline | ||
| Angiotensin II (pg/ml) | RDN | 5.0 (8.0) | 0.6 (4.0) |
| SHAM | 5.5 (10) | 1.0 (4.7) | |
| P value | 0.68 | 0.74 | |
| Renin (pg/ml) | RDN | 7.4 (31.4) | −0.7 (5.8) |
| SHAM | 6.8 (14.7) | 0.3 (4.1) | |
| P value | 0.43 | 0.35 | |
| Aldosterone (pmol/ml) | RDN | 108 (164) | −5 (59)a |
| SHAM | 116 (181) | 12 (65)a | |
| P value | 0.82 | 0.02 | |
| Vasopressin (pg/ml) | RDN | 0.5 (0.3) | 0.0 (0.0) |
| SHAM | 0.6 (0.2) | 0.0 (0.4) | |
| P value | 0.31 | 0.22 |
Values are medians with interquartile range. RDN, renal denervation.
aSignificant difference between groups, P = 0.02 (Mann–Whitney test). Other parameters nonsignificant within and between groups.
Safety data
No procedural complications were reported apart from two cases of self-limiting femoral hematoma. A few patients reported adverse reactions during follow-up. One RDN patient and two SHAM patients were shortly hospitalized during follow-up due to increasing BPs. One SHAM patient suffered a stroke and one SHAM patient had a percutaneous coronary intervention due to unstable angina. Both incidents occurred several weeks after the SHAM procedure and were not considered procedure related. There were no overall changes in renal function in either group. Minor symptoms such as tiredness, headache, atypical chest pain, muscle convulsions and fatigue were reported by five RDN patients and by six SHAM patients. Those patients experiencing a large BP rise had not developed renal arterial stenosis.
DISCUSSION
ReSET is the first single-center randomized SHAM-controlled study to address the potential effect of RDN treatment based on ABPM measurement in patients with pronounced resistant hypertension. We found similar but modest reductions in ABPM parameters at 3 and 6 months with both RDN and SHAM treatment. Although carefully administered by an experienced operator, our study could not show beneficial effects of RDN treatment compared with SHAM treatment with respect to the primary or secondary efficacy endpoints. However, a borderline significant effect on ABPM at 1 month could be observed in favor of RDN treatment, which may reflect a transient effect of RDN. Interestingly, this was accompanied by a significant reduction in plasma levels of aldosterone in the RDN group compared with the SHAM group. A similar effect of RDN on plasma levels of aldosterone has previously been reported [19] and does suggest that some reduction in renal sympathetic afferent nerve activity was actually achieved [20]. However, plasma renin and angiotensin II were unaltered and changes in aldosterone were modest, and because we did not plan for hormone analyses at 3 and 6 months, this finding could not be further elucidated. Nevertheless, our data may support a measurable biological impact of RDN, but the effect did not translate into a sustained BP reduction.
Also, the larger HTN3 trial showed no significant RDN effect on BP when compared with SHAM. Major criticism of this finding was the multicenter design of the trial, and the fact that a large proportion of the RDN procedures were carried out at low-volume centers and by inexperienced operators [10]. Importantly, this criticism is addressed in the ReSET study, as all procedures were carried out by an experienced RDN operator at a single high-volume center.
Although the ablation technique appears simple, a correct spiral location of the applied ablation spots in each renal artery may be crucial for the effect to occur [21]. Moreover, sympathetic nervous fibers may not be located as intimately to the renal arterial wall as suggested [22]. The delivery of sufficient energy also at the distal part of each renal artery may therefore be needed to remove the sympathetic conduction. Unfortunately, there is no established way to document how far renal sympathetic nerve conduction is actually affected during ablation. For any clinical RDN study so far, it is therefore uncertain to what extent renal sympathetic tone has been modulated. However, in our study, the number of technical successful ablations (see methods) in each renal artery was higher compared with the HTN3 trial. This may be important, as the number of achieved successful ablation has been suggested to predict efficacy outcome [22].
Importantly also, patients in our study were evaluated with renal computed axial tomography angiography in advance. This may have avoided a potential pressure on the operator to accept borderline renal artery anatomies for study randomization that otherwise could affect efficacy outcome.
Although it was intended to keep antihypertensive medication unchanged in both treatment arms, almost half of the patients in our study, as in the HTN3 trial, had changes in medication during follow-up. However, most changes were minor and did not account for the neutral outcome of our trial, but underline some important aspects when treating patients with TRH. Due to occasionally very high BPs at follow-up, the physician will often try to intensify drug treatment, and due to drug skepticism and minor adverse reactions, the patient will often desire a drug discontinuation whenever possible. Such bias may have contributed to the overall BP reduction observed in our study. Moreover, a regression toward the mean phenomenon and Hawthorne effects with continuously improved drug compliance after study procedure should be expected to facilitate an artefactual BP reduction during follow-up. As we did not do any drug screening test or had witnessed drug intake, we cannot exclude the possibility that improvement in compliance took place during follow-up, even though our patients did a scheduled intake of antihypertensive medication at baseline. In fact, it was recently described in a meta-analysis that a reduction in SBP of 6–9 mmHg should be expected in placebo arms of randomized and double-blinded hypertension trials due to regression toward the mean and Hawthorne effects [23]. In that respect, the positive outcome of the DENERHTN trial deserves attention [24]. This study compared the effect on ABPM in patients treated with RDN compared with a matched control group. As both groups followed the same standardized stepped-care antihypertensive treatment, this may have avoided some potential confounders concerning drug changes and compliance. However, the study was not blinded and should therefore be interpreted with some caution.
Our patients had at least 1 month of stable antihypertensive medication prior to the baseline ABPM measurement. This was considered sufficient to ensure BP steady state and at the same time, allowed our patients not to be exposed to very high BP levels for an unethically long period of time. The proportion of patients not receiving a diuretic at baseline (14%) may seem high. However, all nine patients had previously been exposed to low-dose thiazide and three of them also to spironolactone, without achieving BP control, and eventually discontinued due to intolerance. These patients therefore appeared to be truly treatment resistant, although not receiving a diuretic at baseline. As the proportion of patients on diuretic therapy did not change during follow-up and especially as the number of patients treated with aldosterone antagonists remained unaltered in both groups from baseline to 6 months (22 and 21% for RDN and SHAM, respectively), the study outcome are unlikely to have been affected significantly by administration of diuretic treatment. Nevertheless, for the interpretation of the study result, it is important to recognize that although apparently drug resistant, the ReSET cohort included some patients who were not in diuretic treatment at inclusion, and that only 70% received either a thiazide diuretic or an aldosterone antagonist. The reason for the relatively high proportion of patients receiving loop diuretics was due to the concomitant use of minoxidil.
We are confident that our patients were sufficiently blinded concerning the study procedure, as a dedicated questionnaire at discharge showed a high blinding index. Because the observed differences in BP reduction between the two treatment arms were indeed minor, our study must be considered conclusively neutral, and not the result of statistical underpowering. Thus, our study does not support the findings from a recently published small SHAM-controlled double-blinded RDN trial in mild TRH, which suggested a positive effect of RDN treatment, but statistically insignificant due to a type 2 error [25].
Some data suggest that patients with isolated systolic hypertension (≥140/<90 mmHg) may respond to less RDN than patients with combined hypertension (≥140/≥90 mmHg) [26]. We allocated nine patients with isolated systolic hypertension to RDN treatment. These patients showed a similar reduction in daytime systolic ABPM compared with the entire RDN group: −8.4 ± 10, −2.7 ± 13.8 and −6.5 ± 14 mmHg at 1, 3 and 6 months, respectively. Thus, the neutral outcome of our study could not be attributed any influence of patients with isolated systolic hypertension.
In conclusion, RDN treatment with the unipolar simplicity flex catheter showed no sustained effect on BP in our study, although the procedure was carried out by an experienced operator in well characterized patients with TRH.
Limitations
Although we had sufficient power to reveal a 10 mmHg difference between groups as hypothesized, the patient number is a limitation to the study, especially for the interpretation of the short term outcome at 1 month follow-up. Our study does, therefore, not exclude the possibility that RDN may contribute with small BP reductions. We used the unipolar Simplicity Flex catheter, although multipolar catheters, constructed to accomplish a higher probability of obtaining a true 360° spiral ablation, became available during the study. Even though our operator did a systematic positioning of each ablation, it is possible that a full circumferential ablation pattern was not always achieved. Also, we have not witnessed drug intake before our AMBP measurements. There is new evidence that more than 20% referred to RDN may not take any medications despite prescription of more than four different antihypertensive drugs [27], but was unrecognized until our study was almost completed. As our study was randomized and blinded, the group differences are not likely to be affected by this, but the observed effects of RDN over time could be overestimated, if noncompliant patients reinitiated their medical treatments after study procedure.
Perspectives
The result of the ReSET trial is in line with the neutral outcome of the HTN3 trial. Thus, there are, to this point, no SHAM-controlled trials to document the BP-lowering effect of RDN. As observational data and open-labeled studies have shown dramatic BP-lowering effects that are likely to be artefactual, we are reminded that open label uncontrolled studies in hypertension have low validity. Likewise, substudies from open trials, exploring the mechanisms for the observed BP reductions should be interpreted cautiously, as the BP drop is likely to have occurred for other reasons than the RDN itself.
However, we cannot exclude that new generation multipolar ablation catheters as well as different denervation techniques could lead to a more complete renal sympathetic denervation and thereby a more substantial and sustained BP-drop. This needs to be examined in randomized double-blind SHAM-controlled clinical trials. For now, RDN should not be offered as a treatment modality in resistant hypertension as there are no reliable SHAM-controlled data to suggest that the procedure is helpful.
ACKNOWLEDGEMENTS
Sources of funding: This study was supported by a grant from The Danish Heart Foundation.
Previous presentation: The main results of the ReSET trial were presented as an oral abstract at the TCT 2015 Congress in San Fransisco, USA.
Disclosures: A.K. has received speaker honoraria from Medtronic.
Conflicts of interest
There are no conflicts of interest.
Reviewers’ Summary Evaluation
Reviewer 1
This is a well designed, small, single centre double blind randomized sham controlled trial assessing the BP-lowering efficacy of renal denervation (RDN) using a single electrode catheter. The main finding is that the BP reduction at 3 and 6 months follow up did not differ between the two groups. The authors conclude that this study confirms the results of Symplicity HTN-3, and that future findings from sham controlled studies using multielectrode catheters are required to ultimately determine a potential role of RDN in the treatment of (resistant) hypertension.
Footnotes
Abbreviations: ABPM, 24-h ambulatory blood pressure monitoring; AVP, arginine–vasopressin; BP, blood pressure; CT, computed axial tomography; DDD, daily defined dose; RDN, renal denervation; TRH, treatment-resistant hypertension
REFERENCES
- 1.Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, et al. Percutaneous renal denervation in patients with treatment resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 2014; 383:622–629. [DOI] [PubMed] [Google Scholar]
- 2.Esler MD, Böhm M, Sievert H, Rump CL, Schmieder RE, Krum H, et al. 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur Heart J 2014; 35:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Böhm M, Mahfoud F, Ukena C, Hoppe UC, Narkiewicz K, Negoita M, et al. First report of the Global SYMPLICITY Registry on the effect of renal artery denervation in patients with uncontrolled hypertension. Hypertension 2015; 65:766–774. [DOI] [PubMed] [Google Scholar]
- 4.Sarafidis P, Bakris G. Resistant hypertension. An overview of evaluation and treatment. J Am Coll Cardiol 2008; 52:1749–1757. [DOI] [PubMed] [Google Scholar]
- 5.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 117:510–526. [DOI] [PubMed] [Google Scholar]
- 6.Persell SD. Prevalence of resistant hypertension in the United States 2003–2008. Hypertension 2011; 57:1076–1080. [DOI] [PubMed] [Google Scholar]
- 7.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension 2011; 57:898–902. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 9.Kjeldsen SE, Fadl Elmula FE, Persu A, Jin Y, Staessen JA. Renal sympathetic denervation in the aftermath of Symplicity HTN-3. Blood Press 2014; 23:256–261. [DOI] [PubMed] [Google Scholar]
- 10.Esler M. Renal denervation for treatment of drug-resistant hypertension. Trends Cardiovasc Med 2015; 25:107–115. [DOI] [PubMed] [Google Scholar]
- 11.Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009; 27:2121–2158. [DOI] [PubMed] [Google Scholar]
- 12.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 13.Fadl Elmula FE, Hoffmann P, Larstorp AC, Fossum E, Brekke M, Kjeldsen SE, et al. Adjusted drug treatment is superior to renal sympathetic denervation in patients with true treatment-resistant hypertension. Hypertension 2014; 63:991–999. [DOI] [PubMed] [Google Scholar]
- 14.Vase H, Mathiassen ON, Kaltoft A, Pedersen EB, Christensen KL, Buus NH, et al. Catheter-based renal denervation for treatment of resistant in hypertension. Dan Med J 2012; 59:A4439. [PubMed] [Google Scholar]
- 15.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen EB, Eiskjaer H, Madsen B, Danielsen H, Egeblad M, Nielsen CB. Effect of captopril on renal extraction of renin, angiotensin II, atrial natriuretic peptide and vasopressin, and renal vein renin ratio in patients with arterial hypertension and unilateral renal artery disease. Nephrol Dial Transplant 1993; 8:1064–1070. [PubMed] [Google Scholar]
- 17.http://www.whocc.no/ddd/definition_and_general_considera/. [Google Scholar]
- 18.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 2003; 326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voskuil M, Verloop WL, Blankestijn PJ, Agostoni P, Stella PR, Doevendans PA. Percutaneous renal denervation for the treatment of resistant essential hypertension; the first Dutch experience. Neth Heart J 2011; 19:319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 1997; 77:75–197. [DOI] [PubMed] [Google Scholar]
- 21.Sakakura K, Ladich E, Cheng Q, Otsuka F, Yahagi K, Fowler DR, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol 2014; 64:635–643. [DOI] [PubMed] [Google Scholar]
- 22.Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J 2015; 36:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel HC, Hayward C, Ozdemir BA, Rosen SD, Krum H, Lyon AR, et al. Magnitude of blood pressure reduction in the placebo arms of modern hypertension trials: implications for trials of renal denervation. Hypertension 2015; 65:401–406. [DOI] [PubMed] [Google Scholar]
- 24.Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015; 385:1957–1965. [DOI] [PubMed] [Google Scholar]
- 25.Desch S, Okon T, Heinemann D, Kulle K, Röhnert K, Sonnabend M, et al. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension 2015; 65:1202–1208. [DOI] [PubMed] [Google Scholar]
- 26.Ewen S, Ukena C, Linz D, Kindermann I, Cremers B, Laufs U, et al. Reduced effect of percutaneous renal denervation on blood pressure in patients with isolated systolic hypertension. Hypertension 2015; 65:193–199. [DOI] [PubMed] [Google Scholar]
- 27.Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H, Toennes SW. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens 2013; 31:766–774. [DOI] [PubMed] [Google Scholar]


