Abstract
Immune responses are initiated and primed by dendritic cells (DCs) that cross-present exogenous antigen. The CD74 (invariant chain) chaperone protein is thought to exclusively promote DC priming in the context of MHC class II. However, we demonstrate herein a CD74-dependent MHC class I cross-presentation pathway in DCs that plays a major role in the generation of MHC class I restricted, cytolytic T lymphocyte (CTL) responses against viral protein- and cell-associated antigens. CD74 associates with MHC class I molecules in the endoplasmic reticulum of DCs and mediates trafficking of MHC class I to endolysosomal compartments for loading with exogenous peptides. We conclude that CD74 plays a hitherto, undiscovered physiological function in endolysosomal DC cross-presentation for priming MHC class I-mediated CTL responses.
Keywords: CD74, MHC class I, cross-presentation, cross-priming, dendritic cell
During primary immune responses, dendritic cells (DCs) are the principal antigen presenting cells (APCs) that initiate adaptive immune responses predominantly through cross-presentation and cross-priming of T cells. This involves extracellular antigen uptake, digestion of cell-associated antigenic fragments and presentation of proteolytic peptide products on both MHC class I and II molecules1. For MHC class I molecules, two main pathways have been described that may explain how this process occurs: the cytosolic pathway2–5 shown to function convincingly in vitro, and the vacuolar pathway, shown to play a major role in vivo for select antigens6–8. The phago-ER model of cross-presentation has been considered a dominant pathway of cross-presentation9. Subsequent data has disputed this conclusion10. One factor contributing to this controversy appears to be the over-interpretation of data that designate intracellular proteins as definitive markers of specific organelles that are often not exclusive but merely enriched during dynamic organelle biogenesis and partitioning. Furthermore, contrasting conclusions may have been inferred from studies using different forms of exogenous antigens and in studies using long-term DC cell lines versus those using freshly isolate DCs.
In the vacuolar pathway, cathepsin S has been identified as a protease that generates antigenic peptides that are loaded onto peptide-receptive MHC class I molecules11. Furthermore, membrane and cytosolic soluble NSF attachment proteins (SNAREs) that control donor and acceptor tethering and docking events during intracellular membrane fusion also appear to play a fundamental role in cross-presentation events 12. However, the source of MHC class I in the cross-priming compartment, the mechanism of its transport and the site of peptide loading remain areas of active study8,13.
Spontaneous internalization of recycling MHC class I into endosomes has been demonstrated14,15. Our previous results support a model in which MHC class I recycling from the plasma membrane to an endolysosomal loading compartment is facilitated through recognition of the tyrosine internalization signal found in the MHC class I cytoplasmic tail8,13. Therefore, recycling MHC class I molecules from the plasma membrane is one source of MHC class I for loading with exogenous antigens destined for participation in cross-presentation8,13. Likewise, transport of MHC class I from the endoplasmic reticulum (ER) to the endocytic compartment has also been proposed. This could occur by a mechanism involving phagosome and ER fusion9. An alternative and potentially complementary hypothesis is that the CD74 (invariant chain) molecule known to associate with MHC class II in the ER thereby preventing premature binding of peptides and mediating trafficking to the endocytic pathway through sorting signals present in the CD74 cytoplasmic tail1,16, could bind MHC class I and deliver a fraction of the MHC class I to the vacuolar-endocytic compartment to function in cross-presentation 17,18. This mechanism would coincidently place peptide-receptive MHC class I in the same or similar compartment with exogenous antigen and MHC class II molecules19, the MIIC compartment, facilitating antigenic peptide loading and binding to MHC class I molecules. This pathway would link MHC class I transport to the vacuolar pathway, as it is unlikely that CD74 would be involved in the cytosolic route of MHC class I exogenous presentation20,21.
The MHC class I interaction with CD74 and their coincident localization in the same compartment was previously demonstrated in human cell lines17–19. Although it was concluded on the basis of older paradigms, that a MHC class I-CD74 interaction was unlikely to control the fate of MHC class I transport to endosomes under physiological conditions22, other contrasting studies demonstrated that CD74-transfected cells exhibited a substantial increase in surface expression of diverse MHC class I alleles suggesting that MHC class I-CD74 interaction might have functional significance23. Here, we have investigated the immunological relevance of MHC class I interaction with CD74 in vivo and describe a clear and critical role for CD74 in cross-presentation of exogenous antigen and subsequent cross-priming by DCs.
RESULTS
CD74 is required for primary anti-viral responses
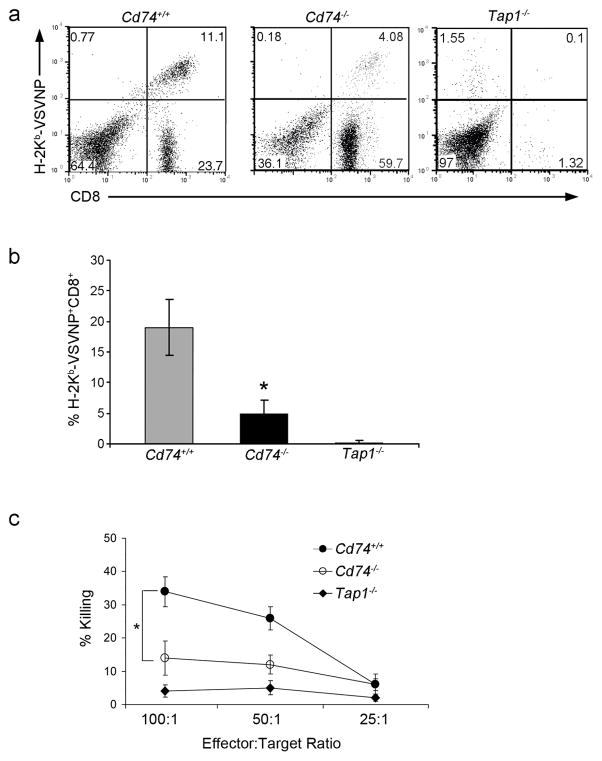
DCs can be directly infected and could therefore utilize classical MHC class I presentation to activate naïve CD8+ T cells. However, during infection with a low viral titer, direct infection of DCs is less likely and DC cross-presentation is the dominant pathway responsible for generation of CD8+ T cell responses8,24. In order to address the role of CD74 in cross-presentation to generate primary anti-viral immune responses, a low dose of Vesicular Stomatitis Virus (VSV) was used to infect wild type (Cd74+/+) and CD74-deficient (Cd74−/−) mice. Tap1−/− mice which are impaired in MHC I assembly and intracellular transport so lack CD8+ T cells due to improper thymic selection, were similarly infected as a negative control (Fig. 1, Supplementary Fig. 1a)25. In this infection, anti-VSV primary and memory CD8+ immune responses can be generated in the absence of CD4+ T cells26,27. In this way, the role of CD74 in cross-presentation can be tested regardless of the role it plays in CD4+ T cell responses. The percentage of CD8+ T cells generated against the VSVNP52-59 immunodominant epitope on MHC class I (H-2Kb) was detected following the VSV infection. Cd74−/− mice had a significantly reduced capacity (5.0% vs. 19.0%; p<0.05) to generate antigen specific CD8+ T cells compared to Cd74+/+ mice (Fig. 1a,b). This resulted in an immune response with reduced CTL killing capacity (Fig. 1c).
Figure 1. Cd74−/− mice generate weak antiviral primary immune responses.
Cd74+/+, Cd74−/− and Tap1−/− mice were infected with a low titer of VSV (2 × 105 TCID50 or dose that infects 50% of a tissue culture cell monolayer/mouse). (a) Six days following viral infection, splenocytes were isolated and after a 5-day stimulation with VSVNP52-59, the number of VSVNP52-59-specific CD8+ T cells generated was assessed. Percentages of VSVNP52-59-specific CD8+ T cells in representative mice are shown. (b) Mean percentages (± SD) of H-2Kb-VSVNP52-59-specific CD8+ T cells of three mice are shown. (c) Standard 51Cr-release assays were performed using CTLs generated following VSV infection and in vitro boosting. Error bars represent SD. Data are representative of at least three separate experiments. * p<0.05.
Bone marrow chimeras were constructed to further exclude a role for T cell help on cross-priming in the VSV infection model class26,27. Additionally, the chimeras would confirm whether the deficiency in generating immune responses is dependent on the hematopoietic-derived DCs ability to cross-present antigen and prime T cells. Normal levels of CD8+ and CD4+ cells were found in the periphery of Cd74+/+ → Cd74+/+ and Cd74−/− → Cd74+/+ mice. However, reduced CD4+ and somewhat increased CD8+ cell numbers were seen in the Cd74−/−→Cd74−/− and Cd74+/+→Cd74−/− mice (Supplementary Fig. 1b,c). This indicated that positive selection in recipient Cd74−/− mice was impaired due to reduced levels of MHC class II in the Cd74−/− thymic epithelium.
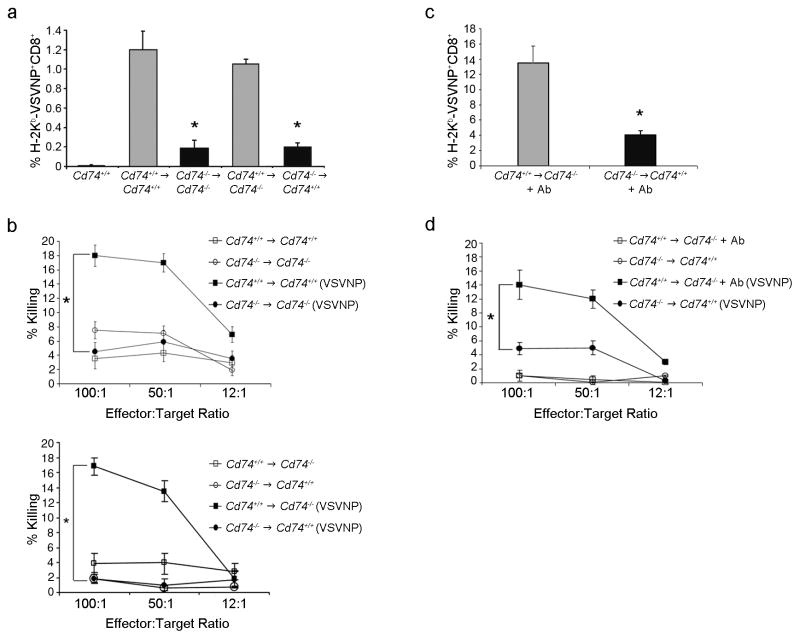
To examine antiviral responses, chimeric mice were infected with a low titer of VSV and assessed for VSVNP52-59 -specific CD8+ T cell generation using tetramer analysis and a CTL killing assay (Fig. 2). Cd74+/+→Cd74−/− mice, with low CD4+ T cell numbers, were able to produce VSVNP52-59-specific CD8+ T cells similar to wild-type Cd74+/+→Cd74+/+ chimeras (1.1% vs. 1.2%, Fig. 2a) resulting in immune responses with comparable killing capacity (16.8% vs. 1.9%; p<0.05, Fig. 2b). However, the Cd74−/− →Cd74+/+ mice were grossly impaired in the generation of VSVNP52-59 specific CD8+ cells (0.2%; p<0.05, Fig. 2a) despite having normal CD4+ T cells resulting in diminished CTL killing responses (18.0% vs 4.5%; p<0.05, Fig. 2b). This suggests that the generation of VSV specific CTL responses is independent of CD4+ T cell numbers. Importantly, bone marrow-derived APCs expressing CD74 were required and allowed Cd74−/− mice to produce a robust antiviral immune response comparable with that of Cd74+/+ mice.
Figure 2. The deficiency of Cd74−/− mice to elicit primary immune responses resides in their APCs and is independent of CD4+ T cells.
(a) Chimeras were injected with 1 × 105 TCID50 VSV and splenocytes were assessed for the generation of VSVNP52-59 -specific CD8+ cells. The mean percentage (± SD) of three mice assayed following in vitro boosting with VSV NP52-59 peptide is shown. (b) Cytotoxicity assays were performed using CTLs generated following in vitro boosting. Data are representative of at least three separate experiments. (c) Cd74+/+→Cd74−/− chimeras were depleted of CD4+ cells by intravenous injection of a CD4 (GK1.5) antibody (+Ab) then assessed for immune function. Mice chimeras infected with VSV were evaluated for the generation of H-2Kb-VSVNP52-59-specific CD8+ T cells. The mean percentage (± SD) of tetramer+CD8+ cells in the spleen of three mice is shown. (d) The lytic activity of the immune response was also assessed. Error bars represent SD. * p < 0.05.
CD4+ cells depletion has no effect on anti-VSV responses
Next, to eliminate the possibility that residual CD4+ cells in the Cd74+/+→Cd74−/− chimeras that result from dysfunctional positive selection in Cd74−/− mice are contributing to the efficiency of anti-viral immune responses, during the course of the infection, the CD4+ cells of Cd74+/+→Cd74−/− chimeras were depleted with CD4 (GK1.5) antibodies. Although CD4+ cells were virtually undetectable over background, CD4+ cell depleted Cd74+/+→Cd74−/− chimeras generated significantly more CD8+ VSVNP52-59 specific T cells than Cd74−/− →Cd74+/+ chimeric mice (13.5% vs 4.1%; p<0.05; Fig. 2c) resulting in an immune response with increased lytic activity (14.0% vs. 4.9%; p<0.05; Fig. 2d). Taken together, these data confirm that Cd74+/+→Cd74−/− chimeras mount stronger anti-VSV responses than Cd74−/− →Cd74+/+. This is independent of CD4+ cells but rather due to the reconstitution of Cd74−/− mice with wild-type DCs that were fully capable of priming anti-viral CD8+ T cells responses.
Cross-priming of cell-associated antigen is CD74 dependent
In order to investigate the role of CD74 in primary immune response to cell-associated antigen, lethally irradiated ovalbumin (OVA)-pulsed DCs or mismatched MHC class I OVA-pulsed DCs were used as a source of cell-associated antigen to activate antigen-specific CTLs in Cd74+/+, CD4+-depleted Cd74+/+ and Cd74−/− mice as well as in reconstituted mouse chimeras. Mice with a wild-type immune system, challenged with cell-associated OVA, were able to induce proliferation of OT-I-derived CD8+ T cells (Supplementary Fig. 2) or activate endogenous H-2Kb-OVA257-264 specific CTLs that were efficient at killing OVA257-264-pulsed target cells (data not shown). However, with the same challenge of cell-associated OVA, mice with the hematopoietic system deficient for CD74 had a substantially reduced ability to stimulate proliferation of OT-I CD8+ T cells and generated fewer endogenous CTLs contributing to a reduced killing ability (Supplementary Fig. 2 and data not shown).
CD74-dependent cross-priming is CD4 T cell independent
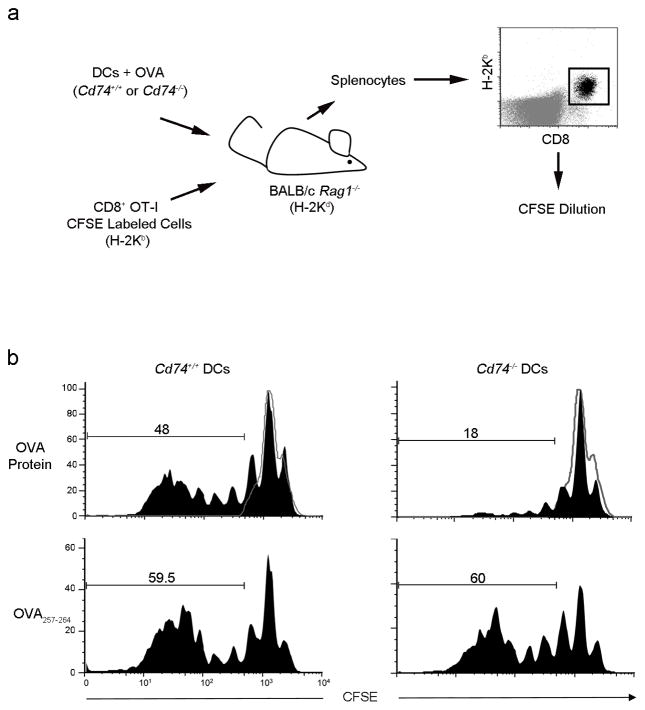
To focus specifically on DC cross-priming defects and eliminate extraneous factors including the requirement for CD4-help, Cd74−/− and Cd74+/+ DCs incubated with OVA protein or OVA257-264 peptide were injected with CFSE-labeled purified OT-I CD8+ T cells into T cell deficient Rag1−/− mice on a BALB/c background. The ability of the DCs to cross-prime the OT-I T cells was assessed (Fig. 3a). Cd74−/− DCs incubated with OVA protein induced significantly less OT-I proliferation in comparison to the Cd74+/+ DCs (18% vs. 48%; Fig. 3B). However, when Cd74−/− DCs were pulsed with OVA257-264 peptide, as a positive control for direct presentation, Cd74−/− DCs were as competent as Cd74+/+DCs to activate the CD8+ OT-I T cells (59.5% vs 60.0%, Fig. 3B).
Figure 3. Cd74−/− DCs are unable to cross-present cell-associated antigens in vivo to prime antigen-specific CD8+ T cells.
(a) OVA protein or OVA257-264 pulsed Cd74−/− or Cd74+/+ BMDCs were injected with purified CD8+ OT-I CFSE-labeled T cells into Rag1−/− mice on a BALB/c background. Three days later, H-2KbCD8+ T cells were assessed for proliferation. (b) Black histograms represents proliferating OT-I derived T cells from the spleens of representative mice (n=3). Grey histograms represent unproliferating OT-I T cells.
In order to address the possible confounding role for CD74 in DC motility and homing28 from the site of injection to the spleen, we assessed the localization of CFSE-labeled DCs after injection intravenously29 (Fig. 3b and Supplementary Fig. 3). Cd74+/+ and Cd74−/− DCs injected intravenously into Rag1−/− mice were found to localize equivalently to the spleen. Therefore, the reduced ability of Cd74−/− DCs to induce T cell proliferation is not due to differences in DC migration but due to reduced antigen processing and presentation ability. We conclude that CD74 plays a critical role in MHC class I cross-presentation of cell-associated antigen and CD8+ T cell priming in vivo and this is unrelated to CD4+ T cell help or CD74-mediated DC motility and homing.
CD74-deficient DCs have impaired cross-priming ability
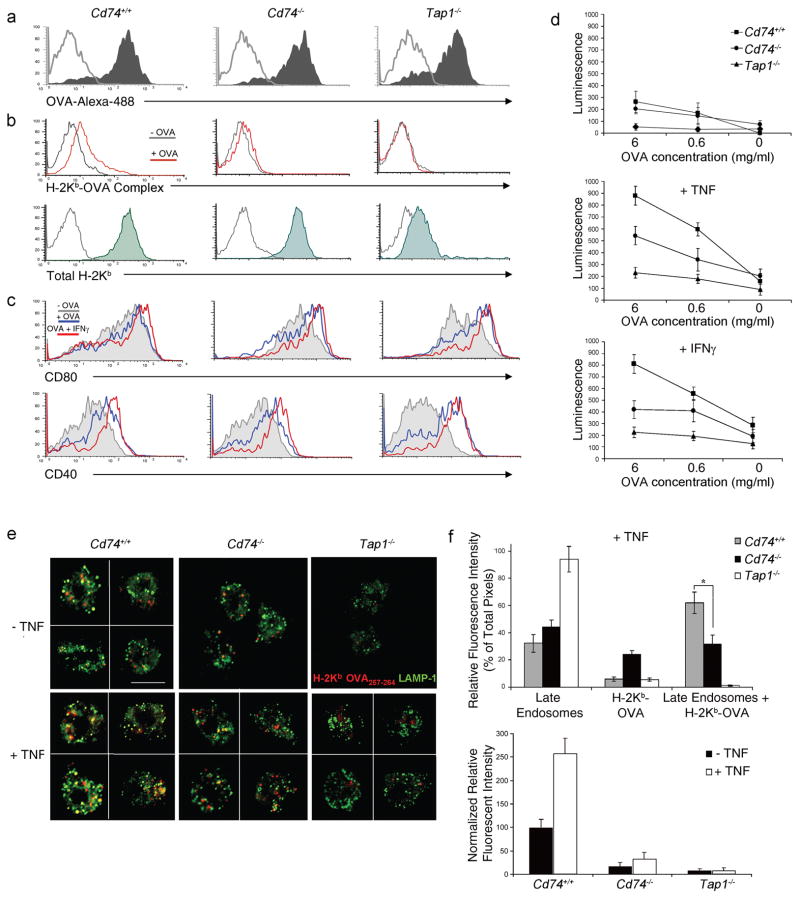
Spleen-derived DCs from different mouse strains were examined for their ability to cross-present the H-2Kb-restricted ovalbumin epitope OVA257-264 in vitro. DCs were incubated with soluble OVA, with or without cytokines, and stained with an antibody specific for the H-2Kb-OVA257-264 complex or co-cultured with B3Z, a T cell hybridoma that is activated following recognition of H-2Kb in association with the OVA257-264 peptide30. Cd74+/+ and Cd74−/− DCs had similar ability to internalize OVA and had comparable levels of total surface MHC class I (Fig. 4a,b). However, Cd74−/− DCs displayed substantially reduced levels of H-2Kb-OVA257-264 complexes following OVA incubation compared to Cd74+/+ DCs (Fig. 4b). It has been shown that cross-priming capacity of DCs is differentially regulated by inflammatory mediators that induce upregulation of costimulatory and MHC molecules, and reduce endocytosis31,32. This results in an increased capacity of T cell priming but lowers the ability of DCs to capture and present soluble antigens. To test T cell activation in a situation resembling in vivo conditions that involves co-stimulation, OVA-pulsed DCs were incubated with B3Z T cells with and without cytokines. In the presence of tumor necrosis factor (TNF) and interferon (IFN)-γ, Cd74+/+ and Cd74−/− DCs had an equal ability to upregulate costimulatory molecules CD80, CD86, and CD40 (Fig. 4c and data not shown) but Cd74−/− DCs had a much reduced capacity to activate B3Z T cells compared to Cd74+/+ DCs (Fig. 4d). As expected, no T cell activation was detected following incubation with OVA-pulsed DCs derived from Tap1−/− in the presence of cytokines. These data support the conclusion that CD74 plays a role in T cell cross-priming and does not affect costimulatory molecule expression.
Figure 4. Cross-presentation and cross-priming is defective in Cd74−/−-derived DCs.
(a) BMDCs were incubated with OVA-Alexa-488 and uptake (shaded) was assessed over background (grey) by flow cytometry. (b) Formation of H-2Kb-OVA257-264 complexes on splenic DCs with (red) or without (grey) incubation with soluble OVA as well as total H-2Kb (shaded) above background (grey) was measured by flow cytometry. (c) Costimulatory receptors CD80 and CD40 on BMDCs were assessed by flow cytometry following incubation with OVA (blue), OVA+IFNγ (red) or media alone (grey). (d) Spleen-derived DCs were incubated with soluble OVA as indicated, in the presence of GM-CSF or GM-CSF and TNF or IFN-γ. Activation of B3Z T cells was measured using a chemiluminescent assay. Data depict means (± SD) of triplicate samples for each OVA concentration. Similar results were observed in 3 separate experiments. (e) Mature spleen-derived DCs incubated with OVA were costained with H-2Kb-OVA257-264 specific antibody (red) and LAMP-1 (green). The figure shows optically merged images representative of the majority of cells examined by ICM. Scale bar, 5 μm. (f) Quantitative assessment was performed comparing H-2Kb-OVA257-264 colocalization with LAMP-1+ late endosomes with or without TNF (top panel). Quantitative assessment comparing fluorescence between Cd74+/+, Cd74−/− and Tap1−/− DCs with TNF treatment (bottom panel). For each analysis >20 DCs per mouse strain were examined. Graph depicts normalized individual color pixel percentages per total pixels ± SD. * p < 0.05.
CD74 mediates endolysosomal MHC class I loading
To better understand the mechanism of the cross-presentation and priming deficiency at a molecular level, comparative immunofluorescent confocal microscopy (ICM) was used to determine the intracellular localization, trafficking and distribution of OVA257-264 loaded MHC class I in Cd74+/+ and Cd74−/− DCs with and without TNF. Intracellular staining was performed with antibodies against H-2Kb-OVA257-264 and the late endosome marker, LAMP1, following incubation with OVA protein. Colocalization with LAMP1 was detectable in a considerable number of the Cd74+/+ splenic DCs staining positive for H-2Kb-OVA257-264 complexes when no TNF was added to the culture (Fig. 4e,f). In the Cd74−/− and Tap1−/− DCs, some H-2Kb-OVA257-264 complexes were identified; however, colocalization with late endosomes was minimal (Fig. 4e,f). The absence of loaded MHC I in the Tap1−/− DCs is consistent with TAP playing a role in cross-presentation, a mechanism that has been previously postulated24,33. Following treatment with TNF, Cd74+/+ DCs demonstrated a significant increase in colocalization of H-2Kb-OVA257-264 complexes with LAMP1 (Fig. 4e,f, p<0.05), but not with the ER marker GRP78, or Golgi marker Giantin (data not shown). In contrast, few H-2Kb-OVA257-264 complexes were observed in late endosomal compartments in Cd74−/−–-derived DCs indicating that the H-2Kb-OVA257-264 complex formation in late endosomes was reduced (Fig. 4f). Comparison of the ICM data indicated that in the presence of TNF, Cd74−/−-derived DCs had significantly less OVA257-264 loaded onto H-2Kb (red) in the late endosomes (green) than in Cd74+/+ DCs (62% vs 32%; p<0.05; Fig. 4f). These data suggest that in DCs a CD74-dependent MHC class I antigen processing pathway exists that is required for the cross-presentation of exogenous antigens.
CD74 directs MHC class I from the ER to the endolysosomes
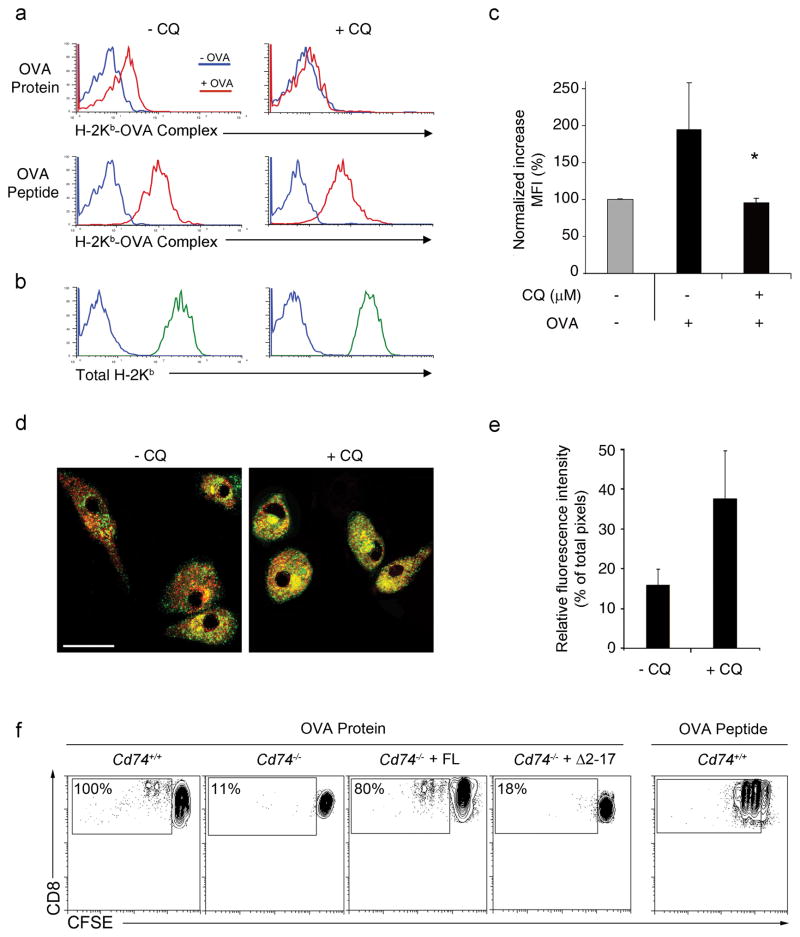
The fact that CD74-deficiency results in fewer H-2Kb-OVA257-264 complexes in late endosomal compartments suggests that CD74 targets MHC class I from the ER into the endolysosomal pathway. Here, CD74 is presumably degraded and MHC class I is loaded with exogenous antigenic peptides. To examine this in more detail, the acidification of endosomes was blocked with chloroquine (CQ) and the CD74-mediated MHC class I cross-presentation pathway was assessed. Following CQ treatment, bone marrow-derived DC (BMDCs) had equivalent MHC I surface expression as untreated controls and displayed H-2Kb-OVA257-264 when pulsed with OVA257-264 peptide; however, when incubated with soluble OVA, CQ-treated DCs had significantly reduced amounts of surface H-2Kb-OVA257-264 (Fig. 5 a–c). ICM analysis of BMDCs following CQ treatment (Fig. 5d,e) showed increased colocalization of H-2Kb (red) and CD74 (green). This indicates that CQ treatment increases the amount of endolysosomal MHC class I molecules presumably by blocking the dissociation of MHC class I and CD74 in the endolysosomes in a similar manner that was reported for the MHC class II pathway 34 and by inhibiting the degradation of recycling MHC class I. The end result is reduced loading of MHC class I with exogenous antigen and subsequently lower surface levels of H-2Kb/OVA257-264. To confirm the finding that CD74 directs MHC class I to an endolysosomal compartment and unequivocally demonstrate that CD74 mediates MHC class I trafficking, CD74-deficient BMDCs were transfected with full length CD74 (FL) or CD74 lacking the cytosolic trafficking domain (Δ2–17) and the ability to present OVA protein or OVA peptide, a positive control that would bypass the need for processing, was assessed. As previously demonstrated, Cd74−/− DCs had impaired cross-priming ability inducing much less OT-I T cell proliferation compared to Cd74+/+ DCs (Fig. 5f). As expected, when Cd74−/− DCs were reconstituted with full length CD74 cross-priming ability was restored and DCs could induce OT-I T cell proliferation with similar ability as wild-type DCs. However, when Δ2–17 CD74 lacking the endosomal trafficking motif was reintroduced into Cd74−/− DCs, cross-priming ability continued to be impaired (Fig. 5f) demonstrating that in the absence of CD74, MHC class I trafficking to endolysosome and cross-priming of OT-I T cells is reduced. Taken together, the data show that CD74 clearly influences MHC class I trafficking to the cross-priming compartment where efficient presentation of exogenous antigen takes place.
Figure 5. Inhibition of CD74-mediated MHC I trafficking in DCs by treatment with chloroquine.
(a) BMDCs were treated with CQ and the formation of H-2Kb-OVA257-264 complexes on DCs following incubation with soluble OVA (red; top panel) or OVA peptide (red; bottom panel) compared to media alone (blue) was measured by flow cytometry. (b) Total H-2Kb (green) above background (blue) was also assessed. (c) The fold increase in surface H-2Kb-OVA257-264 complexes following incubation with soluble OVA was quantified. Graphs depict normalized increases in mean fluorescence intensity (MFI) ± SD. * p < 0.05. (d) Mature BMDCs treated with CQ were costained with H-2Kb (red) and CD74 (green) antibody. The figure shows optically merged images representative of the majority of cells examined by ICM. Scale bar, 5 μm. (e) Quantitative assessment comparing H-2Kb colocalization with CD74. Graph depicts normalized individual color pixel percentages divided per total pixels ± SD. (f) Cd74−/− BMDCs reconstituted with full length (FL) CD74 or truncated (Δ2–17) CD74 lacking the endolysosomal trafficking motif were incubated with soluble OVA and assessed for the ability to induce proliferation in CFSE-labeled OT-I cells. Percentages represent the proportion of proliferating OT-I cells normalized to Cd74+/+ controls.
CD74 and MHC class I molecules form a complex in DCs
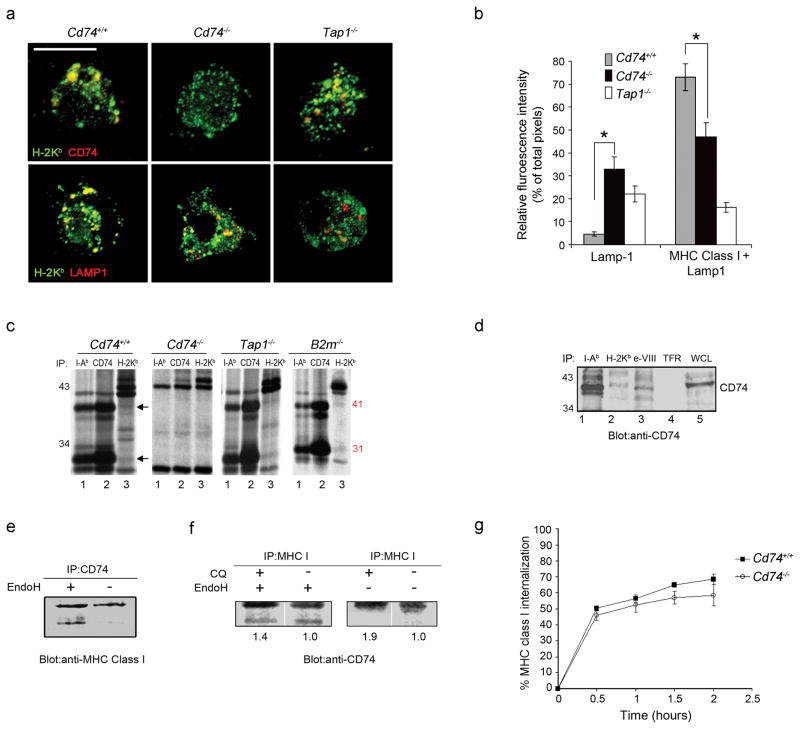
The interaction of CD74 with MHC class I in DCs as a prerequisite of targeting MHC class I to the cross-priming compartment was investigated at the molecular level. Spleen-derived DCs were isolated from Cd74+/+ and Cd74−/− mice for analysis by ICM. DCs were stained with antibodies against H-2Kb (green) and CD74 (red). H-2Kb molecules were distributed at the cell surface and in the cytoplasm where they localized mainly to vesicular-like compartments. CD74 molecules colocalized markedly with these intracellular compartments (Fig. 6a, top left). However, in Tap1−/− DCs, a reduced colocalization of H-2Kb with CD74 was observed, presumably due to the restricted overall availability of H-2Kb pool and their ability to traffic to the endolysosomes (Fig. 6a, top right).
Figure 6. CD74 controls MHC class I ER-to-endolysosome trafficking in DCs.
(a) Mature splenic DCs were stained with H-2Kb (green) and CD74 (red) or LAMP1 (red) antibodies. Representative images as examined by ICM are shown. Scale bar, 5 μm. (b) Quantitative assessment of MHC class I in LAMP+ compartments was performed (50 DCs per mouse strain). Graphs depicts individual color pixel percentages per total pixels (mean ± SD) (c). Immunoprecipitation using H-2Kb, I-A/I-E and CD74 antibodies was performed on [35S]methionine-labeled BMDC. The CD74 41 and 31 k-Da protein bands are indicated. (d) Immunoprecipitation with antibodies against I-Ab, H-2Kb (conformationally dependent), H-2Kb cytoplasmic domain (e-VIII; conformationally independent) or transferrin receptor (TFR) was performed with Cd74+/+ DC lysates. The identity of the co-immunoprecipitated proteins was confirmed by blotting with a CD74 antibody. Whole cell lysate (WCL) was blotted as a control. (e) DC cell lysates were immunoprecipitated with a CD74 antibody and digested with endoglycosidase H. Immunoblotting with a MHC class I antibody was used to assess the MHC class I fraction precipitated by CD74 antibody and to visualize the acquisition of EndoH resistance of the MHC class I subset interacting with CD74. (f) Cells were treated with CQ to enhance CD74 and MHC I interaction and cell lysates were immunoprecipitated with a MHC class I antibody. Western blotting with a CD74 antibody was used to assess the CD74 protein fraction associating with MHC class I. (g) DCs labeled with a H-2Kb antibody were evaluated overtime for MHC class I internalization measured by flow cytometry as a reduction in mean fluorescence intensities over time. * p < 0.05.
To identify the compartment where these molecules colocalize, spleen DCs were co-stained with antibodies recognizing H-2Kb and LAMP1 that detects late endosomes. A considerable proportion of late endosomes contained H-2Kb in Cd74+/+ DCs, confirming that a substantial amount of MHC class I molecules reside in the endocytic compartment 8,21. In contrast, only a small fraction H-2Kb colocalized with late endosomes in Cd74−/− DCs (Fig. 6a, mid panel). This was confirmed by quantification of ICM images and suggests that significantly fewer MHC class I molecules were targeted to the endolysosomal compartment in Cd74−/− vs Cd74+/+ DCs (73% vs 47%; Fig. 6b). Colocalization was even less evident in the Tap1−/− DCs possibly due to the impaired targeting of H-2Kb molecules to endolysosomes in the absence of TAP1. These data suggest that a substantial fraction of MHC class I molecules interact with CD74 facilitating their transport to the endolysosomal compartment of DCs likely from the ER. Demonstration of a direct molecular interaction between MHC class I and CD74 in DCs would further strengthen the argument that this is a yet undescribed pathway of antigen presentation in DCs. To this end, BMDCs from various knock-out and wild-type mice were 35S-labelled, and MHC class I (H-2Kb), MHC class II (I-Ab) or CD74 bound complexes were co-immunoprecipitated and proteins in these complexes were identified based on apparent molecular weight. MHC class II co-immunoprecipitated the abundant 41 and 31 kDa isoforms of CD74 (Fig. 6c left, lane 1). The H-2Kb antibody also co-precipitated these same proteins corresponding to the CD74 isoforms (Fig. 6c left, lane 3) suggesting that at any one time, CD74 is bound to a fraction of the total pool of MHC class I molecules in DC. The two prominent proteins detected between 41 and 31 kDa may be components of a MHC I loading or transporting complex. Their sizes are consistent with the H-2DM or H-2DO but their identities have not yet been conclusively determined. The 41 and 31 kDa forms of CD74 were not present in the Cd74−/− DCs (Fig. 6c middle) demonstrating that they are indeed the previously reported isoforms of CD74 that have been shown to co-immunoprecipitate with MHC class I and MHC class II molecules. In addition, the 41 and 31-kDa CD74 isoforms were co-immunoprecipitated with H-2Kb in Tap1−/− DCs, suggesting that CD74 binding to MHC class I is not dependent on the peptide transporter function of the TAP molecule. Finally, the CD74 isoforms co-precipitated with MHC class I from B2m−/−-derived DCs suggesting that CD74 can bind the folded β2m-associated MHC class I complex (Fig. 6c right) and the β2m-free MHC class I complex.
Immunoblotting was then performed to confirm the identity of the CD74 isoforms bound to MHC class I molecules. Immunoprecipitation with antibodies against I-Ab, H-2Kb and the exon-VIII region of the MHC class I molecule as well as an irrelevant antibody against transferrin receptor (TFR) was followed by blotting with a CD74 antibody (Fig. 6d). As expected, CD74 was found to associate with MHC class II (lane 1) but not with the irrelevant protein, transferrin receptor (lane 4). CD74 was definitively identified to be associated with MHC class I (lane 2,3) confirming that this interaction is clearly detectable and stable under the conditions used in this immunoprecipitation procedure.
CD74- MHC class I complex forms in a pre-Golgi compartment
Next, in order to unequivocally demonstrate the kinetics and origin of the MHC class I-CD74 interaction, we used biochemical means to further deduce the intracellular compartment where the CD74 and MHC class I interaction takes place. Proteins within the secretory pathway acquire Endo H resistance as they traffic from the ER through the Golgi compartment and there, undergo cleavage by mannosidase II 35. It is well accepted that Endo H sensitivity acts as an indication that proteins are localized to the ER or in “transitional elements” between the ER and cis-Golgi. CD74-bound MHC class I was immunoprecipitated from Cd74+/+ BMDCs with a CD74 or MHC class I antibody and treated with Endo H. Immunoblotting was performed with an MHC class I or CD74 antibody to visualize the Endo H sensitivity of the CD74-MHC class I complex. We clearly identified that the MHC class I associated with CD74 is Endo H sensitive (Fig. 6e,f). Furthermore, when treated with CQ, the amount of EndoH resistant CD74 associating with MHC class I was slightly increased as demonstrated by greater protein intensities (Fig. 6f). Overall, these data suggest that the CD74 interaction with MHC class I originates in the ER where the CD74 binds an ‘immature’ fraction of the MHC class I molecules and from here initiates trafficking to an endolysosomal compartment to mediate cross-presentation, T cell priming and primary immune responses8,13.
CD74 does not affect MHC class I internalization
Lastly, to examine the source of MHC class I that binds CD74, the role of CD74-mediated MHC class I trafficking from the plasma membrane was examined. To determine if CD74 functions in surface receptor recycling, we followed the internalization of MHC class I in Cd74+/+ and Cd74−/− DCs. BMDCs were stained with an H-2Kb antibody and flow cytometric analysis was used to follow internalization over time. Cd74+/+ and Cd74−/− DCs have very similar dynamics of MHC class I internalization (Fig. 6g). This indicates that CD74 is not interacting with MHC class I at the cell surface to cause internalization into an intracellular compartment for cross-presentation. This compliments our other studies that demonstrate a tyrosine-based motif in the cytoplasmic domain of MHC class I molecules is crucial for internalizing recycling MHC class I molecules into the endolysosomal cross-priming compartment from the plasma membrane8,13 and thus reveals a unique and distinct pathway of CD74-dependent MHC class I trafficking.
DISCUSSION
The dichotomy of MHC class II molecules presenting exogenous peptides versus class I molecules displaying cytosolic peptides has been revised6,8,36,37. Not only does MHC class I cross-presentation demonstrate the blurring of this division, but it also shows that for specific cell types such as DCs this phenomenon plays a major role in generating primary immune responses in vivo8. In addition, the presentation of endogenously-derived peptides on MHC class II molecules demonstrates that MHC class I and II pathways possibly intersect and that they may share the same antigen-loading compartments38. Although CD74 is classically recognized as a major chaperone in MHC class II presentation, MHC class I and CD74 have also been shown to interact17,18,39,40. However, the physiological contribution of CD74 to MHC class I mediated immune responses in vivo has not been investigated and the previous identification of CD74-MHC class I interaction was largely discounted as a biological curiosity. Here, we demonstrate that CD74 contributes significantly to MHC class I cross-presentation pathways in DCs. These studies demonstrate a major role for CD74-dependent cross-priming in the generation of responses against viral and cell-associated antigen.
To assess CD4+ T cell independent CTL responses generated through DC cross-presentation, we used a low dose VSV infection model. Low viral doses mimic a physiological situation where the majority of DCs would presumably be spared from infection and other infected cells would act as antigenic peptide donors allowing the dissection of direct or endogenous presentation from cross-presentation. The observation that mice lacking CD74 are significantly impaired in their ability to generate MHC class I-restricted CTL responses, particularly against low viral doses where cross-priming is likely to dominate over direct priming by DCs, supports the conclusion that MHC class I cross-presentation is the primary mechanism by which antiviral CD8+ T cell-mediated immunity is generated under physiological conditions in vivo 8,41. We also confirmed the work of others and demonstrated that CTL responses against viruses such as VSV are CD4-independent26,27 and thus independent of the function of MHC class II-CD74 complexes.
The generation of bone marrow chimeras made it possible to study the performance of myeloid Cd74−/− derived DCs on a different host background. These studies led to the conclusion that CD74’s priming defect was of DC origin and indicated that the deficiency lies at the level of DC cross-presentation. Further, CD74-dependent cross-priming was revealed as an important MHC class I antigen presentation pathway as the absence of CD74 resulted in a greater than 50% decrease in the number of anti-VSV CTLs. In addition, the findings obtained by mouse chimeras support the observations that CD74-deficiency impairs the generation of primary immune responses against VSV, independent of reduced CD4+ T cells 26,42. This is in accordance with other recent data that demonstrate that in some cases, CD4 helper cells are required for secondary, but not primary CTL expansion43. Costimulation of the CD8+ CTL by B7 molecules, along with TCR stimulation, can be sufficient to elicit CD8+ CTL without T cell help26. Alternatively, it is entirely possible that two distinct lineages of CD8+ CTLs precursors exist whereby the CD4 helper-independent population provides the predominant response to various viruses resulting in no loss of CTL function in the absence of CD4+ T cells42. Expression of a form of CD74 lacking its endosomal targeting signal, failed to complement DC cross-presentation and priming of T cells. However, reconstitution with a wild-type CD74 molecule containing a functional endosomal targeting signal, restored cross-priming thereby supporting a mechanism where MHC class I is transported from the ER to the endolysosome by CD74. Additionally, the deficient CD8+ T cell activation by Cd74−/− DCs in Rag1−/− mice that completely lack CD4+ T cells, unequivocally demonstrates that the defect in DC cross-priming function is due to the absence of CD74. In our studies, CD74 does not appear to play a role in DC homing and motility in vivo but does mediate a physiologically important CD74-dependent MHC-I dendritic cell cross-priming pathway.
Our studies also provide evidence of an association between MHC class I molecules and CD74 under physiological conditions in DCs. It also suggests that upon CD74 dissociation in endolysosomes, the reassembly of MHC class I heavy chain with β2m and antigenic peptides could then take place in the endolysosomal compartment44. In this context, we have directly demonstrated that the MHC class I-CD74 complex remains assembled in vesicular-like compartments identified as late endosomes. Furthermore, we have established that CD74 influences the presence of MHC class I in endolysosomes confirming previous observations that an MHC class I-CD74 interaction results in targeting of a subset of MHC class I molecules to the endolysosomal pathway17.
In contrast to the cytoplasmic tail tyrosine mutants that affect MHC I recycling into the cross-priming compartment we previously described8,13,45, it is unlikely that a stable interaction between CD74 and MHC class I molecules occurs at the plasma membrane as the absence of CD74 in DCs does not appear to influence MHC class I internalization. Our results support a model whereby both MHC class I recycling from the plasma membrane, directed by a tyrosine internalization signal in the cytoplasmic domain, and MHC class I trafficking from the ER through the binding of the CD74 chaperone contributes to the pool of peptide-receptive MHC class I in the endolysosomal pathway. Thus, in an analogous manner to MHC class II molecules, the MHC class I-CD74 complex is formed in the ER and may be held in a conformation that masks peptide binding as they transit to the cross-priming compartment. Indeed, two independent studies have shown that CD74 peptides, including a smaller peptide derived from the core CLIP peptide, the portion of CD74 bound in the MHC class II binding groove, can be eluted from MHC class I molecules46,47. Such peptides are therefore strong candidates for the MHC class I equivalents of CLIP (MRMATPLLM). This CLIP-derived (CLIPD) peptide may prevent premature peptide binding akin to MHC class II situation46,48. In this model, following CD74 digestion and removal, MHC class I could be loaded with high affinity cathepsin S-derived exogenous peptides11 and progress to the cell surface where they could efficiently prime CD8 T cell precursors to become activated.
In summary, these and previous data8,49 highlight the significance of the endolysosome as a principle compartment for cross-presentation in DCs and the present investigation formally establishes the structural and functional relevance of the CD74-MHC class I interaction on the intracellular routing of MHC class I molecules and cross-priming function of DCs. These observations define a new pathway for priming immune responses and therefore the complete elucidation of this process is of significant importance. These observations have considerable clinical significance and suggest that targeting vaccine candidates to the endolysosomes of DCs will enhance priming for both MHC class I and MHC class II antigens and thereby improve the immunogenicity and efficacy of vaccines.
METHODS AND MATERIALS
Mice
Cd74+/+ (H-2Kb) mice were purchased from Charles River. B2m−/−, Tap1−/−, OT-1 T cell transgenic (H-2Kb), and Rag1−/−(H-2Kd) mice were purchased from Jackson Laboratory. Cd74−/− (H-2Kb) mice were a gift from Diane Mathis (C.U. Strasbourg, France and The Harvard Stem Cell Institute, Boston, MA). For chimeric mice, donor bone marrow was depleted of mature T cells using a Thy1 antibody (Abcam) and injected (1×107) into sublethaly irradiated (1200 rad) recipients. Peripheral T cells subsets were analyzed by flow cytometry following staining with CD8 and CD4 antibodies (Pharmingen). For CD4-depletion, prior to immunization and 48 hours prior to T cell assessment, mice were injected with 100 μg CD4 (GK1.5) antibody50. All studies followed guidelines set by the University of British Columbia’s Animal Care Committee and the Canadian Council on Animal Care.
Viral infections
Vesicular Stomatitis Virus (VSV) was injected intraperitoneally at 1–2 × 105 TCID50 (dose that infects 50% of a tissue culture cell monolayer). Six days post-infection, splenocytes were stained with a CD8 antibody (BD Pharmingen) and H-2Kb-VSVNP52-59 or H-2Kb-OVA257-264 iTAg Tetramer (immunomics-BeckmanCoulter) and analyzed using FACSCalibur (Becton Dickinson) and FlowJo software. Splenocytes were further cultured for 5 days with 1 μM OVA257-264 (SIINFEKL) or VSVNP52-59 (RGYVYQGL) peptide and tetramer staining was performed as above. Cytotoxicity assays were performed as described8.
Uptake assay
Bone marrow DCs (BMDCs) were generated as described8. Cells were incubated with 30 μg/mL ovalbumin (OVA)-Alexa488 (Invitrogen) for 30 minutes on ice or at 37 °C. OVA uptake was analyzed by flow cytometry.
Cross-presentation assays
BMDCs were generated as described8 or splenic DCs were isolated using CD11c+ magnetic beads (Miltenyi Biotech). DCs were incubated with OVA (Worthington) for 15 hours and where indicated 100 μM chloroquine. DCs were stained with Fc block (PharMingen) then H-2Kb, CD80, CD86, CD40 (PharMingen) or H-2Kb-OVA257-264 antibodies (25.D1.16; a gift from John Yewdell, NIH, USA) and analyzed by flow cytometry. For cross-priming assays, DCs were incubated with OVA, 15 ng/mL GM-CSF (Sigma) and 10 ng/mL TNF-α or IFN-γ (R&D Systems). Activation of B3Z T cells (gift from Nilabh Shastri, Berkeley, USA) was assessed as described8.
For in vivo studies, Cd74+/+ and Cd74−/− BMDCs were incubated with 10 mg/ml OVA or OVA257-264 peptide for 2 hours and injected (1 × 107 cells) intravenously into Rag1−/− BALB/c mice. After 24 hours, OT-I T cells were labeled with 2.5 μM CFSE (Molecular Probes) and injected intravenously (5×106 cells). Proliferation of OT-I T cells in the spleen was assessed 3 days later by CFSE dilution using flow cytometry. To confirm localization to spleen, CFSE-labeled DCs were injected intravenously into Rag1−/− BALB/c mice. After 2 hours, the presence of CFSE-positive cells in the spleen was assessed using flow cytometry.
Confocal microscopy
Spleen-derived DCs were isolated, fixed and permeabilized as described8. For analysis of cross-presentation, DCs were incubated with 5 mg/mL OVA for 10 hours with or without 10 ng/mL TNFα. As indicated, DCs were treated with 50 μM chloroquine for 72 hours prior to processing34. Cells were stained with antibodies against H-2Kb (BD Biosciences), CD74 (Fitzgerald), LAMP-1 (Santa Cruz Biotechnology) or H-2Kb-OVA257-264. Rabbit anti-mouse or goat Alexa-488 or 568, or goat anti-mouse Alexa-488 (Molecular Probes) were used as secondary antibodies. Images were acquired using a Nikon-C1, TE2000-U ICM and EZ-C1 software. Data were analyzed using ImageJ.1, Openlab and Adobe Photoshop. The relative fluorescence intensity of individual colors is expressed as percent of total fluorescence intensity.
Proliferation assay
C3H-derived BMDCs (H-2Kk) were incubated for 15 hours with 10 mg/ml OVA and injected (5 × 106 cells) intraperitoneally. OT-I T cells were labeled and intravenously injected as above. Proliferation of OT-I T cells was assessed by CFSE dilution 3 days later using flow cytometry.
Transfection
Immature BMDCs were transfected with full-length murine CD74 (p31 isoform; FL) or CD74 lacking amino acids 2–17 (Δ2–17) in the pBabe vector (a kind gift from Idit Shachar of the Weizmann Institute of Sciences, Israel) using the Amaxa Mouse Dendritic Cell Nucleofector Kit. One day post-electroporation, DCs were incubated with 20mg/ml OVA or 1 μM OVA257- 264 peptide for 8 hours then with OT-I CFSE labeled CD8+ T cells for 3 days. CFSE dilution was assessed by flow cytometry.
Immunoprecipitation
BMDCs were starved in methionine and cysteine-free media for 1 hour, pulsed with 300 uCi/mL [35S]methionine for 30 minutes then lysed in 0.5% Nonidet P-40 buffer (120 mM NaCl, 4 mM MgCl2, 20mM Tris-HCl pH 7.6) containing protease inhibitor cocktail (Roche) and 40 μg/mL PMSF. Where indicated, DCs were incubated with 100μM chloroquine overnight before lysis. Cell lysate were precleared overnight with normal rabbit serum and Protein A-sepharose (Pharmacia). Immunoprecipitation was performed using the H-2Kb (AF6.88.5, BD Pharmingen) antibody recognizing fully-folded MHC class I, exon-VIII antibody (a kind gift of Professors David Williams and Brian Barber, University of Toronto, Canada) that recognizes all MHC class I, I-A/E antibody (M5/114.15.2, Becton Dickinson CA), CD74 antibody (ln-1, Fitzgerald) or transferrin receptor antibody (Invitrogen). Samples were analyzed on a 10–12% SDS polyacrylamide gel electrophoresis (PAGE). The gels were fixed, enhanced with Amplify (Amersham Biosciences), dried and exposed to Kodak XMR autoradiographic film. Alternatively, samples were transferred to a nitrocellulose membrane and immunoblotted with a CD74 or MHC class I antibody (KH95; SantaCruz Biotechnology). Endoglycosidase Hf digestions were performed per manufacturer’s protocol (New England Biolabs). Whole cell lysate was blotted as a positive control. Donkey anti-mouse IgG (Li-Cor Biosciences) or goat anti-rat IgG (Invitrogen) were used as a secondary antibody. Blots were visualized using the Odyssey Infrared Imaging.
MHC class I internalization
BMDCs were stained with Fc block (BD PharMingen) then labeled with biotinylated H-2Kb (AF6-88.5) antibody for 30 minutes at 0°C. Samples were placed at 37°C or 0°C. At indicated times, DCs were fixed in 2% paraformaldehyde, labeled with streptavidin-PE then examined by flow cytometry. Data were analyzed using FlowJo software to calculate the amount of internalized MHC class I.
Statistical analysis
Student’s t-test was used to compare the difference between populations. The difference was considered statistically significant if p < 0.05 (two-tailed).
Supplementary Material
Acknowledgments
We would like to thank Dr. N. Shastri of the University of California, Berkeley for providing the B3Z T-cell Hybridoma cell line, Drs. R. Germain and J. Yewdell of the National Institutes of Health, Bethesda, MD for providing the 25.D1.16 antibody, Professor I. Shachar of the Weizmann Institute of Sciences for the kind and timely gift of the CD74 constructs and Professors B. Barber and D. Williams of the University of Toronto for providing the antisera directed against the protein region encoded by exon-8 of H-2Kb. These studies were supported by two grants to WAJ from the Canadian Institutes of Health Research (CIHR; MOP-77631 and MOP-86739), and a graduate fellowship to KO from Canadian Institutes of Health Research.
Footnotes
This work is dedicated to the memory of Dr. Ralph Steinman.
CONTRIBUTIONS
GB and KO designed, performed and analyzed experiments. ACS performed DC transfection experiments. ATR performed experiments and provided intellectual input. NL provided intellectual input. KBC performed experiments. WAJ conceptualized the research project, designed experiments, supervised the research and analyzed the data. GB, KO and WAJ wrote the manuscript.
References
- 1.Guagliardi LE, et al. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990;343:133–139. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- 2.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 3.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci U S A. 2003;100:12889–12894. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guermonprez P, et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 5.Houde M, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 6.Pfeifer JD, et al. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 7.Song R, Harding CV. Roles of proteasomes, transporter for antigen presentation (TAP), and beta 2-microglobulin in the processing of bacterial or particulate antigens via an alternate class I MHC processing pathway. J Immunol. 1996;156:4182–4190. [PubMed] [Google Scholar]
- 8.Lizee G, et al. Control of dendritic cell cross-presentation by the major histocompatibibilty complex class I cytoplasmic domain. Nature Immunology. 2003;4:1065–1073. doi: 10.1038/ni989. [DOI] [PubMed] [Google Scholar]
- 9.Gagnon E, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–131. doi: 10.1016/s0092-8674(02)00797-3. S0092867402007973 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Touret N, et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell. 2005;123:157–170. doi: 10.1016/j.cell.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–165. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Cebrian I, et al. Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell. 2011;147:1355–1368. doi: 10.1016/j.cell.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Basha G, et al. MHC class I endosomal and lysosomal trafficking coincides with exogenous antigen loading in dendritic cells. PLoS One. 2008;3:e3247. doi: 10.1371/journal.pone.0003247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu I, Davis DM, Strominger JL. Trafficking of spontaneously endocytosed MHC proteins. Proc Natl Acad Sci U S A. 1999;96:13944–13949. doi: 10.1073/pnas.96.24.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reid PA, Watts C. Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature. 1990;346:655–657. doi: 10.1038/346655a0. [DOI] [PubMed] [Google Scholar]
- 16.Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 17.Sugita M, Brenner MB. Association of the invariant chain with major histocompatibility complex class I molecules directs trafficking to endocytic compartments. J Biol Chem. 1995;270:1443–1448. doi: 10.1074/jbc.270.3.1443. [DOI] [PubMed] [Google Scholar]
- 18.Vigna JL, Smith KD, Lutz CT. Invariant chain association with MHC class I: preference for HLA class I/beta 2-microglobulin heterodimers, specificity, and influence of the MHC peptide-binding groove. J Immunol. 1996;157:4503–4510. [PubMed] [Google Scholar]
- 19.Kleijmeer MJ, et al. Antigen loading of MHC class I molecules in the endocytic tract. Traffic. 2001;2:124–137. doi: 10.1034/j.1600-0854.2001.020207.x. [DOI] [PubMed] [Google Scholar]
- 20.Zwickey HL, Potter TA. Antigen secreted from noncytosolic Listeria monocytogenes is processed by the classical MHC class I processing pathway. J Immunol. 1999;162:6341–6350. [PubMed] [Google Scholar]
- 21.MacAry PA, et al. Mobilization of MHC class I molecules from late endosomes to the cell surface following activation of CD34-derived human Langerhans cells. Proc Natl Acad Sci U S A. 2001;98:3982–3987. doi: 10.1073/pnas.071477498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tourne S, et al. Biosynthesis of major histocompatibility complex molecules and generation of T cells in Ii TAP1 double-mutant mice. Proc Natl Acad Sci U S A. 1996;93:1464–1469. doi: 10.1073/pnas.93.4.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reber AJ, Turnquist HR, Thomas HJ, Lutz CT, Solheim JC. Expression of invariant chain can cause an allele-dependent increase in the surface expression of MHC class I molecules. Immunogenetics. 2002;54:74–81. doi: 10.1007/s00251-002-0446-8. [DOI] [PubMed] [Google Scholar]
- 24.Vitalis TZ, et al. Using the TAP component of the antigen-processing machinery as a molecular adjuvant. PLoS Pathog. 2005;1:e36. doi: 10.1371/journal.ppat.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8 + T cells. Cell. 1992;71:1205. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 26.McAdam AJ, Farkash EA, Gewurz BE, Sharpe AH. B7 costimulation is critical for antibody class switching and CD8(+) cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J Virol. 2000;74:203–208. doi: 10.1128/jvi.74.1.203-208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marzo AL, et al. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol. 2004;173:969–975. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- 28.Faure-Andre G, et al. Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science. 2008;322:1705–1710. doi: 10.1126/science.1159894. 322/5908/1705 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Benvenuti F, et al. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. doi: 10.1126/science.1099159305/5687/1150. [pii] [DOI] [PubMed] [Google Scholar]
- 30.Shastri N, Gonzalez F. Endogenous generation and presentation of the ovalbumin peptide/Kb complex to T cells. J Immunol. 1993;150:2724–2736. [PubMed] [Google Scholar]
- 31.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–1599. [PMC free article] [PubMed] [Google Scholar]
- 33.Merzougui N, Kratzer R, Saveanu L, van Endert P. A proteasome-dependent, TAP-independent pathway for cross-presentation of phagocytosed antigen. EMBO reports. 2011 doi: 10.1038/embor.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loss GE, Jr, Sant AJ. Invariant chain retains MHC class II molecules in the endocytic pathway. Journal of immunology. 1993;150:3187–3197. [PubMed] [Google Scholar]
- 35.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 36.Rock KL, Gamble S, Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 1990;249:918–921. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- 37.van Lith M, van Ham M, Neefjes J. Stable expression of MHC class I heavy chain/HLA-DO complexes at the plasma membrane. Eur J Immunol. 2003;33:1145–1151. doi: 10.1002/eji.200323472. [DOI] [PubMed] [Google Scholar]
- 38.Nuchtern JG, Biddison WE, Klausner RD. Class II MHC molecules can use the endogenous pathway of antigen presentation. Nature. 1990;343:74–76. doi: 10.1038/343074a0. [DOI] [PubMed] [Google Scholar]
- 39.Cerundolo V, Elliott T, Elvin J, Bastin J, Townsend A. Association of the human invariant chain with H-2 Db class I molecules. Eur J Immunol. 1992;22:2243–2248. doi: 10.1002/eji.1830220910. [DOI] [PubMed] [Google Scholar]
- 40.Powis SJ. CLIP-region mediated interaction of Invariant chain with MHC class I molecules. FEBS Lett. 2006;580:3112–3116. doi: 10.1016/j.febslet.2006.04.060. S0014-5793(06)00502-3 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Sigal LJ, Rock KL. Bone marrow-derived antigen-presenting cells are required for the generation of cytotoxic T lymphocyte responses to viruses and use transporter associated with antigen presentation (TAP)-dependent and -independent pathways of antigen presentation. J Exp Med. 2000;192:1143–1150. doi: 10.1084/jem.192.8.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buller RM, Holmes KL, Hugin A, Frederickson TN, Morse HC., 3rd Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987;328:77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- 43.Janssen EM, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. nature01441 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Machold RP, Ploegh HL. Intermediates in the assembly and degradation of class I major histocompatibility complex (MHC) molecules probed with free heavy chain-specific monoclonal antibodies. J Exp Med. 1996;184:2251–2259. doi: 10.1084/jem.184.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reinicke AT, Omilusik KD, Basha G, Jefferies WA. Dendritic cell cross-priming is essential for immune responses to Listeria monocytogenes. PLoS One. 2009;4:e7210. doi: 10.1371/journal.pone.0007210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luckey CJ, et al. Differences in the expression of human class I MHC alleles and their associated peptides in the presence of proteasome inhibitors. J Immunol. 2001;167:1212–1221. doi: 10.4049/jimmunol.167.3.1212. [DOI] [PubMed] [Google Scholar]
- 47.Kruger T, et al. Lessons to be learned from primary renal cell carcinomas: novel tumor antigens and HLA ligands for immunotherapy. Cancer Immunol Immunother. 2005;54:826–836. doi: 10.1007/s00262-004-0650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Busch R, Cloutier I, Sekaly RP, Hammerling GJ. Invariant chain protects class II histocompatibility antigens from binding intact polypeptides in the endoplasmic reticulum. EMBO J. 1996;15:418–428. [PMC free article] [PubMed] [Google Scholar]
- 49.Savina A, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. S0092-8674(06)00761-6 [pii] [DOI] [PubMed] [Google Scholar]
- 50.Rashid A, Auchincloss H, Jr, Sharon J. Comparison of GK1.5 and chimeric rat/mouse GK1.5 anti-CD4 antibodies for prolongation of skin allograft survival and suppression of alloantibody production in mice. J Immunol. 1992;148:1382–1388. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.