Abstract
Amputations are common after severe frostbite injuries, often mediated by postinjury arterial thrombosis. Since 1994, the authors have performed angiography to identify perfusion deficits in severely frostbitten digits and treated these lesions with intraarterial infusion of thrombolytic agents, usually combined with papaverine to reduce vasospasm. A retrospective review was performed of patients admitted to the regional burn center with frostbite injury from 1994 to 2007. Patients with severe frostbite, without contraindications to thrombolytic therapy, underwent diagnostic angiography of the affected extremities. Limbs with perfusion defects received intraarterial thrombolytic therapy according to protocol and the response was documented. Delayed amputation was performed for mummified digits. Angiogram results and amputation rates were tabulated. In this 14-year review, 114 patients were admitted for frostbite injuries. There was a male predominance (84%) and the mean age was 40.4 years. Of this group, 69 patients with severe frostbite underwent angiography; 66 were treated with intraarterial thrombolytic therapy. Four treated were excluded due to incomplete data. In the remaining 62 patients, angiography identified 472 digits with frostbite injury and impaired arterial perfusion. At the termination of thrombolytic infusion, a completion angiogram was performed. Partial or complete amputations were performed on only four of 198 digits (2.0%) with distal vascular blush, and in 71 of 75 digits (94.7%) with no improvement. Amputations occurred in 73 of 199 digits (36.7%) with partially restored flow. Overall complete digit salvage rate was 68.6%. Angiography after severe frostbite is a sensitive method to detect impaired arterial blood flow and permits catheter-directed treatment with thrombolytic agents. Improved perfusion after such treatment decreases late amputations following frostbite injury.
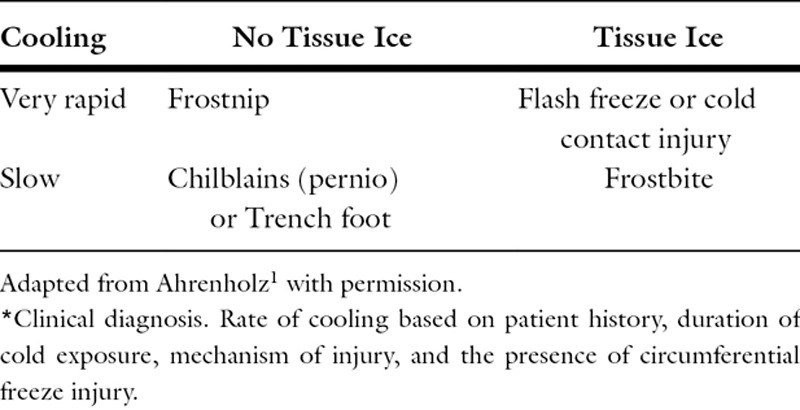
Local cold injury is poorly understood by most physicians and clinical presentations can include frostnip, chilblains, flash freeze injury, and cold contact injury, as well as frostbite (Table 1). Frostnip and chilblains (sometimes referred to as near-freeze injuries) do not blister or necrose tissue, and do not require surgical intervention. Flash-freeze injury and cold contact injuries are rarely circumferential and initiate such rapid cooling that large intracellular ice crystals form which rupture and immediately kill the affected cells. Frostbite, the most common form of injury, occurs with slower rates of cooling and can mimic laboratory cryopreservation, where small ice crystals form initially in the extravascular space.1 Unlike flash freeze and cold contact injuries, soft tissue cells can potentially survive after thawing, if vascular infarction can be prevented.
Table 1.
Classification of local cold injury*
Length of exposure to cold, duration of ice in tissue, and rapidity of rewarming are important determinants of tissue damage in frostbite patients. Rapid rewarming of frozen tissue is the treatment that consistently reduces the severity of frostbite injury.2–4 Most U.S. burn centers then follow a wound protocol adapted from McCauley et al,5,6 and the thawed digits are observed for signs of injury (Table 2). Either perfusion is restored and maintained in the affected digits, or progressive ischemia leads to tissue necrosis. Multiple interventions have failed to prevent frostbite thrombosis, including heparin,7,8 dextran,9,10 intraarterial (IA) reserpine (sympathetic block),11 and surgical sympathectomy.12–14 Thrombolytic therapy using IA infusion of tissue plasminogen activator (tPA) has shown promise in animal models,15,16 and at least two clinical case series17,18; however, recent published results of IV thrombolytic infusion are less impressive.19
Table 2.
Regions hospital conservative treatment protocol for frostbite

In 1994, our burn center implemented an innovative IA thrombolytic therapy (IATT) protocol, which we have shared extensively with other burn centers. Patients with acute extremity frostbite follow a standard frostbite algorithm with bed rest, extremity elevation, deflation of bullae, and oral ibuprofen after rapid rewarming, and are then evaluated for severity of injury and evidence of ischemic changes. Patients are screened for contraindications to thrombolytics, and failure to restore or maintain digit perfusion will prompt initiation of the IATT protocol. Our protocol was modified during the course of this study as we noted poor response in patients with delayed presentation or freeze-thaw-refreeze injuries, and as the availability of individual lytic agents changed at our institution. This review documents the outcomes of our IATT frostbite patients as the protocol evolved over 14 years, using four different thrombolytic agents, and compares our results to other published series of thrombolytic therapy for frostbite.
METHODS
This is a retrospective, observational cohort study of patients admitted with local cold injury to the Burn Center at Regions Hospital between 1994 and 2007. All charts with a discharge diagnosis of frostbite (ICD-9 codes 991.0, 991.1, 991.2, and 991.3) were reviewed after study approval by our institutional review board.
Tabulated data for these patients included age, sex, and race of the patient, time between injury and presentation, admission core temperature, comorbid conditions, history of alcohol, tobacco and other illicit drug abuse, extremities affected, number and location of digits with perfusion deficits, outcomes of thrombolytics used (for IATT group), number of digits amputated, total hospital charges, and complications.
Primary endpoints of this study included angiographic evidence of arterial reperfusion, correlation of perfusion level with incidence of amputation for previously occluded distal digital vascular beds, and the total number of amputations (digital, transmetacarpal, transmetatarsal, or below the knee or elbow). Secondary endpoints included acute length of stay (excluding readmission), total hospital charges, and complications. Data analysis was conducted using SAS statistical analysis software (SAS Institute, Cary, North Carolina). For categorical responses, Fisher’s exact test was used to determine differences between therapy types. For continuous outcome variables, two-sample Wilcoxon tests were used to determine differences between treatment groups. Statistical significance was P < .05.
IATT Protocol
After rewarming, patients with frostbite injuries are treated with a conservative treatment protocol including bed rest, oral ibuprofen, and deflation of bullae. Patients with poor prognostic findings within 24 hours (Table 3) are considered for selective diagnostic angiography. Indications include severe acute extremity frostbite with poor or absent digit perfusion after rewarming, or presence of hemorrhagic bullae (Figure 1). Relative contraindications are listed in Table 4. Figure 2 demonstrates the appearance of a normal angiogram, and these patients do not receive thrombolytics. If the angiogram is abnormal, the level of arterial occlusion is recorded for each involved digit and thrombolytic therapy is initiated.
Table 3.
Prognostic findings within 12 hours after rewarming

Figure 1.

Frostbite to hands demonstrating hemorrhagic blebs on the right vs clear blebs on the left.
Table 4.
Relative contraindications to IATT for frostbite therapy

Figure 2.

Normal angiogram showing patent digital arteries and distal vascular blush.
The protocol is illustrated in Figure 3. Diagnostic angiograms are performed via a single femoral port. The thrombolytic agent and a vasodilatory agent, currently papaverine, are infused coaxially into the involved limb(s) proximal to the popliteal or antecubital fossa to include proximal origins of outflow vessels and minimize local vasospasm. If more than one limb requires therapy, additional catheters are placed via femoral or proximal brachial sites and the thrombolytic dose is divided with preference given to the upper extremities. Papaverine is infused coaxially at 30 mg/hr in each extremity via the inner infusion catheter to diminish vasospasm. Heparin is infused to prevent new clots and to halt extension of existing thrombi in the injured endothelium. Unfractionated intravenous (IV) heparin is infused at 500 units/hr, but is reduced if the partial thromboplastin time exceeds 60 seconds, which is checked every 12 hours. Patients are monitored in the burn intensive care unit during infusion. Follow-up arteriography is performed at 24-hour interval. The expected maximum infusion period is 72 hours and is only extended if the patient shows continued improvement of perfusion. Thrombolytic infusion is terminated if perfusion has not improved on successive angiograms or when distal blush is documented in all digits. When the arterial catheters are removed, the IV heparin infusion is continued to maintain vascular perfusion. The patient is offered an oral anticoagulant or antiplatelet therapy at discharge. If the IATT is unsuccessful, we perform delayed amputations for mummified digits.
Figure 3.

The Burn Center at Regions Hospital IATT Algorithm. IATT, intraarterial thrombolytic therapy.
During this study, choice of thrombolytic agent was determined by availability from the inpatient hospital pharmacy. Initially, urokinase was used with therapeutic heparin and a coaxially infused IA vasodilator, either papaverine or nitroglycerine. When urokinase was removed from the market, IA tPA at 1 mg/hr with IA papaverine and IV heparin were substituted. After tPA was discontinued at our hospital, the protocol was changed to reteplase at 0.15 to 0.5 mg/hr with sub-therapeutic IV heparin. Most recently, we have used tenecteplase (TNKase) infused at 0.25 to 0.5 mg/hr with IV heparin.
Full response to IATT was defined as a digit with arterial filling defects (narrowing or occlusion) seen on initial angiogram that had restoration of the distal vascular blush on final angiogram (Figure 4). Partial response was defined as a digit where the blockage point or stenosis had progressed distally compared with the initial angiogram. No response was tabulated if a digit had no improvement in distal blood flow at the completion of IATT.
Figure 4.

Patient from Figure 1. A. Angiogram HD 1. Perfusion defect proximal to right palmar arch and at level of left wrist. B. Final angiogram after IATT. HD 4. Distal blush present on thumb and the third through fifth digits bilaterally and flow to the distal interphalangeal joint on both indices. C. Hands after completion of thrombolytic therapy. D. Several weeks post-IATT. No amputations were performed. HD 1, hospital day 1; IATT, intraarterial thrombolytic therapy
RESULTS
Patient Demographics
From 1994 to 2007, 114 patients (mean = 8.1/yr) were admitted with frostbite injuries to the Burn Center at Regions Hospital, St. Paul, MN, a teaching hospital affiliated with the University of Minnesota. Fifty-two patients were managed with conservative therapy alone; 47 had superficial frostbite injury and five had a contraindication to thrombolytic therapy. After obtaining informed consent, the remaining 69 patients with severe frostbite underwent selective extremity angiography. Three with normal angiograms were treated without lytic agents; 66 had documented perfusion defects and were treated with IATT. Four IATT patients had incomplete angiographic data charted and are excluded from this study. In the remaining 62 patients, we identified 472 digits with frostbite injury and impaired arterial perfusion (Figure 5).
Figure 5.

IATT treatment population. IATT, intraarterial thrombolytic therapy; FB, frostbite; C/I, contraindications.
Demographics of IATT Patients
Demographics of our IATT frostbite patients are listed in Table 5. Age ranged from 4.7 to 89 years of age, with a mean of 40.4 years with a male predominance (84%).
Table 5.
Characteristics of frostbite patients

Risk factors associated with frostbite injuries are listed in Table 6. Alcohol intoxication was a significant factor in 31% (n = 19) of injuries, and 50% (n = 32) had a measurable blood alcohol level. Twenty-three percentage (n = 14) of injuries were secondary to activities of winter months in a snowy climate and 13% of patients (n = 8) were homeless. Nine percentage of the frostbite cases (n = 6) had cold exposure due to cognitive impairments, such as dementia or developmental delay.
Table 6.
Causal factors and substance abuse associated with frostbite

Of the 62 patients reviewed after IATT, angiograms were obtained of 123 extremities and thrombolytics administered to 116 extremities; 19 patients had one extremity studied, 36 patients had two extremities studied, three patients had three extremities studied, and four patients had all four extremities studied. Both groin and axillary access sites were used when necessary. Angiography identified 472 digits with perfusion deficits, characterized by stenosis, discontinuous filling defects, or absent distal perfusion that were considered at risk for digital necrosis and amputation.
Thrombolytic Treatment Results
Among 62 patients with perfusion defects who received IATT, only 20 required one or more amputations (range 1–14; Tables 7 and 8). A total of 148 digit amputations were performed; 75 digits were distal amputations, and eight patients underwent transmetacarpal or transmetatarsal amputation of 53 digits. A single patient required bilateral AK amputations and another bilateral BK amputations (20 digits total).
Table 7.
Predictive value of final angiogram for amputation

Table 8.
Relationship between patient clinical outcome and final angiogram

The location of injuries in the IATT group is listed in Table 5, with a slight majority of lower extremity frostbite injuries. The mean duration of thrombolytic therapy was 52 hours (SD 24.6, range 14–120). Six patients who showed slow but progressive response to IATT received prolonged infusions from 80 to 120 hours with no complications, and none required amputations.
Table 6 lists the incidence of substance abuse among our frostbite patients. Sixty-three percentage (39) of our population used tobacco products and 12 required amputations of 93 digits (63% of all digits amputated). Almost 50% patients (n = 32) admitted with frostbite who underwent IATT had an elevated blood alcohol level and accounted for 69 digit amputations (47% of all digits amputated) in eight patients. Three of four methamphetamine users had amputations (a total of 30 digits, 20.3% of amputations performed). Thirteen percentage of patients (n = 8) presented more than 24 hours after their injury (Table 5) and three of those required amputations.
Table 7 contains the completion angiography results for digits treated with IATT. The positive predictive value for amputation in patients with no digit flow on final angiogram was 94.7% (95% CI: 86.9–98.5%). The positive predictive value of angiography for digit salvage in a patient with distal vascular blush on the final angiogram was 98.0% (95% CI: 95.0–99.4%).
Table 8 links the patient outcome to the final angiogram findings. A full clinical patient response to IATT was defined as those patients with no amputations after IATT. A partial clinical response described those with some improvement of vascular flow on completion angiogram. These 13 patients had more distal amputations than initially predicted, salvaging 86 joint spaces of the upper extremities and 17 joint spaces of the lower extremities. There were seven patients with no improvement in flow (nonresponders) that accounted for 44% (65/148) of all amputations, including 24 digits with partial flow and 41 digits with no flow on completion angiogram. Each “nonresponder” had one of the following characteristics: methamphetamine use (three), greater than 24 hours of warm ischemia (two), or presentation with freeze-thaw-refreeze injuries (two).
The response rates for patients treated with each thrombolytic agent are shown in Table 9. No significant difference in response was found between groups treated with the individual thrombolytic agents (P = .86).
Table 9.
Response rates for thrombolytic agents

Hospital charges were available for 41 of 52 patients who were treated without thrombolytics and 45 of 62 patients treated with IATT from the same time period. Although the groups do not represent comparably severe injuries, median (IQR) acute hospital charges including intensive care unit stay and medication infusion were significantly higher for IATT $56,426 (IQR: $32,514–$96,735) vs $16,242 (IQR: $7881–$33,176) (P < .0001) for non-IATT. The groups are not equivalent, because the majority of non-IATT patients had mild to moderate frostbite. The median length of stay (IQR) was 8 (range 5–12) days for IATT vs 4 (2–9) days for conservative therapy (P = .005). We did not review readmission charges for either group.
DISCUSSION
Many factors increase the risk of cold injury.1,20,21 Alcohol and sedative drugs decrease the awareness of cold and impair the judgment necessary to seek shelter.22 Alcohol also inhibits shivering and causes cutaneous vasodilatation,23 precipitating frostbite at warmer temperatures.24 Many schizophrenic patients exhibit acrocyanosis,25 and their ability to assess tissue cooling or comprehend cold injury is often impaired. Any behavior that prolongs cold exposure will worsen the prognosis.26 The majority of civilian frostbite injuries are associated with mental impairment related to alcohol consumption (35–53%),17,21,24,27 mental illness (10–100%),27,28 or drug use (4%).17 Smoking has also been implicated as increasing the risk of frostbite.29 In our frostbite population, 47 (41%) were admitted with an elevated blood alcohol level, 28 (25%) had major mental health diagnoses, and 28 (25%) were positive for drugs of abuse. In addition, 21 (18%) were homeless. Thirty-nine (63%) were smokers. Our study population was similar to other frostbite series except that drug use was six times greater in our population compared with another series published in 1993.17
The pathophysiology of local cold injury has been reviewed extensively elsewhere.1,20,30 Brief exposure to near-freezing skin temperatures causes frostnip with pink skin but little acute damage. Rapid tissue cooling to freezing temperatures (flash freeze or cold contact injury) forms large intracellular and extracellular ice crystals resulting in cell rupture and irreversible damage to the skin. Physicians have not traditionally recognized that rapid freezing is very different than the more common true frostbite injuries induced by slow tissue cooling.
Frostbite occurs when intense vasospasm limits blood inflow and the cold injury progresses distally to proximally. This slow cooling produces small extracellular ice crystals preferentially in the extracellular fluid where protein concentrations are low. The extracellular electrolytes become concentrated, osmotically drawing water from the adjacent cells. These deflated cells are less easily injured by the expanding extracellular ice crystals. Cellular metabolism slows and the skin can tolerate decreased or absent blood flow for some period of time. Under optimal in vitro conditions, explanted human skin can be frozen and maintained at −70°C for years with excellent viability. Frostbite may mimic cryogenic freezing and not irreversibly damage the skin.
Blood flow usually resumes after thawing, but may be diminished by edema, vasospasm, or thrombosis. Damaged endothelium in the arterioles will desquamate, occluding the capillary bed and leaving a thrombogenic basement membrane. The endothelium also releases vasoactive inflammatory mediators including thromboxane A2, prostaglandin F2-alpha, bradykinin, and histamine.26,31 Progressive thrombosis with occlusion of the small arterial collaterals precedes demarcation of tissue infarction. And if the injured tissue refreezes, the swollen and very fragile cells quickly rupture, making the patients poor candidates for thrombolytic therapy.
Because an anoxic reperfusion injury is likely if frozen tissue thaws slowly,1,18,32 the most effective treatment for acute frostbite is rapid rewarming,2 but 25% of our patients presented after thawing the extremity. Clinically, return of blood flow is indicated by pink skin and warm digits. Large blisters will form beneath the damaged epidermis only where fluid leaks from perfused capillaries. Minimal blisters form where blood flow is absent, and the distal digits become gray or cyanotic, often with hemorrhagic blisters forming proximally.2,30 These patients are candidates for thrombolytic therapy.
Table 10 summarizes the results of all other publications regarding the clinical use of thrombolytics in frostbite. During the study period, we have made oral presentations of our results of IATT,33,34 and freely shared our treatment protocol with other centers.35,36
Table 10.
Review of current thrombolytic studies that utilize thrombolytic therapy for frostbite

In 2005, Twomey et al presented their experience with frostbite injury. They studied 16 frostbite patients treated without thrombolytic agents to show that acute bone scans predicted level of amputation in these conservatively treated patients. The data for the subsequent 19 frostbite patients treated with tPA were presented only in aggregate. All were evaluated with bone scans after thawing, and three successive treatment groups were mentioned. First were six patients treated with IA tPA. There was no report of diagnostic angiograms before or after treatment, rates or duration of IA-tPA infusion, or results in these patients. Two patients had complications, one with hematuria and another with bleeding from arterial puncture sites. The next seven patients were treated with varying but unspecified does of IV tPA. Although the outcomes for this cohort are not detailed, from these seven patients, the authors estimated that the optimal dose was 0.15 mg/kg/min to a maximum dose of 100 mg of IV tPA, not to exceed 8 hours of infusion. Six additional patients were treated with this optimized protocol. The authors reported that 16 of 19 patients responded to the lytic treatment. Complete or partial amputations were required in 53 of 174 digits identified with impaired blood flow. One patient rethrombosed his extremities after lytic therapy requiring 10 digit amputations and another, who was frozen for at least 60 hours, had no response to lytics and had multiple digits amputated (number not specified).37
This optimized IV-tPA protocol was used in an additional 11 frostbite patients reported by Johnson et al from the same institution. All showed impaired blood flow on acute bone scan and were evaluated by repeat bone scan at 24 to 72 hours posttreatment. They found of 73 digits with impaired blood flow on the initial bone scan, 43 (59%) required amputation, although impaired blood flow was identified in 67 (93%) digits on the posttreatment bone scan, implying this test has very limited predictive value when performed 24 to 72 hours postinfusion.37
Bruen et al used an IA tPA protocol significantly modified from our own, infusing IA-tPA for a shorter period in six of seven treated patients. The six patients had 13 extremities with frostbite, although they do not report the number of ischemic digits or level of impaired blood flow either pre- or posttreatment. After IATT, there were no proximal amputations and only three digit amputations. The authors compared these outcomes to the results of 25 patients who received traditional treatment and served as historical controls. There was no documentation of the level of impaired blood flow in 23 patients; two more had no angiographic evidence of ischemia, were not treated with tPA, and were included in the control group. They also chose to move to the control group the additional patient who received IA-tPA 37 hours postinjury, who did not respond to treatment. Of 57 affected extremities, there were 14 proximal extremity amputations and an additional 18 digit amputations (including size digits in the nonresponding patient). The rate of digit amputation was 10% in the optimal IA thrombolytic group vs 41% in the control plus late treatment group (P < .05).35
In a Letter to the Editor which is remarkable for its brevity (less than 700 words, plus references and two tables), Cauchy et al presented the results of a randomized trial of 44 frostbite patients in which one of three of them received IV recombinant tPA (rt-PA) in combination with aspirin and iloprost. The other groups received either 1) aspirin plus buflomedil or 2) aspirin plus iloprost only. All received rapid rewarming. No benefit was found in the patients who received rt-PA compared with the other two groups.38 The weakness of the paper is its brevity and the fact that there was no attempt to define the level of blood flow in the digits until day 8 postinjury, when bone scans were found to accurately predict the level of subsequent amputation. The severity of injury in the table was stratified using the unique staging system previously presented by the authors,39 which only records for each digit the most proximal level of injury (defined as cyanosis and grayness after rewarming). The staging system was derived by selecting 70 of 719 total frostbite patients, 68 of whom were injured while climbing Mount-Blanc at an altitude above 2000 m, presumably wearing highly protective footwear and not consuming alcohol. All 70 patients were rapidly rewarmed, and treated with a variety of interventions including unspecified combinations of hemodilution, aspirin, ketanserin, buflomedil, iloprost, streptokinase, urokinase, or rt-PA. This classification system has not been validated for any other group of frostbite patients. Unfortunately, of the two other agents studied, buflomedil was removed from the market in Europe and iloprost is available in the U.S. only as an inhaled agent via nebulizer.
The three case reports discuss diverse treatment regimens.36,40,41 Angiograms identified perfusion defects and IA infusion of tPA was used in each case. There was one groin hematoma and another patient required split thickness skin grafts on two digits. No amputations were performed.
The combined experience with thrombolytic therapy for these institutions was a 25% digit amputation rate for severe frostbite (Table 10). We performed IATT on 62 frostbite patients with a digit amputation rate of 31%. Complete vascular reperfusion after thrombolytics was demonstrated by a distal blush on final angiography in approximately two-thirds of patients (n = 42), which was associated with a 98% digit salvage rate. Overall, 89% (n = 55) had at least partial response, as indicated by return of vascular flow beyond the next joint level for one or more digits. For patients with frostbite of the hands, partial response to IATT salvaged 86 joints in nine patients (ie, these patients received a more distal amputation). For patients with lower extremity frostbite, partial response to lytics salvaged 17 joint spaces in 17 digits in four patients. The most significant impact was demonstrated in two patients; one had complete bilateral mid-calf arterial occlusions and the second had similar unilateral occlusions. After IATT, the patients required only transmetatarsal amputations. Although these patients account for 15 digit amputations in the partial responder group, all were able to ambulate and avoided disabling BK amputations.
In the initial years of our study, lytic therapy was given to patients with greater than 24 hours of warm ischemia (two), methamphetamine use (three), or freeze-thaw-refreeze injury without bullae (two). None of these seven patients responded to IATT and accounted for 65 digit amputations (44% of all amputations). This experience suggests these patients derive little benefit from IATT, and we have not discovered an alternative treatment option. In the subset of all treated patients who did not have a relative IATT contraindication, 80% of digits remained intact.
Bruen et al identified a number of limitations to their retrospective review—it is impossible to compare treated and untreated groups of equal severity, there are no data on functional outcomes, because most of these patients are quickly lost to follow-up, and it is possible that patients who receive tPA might have improved without thrombolytic therapy.
We share these limitations within our own study. The literature of frostbite illustrates that the clinical presentation often does not correlate with the final outcome. Some patients progress to amputation while others almost inexplicably heal. We have attempted to obtain objective evidence of arterial injury in our patients before initiating thrombolytic therapy. It is possible that serial angiograms permit the identification of nonresponse to conservative therapy vs nonresponse to thrombolytics. Nevertheless, we designed a protocol to use angiography to document as precisely as possible the severity of impaired blood flow, using vasodilators to specifically reduce the contribution of any transient vasospasm which can so easily impair the accuracy of bone scans.35 Although two or three phase bone scanning and magnetic resonance imaging/angiography have been used for diagnostic and prognostic purposes in frostbite,1,26,42 they also are costly and not therapeutic. Reliability of bone scanning is greatest 7 days after injury.42 Despite the data from Twomey et al, others report the predictive accuracy varies with time postinjury, possibly because early vasoconstriction limits blood flow in acute injuries.30,42
Our study also suffers from small patient numbers in each treatment group. The thrombolytic agent changed more than once since 1994 for several reasons. Urokinase was removed from the market and reteplase was associated with bleeding complications. Today we use TNKase, which is a derivative of tPA, but has a greater fibrin specificity, greater plasma stability, and reduced clearance.43 These pharmacokinetic features are beneficial where the thrombolytic agent is infused proximally into the limb. We did not detect significant differences in the efficacy of the different agents in this study, but patient numbers are small in each group.
Five percentage of our IATT patients had transient complications from which they all recovered (Table 11). Each of these patients benefited from the IA thrombolytics—only one of the 35 digits at risk required an amputation. The hospital charges incurred after thrombolytic therapy are substantial ($33,000–$97,000). Because we were unable to collect data regarding the long-term sequellae of frostbite injury in our patients, additional studies are need to perform a cost–benefit analysis of this treatment. Early use of catheter-directed thrombolytics offers the hope of restored blood flow and tissue salvage to severely frostbitten digits, but patients should be carefully screened to optimize the benefits of this costly intervention.
Table 11.
IATT complications

CONCLUSIONS
In patients with severe frostbite injury, when angiograms demonstrate severe perfusion defects, continuous infusion of thrombolytic agents may partially or completely restore blood flow to nonperfused digits, and risk of amputation is minimized when distal blood flow is restored. The optimal thrombolytic agent, duration of infusion, and total drug dosages remain undefined. IATT decreases the number of amputations in patients with frostbite injury and leads to increased limb and digit salvage.
ACKNOWLEDGMENTS
The authors thank George R. Edmonson, MD (Interventional Radiology, Regions Hospital).
Footnotes
Presented at the 41st Annual Meeting of the American Burn Association, San Antonio, Texas, March 24–27, 2009.
REFERENCES
- 1.Ahrenholz DH. Frostbite. Problems Gen Surg. 2003;20(1):129–37. [Google Scholar]
- 2.McCauley RL, Hing DN, Robson MC, Heggers JP. Frostbite injuries: a rational approach based on the pathophysiology. J Trauma. 1983;23:143–7. [PubMed] [Google Scholar]
- 3.Biem J, Koehncke N, Classen D, Dosman J. Out of the cold: management of hypothermia and frostbite. CMAJ. 2003;168:305–11. [PMC free article] [PubMed] [Google Scholar]
- 4.Purdue GF, Hunt JL. Cold injury: a collective review. J Burn Care Rehabil. 1986;7:331–42. doi: 10.1097/00004630-198607000-00007. [DOI] [PubMed] [Google Scholar]
- 5.McCauley RL, Heggers JP, Robson MC. Frostbite: methods to minimize tissue loss. Postgraduate Med. 1990;88(8):67–77. doi: 10.1080/00325481.1990.11704769. [DOI] [PubMed] [Google Scholar]
- 6.Smith D, Robson M, Heggers J. Frostbite and other cold-induced injuries. 2nd ed. St. Louis: C.V. Mosby; 1989. [Google Scholar]
- 7.Lewis RB, Walker WB, Pichotka J. The use of anticoagulant therapy in frostbite with special reference to heparin. Q Res Rep. 1948;60:21. [PubMed] [Google Scholar]
- 8.Pichotka J, Lewis RB. Use of heparin in treatment of experimental frostbite. Proc Soc Exp Biol Med. 1949;72(1):130–136. doi: 10.3181/00379727-72-17354. [DOI] [PubMed] [Google Scholar]
- 9.Kapur BM, Gulati SM, Talwar JR. Low molecular dextran in the management of frostbite in monkeys. Indian J Med Res. 1968;56:1675–81. [PubMed] [Google Scholar]
- 10.Welch GS, Gormly PJ, Lamb DW. Frostbite of the hands. Hand. 1974;6:33–9. doi: 10.1016/0072-968x(74)90007-2. [DOI] [PubMed] [Google Scholar]
- 11.Bouwman DL, Morrison S, Lucas CE, Ledgerwood AM. Early sympathetic blockade for frostbite–is it of value? J Trauma. 1980;20:744–9. [PubMed] [Google Scholar]
- 12.Flatt AE. Digital artery sympathectomy. J Hand Surg Am. 1980;5:550–6. doi: 10.1016/s0363-5023(80)80104-3. [DOI] [PubMed] [Google Scholar]
- 13.Martinez A, Golding M, Sawyer PN, Wesolowski SA. The specific arterial lesions in mild and severe frostbite: effect of sympathectomy. J Cardiovasc Surg (Torino) 1966;7:495–503. [PubMed] [Google Scholar]
- 14.Golding MR. Protection from the early and late sequela of frostbite by regional sympathectomy: mechanisms of “cold sensitivity” following frostbite. Surgery. 1963;53:303–8. [PubMed] [Google Scholar]
- 15.Salimi Z, Wolverson MK, Herbold DR, Vas W, Salimi A. Treatment of frostbite with i.v. streptokinase: an experimental study in rabbits. AJR Am J Roentgenol. 1987;149:773–6. doi: 10.2214/ajr.149.4.773. [DOI] [PubMed] [Google Scholar]
- 16.Zdeblick TA, Field GA, Shaffer JW. Treatment of experimental frostbite with urokinase. J Hand Surg Am. 1988;13:948–53. doi: 10.1016/0363-5023(88)90278-x. [DOI] [PubMed] [Google Scholar]
- 17.Valnicek SM, Chasmar LR, Clapson JB. Frostbite in the prairies: a 12-year review. Plast Reconstr Surg. 1993;92:633–41. doi: 10.1097/00006534-199309001-00012. [DOI] [PubMed] [Google Scholar]
- 18.Su CW, Lohman R, Gottlieb LJ. Frostbite of the upper extremity. Hand Clin. 2000;16:235–47. [PubMed] [Google Scholar]
- 19.Johnson AR, Jensen HL, Peltier G, DelaCruz E. Efficacy of intravenous tissue plasminogen activator in frostbite patients and presentation of a treatment protocol for frostbite patients. Foot Ankle Spec. 2011;4:344–8. doi: 10.1177/1938640011422596. [DOI] [PubMed] [Google Scholar]
- 20.Ingram BJ, Raymond TJ. Recognition and treatment of freezing and nonfreezing cold injuries. Curr Sports Med Rep. 2013;12:125–30. doi: 10.1249/JSR.0b013e3182877454. [DOI] [PubMed] [Google Scholar]
- 21.Urschel J. Frostbite: predisposing factors and predictors of poor outcome. J Trauma. 1990;30(3):340–343. doi: 10.1097/00005373-199003000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Bracker MD. Environmental and thermal injury. Clin Sports Med. 1992;11:419–36. [PubMed] [Google Scholar]
- 23.Christenson C, Stewart C. Frostbite. Am Fam Physician. 1984;30:111–22. [PubMed] [Google Scholar]
- 24.Ervasti O, Hassi J, Rintamäki H, et al. Sequelae of moderate finger frostbite as assessed by subjective sensations, clinical signs, and thermophysiological responses. Int J Circumpolar Health. 2000;59:137–45. [PubMed] [Google Scholar]
- 25.Dalton J, Robertson M. Cold injury caused by psychiatric illness: six case reports. Br J Psychiatry. 1982;140:615–8. doi: 10.1192/bjp.140.6.615. [DOI] [PubMed] [Google Scholar]
- 26.Murphy JV, Banwell PE, Roberts AH, McGrouther DA. Frostbite: pathogenesis and treatment. J Trauma. 2000;48:171–8. doi: 10.1097/00005373-200001000-00036. [DOI] [PubMed] [Google Scholar]
- 27.Miller BJ, Chasmar LR. Frostbite in Saskatoon: a review of 10 winters. Can J Surg. 1980;23:423–6. [PubMed] [Google Scholar]
- 28.Pinzur MS, Weaver FM. Is urban frostbite a psychiatric disorder? Orthopedics. 1997;20:43–5. doi: 10.3928/0147-7447-19970101-09. [DOI] [PubMed] [Google Scholar]
- 29.Burgess JE, Macfarlane F. Retrospective analysis of the ethnic origins of male British army soldiers with peripheral cold weather injury. J R Army Med Corps. 2009;155:11–5. doi: 10.1136/jramc-155-01-04. [DOI] [PubMed] [Google Scholar]
- 30.Mohr WJ, Jenabzadeh K, Ahrenholz DH. Cold injury. Hand Clin. 2009;25:481–96. doi: 10.1016/j.hcl.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Britt LD, Dascombe WH, Rodriguez A. New horizons in management of hypothermia and frostbite injury. Surg Clin North Am. 1991;71:345–70. doi: 10.1016/s0039-6109(16)45384-3. [DOI] [PubMed] [Google Scholar]
- 32.Reamy BV. Frostbite: review and current concepts. J Am Board Fam Pract. 1998;11:34–40. doi: 10.3122/15572625-11-1-34. [DOI] [PubMed] [Google Scholar]
- 33.Jenabzadeh K, Mohr W, Ahrenholz DH. Frostbite: a single institution’s twenty year experience with intra- arterial thrombolytic therapy [abstract]. J Burn Care Res. 2009;30(2):S103. [Google Scholar]
- 34.Edmonson GR, Bretzman PA, Mohr WJ, Ahrenholz DH. Intra-arterial thrombolytic therapy for limb salvage in severe frostbite. J Vasc Interv Radiol. 2008;19(2):S21–2. [Google Scholar]
- 35.Bruen KJ, Ballard JR, Morris SE, Cochran A, Edelman LS, Saffle J. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142:546–53. doi: 10.1001/archsurg.142.6.546. [DOI] [PubMed] [Google Scholar]
- 36.Sheridan RL, Goldstein MA, Stoddard FJ, Jr, Walker TG. Case records of the Massachusetts General Hospital. Case 41–2009. A 16-year-old boy with hypothermia and frostbite. N Engl J Med. 2009;361(27):2654–62. doi: 10.1056/NEJMcpc0910088. [DOI] [PubMed] [Google Scholar]
- 37.Twomey JA, Peltier GL, Zera RT. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma. 2005;59:1350–4. doi: 10.1097/01.ta.0000195517.50778.2e. discussion 1354–5. [DOI] [PubMed] [Google Scholar]
- 38.Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med. 2011;364:189–90. doi: 10.1056/NEJMc1000538. [DOI] [PubMed] [Google Scholar]
- 39.Cauchy E, Chetaille E, Marchand V, Marsigny B. Retrospective study of 70 cases of severe frostbite lesions: a proposed new classification scheme. Wilderness Environ Med. 2001;12(4):248–55. doi: 10.1580/1080-6032(2001)012[0248:rsocos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Saemi AM, Johnson JM, Morris CS. Treatment of bilateral hand frostbite using transcatheter arterial thrombolysis after papaverine infusion. Cardiovasc Intervent Radiol. 2009;32:1280–3. doi: 10.1007/s00270-009-9584-9. [DOI] [PubMed] [Google Scholar]
- 41.Wagner C, Pannucci CJ. Thrombolytic therapy in the acute management of frostbite injuries. Air Med J. 2011;30:39–44. doi: 10.1016/j.amj.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cauchy E, Marsigny B, Allamel G, Verhellen R, Chetaille E. The value of technetium 99 scintigraphy in the prognosis of amputation in severe frostbite injuries of the extremities: a retrospective study of 92 severe frostbite injuries. J Hand Surg Am. 2000;25:969–78. doi: 10.1053/jhsu.2000.16357. [DOI] [PubMed] [Google Scholar]
- 43.Tanswell P, Modi N, Combs D, Danays T. Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet. 2002;41:1229–45. doi: 10.2165/00003088-200241150-00001. [DOI] [PubMed] [Google Scholar]


