Abstract
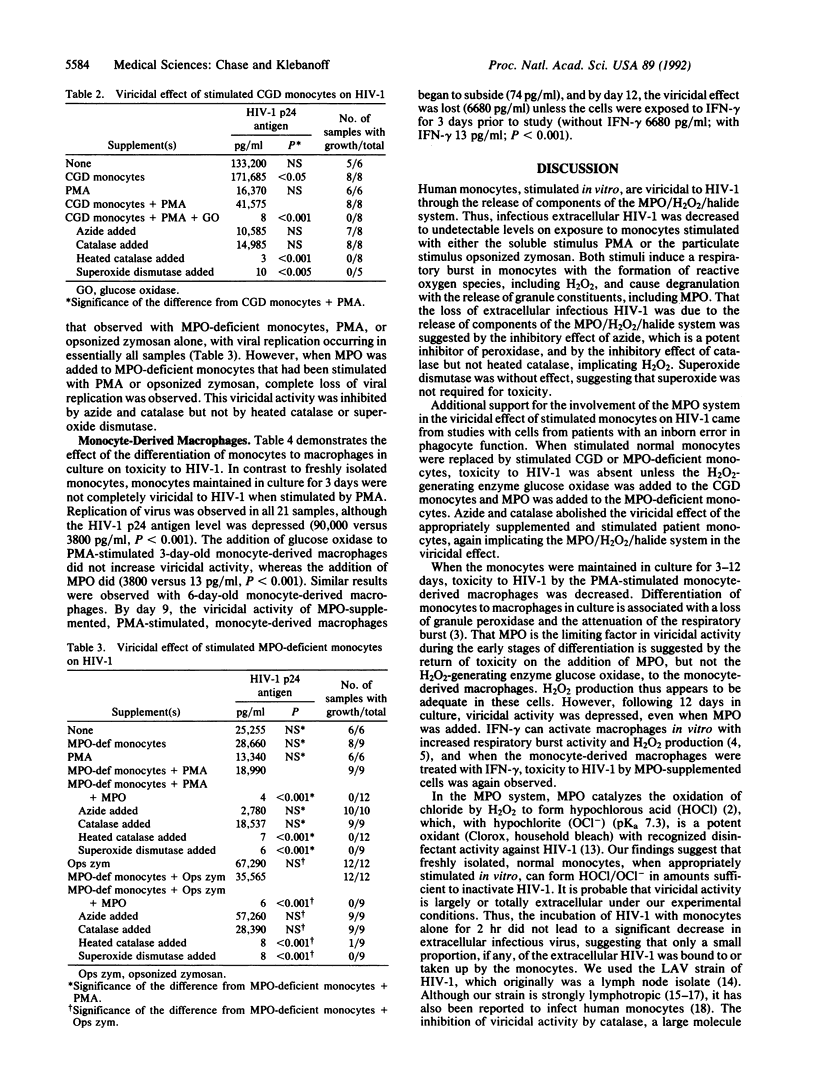
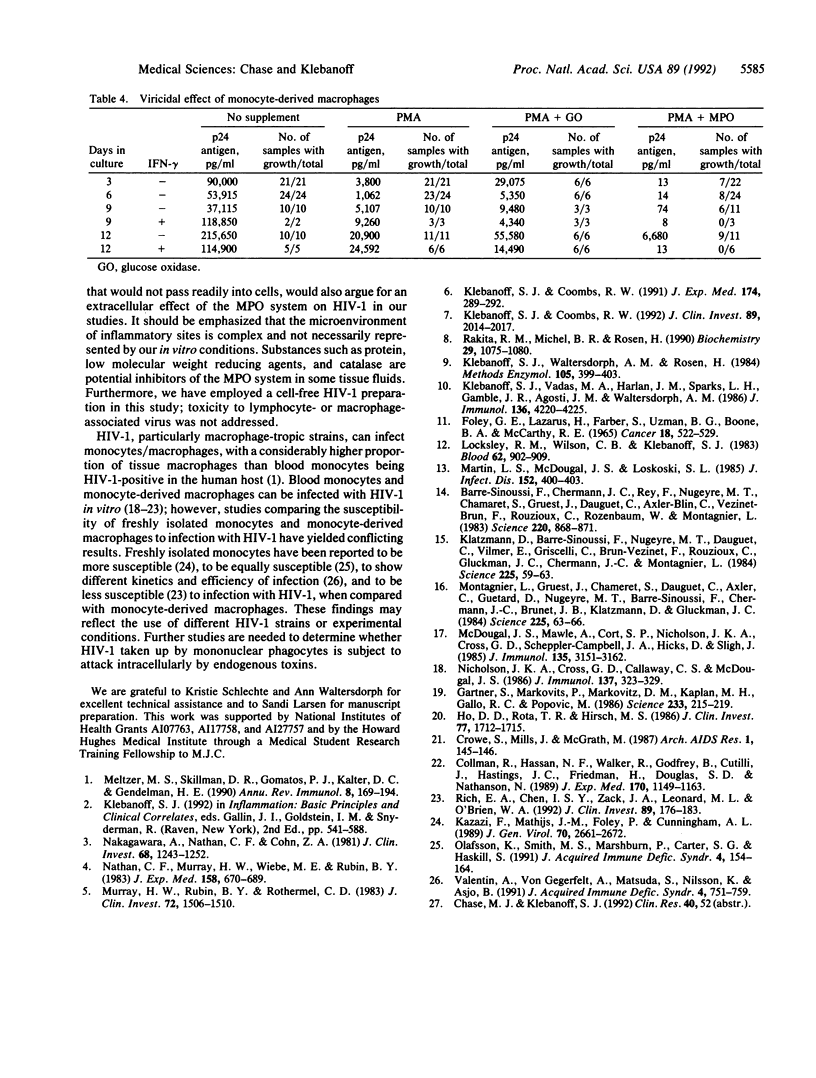
Human monocytes stimulated with phorbol 12-myristate 13-acetate or opsonized zymosan in vitro were viricidal to human immunodeficiency virus type 1 (HIV-1) as measured by the inability of the virus to replicate in CEM cells. Monocytes, when stimulated, release myeloperoxidase (MPO) and produce H2O2; MPO reacts with H2O2 and chloride to form hypochlorous acid, a known microbicidal agent. The viricidal activity of stimulated monocytes was inhibited by the peroxidase inhibitor azide, implicating MPO, and by catalase but not heated catalase or superoxide dismutase, implicating H2O2. Stimulated monocytes from patients with chronic granulomatous disease (CGD) or hereditary MPO deficiency were not viricidal to HIV-1 unless they were supplemented with the H2O2-generating enzyme glucose oxidase or MPO, respectively. The viricidal activity of stimulated, glucose oxidase-supplemented CGD monocytes and MPO-supplemented MPO-deficient monocytes, like that of normal stimulated monocytes, was inhibited by azide and catalase. Monocytesmaintained in culture differentiate into macrophages with loss of MPO and decreased H2O2 production. The viricidal activity of 3- to 9-day monocyte-derived macrophages was decreased unless MPO was added, whereas the loss of viricidal activity by 12-day-old monocyte-derived macrophages was not reversed by MPO unless the cells were pretreated with gamma-interferon. These findings suggest that stimulated monocytes can be viricidal to HIV-1 through the release of the MPO/H2O2/chloride system and that the decreased viricidal activity on differentiation to macrophages results initially from the loss of MPO and, with more prolonged culture, also from a decreased respiratory burst that can be overcome by gamma-interferon.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Collman R., Hassan N. F., Walker R., Godfrey B., Cutilli J., Hastings J. C., Friedman H., Douglas S. D., Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989 Oct 1;170(4):1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe S., Mills J., McGrath M. S. Quantitative immunocytofluorographic analysis of CD4 surface antigen expression and HIV infection of human peripheral blood monocyte/macrophages. AIDS Res Hum Retroviruses. 1987 Summer;3(2):135–145. doi: 10.1089/aid.1987.3.135. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazi F., Mathijs J. M., Foley P., Cunningham A. L. Variations in CD4 expression by human monocytes and macrophages and their relationships to infection with the human immunodeficiency virus. J Gen Virol. 1989 Oct;70(Pt 10):2661–2672. doi: 10.1099/0022-1317-70-10-2661. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Coombs R. W. Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J Exp Med. 1991 Jul 1;174(1):289–292. doi: 10.1084/jem.174.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Coombs R. W. Viricidal effect of polymorphonuclear leukocytes on human immunodeficiency virus-1. Role of the myeloperoxidase system. J Clin Invest. 1992 Jun;89(6):2014–2017. doi: 10.1172/JCI115810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Klebanoff S. J., Waltersdorph A. M., Rosen H. Antimicrobial activity of myeloperoxidase. Methods Enzymol. 1984;105:399–403. doi: 10.1016/s0076-6879(84)05055-2. [DOI] [PubMed] [Google Scholar]
- Locksley R. M., Wilson C. B., Klebanoff S. J. Increased respiratory burst in myeloperoxidase-deficient monocytes. Blood. 1983 Oct;62(4):902–909. [PubMed] [Google Scholar]
- Martin L. S., McDougal J. S., Loskoski S. L. Disinfection and inactivation of the human T lymphotropic virus type III/Lymphadenopathy-associated virus. J Infect Dis. 1985 Aug;152(2):400–403. doi: 10.1093/infdis/152.2.400. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Meltzer M. S., Skillman D. R., Gomatos P. J., Kalter D. C., Gendelman H. E. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- Montagnier L., Gruest J., Chamaret S., Dauguet C., Axler C., Guétard D., Nugeyre M. T., Barré-Sinoussi F., Chermann J. C., Brunet J. B. Adaptation of lymphadenopathy associated virus (LAV) to replication in EBV-transformed B lymphoblastoid cell lines. Science. 1984 Jul 6;225(4657):63–66. doi: 10.1126/science.6328661. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K., Cross G. D., Callaway C. S., McDougal J. S. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J Immunol. 1986 Jul 1;137(1):323–329. [PubMed] [Google Scholar]
- Olafsson K., Smith M. S., Marshburn P., Carter S. G., Haskill S. Variation of HIV infectibility of macrophages as a function of donor, stage of differentiation, and site of origin. J Acquir Immune Defic Syndr. 1991;4(2):154–164. [PubMed] [Google Scholar]
- Rakita R. M., Michel B. R., Rosen H. Differential inactivation of Escherichia coli membrane dehydrogenases by a myeloperoxidase-mediated antimicrobial system. Biochemistry. 1990 Jan 30;29(4):1075–1080. doi: 10.1021/bi00456a033. [DOI] [PubMed] [Google Scholar]
- Rich E. A., Chen I. S., Zack J. A., Leonard M. L., O'Brien W. A. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J Clin Invest. 1992 Jan;89(1):176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin A., Von Gegerfelt A., Matsuda S., Nilsson K., Asjö B. In vitro maturation of mononuclear phagocytes and susceptibility to HIV-1 infection. J Acquir Immune Defic Syndr. 1991;4(8):751–759. [PubMed] [Google Scholar]



