Abstract
Background and Purpose:
This study aimed to investigate the effect of ceftriaxone on oxidative stress and gap junction protein (connexin 43, Cx-43) expression in pentylenetetrazole (PTZ) induced kindling model.
Methods:
Twenty four Sprague dawely rats were divided into 3 equal groups (a) normal group: normal rats. (b) PTZ kindled group: received PTZ at the dose of 50 mg/kg via intraperitoneal injection (i.p.) every other day for 2 weeks (c) ceftriaxone treated group: received ceftriaxone at the dose 200 mg\kg/12 hrs via i.p. injection daily from the 6th dose of PTZ for 3 days. Racine score, latency before beginning the first myoclonic jerk and duration of the jerks used as parameters of behavioral assessment. Immunohistopathological study for Cx-43 expression in hippocampus and measurement of markers of oxidative stress (malondialdehyde [MDA], low reduced glutathione [GSH] and catalase [CAT]) in hippocampal neurons were done.
Results:
PTZ kindling was associated with behavioral changes (in the form high stage of Racine score, long seizure duration and short latency for the first jerk), enhanced oxidative stress state (as demonstrated by high MDA, low GSH and CAT) and up regulation of Cx43 in hippocampal regions. While, ceftriaxone treatment ameliorated, significantly, PTZ-induced convulsions and caused significant improvement in oxidative stress markers and Cx-43 expression in hippocamal regions (p < 0.05).
Conclusions:
These findings support the anticonvulsive effects of some beta-lactams antibiotics which could offer a possible contributor in the basic treatment of temporal lobe epilepsy. This effect might be due to reduction of oxidative stress and Cx43 expression.
Keywords: Pentylenetetrazole, Ceftriaxone, Connexin 43, Epilepsy, Malondialdehyde (MDA), Rats
Introduction
Out of all neurodegenerative disorders, epilepsy is one of the most poignant and most severe. Seeing somebody falling into relentless convulsions is tragic. We can say that this person has epilepsy if he or she develops at least two unprovoked seizures with more than 24 hrs apart or one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years or if he or she is diagnosed with epilepsy syndrome.1 It is an increasing burden on the world with about 0.5–2% of population has epilepsy worldwide.2 Bad news is that approximately 30% of the patients have refractory epilepsy which isn’t relieved even after accurate diagnosis and treatment.3
To understand the process of epileptogenesis, kindling model is implicated. It is a chronic animal model of epilepsy produced by chemical or electrical stimuli. Of the chemical stimuli, pentylenetetrazol (PTZ) kindling is a widely used method by using subconvulsive doses that is applied intermittently and repetitively to produce full-blown convulsions.4 PTZ kindled rat model simulates human temporal lobe epilepsy (TLE) signs: hippocampal atrophy, loss of neurons in the limbic areas: CA1, CA3, and dentate gyrus of the hippocampus, glass and growth of neuronal connections. Mechanisms underlying pathogenesis of epilepsy might include oxidative stress and altered gap junction communication between neurons. Gupta et al reported a role for reactive oxygen species (ROS) and oxidative stress in the epileptogenesis.5 This oxidative stress could facilitates more connexins to reach the cell membrane due to a decrease in the endoplasmic reticulum associated degradation of connexins.6,7 The role of gap junction proteins (connexins) in epileptogenesis has been investigated by many experimental studies.8,9 Connexins-32 (Cx-32) and Cx-43 expression in the rats hippocampus and cortex was investigated by Zhao et al., the results demonstrated an evident expression after 8 hrs of PTZ administration where the first expression noted after only 2 hrs.10 It has been documented that pharmacological inhibition of gap junction by drugs such as carbenoxolone and glycyrrhetinic acid may be effective anticonvulsant therapy.11
The relationship between beta-lactam antibiotics and epilepsy has been studied several times. Some laboratory and clinical evidences showed that some of these drugs have epileptogenic tendencies with proposed mechanism of inhibiting post-synaptic inhibitory responses of GABA-A receptors.12 On the other hand, Jelenkovic et al. have got positive results on the frequency and latency of generalized clonic convulsions (GCCs) after i.p. administration of 200 mg/kg ceftriaxone on the PTZ-kindled mice.13 Down-regulation of glutamate transporter-1 (GLT-1) and accumulation of glutamate in the brain have been documented in many neurological disorders such as amyotrophic lateral sclerosis,14,15 Alzheimer disease,16 several forms of epilepsy,17 ischemia/stroke and traumatic brain injury.18 Recently, it has been demonstrated found that ceftriaxone enhances glutamate re-uptake by up regulating glutamate transporter-1 (GLT-1)19 suggesting that it may have an anti-epileptic effect. So, in the present study we investigated the possible neuroprotective effect of ceftriaxone in PTZ-induced seizure model as well as its effects on the markers of oxidative stress and expression of CX-43 in hippocampus of PTZ kindled animal model.
Methods
Experimental animals
Twenty four male Sprague-Dawely rats weighing 180–200 g were individually housed in standard cages at Department of Physiology, Mansoura Faculty of Medicine. Animals fed on standard diet and water adlibitum. All experimental procedures were done according to the international guidelines for the care of the experimental animals and were approved by our local committee of ethics for animal use and care.
Experimental Design
Rats were randomly divided into 3 equal groups (each contains 8 rats) as follows:
Normal group: normal animals received 0.2 mL saline i.p. via intraperitoneal injection (i.p.)
PTZ group: rats received PTZ (50 mg/kg i.p in 0.2 mL) via i.p. injection on alternate day for 2 weeks (7 doses of PTZ)
Ceft group: as PTZ group but rats received ceftriaxone (200 mg/kg/12 hrs i.p.) from 6th dose of PTZ for 3 days (at the end)
Induction of PTZ-induced Kindling Rat Model and behavioral assessment
PTZ was dissolved in saline and given at 50 mg/kg i.p. on alternate day for 14 days i.e. 7 doses were given to every rat. Full animal kindling started after 6th dose o PTZ. After each injection of PTZ, the rats were placed singly in isolated transparent Plexiglas cages and their convulsive behavior was observed and videotaped for 30 min. The latency of the onset of seizure (seconds), seizure duration (seconds) and severity of seizures was scored according to Racine’s scale20 as follows: 0 = normal, nonepileptic activity, 1 = mouth and facial movements, hyperactivity, grooming, sniffing, scratching, wet dog shakes, 2 = head nodding, staring, tremor, 3 = forelimb clonus, forelimb extension, 4 = rearing, salivating, tonic clonic activity and 5 = falling, status epilepticus
Collection of samples
When the animal became fully kindled (exhibited stage 4 or 5 of seizure score on three consecutive trials), on the next day, it was sacrificed by overdose of intraperitoneal Na+ thiopental (120 mg/kg) anesthesia. Then 1 mL blood was collected from ophthalmic capillary plexus by Pasteur petite. Brain was perfused transcardially with 100 mL heparinized saline followed by 150 mL of 10% formalin. Then, collected brains were postfixed in 10% paraformaldehyde for 4 h and stored in a 25% sucrose plus 0.1% sodium azide solution till time of processing. For biochemical assay of markers of oxidative stress brain was collected after saline perfusion only.
Assay of lipid peroxidations marker (MDA) and antioxidants (GSH activity) in brain tissues
About 50–100 mg of brain tissues from 4 rats was homogenized in 1–2 mL cold buffer (50 mM potassium phosphate, pH 7.5,1 mM EDTA) using mortar and pestle then centrifuged at 4,000 rpm for 15 minutes at 4°C. The supernatant was kept at − 20°C until it was used for analysis of oxidant and antioxidants. Malondialdhyde (MDA), reduced glutathione (GSH) and catalase enzyme activity in the supernatant of kidney homogenates were measured using a colorimetric method according to the manufacturer’s instructions (Bio-Diagnostics, Dokki, Giza, Egypt).
Measurement of expression of connexins 43 by immunohistochemistry in hippocampal neurons
Serial coronal sections (40 μm) of brain from 4 rats were cut using a freezing sledge microtome and a 1:6 series was used for all quantitative immunohistochemistry. Peroxidase-based immunostaining staining was completed as described previously.21 In brief, following quenching of endogenous peroxidase activity where appropriate (using a solution of 3% hydrogen peroxide/10% methanol in distilled water) and blocking of nonspecific secondary antibody binding (using 3% normal serum in Tris-buffered saline (TBS) with 0.2% Triton X-100 at room temperature for 1 h), sections were incubated overnight at room temperature with the appropriate primary antibody diluted in 1% normal serum in TBS with 0.2% Triton X-100; polyclonal rabbit anti-Cx43 antiserum (Zymed); 1:1,000. Sections were then incubated for 3 h at room temperature with peroxidase-based immunohistochemical staining, in a biotinylated secondary antibody for 3 h (horse anti-mouse, BA2001, 1:200; Vector, Burlingame, CA, USA), followed by a 2-h incubation in streptavidin–biotin–horseradish peroxidase solution (Vector. For peroxidase-based immunohistochemistry, sections were developed in 0.5% solution of diaminobenzidine tetrahydrochloride (DAB; Sigma–Aldrich) in Tris-buffer containing 0.3 lL/mL of hydrogen peroxide. Sections were mounted on gelatin-coated microscope slides, dehydrated in ascending concentrations of alcohols, cleared in xylene and cover-slipped using DPX mountant (BDH chemicals).
Image Quantification
Slides were photographed using Olympus® digital camera installed on Olympus® microscope with 1/2 X photo adaptor, using 40 X objective. Image analyses were performed with ImageJ software (National Institute of Mental Health, Bethesda, Maryland, USA) as previously described,22 employing approximately 30 hippocampal slices from 4 animals (6–8 hippocampal slices from each animal) in each group. After channel separation (RGB) of the color images, we performed quantification of the mean pixel intensity, where values correspond to the brightness of the pixels of delimited areas corresponding to the cell nuclei. For Cx43 analysis, after channel separation (RGB) of color images, we performed quantification of the mean area of interest (AOI) in hippocampal regions.
Statistical analysis
SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Mann-Whitney test and Wilcoxon test were used for statistical evaluation of seizure scores, while independent T test, paired T test and One ANOVA with Tukey’s posthoc test were used for other quantitative data. The differences were considered statistically significant at probability level p ≤ 0.05.
Results
Effects of ceftriaxone on Racine’s score in PTZ-induced seizures
Racine’s stage score showed gradual increase in PTZ and Ceft groups among all trials compared to their basal values which became significant in last 3 records in PTZ group and on 5th trial in Ceft group (p < 0.01). Also, Ceft group showed non-significant decrease in Racine’s score in the last 2 trials compared to 5th trial record. On other hand, there were significant decrease in Racine’s score in Ceft group compared to PTZ group in last 2 trial records (p < 0.01) (Table 1).
Table 1.
Racine’s stage score in PTZ and ceftriaxone groups at different trial records
| Record | Group | p-value* | ||
|---|---|---|---|---|
|
| ||||
| PTZ group (n = 8) | Ceft group (n = 8) | |||
| 1st | Median | 2.33 | 2.167 | 0.863 |
| Minimum | 2.0 | 1.0 | ||
| Maximum | 3.0 | 3.0 | ||
| 2nd | Median | 2.50 | 2.33 | 0.789 |
| Minimum | 2.0 | 1.0 | ||
| Maximum | 3.0 | 3.0 | ||
| 3rd | Median | 3.00 | 3.167 | |
| Minimum | 2.0 | 2.0 | 0.733 | |
| Maximum | 4.0 | 4.0 | ||
| 4th | Median | 3.50 | 3.33 | 0.575 |
| Minimum | 3.0 | 3.0 | ||
| Maximum | 4.0 | 4.0 | ||
| 5th | Median | 4.00† | 4.167† | 0.733 |
| Minimum | 3.0 | 3.0 | ||
| Maximum | 5.0 | 5.0 | ||
| 6th | Median | 4.167† | 3.00‡ | 0.022 |
| Minimum | 3.0 | 2.0 | ||
| Maximum | 5.0 | 4.0 | ||
| 7th | Median | 4.167† | 3.00‡ | 0.044 |
| Minimum | 3.0 | 2.0 | ||
| Maximum | 5.0 | 4.0 | ||
Data were expressed as median and minimum and maximum.
PTZ = pentylenetetrazole; Ceft = ceftriaxone.
p = Mann-Whitney’s test for comparison between 2 groups at the same time. Wilcoxon test for comparison between 2 time intervals in the same group;
Significant vs. 1st record of the same time group (p ≤ 0.041);
Significant vs. PTZ group of the same time period (p ≤ 0.041).
Effects of ceftriaxone on onset latency (sec) and duration (sec) of PTZ-induced seizure
PTZ and Ceft groups showed significant gradual decrease in seizure onset latency with significant increase in seizure duration among all trials compared to their basal values (p < 0.001). Moreover, Ceft group showed significant increase in latency onset with significant decrease in seizure duration in the last 2 trials compared to previous ones (p < 0.001). Also, Ceft group showed significant increase in seizure latency onset with significant decrease in seizure duration compared to PTZ group in the last 2 doses (p < 0.01) (Table 2).
Table 2.
Latency of PTZ-induced seizure (sec) and duration of PTZ-induced seizure (sec) in PTZ and ceftriaxone groups in different trial records
| Record Group | 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | |
|---|---|---|---|---|---|---|---|---|
| Latency of Seizure (sec) | ||||||||
| PTZ group (n = 8) | 327.0 ± 28.50 | 174.0 ± 49.04* | 75.70 ± 12.50*§ | 56.20 ± 11.00*§‖ | 25.20 ± 10.43*§‖† | 23.11 ± 9.80*§‖† | 24.70 ± 8.60*§‖† | |
| Ceft- group (n = 8) | 319.01 ± 35.05 | 182.0 ± 38.60* | 89.20 ± 15.45*§ | 49.70 ± 14.30*§‖ | 29.10 ± 8.15*§‖† | 110.50 ± 25.90*§‖†‡¶ | 129.06 ± 15.60*§‖†‡¶ | |
| Duration of Seizure (sec) | ||||||||
| PTZ group (n = 8) | 4.10 ± 0.63 | 21.40 ± 1.95* | 104.50 ± 39.10*§ | 165.0 ± 28.80*§ | 185.50 ± 33.50*§† | 211.50 ± 29.70*§‖†‡ | 218.50 ± 25.40*§‖†‡ | |
| Ceft- group (n = 8) | 5.10 ± 0.95 | 29.0 ± 7.80* | 124.0 ± 28.60*§ | 159.50 ± 42.50*§ | 178.60 ± 26.80*§† | 115.50 ± 25.70*§‖†‡¶ | 101.50 ± 11.30*§‖†‡¶ |
Data expressed as mean ± SD. Independent T test for comparisons between 2 groups of the same time and paired T test for comparison between 2 trials of the same group.
PTZ, pentylenetetrazole; Ceft, ceftriaxone.
Significant vs. 1st trial;
Significant vs. 4th trial;
Significant vs. 5th trial;
Significant vs. 2nd trial;
Significant vs. 3rd trial;
Significant vs. PTZ group of the same time period.
Effects of ceftriaxone on oxidative stress markers (MDA, GSH and catalase activity) in rat hippocampus
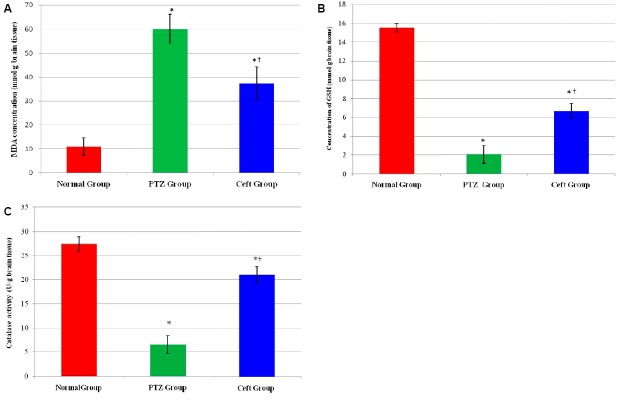
Assessment of oxidative stress markers in hippocampal region showed significant increase in MDA and significant decrease in (GSH and catalase activity) in PTZ group compared to normal group (p < 0.01). On the other hand, Ceft group showed significant increase in GSH concentration and catalase activity with significant decrease in MDA concentration compared to PTZ group (p < 0.01) (Fig. 1).
Figure 1.
Markers of oxidative stress in hippocamal regions of rat’s brain in different groups. Malondialdehyde (MDA) (nmol/g brain tissues) (A), reduced glutathione (GSH) concentration mmol/g brain tissue (B) and catalase activity (U/g brain tissues) (C). Ceft, ceftriaxone. *Significant vs. normal group (p < 0.01); †Significant vs. pentylenetetrazole (PTZ) group (p < 0.05).
Effects of ceftriaxone on Cx43 protein expression in CA3 region of hippocampus
Immunohistochemical examination showed significant increase in the mean area of interest (AOI) of Cx43 positive cells in CA3 region of hippocampus in PTZ and Ceft groups compared to normal group (p < 0.001). Also, Ceft group showed significant decrease in Cx43 score compared to PTZ group (Fig. 2A) (p < 0.001). Brain sections obtained from normal group showed negative expression of Cx43 in normal group (Fig. 2B), high membranous expression in PTZ group (Fig. 2C, D), and low expression (negative) in Ceft group (Fig. 2E).
Figure 2.
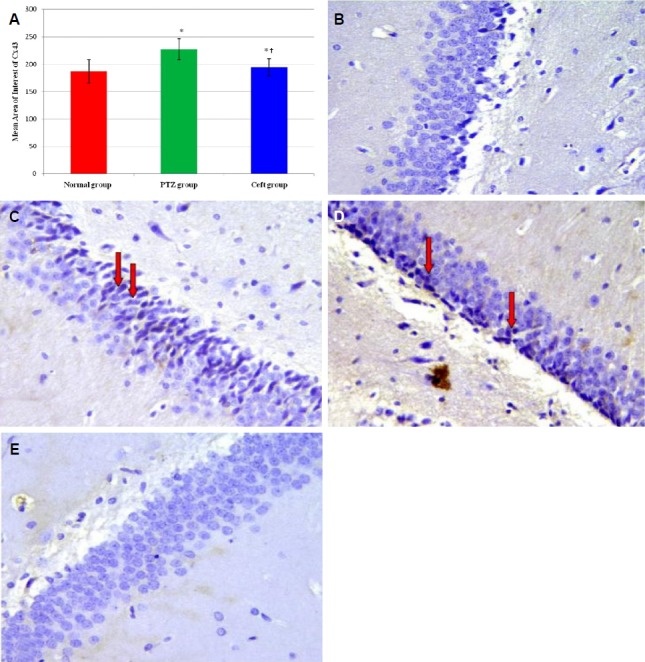
Mean area of interest of Cx43 positivity in CA3 region of hippocampus from different groups (A). Section of brain in CA3 region of hippocampus showing negative expression for Cx43 (normal group) (B). High membranous brown staining for Cx43 (arrows) (pentylenetetrazole [PTZ] group) (C, D) and negative expression for Cx43 (Ceft-group) (E) (400×). Ceft, ceftriaxone. *Significant vs. normal group (p < 0.01); †Significant vs. PTZ group (p < 0.05).
Discussion
The present study demonstrated neuroprotective effects for beta lactam antibiotic (ceftriaxone) against PTZ induced seizures in the form of reduction in seizure stage and duration and prolongation of seizure latency as well as attenuation of oxidative stress and down regulation of Cx-43 expression in CA3 region of hippocampus. Kindling means giving subsequent subconvulsive doses until generalized clonic convulsions are detected with the continuity of such hyper-excitable state.
On the footsteps of Goddard who first studied kindling model in 1967, a lot of scientists used kindling model (whether electrical as Goddard did or chemical by using PTZ for example as we did) to study either the possible neuromechanisms of epileptogenesis or the neuroprotective effects of some drugs on this model as a step on the way of developing safe and effective antiepileptic drugs.23 In the present study a dose of 50 mg/kg PTZ was given every other day to develop a model of chronic PTZ-induced epilepsy. The present study demonstrated that PTZ administration caused significant increase in Racine score and duration of seizure with significant decrease in onset of developing seizure. These findings are in agreement with previous studies.24,25 Rajabzadeh et al developed a rat model of PTZ-induced chronic epilepsy by i.p. injection of 40 mg/Kg PTZ- every 48 hrs with total a total of 12–15 doses of PTZ were given to each rat.24
Also, the present study demonstrated that treatment with ceftriaxone in PTZ kindling model greatly reduced tonic-clonic convulsions and duration of these convulsions and prolonged the latency time for their erupting. The relationship between beta lactam antibiotics and epilepsy captured the attention of scientists in the last few years; the first convulsant effect of beta lactam antibiotic studied was that of penicillin however, other members of this generation showed comparative convulsant actions.26 Chen et al used systemic penicillin as an experimental model of epilepsy in 1986.27 Convulsant action of carbapenem also was studied by Shimada et al in 1992 in one study, seven different cephalosprins are entangled in an in-vivo and in-vitro models to demonstrate whether this convulsive activity is mediated through GABA-A receptor inhibition or through NMDA receptor modulation.28,12 The results of a blinded screen of 1,040 FDA approved drugs carried out by Rothstein et al in 2005.29 In agreement with the finding of the present study, Jelenkovic et al, reported an anticonvulsant effect of ceftriaxone using PTZ kindling model in two different mice strains in their young age and after adulthood.13
It was postulated that oxidative stress and ROS play an important role in development of epilepsy because oxidative stress resulting from mitochondrial dysfunction gradually disrupts the intracellular calcium homeostasis, which modulates neuronal excitability and synaptic transmission making neurons more vulnerable to additional stress, and leads to neuronal loss in epilepsy.30 In consistence with this hypothesis, the present study demonstrated significant increase in oxidative stress in hippocampus of rats treated with PTZ as evidenced by significant increase in MDA and significant decrease in GSH and catalase activities in rat hippocampus. In line with these findings, Shin et al demonstrated that kainate (KA)-induced increased seizure susceptibility is associated increased mitochondrial lipid peroxidation and protein oxidation and mitochondrial loss of glutathione homeostasis in rat hippocampus.31 Also, we demonstrated in the present study that pretreatment with ceftriaxone showed significant attenuation of oxidative stress markers in CA3 region of hippocampus suggesting antioxidant effects for ceftriaxone. These findings suggest neuroprotective and antioxidant for ceftriaxone antibiotic probably by upregulation of glutamate transporter-1 resulting in reduction in glutamate chemical transmitter and Ca+2 overload which are the main mechanisms involved in enhanced production of ROS in hippocampus during epileptic seizure.32 The neuroprotective and antioxidant effect for ceftriaxone was demonstrated also, by Altaş et al, who demonstrated that ceftriaxone treatment caused significant increase in glutathione peroxidase and SOD activities with significant decrease in MDA in rat’s brain exposed to ischemia.33
The role of gap junction proteins (connexins, Cx) in development of epilepsy has been documented in several studies. Laura et al. demonstrated an increase in the protein expression of Cx-32 and Cx-43 in oligodendrocytes in the right dentate gyrus and in principal cells of the hippocampus in 4-aminopyrine –induced seizure within 60 min.34 In the present study, we demonstrated significant increase in Cx-43 protein in CA3 region of hippocampus in rats treated with PTZ. These results agree with previous studies in which an increase in Cx-43 protein was observed in hippocampal slices treated with pilocarpine and bicuculline.35,36 In addition, an increase in the mRNA of Cx 43 has been observed in the hippocampus of patients with temporal lobe epilepsy (TLE).37 Also, Zhao et al. demonstrated significant increase in the expression of Cx-32 and Cx-43 in the cortex and hippocampus of rats 2 h after PTZ administration and their expression became more obvious 8 h after PTZ administration.10 Upregulation of Cx-43 might be explained by enhanced oxidative stress which could facilitates more connexins to reach the membrane due to reduction in connexin degradation by endoplasmic reticulum.6,7
Also, we demonstrated, in the present study, that ceftriaxone treatment caused significant reduction in Cx-43 expression in hippocamal regions especially in pretreatment. Down regulation of Cx-43 expression in ceftriaxone treated group might be due to reduction of oxidative stress in brain tissues. Studies have shown that the opening of Cx-43 hemichannels is promoted by positive transmembrane voltages and reduced concentrations of extracellular divalent cations such as Ca2+ and its closure is promoted by high intracellular H+ and phosphorylation of Cx-43.38–41 Also, Lin et al. demonstrated that ceftriaxone dramatically inhibited glutamate release from synaptosomes by suppression of voltage-dependent Ca2+ entry through Cav2.2 and Cav2.1 channels, suggesting that ceftriaxone is an effective inhibitor of presynaptic glutamate release.42 So, inactivation of Cx-43 hemichannels might be another possible mechanism for the anti-convulsive action of ceftriaxone. However we did not investigate this mechanism in our study.
In conclusion, this study represents a forward step on the way of anticonvulsant effect of ceftriaxone on behavioral changes, oxidative stress markers and Cx-43. Of course it will be a myopic view to use these data as a full evidence to engage ceftriaxone in treating epilepsy, further studies needed in other animal species and measuring other markers as GLT-1 levels and other oxidative stress markers and the possible side effects of such dose of ceftriaxone.
References
- 1.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 2.Naseer MI, Shupeng L, Kim MO. Maternal epileptic seizure induced by pentylenetetrazol: apoptotic neurodegeneration and decreased GABAB receptor expression in prenatal rat brain. Mol Brain. 2009;2:20. doi: 10.1186/1756-6606-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picot MC, Baldy-Moulinier M, Daurès JP, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a Western European country. Epilepsia. 2008;49:1230–8. doi: 10.1111/j.1528-1167.2008.01579.x. [DOI] [PubMed] [Google Scholar]
- 4.Dhir A. Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci. 2012 doi: 10.1002/0471142301.ns0937s58. Chapter 9:Unit9.37. [DOI] [PubMed] [Google Scholar]
- 5.Gupta YK, Veerendra Kumar MH, Srivastava AK. Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacol Biochem Behav. 2003;74:579–85. doi: 10.1016/s0091-3057(02)01044-4. [DOI] [PubMed] [Google Scholar]
- 6.VanSlyke JK, Musil LS. Dislocation and degradation from the ER are regulated by cytosolic stress. J Cell Biol. 2002;157:381–94. doi: 10.1083/jcb.200111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su V, Lau AF. Connexins: mechanisms regulating protein levels and intercellular communication. FEBS Lett. 2014;588:1212–20. doi: 10.1016/j.febslet.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakase T, Naus CC. Gap junctions and neurological disorders of the central nervous system. Biochim Biophys Acta. 2004;1662:149–58. doi: 10.1016/j.bbamem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Mylvaganam S, Ramani M, Krawczyk M, Carlen PL. Roles of gap junctions, connexins, and pannexins in epilepsy. Front Physiol. 2014;5:172. doi: 10.3389/fphys.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Xh, Cao Ll, Wang Sj, Zhang Tx, Liu XW, Chi ZF. Role of gap junction in pentylenetetrazol-induced epilepsy in rats. Chin J Pathophysiol. 2012;1:118–23. [Google Scholar]
- 11.Nemani VM, Binder DK. Emerging role of gap junctions in epilepsy. Histol Histopathol. 2005;20:253–59. doi: 10.14670/HH-20.253. [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto M, Uchida I, Mashimo T, et al. Evidence for the involvement of GABA(A) receptor blockade in convulsions induced by cephalosporins. Neuropharmacology. 2003;45:304–14. doi: 10.1016/s0028-3908(03)00188-6. [DOI] [PubMed] [Google Scholar]
- 13.Jelenkovic AV, Jovanovic MD, Stanimirovic DD, Bokonjic DD, Ocic GG, Boskovic BS. Beneficial effects of ceftriaxone against pentylenetetrazole-evoked convulsions. Exp Biol Med (Maywood) 2008;233:1389–94. doi: 10.3181/0803-RM-83. [DOI] [PubMed] [Google Scholar]
- 14.Trotti D, Rolfs A, Danbolt NC, Brown RH, Jr, Hediger MA. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat Neurosci. 1999;2:427–33. doi: 10.1038/8091. [DOI] [PubMed] [Google Scholar]
- 15.Trotti D, Aoki M, Pasinelli P, et al. Amyotrophic lateral sclerosis-linked glutamate transporter mutant has impaired glutamate clearance capacity. J Biol Chem. 2001;276:576–82. doi: 10.1074/jbc.M003779200. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Mallory M, Alford M, Tanaka S, Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol. 1997;56:901–11. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K, Watase K, Manabe T, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 18.Martin LJ, Brambrink AM, Lehmann C, et al. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol. 1997;42:335–48. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- 19.Abulseoud OA, Camsari UM, Ruby CL, Kasasbeh A, Choi S, Choi DS. Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology. 2014;39:1674–84. doi: 10.1038/npp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 21.Moloney TC, Rooney GE, Barry FP, Howard L, Dowd E. Potential of rat bone marrow-derived mesenchymal stem cells as vehicles for delivery of neurotrophins to the Parkinsonian rat brain. Brain Res. 2010;1359:33–43. doi: 10.1016/j.brainres.2010.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Kinjo ER, Higa GS, de Sousa E, et al. A possible new mechanism for the control of miRNA expression in neurons. Exp Neurol. 2013;248:546–58. doi: 10.1016/j.expneurol.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Goddard GV. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214:1020–1. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- 24.Rajabzadeh A, Bideskan AE, Fazel A, Sankian M, Rafatpanah H, Haghir H. The effect of PTZ-induced epileptic seizures on hippocampal expression of PSA-NCAM in offspring born to kindled rats. J Biomed Sci. 2012;19:56. doi: 10.1186/1423-0127-19-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saha L, Bhandari S, Bhatia A, Banerjee D, Chakrabarti A. Anti-kindling effect of bezafibrate, a peroxisome proliferator-activated receptors alpha agonist, in pentylenetetrazole induced kindling seizure model. J Epilepsy Res. 2014;4:45–54. doi: 10.14581/jer.14011. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Horiuchi M, Kimura M, Tokumura M, Hasebe N, Arai T, Abe K. Absence of convulsive liability of doripenem, a new carbapenem antibiotic, in comparison with b-lactam antibiotics. Toxicology. 2006;222:114–24. doi: 10.1016/j.tox.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Chen RC, Huang YH, How SW. Systemic penicillin as an experimental model of epilepsy. Exp Neurol. 1986;92:533–40. doi: 10.1016/0014-4886(86)90295-5. [DOI] [PubMed] [Google Scholar]
- 28.Shimada J, Hori S, Kanemitsu K, Shoji Y, Nakashio S, Yanagawa A. A comparative study on the convulsant activity of carbapenems and beta-lactams. Drugs Exp Clin Res. 1992;18:377–81. [PubMed] [Google Scholar]
- 29.Rothstein JD, Patel S, Regan MR, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 30.Chang SJ, Yu BC. Mitochondrial matters of the brain: mitochondrial dysfunction and oxidative status in epilepsy. J Bioenerg Biomembr. 2010;42:457–59. doi: 10.1007/s10863-010-9317-4. [DOI] [PubMed] [Google Scholar]
- 31.Shin EJ, Jeong JH, Bing G, et al. Kainate-induced mitochondrial oxidative stress contributes to hippocampal degeneration in senescence-accelerated mice. Cell Signal. 2008;20:645–58. doi: 10.1016/j.cellsig.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Puttachary S, Sharma S, Stark S, Thippeswamy T. Seizure-induced oxidative stress in temporal lobe epilepsy. Biomed Res Int. 2015;2015:745613. doi: 10.1155/2015/745613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altaş M, Meydan S, Aras M, et al. Effects of ceftriaxone on ischemia/reperfusion injury in rat brain. J Clin Neurosci. 2013;20:457–61. doi: 10.1016/j.jocn.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 34.Laura MC, Xóchitl FP, Anne S, Alberto MV. Analysis of connexin expression during seizures induced by 4-aminopyridine in the rat hippocampus. J Biomed Sci. 2015;22:69. doi: 10.1186/s12929-015-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su M, Tong XX. Astrocytic gap junction in the hippocampus of rats with lithium pilocarpine-induced epilepsy. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:2738–41. [PubMed] [Google Scholar]
- 36.Samoilova M, Li J, Pelletier MR, et al. Epileptiform activity in hippocampal slice cultures exposed chronically to bicuculline: increased gap junctional function and expression. J Neurochem. 2003;86:687–99. doi: 10.1046/j.1471-4159.2003.01893.x. [DOI] [PubMed] [Google Scholar]
- 37.Fonseca CG, Green CR, Nicholson LF. Upregulation in astrocytic connexin 43 gap junction levels may exacerbate generalized seizures in mesial temporal lobe epilepsy. Brain Res. 2002;929:105–16. doi: 10.1016/s0006-8993(01)03289-9. [DOI] [PubMed] [Google Scholar]
- 38.Chang M, Werner R, Dahl G. A role for an inhibitory connexin in testis? Dev Biol. 1996;175:50–6. doi: 10.1006/dbio.1996.0094. [DOI] [PubMed] [Google Scholar]
- 39.Bennett MV, Contreras JE, Bukauskas FF, Sáez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–7. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sáez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta. 2005;1711:215–24. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verselis VK, Srinivas M. Divalent cations regulate connexin hemichannels by modulating intrinsic voltage-dependent gating. J Gen Physiol. 2008;132:315–27. doi: 10.1085/jgp.200810029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin TY, Lu CW, Huang SK, Wang SJ. Ferulic acid suppresses glutamate release through inhibition of voltage-dependent calcium entry in rat cerebrocortical nerve terminals. J Med Food. 2013;16:112–9. doi: 10.1089/jmf.2012.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]