Abstract
The oxidation of guanosine to 8-oxo-2'-deoxyguanosine (8-oxo-dG) in DNA is closely associated with induction of various diseases, but the endogenous oxidant species involved remains unclear. Hydrogen peroxides (H2O2) have been considered to be the oxidant, while lipid peroxides are another possible oxidant because generated easily in bio-membranes surrounding DNA. The oxidant potency was compared between lipid peroxides and H2O2. Linoleic acid hydroperoxides (LOOH) formed 8-oxo-dG at a higher level than H2O2 in guanosine or double-stranded DNA. In the presence of a physiological concentration of Fe2+ to produce hydroxyl radicals, LOOH was also a stronger oxidant. In a lipid micelle, LOOH markedly produced 8-oxo-dG at a concentration one-tenth of that of H2O2. Upon adding to rat hepatic mitochondria, phosphatidylcholine hydroperoxides produced 8-oxo-dG abundantly. Employing HepG2 cells after pretreated with glutathione peroxidase inhibitor, LOOH formed 8-oxo-dG more abundantly than H2O2. Then, antioxidants to suppress the 8-oxo-dG formation were examined, when the nuclei of pre-incubated HepG2 with antioxidants were exposed to LOOH. Water-soluble ascorbic acid, trolox, and N-acetyl cysteine showed no or weak antioxidant potency, while lipid-soluble 2,6-dipalmitoyl ascorbic acid, α-tocopherol, and lipid-soluble phytochemicals exhibited stronger potency. The present study shows preferential formation of 8-oxo-dG upon LOOH and the inhibition by lipid-soluble antioxidants.
Keywords: 8-OHdG, lipid peroxides, hydrogen peroxide, oxidation of guanosine, lipid-soluble antioxidants
Introduction
The oxidative injury of DNA is closely associated with the induction of degenerative diseases including cancer. Among DNA bases, deoxyguanosine (dG) is most easily oxidized and forms 8-oxo-2'-deoxyguanosine (8-oxo-dG) because its 8-position is substitutable and has very low redox potential.(1) The formed 8-oxo-dG can pair with adenine and lead to G:C→T:A transversion mutations unless repaired before replication.(2,3) Indeed, 8-oxo-dG has been frequently detected in mutated genes, carcinomas, and tumors cells,(4–8) and the 8-oxo-dG levels have been adopted as a biomarker of oxidative stress, which causes degenerative diseases.(1,9–11) Thus, the formation of 8-oxo-dG in DNA has been considered to cause the induction of severe diseases. However, some issues regarding the mechanism of formation of 8-oxo-dG remain unclear and particularly the endogenous oxidants that generate 8-oxo-dG are unclear.(12,13) Knowledge of such endogenous oxidants will promote our understanding of antioxidants that can prevent disease.
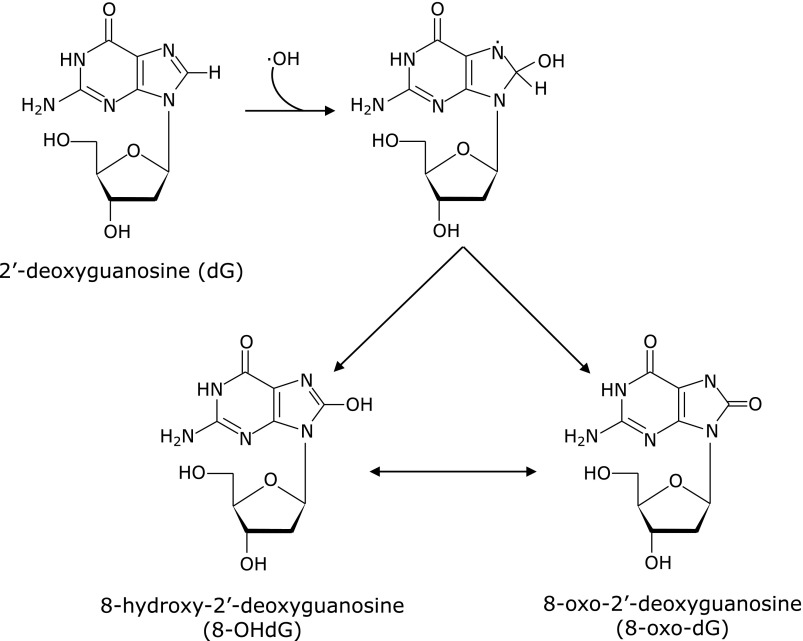
Endogenous oxidants are superoxide anion and its derivatives, such as H2O2, lipid peroxides, OH radical, and peroxynitrite.(14–16) Another reactive oxygen species, singlet oxygen, is electronically excited molecular oxygen that is produced by photochemical reactions or biological dark reactions with chloroperoxidase, lactoperoxidase, and lipoxygenase, and is also generated in humans but is not frequently.(17,18) It has been generally recognized that superoxide anion does not attack dG directly and that it can attack guanosine after being dis-proportionated to H2O2 and decomposed to OH radical.(19) Peroxynitrite cannot so frequently participate in the formation of 8-oxo-dG.(20,21) Thus, candidates for the endogenous oxidants that form 8-oxo-dG would be H2O2, lipid peroxides, and/or OH radical, generating it from peroxides, and the formation of 8-oxo-dG by OH radical has been considered as shown in Fig. 1.(22)
Fig. 1.
Formation of 8-oxo-2'-deoxyguanosine (8-oxo-dG) from 2'-deoxyguanosine by OH radicals. C8-OH-adduct radical that formed by an attack of OH radical to 2'-deoxyguanosine is followed by oxidation to 8-OHdG or its tautomer 8-oxo-dG.
OH radical is generated endogenously through Fenton’s reaction from H2O2. Fenton’s reaction requires transition metals such as Cu+ and Fe2+.(23,24) Additionally, the half-life of the OH radical generated is very short, approximately 10−9 s.(25) These factors indicate that transition metals and H2O2 should coexist near DNA for 8-oxo-dG production, and such coexistence is highly limited in living cells. In contrast, lipid peroxides are easily generated by various reactive oxygen species and increase in the concentration in membranous phospholipids, and their half-life is markedly longer than that of OH radical, at 7 s.(26,27) Additionally, the second position of membranous phospholipids is composed abundantly of polyunsaturated fatty acids. Thus, lipid peroxides can arise in nuclear and mitochondrial membranes. Particularly in the inner mitochondrial membrane, the mitochondrial electron transfer system easily generates superoxide anion and peroxidizes membranous lipid.(14,16) The lipid peroxides generated are assumed to attack mitochondrial DNA readily because of being located close by in the inner membrane. Hruszkewycz et al.(28) showed that isolated mitochondria produced 8-oxo-dG by the induction of lipid peroxidation. Park et al.(26) showed that calf thymus DNA produced 8-oxo-dG when mixed with peroxidizing lipids. Additionally, in our previous study, the formation of 8-oxo-dG from dG required the coexistence of thymidine and the formation of peroxide on the C-5 methyl of thymidine.(13) Thus, the lipid peroxides may be the strongest candidate for an endogenous oxidant to produce 8-oxo-dG.
In the present study, we prepared linoleic acid hydroperoxides (LOOH) and phosphatidylcholine hydroperoxides (PCOOH) for the lipid peroxides, and compared them in terms of the production potency of 8-oxo-dG with H2O2. In addition, dietary bioavailable antioxidants were examined in terms of suppressing the formation of 8-oxo-dG.
Materials and Methods
Chemicals
dG was purchased from Wako Pure Chem. Ind., Ltd. (Osaka, Japan), which contained 8-oxo-dG at a level of 0.60 ± 0.15 per 105 dG. Standard 8-oxo-dG and calf thymus DNA (type I, highly polymerized) were obtained from Sigma (St. Louis, MO). Ascorbic acid, dl-α-tocopherol, and N-acetyl-l-cysteine (NAC) were purchased from Nacalai Tesque (Kyoto, Japan). All other reagents used were of the highest grade available from commercial sources.
Preparation of lipid peroxides
LOOH was prepared from auto-oxidized linoleic acid, as mentioned previously.(29) Briefly, the auto-oxidized linoleic acid was first purified by silica gel column chromatography and then HPLC equipped with a column of SILICA (5 µm mesh and φ 4.6 × 250 mm, SG120; Shiseido, Tokyo, Japan) eluting with a mixed mobile solvent of 97.3% n-hexane, 2.5% isopropyl alcohol, and 0.2% acetic acid (v/v). The fraction of four LOOH isomers, 13-hydroperoxy-(9Z,11E)- and 13-hydroperoxy-(9E,11E)-octadeca-9,11-dienoic acid, and 9-hydroperoxy-(10E,12Z)- and 9-hydroperoxy-(10E,12E)-octadeca-10,12-dienoic acid, were collected, and they were characterized in terms of the peroxide value (PV) and UV absorption at 233 nm for conjugated diene after drying under a nitrogen gas stream.(30,31) The PV was 3.32 µeq/kg in 0.16 mg of dried LOOH (M.W., 294) and the concentration of conjugated diene was 0.53 µmol/L in 0.16 mg; the purity of LOOH was thus calculated to be more than 97% as linoleic acid hydroperoxides. LOOH at a concentration of 100 mmol/L in methanol was stored at −30°C in the dark until use.
Phosphatidylcholine hydroperoxides (PCOOH) were prepared and purified according to the method of Wrona.(32) Briefly, phosphatidylcholine at 20 mg was dissolved in 2 ml of chloroform/methanol (1:1) and autoxidized at 37°C for 5 days. After being dried and dissolved in 1 ml of methanol, the autoxidized phosphatidylcholine was purified by HPLC under the following conditions: column, Capcell pak C18 MG maintained at 35°C; mobile phase, a mixed solvent of 95% methanol and 5% water; and flow rate, 2.0 ml/min. The collected fraction of PCOOH was characterized by PV and UV spectrophotometry similarly to LOOH and determined to be more than 96% pure.
Exposure of dG and calf thymus DNA to oxidants
dG at 250 µmol/L or calf thymus DNA at 10 µg was added to 1 ml of TE buffer (10 mM Tris-HCl containing 1 mM EDTA at pH 7.4) and 1 mM ethylene diamine tetraacetate (EDTA) and mixed in 50 µmol/L LOOH or H2O2, followed by incubation with or without FeSO4 at 37°C for 1 h. The dG mixture was subsequently subjected to determination of the production of 8-oxo-dG by HPLC. In the case of calf thymus mixture, the DNA was precipitated by adding 110 µl of 1 M NaI and 750 µl of ice-cold 2-propanol and centrifuged at 20,600 × g for 15 min at 4°C after the 1 h incubation. The precipitate was washed with 70% ethanol twice and then stored at −80°C until analysis.
Oxidation of dG in a lipid micelle containing oxidants
A lipid micelle was prepared according to a method described previously.(33) Briefly, 19.4 mg of taurocholic acid sodium salt hydrate in 500 µl of ethanol was dried under a nitrogen gas stream and added to a mixture of 100 µl of potassium phosphate buffer (10 mM, pH 7.4) containing 12.5 mmol/L lysophosphatidylcholine, 25 mmol/L mono-olein, and 10 µl of methanol solution containing 150 mmol/L oleic acid or linoleic acid, or a mixture of 145 mmol/L linoleic acid and 5.0 mmol/L LOOH. This mixture was added to 50 µl of 250 µmol/L dG in 10 mM potassium phosphate buffer (pH 7.4) and with or without FeSO4 and 1.0 mmol/L H2O2, and was then vortexed for 1 min and filled up to 1 ml with n-hexane. After incubation at 37°C for 1 h, the micelle suspension was extracted with 250 µl of TE buffer and a 25 µl aliquot of it was analyzed by HPLC to determine 8-oxo-dG.
Treatment of rat hepatic mitochondria with oxidants
The animal study was approved by the Institutional Animal Care and Use Committee (permission number 22-05-27) and carried out according to Kobe University Animal Experimentation Regulations. Male Wistar-ST rats, 11 weeks old, 320–340 g in body weight, were obtained from Japan SLC (Shizuoka, Japan) and housed under a 12 h light/dark cycle at a constant temperature of 22 ± 2°C for 1 week, allowing free access to a rodent diet (PMI Nutrition International, St. Louis, MO) and water. After anesthesia with 5% pentobarbital, the liver was isolated and perfused with ice-cold 1.15% KCl, and immediately frozen in liquid nitrogen and stored at −80°C until the following mitochondrial experiments.
The hepatic mitochondria were prepared at 4°C according to a previously described method.(34) Around 2 g of liver was cut into small pieces and homogenized in 12 ml of sucrose buffer containing 0.25 M sucrose, 5 mM N-(2-hydroxyethyl)piperazine-N'-(2-ethanesulfonic acid) (HEPES), and 0.5 mM ethylene glycol-bis(β-aminoethyl ether)N,N,N',N'-tetraacetic acid (pH 7.5) using Multi-Beads shocker (YASUI KIKAI, Osaka, Japan), and then centrifuged at 100 × g for 5 min. The supernatant was centrifuged again at 600 × g for 10 min, and the supernatant was further centrifuged at 5,500 × g for 20 min. The pellet was suspended in 5 ml of sucrose buffer and washed with centrifugation at 6,000 × g for 20 min twice. Then, the pellet was incubated with 100 µmol/L LOOH or H2O2 in 1 ml of HEPES buffer containing 10 mM HEPES and 0.15 M NaCl (pH 7.4) at 37°C for 1 h, and then subjected to the following DNA preparation.
The mitochondria were suspended in 200 µl of buffer of 50 mM glucose and 25 mM Tris-HCl containing 10 mM EDTA (pH 8.0) on ice, and mixed in 400 µl of 0.2 M NaOH containing 1% sodium dodecyl sulfate (SDS), stirring thoroughly. After standing on ice for 5 min, the mitochondria were added to 300 µl of 3 M potassium acetate and allowed to stand at −80°C for 2 min. The suspension was centrifuged at 10,000 × g for 10 min, and 750 µl of clear supernatant was mixed in 750 µl of 2-propanol standing at −80°C for 5 min. The precipitated DNA was collected by centrifugation at 20,600 × g for 15 min and washed with 70% ethanol twice, and then was stored at −80°C until 8-oxo-dG determination.
Treatment of HepG2 cells with oxidants
The human hepatocarcinoma cell line HepG2 was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Nissui Pharmaceutical Co., Tokyo, Japan), seeded at a density of 5 × 105 cells/ml on a 100-mm dish, and cultured for 3 days, as described previously.(35) The medium was changed to fresh serum-free medium containing 100 µmol/L LOOH or H2O2 for 2 h at 37°C, and then the cells were harvested with 0.25% trypsin and suspended in 800 µl of 0.5 M HEPES-KOH buffer (pH 8.0) including 2 M KCl and 0.5 M MgCl2. The suspension was placed on ice for 10 min and homogenized gently using a pellet mixer. After centrifugation at 1,300 × g for 5 min at 4°C, the pellet was re-suspended in 5 ml of 0.25 M sucrose buffer containing 2 M Tris-HCl (pH 7.9), 0.5 M MgCl2, and 10% TritonX-100 and centrifuged at 1,300 × g for 5 min at 4°C. The precipitated nuclei were subjected to 8-oxo-dG determinations.
Alternatively, 3-day-cultured cells were pre-incubated with 500 µmol/L mercaptosuccinic acid, an inhibitor of glutathione peroxidase (GPx),(36) in fresh medium containing 2% FBS for 24 h, and then were mixed in LOOH or H2O2.
In cells pre-treated with or without mercaptosuccinate, GPx activity was determined by a partly modified version of a method described previously.(37) Briefly, HepG2 cells were washed twice with ice-cold PBS and scraped with 100 µl of 0.1 M potassium phosphate buffer (pH 6.5) containing 1 mol/L phenylmethylsulfonyl fluoride. After 5-s sonication with an ultrasonic cell disruptor (Microson, NY) on ice 6 times, the lysate was centrifuged at 12,000 × g for 20 min at 4°C. A total of 100 µl of the supernatant was mixed with 1 ml of 0.1 M phosphate buffer (pH 7.0) containing 4 mM EDTA, 1.2 ml of distilled water, 0.2 ml of 10 µM sodium azide, 0.2 ml of 10 µM glutathione, 0.2 ml of 1.5 mmol/L NADPH, and 6 µl of 250 U/ml glutathione reductase. The mixture was incubated at 37°C for 1 min, and was added to 0.1 ml of 1.5 mM t-butyl hydroperoxide. Then, the oxidation of NADPH was measured at 340 nm, and GPx activity was expressed as nmoles of the oxidized NADPH per minute per mg of protein after determining the protein amounts by the Lowry method.
Evaluation of antioxidant potency in HepG2 cells
Four-day-cultured HepG2 cells were pre-incubated with 10 µmol/L ascorbic acid, α-tocopherol, NAC, or flavonoids, or 3 µmol/L carotenoids in fresh serum-free medium for 1 h at 37°C, and the nuclei were isolated. After treatment with 100 µmol/L LOOH in PBS for another 1 h, the nuclei were washed twice with PBS and lysed with 400 µl of TE buffer containing 0.5% SDS. The lysate was treated with a final concentration of 0.5 mg/ml ribonuclease A (Sigma) for 30 min at 50°C, followed by treatment with 0.5 mg/ml proteinase K (Sigma) for 1 h at 50°C. DNA was precipitated with 0.7 M NaI and 50% 2-propanol, and centrifuged at 17,000 × g for 15 min at 4°C. The precipitated nuclei were subjected to 8-oxo-dG determinations.
Determination of 8-oxo-dG
Before subjecting 8-oxo-dG to HPLC, calf thymus DNA, precipitated nuclei of HepG2 cells, and precipitated DNA of mitochondria were treated as follows: they were denatured in 200 µl of 1 mM EDTA by heating at 95°C for 5 min and were immediately cooled on ice for 5 min. The DNA was hydrolyzed with 2.5 units of nuclease P1 (Wako) for 30 min at 37°C and the hydrolysis was stopped by mixing in 0.1 M Tris-HCl (pH 7.4). Then, the DNA was treated with 3 units of alkaline phosphatase (Sigma) for 1 h at 37°C and centrifuged at 17,000 × g for 10 min at 4°C. The supernatant was filtered in a Vivaspin 500 microconcentrator (Sartorius Stedim Biotech, Goettingen, Germany) by centrifugation at 15,000 × g for 20 min at 4°C. An aliquot of 25 µl was subjected to the following HPLC.
8-Oxo-dG was determined as described previously.(38) Briefly, the HPLC conditions were as follows: column, Capcell pak C18 UG120 (5 µm mesh and φ 4.6 × 250 mm; Shiseido, Tokyo, Japan) maintained at 35°C; mobile phase, 6.5% methanol and 93.5% 20 mM potassium phosphate buffer (pH 4.5) containing 0.1 mM EDTA; and flow rate, 1.0 ml/min. 8-Oxo-dG was determined with an electrochemical detector (Nanospace SI-2; Shiseido) at +600 mV, and simultaneously dG was measured by the UV detector (L-7420; Hitachi, Tokyo, Japan). In this analysis, the determination limit was 2.5 pmol for 8-oxo-dG. The determined 8-oxo-dG levels are expressed as mean ± SD of the number of 8-oxo-dG molecules per 105 dG.
Statistical analysis
The data are reported as the mean ± SD. Statistical analysis was performed using ANOVA and Tukey-Kramer. Probability values of <0.05 were considered to be statistically significant.
Results
Comparison of the oxidant potency between H2O2 and lipid peroxides
The oxidant species forming 8-oxo-dG has been considered to be mainly H2O2, while lipid peroxides may also be included among such oxidant species because bio-membrane enclosing DNA has abundant polyunsaturated fatty acids that can be frequently peroxidized to lipid peroxides. Thus, the oxidant potency was compared between H2O2 and LOOH.
Table 1 shows that LOOH oxidized dG to 8-oxo-dG at the level of 3.35 ± 0.65/105 dG and was a significantly stronger oxidant than H2O2 (1.91 ± 0.39/105 dG) at a concentration of 50 µmol/L. The addition of Fe2+ for Fenton’s reaction at a physiological concentration of 0.5 µmol/L significantly increased the oxidant potency of LOOH but did not increase that of H2O2.(39,40) The physiologically excessive amount of Fe2+ of 10 µmol/L increased the formation by both LOOH and H2O2. Similarly, in Table 2 for calf thymus double-stranded DNA, LOOH exhibited significantly stronger potency as an oxidant than H2O2 in the absence and presence of a physiological concentration of Fe2+, and produced an amount of 8-oxo-dG similar to that of H2O2 after the addition of a physiologically excessive amount of Fe2+. Thus, LOOH was a stronger oxidant of dG and thymus DNA under physiological conditions.
Table 1.
Production of 8-oxo-dG from dG by H2O2 or LOOH with or without Fe2+
| Substrate* | Fe2+ (µmol/L) | Oxidant (50 µmol/L) |
||
|---|---|---|---|---|
| Vehicle | H2O2 | LOOH | ||
| Produced number of 8-oxo-dG per 105 dG** | ||||
| dG (250 µmol/L) | 0 | 0.67 ± 0.30a | 1.91 ± 0.39a | 3.35 ± 0.65b |
| 0.5 | 1.27 ± 0.06a | 1.89 ± 0.20a | 3.59 ± 0.47b | |
| 10 | 5.04 ± 0.29c | 11.55 ± 0.82d | 8.73 ± 1.86e | |
*dG was incubated with oxidants as shown in Materials and Methods. The commercial dG originally contained 8-oxo-dG at levels of 0.58 ± 0.08 number per 105 dG, and the present results include the original amounts of 8-oxo-dG. **Values are mean ± SD (n = 6) and different superscript letters indicate statistically significant differences (p<0.05).
Table 2.
Production of 8-oxo-dG in calf thymus DNA by H2O2 or LOOH with or without Fe2+
| Substrate* | Fe2+ (µmol/L) | Oxidant (50 µmol/L) |
||
|---|---|---|---|---|
| Vehicle | H2O2 | LOOH | ||
| Produced number of 8-oxo-dG per 105 dG** | ||||
| Calf thymus DNA (10 µg/ml) | 0 | 0.94 ± 0.23a | 9.28 ± 1.79b | 27.16 ± 1.88c |
| 0.5 | 0.67 ± 0.13a | 11.20 ± 1.59b | 31.29 ± 1.20c | |
| 10 | 1.62 ± 0.68a | 56.64 ± 6.71d | 62.10 ± 7.01e | |
*Calf thymus DNA was incubated with oxidants as shown in Materials and Methods. The commercial calf thymus DNA originally contained 8-oxo-dG at levels of 0.60 ± 0.15 number per 105 dG, and the present results include the original amounts of 8-oxo-dG. **Values are mean ± SD (n = 6) and different superscript letters indicate statistically significant differences (p<0.05).
Bio-membranes enclosing DNA such as the nuclear membrane and the mitochondrial membrane frequently contain lipid peroxides. Here, lipid micelles including oxidants were prepared and compared in terms of their oxidant potency, as shown in Table 3. A few 8-oxo-dG molecules were detected in the micelles composed of a non-oxidized mono-unsaturated fatty acid, oleic acid, and a di-unsaturated fatty acid, linoleic acid. The addition of H2O2 at 50 µmol/L slightly increased the level of 8-oxo-dG. In contrast, the addition of LOOH at one-tenth of that level (5 µmol/L) significantly enhanced the production of 8-oxo-dG compared with that by H2O2. The presence of a physiological concentration of Fe2+ facilitated the production in LOOH micelles more than in H2O2 micelles.
Table 3.
Production of 8-oxo-dG by H2O2 or LOOH in lipid micelles containing dG
| Fe2+ (µmol/L)1 | Components of micelles2 |
|||
|---|---|---|---|---|
| Oleic acid (150 µmol/L) | Linoleic acid (150 µmol/L) | Linoleic acid (145 µmol/L) and LOOH (5.0 µmol/L) | H2O2 (50 µmol/L)1 | |
| Produced number of 8-oxo-dG per 105 dG3 | ||||
| 0 | 0.82 ± 0.15a | 0.47 ± 0.07a | 6.04 ± 0.56b | 2.17 ± 0.36a |
| 0.5 | 0.97 ± 0.18a | 0.70 ± 0.04a | 11.34 ± 0.38c | 6.93 ± 1.66b |
| 10 | — | — | 9.30 ± 2.21d | 9.29 ± 1.26d |
1FeSO4 and H2O2 were added to the liposomal mixture prepared as shown in Materials and Methods. 2Oleic acid, linoleic acid, or a mixture of linoleic acid and LOOH was mixed in a suspension of lysophosphatidylcholine and mono-olein as shown in Materials and Methods. 3Values are mean ± SD (n = 6) and different letters indicate statistically significant differences (p<0.05). The commercial dG originally contained 8-oxo-dG at levels of 0.58 ± 0.08 number per 105 dG, and the present results include the original amounts of 8-oxo-dG.
Table 4 shows the 8-oxo-dG production in mitochondria prepared from rat liver after treatment with 100 µmol/L H2O2, LOOH, or PCOOH. Every oxidant produced considerable amounts of 8-oxo-dG compared with the vehicle controls. LOOH showed the greater production than H2O2, and PCOOH produced even more 8-oxo-dG, but significant differences were not found among these three oxidants.
Table 4.
Production of 8-oxo-dG in mitochondria of rat hepatocytes upon exposure to oxidants
| Vehicle | Oxidant (100 µmol/L) |
||
|---|---|---|---|
| H2O2 | LOOH | PCOOH | |
| Produced number of 8-oxo-dG per 105 dG1 | |||
| ud2 | 23.96 ± 8.54 | 34.43 ± 10.18 | 75.55 ± 71.3 |
1Values are mean ± SD (n = 6). 2“ud” shows that the number of 8-oxo-dG is under the detection limit of 2.5 pmol.
Production of 8-oxo-dG in HepG2 cell
As one of the models of living cells, HepG2 cells were employed and treated with H2O2 or LOOH using the oxidants at the physiologically excess concentrations, 100 µmol/L, in order to compare in their oxidant potency. Table 5 shows that both oxidants slightly increased 8-oxo-dG levels, and the production by H2O2 was significant while that by LOOH was not significant compared to that of vehicle control. Living cells have been shown to possess antioxidant enzymes that can remove peroxides such as GPx.(36) Thus, HepG2 cells were pretreated with an inhibitor of GPx, mercaptosuccinate, before adding the oxidants. H2O2 and LOOH significantly elevated the levels of 8-oxo-dG in the cells, and LOOH produced more 8-oxo-dG than H2O2. Thus, LOOH was preferential oxidant for the cellular production of 8-oxo-dG under such conditions that GPx was not working.
Table 5.
Production of 8-oxo-dG by H2O2 or LOOH in HepG2 cells
| Pretreatment with | Oxidant (100 µmol/L) |
||
|---|---|---|---|
| Vehicle | H2O2 | LOOH | |
| Produced number of 8-oxo-dG per 105 dG1 | |||
| None | 0.08 ± 0.04a | 0.38 ± 0.15b | 0.23 ± 0.12a,b,c,d |
| Mercaptosuccinate2 | 0.11 ± 0.03a,c | 0.34 ± 0.11b,d | 0.61 ± 0.04e |
1Values are mean ± SD (n = 6) and different letters indicate statistically significant differences (p<0.05) in the same line. 2HepG2 cells were pre-incubated with 500 µmol/L mercaptosuccinate and then were exposed to H2O2 or LOOH.
Suppressing effects of dietary antioxidants on 8-oxo-dG formation
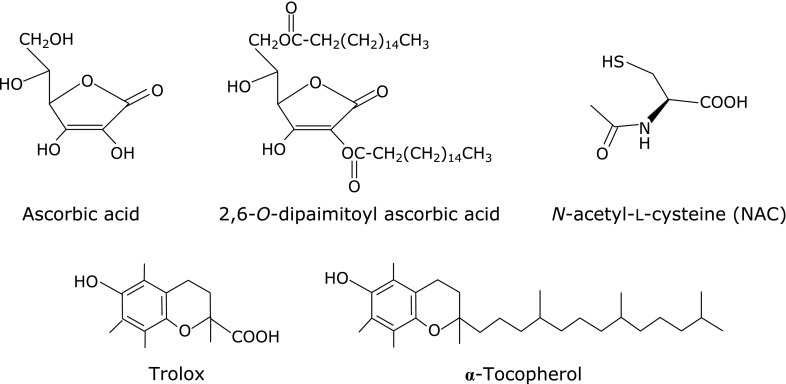
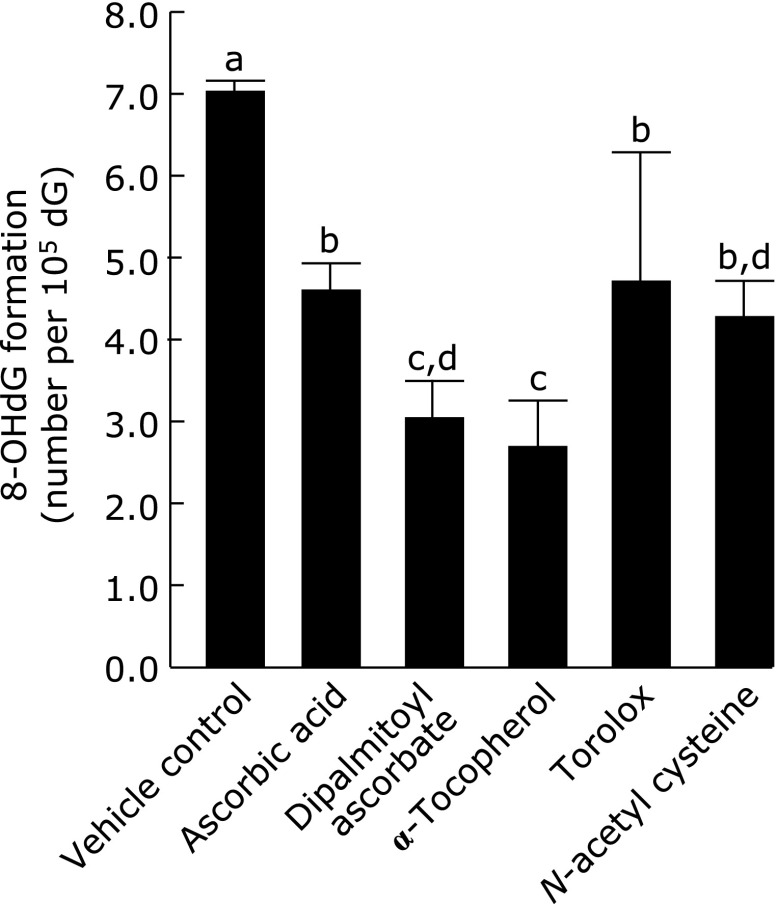
Antioxidants were added to the HepG2 cells for 1 h, and then the nuclei were isolated. After treated with 100 µmol/L LOOH, the nuclei were determined in the formed amounts of 8-oxo-dG. First, using chemicals as shown in Fig. 2, the antioxidant potency was compared between water-soluble and lipid-soluble antioxidants. Fig. 3 shows that water-soluble ascorbic acid, torolox and NAC significantly suppressed 8-oxo-dG production to 66%, 68% and 62%, respectively, compared to the production of 8-oxo-dG in a control not treated with antioxidants. In contrast, lipid-soluble 2,6-dipalmitoyl ascorbic acid and α-tocopherol inhibited the production more, to 44% and 37%, respectively, than the water-soluble antioxidants.
Fig. 2.
Chemical structures of water- and lipid-soluble antioxidants employing in Fig. 3.
Fig. 3.
Suppressing activity of water- and lipid-soluble vitamins on 8-oxo-dG production induced by LOOH in HepG2 cells. HepG2 cells were pre-incubated with 10 µmol/L of the presented chemicals at 37°C for 1 h, and then the nuclei were isolated and exposed to 100 µmol/L LOOH for 1 h. The nuclei were determined in the produced 8-oxo-dG as mentioned in Materials and Methods. Figures are mean ± SD (n = 6) of the number of 8-oxo-dG molecules per 105 dG. The most left bar is a control when HepG2 cells were incubated with vehicle (7.08 ± 0.07 per 105 dG). The different superscript letters indicate statistically significant differences (p<0.05).
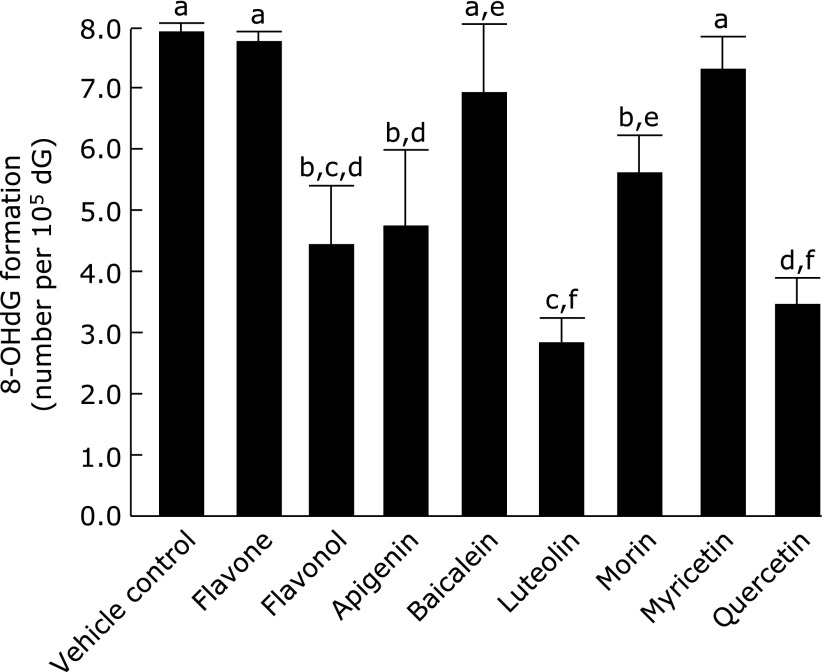
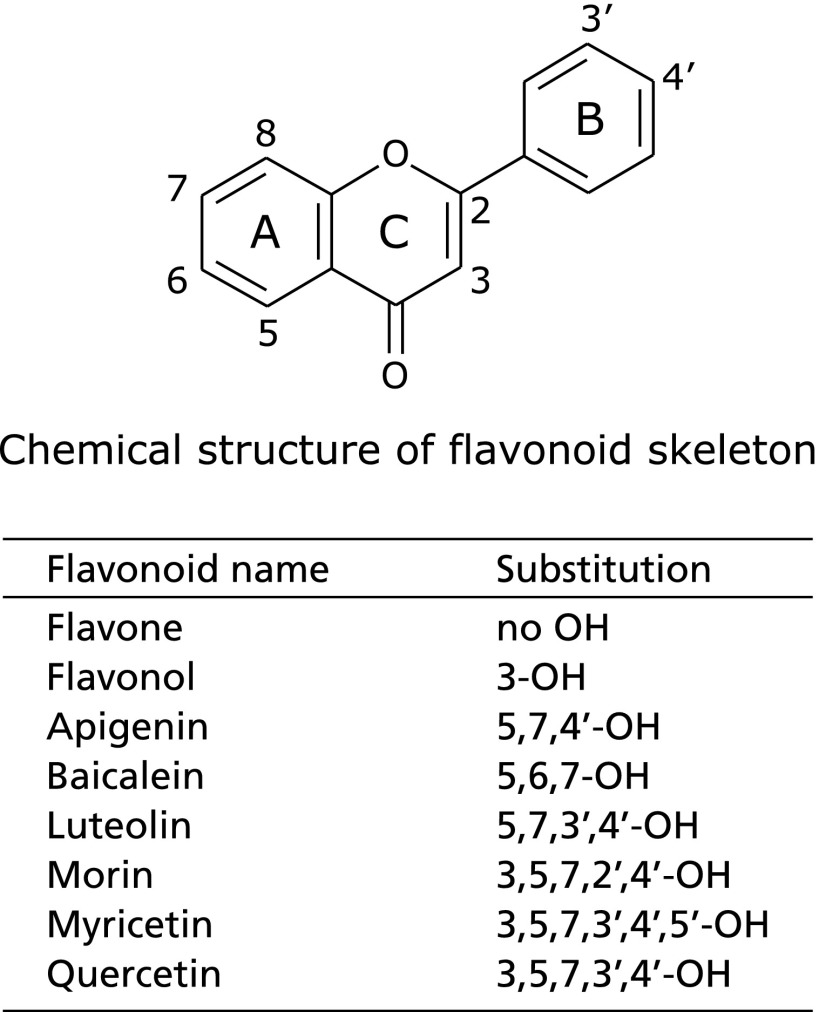
Fig. 4 shows the antioxidant potency of flavonoids as shown the chemical structures in Fig. 5 on the 8-oxo-dG formation in nuclei of HepG2 cells exposed to LOOH. Flavone having no OH groups did not exhibit potency, while a flavonol, 3-OH flavone, significantly suppressed the 8-oxo-dG formation to 56% compared to the production in the control. Baicalein, a flavone with 5,6,7-OH groups, and myricetin, a flavonol with 5,7,3',4',5'-OH groups, did not exhibit the activity. Apigenin, a flavone with 5,7,4'-OH groups, significantly suppressed it to 61%, while morin, a flavonol with 5,7,2',4'-OH groups, did so to 72%, and luteolin, a flavone with 5,7,3',4'-OH groups, and quercetin, a flavonol with 5,7,3',4'-OH groups, exhibited significantly stronger suppression, to 36% and 44%, respectively.
Fig. 4.
Suppressing activity of flavonoids on 8-oxo-dG production induced by LOOH in HepG2 cells. After the HepG2 cells had been pre-incubated with 10 µmol/L of the presented flavonoids at 37°C for 1 h, the nuclei were treated as mentioned in Fig. 1. Figures are mean ± SD (n = 6) of the number of 8-oxo-dG molecules per 105 dG. The most left bar is a control when HepG2 cells were incubated with vehicle (7.95 ± 0.14 per 105 dG). The different superscript letters indicate statistically significant differences (p<0.05).
Fig. 5.
Chemical structures of flavonoids comparing in the antioxidant potency in Fig. 4.
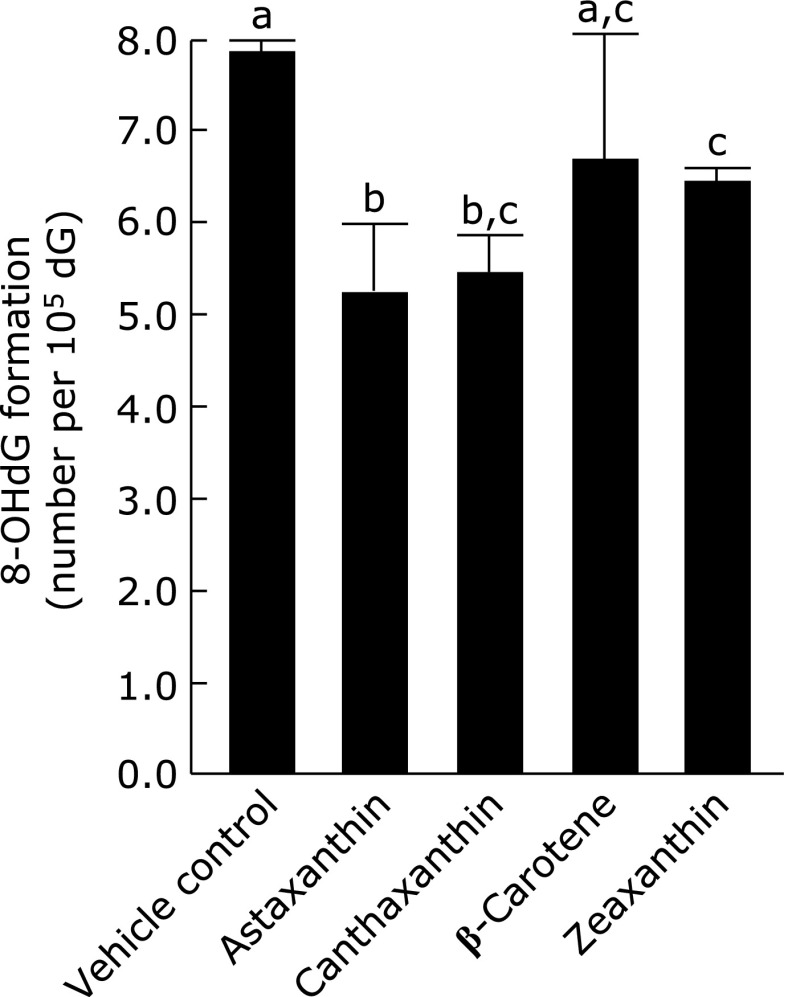
Fig. 6 shows the antioxidant potencies of carotenoids, showing their chemical structures in Fig. 7. β-Carotene did not exhibit such activity, but xanthophylls, oxygen-containing carotenoids, namely, astaxanthin, canthaxanthin, and zeaxanthin, showed weak but significant antioxidant potency, with suppression to 68%, 70%, and 82%, respectively, compared to the production in the control.
Fig. 6.
Suppressing activity of carotenes on 8-oxo-dG production induced by LOOH in HepG2 cells. HepG2 cells were pre-incubated with 3 µmol/L of the presented carotenoids at 37°C for 1 h, and the 8-oxo-dG levels in nuclei were determined as mentioned in Fig. 1. Figures are mean ± SD (n = 6) of the number of 8-oxo-dG molecules per 105 dG. The most left bar is a control when HepG2 cells were incubated with vehicle (7.85 ± 0.52 per 105 dG). The different superscript letters indicate statistically significant differences (p<0.05).
Fig. 7.
Chemical structures of carotenoids used in Fig. 6.
Discussion
In living cells, H2O2 has generally been considered to be the most contributable oxidant species because it easily produces OH radicals. On the other hand, lipid peroxides such as LOOH are easily generated in polyunsaturated fatty acids of membranous phospholipids.(14,16,28) The present study compared the oxidant potency between H2O2 and LOOH, and found that LOOH markedly oxidizes dG to 8-oxo-dG in DNA, and that lipid-soluble antioxidants could prevent such oxidation.
LOOH is a stronger oxidant for forming 8-oxo-dG than H2O2 on dG and double-stranded DNA (Table 1 and 2). Since H2O2 requires Cu+ or Fe2+ to produce the stronger oxidant species of OH radical,(23,24) Fe2+ was added to the solutions of dG and DNA. Surprisingly, H2O2 produced little 8-oxo-dG but LOOH produced significant amounts of it under conditions with 0.5 µmol/L Fe2+, which is the physiological concentration of Fe2+.(39,40) Under conditions of physiologically excessive amounts of Fe2+, both H2O2 and LOOH produced considerable amounts of 8-oxo-dG. Then, lipid micelles enclosing dG were prepared by adding the oxidants to the micelles (Table 3). LOOH produced a large amount of 8-oxo-dG at a concentration one-tenth of that of H2O2, and thus was a more reactive oxidant on dG than H2O2 at a physiological concentration of Fe2+. These results clearly demonstrate that lipid peroxides like LOOH is preferential oxidant species for the formation of 8-oxo-dG. The preferential point for lipid peroxides as the endogenous oxidants is considered to be more easily mixed in lipid membrane than water-soluble H2O2 and to approach to the guanosine residue (Table 3). The formation mechanism of 8-oxo-dG is suggested to be a direct addition of lipid peroxyl radicals on C8 position of guanosine as reported in our previous paper,(13) though the details should be examined.
Next, the oxidant potency was compared in hepatic mitochondria among lipid peroxides, LOOH and PCOOH, and H2O2 (Table 4). PCOOH produced large amounts of 8-oxo-dG, but the difference was not significant, indicating that the lipid peroxides were more reactive than H2O2. PCOOH generated in the mitochondrial membranes seems likely to attack the mitochondrial DNA more frequently than H2O2 because the mitochondrial membranes enclosing the DNA are composed of phospholipids such as PC and easily form the peroxides of unsaturated fatty acids in the second position of PC.(14,16,28)
As a living cell model to evaluate the oxidant potency, HepG2 cells were employed (Table 5). The addition of H2O2 and LOOH increased slightly 8-oxo-dG levels in the cells. Living cells have been known to possess antioxidant enzymes such as GPx. Then, the cells were pretreated with enzyme inhibitor before adding the oxidants. LOOH produced significantly larger amounts of 8-oxo-dG than H2O2, showing that the lipid peroxides oxidized DNA in the cells but not H2O2 under the conditions of decreasing antioxidant enzyme activity. Enzyme activity has been recognized to decrease or to exceed the capacity when living cells undergo oxidative stress in which reactive oxygen species are excessively generated. Such oxidative stress is induced under the following conditions: excessive activity of the mitochondrial electron transfer system after surplus intake and leakage of electrons to oxygen,(41) full production of superoxide anions by cytochrome P450 monooxygenase for the detoxification of xenobiotics and/or alcohol,(42,43) and neutrophils removing invading pathogens using reactive oxygen species such as perchloric acid formed by myeloperoxidase,(44,45) among others.(46) Under oxidative stress conditions, dG in DNA will be oxidized to 8-oxo-dG. Against this background, next, the bioavailable antioxidants that can suppress the formation of 8-oxo-dG were examined using HepG2 cells.
After HepG2 cells had been pre-incubated with the antioxidants, their nuclei were isolated and treated with LOOH, and then the formed amounts of 8-oxo-dG were compared among the antioxidants. NAC is a water-soluble antioxidant, and trolox is a modified chemical that becomes water-soluble by removing the lipid-soluble side chain from α-tocopherol (Fig. 2). 2,6-Dipalmitoyl ascorbic acid is modified to be lipid-soluble by esterification with palmitic acid on water-soluble ascorbic acid (Fig. 2). Ascorbic acid, trolox, and NAC showed no or weak antioxidant potency on the 8-oxo-dG formation induced by LOOH, and 2,6-dipalmitoyl ascorbic acid and α-tocopherol exhibited stronger antioxidant potency (Fig. 3). Thus, lipid-soluble antioxidants suppressed the 8-oxo-dG formation induced by lipid peroxides more effectively than water-soluble ones.
Most flavonoids are not water-soluble, and especially flavones and flavonols are insoluble in water and partially soluble in lipids.(47) In terms of the 8-oxo-dG formation inducing by lipid peroxides, several flavones and flavonols are clear antioxidants (Fig. 4). Flavone possessing no OH groups (Fig. 5), baicalein with three OH groups, and myricetin with six OH groups did not show antioxidant activity, while flavonol having one OH group, apigenin with three, luteolin with four, morin with five, and quercetin with five exhibited significant antioxidant potency. Thus, the antioxidant activity of flavonoids did not depend on the number of OH groups. Luteolin and quercetin exhibited greater activity than the other flavonoids, and both flavonoids possess a catechol structure in the B-ring of the flavonoid skeleton (Fig. 5). Catechol flavonoids have been recognized to be strong antioxidants,(38) and quercetin has been reported to be a bioavailable antioxidant, although the flavonoids when ingested undergo conjugation in the intestinal absorption process.(48) The conjugation is a masking reaction with glucuronide or sulfate and occurs on one of the OH groups of the flavonoid skeleton. After undergoing the conjugation, flavonoids retaining the catechol structure can exhibit antioxidant potency, and there is a probability of 2/3 of quercetin retaining the catechol structure. Moon et al.(49) reported that 2/3 of quercetin in plasma exhibited antioxidant activity after quercetin had been ingested and incorporated into the blood circulation.
Carotenoids have been considered to be quenchers of singlet oxygen and to be weak antioxidants against lipid peroxides.(50) Astaxanthin, canthaxanthin, and zeaxanthin are oxygen-containing carotenoids, namely, xanthophylls, and are lipid-soluble and partially soluble in water. β-Carotene did not show antioxidant potency, but the xanthophylls exhibited such activity on the 8-oxo-dG formation induced by the lipid peroxides (Fig. 6). The xanthophylls possess one or two OH groups on their β-ionone ring (Fig. 7), which seem to contribute to their antioxidant activity.(51) Dietary β-carotene has been recognized to be incorporated into the blood circulation and then into the cells of the body.(52) Dietary xanthophylls have a lower incorporation rate, but can partly circulate in the body.(53–55) Xanthophylls are probably bioavailable antioxidants acting against 8-oxo-dG production. Thus, several lipid-soluble flavonoids and xanthophylls were able to suppress the formation of 8-oxo-dG in the living cells, which was probably attributable to ease of mixing with and entering into the bio-membranes.(47,56)
It is concluded that oxidative injury of DNA is induced more abundantly by lipid peroxides than by H2O2, so lipid-soluble antioxidants such as vitamin E, 2,6-dipalmitoyl vitamin C, quercetin, and several xanthophylls could be available to prevent the oxidative injury of DNA.
Acknowledgments
This work was supported in part by a Grant-in-Aid for research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (23580167).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Boiteux S, Radicella JP. Base excision repair of 8-hydroxyguanine protects DNA from endogenous oxidative stress. Biochimie. 1999;81:59–67. doi: 10.1016/s0300-9084(99)80039-x. [DOI] [PubMed] [Google Scholar]
- 2.Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992;267:166–172. [PubMed] [Google Scholar]
- 3.Hsu GW, Ober M, Carell T, Beese LS. Error-prone replication of oxidatively damaged DNA by a high-fidelity DNA polymerase. Nature. 2004;431:217–221. doi: 10.1038/nature02908. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto K, Toyokuni S, Uchida K, et al. Formation of 8-hydroxy-2'-deoxyguanosine and 4-hydroxy-2-nonenal-modified proteins in human renal-cell carcinoma. Int J Cancer. 1994;58:825–829. doi: 10.1002/ijc.2910580613. [DOI] [PubMed] [Google Scholar]
- 5.Musarrat J, Arezina-Wilson J, Wani AA. Prognostic and aetiological relevance of 8-hydroxyguanosine in human breast carcinogenesis. Eur J Cancer. 1996;32A:1209–1214. doi: 10.1016/0959-8049(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 6.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res. 1997;387:147–163. doi: 10.1016/s1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 7.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 8.Olinski R, Gackowski D, Rozalski R, Foksinski M, Bialkowski K. Oxidative DNA damage in cancer patients: a cause or a consequence of the disease development? Mutat Res. 2003;531:177–190. doi: 10.1016/j.mrfmmm.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Malins DC, Holmes EH, Polissar NL, Gunselman SJ. The etiology of breast cancer. Characteristic alteration in hydroxyl radical-induced DNA base lesions during oncogenesis with potential for evaluating incidence risk. Cancer. 1993;71:3036–3043. doi: 10.1002/1097-0142(19930515)71:10<3036::aid-cncr2820711025>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Hussain SP, Harris CC. Molecular epidemiology of human cancer: contribution of mutation spectra studies of tumor suppressor genes. Cancer Res. 1998;58:4023–4037. [PubMed] [Google Scholar]
- 11.Foksinski M, Kotzbach R, Szymanski W, Oinski R. The level of typical biomarker of oxidative stress 8-hydroxy-2'-deoxyguanosine is higher in uterine myomas than in control tissue and correlates with size of the tumor. Free Radic Biol Med. 2000;29:597–601. doi: 10.1016/s0891-5849(00)00358-0. [DOI] [PubMed] [Google Scholar]
- 12.Djuric Z, Heilbrun LK, Lababidi S, Berzinkas E, Simon MS, Kosir MA. Levels of 5-hydroxymethyl-2'-deoxyuridine in DNA from blood of women scheduled for breast biopsy. Cancer Epidemiol Biomarkers Prev. 2001;10:147–149. [PubMed] [Google Scholar]
- 13.Goto M, Ueda K, Hashimoto T, et al. A formation mechanism for 8-hydroxy-2'-deoxyguanosine mediated by peroxidized 2'-deoxythymidine. Free Radic Biol Med. 2008;45:1318–1325. doi: 10.1016/j.freeradbiomed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Henderson JR, Swalwell H, Boulton S, Manning P, McNeil CJ, Birch-Machin MA. Direct, real-time monitoring of superoxide generation in isolated mitochondria. Free Radic Res. 2009;43:796–802. doi: 10.1080/10715760903062895. [DOI] [PubMed] [Google Scholar]
- 15.Yeh GC, Henderson JP, Byun J, André d’Avignon D, Heinecke JW. 8-Nitroxanthine, a product of myeloperoxidase, peroxynitrite, and activated human neutrophils, enhances generation of superoxide by xanthine oxidase. Arch Biochem Biophys. 2003;418:1–12. doi: 10.1016/s0003-9861(03)00256-x. [DOI] [PubMed] [Google Scholar]
- 16.Jezek P, Hlavatá L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Devasagayam TP, Steenken S, Obendorf MS, Schultz WA, Sies H. Formation of 8-hydroxy(deoxy)guanosine and generation of strand breaks at guanine residues in DNA by singlet oxygen. Biochemistry. 1999;30:6283–6289. doi: 10.1021/bi00239a029. [DOI] [PubMed] [Google Scholar]
- 18.Terao J. Cholesterol hydroperoxides and their degradation mechanism. Subcell Biochem. 2014;77:83–91. doi: 10.1007/978-94-007-7920-4_7. [DOI] [PubMed] [Google Scholar]
- 19.Henle ES, Luo Y, Gassmann W, Linn S. Oxidative damage to DNA constituents by iron-mediated fenton reactions. The deoxyguanosine family. J Biol Chem. 1996;271:21177–21186. [PubMed] [Google Scholar]
- 20.Niles JC, Wishnok JS, Tannenbaum SR. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: structures and mechanisms of product formation. Nitric Oxide. 2006;14:109–121. doi: 10.1016/j.niox.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Shukla PK, Mishra PC. Catalytic involvement of CO2 in the mutagenesis caused by reactions of ONOO− with guanine. J Phys Chem B. 2008;112:4779–4789. doi: 10.1021/jp710418b. [DOI] [PubMed] [Google Scholar]
- 22.Valavanidis A, Vlachogianni T, Fiotakis C. 8-Hydroxy-2'-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 23.Niki E. Free radical initiators as source of water- or lipid-soluble peroxyl radicals. Methods Enzymol. 1990;186:100–108. doi: 10.1016/0076-6879(90)86095-d. [DOI] [PubMed] [Google Scholar]
- 24.Morier-Teissier E, Bernier JL, Lohez M, Catteau JP, Hénichart JP. Free radical production and DNA cleavage by copper chelating peptide-anthraquinones. Anticancer Drug Des. 1990;5:291–305. [PubMed] [Google Scholar]
- 25.Pryor WA. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annu Rev Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
- 26.Park JW, Floyd RA. Lipid peroxidation products mediate the formation of 8-hydroxydeoxyguanosine in DNA. Free Radic Biol Med. 1992;12:245–250. doi: 10.1016/0891-5849(92)90111-s. [DOI] [PubMed] [Google Scholar]
- 27.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 28.Hruszkewycz AM, Bergtold DS. The 8-hydroxyguanine content of isolated mitochondria increases with lipid peroxidation. Mutat Res. 1990;244:123–128. doi: 10.1016/0165-7992(90)90060-w. [DOI] [PubMed] [Google Scholar]
- 29.Kanazawa K, Ashida H. Dietary hydroperoxides of linoleic acid decompose to aldehydes in stomach before being absorbed into the body. Biochim Biophys Acta. 1998;1393:349–361. doi: 10.1016/s0005-2760(98)00089-7. [DOI] [PubMed] [Google Scholar]
- 30.Takagi T, Mitsuno Y, Masumura M. Determination of peroxide value by the colorimetric iodine method with protection of iodine as cadmium complex. Lipids. 1977;13:147–151. [Google Scholar]
- 31.Little C, O'Brian PJ. The effectiveness of lipid peroxide in oxidizing protein and non-protein thiols. Biochem J. 1968;106:419–423. doi: 10.1042/bj1060419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wrona M, Korytowski W, Rózanowska M, Sarna T, Truscott TG. Cooperation of antioxidants in protection against photosensitized oxidation. Free Radic Biol Med. 2003;35:1319–1329. doi: 10.1016/j.freeradbiomed.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Kim SJ, Nara E, Kobayashi H, Terao J, Nagao A. Formation of cleavage products by autoxidation of lycopene. Lipids. 2001;36:191–199. doi: 10.1007/s11745-001-0706-8. [DOI] [PubMed] [Google Scholar]
- 34.Qu B, Li QT, Wong KP, Ong CN, Halliwell B. Mitochondrial damage by the “pro-oxidant” peroxisomal proliferator clofibrate. Free Radic Biol Med. 1999;27:1095–1102. doi: 10.1016/s0891-5849(99)00143-4. [DOI] [PubMed] [Google Scholar]
- 35.Azuma Y, Hashimoto T, Nomura H, Das SK, Ozaki Y, Kanazawa K. Fucoxanthin induced apoptosis in human hepatocarcinoma HepG2 cells. J Clin Biochem Nutr. 2008;43(Suppl 1):273–276. [Google Scholar]
- 36.Steinbrenner H, Bilgic E, Alili L, Sies H, Brenneisen P. Selenoprotein P protects endothelial cells from oxidative damage by stimulation of glutathione peroxidase expression and activity. Free Radic Res. 2006;40:936–943. doi: 10.1080/10715760600806248. [DOI] [PubMed] [Google Scholar]
- 37.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 38.Kanazawa K, Uehara M, Yanagitani H, Hashimoto T. Bioavailable flavonoids to suppress the formation of 8-OHdG in HepG2 cells. Arch Biochem Biophys. 2006;455:197–203. doi: 10.1016/j.abb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes. Free Radic Biol Med. 2002;33:1037–1046. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- 40.Gackowski D, Kruszewski M, Bartlomiejczyk T, Jawien A, Ciecierski M, Olinski R. The level of 8-oxo-7,8-dihydro-2'-deoxyguanosine is positively correlated with the size of the labile iron pool in human lymphocytes. J Biol Inorg Chem. 2002;7:548–550. doi: 10.1007/s00775-001-0335-x. [DOI] [PubMed] [Google Scholar]
- 41.Sohal RS, Forster MJ. Caloroc restriction and the aging process: a critique. Free Radic Biol Med. 2014;73:366–382. doi: 10.1016/j.freeradbiomed.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werck-Reichhart D, Feyereisen R.Cytochromes P450: a success story Genome Biol 20001: REVIEWS3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loquercio C, Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med. 2003;34:1–10. doi: 10.1016/s0891-5849(02)01167-x. [DOI] [PubMed] [Google Scholar]
- 44.Postma NS, Mommers EC, Eling WN, Zuidema J. Oxidative stress in malaria; implications for prevention and therapy. Pharm World Sci. 1996;18:121–129. doi: 10.1007/BF00717727. [DOI] [PubMed] [Google Scholar]
- 45.Tao F, Gonzalez-Flecha B, Kobzik L. Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radic Biol Med. 2003;35:327–340. doi: 10.1016/s0891-5849(03)00280-6. [DOI] [PubMed] [Google Scholar]
- 46.Muralikrishna AR, Hatcher JF. Phospholipase A2, reactive oxugen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40:376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 47.Murota K, Matsuda N, Kashino Y, et al. α-Oligoglucosylation of a sugar moiety enhances the bioavailability of quercetin glucosides in humans. Arch Biochem Biophys. 2010;501:91–97. doi: 10.1016/j.abb.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 48.Murota K, Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch Biochem Biophys. 2003;417:12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- 49.Moon JH, Tsushida T, Nakahara K, Terao J. Identification of quercetin 3-O-β-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic Biol Med. 2001;30:1274–1285. doi: 10.1016/s0891-5849(01)00522-6. [DOI] [PubMed] [Google Scholar]
- 50.Ouchi A, Aizawa K, Iwasaki Y, et al. Kinetic study of the quenching reaction of singlet oxygen by carotenoids and food extracts in solution, development of a singlet oxygen absorption capacity (SOAC) assay method. J Agric Food Chem. 2010;58:9967–9978. doi: 10.1021/jf101947a. [DOI] [PubMed] [Google Scholar]
- 51.Terao J. Antioxidant activity of β-carotene-related carotenoids in solution. Lipids. 1989;24:659–661. doi: 10.1007/BF02535085. [DOI] [PubMed] [Google Scholar]
- 52.Cutler RG. Carotenoids and retinol: their possible importance in determining longevity of primate species. Proc Natl Acad Sci U S A. 1984;81:7627–7631. doi: 10.1073/pnas.81.23.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rüfer CE, Moeseneder J, Briviba K, Rechkemmer G, Bub A. Bioavailavility of astaxanthin stereoisomers from wild (Oncorhynchus spp.) and aquacultured (Salmo salar) salmon in healthy men: a randomized, double-blind study. Br J Nutr. 2008;99:1048–1054. doi: 10.1017/S0007114507845521. [DOI] [PubMed] [Google Scholar]
- 54.White WS, Stacewicz-Sapuntzakis M, Erdman JW, Jr, Bowen PE. Pharmacokinetics of beta-catotene and canthaxanthin after ingestion of individual and combined doses by human subjects. J Am Coll Nutr. 1994;13:665–671. doi: 10.1080/07315724.1994.10718463. [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto T, Ozaki Y, Mizuno M, et al. Pharmacokinetics of fucoxanthinol in human plasma after the oral administration of kombu extract. Br J Nutr. 2012;107:1566–1569. doi: 10.1017/S0007114511004879. [DOI] [PubMed] [Google Scholar]
- 56.Sinha R, Joshi A, Joshi UJ, Srivastava S, Govil G. Localization and interaction of hydroxyflavones with lipid bilayer model membranes: a study using DSC and multinuclear NMR. Eur J Med Chem. 2014;80:285–294. doi: 10.1016/j.ejmech.2014.04.054. [DOI] [PubMed] [Google Scholar]