ABSTRACT
Introduction:
Administration of high ratios of plasma to packed red blood cells is a routine practice for in-hospital trauma resuscitation. Military and civilian emergency teams are increasingly carrying prehospital blood products (PHBP) for trauma resuscitation. This study systematically reviewed the clinical literature to determine the extent to which the available evidence supports this practice.
Methods:
Bibliographic databases and other sources were searched to July 2015 using keywords and index terms related to the intervention, setting, and condition. Standard systematic review methodology aimed at minimizing bias was used for study selection, data extraction, and quality assessment (protocol registration PROSPERO: CRD42014013794). Synthesis was mainly narrative with random effects model meta-analysis limited to mortality outcomes.
Results:
No prospective comparative or randomized studies were identified. Sixteen case series and 11 comparative studies were included in the review. Seven studies included mixed populations of trauma and non-trauma patients. Twenty-five of 27 studies provided only very low quality evidence. No association between PHBP and survival was found (OR for mortality: 1.29, 95% CI: 0.84–1.96, P = 0.24). A single study showed improved survival in the first 24 h. No consistent physiological or biochemical benefit was identified, nor was there evidence of reduced in-hospital transfusion requirements. Transfusion reactions were rare, suggesting the short-term safety of PHBP administration.
Conclusions:
While PHBP resuscitation appears logical, the clinical literature is limited, provides only poor quality evidence, and does not demonstrate improved outcomes. No conclusions as to efficacy can be drawn. The results of randomized controlled trials are awaited.
Keywords: Blood component transfusion, emergency medical services, erythrocyte transfusion, haemorrhage, meta-analysis, military medicine, plasma, wounds and injuries
INTRODUCTION
Liberal blood product resuscitation has probably contributed to improved casualty survival in recent conflicts (1, 2). Early administration of plasma in high ratios to packed red blood cells (PRBC) is a characteristic (3). The reintroduction of military prehospital blood product (PHBP) resuscitation was a logical evolution and is increasingly mirrored in civilian practice. However, the evidence supporting plasma-rich resuscitation is limited to systematic reviews of predominantly retrospective, observational studies (4, 5). A Cochrane review of plasma in massive transfusion is yet to be published (6), whereas a review of plasma transfusion in the critically ill failed to identify any relevant randomized studies (7). A recent observational study (8) associated early plasma administration with improved 30-day survival (9). However, the PROPPR trial found that despite achieving earlier haemostasis, resuscitation with plasma, platelets and PRBC in 1:1:1 ratios did not improve overall survival compared with 1:1:2 (10).
PHBP were used during the Vietnam War (11), with civilian prehospital PRBC administration reported in 1985 (12). In 2008, plasma and PRBC were added to the capabilities of the British Military's Medical Emergency Response Team (Enhanced) (MERT(E)) (13). Other nations have implemented similar strategies (14, 15). Retrieval by MERT(E) is associated with improved survival after major injury (16). However, blood product administration is not unequivocally benign; in addition to transfusion reactions, increasing blood product receipt after trauma has been independently associated with ARDS (17), multi-organ failure (18), and mortality (19–21). This suggests a context-specific balance of risks and benefits. In addition, widespread implementation of PHBP resuscitation (especially plasma) in civilian practice is challenging. Only 4% of US and UK donor pools are universal (group AB) plasma donors and the shelf-life of thawed plasma is only 24 h. Nonetheless, various PHBP combinations have been delivered with minimal wastage (22–28).
The aim of this systematic review was to determine the extent to which PHBP resuscitation for trauma is supported by clinical evidence.
METHODS
The study was registered with PROSPERO (CRD42014013794), was conducted according to the published protocol (29), and is reported according to PRISMA guidelines (30) (Checklist, Supplementary Digital Content 1). Relevant studies were sought from bibliographic databases (monthly searches to July 2015) and other relevant sources; see protocol (30) for full details and Medline search strategy (see also Text, Supplementary Digital Content 2, EMBASE search strategy, at). Standard systematic review methodology aimed at minimizing bias was used for study selection and data extraction. Studies were eligible if they evaluated blood products (case-series) or compared these to other resuscitative fluids (controlled studies); were in patients aged ≥16 years with traumatic haemorrhage; and were conducted in a military or civilian setting. There was no restriction by outcome. Data not included in published manuscripts or abstracts were sought from the relevant authors.
Ten studies that met selection criteria were not taken forward for analysis (see Table, Supplementary Digital Content 3, relevant studies excluded, at). Seven reported no patient outcomes. Three reported PHBP as an inconsistent component of a care bundle; no association between PHBP receipt and outcomes could be determined.
Risk of bias assessments was made using the Newcastle-Ottawa Scale (31) for comparative studies. Case series and uncontrolled before-and-after series were assessed with appropriate tools (32, 33). The quality of evidence provided by each study was reported using the GRADE method (34). GRADE allows ratings to be upgraded due to strengths or downgraded due to limitations. In this review studies were downgraded for important disparities between cohorts, lack of control for injury burden, and significant loss to follow-up. Given the inherent limitations of observational studies, merely meeting most or all design quality criteria was insufficient to merit upgrading; no studies were upgraded.
Two cohort studies reported additional subgroup analyses (35-i, 36-i). One reported matched patients and primary retrievals (patients transported directly from the incident scene to the trauma center) (35-ii, 36-iii). The second reported primary retrievals (36-ii). Data from either main or sub-studies were included as appropriate and are indicated accordingly.
Due to the disparate nature of populations, interventions, and outcomes, only limited meta-analysis was possible. Consequently, a narrative synthesis of the available evidence was constructed. Evidence for the following outcomes was considered: long-term mortality (30 days or in-hospital), early mortality (prehospital or at 24 h), in-hospital transfusion requirements, vital signs, and biochemical/haematological indices up to and at Emergency Department arrival.
Pooled estimates of mortality were calculated using inverse weighting and mixed models to reflect heterogeneity between studies. Meta-analysis of 30 day/index admission survival was performed using the Mantel–Haenszel method with a random effects model. The principal summary statistic was the odds ratio. Statistics were computed with Review Manager 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark) and R 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study selection is shown in Figure 1. Sixteen case series and 11 comparative studies (one case control, 10 retrospective cohorts) were included. Nine studies considered military trauma patients. Eighteen considered civilian patients, of which seven pooled trauma and non-trauma patients. The aims of case series were varied; frequent themes were feasibility, process description, or characterization of PHBP recipients. Comparative studies examined associations between PHBP receipt and physiological parameters or clinical outcomes.
Fig. 1.
PRISMA diagram for selection of included studies.
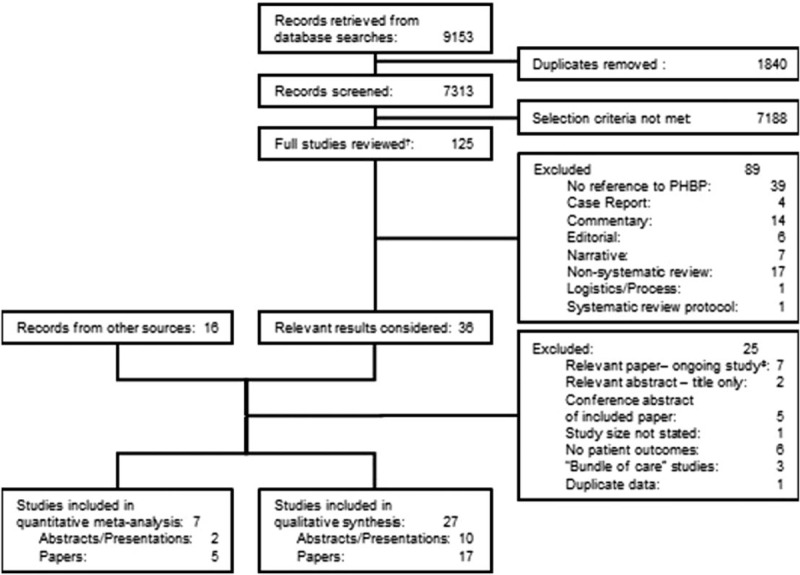
†Including studies only available in abstract; ‡trial design or authors blinded to allocations.
Both arms of one cohort study (37) formed part of a case series (38) which formed one arm of a second cohort study (39). As each study reported different aspects of PHBP resuscitation, each was considered individually. Only the final study was included in summary measures. One military study (40) contained an intervention cohort drawn from a larger case series (41).
Tables 1 and 2 summarize the various study and population characteristics. For interventions and important differences between cohorts, see Table, Supplementary Digital Content 4, Study Interventions and Differences, at. In total, 1080 of 4714 (23%) patients in comparative studies received PHBP; 2668 PHBP recipients were reported in case series, of whom 1463 (55%) had sustained trauma.
Table 1.
Case series—study and patient characteristics
| Authors | Full text/abstract (timing) | Purpose of study | Context1 trauma/mixed (secondary transfers) | Patients in study (%male) | Age | Mechanism of injury | Injury burden | Intervention |
| Dalton (12) Portland, OR | Full text (retrospective) | Demonstrate safety | Civilian trauma (unknown) | 112 (unknown) | RTA: 81 (72%) | Mean ISS: 32 | PRBC: 416 mL | |
| Penetrating: 16 (14%) | [R: 100–1250] | |||||||
| Other: 15 (13%) | ||||||||
| Berns and Zietlow (59) Rochester, MN | Full text (retrospective) | Describe protocols and experience | Civilian mixed (trauma: 48%) (transfers: 91%) | 94 (75%) | 51–60 (21–30 to 71–80) | Unknown | Unknown | “Average” 2u PRBC |
| Prause et al. (52) Graz, Austria | Full text (retrospective) | Description of process | Trauma (transfers: 0%) | 26 (unknown) | Unknown | Polytrauma: 12 | Not specified | |
| Amputations: 4 | ||||||||
| Torso trauma: 6 | ||||||||
| Craniocerebral: 2 | ||||||||
| Unspecified: 2 | ||||||||
| Badjie et al. (38) Rochester, MN | Abstract (retrospective) | Evaluate the impact of using thawed plasma on board | Civilian mixed (trauma: 48%) (transfers: 91%) | 81 (48%) | Unknown | Unknown | PRBC: 3u | |
| Plasma: 2u | ||||||||
| Higgins et al. (55) Portland, ME | Full text (retrospective) | Describe the PHBP experience, focussing on protocol compliance, provider safety, patient outcomes and transfusion complications. | Civilian mixed (trauma 71%) (transfers: 68%) | 45 (unknown) | Unknown | Unknown | PRBC: mean 1.4u | |
| (SD: 0.23u) | ||||||||
| Chew et al. (51) Victoria, Australia | Abstract (retrospective) | Report PHBP supply procedures to audit supply procedures and use | Civilian mixed (trauma >78%) (transfers: 12%) | 59 (58%) | Median 37 | RTC: 46 (78%) | Unknown | PRBC: 2u |
| Range: 16–81 | Other trauma or medical: 13 (22%) | (IQR: 2–4u) | ||||||
| Mena-Munoz et al. (74) Pittsburgh, PA | Abstract (retrospective) | Characterise PHBP recipients | Civilian mixed (trauma 25%) (transfers: 92%) | 1441 (unknown) | Unknown | Unknown | Up to 2u PRBC | |
| Sherren and Burns (25) Sydney, Australia | Abstract (retrospective) | Unclear | Civilian trauma (transfers: 0%) | 147 (69%) | 34.5 (22–52) | Blunt: 121 (82%) | RTS: 5.967 (4.083–6.904) | PRBC: 3u |
| Penetrating: 9 (6%) | (range: 1–6u) | |||||||
| Other: 17 (12%) | ||||||||
| Weaver et al. (23) London, UK | Abstract (prospective) | Examine the impact of on-scene blood transfusion for seriously injured patients | Civilian trauma (transfers: 0%) | 50 | Mean: 35 | Unknown | Unknown | PRBC: mean 2.8u |
| Bodnar et al. (50) Queensland, Australia | Full text (retrospective) | Describe the characteristics, clinical interventions and outcomes of PHBP recipients | Civilian trauma (transfers: 0%) | 71 (79%) | 39.6 (SD = 16.7) | Blunt: 52 (73%) | ISS: 32.11 (18.19) | PRBC: mean 1.8u (SD: 0.7u) |
| Penetrating: 19 (27%) | RTS: 4.7 (2.73) | |||||||
| TRISS: 0.573 (0.396) | ||||||||
| Sunde et al. (53) Bergen, Norway | Full text (retrospective) | Evaluate feasibility of introducing FDP and PRBC | Civilian mixed (trauma 56%) (transfers: 0%) | 16 (88%) | Range 23–51 | Blunt: 5 (31%) | Unknown | FDP: 200 mL (range: 100–200 mL) |
| Penetrating: 4 (25%) | PRBC “given to 4 patients” | |||||||
| Non-trauma: 7 (44%) | ||||||||
| Barkana et al. (14) Israel | Full text (retrospective) | “Characterize aspects” of PHBP use and “evaluate potential effects on morbidity & mortality” | Military trauma (Transfers: 0%) | 40 (unknown) | Range: 18–37 | Blast: 19 (47.5%) | ISS: 18 (11.5–25) | PRBC: 1u |
| Penetrating: 12 (30%) | (IQR: 1–2) [R: 1–4] | |||||||
| Blunt: 9 (22.5%) | ||||||||
| Malsby et al. (15) Afghanistan | Full text (retrospective) | Process refinement | Military trauma (transfers: 0%) | 15 (100%) | Explosive: 13 (87%) | Unknown | Median 1u blood products (IQR: 0.5–1.5u) [R: 0–2] (Various combinations of PHBP administered) | |
| GSW: 2 (13%) | ||||||||
| Glassberg et al. (67) Israel | Full text (retrospective) | Description of initial experience with prehospital lyophilized plasma | Military trauma (transfers: 0%) | 10 (unknown) | Penetrating: 8 (80%) | ISS: 19 (17.5–23.5) | FDP: 1.5u (IQR: 1–2) | |
| Other 2 (20%) | PRBC transfusion implied | |||||||
| O’Reilly et al. (41) Afghanistan | Full text (retrospective) | Description of initial experience with PHBP | Military trauma (transfers: 0%) | 310 (97%) | 24 (21–27) | Explosive: 226 (73%) | mISS 20 (16–29) | PRBC: 2u (IQR: 1–2) |
| GSW: 80 (26%) | mNISS 29 (18–48) | [range: 0–4] | ||||||
| Blunt: 3 (1%) | Plasma: 2u (IQR: 1–2) | |||||||
| Burn: 1 (0.3%) | [range: 0–4] | |||||||
| Chen (75) Israel | Abstract (retrospective) | Unclear | Military trauma (transfers: 22%) | 90 (80%) | 28 Range: 12–60 | Explosive: 20 (22%) | Unknown | PRBC: mean 1.2u |
| RTC: 26 (29%) | 392 mL (SD: 322) | |||||||
| GSW: 32 (36%) | ||||||||
| Stab: 5 (5%) | ||||||||
| Other: 7 (8%) | ||||||||
| Powell-Dunford et al. (54) Afghanistan | Full text (retrospective) | Description of process risk mitigation | Military trauma (transfers: 0%) | 61 (98%) | 24 (20–28) | Explosive: 45 (74%) | Unknown | PRBC: 1u (IQR: 1–1) |
| GSW: 16 (26%) | [range: 1–2] | |||||||
| Plasma: 0u (IQR: 0–0) | ||||||||
| [range: 0–1] |
1“Military”: casualties of armed conflict.
FDP indicates Freeze Dried Plasma; mISS and mNISS, ISS and NISS derived from the military edition of the Abbreviated Injury Scale (2005).
Table 2.
Comparative studies: study and patient characteristics (all trauma except for Badjie et al. (2013))
| Authors | Study type paper/abstract (timing context) | Purpose of study | Group (secondary transfers) | Patients in study arm (% male) | Age | Mechanism of injury | Injury Burden | Intervention |
| Price et al. (49) Portland, OR | Matched cohort | Compare efficacy of early blood transfusion | Non-recipients (Unknown) | 162 | Unknown | Unknown | Unknown | |
| abstract (retrospective civilian) | PHBP recipients (unknown) | 84 | Unknown | Unknown | Unknown | PRBC: 426 ml | ||
| Sumida et al. (48) Chattanooga, TN, Hartford, CN | Cohort full text (retrospective civilian) | Analyze the effect of PHBP on physiologic parameters and outcomes | Non-recipients (unknown) | 31 (Unknown) | 30.4 | Unknown | ISS: 27.8 | |
| RTS: 7.0 | ||||||||
| TRISS: 0.669 | ||||||||
| PHBP recipients (unknown) | 17 (Unknown) | 31.2 | Unknown | ISS: 28.0 | “blood”: 711 mL | |||
| RTS: 6.3 | ||||||||
| TRISS: 0.524 | ||||||||
| Kim et al. (37) Rochester, MN | Cohort full text (retrospective civilian) | Will delivery of prehospital plasma improve coagulopathy | PRBC only (Transfers: 54%) | 50 (60%) | 41 | Penetrating: 9 (18%) | ISS: 23 | PRBC: 1u |
| TRISS: 0.66 | ||||||||
| PRBC + Plasma (transfers: 100%) | 9 (100%) | 54 | Penetrating: 3 (33%) | ISS: 27 | PRBC: 2.5u | |||
| TRISS: 0.24* | Plasma: 2.1u | |||||||
| Badjie et al. (39) Rochester, MN | Cohort abstract (retrospective civilian) | To evaluate mortality rates of patients who received a 1:1 FFP: RBC ratio en-route | PRBC: plasma 2:1 (unknown) | 79 (Unknown) | Unknown but “comparable” | Reasons for transport not stated but “comparable” | Unknown | Up to 2u PRBC + 2u Plasma + 2u |
| PRBC OR 2u Plasma + 4u PRBC | ||||||||
| PRBC: Plasma 1:1 (unknown) | 79 (Unknown) | Unknown | Up to 3u plasma + 3u PRBC | |||||
| PHBP recipients (transfers: 0%) | 66 (61%) | Median 40 | Unknown | Not specified | ||||
| Brown et al. (35-i) Pittsburgh, PA | Cohort | Is pretrauma center RBC transfusion associated with reduced mortality and early TIC? | Non-recipients (transfers: 4%) | 1365 (67%) | 41 (26–54) | Unknown | ISS: 33 (22–41) | |
| full text (retrospective civilian) | PHBP recipients (transfers: 48%) | 50 (64%) | 41 (28–52) | Unknown | ISS: 37 (24–43) | PRBC: 1.3u (1.0–2.3) | ||
| Brown et al. (35-ii) Pittsburgh, PA | Matched cohort full text (retrospective civilian) | Is pretrauma center RBC transfusion associated with reduced mortality and TIC in a matched cohort? | Non-recipients (transfers: 24%) | 78 (72%) | 37 (24–55) | Unknown | ISS: 30 (23–43) | |
| PHBP recipients (transfers: 29%) | 35 (60%) | 36 (28–52) | Unknown | ISS: 34 (18–43) | PRBC: 1.2u (1.0–2.0) | |||
| Brown et al. (36-i) Pittsburgh, PA | Cohort full text (retrospective civilian) | Is pretrauma center RBC transfusion associated with reduced 24-h mortality, TIC, shock and Tx requirements in air medical transport | Non-recipients (transfers: 75%) | 480 (67%) | 49 (31–68) | Blunt: 395 (82%) Penetrating: 85 (18%) | ISS: 17 (9–27) | |
| PHBP recipients (transfers: 68%) | 240 (69%) | 49 (28–71.5) | Blunt: 191 (80%) Penetrating: 49 (20%) | ISS: 18 (10–29) | PRBC: 300 mL (IQR: 200–500) | |||
| Brown et al. (36-ii) Pittsburgh, PA | Cohort full text (retrospective civilian) | Is pretrauma center RBC transfusion associated with reduced 24-h mortality, TIC, shock and Tx requirements in patients transported from scene | Non-recipients (transfers: 0%) | 142 (68%) | 37 (25–65) | Blunt: 98 (69%) | ISS: 22 (13–29) | |
| PHBP recipients (transfers: 0%) | 71 (83%) | 42 (24–55) | Penetrating: 44 (31%) | ISS: 22 (10–34) | PRBC: 300 mL | |||
| Blunt: 98 (69%) | (IQR: 200–500) | |||||||
| Penetrating: 44 (31%) | ||||||||
| Wheeler et al. (57) Lebanon, NH | Case-control Full Text (Retrospective Civilian) | Identify factors associated with hypothermia | Non-hypothermic (transfers: 0%) | 647 (68%) | 39 (SD: 19) | Unknown | ISS: 16 (SD: 11) | PRBC given to 3% of subjects |
| RTS: 7.34 (SD: 1.19) | ||||||||
| TRISS: 0.93 (SD: 0.16) | ||||||||
| Hypothermic (<35°C) (Transfers: 0%) | 60 (68%) | 41 (SD: 20) | Unknown | ISS: 26 (SD: 12) | Up to 3u PRBC given to 17% of subjects | |||
| RTS: 5.86 (SD: 1.85) | ||||||||
| TRISS: 0.75 (SD: 0.29) | ||||||||
| O’Reilly et al. (40) Afghanistan | Matched cohort full text (retrospective military) | “PHBP will be associated with reduction in mortality” | Non-recipients | 97 (100%) | 23 (21–28) | Explosive: 48 (49%) | mISS: 16 (9–25) | |
| GSW: 46 (47%) | mNISS: 21 (14–34) | |||||||
| Blunt: 3 (3%) | ||||||||
| PHBP recipients | 97 (98%) | 24 (20–28) | Explosive: 50 (52%) | mISS: 16 (9–25) | PRBC: 1u (IQR: 1–2) | |||
| GSW: 46 (47%) | mNISS: 22 (15–33) | [R: 0–4] | ||||||
| Blunt: 1 (1%) | Plasma: 2u (IQR: 1–2) | |||||||
| [R: 0–4] | ||||||||
| Smith et al. (46) Afghanistan | Cohort abstract (full data available) (retrospective military) | Is PHBP receipt associated with reduced mortality or coagulopathy? | Non-recipients | 775 (96.6%) | Median band: 17–24 | Explosive: 423 (55%) | mISS: 18 (14–26) | |
| GSW: 274 (35%) | mNISS: 25 (18–34) | |||||||
| MVC: 46 (6%) | ||||||||
| Burn: 11 (1%) | ||||||||
| Other: 21 (3%) | ||||||||
| PHBP recipients | 272 (98.5%) | Median band: 17–24 | Explosive: 250 (92%) | mISS: 26 (18–30) | PRBC: 2u (IQR: 1–2) | |||
| GSW: 19 (7%) | mNISS: 41 (29–54) | [R: 0–4] | ||||||
| MVC: 3 (1%) | Plasma: 2u (IQR: 1–2) | |||||||
| [R: 0–4] | ||||||||
| Gross et al. (56) Afghanistan | Conference poster (retrospective military) | Not stated | Non-recipients | 54 (Unknown) | 25 (22–28) | Unknown | Unknown | |
| PHBP recipients | 66 (Unknown) | 25 (24–29) | Unknown | Unknown | not specified |
mISS and mNISS indicates ISS and NISS derived from the military edition of the Abbreviated Injury Scale (2005).
No blinded or randomized studies were identified—other than one prospective case series, all were retrospective observational studies. Only two studies provided more than “very low” quality evidence (see Table, Supplementary Digital Content 5, Risk of bias assessments, at). Most comparative studies were limited by differences between groups (injury burden, additional in-transit interventions, or in-hospital treatment) without control by case matching or statistical methods. Common limitations of case series included lack of a clear research question, pooling of trauma and non-trauma patients, small numbers, and lack of robust clinical outcome measures.
Long-term mortality
Long-term mortality among PHBP recipients varied from 8% to 52% (Fig. 2A). This analysis included unpublished absolute survival data for one cohort study (35-i) (J. Brown. 2015, pers. comm. June 08). One study reported 67% mortality among six subjects, but was excluded from analysis due to 60% loss to follow-up (15). Early studies reported loss to follow-up of 18% (12) and 20% (14). Later studies either minimized such losses through design or improved record keeping or (particularly when published in abstract) had insufficient information to allow loss to follow-up to be assessed. In studies from military operations in Afghanistan survival of non-coalition casualties was reported up to point of transfer to host nation medical facilities (up to 47% of study population). Significant post-transfer mortality was considered unlikely as patients were only transferred once in established recovery (42, 43). The pooled mortality estimate of 32% (95% CI: 26%–38%) exceeds the 23% mortality reported in profoundly hypotensive (SBP < 90 mm Hg) trauma patients treated without PHBP (44, 45) and provides no obvious evidence of benefit. Meta-analysis of uncorrected mortality data was performed, using matched data where available. PHBP receipt was not associated with reduced mortality (OR for mortality: 1.29, 95% CI: 0.84–1.96) (Fig. 3A). Heterogeneity was substantial (I2 = 63%). Limiting the meta-analysis to matched studies provided no evidence of benefit (Fig. 3B). Only three studies reported mortality adjusted for confounders (Fig. 4A)(35, 36, 46). These were not combined statistically.
Fig. 2.
Mortality among PHBP recipients.
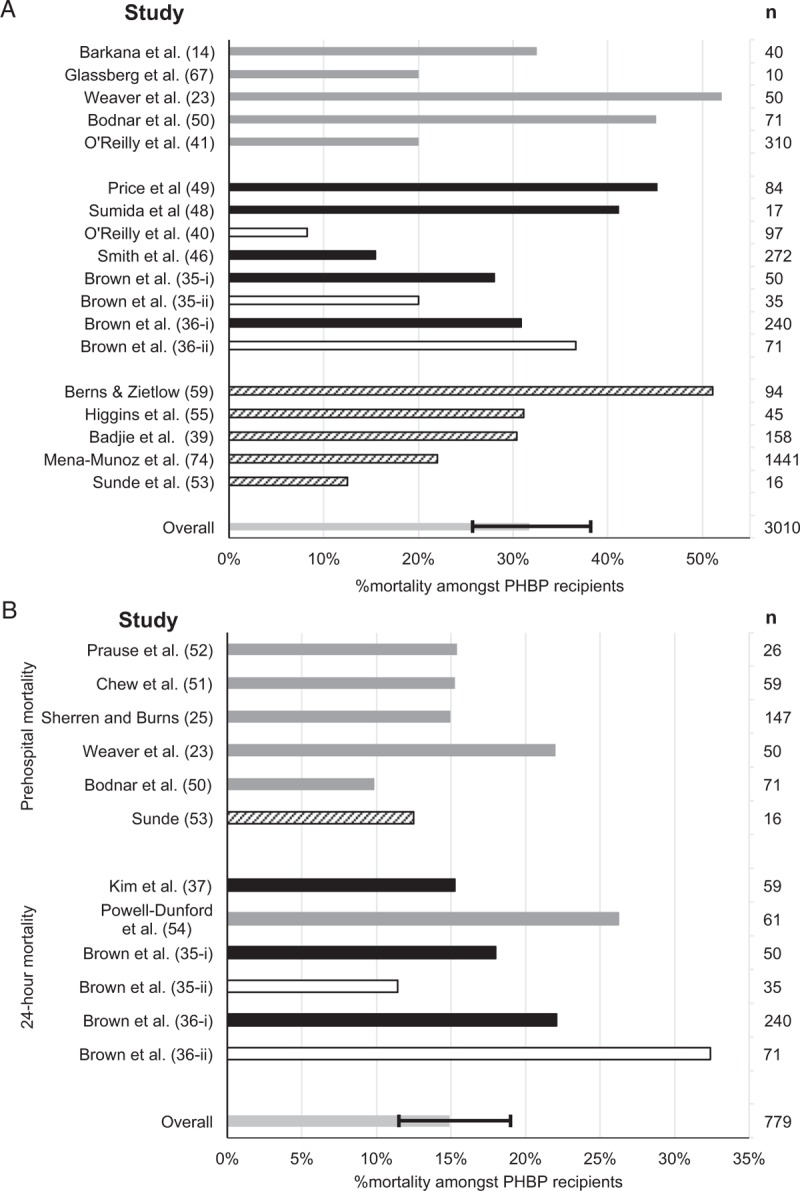
A, Overall; B, within 24 h. Gray bars: case series. Black bars: cohort studies. Solid bars: trauma patients. Hashed bars: studies including both trauma and non-trauma patients. Unfilled bars: subgroup analyses or patients drawn from a larger series, published separately (not included in estimation of mortality). Pooled estimate of mortality shown with 95% confidence interval. PHBP indicates prehospital blood products.
Fig. 3.
Meta-analysis of unadjusted risk of mortality.
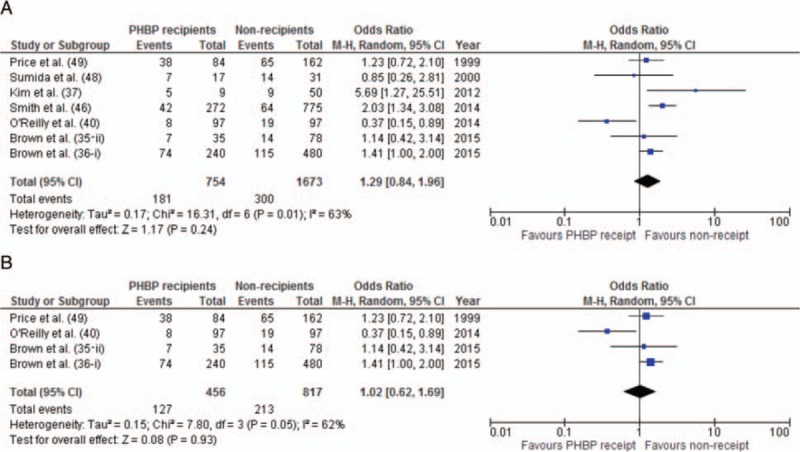
A, All comparative studies. B, Studies with matched cohorts.
Fig. 4.
Forest plot of adjusted mortality.
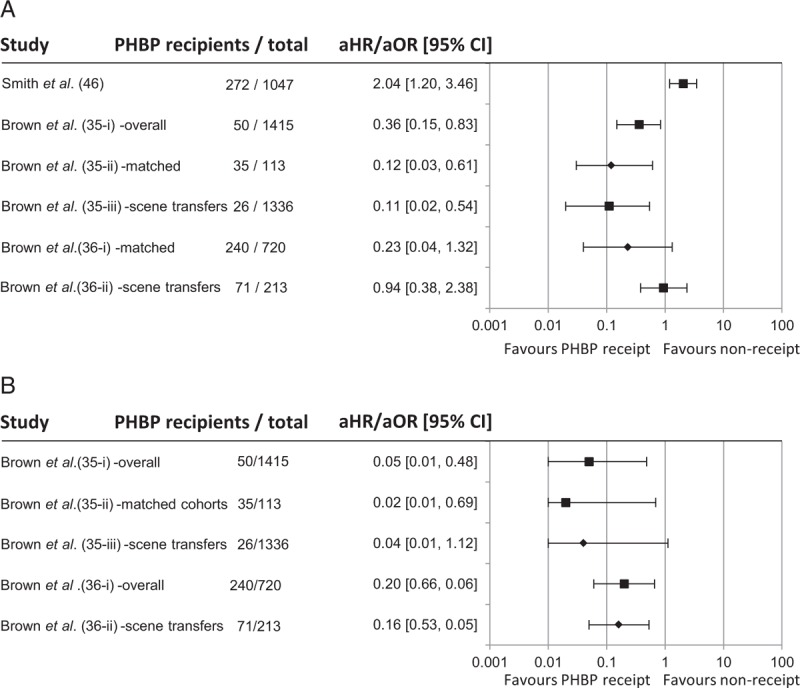
A, Overall; B, at 24 h. Data shown for adjusted odds ratios, other than Brown et al. (35) which shows hazard ratio. ▪: data from main study; ♦: data from subgroup analysis.
Matched cohort studies (35-ii, 40) reported markedly lower mortality among PHBP recipients than the unmatched PHBP cohorts from which they were drawn (35-i, 41). This may indicate tasking of more capable assets to casualties with more severe injuries, resulting in fewer non-recipient matches as injury burden increases. If so, matched studies will underestimate mortality among PHBP recipients but may also underestimate the potential effect size of PHBP due to the exclusion of patients at greater risk of death, among whom a survival benefit might be more evident.
Seven cohort studies reported mortality (Fig. 3A). Only one study found an association between PHBP receipt and absolute survival (40), whereas three reported increased absolute mortality (35-i (unpublished data), 37, 46). However, the mortality difference reported in the first of these (35-i) was lost when only matched patients were considered (35-ii).
An absolute mortality reduction of 11% was reported among battlefield casualties matched by injuries to historical controls from the same facility (40). Acknowledged confounders included limited in-hospital plasma and PRBC transfusions received by both cohorts—75% of non-recipients received no blood products after hospital arrival. Transfusion practice at this facility became more liberal over time (47); reflected in larger in-hospital transfusion volumes received by the later PHBP cohort. Other differences included shorter transport times, more frequent prehospital airway support, more tranexamic acid, and higher in-hospital transfusion ratios (FFP:PRBC 1:1 vs. 0.46:1) among PHBP recipients. Recent data from this facility show a stepwise annual survival improvement at all levels of injury (2), suggesting that comparison with this historical cohort will have introduced significant confounding.
A contemporaneous cohort study of battlefield casualties with major trauma (New Injury Severity Score≥16) treated at the above facility (46) found an independent association between PHBP receipt and mortality in multivariate analysis. However, marked differences in injury mechanisms, wounding patterns, and especially injury burden probably defied statistical correction. These military studies were limited by frequent nonavailability of prehospital vital signs; hence pretransfusion physiological status could not be assessed.
Significant baseline differences are found in two smaller civilian cohort studies (37, 48). The former compared 50 injured prehospital PRBC recipients with nine patients who also received plasma. Indications for plasma transfusion included known pharmaceutical anticoagulation. Plasma recipients had a pretransfusion INR of 2.6 (vs. 1.5 among non-recipients) and this remained higher at hospital arrival. In-hospital treatment also differed; plasma recipients received transfusion ratios closer to 1:1 and less crystalloid. Plasma recipients had a higher Trauma and Injury Severity Score (TRISS)-predicted mortality and over 50% died, despite more aggressive blood product resuscitation. The latter study (in subjects well matched by injury burden) found no survival difference, although PHBP recipients had longer prehospital times (mean 30 min) than non-recipients (mean 12 min) (48). Neither study was adequately powered to detect a mortality difference.
The earliest matched cohort study identified that PHBP recipients received almost four times more prehospital crystalloid, were intubated more frequently, and received 50% more PRBC during in-hospital resuscitation than non-recipients (49). No survival benefit was found. The authors speculated that PHBP “may have compensated for…longer transport times and possibly more gravely injured patients.”
The most robust studies to date are two contemporaneous cohort studies (35, 36). The first compared 50 blunt trauma patients who received a median of 1.3u pretrauma center (PTC) PRBC to 1365 non-recipients. Despite similar injury burdens, unadjusted mortality in PHBP recipients was 28% versus 16% in non-recipients (P = 0.02) (J Brown 2015, pers. comm. June 08). PHBP recipients were more often secondary transfers (48%) than non-recipients (4%)—introducing a high risk of selection bias due to the probability that more “unavoidable” early deaths were included among non-recipients. As in military studies, PHBP recipients were managed more aggressively, receiving 2.5 times more PTC crystalloid, more in-hospital PRBC, and more platelet transfusions. However, in regression analysis PHBP receipt was associated with reduced 30-day mortality. Thirty-five PHBP recipients were propensity matched with 78 non-recipients. PHBP recipients were less frequently hypotensive at hospital arrival and the median PRBC transfusion was 69% greater than for non-recipients. Regression analysis again found an association between PHBP receipt and improved 30-day survival. However, whether statistics can correctly adjust for very different transfusion strategies in a relatively small study is uncertain. In contrast, the same group's larger study comparing 240 PHBP recipients to 480 non-recipients, transported by a single service to one trauma center, found no overall survival benefit from PTC PRBC (36-i).
Early mortality
Six case series reported prehospital mortality (23, 25, 50–53). Three cohort studies and one case series reported 24-h mortality (Fig. 2B) (35–37, 54). Two of the latter reported adjusted odds ratios, including three subgroup analyses (Fig. 4B) (35, 36). These suggest an effect on early mortality, but are limited by the small proportion of PHBP recipients. Of note, mortality among PHBP recipients is almost 50% greater when only primary retrievals are considered (36), suggesting that these are a different population from secondary transfers. This may lead to marked selection bias when proportions of primary retrievals and secondary transfers differ between cohorts (35-i). However, early survival benefits remained when matched cohorts containing similar proportions of secondary transfers were considered (35-ii). Statistical significance was lost when primary retrievals alone were considered (35-iii).
In-hospital transfusion
Six studies reported in-hospital blood product resuscitation (Fig. 5) (35–37, 40, 46, 49). Four studies matched by injury burden (35, 36, 40, 49), two did not (37, 46). In military studies PHBP recipients received more in-hospital transfusions (40, 46). The former reflects changes in transfusion practice over time, while the latter studies are confounded by differences in injury. No study provided evidence of reduced in-hospital transfusion requirements.
Fig. 5.
In-hospital transfusion requirements for (A) PRBC and (B) plasma.
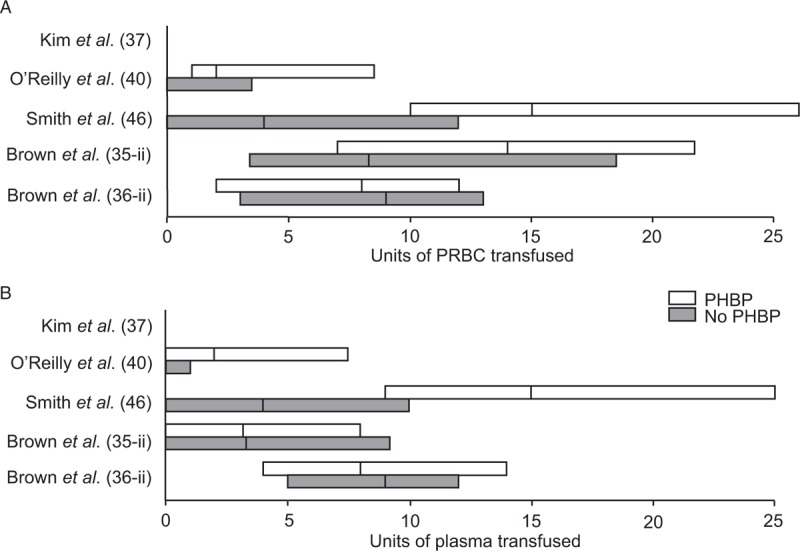
O’Reilly et al. (40) and Smith et al. (46) reported total transfusion data from primary receiving hospital. Brown et al. (35, 36) and Kim et al. (37) reported transfusion data within 24 h of admission. Data shown as median (IQR) except for Kim et al. (37) (median only). Δ: median transfusion for PHBP recipients, X: median transfusion for non-recipients. Price et al. (49) also reported statistically significantly greater in-hospital transfusion volumes for PHBP recipients (mean 1414 mL (SD: 1660 mL)) vs. non-recipients (1007 mL (SD: 935 mL)).
Vital signs
Four case series report an increase in SBP (12, 53, 54) or decrease in HR or Shock Index (54, 55) associated with PHBP receipt. Among military casualties PHBP receipt was associated with a significantly greater correction in Shock Index (56). However, PHBP recipients were significantly more haemodynamically compromised prior to transport, thus had greater scope for correction. Consequently, reporting absolute correction biases the study in favor of PHBP. Two-thirds of eligible patients were excluded due to nonavailability of pre- and post-transfusion vital signs. This may indicate selection bias if vital signs were unrecordable or interventions prioritized in the sickest patients.
In a matched subgroup analysis prehospital hypotension was more common in PHBP recipients but was less common at hospital arrival (35). However, in a larger study, although prehospital SBP were similar, PHBP recipients were more frequently shocked on arrival (36). The final civilian cohort study identified no difference in haemodynamic changes between PHBP recipients and non-recipients (48). In a case-control study, patients hypothermic at ED arrival were more likely to have received PHBP (57). However, the significance of this is unclear, as crystalloids were warmed before administration whereas PRBC were not (F. M. von Recklinghausen (2015) pers. comm. June 23). Collectively, the published data provide no evidence that PHBP improves physiology compared to crystalloids.
Coagulopathy and acid–base
Two overlapping studies report correction of predominantly warfarin-related anticoagulation with prehospital plasma. In a case series of mixed trauma and non-trauma patients, INR reduced from 4 to 2 (38). In a cohort study—whose pooled subjects formed part of that series—greater absolute correction (INR 2.6 to 1.6) was seen in plasma recipients than non-recipients (INR 1.5 to 1.3) (37). However, pharmaceutical anticoagulation is not analogous to trauma-induced coagulopathy (TIC); thus these papers demonstrate only that plasma-mediated reversal of pharmaceutical anticoagulation can be delivered prehospital and should not be extrapolated to suggest a benefit in the treatment of TIC. In blunt trauma patients, PHBP were associated with reduced odds of TIC; however, the PHBP group also received greater volumes of crystalloid (35). The association was not found in the same group's larger study in which both cohorts received comparable crystalloid volumes (36). It is possible that greater crystalloid loading reduced TIC-inducing hypoperfusion. In military data, PHBP receipt was independently associated with TIC (46) but this probably reflects vastly greater tissue disruption in PHBP recipients.
PHBP receipt has been associated with greater acidosis at hospital arrival compared with non-recipients with comparable injury burdens (48). PHBP recipients had mean flight times of 34 min versus 12 min for non-recipients. This provided greater opportunity for PHBP administration, but potentially longer uncontrolled bleeding. In contrast, PHBP receipt was associated with a non-significant trend to lower serum lactate concentration when prehospital times were less than 150 min (58). However, no details of study size or blood products administered were available.
Adverse events
Among 759 PHBP recipients in studies that specifically reported presence or absence of transfusion reactions (12, 14, 25, 36, 38, 55, 59), only three possible reactions were noted. One patient suffered transient shortness of breath after infusion of 5L crystalloid and 900 mL PRBC (12), although this was probably secondary to volume overload, one patient developed a “fine [truncal] rash” following one unit of PRBC (14) and one patient had a reaction during a subsequent in-hospital transfusion (36). These studies suggest that PHBP receipt is associated with a minimal risk of transfusion-related adverse events.
DISCUSSION
PHBP resuscitation is increasingly employed to try to reduce the 23% mortality among hypotensive trauma patients (44, 45). However, provision of universal PHBP components to all trauma networks involves substantial clinical, logistical, and fiscal costs. In this first systematic review of the topic, we evaluated the clinical evidence around PHBP for trauma. We identified 27 observational studies that reported relevant clinical outcomes. Twenty-six of 27 were retrospective. Twenty-five of 27 provided very poor quality evidence. Common limitations were the lack of a control group or a control group that differed significantly from PHBP recipients. Most comparative studies were too small to permit adjustment for confounders. Studies frequently pooled primary retrievals with secondary transfers, despite these being distinct populations. While PHBP resuscitation is achievable with minimal wastage of universal donor components, and with short-term safety, no more than low-quality evidence supports this as a “standard of care.” This review did not identify an overall survival benefit. Evidence for improved survival at 24 h is derived from only two observational studies and, even if a true effect, may not translate to improved long-term outcomes.
Differences between patients and/or treatment pathways further limited the studies considered in this review. Even when subjects were matched, PHBP recipients received more in-hospital transfusions. Consequently, even where associations between PHBP and improved survival are found after statistical correction, this improvement cannot be confidently attributed to PHBP receipt.
The available clinical data show no evidence that PHBP reduces in-hospital transfusion. This is consistent with recent animal modelling of prehospital resuscitation (60). Although TIC was reduced by blood products in various ratios compared with saline, transfusion requirements over the subsequent 150 min of “hospital” resuscitation were similar in all groups. Similarly, a previous animal model of uncontrolled splenic haemorrhage showed that while Hextend increased blood loss compared with blood products—potentially reflecting the previously reported exacerbation of TIC produced by hetastarches (61)—there was no difference in post-resuscitation blood loss between blood product resuscitation and Hartmann's solution (62). The combination of lyophilized plasma and PRBC in a 1:1 ratio has been shown to reduce total blood loss in a swine polytrauma model compared with both plasma alone and with 1:1 FFP:PRBC resuscitation (63). Short-term survival was not improved by resuscitation with blood products compared with crystalloid. Long-term animal survival studies would be ethically challenging and have not been performed.
As with our findings from the clinical literature, a swine model of PHBP resuscitation did not improve acid–base status. A non-significant trend to less extreme maxima for serum lactate and pH among “haemostatically resuscitated” animals was found; however, there were fewer than 10 animals per group (60). In other animal studies, neither plasma lactate concentration (63) nor acid–base status (62) has been influenced by different blood product ratios. Any metabolic benefit from PHBP remains uncertain.
Strengths and limitations
The searches for this review were not restricted by language nor by date and included all major citation databases, specialist resources, and reference lists from included studies. It is unlikely that material that would significantly change the findings has been overlooked.
The most significant weakness of the study is the low quality of evidence on which the review could draw. Consequently, no conclusions about the efficacy of PHBP resuscitation can be drawn. The extent to which this review makes use of “gray literature” reflects the poor state of evidence in this area. This material has not been subjected to the same degree of peer review as that in published papers, but is nonetheless recognized as being an essential component of a systematic review (64).
These considerations limited the possible statistical syntheses to unadjusted mortality alone, with no indication identified of improved long-term survival after PHBP receipt. However, the marked differences between the populations in included studies render this finding tenuous. These difficulties are consistent with previous reviews of blood product resuscitation for trauma (65, 66). Meta-analysis produces not only an estimate of overall effect size, but a measure of heterogeneity from which the consistency of the literature can be assessed. In meta-analysis of both unmatched and matched studies, heterogeneity was present and significant, demonstrating the degree of uncertainty that exists about a measurable benefit of PHBP resuscitation.
This review considered both military and civilian studies. The validity of extrapolating from studies of predominantly younger, massively traumatized males to the civilian population is questionable. However, the inclusion of military case series illustrates the marked change in resuscitation practice over the last decade and thus further factors that must be considered when interpreting the existing literature. Transfusion criteria used by the Israeli military initially required 2L crystalloid administration prior to administration of PRBC, with casualties receiving an average of 4.4L of prehospital crystalloid (14). Lyophilized plasma has now replaced crystalloid in Israeli retrieval missions (67), such that “crystalloid infusion was minimized” (15). Similar practices have been adopted by the UK military, with casualties retrieved by MERT(E) in Afghanistan receiving up to 4u PRBC and 4u plasma (41) with crystalloid minimized (3). This is borne out in data examined in this review (46). In contrast, civilian studies continue to include failure to respond to 2L intravenous crystalloid as an indication for PHBP. This is despite good quality evidence that aggressive clear fluid administration increases mortality and morbidity after penetrating trauma (68). Prehospital cannulation (as a surrogate for fluid administration) was associated with greater mortality in every patient subgroup examined in a registry study, other than those with Injury Severity Scores <9 (69), while more than 1L of prehospital fluid has been shown to be an independent risk factor for death in patients without severe traumatic brain injury (70). High ratios of crystalloid to PRBC given in-hospital increase morbidity (71). Whether PHBP are associated with similar volume effects is unknown. It is possible that the negative impact of crystalloid loading prior to PHBP administration has masked benefit from PHBP in many studies to date.
Safety
Very few PHBP-related adverse events were identified, implying transfusion safety. However, blood transfusions suppress the immune system and are associated with a stepwise increase in infectious complications for each unit of PRBC transfused, starting with single-unit transfusions (72). Similarly, a dose–response relationship exists between transfusion and development of multi-organ failure (73). This is a concern given the frequency with which patients in this review received PHBP but little or no in-hospital transfusion, calling into question their need for PHBP transfusion. No study in this review associated PHBP with reduced in-hospital transfusion. However, if administered inappropriately liberally, PHBP may lead to excess morbidity.
To address these various questions, four randomized clinical trials and one cohort study comparing various combinations of blood products and crystalloid are underway (see Table, Supplementary Digital Content 6, ongoing studies, at). If PHBP trauma resuscitation is beneficial, universal provision should be advocated. However, robust evidence is required to justify the clinical, logistical, and financial costs of making PHBP “standard care.” This review demonstrates the lack of such evidence and makes ongoing support for these studies imperative.
Military and expedition settings require the consideration of factors specific to austere environments. Although evacuation times in recent operations have typically been short, future conflicts may require prolonged pre-evacuation field and en-route care. These timelines may necessitate PHBP support. Data collection on future operations will be essential to establish the place of PHBP in “Remote Damage Control Resuscitation.”
CONCLUSIONS
The literature reporting PHBP for trauma resuscitation is contradictory and provides only poor-quality evidence. Evidence-based conclusions to guide practice cannot be drawn. While PHBP resuscitation appears logical the potential harms of this practice must be recognized. More rigorous evidence of benefit is required to justify universal adoption. Whether PHBPs improve survival despite these competing risks is unknown. The only satisfactory way to answer this outstanding question of benefit from PHBP-based resuscitation for major traumatic haemorrhage is by randomized controlled trials.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors thank the following for assistance with retrieval and translation of non-English language literature: Lt Cdr Timothy Castrinoyannakis Royal Navy, Capt Viktor Reva, Army of the Russian Federation, Dr Robert Helling, University of Munich, and Mrs Alison Smith. Dr Jon Bishop, University of Birmingham, is thanked for statistical advice.
Footnotes
Revised 16 November, 2015
Accepted 12 January, 2016
No financial support was received in relation to this study.
These data and the views expressed are the authors’ own and do not necessarily reflect those of the Ministry of Defence, National Health Service, National Institute for Health Research or Department of Health.
IS and MJM are investigators for the RePHILL Trial, funded by NIHR EME Project grant 14/152/14.
REFERENCES
- 1.Bailey JA, Morrison JJ, Rasmussen TE. Military trauma system in Afghanistan: lessons for civil systems? Curr Opin Crit Care 2013; 19 6:569–577. [DOI] [PubMed] [Google Scholar]
- 2.Penn-Barwell JG, Roberts SA, Midwinter MJ, Bishop JR. Improved survival in UK combat casualties from Iraq and Afghanistan: 2003-2012. J Trauma Acute Care Surg 2015; 78 5:1014–1020. [DOI] [PubMed] [Google Scholar]
- 3.Dawes R, Thomas GO. Battlefield resuscitation. Curr Opin Crit Care 2009; 15 6:527–535. [DOI] [PubMed] [Google Scholar]
- 4.Bhangu A, Nepogodiev D, Doughty H, Bowley DM. Meta-analysis of plasma to red blood cell ratios and mortality in massive blood transfusions for trauma. Injury 2013; 44 12:1693–1699. [DOI] [PubMed] [Google Scholar]
- 5.Murad MH, Stubbs JR, Gandhi MJ, Wang AT, Paul A, Erwin PJ, Montori VM, Roback JD. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion 2010; 50 6:1370–1383. [DOI] [PubMed] [Google Scholar]
- 6.Haas B, Gomez D, Steel A, Nathens A. Ratio of units of red blood cells to fresh frozen plasma for severely injured patients undergoing massive transfusion (Protocol). Cochrane Database Syst Rev (3):CD009033, 2011. Available at: http://dx.doi.org/10.1002/14651858.CD009033 Accessed August 22, 2015. [Google Scholar]
- 7.Karam O, Tucci M, Combescure C, Lacroix J, Rimensberger PC: Plasma transfusion strategies for critically ill patients. Cochrane Database Syst Rev (12):CD010654, 2013. Available at: http://dx.doi.org/10.1002/14651858.CD010654.pub2 Accessed August 22, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. the PROMMTT Study Group. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg 2013; 148 2:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Junco DJ, Holcomb JB, Fox EE, Brasel KJ, Phelan HA, Bulger EM, Schreiber MA, Muskat P, Alarcon LH, Cohen MJ, et al. the PROMMTT Study Group. Resuscitate early with plasma and platelets or balance blood products gradually: findings from the PROMMTT study. J Trauma Acute Care Surg 2013; 75 (1 Suppl 1):S24–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. the PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA 2015; 313 5:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiel F. Development of a blood program in Vietnam. Mil Med 1966; 131 12:1469–1482. [PubMed] [Google Scholar]
- 12.Dalton AM. Use of blood transfusions by helicopter emergency medical services: is it safe? Injury 1993; 24 8:509–510. [DOI] [PubMed] [Google Scholar]
- 13.Calderbank P, Woolley T, Mercer S, Schrager J, Kazel M, Bree S, Bowley DM. Doctor on board? What is the optimal skill-mix in military pre-hospital care? Emerg Med J 2011; 28 10:882–883. [DOI] [PubMed] [Google Scholar]
- 14.Barkana Y, Stein M, Maor R, Lynn M, Eldad A. Prehospital blood transfusion in prolonged evacuation. J Trauma 1999; 46 1:176–180. [DOI] [PubMed] [Google Scholar]
- 15.Malsby RF, Quesada J, Powell-Dunford N, Kinoshita R, Kurtz J, Gehlen W, Adams C, Martin D, Shackelford S. Prehospital blood product transfusion by U.S. army MEDEVAC during combat operations in Afghanistan: a process improvement initiative. Mil Med 2013; 178 7:785–791. [DOI] [PubMed] [Google Scholar]
- 16.Morrison JJ, Oh J, DuBose JJ, O’Reilly DJ, Russell RJ, Blackbourne LH, Midwinter MJ, Rasmussen TE. En-route care capability from point of injury impacts mortality after severe wartime injury. Ann Surg 2013; 257 2:330–334. [DOI] [PubMed] [Google Scholar]
- 17.Chaiwat O, Lang JD, Vavilala MS, Wang J, MacKenzie EJ, Jurkovich GJ, Rivara FP. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology 2009; 110 2:351–360. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JL, Moore EE, Kashuk JL, Banerjee A, Cothren CC, Biffl WL, Sauaia A. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg 2010; 145 10:973–977. [DOI] [PubMed] [Google Scholar]
- 19.Malone DL, Dunne J, Tracy JK, Putnam AT, Scalea TM, Napolitano LM. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma 2003; 54 5:898–905. [DOI] [PubMed] [Google Scholar]
- 20.Dunne JR, Malone DL, Tracy JK, Napolitano LM. Allogenic blood transfusion in the first 24 hours after trauma is associated with increased systemic inflammatory response syndrome (SIRS) and death. Surg Infect 2004; 5 4:395–404. [DOI] [PubMed] [Google Scholar]
- 21.Robinson WP, Ahn J, Stiffler A, Rutherford EJ, Hurd H, Zarzaur BL, Baker CC, Meyer AA, Rich PB. Blood transfusion is an independent predictor of increased mortality in nonoperatively managed blunt hepatic and splenic injuries. J Trauma 2005; 58 3:437–445. [DOI] [PubMed] [Google Scholar]
- 22.Holcomb JB, Donathan DP, Cotton BA, Del Junco DJ, Brown G, Wenckstern TV, Podbielski JM, Camp EA, Hobbs R, Bai Y, et al. Prehospital transfusion of plasma and red blood cells in trauma patients. Prehosp Emerg Care 2015; 19 1:1–9. [DOI] [PubMed] [Google Scholar]
- 23.Weaver AE, Eshelby S, Norton J, Lockey DJ. The introduction of on-scene blood transfusion in a civilian physician-led pre-hospital trauma service. Scand J Trauma Resusc Emerg Med 2013; 21 suppl 1:S27. [Google Scholar]
- 24.Zielinski MD, Smoot DL, Stubbs JR, Jenkins DH, Park MS, Zietlow SP. The development and feasibility of a remote damage control resuscitation prehospital plasma transfusion protocol for warfarin reversal for patients with traumatic brain injury. Transfusion 2013; 53 suppl 1:59S–64S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherren PB, Burns B. Prehospital blood transfusion: 5-year experience of an Australian helicopter emergency medical service. Crit Care 2013; 17:S112. [Google Scholar]
- 26.Bodnar D, Rashford S, Williams S, Enraght-Moony E, Parker L, Clarke B. The feasibility of civilian prehospital trauma teams carrying and administering packed red blood cells. Emerg Med J 2014; 31 2:93–95. [DOI] [PubMed] [Google Scholar]
- 27.Chapman MP, Moore EE, Chin TL, Ghasabyan A, Chandler J, Stringham J, Gonzalez E, Moore HB, Banerjee A, Silliman CC, et al. COMBAT: initial experience with a randomized clinical trial of plasma-based resuscitation in the field for traumatic hemorrhagic shock. Shock 2015; 44 suppl 1:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wild G, Anderson D, Lund P. Round Afghanistan with a fridge. J R Army Med Corps 2013; 159 1:24–29. [DOI] [PubMed] [Google Scholar]
- 29.Dretzke J, Smith IM, James RH, Midwinter MJ. Protocol for a systematic review of the clinical effectiveness of pre-hospital blood components compared to other resuscitative fluids in patients with major traumatic haemorrhage. Syst Rev 2014; 3 1:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8 5:336–341.[Erratum appears in Int J Surg 8(8):658, 2010]. [DOI] [PubMed] [Google Scholar]
- 31.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2008. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed October 14, 2014. [Google Scholar]
- 32.National Heart, Lung and Blood Institute. Quality assessment tool for Before-After (Pre-Post) studies with no control group. Bethesda, NHLBI, 2014. Available at: http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/before-after Accessed January 14, 2015. [Google Scholar]
- 33.National Heart, Lung and Blood Institute. Quality assessment tool for case series studies. Bethesda, NHLBI, 2014. Available at: http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/case_series Accessed January 14, 2015. [Google Scholar]
- 34.Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P. Eastern Association for the Surgery of Trauma Practice Management Guidelines Ad Hoc C. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg 2012; 73 (5 Suppl 4):S283–S287. [DOI] [PubMed] [Google Scholar]
- 35.Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, Peitzman AB, Moore EE, Cuschieri J, Sperry JL. Pretrauma center red blood cell transfusion is associated with reduced mortality and coagulopathy in severely injured patients with blunt trauma. Ann Surg 2015; 261 5:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JB, Sperry JL, Fombona A, Billiar TR, Peitzman AB, Guyette FX. Pre-trauma center red blood cell transfusion is associated with improved early outcomes in air medical trauma patients. J Am Coll Surg 2015; 220 5:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim BD, Zielinski MD, Jenkins DH, Schiller HJ, Berns KS, Zietlow SP. The effects of prehospital plasma on patients with injury. J Trauma Acute Care Surg 2012; 73 (2 Suppl 1):S49–S53. [DOI] [PubMed] [Google Scholar]
- 38.Badjie KS, Berns KS, Button LM, Stubbs JR. The impact of thawed plasma usage during emergency medical helicopter transport. Transfusion 2012; 52 suppl 3:197A–198A. [Google Scholar]
- 39.Badjie KS, Berns KS, Bryant SC, Button LM, Kreuter J, Van Buskirk CM. 1:1 Fresh frozen plasma: red blood cell (FFP: RBC) ratio improves patient survival during emergency medical air helicopter transport. Transfusion 2013; 53 suppl 2:197A. [Google Scholar]
- 40.O’Reilly DJ, Morrison JJ, Jansen JO, Apodaca AN, Rasmussen TE, Midwinter MJ. Prehospital blood transfusion in the en route management of severe combat trauma: a matched cohort study. J Trauma Acute Care Surg 2014; 77 (3 Suppl 2):S114–S120. [DOI] [PubMed] [Google Scholar]
- 41.O’Reilly DJ, Morrison JJ, Jansen JO, Nordmann G, Rasmussen TE, Midwinter MJ, Doughty H. Initial UK experience of prehospital blood transfusion in combat casualties. J Trauma Acute Care Surg 2014; 77 (3 Suppl 2):S66–S70. [DOI] [PubMed] [Google Scholar]
- 42.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg 2012; 147 2:113–119. [DOI] [PubMed] [Google Scholar]
- 43.Smith IM, Beech ZKM, Lundy JB, Bowley DM. A prospective observational study of abdominal injury management in contemporary military operations: damage control laparotomy is associated with high survivability and low rates of fecal diversion. Ann Surg 2015; 261 4:765–773. [DOI] [PubMed] [Google Scholar]
- 44.Hasler RM, Nuesch E, Juni P, Bouamra O, Exadaktylos AK, Lecky F. Systolic blood pressure below 110mmHg is associated with increased mortality in blunt major trauma patients: multicentre cohort study. Resuscitation 2011; 82 9:1202–1207. [DOI] [PubMed] [Google Scholar]
- 45.Hasler RM, Nuesch E, Juni P, Bouamra O, Exadaktylos AK, Lecky F. Systolic blood pressure below 110 mmHg is associated with increased mortality in penetrating major trauma patients: multicentre cohort study. Resuscitation 2012; 83 4:476–481. [DOI] [PubMed] [Google Scholar]
- 46.Smith IM, Bishop JR, Streets CG, Woolley T, Midwinter MJ. Pre-hospital blood product resuscitation: benefits unclear. Paper presented at Military Health System Research Symposium. 2014. Fort Lauderdale, FL. [Google Scholar]
- 47.Jansen JO, Morrison JJ, Midwinter MJ, Doughty H. Changes in blood transfusion practices in the UK Role 3 Medical Treatment Facility in Afghanistan, 2008-2011. Transfus Med 2014; 24 3:154–161. [DOI] [PubMed] [Google Scholar]
- 48.Sumida MP, Quinn K, Lewis PL, Jones Y, Barker DE, Ciraulo DL, Cowell V, Luk S, Murphy D, Jacobs L. Prehospital blood transfusion versus crystalloid alone in the air medical transport of trauma patients. Air Med J 2000; 19 4:140–143. [DOI] [PubMed] [Google Scholar]
- 49.Price DD, Norton RL, Zechnich AD, Eldurkar J, Chok J, Mann NC. Out-of-hospital blood administration for critically injured patients transported by helicopter. Ann Emerg Med 1999; 34 (4 Part 2):S50–S51. [Google Scholar]
- 50.Bodnar D, Rashford S, Hurn C, Quinn J, Parker L, Isoardi K, Williams S. Characteristics and outcomes of patients administered blood in the prehospital environment by a road based trauma response team. Emerg Med J 2014; 31 7:583–588. [DOI] [PubMed] [Google Scholar]
- 51.Chew E, Gilbertson M, Kelsey G, Hogan C, Hammer F, Barkmeyer M, Haeusler M. An Australian experience in out-of-hospital transfusion of red cell concentrates by air ambulance personnel. Vox Sang 2013; 105 (Suppl s1):26. [Google Scholar]
- 52.Prause G, Dacar D, Gimpl R, Vadon M, Lanzer G. Prehospital use of packed red cells: preparation, storage and first experience [German]. Die applikation von ery-konzentraten am notfallort - Vorbereitung, lagerung und erste erfahrungen im hubschrauberdienst. Notarzt 1999; 15 1:9–12. [Google Scholar]
- 53.Sunde GA, Vikenes B, Strandenes G, Flo KC, Hervig TA, Kristoffersen EK, Heltne JK. Freeze dried plasma and fresh red blood cells for civilian prehospital hemorrhagic shock resuscitation. J Trauma Acute Care Surg 2015; 78 (6 Suppl 1):S26–S30. [DOI] [PubMed] [Google Scholar]
- 54.Powell-Dunford N, Quesada JF, Malsby RF, Chou V, Gerhardt RT, Gross KR, Shackelford SA. Risk management analysis of air ambulance blood product administration in combat operations. Aviat Space Environ Med 2014; 85 11:1130–1135. [DOI] [PubMed] [Google Scholar]
- 55.Higgins GL, 3rd, Baumann MR, Kendall KM, Watts MA, Strout TD. Red blood cell transfusion: experience in a rural aeromedical transport service. Prehosp Disaster Med 2012; 27 3:231–234. [DOI] [PubMed] [Google Scholar]
- 56.Gross K, Shackelford S, Powell-Dunford N, Orman J, Howard J, Quesada J, Bailey J. En route blood transfusion from point of injury in a theater of operations positively impacts shock index. Poster presented at Military Health System Research Symposium. 2014. Fort Lauderdale, FL. [Google Scholar]
- 57.Wheeler R, von Recklinghausen FM, Brozen R. Blood administration in helicopter emergency medical services patients associated with hypothermia. Air Med J 2013; 32 1:47–51. [DOI] [PubMed] [Google Scholar]
- 58.Bates C, Hawthorn B, Iacano I. Impact of prehospital blood transfusion on serum lactate levels in the trauma patient. Air Med J 2012; 31 6:260. [Google Scholar]
- 59.Berns KS, Zietlow SP. Blood usage in rotor-wing transport. Air Med J 1998; 17 3:105–108. [DOI] [PubMed] [Google Scholar]
- 60.Watts S, Nordmann G, Brohi K, Midwinter M, Woolley T, Gwyther R, Wilson C, Poon H, Kirkman E. Evaluation of prehospital blood products to attenuate acute coagulopathy of trauma in a model of severe injury and shock in anesthetized pigs. Shock 2015; 44 suppl 1:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alam HB, Bice LM, Butt MU, Cho SD, Dubick MA, Duggan M, Englehart MS, Holcomb JB, Morris MS, Prince MD, et al. Hemostatic Resuscitation Research Group. Testing of blood products in a polytrauma model: results of a multi-institutional randomized preclinical trial. J Trauma 2009; 67 4:856–864. [DOI] [PubMed] [Google Scholar]
- 62.Sondeen JL, Prince MD, Kheirabadi BS, Wade CE, Polykratis IA, de Guzman R, Dubick MA. Initial resuscitation with plasma and other blood components reduced bleeding compared to hetastarch in anesthetized swine with uncontrolled splenic hemorrhage. Transfusion 2011; 51 4:779–792. [DOI] [PubMed] [Google Scholar]
- 63.Spoerke N, Zink K, Cho SD, Differding J, Muller P, Karahan A, Sondeen J, Holcomb JB, Schreiber M. Lyophilized plasma for resuscitation in a swine model of severe injury. Arch Surg 2009; 144 9:829–834. [DOI] [PubMed] [Google Scholar]
- 64.Lefebvre C, Manheimer E, Glanville J. Chapter 6, Section 2.2.4: Searching for studies: Conference Abstracts and Proceedings. In Higgins JPT and Green S (eds): Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at: http://handbook.cochrane.org/chapter_6/6_2_2_4_conference_abstracts_or_proceedings.htm Accessed December 21, 2015. [Google Scholar]
- 65.Curry N, Hopewell S, Doree C, Hyde C, Brohi K, Stanworth S. The acute management of trauma hemorrhage: a systematic review of randomized controlled trials. Crit Care 2011; 15 2:R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Curry N, Stanworth S, Hopewell S, Doree C, Brohi K, Hyde C. Trauma-induced coagulopathy: a review of the systematic reviews: is there sufficient evidence to guide clinical transfusion practice? Transfus Med Rev 2011; 25 3:217–231. [DOI] [PubMed] [Google Scholar]
- 67.Glassberg E, Nadler R, Gendler S, Abramovich A, Spinella PC, Gerhardt RT, Holcomb JB, Kreiss Y. Freeze-dried plasma at the point of injury: from concept to doctrine. Shock 2013; 40 6:444–450. [DOI] [PubMed] [Google Scholar]
- 68.Bickell WH, Wall MJ, Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med 1994; 331 17:1105–1109. [DOI] [PubMed] [Google Scholar]
- 69.Haut ER, Kalish BT, Cotton BA, Efron DT, Haider AH, Stevens KA, Kieninger AN, Cornwell EE, 3rd, Chang DC. Prehospital intravenous fluid administration is associated with higher mortality in trauma patients: a National Trauma Data Bank analysis. Ann Surg 2011; 253 2:371–377. [DOI] [PubMed] [Google Scholar]
- 70.Hussmann B, Heuer M, Lefering R, Touma A, Schoeneberg C, Keitel J, Lendemans S: Prehospital volume therapy as an independent risk factor after trauma. Biomed Res Int, 2015. Article ID: 354367. Available at: http://dx.doi.org/10.1155/2015/354367 Accessed May 27, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neal MD, Hoffman MK, Cuschieri J, Minei JP, Maier RV, Harbrecht BG, Billiar TR, Peitzman AB, Moore EE, Cohen MJ, et al. Crystalloid to packed red blood cell transfusion ratio in the massively transfused patient: when a little goes a long way. J Trauma Acute Care Surg 2012; 72 4:892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Claridge JA, Sawyer RG, Schulman AM, McLemore EC, Young JS. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner. Am Surg 2002; 68 7:566–572. [PubMed] [Google Scholar]
- 73.Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg 1997; 132 6:620–624. [PubMed] [Google Scholar]
- 74.Mena-Munoz J, Srivastava U, Martin-Gill C, Suffoletto B, Guyette F. Characterization of out of hospital resuscitation utilizing blood transfusion. Circulation 2013; 128:A315. [Google Scholar]
- 75.Chen J. Prehospital blood transfusions during aeromedical evacuations of trauma patients in Israel. Paper presented at Military Health System Research Symposium. 2014. Fort Lauderdale, FL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


