Abstract
Background:
The beneficial effects of endovascular treatment with new-generation mechanical thrombectomy devices compared with intravenous thrombolysis alone to treat acute large-artery ischemic stroke have been shown in randomized controlled trials (RCTs). This study aimed to estimate the cost utility of mechanical thrombectomy compared with the established standard of care.
Methods:
We developed a Markov decision process analytic model to assess the cost-effectiveness of treatment with mechanical thrombectomy plus intravenous thrombolysis versus treatment with intravenous thrombolysis alone from the public payer perspective in Canada. We conducted comprehensive literature searches to populate model inputs. We estimated the efficacy of mechanical thrombectomy plus intravenous thrombolysis from a meta-analysis of 5 RCTs, and we used data from the Oxford Vascular Study to model long-term clinical outcomes. We calculated incremental cost-effectiveness ratios (ICER) using a 5-year time horizon.
Results:
The base case analysis showed the cost and effectiveness of treatment with mechanical thrombectomy plus intravenous thrombolysis to be $126 939 and 1.484 quality-adjusted life-years (QALYs), respectively, and the cost and effectiveness of treatment with intravenous thrombolysis alone to be $124 419 and 1.273 QALYs, respectively. The mechanical thrombectomy plus intravenous thrombolysis strategy was associated with an ICER of $11 990 per QALY gained. Probabilistic sensitivity analysis showed that the probability of treatment with mechanical thrombectomy plus intravenous thrombolysis being cost-effective was 57.5%, 89.7% and 99.6% at thresholds of $20 000, $50 000 and $100 000 per QALY gained, respectively. The main factors influencing the ICER were time horizon, extra cost of mechanical thrombectomy treatment and age of the patient.
Interpretation:
Mechanical thrombectomy as an adjunct therapy to intravenous thrombolysis is cost-effective compared with treatment with intravenous thrombolysis alone for patients with acute large-artery ischemic stroke.
A cute ischemic stroke is caused by occlusion of a cerebral artery.1 This condition carries a high burden of disability and death, and an economic burden due to hospitalization, long-term disability and productivity loss. In Canada, 62 000 strokes occur per year, and there are more than 400 000 stroke survivors who are living with long-term stroke disability.2,3 About 87% of strokes are ischemic and 20% of those are caused by large-vessel occlusion in the internal carotid artery and middle cerebral artery.4 Therefore, about 8700 people per year may be eligible for endovascular treatment in Canada.
Hyperacute treatment of acute ischemic stroke includes time-sensitive therapies designed to open the occluded blood vessels to re-establish blood flow. This can be accomplished through either intravenous thrombolysis and/or endovascular treatment via mechanical thrombectomy with retrievable stents and thrombus aspiration.5 Before 2015, intravenous thrombolysis was the standard of care for treating acute ischemic stroke. However, intravenous thrombolysis has several limitations, including a narrow therapeutic time window, applicability to only a subset of stroke patients and relative ineffectiveness for stroke resulting from proximal large-artery occlusion or large-clot burden.6
Efforts to improve recanalization rates in patients with a large-artery occlusion were explored with intra-arterial therapy and endovascular treatment with first-generation mechanical thrombectomy devices. Although these therapies failed to show clinical benefit in randomized controlled trials (RCTs),7-9 trials using new-generation mechanical thrombectomy devices (i.e., stent retriever and thromboaspiration) have shown more promising results. A recent systematic review and meta-analysis based on 5 RCTs10-14 of new-generation mechanical thrombectomy reported a clinically significant increase in functional independence for patients who were treated with mechanical thrombectomy employing retrievable stents or thromboaspiration devices (with or without intravenous thrombolysis) compared with those treated with intravenous thrombolysis and/or best medical treatment.15 These trials were characterized by a triad of very fast treatment, advanced imaging-based patient selection and high rates of early brain reperfusion. This therapy has now been recommended as the new standard of care for acute ischemic stroke due to large-artery occlusion.16 We conducted a cost-utility analysis from the public payer perspective to determine the health economic impact of mechanical thrombectomy for treatment of patients with large-artery acute ischemic stroke in Canada. The cost-utility analysis was requested by Health Quality Ontario to inform its recommendations regarding public funding of mechanical thrombectomy. Health Quality Ontario is a provincial agency with a broad mandate that includes making recommendations about the Government of Ontario's funding of health care services.
Methods
Overview
Based on the efficacy determined by 5 RCTs10-14 that examined endovascular treatment with new-generation mechanical thrombectomy devices for patients with acute ischemic stroke in a large artery, we developed a decision analytic model to address the cost-effectiveness of this type of treatment. We modelled treatment with mechanical thrombectomy plus intravenous thrombolysis versus intravenous thrombolysis alone as the expected treatments. The mean age of patients ranged from 65 to 71 years of age, and there was an equal proportion of men and women.10-14 Patients had occlusion of either an internal carotid artery or middle cerebral artery, and eligibility for mechanical thrombectomy was confirmed by imaging and established clinical criteria.14 Patients were functioning independently in the community before the stroke. We based clinical outcomes for the first 90 days on evidence from a systematic review and meta-analysis.15 Long-term outcomes (after 3 mo) were based on data from the Oxford Vascular Study involving a large cohort of patients with stroke in the United Kingdom.17,18 We conducted a comprehensive literature search to obtain the most appropriate inputs of health utility and cost for the cost-utility analysis.
The Markov decision process analytic model
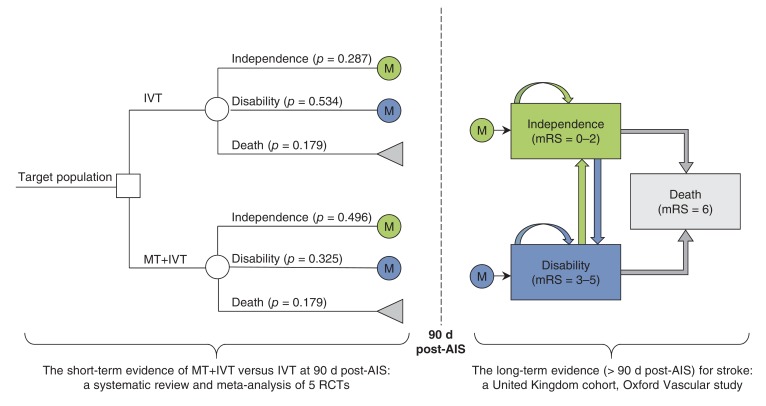
We developed a Markov decision process analytic model to assess the long-term clinical and economic outcomes of treatment with mechanical thrombectomy plus intravenous thrombolysis versus treatment with intravenous thrombolysis alone (Figure 1). The model combined a decision tree for the first 90 days after acute ischemic stroke and the Markov model for those at risk after 3 months. The Markov model consisted of 3 mutually exclusive health states, functional independence (modified Rankin Scale [measurement of degree of disability] = 0-2), disability (modified Rankin Scale = 3-5) and death (modified Rankin Scale = 6). Target patients received treatment with mechanical thrombectomy plus intravenous thrombolysis or intravenous thrombolysis alone, and they would be either functionally independent, disabled or dead at 90 days after acute ischemic stroke. Survivors at 90 days would join the corresponding health state in the Markov model. Patients could transfer between health states or stay in the same health states at the end of each monthly cycle, with assigned probabilities. In the model, patients could recover from disability to functional independence during the first year after a stroke but not after the first year.
Figure 1.
Decision analytic model of mechanical thrombectomy plus intravenous thrombolysis versus intravenous thrombolysis alone for acute ischemic stroke. AIS = acute ischemic stroke, IVT = intravenous thrombolysis, M = Markov model, mRS = modified Rankin Scale, MT = mechanical thrombectomy, RCT = randomized controlled trial.
Principal assumptions
The following assumptions were made for the base case analysis:
Compared with treatment with intravenous thrombolysis alone, mechanical thrombectomy plus intravenous thrombolysis treatment can reduce the risk of disability at 90 days but not mortality,15 because only 1 of the 5 RCTs showed a reduction in mortality.14
Patients' long-term health outcomes (i.e., more than 3 mo after a major stroke) would be conditional on their health status at 90 days (i.e., functional independence or disability).
Disability was associated with increased risk of mortality and reduced health-related quality of life.19
The two treatments were associated with a similar risk of symptomatic intracerebral hemorrhage within 90 days after stroke.15 We ignored it in the model because it would not impact the incremental cost-effectiveness ratio.
Model input parameters
Data were obtained from the best available evidence (Table 1 and Table 2). When necessary, we contacted authors to clarify questions we had regarding their publications. When we could not obtain the desired estimates, we adapted available data after discussion with clinical experts. We also consulted experts to validate our parameter estimates.
Table 1: Model inputs for base case. Table 1: Model inputs for base case.
| Description | Mean (95% CI) | Distribution (parameter 1, parameter 2) for PSA | Reference |
|---|---|---|---|
| Efficacy of treatment at 90 d post-AIS | |||
| IVT alone strategy, % | |||
| All-cause mortality | 17.86 (13.89-21.82) | β (64.03, 294.50) | 10-14 |
| Functional independence | 28.74 (21.80-35.67) | β (46.68, 115.75) | 10-14 |
| MT plus IVT strategy | |||
| All-cause mortality, % | 17.86 (13.89-21.82) | β (64.03, 294.50) | 10-14 |
| Odds ratio of functional independence, MT plus IVT v. IVT alone* | 2.39 (1.88-3.04) | Log-normal (0.8713, 0.1226) | 10-14 |
| Difference in health utility relative to IVT | 0.0735 (0.0137-0.1333) | Normal (0.0735, 0.0305) | 10,12 |
| Calibrated monthly transition probabilities of natural history for > 3 mo after stroke | Best fitting parameter set | 1000 convergent parameter sets | |
| From functional independence to disability, mo | |||
| 4-6 | 0.0321 | - | 17,18,20,21 |
| 7-12 | 0.0220 | - | 17,18,20,21 |
| 13-24 | 0.0134 | - | 17,18,20,21 |
| 25-36 | 0.0111 | - | 17,18,20,21 |
| 37-48 | 0.0093 | - | 17,18,20,21 |
| 49-60 | 0.0077 | - | 17,18,20,21 |
| From disability to functional independence, mo | |||
| 4-6 | 0.0372 | - | 20 |
| 7-12 | 0.0156 | - | 20 |
| 13-60 | 0 | - | Assumption** |
| From functional independence to death, mo | |||
| 4-12 | 0.0080 | - | 17,18,20,21 |
| 13-24 | 0.0034 | - | 17,18,20,21 |
| 25-36 | 0.0039 | - | 17,18,20,21 |
| 37-48 | 0.0043 | - | 17,18,20,21 |
| 49-60 | 0.0047 | - | 17,18,20,21 |
| From disability to death, mo | |||
| 4-12 | 0.0229 | - | 17,18,20,21 |
| 13-24 | 0.0096 | - | 17,18,20,21 |
| 25-36 | 0.0108 | - | 17,18,20,21 |
| 37-48 | 0.0122 | - | 17,18,20,21 |
| 49-60 | 0.0131 | - | 17,18,20,21 |
| Health care costs, $CAD† | |||
| First 3 mo after stroke | |||
| Extra cost of MT treatment | 15 000 | γ (25, 600) | 22-28 |
| Functional independence (mRS of 0-2) | 18 852 | γ (25, 754.08) | 29 |
| Disability (mRS of 3-6) | 57 382 | γ (25, 2295.28) | 29 |
| IVT alone, weighted by health status‡ | 46 308 | - | 22-29 |
| MT + IVT, weighted by health status§ | 53 471 | - | 22-29 |
| Greater than 3 mo after stroke | |||
| Functional independence (mRS of 0-2) | 1 384 per month | γ (25, 55.36) | 29 |
| Disability (mRS of 3-5) | 3 080 per month | γ (25, 123.2) | 29 |
| Health utility for > 3 mo after stroke | |||
| Functional independence (mRS of 0-2) | 0.71 (0.68-0.74) | β (623.29, 254.58) | 30 |
| Disability (mRS of 3-5) | 0.31 (0.29-0.34) | β (407.26, 906.49) | 30 |
Note: AIS = acute ischemic stroke, $CAD = Canadian dollars in 2015, CI = confidence interval, IVT = intravenous thrombolysis, mRS = modified Rankin Scale, MT = mechanical thrombectomy, PSA = probabilistic sensitivity analysis.
*Given the odds ratio of 2.39 and pooled proportion of functional independence of 0.2874, we estimated that the proportion of functional independence was 0.4908.
†Economic Burden of Ischemic Stroke study29 did not report the 95% CI or standard error (SE) of their cost estimates. We assumed the SE was equal to 20% of the mean in the probabilistic sensitivity analysis.
‡$18 852 × 0.2874 + $57 382 × 0.7126 = $46 308.
§$15 000 + $18 852 × 0.4908 + $57 382 × 0.5092 = $53 471.
¶Unless stated otherwise. **Evidence suggests that the chance of patients recovering from disability to functional independence after 1 yr after acute stroke is small.20
Table 2: Model inputs for scenario and sensitivity analyses.
| Description | Mean | Reference |
|---|---|---|
| Inputs from ESCAPE trial, efficacy of treatment at 90 d post AIS | ||
| IVT alone strategy, % | ||
| All-cause mortality | 19.0 | 14 |
| Functional independence | 29.3 | 14 |
| Cost in first 3 mo, weighted by health status; $CAD | 46 093 | 14,29 |
| MT + IVT strategy, % | ||
| All-cause mortality | 10.4 | 14 |
| Functional independence | 53.0 | 14 |
| Cost in first 3 mo, weighted by health status; $CAD | 51 961 | 14,29 |
| Inputs from Broderick and colleagues, severe stroke (National Institutes of Health Stroke Scale score ≥ 20) | 31 | |
| IVT alone strategy, % | ||
| All-cause mortality | 34 | 31 |
| Functional independence | 14 | 31 |
| Cost in first 3 mo, weighted by health status; $CAD | 51 988 | 29,31 |
| MT + IVT strategy | ||
| All-cause mortality, % | 34 | Assumption* |
| Odds ratio of functional independence, MT + IVT v. IVT alone | 1.97 | 31 |
| Cost in first 3 mo, weighted by health status; $CAD | 63 026 | 29,31 |
| Odds ratio of functional independence in subgroup patients, MT + IVT v. IVT; yr | ||
| ≤ 70 | 3.02 | 10-14 |
| > 70 | 1.79 | 10-14 |
| Including cost for cost of end-of-life care | ||
| Cost of end-of-life care for death after 90 d poststroke; $CAD | 50 892 | 32 |
Note: AIS = acute ischemic stroke, $CAD = Canadian dollars in 2015, IVT = intravenous thrombolysis, mRS = modified Rankin Scale, MT = mechanical thrombectomy.
*This study31 did not find a significant difference in mortality between groups (28.8% mRS 6 in the endovascular treatment group v. 34% in the IT group). Therefore, we assumed no survival benefit for MT in this scenario analysis.
Intervention summary estimates (the first 90 d)
We conducted a meta-analysis to estimate the proportion of functional independence and mortality in the intravenous thrombolysis only arm at 90 days, and estimated these parameters for the mechanical thrombectomy plus intravenous thrombolysis arm at the given odds ratio (OR) from meta-analysis.15 The pooled estimate of the adjusted β coefficient in the linear regression in 2 of the 5 RCTs10,12 showed that treatment with mechanical thrombectomy plus intravenous thrombolysis increased EuroQol five dimensions questionnaire (EQ-5D) utility by 0.074 (95% confidence interval [CI] 0.014-0.133) at 90 days compared with treatment with intravenous thrombolysis alone. We assumed that the two arms had the same utility at base, but the difference in utility linearly increased over time reaching 0.074 at 90 days after stroke. As a result, the mechanical thrombectomy arm would lead to a quality-adjusted life years (QALY) gain of 0.008 in the first 90 days, i.e., {[(0 + utility increase at 90 days)/2] × 0.25} × (1 - probability of death in 90 days).
Natural history (3 mo after stroke)
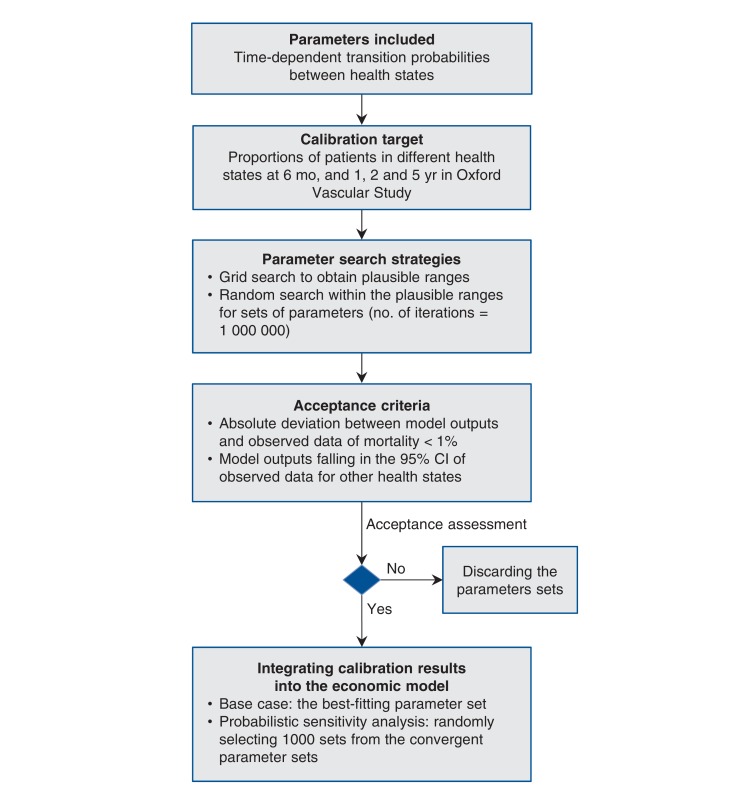
The evidence for long-term outcomes for acute ischemic stroke is sparse in Canada. For our model inputs, we used evidence from the Oxford Vascular Study, involving a large cohort study in the UK.17,18 We calibrated the parameters for the Markov model using a 7-step approach introduced by Vanni and colleagues.33 We summarize the calibration process in Figure 2 (more details in Appendix 1, available at www.cmajopen.ca/content/4/2/E316/suppl/DC1). We defined the parameters for estimating time-dependent transition probabilities and selected the proportions of mortality, functional independence and disability at 6 months and 1, 2 and 5 years in the moderate stroke group of the Oxford Vascular Study as the calibration targets.17,18 We used a grid search to obtain plausible ranges for each parameter and then simulated 1 000 000 parameter sets by sampling values from the plausible ranges. We assessed the goodness of fit (i.e., absolute deviation and sum of squared errors) for the model output produced by each parameter set. The best fitting parameter set (i.e., minimal sum of squared errors) was used as the base case, and 1000 parameter sets were randomly selected from those meeting the acceptance criteria for probabilistic sensitivity analysis.
Figure 2.
Flow chart of calibration for time-dependent transition probabilities. CI = confidence interval.
Costs
Costs for stroke were based on the Economic Burden of Ischemic Stroke study29 and are expressed in April 2015 Canadian dollars.34 The Economic Burden of Ischemic Stroke study was a prospective cohort study involving patients with ischemic stroke in 12 Canadian stroke centres. The authors stratified the costs for disability status (modified Rankin Scale = 0-2 and modified Rankin Scale = 3-5) measured at discharge. They also divided costs into direct (e.g., emergency services, hospitalization, rehabilitation, physician services, diagnostics, medications) and indirect (e.g., productivity loss and resource use for unpaid caregivers) costs. We considered direct costs from the Economic Burden of Ischemic Stroke study29 in the base case analysis. We did not include stroke recurrence as a separate event in our model, but the health care costs of recurrence were accounted for in the cost estimates.
It is difficult to make a precise estimate of the additional cost of mechanical thrombectomy plus intravenous thrombolysis intervention relative to intravenous thrombolysis; apart from the materials and staffing, mechanical thrombectomy may also impact intensive care unit time, angiography suite time, diagnostics, physician time and rehabilitation. According to the published health economic studies of mechanical thrombectomy (with or without intravenous thrombolysis) versus intravenous thrombolysis (or best medical treatment), the extra cost of mechanical thrombectomy treatment versus control in most studies ranged from $10 000 to $20 000.23-29 (Appendix 2, available at www.cmajopen.ca/content/4/2/E316/suppl/DC1). We estimated that the additional cost of mechanical thrombectomy treatment in Ontario lies somewhere in the middle at $15 000.
Health utilities
Several different factors significantly affect the health utility of stroke patients, including stroke severity, comorbidity and age.18 For simplicity, we used the EQ-5D utilities found in an article by Dorman and colleagues, which only considered the stroke severity of functional independence and disability.30
Statistical analysis
Using our Markov decision process analytic model, we compared the cost-effectiveness of the 2 treatment strategies. Our main outcome was the incremental cost-effectiveness ratio, measured as incremental cost per QALY gained. Because there are considerable uncertainties of long-term outcomes for both treatment strategies, we selected a 5-year time horizon as the base case scenario. An annual discount rate of 5% was applied to both costs and QALYs.
We conducted a scenario analysis using inputs from the Endovascular Treatment for Small Core and Proximal Occulsion Ischemic Stroke (ESCAPE) trial.14 Eleven health centres in Canada participated in this trial. This study included patients with proximal occlusions and contraindications to intravenous tissue plasminogen activator, representing 25% of the trial participants and the group of patients who may obtain the most benefit from mechanical thrombectomy treatment. We also analyzed the scenario involving stroke patients with severe neurologic deficit (National Institutes of Health Stroke Scale score, ≥ 20) based on pooled results from the Interventional Management of Stroke III and Multicenter Randomized Clinical Trial of Endovascular Therapy for Acute Ischemic Stroke (Netherlands) trials.31 We also conducted 1- and multi-way sensitivity analyses to assess factors that affect the incremental cost per QALY gained. Given no significant differences in functional independence were found among subgroups of status of intravenous thrombolysis (p = 0.72) and occlusion site (p = 0.94), the analyses for those subgroups were not conducted. Additionally, we conducted probabilistic sensitivity analysis by assigning probability distributions to model parameters (N iterations = 1000). Distributions of inputs are listed in Table 1 and Table 2.
We conducted the economic analyses and calibration using SAS version 9.4 (SAS Institute Inc.). We also used R version 3.1.2 (R Development Core Team, Vienna, Austria) for meta-analysis (metafor package in R) and simultaneous CI for multinomial proportion (MultinomialCI package in R, available at http://CRAN.R-project.org/package=MultinomialCI; and CoinMinD package in R, available at http://CRAN.R-project.org/package=CoinMinD). The model validation can be found in Appendix 3, available at www.cmajopen.ca/content/4/2/E316/suppl/DC1.
Results
Base case and scenario analyses
Based on the model proposed in Figure 1 and using the parameter estimates given in Table 1 and Table 2, the mechanical thrombectomy plus intravenous thrombolysis strategy was associated with an incremental cost-effectiveness ratio of $11 990 per QALY gained over 5 years compared with intravenous thrombolysis alone in the base case (Table 3). Compared with the base case, inputs from the ESCAPE Trial resulted in greater QALYs gained (0.348 QALYs) with higher incremental cost ($9324), corresponding to an incremental cost-effectiveness ratio of $26 815 per QALY gained. Although there is no universally accepted maximum willingness-to-pay threshold in Canada, the mechanical thrombectomy plus intravenous thrombolysis strategy is highly likely to be cost-effective if the willingness-to-pay threshold were $50 000 per QALY or higher. For patients with severe stroke, assuming no improvement in mortality, the incremental cost-effectiveness ratio was increased to $81 651 with QALY gained of 0.106 and an incremental cost of $8691.
Table 3: Main results (time horizon = 5 yr).
| Strategy | Average total costs ($CAD) | Incremental cost ($CAD) | QALY | QALYs gained | ICER* ($CAD) |
|---|---|---|---|---|---|
| Base case | |||||
| IVT | 124 419 | - | 1.273 | - | - |
| MT + IVT | 126 939 | 2 520 | 1.484 | 0.210 | 11 990 |
| Efficacy based on ESCAPE trial14 | |||||
| IVT | 122 901 | - | 1.265 | - | - |
| MT + IVT | 132 224 | 9 323 | 1.613 | 0.348 | 26 815 |
| Severe stroke | |||||
| IVT | 116 826 | - | 0.933 | - | - |
| MT + IVT | 125 517 | 8 691 | 1.039 | 0.106 | 81 651 |
Note: Numbers may appear inexact because of rounding. $CAD = Canadian dollars in 2015, ICER = incremental cost-effectiveness ratio, IVT = intravenous thrombolysis, MT = mechanical thrombectomy, QALY = quality-adjusted life-year.
*Incremental cost per QALY gained.
Deterministic sensitivity analysis
We examined several factors that could affect the incremental cost-effectiveness ratio of mechanical thrombectomy plus intravenous thrombolysis versus intravenous thrombolysis alone (Table 4). When the model inputs were varied, the mechanical thrombectomy plus intravenous thrombolysis approach remained cost-effective in most scenarios. The main factors influencing incremental cost-effectiveness ratio were time horizon, additional cost of mechanical thrombectomy and age. The incremental cost-effectiveness ratio decreased, with longer follow-up time in the first 4 years (Appendix 4, available at www.cmajopen.ca/content/4/2/E316/suppl/DC1), and was relatively stable at about $10 000 per QALY gained at a follow-up of 5 years or longer.
Table 4: One-way or 2-way sensitivity analysis results.
| Scenario | Incremental cost per QALY gained ($CAD) |
|---|---|
| Base case analysis (reference) | 11 990 |
| Time horizon, yr | |
| 1 | 91 080 |
| 3 | 20 540 |
| 10 | 11 491 |
| 15* | 12 877 |
| MT with reduced mortality risk (odds ratio of mortality, MT + IVT v. IVT alone, 0.80) | 22 891 |
| Extra cost of MT treatment, $CAD | |
| 10 000 | Dominant |
| 20 000 | 35 779 |
| Age group, yr | |
| ≤ 70 | 4 429 |
| > 70 | 29 899 |
| Health utility in functional independence and disability states | |
| Lower limit of 95% CI | 12 366 |
| Upper limit of 95% CI | 11 809 |
| Annual discount rate for both cost and utility, % | |
| 0 | 10 028 |
| 3 | 11 205 |
| 10 | 13 943 |
| Including cost for end-of-life care for those who survive at 90 d after an acute ischemic stroke | 4 212 |
Note: $CAD = Canadian dollars in 2015, CI = confidence interval, IVT = intravenous thrombolysis, MT = mechanical thrombectomy, QALY = quality-adjusted life-year.
*About 5.4% and 6.9% of patients were alive in the IVT alone and IVT + MT arms, respectively, at 15 yr follow-up in our model.
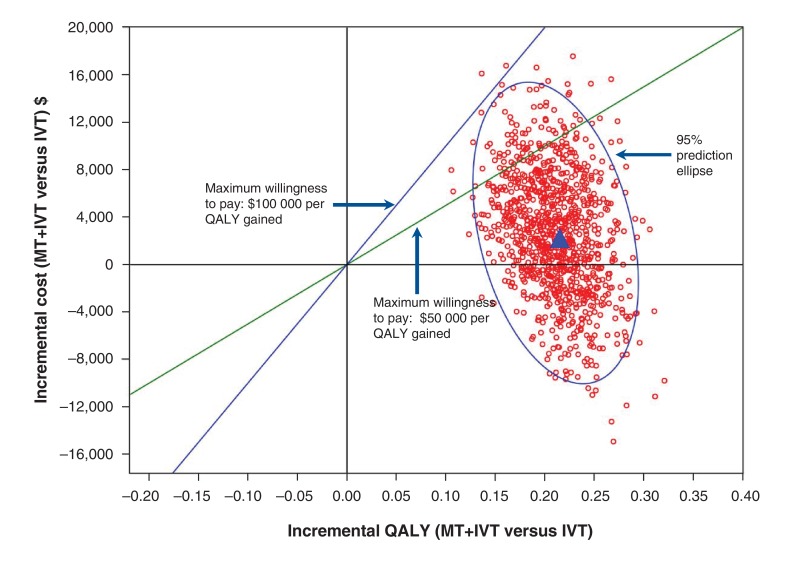
Probabilistic sensitivity analysis
Results of the Monte Carlo simulations were consistent with those in the base case (Figure 3). The probability of mechanical thrombectomy plus intravenous thrombolysis dominating intravenous thrombolysis alone (i.e., mechanical thrombectomy plus intravenous thrombolysis with lower costs and higher QALYs) was 0.286. The cost-effectiveness acceptability curve showed that the probability of mechanical thrombectomy plus intravenous thrombolysis being cost-effective was 57.5%, 89.7% and 99.6%, respectively, at thresholds of $20 000, $50 000, and $100 000 per QALY gained, respectively (Appendix 5, available at www.cmajopen.ca/content/4/2/E316/suppl/DC1).
Figure 3.
Cost-effectiveness plane: the incremental cost and QALY gained of mechanical thrombectomy plus intravenous thrombolysis versus intravenous thrombolysis alone. The blue triangle in the centre of the circle indicates the base case scenario. Each red circle surrounding the blue triangle represents a single result from the simulation, presenting the incremental effects and incremental costs of mechanical thrombectomy plus intravenous thrombolysis compared with intravenous thrombolysis alone. IVT = intravenous thrombosis, MT = mechanical thrombectomy, QALY = quality-adjusted life-year.
Interpretation
Our economic analysis showed that mechanical thrombectomy is highly likely to be cost-effective according to commonly cited cost-effectiveness thresholds. This is concordant with the large clinical effect size observed in the randomized trials, and sensitivity analyses suggested that these findings are robust to a range of assumptions.
Our findings were consistent with the most recent published economic evaluation from the United States,22 which evaluated new-generation devices and used efficacy estimates from a single RCT.10 In that evaluation, mechanical thrombectomy (with or without intravenous thrombolysis) resulted in an incremental cost-effectiveness ratio of US$14 137 per QALY gained.
We used the long-term outcome of a subgroup of patients in the Oxford Vascular Study17,18 in the UK to project the long-term outcomes of our target population in Canada. About 83% the patients in the UK cohort were patients with ischemic stroke. The treatments and age of patients were different between the cohort in the Oxford Vascular Study and the control arms in our model, but the disease severity in mortality (about 20%) and disability rate (about 60% for survivors) at 90 days after stroke were similar. In addition, the UK and Canada have centrally planned and publicly funded health systems. We believe that it is reasonable to expect our target patients in Canada to have a similar trajectory to those in the UK.
Strengths and limitations
The strengths of our study include using high-quality evidence from the meta-analysis of 5 RCTs;15 using monthly compared with yearly cycles in the Markov model, which models disease progression more accurately; and the transitions from functional independence to disability, as well as from disability to functional independence (i.e., recovery), which models the progression of patients with stroke more naturally. Furthermore, we used a calibration approach to provide parameter estimates for the economic model, and experts validated model assumptions and inputs.
Our study also has several limitations that merit emphasis. First, the conclusions are limited by the short-interval follow-up (90 days) in the 5 RCTs identified, and the need to combine results with a cohort study to model longer-term outcomes. Second, many of our parameter estimates came from studies conducted outside Ontario; parameter estimates may have been different if data from Ontario were available. However, our findings were robust to a range of plausible assumptions. Third, we caution that we did not model specific endovascular devices or patient imaging-selection strategies, and cost-effectiveness may be influenced by these choices, as well as by costs incurred by system-level changes implemented to change stroke care. In addition, future RCTs that examine long-term outcomes in patients with stroke would help validate several important parameters in our model.
Conclusion
Treatment with mechanical thrombectomy as an adjunct therapy to intravenous thrombolysis is cost-effective compared with intravenous thrombolysis alone in patients with acute large-artery ischemic stroke. In publicly funded health care systems such as Canada's, the use of mechanical thrombectomy is likely to represent good value for money and should be supported.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/4/2/E316/suppl/DC1
Supplementary Material
Footnotes
Competing interests: Xuanqian Xie, Anna Lambrinos, Brian Chan, Irfan Dhalla and Nancy Sikich receive a personal salary from Health Quality Ontario. Leanne Casaubon received consultant fees from Medtronic Canada, speaker fees from Bayer and compensation as a member of the Advisory Board of Covidien Canada. Aditya Baratha received a grant from the ESCAPE trial (University of Calgary). Michael Hill received compensation from Merck; in-kind nonfinancial support from Hoffmann-La Roche Canada Ltd.; grants from Covidien Canada (ESCAPE trial), Alberta Innovates Health Solutions, Canadian Institutes of Health Research, Heart & Stroke Foundation of Canada and National Institute of Neurological Disorders and Stroke; he has a patent pending with the US Patent Office (no. 62/086,077) and he owns stock in Calgary Scientific Incorporated (medical imaging software).
Disclaimer: The views and opinions expressed by the authors in this publication are those of the authors and do not necessarily reflect those of Health Quality Ontario.
References
- 1.van der Worp HB, van Gijn J. Clinical practice. Acute ischemic stroke. N Engl J Med. 2007;357:572–9. doi: 10.1056/NEJMcp072057. [DOI] [PubMed] [Google Scholar]
- 2.Access to stroke care: the critical first hours. Ottawa: Heart and Stroke Foundation of Canada. 2016. [accessed 2016 Apr. 28]. Available http://www.heartandstroke.com/atf/cf/%7B99452d8b-e7f1-4bd6-a57d-b136ce6c95bf%7D/HSF_2015_STROKE_REPORT_FINAL.pdf.
- 3.Statistics. Ottawa: Heart and Stroke Foundation of Canada. 2016. [accessed 2016 Apr. 28]. Available http://www.heartandstroke.com/site/c.ikIQLcMWJtE/b.3483991/k.34A8/Statistics.htm.
- 4.Types of stroke - ischemic strokes (clots). Dallas: American Heart Association. 2015. [accessed 2015 Nov. 3]. Available www.strokeassociation.org/STROKEORG/AboutStroke/TypesofStroke/IschemicClots/Ischemic-Strokes-Clots_UCM_310939_Article.jsp.
- 5.Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 6.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–35. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–13. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–23. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intra-arterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 11.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 12.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 13.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 14.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 15.Mechanical thrombectomy in patients with acute ischemic stroke: a health technology assessment. Toronto: Health Quality Ontario. 2015. [accessed 2016 Apr. 20]. Available www.hqontario.ca/Portals/0/Documents/evidence/reports/hta-mechanical-thrombectomy-1602-en.pdf. [PMC free article] [PubMed]
- 16.Casaubon LK, Boulanger JM, Blacquiere D, et al. Canadian Stroke Best Practice Recommendations: Hyperacute Stroke Care guidelines, update 2015. Int J Stroke. 2015;10:924–40. doi: 10.1111/ijs.12551. [DOI] [PubMed] [Google Scholar]
- 17.Luengo-Fernandez R, Paul NL, Gray AM, et al. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke. 2013;44:2854–61. doi: 10.1161/STROKEAHA.113.001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luengo-Fernandez R, Gray AM, Bull L, et al. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology. 2013;81:1588–95. doi: 10.1212/WNL.0b013e3182a9f45f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hankey GJ. Long-term outcome after ischaemic stroke/transient ischaemic attack. Cerebrovasc Dis. 2003;16(Suppl 1):14–9. doi: 10.1159/000069936. [DOI] [PubMed] [Google Scholar]
- 20.Hankey GJ, Spiesser J, Hakimi Z, et al. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007;68:1583–7. doi: 10.1212/01.wnl.0000260967.77422.97. [DOI] [PubMed] [Google Scholar]
- 21.The human mortality database. Life tables in 2004 in the United Kingdom. Berkeley: The Human Mortality Database Research Teams. 2013. [accessed 2015 Jun. 1]. Available www.mortality.org/
- 22.Leppert MH, Campbell JD, Simpson JR, et al. Cost-effectiveness of intra-arterial treatment as an adjunct to intravenous tissue-type plasminogen activator for acute ischemic stroke. Stroke. 2015;46:1870–6. doi: 10.1161/STROKEAHA.115.009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rai AT, Evans K. Hospital-based financial analysis of endovascular therapy and intravenous thrombolysis for large vessel acute ischemic strokes: the 'bottom line.'. J Neurointerv Surg. 2015;7:150–6. doi: 10.1136/neurintsurg-2013-011085. [DOI] [PubMed] [Google Scholar]
- 24.Simpson KN, Simpson AN, Mauldin PD, et al. Drivers of costs associated with reperfusion therapy in acute stroke: The Interventional Management of Stroke III trial. Stroke. 2014;45:1791–8. doi: 10.1161/STROKEAHA.113.003874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M. Cost-effectiveness of endovascular therapy for acute ischemic stroke. Neurology. 2012;79:S16–21. doi: 10.1212/WNL.0b013e31826957df. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen-Huynh MN, Johnston SC. Is mechanical clot removal or disruption a cost-effective treatment for acute stroke? AJNR Am J Neuroradiol. 2011;32:244–9. doi: 10.3174/ajnr.A2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim AS, Nguyen-Huynh M, Johnston SC. A cost-utility analysis of mechanical thrombectomy as an adjunct to intravenous tissue-type plasminogen activator for acute large-vessel ischemic stroke. Stroke. 2011;42:2013–8. doi: 10.1161/STROKEAHA.110.606889. [DOI] [PubMed] [Google Scholar]
- 28.Patil CG, Long EF, Lansberg MG. Cost-effectiveness analysis of mechanical thrombectomy in acute ischemic stroke. J Neurosurg. 2009;110:508–13. doi: 10.3171/2008.8.JNS08133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mittmann N, Seung SJ, Hill MD, et al. Impact of disability status on ischemic stroke costs in Canada in the first year. Can J Neurol Sci. 2012;39:793–800. doi: 10.1017/s0317167100015638. [DOI] [PubMed] [Google Scholar]
- 30.Dorman P, Dennis M, Sandercock P. Are the modified "simple questions" a valid and reliable measure of health related quality of life after stroke? United Kingdom Collaborators in the International Stroke Trial. J Neurol Neurosurg Psychiatry. 2000;69:487–93. doi: 10.1136/jnnp.69.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broderick JP, Berkhemer OA, Palesch YY, et al. Endovascular therapy is effective and safe for patients with severe ischemic stroke: pooled analysis of interventional management of stroke III and multicenter randomized clinical trial of endovascular therapy for acute ischemic stroke in the Netherlands data. Stroke. 2015;46:3416–22. doi: 10.1161/STROKEAHA.115.011397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pham B, Krahn M. End-of-life care interventions: an economic analysis. Ont Health Technol Assess Ser. 2014;14:1–70. [PMC free article] [PubMed] [Google Scholar]
- 33.Vanni T, Karnon J, Madan J, et al. Calibrating models in economic evaluation: a seven-step approach. Pharmacoeconomics. 2011;29:35–49. doi: 10.2165/11584600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Consumer Price Index, health and personal care, by province (monthly). Ottawa: Statistics Canada. 2015. [accessed 2015 June 1]. Available www.statcan.gc.ca.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.