Abstract
Key points
Excessive exercise-induced shortness of breath is a common complaint. For some, exercise-induced bronchoconstriction is the primary cause and for a small minority there may be an alternative organic pathology. However for many, the cause will be simply reaching their physiological limit or be due to a functional form of dysfunctional breathing, neither of which require drug therapy.
The physiological limit category includes deconditioned individuals, such as those who have been through intensive care and require rehabilitation, as well as the unfit and the fit competitive athlete who has reached their limit with both of these latter groups requiring explanation and advice.
Dysfunctional breathing is an umbrella term for an alteration in the normal biomechanical patterns of breathing that result in intermittent or chronic symptoms, which may be respiratory and/or nonrespiratory. This alteration may be due to structural causes or, much more commonly, be functional as exemplified by thoracic pattern disordered breathing (PDB) and extrathoracic paradoxical vocal fold motion disorder (pVFMD).
Careful history and examination together with spirometry may identify those likely to have PDB and/or pVFMD. Where there is doubt about aetiology, cardiopulmonary exercise testing may be required to identify the deconditioned, unfit or fit individual reaching their physiological limit and PDB, while continuous laryngoscopy during exercise is increasingly becoming the benchmark for assessing extrathoracic causes.
Accurate assessment and diagnosis can prevent excessive use of drug therapy and result in effective management of the cause of the individual’s complaint through cost-effective approaches such as reassurance, advice, breathing retraining and vocal exercises.
This review provides an overview of the spectrum of conditions that can present as exercise-induced breathlessness experienced by young subjects participating in sport and aims to promote understanding of the need for accurate assessment of an individual’s symptoms. We will highlight the high incidence of nonasthmatic causes, which simply require reassurance or simple interventions from respiratory physiotherapists or speech pathologists.
Short abstract
Breathlessness: accurate assessment and diagnosis is essential in order to provide correct advice and assistance http://ow.ly/4nrW8z
Introduction
Perceived excessive breathlessness during exertion or exercise-induced dyspnoea (EID) is reported to be the commonest symptom limiting performance and/or enjoyment when participating in sporting activity among children, adolescents and young adults [1, 2]. Indeed for some the sensation of excessive breathlessness is given as a reason for not participating in physical activity. This is observed across the spectrum from occasional recreational participation to elite athletes. Clarifying the cause of the perceived symptoms requires careful assessment with a wide range of factors potentially contributing to the reported symptoms.
Asthma and exercise-induced bronchoconstriction (EIB) are relatively common causes of EID. However, a presumptive diagnosis of asthma is often made based on a subjective history without objective confirmation and as a result over diagnosis is common. In part this is attributable to a lack of awareness of possible alternative diagnoses or comorbidities that may be responsible for the reported breathlessness. This has led to many individuals receiving inappropriately prescribed “asthma” medications for EID caused by other conditions, or receiving excessively high doses as comorbidities are not recognised. Failure to clearly identify the cause of the individual’s symptoms also deprives them of appropriate management resulting in ongoing impairment of their quality of life (QoL) and unnecessary healthcare utilisation.
This article considers the range of problems that may masquerade as, or coexist with, exercise-induced asthma in causing dyspnoea. While serious cardiovascular, pulmonary or upper airways pathology can cause EID, it is much more likely the cause is “benign” or functional. From studies of symptomatic subjects it would appear that reaching their physiological limit or experiencing a form of “dysfunctional breathing” are the commonest causes [1, 2]. The following sections aim to help clinicians recognise when referral to a respiratory physiotherapist or speech pathologist is useful, and highlights the need for awareness of rare but important structural or pathological causes.
Conditions causing EID
Individuals reporting EID are frequently looked after by nonspecialists in the community, although many are referred on to respiratory clinics with “difficult asthma” when they fail to respond to standard therapy. Others present to otolaryngologists when an upper airways cause is suspected or to psychologists with “hyperventilation syndrome” (HVS).
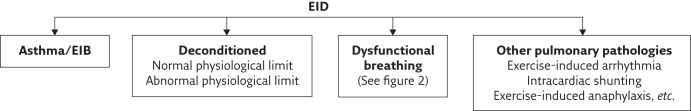
Figure 1 outlines the range of conditions that may present with EID and provides a possible framework for clinicians when presented with a complaint of perceived excessive breathlessness during physical activity. In studies exploring the causes of EID in young people, those with asthma are usually in the minority [1–5]. The most common diagnosis appears to be that the breathlessness is appropriate and that individuals are reaching their physiological limit. This can apply to those attempting to compete at the highest levels as well as those who are “deconditioned” due to lack of adequate training, obesity or indeed recent severe illness. Also common are the conditions grouped under the umbrella term dysfunctional breathing, which can be due to thoracic or extrathoracic problems and include both structural and functional causes. This area is perhaps the least well understood and researched. Patients with chronic pulmonary disease may manifest any of these as a comorbidity and this needs to be borne in mind while accepting that the disease alone can be responsible for reported EID. The prevalence of the various causes of EID in the community has not been determined.
Figure 1.
Conditions that can contribute to EID.
Exercise-induced asthma and EIB
The impact of exercise on asthmatic subjects is well recognised, with bronchoconstriction occurring in asthmatic subjects following 5–8 min of intense exercise and nonselective β-agonists preventing or greatly lessening the resultant fall in forced expiratory volume in 1 s (FEV1) [6]. Given this “typical” pattern, the cause of the EIB is unlikely to be bronchoconstriction when individuals report that symptoms commence shortly after initiating vigorous activity. It is recognised that some individuals experience EIB associated with chest tightness, cough and wheeze without other interval symptoms of asthma and hence many consider that it can exist independent of “classic” asthma [7, 8].
EIB appears to be common in athletes [6, 8], more so in those that: are endurance athletes, have allergic rhinitis, are female, or who exercise in cold weather or in chemical environments such as swimming pools [7–10].
The vast majority of individuals with exercise-induced asthma/EIB can be managed very effectively with inhaled corticosteroids and pre-exercise β-agonists. Failure to control symptoms suggests nonadherence or an alternative diagnosis as the cause of symptoms in all but a very small minority.
Deconditioning and the physiological limit
The term deconditioning is used interchangeably to describe different physical presentations. Frequently, deconditioning is used to refer to a lower than expected physiological limit to exercise. True physiological deconditioning occurs after prolonged illness, bed rest or a sedentary lifestyle where associated cardiovascular and neuromuscular physiological changes lead to a reduction in maximal oxygen uptake and reduced cardiac output with exercise [11, 12]. This is different to the individual with a complaint of breathlessness and fatigue with exercise, but who is still able to reach their predicted physiological limit without ventilatory or cardiac limitation (figure 1).
Individuals presenting with excessive breathlessness may be truly deconditioned, as in the case of a post-critical illness patient, or have a perception that their breathlessness with exercise is abnormal despite normal physiological responses to skeletal and respiratory effort during cardiopulmonary exercise testing (CPET) [1, 2]. Perceived breathlessness for any given task or activity can vary widely between individuals with no association found between the level of perception of dyspnoea and the level of physical activity [13]. The athlete with EID may report excessive breathlessness due to a hypersensitivity to their normal physiological response. Likewise, the “unfit” person may have a normal CPET but be “unconditioned” rather than “deconditioned” in their perceived response to exercise.
Reports of deconditioning in the literature as a cause of EID are difficult to ascertain with variability in its definition and meaning. However, it should not be excluded as a significant contributor to EID in otherwise healthy young people. CPET studies on healthy adolescents and adults who report EID have reported low fitness levels or deconditioning caused EID in 23–67% of cases, exceeding any other contributing diagnosis to EID [1, 2]. It is also important to recognise that not all individuals with obesity are deconditioned. Young people who are overweight may display expiratory flow limitation at sub-maximal exercise [14]. In some, compensatory breathing strategies can limit breathlessness, but for others there is an increased perception of dyspnoea [14, 15]. Although objective investigation into the causes of EID is recommended [16] it would appear that few healthy young adults seeking medical advice for breathlessness undergo diagnostic testing [7, 9].
Dysfunctional breathing
Dysfunctional breathing can be considered an umbrella term that describes: “An alteration in the normal biomechanical patterns of breathing that result in intermittent or chronic symptoms which may be respiratory and/or nonrespiratory” [17]. Patients with dysfunctional breathing present with a variety of respiratory and nonrespiratory symptoms, including EID, shortness of breath at rest, “wheeze”, stridor, throat tightness, sighing, chest pain, throat clearing, “air hunger” (a sense of being unable to get a complete breath in even after a maximal inspiratory manoeuvre), tingling, dizziness and general fatigue [17, 18]. Frequently a number of symptoms coexist, although one may predominate.
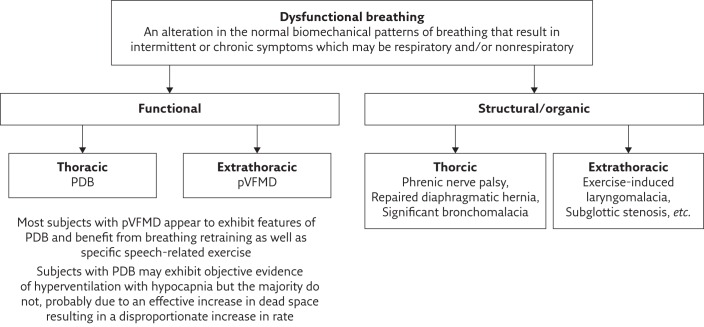
The biomechanical patterns of breathing typified by dysfunctional breathing may be altered by structural or functional factors (figure 2). An awareness of structural causes such as repaired diaphragmatic hernia and abnormalities of the vocal cords is important. Recognition of exercise-induced laryngomalacia with the advent of continuous laryngeal examination to characterise the cause of upper obstruction occurring during intense exercise, represents a significant advance in this field [19, 20]. This structural abnormality is important in that surgery can be undertaken and is increasingly being carried out for competitive sports men and women [19].
Figure 2.
Classification of dysfunctional breathing.
This review will focus on the more common functional dysfunctional breathing, which appears to represent habituated alterations in breathing patterns, and can be further classified as thoracic or extrathoracic. Thoracic dysfunctional breathing presents as alterations in the pattern of respiratory muscle activity (pattern disordered breathing (PDB)), which may or may not be associated with hyperventilation, while extrathoracic dysfunctional breathing describes conditions affecting the upper airway, such as paradoxical vocal fold motion disorder (pVFMD), in addition to PDB.
Causes of “functional” dysfunctional breathing
Patients appear to develop symptoms of dysfunctional breathing when abnormal breathing patterns (PDB) become habitual. This PDB can be constant or happen intermittently when provoked by a physical or psychological stress [17]. Dysfunctional breathing appears to frequently coexist with respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD) [21], although the nature of the relationship (causal or coincidental) remains unclear. The mechanisms leading to such characteristically “abnormal” patterns of breathing are also unclear, although the condition does appear to be amenable to “breathing retraining” where it is possible to exert a level of voluntary control over the pattern and rate of respiration.
Alterations in breathing pattern, rate and regularity are normal responses to physical, mental or emotional stress, driven largely through the autonomic system [18, 22]. The change in respiration in response to acute stress is characterised by fast, relatively shallow breathing with little contribution from the diaphragm. Fluoroscopic studies have demonstrated that when a person is exposed to emotional stress the diaphragm becomes flattened, hypertonic and relatively immobile, causing intercostal and accessory breathing muscles to contribute more to respiration [18]. From this perspective the development of dysfunctional breathing can be seen as an unconsciously learned, habitual change in the normal patterns of breathing, which may become apparent at rest or only when stressed, and would explain the link between dysfunctional breathing and psychological correlates [17]. This pattern of breathing may also potentially explain why many with thoracic dysfunctional breathing do not meet the criteria for HVS, with the characteristic excessively rapid and shallow breathing generating a relatively high dead space/tidal volume preventing falls in arterial carbon dioxide (characteristic of true alveolar hyperventilation), a situation commonly observed during CPET in our clinic.
In our experience of treating children and adolescents with dysfunctional breathing, it appears to be common in individuals who set themselves very high goals or standards, providing an internal source of stress. They may be an athlete or “A” grade student, but equally may be someone struggling academically who is desperately trying to please parents by meeting standards they think are expected of them. It has been suggested that in many cases the trigger initiating a transition from an intermittent, appropriate adoption of an altered breathing pattern during periods of stress, to an inappropriate maintenance of this PDB, can sometimes be related to a specific episode such as excessive aerobic training, bereavement or a health-related illness [18]. While the initial response may have been appropriate, PDB might be seen as a failure to return to a baseline, efficient pattern of relaxed, slow abdominal breathing pattern [18].
As with any chronic “disease” there is a vast spectrum of severity and presentations. At the most severe end of the functional dysfunctional breathing spectrum it appears to form part of post-traumatic stress disorders with some of the earliest descriptions in soldiers after the American civil war and the First World War [23]. However, while post-traumatic stress was a prominent feature in early descriptions of dysfunctional breathing, extreme triggers are uncommon in routine practice.
Thoracic dysfunctional breathing
The role of the diaphragm is critical in the development of the disordered breathing pattern (PDB) seen in thoracic dysfunctional breathing. The diaphragm is well documented as a muscle of respiration and as having a role in musculoskeletal stabilisation. The diaphragm acts as a “roof” to the abdominal cavity, has an attachment to the spinal musculature and plays a key role in the generation and maintenance of abdominal cavity pressure and trunk stabilisation [18, 24–26]. When a system is under stress, respiration will dominate at the expense of voice, locomotion and postural control [24, 26]. It is not unreasonable therefore to consider that respiratory disease, musculoskeletal dysfunction, pain, altered chest wall shape or indeed physiological stress related to competitive exercise, might trigger the diaphragm into an abnormal and habitual pattern of breathing with a subsequent increase in use of respiratory accessory muscles. In the individual with PDB, the respiratory demand required by exercise on a background of an inefficient breathing pattern makes it unsurprising that they may present with breathlessness.
Particularly in those with pulmonary diseases such as COPD, habitual PDB can result in a more fixed upper chest breathing pattern, flattening of the diaphragm and alteration in the length–tension relationship of respiratory muscles. This can lead to an increase in expiratory reserve volume and a reduction in inspiratory reserve volume, suggesting a dynamic hyperinflation pattern. It can also occur as a result of “breath stacking” in HVS and asthma, where incomplete exhalation leads to an overall increase in the volume of air within the lungs with each inhalation causing over inflation [24].
The symptom of hyperventilation falls under the umbrella of “thoracic dysfunctional breathing”. True HVS that involves physiological inappropriate alveolar hyperventilation has been the cause of much misdiagnosis and over diagnosis, particularly in children [17]. No clear pathophysiological mechanism has been identified. The popular Nijmegen score, which has been used in the past to aid diagnosis, has not been validated in children, adolescents or asthmatics [17], and was recently suggested as a score of “functional respiratory complaints” rather than a diagnostic tool [27]. Likewise the “hyperventilation test” is considered by some to be nonspecific and insensitive in identifying significant hypocapnia in many patients with HVS type symptoms [17]. HVS may be isolated in the presence of normal cardiorespiratory function, but can occur in patients with other respiratory diseases (e.g. asthma), further complicating diagnosis. True hyperventilation can be considered part of the thoracic dysfunctional breathing spectrum with many of the respiratory symptoms being attributed to the PDB and the nonpulmonary symptoms to hypocapnia [17].
Extrathoracic dysfunctional breathing
Extrathoracic causes for dysfunctional breathing include pVFMD and exercise-induced laryngomalacia [28]. They were previously known under the banner of “vocal cord dysfunction” but have been renamed to differentiate them from more permanent structural abnormality. pVFMD occurs when there is intermittent, abnormal, paradoxical adduction of the vocal folds with respiration, causing variable upper airway obstruction that is not related to a permanent deficit in vocal cord mobility [7, 29]. Likewise, exercise-induced laryngomalacia is intermittent causing obstruction due to collapse of supraglottic tissues during inspiration when a subject is participating in vigorous exercise and generating large negative intrathoracic pressures [28]. Symptoms for both include EID, variable and/or sudden shortness of breath, inspiratory stridor, choking, throat tightness, voice changes and/or cough [7, 28, 29]. If triggered with exercise, it quickly resolves with exercise cessation. Aetiology is unknown but it has been suggested that “hypersensitivity” of the larynx is the initiating factor for many. Some authors have proposed that conditions such as gastro-oesophageal reflux, allergic rhinitis and “post-nasal drip” are triggers; however, there are no studies convincingly correlating these. Frequently extrathoracic dysfunctional breathing coexists with, or can lead to, thoracic PDB.
Impact of dysfunctional breathing
The literature reports prevalence of dysfunctional breathing (both thoracic and extrathoracic dysfunctional breathing) to be common, but prevalence is clouded by a lack of consistency of definition, diagnosis and comorbidity inclusion criteria. Under reporting is likely with patients not seeking medical support, the over diagnosis of asthma, and the outdated belief that symptoms solely represent psychological disturbance and stress rather than biomechanical dysfunction.
Dysfunctional breathing is associated with significant morbidity and health-related costs. Higher health-related costs have been reported in the management of people with diagnosed forms of dysfunctional breathing such as pVFMD, HVS and PDB, when compared with moderate asthmatic patients [30]. As well as the economic cost to healthcare delivery, the burden to the individual with dysfunctional breathing is significant, with a reduction in health-related QoL affecting school and work attendance, hospital presentations and happiness [4, 25, 31–33].
The average length of time of misdiagnosis or delayed diagnosis in dysfunctional breathing causes of EID has been reported as 2–7 years [25, 28, 31, 33]. A likely reason for this is that clinically relevant comorbidities such as asthma are often found with dysfunctional breathing, and although the asthma is managed, the individual may continue to experience symptoms due to the unmanaged concomitant dysfunctional breathing.
While respiratory physiotherapists are frequently well aware of the importance of PDB and interested speech pathologists recognise the importance of dealing with pVFMD, many clinicians do not have a conceptual model on which they can build to identify and appropriately refer such patients. The use of multiple terms for the same conditions adds to the confusion and deters clinician’s from developing an insight into the presentation, causes and management, which is unfortunate as patients can often be cured or have their QoL greatly enhanced without drug therapy using simple interventions delivered via an interested health professional. It is probably only at the most extreme end of the spectrum that input from a psychologist is required and indeed they will often use breathing retraining as part of their approach. Of note many patients with dysfunctional breathing who have been treated with ever increasing medication improve significantly when the condition is explained to them, taking away the fear that “it must be something terrible because the doctors do not have an answer”.
Unusual pathogenic causes
While this review has focused on the more common and benign causes of EID, it is important to consider the unusual and rare in the differential diagnosis of unexplained breathlessness not responsive to asthma medication. Intrapulmonary or intracardiac shunting due to conditions such as the hepatopulmonary syndrome, significant arteriovenous malformation and some forms of intracardiac shunting that increase with exercise may result in rapid onset of symptoms with desaturation. Exercise-induced arterial hypoxaemia is reported in some well-trained athletes in the absence of identified shunting, although the mechanism is not fully understood. Exercise-provoked cardiac arrhythmias such as supraventricular tachycardia may be difficult to identify from the history unless actively sought. Exercise-induced anaphylaxis is a potentially fatal rare cause that has been recently reviewed, and case studies have been reported of swimming induced pulmonary oedema and pulmonary embolus in athletes [7, 34]. Similarly increased fatigue and reduced exercise tolerance are common among those developing anaemia and chronic thromboembolic disease, and both are easily overlooked by the respiratory physician. Thus, being conscious of rare but very important pathologies that can impact on exercise tolerance is very important.
A clinical approach to the patient with EID
Inaccurate assessment of the individual with EID may result in inappropriate therapy and failure to provide symptom relief. As with any presenting complaint, a careful history is essential. Examination can be helpful and appropriate use of investigations may be necessary to provide a definitive diagnosis. While investigations such as CPET and continuous laryngoscopy during exercise (CLE) may be relatively expensive to undertake, they may save the healthcare provider money by preventing unnecessary interventions and follow-up, while ensuring the best possible outcome for the individual. The following will concentrate on nonasthmatic causes of EID.
History
It is important to take a history from a position of having a comprehensive conceptual model of potential causes (figure 1). EID can present on its own or with a number of other symptoms such as cough, chest tightness and wheeze. A history may elicit typical symptoms of asthma with a clear family history, but the diagnosis only remains probable until the patient reports a clear unequivocal and dramatic response to therapy [35].
Clues that EIB with or without coexisting “asthma” is not the primary cause include reported poor response to β-agonists taken before exercise or immediately after onset of symptoms; onset within a short time of starting exercise; episodic shortness of breath when at rest; few, if any, nocturnal symptoms; and lack of a refractory period with symptoms starting shortly after resuming exercise post-rest.
Symptoms suggestive of thoracic “functional” dysfunctional breathing include excessive sighing; “air hunger” (a sensation of being unable to get a full breath in despite maximum effort); chest pain/discomfort in the absence of severe respiratory distress; and episodes of shortness of breath at rest, such as when watching TV. Generally there are no symptoms during sleep.
Extrathoracic “functional” dysfunctional breathing symptoms include stridor, this can be biphasic if the cords remain closed during exhalation and is often relatively quiet (which is often misreported as wheeze); a sensation that the throat is closing or tightness in the throat; and during an “acute episode”, normal oxygen saturations despite dramatic symptoms.
It should be noted that most asthmatics find breathing “in” more difficult than exhaling when symptomatic, even though doctors expect it to be the reverse; hence difficulty breathing in per se is not a discriminating feature for asthma and dysfunctional breathing. This is presumably because exhalation is passive and does not require effort, while inhalation when hyperinflated requires increased effort.
For the rare, more serious conditions, a history of colour change or marked tachycardia (>200 beats per min) accompanying the exercise may be present.
Examination
If seen when symptomatic, it may be possible to distinguish true wheeze (a musical expiratory sound generated by vibration of central airways that occurs with flow limitation) from stridor. In this context mobile phones can be invaluable to capture episodes. Respiratory physiotherapists are skilled at identifying the characteristic breathing pattern with dysfunctional breathing involving limited excursion of the diaphragm with excessive upper chest wall movement and use of accessory muscles. Auscultation is commonly unremarkable when the patient is seen in clinic, but occasionally may be helpful.
Investigations
Spirometry is generally the minimum investigation that should be undertaken and a diagnosis of asthma may be confirmed should there be significant bronchodilator responsiveness. A discussion of the various direct and indirect challenge tests available to help confirm the presence of significant bronchial hyperresponsiveness is beyond the remit of this review, but has been reviewed recently [35].
When the aetiology of symptoms is unclear, the importance of observing and assessing an individual exposed to their usual provocation has long been recognised. Since the early 1990s, CPET has increasingly been used to help distinguish those with dysfunctional breathing or who are reaching their physiological limit (be they “deconditioned” or fit) from more serious pathologies related to a cardiac or ventilatory limitation [2, 12, 36–39]. Numerous studies using CPET as a research outcome tool, have reported on misdiagnoses, incorrect management of EID and in some cases identification of serious pathology [1, 5, 19, 36, 37]. In the clinical setting, CPET can be used to provide a provocation test in EIB and exercise-induced asthma with symptoms expected after 5 min of exercise and a fall in FEV1 post-test that is slow to resolve without bronchodilator therapy. This is different to the symptoms experienced in pVFMD where symptoms can be immediate and quickly resolve at the cessation of exercise. The characteristics of PDB during CPET have been less well described and appear to be, in part, purely observational of breathing patterns, but the interest and practice of using CPET to objectively describe PDB as a contributor to EID is increasing [39].
“True” deconditioning can be differentiated on CPET as presentation of a low anaerobic threshold and elevated or excessive heart response to relative oxygen uptake, even with sub-maximal exercise testing [1, 2, 16]. Both deconditioning and poor effort can produce a lower peak oxygen uptake in the absence of other abnormalities. In the context of an EID presentation, a completely normal CPET could simply mean a high sensitivity of the individual to their normal responses when reaching a physiological limit, or an athlete who has reached peak performance but wishes for more.
While an abnormal CPET is useful in determining the cause of EID, a normal or near normal CPET is equally useful in providing some reassurance to the individual and clinician. This in itself may provide a benefit to treatment, particularly with children and adolescents in whom a high proportion of those who present with EID are in fact just reaching normal physiological limits [2, 3, 5].
Treatment pathways for functional breathing abnormalities
An increasing number of literature reviews and randomised controlled trials demonstrate the emergence of recognition that not all breathlessness in otherwise well individuals is asthma [1, 2, 5, 16–18, 23, 25, 30, 40]. When an individual does not respond to asthma medication, or does not respond as well as expected, comorbidities such as dysfunctional breathing, simple deconditioning or sensitivity to a normal physiological response to exercise need to be considered.
There appear to be common factors contributing to the onset of extrathoracic and thoracic “functional” dysfunctional breathing. Both are frequently found in the self-driven person and athlete, and both appear to be helped with breathing retraining. Classification can assist treatment with referral to a speech pathologist for management of extrathoracic dysfunctional breathing and to a respiratory physiotherapist for thoracic dysfunctional breathing. Those with a combination of extrathoracic dysfunctional breathing and PDB may best be managed by both professions and could be considered to be a more severe form of the same basic condition. As with management of many conditions it is important that the therapist to whom the patient is referred has an interest in the condition.
Recommendations for the management of thoracic dysfunctional breathing are found in recent guidelines for physiotherapists [41]. Published research protocols generally follow treatment principles that focus on education about the condition, reassurance, abdominal breathing retraining, breathing rate and depth control, breathing retraining in progressively taxing postures such as walking, recognition of triggers, and control of symptoms during an episode and manual therapy [18, 24, 25]. Studies have demonstrated a reduction in respiratory symptoms and hospital visits and an improvement in health-related QoL and athletic performance over relatively short periods of two to eight clinical visits with a physiotherapist [25, 33, 42–44]. Furthermore, benefits have been maintained in some groups from 6 months to 5 years [33, 42]. Treatment practices often add manual therapy to address musculoskeletal dysfunction; however, adding manual therapy in addition to breathing retraining techniques has not yet been proven to add further benefit to the relief of symptoms [25].
Treatment for deconditioning should include individualised exercise prescription and management of associated musculoskeletal dysfunction; however, reassurance and education is also required. In a study intended to provide breathing retraining, a large number of children with EID, who performed a normal CPET, went on to decline intervention, leading the authors to suggest that in children reassurance in itself from conclusive objective testing may be a beneficial treatment [2].
While some studies have suggested children with EID report similar activity levels to their healthy peers [4], of concern is the early termination of activity in others [3]. A cycle of perceived excessive EID, regardless of cause, will perhaps lead to a cycle of activity avoidance and deconditioning. In the modern world of increasing sedentary behaviour and a health-related obesity crisis, failing to identify and treat benign EID in children and adolescents may have long lasting cost and health impacts.
Clinics and multidisciplinary teams established to manage the complex patient with breathlessness are becoming more common. A recent report from the UK’s first paediatric respiratory physiotherapist run dysfunctional breathing clinic indicated dramatic improvements in QoL that were sustained when subjects were re-evaluated 6 months after the last of their four sessions at the clinic [45]. Another recent report describes the experience of a specialised “airways clinic” in the UK, in which patients referred with complex breathlessness are seen by a physician and have access to experienced speech pathologists and physiotherapists attached to their team. This clinic reports a significant number of their patients with severe asthma or chronic lung disease to also have PDB and/or vocal cord dysfunction. Despite a protracted history of symptoms in most, patients reported clinical improvement with multidisciplinary team intervention addressing their dysfunctional breathing [21].
Conclusion
EID is a common symptom reported in young people and adults and can affect QoL and engagement in physical activity. Bothersome EID may be a symptom of serious cardiovascular or pulmonary disease, or of a benign cause. Differential diagnosis of EID is important to guide clinical management that maximises treatment effect and minimises unnecessary medication prescription. The clinician should “seek to do no harm”, harm in the context of EID could be failure to diagnose the cause accurately and the administration of unnecessary and potentially harmful drugs, while failing to deal with the underlying problem and hence perpetuating a condition that is impacting negatively on the patient’s QoL.
Careful clinical evaluation based on a clear conceptual map of potential causes linked to appropriate testing should be the standard. Lung function testing with appropriate use of challenge testing, CPET testing and/or CLE should form part of an integrated approach to the athlete (at whatever level) who is reporting limitation of exercise due to their breathing.
Thoracic dysfunctional breathing appears, based on the limited data available, to affect up to 10% of the population and 30% of the asthmatic population, yet few individuals are being referred to respiratory physiotherapists. This reflects a lack of awareness of the nature, prevalence and impact of the condition among many clinicians. Consequently funding bodies do not appear to recognise it as an important cause of morbidity worthy of funding, thus perpetuating this situation. Clarity in our approach to dysfunctional breathing is vital if funding is to be made available for high quality studies designed to identify the prevalence and the potential healthcare cost saving and improvements in QoL that would follow from accurate assessment and intervention.
Self-evaluation questions
- Which of the following are typical of EIB?
- a) Rapid onset after starting to train or compete
- b) Some response to a short-acting β-agonist
- c) Re-occurrence when restarting activity after rest
- d) Difficulty breathing out
- e) None of the above
- Which of the following apply to PDB (thoracic dysfunctional breathing)?
- a) Can be identified on examination
- b) May cause breathlessness at rest
- c) Is only present during periods of stress
- d) Is due to excessive diaphragmatic activity
- e) None of the above
- Which of the following apply to upper airways dysfunctional breathing?
- a) Always results from underlying stress
- b) Can be accurately assessed during an asthma exercise test
- c) Only causes inspiratory stridor
- d) When functional is quite distinct from pattern disordered breathing
- e) None of the above
- Which of the following are frequently reported symptoms due to PDB?
- a) Difficulty getting a full breath in
- b) Chest pain
- c) Frequent sighing
- d) Onset when sitting quietly
- e) None of the above
Suggested answers
e
e
e
a–d
Footnotes
Conflict of interest None declared.
References
- 1.Abu-Hasan M, Tannous B, Weinberger M. Exercise-induced dyspnea in children and adolescents: if not asthma then what? Ann Allergy Asthma Immunol 2005; 94: 366–371. [DOI] [PubMed] [Google Scholar]
- 2.Mahut B, Fuchs-Climent D, Plantier L, et al. Cross-sectional assessment of exertional dyspnea in otherwise healthy children. Pediatr Pulmonol 2014; 49: 772–781. [DOI] [PubMed] [Google Scholar]
- 3.De Baets F, Bodart E, Dramaix-Wilmet M, et al. Exercise-induced respiratory symptoms are poor predictors of bronchoconstriction. Pediatr Pulmonol 2005; 39: 301–305. [DOI] [PubMed] [Google Scholar]
- 4.Johansson H, Norlander K, Hedenström H, et al. Exercise-induced dyspnea is a problem among the general adolescent population. Respir Med 2014; 108: 852–858. [DOI] [PubMed] [Google Scholar]
- 5.Seear M, Wensley D, West N. How accurate is the diagnosis of exercise induced asthma among Vancouver schoolchildren? Arch Dis Child 2005; 90: 898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons JP, Hallstrand TS, Mastronarde JG, et al. An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med 2013; 187: 1016–1027. [DOI] [PubMed] [Google Scholar]
- 7.Krey D, Best T. Dyspneic athlete. Curr Rev Musculoskelet Med 2014; 7: 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns J, Mason C, Mueller N, et al. Asthma prevalence in Olympic summer athletes and the general population: an analysis of three European countries. Respir Med 2015; 109: 813–820. [DOI] [PubMed] [Google Scholar]
- 9.Newsham KR, Frese EM, McGuire RA, et al. Exercise-induced dyspnea in college-aged athletes. Int J Allied Health Sci Pract 2013; 11: 6. [Google Scholar]
- 10.Boulet LP. Cough and upper airway disorders in elite athletes: a critical review. Br J Sports Med 2012; 46: 417–421. [DOI] [PubMed] [Google Scholar]
- 11.Kisner C, Colby LA. Therapeutic exercise: foundations and techniques. Philadelphia, F.A. Davis, 2012. [Google Scholar]
- 12.Bhatt DV, Kocheril AG. Submaximal cardiopulmonary exercise testing for the evaluation of unexplained dyspnea. South Med J 2014; 107: 144–149. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler B, Fernandes AK, Sanches PRS, et al. Variability of the perception of dyspnea in healthy subjects assessed through inspiratory resistive loading. J Bras Pneumol 2015; 41: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson N, Johnston K, Bear N, et al. Expiratory flow limitation and breathing strategies in overweight adolescents during submaximal exercise. Int J Obes 2014; 38: 22–26. [DOI] [PubMed] [Google Scholar]
- 15.Carpio C, Villasante C, Galera R, et al. Systemic inflammation and higher perception of dyspnea mimicking asthma in obese subjects. J Allergy Clin Immunol 2016; 137: 718–726. [DOI] [PubMed] [Google Scholar]
- 16.Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012; 185: 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barker N, Everard ML. Getting to grips with ‘dysfunctional breathing’. Paediatr Respir Rev 2015; 16: 53–61. [DOI] [PubMed] [Google Scholar]
- 18.Courtney R. The functions of breathing and its dysfunctions and their relationship to breathing therapy. Int J Osteopath Med 2009; 12: 78–85. [Google Scholar]
- 19.Nielsen EW, Hull JH, Backer V. High prevalence of exercise-induced laryngeal obstruction in athletes. Med Sci Sports Exerc 2013; 45: 2030–2035. [DOI] [PubMed] [Google Scholar]
- 20.Norlander K, Christensen PM, Maat RC, et al. Comparison between two assessment methods for exercise-induced laryngeal obstructions. Eur Arch Otorhinolaryngol 2016; 273: 425–430. [DOI] [PubMed] [Google Scholar]
- 21.Haines J, Vyas A, Slinger C, et al. M13 Clinical characteristics and management of patients presenting to the “Airways Clinic”; a specialised tertiary multi-disciplinary respiratory service. Thorax 2015; 70: Suppl. 3, A232. [Google Scholar]
- 22.Lum LC. Hyperventilation syndromes in medicine and psychiatry: a review. J R Soc Med 1987; 80: 229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaitow L, Gilbert C, Bradley D. What are breathing pattern disorders? In: Chaitow L, Gilbert C, Bradley D, eds. Recognizing and treating breathing disorders: a multidisciplinary approach. London, Churchill Livingstone, 2014; pp. 1–10. [Google Scholar]
- 24.CliftonSmith T, Rowley J. Breathing pattern disorders and physiotherapy: inspiration for our profession. Phys Ther Rev 2011; 16: 75–86. [Google Scholar]
- 25.Jones M, Troup F, Nugus J, et al. Does manual therapy provide additional benefit to breathing retraining in the management of dysfunctional breathing? A randomised controlled trial. Disabil Rehabil 2015; 37: 763–770. [DOI] [PubMed] [Google Scholar]
- 26.Massery M. Multisystem consequences of impaired breathing mechanics and/or postural control. In: Frownfelter D, Dean E, eds. Cardiovascular and Pulmonary Physical Therapy Evidence and Practice. 4th Edn. St Louis, Elsevier Health Sciences, 2006; pp. 695–717. [Google Scholar]
- 27.van Dixhoorn J, Folgering H. The Nijmegen Questionnaire and dysfunctional breathing. ERJ Open Res 2015; 1: 00001–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shembel AC, Sandage MJ, Verdolini Abbott K. Episodic laryngeal breathing disorders: literature review and proposal of preliminary theoretical framework. J Voice 2016. [in press; DOI: 10.1016/j.jvoice.2015.11.027]. [DOI] [PubMed] [Google Scholar]
- 29.Matrka L. Paradoxic vocal fold movement disorder. Otolaryngol Clin North Am 2014; 47: 135–146. [DOI] [PubMed] [Google Scholar]
- 30.D’Alba I, Carloni I, Ferrante AL, et al. Hyperventilation syndrome in adolescents with and without asthma. Pediatr Pulmonol 2015; 50: 1184–1190. [DOI] [PubMed] [Google Scholar]
- 31.Chenivesse C, Similowski T, Bautin N, et al. Severely impaired health-related quality of life in chronic hyperventilation patients: exploratory data. Respir Med 2014; 108: 517–523. [DOI] [PubMed] [Google Scholar]
- 32.Ten Voorde M, van der Zaag-Loonen HJ, van Leeuwen RB. Dizziness impairs health-related quality of life. Qual Life Res 2012; 21: 961–966. [DOI] [PubMed] [Google Scholar]
- 33.Hagman C, Janson C, Emtner M. Breathing retraining - a five-year follow-up of patients with dysfunctional breathing. Respir Med 2011; 105: 1153–1159. [DOI] [PubMed] [Google Scholar]
- 34.Ansley L, Bonini M, Delgado L, et al. Pathophysiological mechanisms of exercise-induced anaphylaxis: an EAACI position statement. Allergy 2015; 70: 1212–1221. [DOI] [PubMed] [Google Scholar]
- 35.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. [DOI] [PubMed] [Google Scholar]
- 36.Martinez FJ, Stanopoulos I, Acero R, et al. Graded comprehensive cardiopulmonary exercise testing in the evaluation of dyspnea unexplained by routine evaluation. Chest 1994; 105: 168–174. [DOI] [PubMed] [Google Scholar]
- 37.McNicholl DM, Megarry J, McGarvey LP, et al. The utility of cardiopulmonary exercise testing in difficult asthma. Chest 2011; 139: 1117–1123. [DOI] [PubMed] [Google Scholar]
- 38.Forman DE, Myers J, Lavie CJ, et al. Cardiopulmonary exercise testing: relevant but underused. Postgrad Med 2010; 122: 68–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benzrath S, Schlegtendal A, Brinkmann F, et al. Dyspnoe due to exercise-induced dysfunctional breathing – Frequent entity in adolescents but poorly understood? Eur Respir J 2015; 46: Suppl. 59, PA1346. [Google Scholar]
- 40.Niggemann B. How to diagnose psychogenic and functional breathing disorders in children and adolescents. Pediatr Allergy Immunol 2010; 21: 895–899. [DOI] [PubMed] [Google Scholar]
- 41.Bott J, Blumenthal S, Buxton M, et al. Guidelines for the physiotherapy management of the adult, medical, spontaneously breathing patient. Thorax 2009; 64: Suppl, i1–i51. [DOI] [PubMed] [Google Scholar]
- 42.Thomas M, McKinley R, Freeman E, et al. Breathing retraining for dysfunctional breathing in asthma: a randomised controlled trial. Thorax 2003; 58: 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laurino RA, Barnabé V, Saraiva-Romanholo BM, et al. Respiratory rehabilitation: a physiotherapy approach to the control of asthma symptoms and anxiety. Clinics (Sao Paulo) 2012; 67: 1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vickery RL. The effect of breathing pattern retraining on performance in competitive cyclists. Auckland, Auckland University of Technology, 2008. [Google Scholar]
- 45.Barker N, Everard M. Breathing retraining as a treatment modality for dysfunctional breathing in children. Eur Respir J 2014; 44: Suppl. 58, 4680. [Google Scholar]