Abstract
Fucoidan is a sulfated polysaccharide from brown sea algae. In the present study, it was demonstrated that oral administration of F-fucoidan from Saccharina japonica possessed anti-allergic effects using the passive cutaneous anaphylaxis reaction, but not by intraperitoneal administration. The inhibitory mechanism was dependent on galectin-9, which belongs to a soluble lectin family that recognizes β-galactoside and prevents IgE binding to mast cells. The anti-allergy properties of F-fucoidan were cancelled by an intravenous dose of anti-galectin-9 antibody or lactose, which bind competitively with galectin-9 before the passive cutaneous anaphylaxis reaction. F-fucoidan increased the expression level of galectin-9 mRNA in intestinal epithelial cells and serum galectin-9 levels. Oral treatment with F-fucoidan suppressed allergic symptoms through the induction of galectin-9. This is the first report that F-fucoidan can induce the secretion of galectin-9.
Keywords: F-fucoidan, allergy, galectin-9
Introduction
Galectins are a family of soluble lectins that bind β-galactoside, and there are identified 15 members of the galectin family in mammals. Each galectin has different binding capacity to oligosaccharides, although all galectins recognize galactose-containing glycan.(1) Galectins have various biological functions such as induction of apoptosis, cell growth arrest, cell adhesion, migration, and modulation of immune responses, including antitumor immunity.(2,3) Galectin-9, one of the galectin family, has two carbohydrate recognition domains and is widely distributed.(4) Although galectin-9 was originally found as a potent eosinophil chemoattractant, it has complicated functions in immune modulation. For example, galectin-9 has been reported to exhibit divergent activities such as preventive effects on autoimmune disease through the suppression of Th1 and Th17 cells and the induction of regulatory T cells(5) and effects on enhancement antitumor immunity by the induction of dendritic cell (DC) maturation and the promotion of Th1 immune responses.(6) Some reports suggested that galectin-9 levels increase in mouse models of allergic airway,(7) food allergy,(8) and atopic dermatitis.(9) However, other reports indicated that exogenous galectin-9 diminished allergic inflammation in a mouse model of allergic asthma.(10) In addition to T-cell immunoglobulin and mucin-containing protein-3 (TIM-3) and CD44, which are reported as the major targets of galectin-9,(10,11) galectin-9 has been reported recently to interact with immunoglobulin E (IgE), whereby mast cell degranulation was suppressed.(12) Thus, conflicting data have been reported regarding galectin-9 effects on allergy.
Fucoidan is a sulfated polysaccharide found in brown sea algae. Although the structure of fucoidan from different brown sea algae varies between species, it is mainly composed of fucose and sulfated fucose.(13) Besides these, other fucoidans from different brown sea algae also contain specific sugars such as glucose, galactose, xylose and uronic acid.(13) Fucoidan has various antitumor(14) and anticoagulant(15,16) activities through the modulation of immune responses. Therefore, it was supposed that fucoidan is able to suppress allergic symptoms caused by immune disorders. Although fucoidan has been reported to have anti-allergic effects,(17–19) there are few reports on the anti-allergic mechanism when fucoidan was administered orally. Fucoidan shows little or no absorption from the intestine;(16,20) therefore, it was presumed that fucoidan exhibits diverse activities against type I allergy irrespective of the administration pathway. We reported previously that dietary F-fucoidan from Saccharina japonica (S. japonica), which consists of fucose and sulfated fucose, has exhibited anti-thrombotic effects and was eliminated without being completely absorbed.(16) This indicated that F-fucoidan can show activity without being absorbed into the body.
In this study, the effect of oral intake of F-fucoidan from S. japonica on type I allergy was investigated. Our results indicated that F-fucoidan induced the expression of galectin-9 in intestinal epithelial cells (IECs) and secretion of galectin-9 into blood, resulting in the prevention of type I allergic symptoms by galectin-9 binding to IgE and preventing the interaction of IgE and mast cells.
Materials and Methods
Reagents
Mouse anti-2,4,6-trinitrophenyl (TNP) monoclonal IgE was purchased from BD Biosciences (San Jose, CA). 2,4,6-trinitrochlorobenzene was purchased from Tokyo Chemical Industry (Tokyo, Japan). Ovalbumin (OVA), and Al(OH)3 were purchased from Sigma-Aldrich (Saint Louis, MO). Recombinant mouse galectin-9 was purchased R&D Systems (Minneapolis, MN), and an anti-mouse galectin-9 antibody was purchased from Santa Cruz Biotechnology (Dallas, TX) and R&D Systems. Anti-mouse IgE and anti-goat IgG-HRP antibodies were purchased from Bethyl Laboratories (Montgomery, AL).
Preparation of F-fucoidan
Crude F-fucoidan was extracted from powdered S. japonica using sodium acetate buffer (pH 4.6). Crude F-fucoidan was divided into F-fucoidan and alginic acid on an anion-exchange column (Toyopearl DEAE-650, TOSOH, Tokyo, Japan).
Mice
Female BALB/c mice (4 weeks old) were purchased from Japan SLC (Shizuoka, Japan). Mice were housed in an air-conditioned animal room at a temperature of 23 ± 2°C and a humidity of 55 ± 10%. Mice were acclimatized with ad libitum supply of food and drinking water. This study was approved by the Institutional Animal Care and Use Committee and carried out according to the Kobe University Animal Experimentation Regulations (permission number: 25-06-04).
Passive cutaneous anaphylaxis reaction
Mice were intravenously sensitized with anti-TNP IgE and then challenged with application of 1.6% 2,4,6-trinitrochlorobenzene in acetone:olive oil (1:1) as an antigen on the ear at 30 min after sensitization. Ear thickness was measured before and 2 h after antigen challenge using a micrometer (Ozaki MFG, Tokyo, Japan), and the difference in ear thickness before and after application of the antigen was defined as an edema. Before the passive cutaneous anaphylaxis (PCA) reaction, mice were orally or intraperitoneally administered crude F-fucoidan (100, 200 or 400 µg/day) everyday for 4 days. To investigate the contribution of galectin-9 to the anti-allergy properties of F-fucoidan, mice were injected intravenously with an anti-galectin-9 antibody (50 µg) 1 h before the PCA reaction. To evaluate whether galectin-9 induced by F-fucoidan prevented type I allergy through binding IgE, mice were injected intravenously with lactose (100 mM) 10 min before the PCA reaction. Sucrose was used as a negative control.
Sampling of tissues, RNA isolation and real-time PCR
Harvested small intestine was washed in ice-cold PBS to remove feces and then cut longitudinally. IECs were isolated by scraping using a glass slide. Total RNA was extracted from the tissues using Sepasol RNA I super G (Nacalai Tesque, Kyoto, Japan) in accordance with the manufacturer’s protocol. cDNA synthesis was performed using a High-Capacity cDNA Reverse Transcription kit (Life Technologies, Carlsbad, CA). Quantitative PCR was performed using a 7500 Fast Real Time PCR system (Life Technologies) using TaqMan® Fast Universal PCR Master Mix (Life Technologies), and Gene Expression Assays for mouse galectin-9 and β-actin (Life Technologies), in accordance with the manufacturer’s protocol.
Immunoprecipitation, SDS-PAGE and western blotting
Recombinant mouse galectin-9 (250 ng) and lactose (100 mM) were mixed and left on ice for 15 min. Sucrose (100 mM) was used as a negative control. Anti-TNP IgE (50 ng) was added and left on ice for 15 min. Then, the mixtures were incubated with anti-mouse IgE antibodies bound to protein G Dynabeads (Life Technologies) at room temperature for 10 min. SDS sample buffer (62.5 mM Tris-HCl (pH 6.8), 1% SDS, 10% glycerol, 0.005% bromophenol blue, and 2-mercaptoethanol) was added to each of the supernatants and beads, then heated at 70°C for 10 min. Samples were subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride membranes (General Electric, Fairfield, CT). Anti-mouse galectin-9 or anti-mouse IgE antibodies were reacted as the primary antibody at 4°C overnight, followed by anti-goat IgG-HRP antibody as the secondary antibody at 4°C for 1 h. All signals were detected by enhanced chemiluminescence using Chemi-Lumi One (Nacalai Tesque). In the case of detection of galectin-9 in serum, SDS-PAGE and western blotting were conducted in the same manner as above except the detection reagent. BCIP/NBT Phosphatase Substrate and PhosphaGLO ReserveTM AP Substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) were used as the detection reagent.
Ovalbumin-induced allergy model
Mice were sensitized by intraperitoneal injection of 10 µg OVA with 1 mg Al(OH)3 adjuvant and evoked by injection of 10 µg OVA with 0.5 mg Al(OH)3 adjuvant every 5 days for 4 weeks after sensitization. Oral administration of crude F-fucoidan (200 µg/day) was started on the 7th day before first injection of OVA. One day before the injection of OVA, blood samples were collected from the tail vein, and on the final day, whole blood was collected by cardiac puncture. The blood samples were centrifuged at 10,000 rpm for 10 min after incubation at room temperature for 1 h and at 4°C overnight for serum. The amount of total IgE, OVA-specific IgE and OVA-specific IgG1 were measured from serum by using an ELISA kit (BD Biosciences, San Jose, CA, DS Pharma Biomedical, Osaka, Japan, and Cayman Chemical, Ann Arbor, MI, respectively).
Statistical analysis
Data were expressed as means ± SE. Statistical analysis were performed by Tukey-Kramer test. P˂0.05 was considered statistically significant.
Results
Effect of F-fucoidan on passive cutaneous anaphylaxis reaction
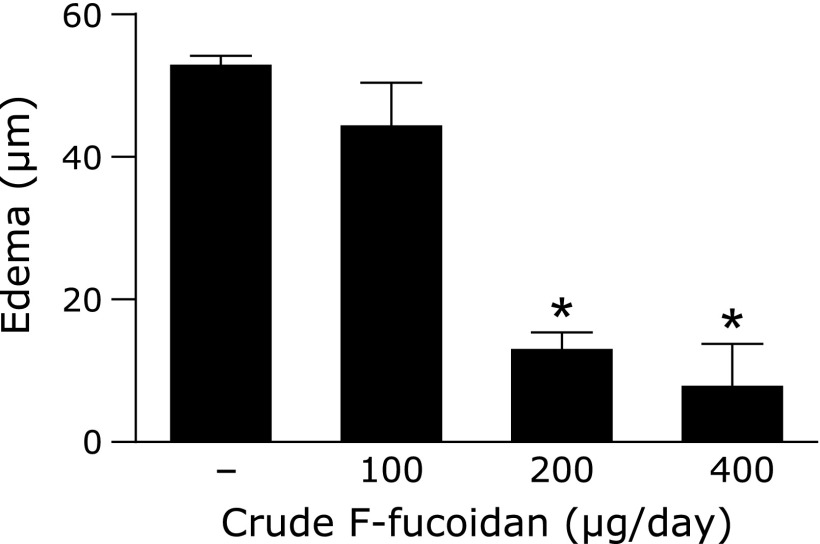
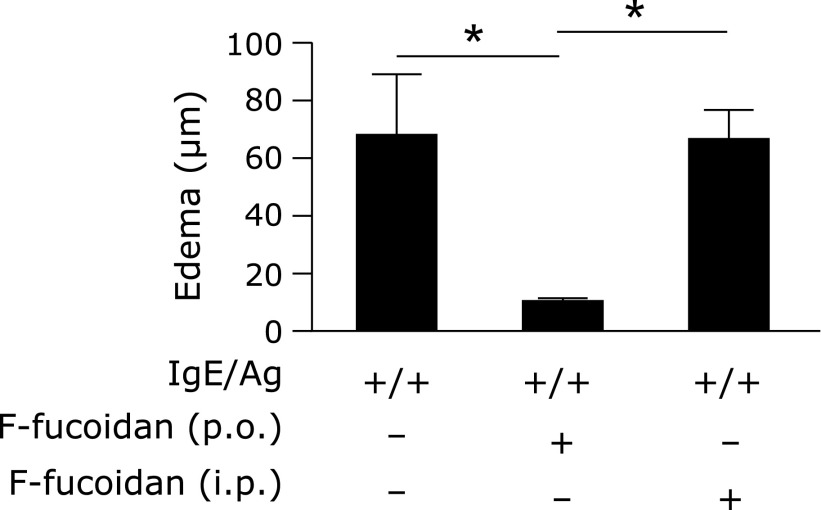
To investigate whether oral administration of F-fucoidan could inhibit a type I allergic reaction, PCA reaction was conducted after oral administration of crude F-fucoidan. Oral administration of F-fucoidan prevented ear edema induced by IgE/antigen at a concentration of more than 200 µg/day (Fig. 1). To confirm whether it is essential that F-fucoidan goes through intestine to exert its effect, mice were orally or intraperitoneally administered F-fucoidan. Although ear edema was inhibited in the group administered oral F-fucoidan, it was not absolutely inhibited in the group that received intraperitoneal crude F-fucoidan (Fig. 2). These data suggested that F-fucoidan shows an inhibitory effect on type I allergy only the through intestine.
Fig. 1.
Effect of F-fucoidan on ear edema induced by the passive cutaneous anaphylaxis reaction. Mice were administered F-fucoidan orally (100, 200, or 400 µg/day) for 4 days and conducted a passive cutaneous anaphylaxis reaction. Ear edema was evaluated 2 h after antigen challenge. *p<0.05, significantly different from the values of the group that did not receive crude F-fucoidan. Values represent the means ± SE of 3 mice in each group.
Fig. 2.
Difference in the pathway of administration of F-fucoidan. Mice were orally (p.o.) or intraperitoneally (i.p.) administered F-fucoidan (200 µg/day) for 4 days and induced allergic symptoms by passive cutaneous anaphylaxis reaction. Ear edema was evaluated 2 h after antigen challenge. *p<0.05. Values represent the means ± SE of 3 mice in each group.
Galectin-9 content in blood of mice administered F-fucoidan
It has been reported that galectin-9 prevented mast cell degranulation and ear edema induced by PCA reaction through an interaction with IgE.(12) Therefore, to investigate the involvement of galectin-9 in the inhibitory effect of F-fucoidan on the PCA reaction, galectin-9 in the blood of mice administered F-fucoidan was detected by western blotting. In serum of mice administered F-fucoidan, a band corresponding to galectin-9 was detected (Fig. 3). To confirm the identity of this band, TOF-MS was performed and identified it as galectin-9. Therefore, the administration of fucoidan increased galectin-9 content in the blood.
Fig. 3.
Increase in galectin-9 in serum of mice administered F-fucoidan. Mice were administered F-fucoidan orally (200 µg/day) for 4 days and induced allergic symptoms by passive cutaneous anaphylaxis reaction. After evaluation of ear edema, blood was collected. Galectin-9 in serum was detected using an anti-mouse galectin-9 antibody.
Association between suppression of the passive cutaneous anaphylaxis reaction and galectin-9 induction by oral administration of F-fucoidan
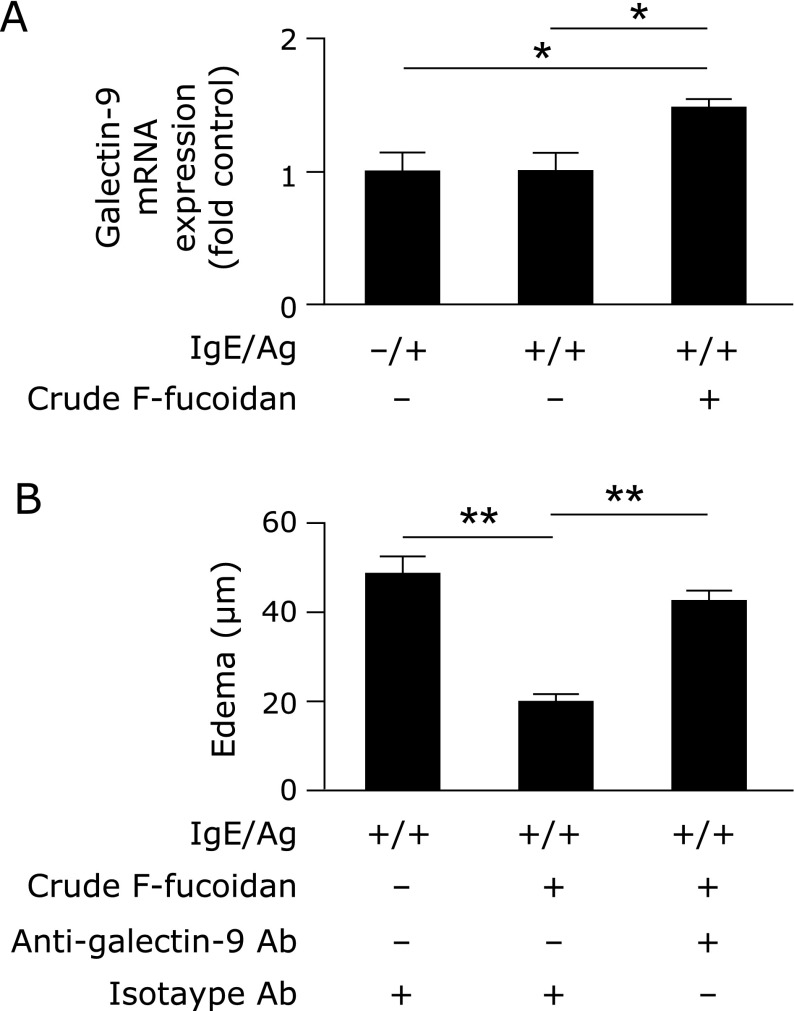
It has been reported that high expression levels of galectin-9 in the intestine led to the suppression of food allergy.(21) Therefore, to examine whether expression of galectin-9 in the intestine was increased when mice were administered F-fucoidan orally, galectin-9 mRNA expression levels in IECs of mice administered F-fucoidan were measured. Galectin-9 mRNA expression levels did not show any change in response to IgE/Ag challenge compared with just an IgE challenge. However, in the group administered crude F-fucoidan, the expression level of galectin-9 mRNA was increased significantly (Fig. 4A). To examine the requirement for galectin-9 in the anti-allergic properties of F-fucoidan, mice administered crude F-fucoidan were injected intravenously with an anti-galectin-9 antibody 1 h before the PCA reaction. As shown in Fig. 4B, ear edema, which was suppressed by oral administration of F-fucoidan, were canceled by injection of the anti-galectin-9 antibody.
Fig. 4.
Involvement of galectin-9 in the inhibitory effect of F-fucoidan on type I allergy. (A) Mice were administered F-fucoidan orally (200 µg/day) for 4 days and induced allergic symptoms by passive cutaneous anaphylaxis (PCA) reaction. Galectin-9 mRNA expression in intestinal epithelial cells after PCA reaction was measured. (B) Mice were administered F-fucoidan orally (200 µg/day) for 4 days and intravenously injected with anti-galectin-9 antibody or isotype IgG 1 h before PCA reaction. Ear edema was evaluated 2 h after the antigen challenge. *p<0.05, **p<0.01. Values represent the means ± SE of 3 mice in each group.
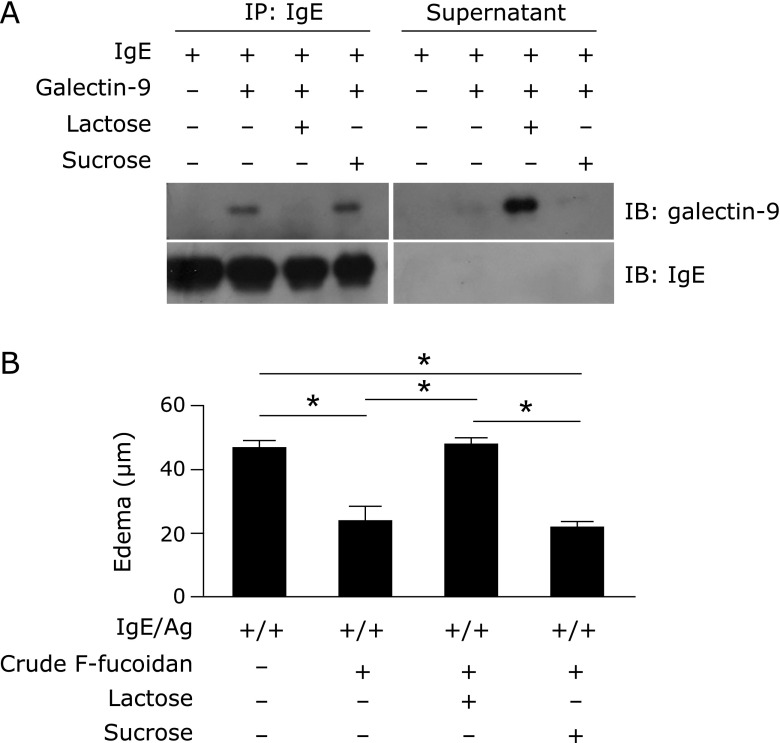
Involvement of binding between galectin-9 and IgE in the inhibitory properties of F-fucoidan in the passive cutaneous anaphylaxis reaction
It has been reported that the interaction of galectin-9 with IgE was competitively inhibited by lactose.(12) Indeed, lactose, but not sucrose, inhibited co-immunoprecipitation of galectin-9 with IgE, and galectin-9 was detected in the supernatant (Fig. 5A). To confirm the effect of lactose in vivo, mice administered crude F-fucoidan were intravenously injected with lactose as well as an anti-galectin-9 antibody. As shown in Fig. 5B, mice administered lactose after administration of crude F-fucoidan and before injection of IgE formed ear edema equivalent to mice challenged with IgE/antigen without the administration of F-fucoidan. However, in mice administered sucrose, which did not contain galactose, instead of lactose, ear edema was suppressed to the same level as mice administered crude F-fucoidan. These data indicated that oral treatment with F-fucoidan may increase galectin-9 content in the blood and prevent the interaction of IgE and mast cells.
Fig. 5.
Lactose-mediated inhibition of the interaction between galectin-9 and IgE and the effect of F-fucoidan on ear edema induced by IgE/antigen. (A) IgE and galectin-9 reacted with or without lactose, followed by immunoprecipitation with an anti-mouse IgE antibody and western blotting with an anti-mouse galectin-9 antibody. The data are representative of triplicate experiments. (B) Mice were administered F-fucoidan orally (200 µg/day) for 4 days and intravenously injected with lactose or sucrose 10 min before passive cutaneous anaphylaxis reaction. Ear edema measured 2 h after antigen challenge. *p<0.01. Values represent the means ± SE of 3 mice in each group.
The immune-modulatory effect of oral administration of F-fucoidan
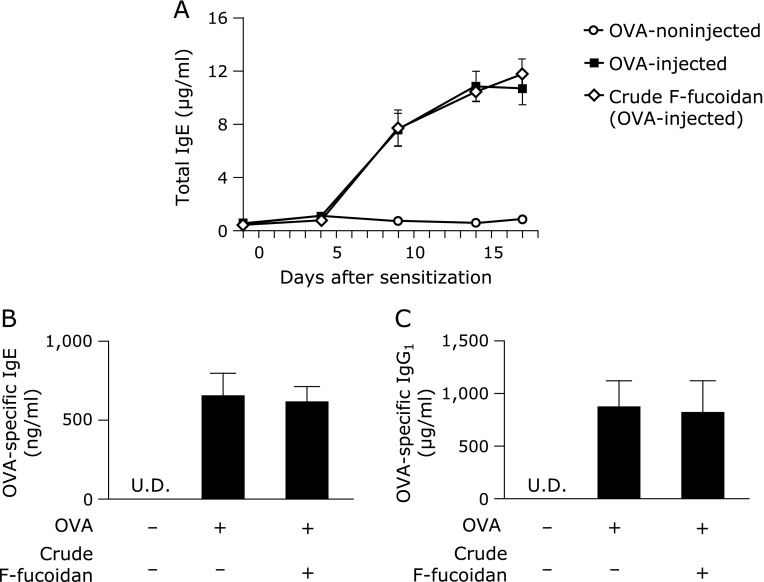
It has been shown that serum IgE and IgG1 contents are increased when Th1/Th2 balance is deflected to Th2, and Th2 cytokine levels, such as those of IL-4 and IL-13, are increased. Indeed, the anti-allergic properties of fucoidan from Undaria pinnatifida were caused by modulation of Th1/Th2.(19) Therefore, to confirm that F-fucoidan affected the Th1/Th2 balance, allergy was induced by the injection of OVA and the amount of serum IgE and IgG1 was measured. Oral administration of crude F-fucoidan did not decrease serum total IgE content increased by OVA injection (Fig. 6A). The increase in antigen-specific IgE and IgG1 was not affected by administration of crude F-fucoidan (Fig. 6B and C). These data suggested that the anti-allergic properties of F-fucoidan from S. japonica were due to suppression of mast cell activation but not the modulation of Th1/Th2 balance.
Fig. 6.
No effect of F-fucoidan on the increase in IgE and IgG1 induced by ovalbumin injection. Mice were administered F-fucoidan orally (200 µg/day) for 7 days and immunized with ovalbumin (OVA) as described in the Materials and Methods. Amount of total IgE (µg/ml) in serum collected from the tail vein 1 day before injection OVA (A). Serum OVA-specific IgE (ng/ml) (B) and OVA-specific IgG1 (µg/ml) (C) content on the final day. Values represent the means ± SE of 6–7 mice in each group. U.D. indicates under detection.
Discussion
Development of type I allergy starts from phagocytosis of foreign antigens by antigen-presentation cells and presentation of the antigen to naïve T cells followed by differentiation of naïve T cells into Th2 cells, which produce Th2 cytokines such as IL-4 and IL-13. These cytokines induce immunoglobulin class switching to IgE in B cells,(22) whereby blood IgE level is increased. Increased IgE binds to IgE receptors on mast cells. Subsequently, when the antigen reenters the body, the antigen bridges IgE molecules on mast cells and the mast cells are activated. Activated mast cells undergo degranulation and release chemical mediators such as histamine followed by the production of eicosanoid and cytokine.(23) In previous reports on the anti-allergic effects of fucoidan, it inhibits allergic symptoms through modulating the polarized immune response and inhibiting the production of IgE.(19,24) Briefly, fucoidan has been thought to mainly exert an inhibitory effect on allergy by modulation of the Th1/Th2 balance. However, in this study, although F-fucoidan inhibited ear edema, which was caused by mast cell degranulation, F-fucoidan did not completely suppress the increased in serum IgE and IgG1 (Fig. 1 and 6). Moreover, to further investigate the immune-modulatory effect of F-fucoidan, IFN-γ and IL-4 production of typical Th1 and Th2 cytokines in splenocytes from OVA-induced allergy model mouse was examined. When the splenocytes were restimulated with OVA for 72 h, F-fucoidan did not affect production of Th1 and Th2 cytokines in splenocytes of OVA-induced allergy model (data not shown). These results suggested that F-fucoidan can inhibit mast cell degranulation but not polarization to Th2. Moreover, it was indicated that the inhibitory effect on type I allergy by oral administration of F-fucoidan is not an immune-modulatory effect, which was previously reported as an effect of fucoidan.
In this study, intravenous administration of an anti-galectin-9 antibody prevented the inhibitory effect of F-fucoidan on ear edema (Fig. 4B). It was ascertained that the inhibitory effect of F-fucoidan on ear edema induced by IgE/antigen reaction depended on galectin-9. Galectin-9 binds to IgE and prevents binding of IgE to IgE receptor FcεRI, resulting in the inhibition of mast cell activation.(12) Moreover, administration of F-fucoidan increased the expression level of the galectin-9 mRNA in IECs (Fig. 4A) and the content of galectin-9 in serum (Fig. 3). The inhibitory effect of F-fucoidan on ear edema was shown only when F-fucoidan was administered orally (Fig. 2). Taking into consideration these results, it was predicated that oral administration of F-fucoidan influences IECs and promotes the expression and secretion of galectin-9 into blood. As shown Fig. 4, the inhibitory effect of F-fucoidan on ear edema was also prevented by intravenous administration of lactose, which binds to galectin-9 and suppresses the interaction of galectin-9 with IgE.(12) These results suggested that secreted galectin-9 bound to IgE in blood and prevented ear edema.
The result that F-fucoidan inhibited ear edema but did not suppress the increase of serum IgE and IgG1 (Fig. 1 and 6) was consistent with a previous report that the exogenous galectin-9 could suppress ear swelling induced by IgE/antigen, although it did not affect the serum contents of IgE and IgG1.(12) Besides, while it has been reported that mast cells expressed TIM-3, which is one of the major targets of galectin-9,(25,26) activation of TIM-3 on mast cells did not affect mast cell degranulation induced by IgE/antigen.(26) These reports and the results in this study suggested that galectin-9 induced by F-fucoidan interacted with IgE but not TIM-3 and prevented allergic symptoms.
Galectin-9 has been reported to have the inhibitory properties against allergy(10,12,27) and various autoimmune diseases.(5,11,28,29) However, in these studies mice were administered exogenous galectin-9 or overexpressed galectin-9. The substances that can induce galectin-9 are less understood, although there was a report that combination of oligosaccharides in human milk with bifidobacteria can increase galectin-9 in IECs and blood, whereby food allergy was suppressed.(21) In this study, we demonstrated that F-fucoidan from S. japonica possessed an ability to increase expression of galectin-9 in IECs (Fig. 4A). Furthermore, administration of F-fucoidan increased the amounts of galectin-9 in blood (Fig. 3). These data indicated that F-fucoidan is a novel inducer of galectin-9. Moreover, the inhibitory effect of F-fucoidan on type I allergy through the induction of galectin-9 was observed only when F-fucoidan was administered orally (Fig. 2). This suggested that dietary intake of S. japonica could be useful for the induction of galectin-9, followed by preventive effects on allergic symptoms.
Our laboratory reported previously that F-fucoidan is not completely absorbed into the body and all F-fucoidan is excreted in the feces.(16) Therefore, it was assumed that F-fucoidan interacts with factors on IECs or DCs passing their dendrites between IECs into the gut lumen and promotes the expression and secretion of galectin-9. Fucoidan has been reported to interact with several lectins,(30–33) and IECs express various lectins.(34–36) Therefore, fucoidan could be recognized through one of the lectins on IECs. In addition, DCs passing their dendrites between IECs have been reported to have DC-specific intracellular adhesion molecule 3-grabbing non-integrin (DC-SIGN), which recognizes fucose.(37) It is known that DCs can protrude dendrites to the luminal side, and DC-SIGN is also expressed on protruding dendrites.(38) Thus, F-fucoidan might be recognized by DCs using intraepithelial dendrites but not by IECs. However, the receptor that recognizes F-fucoidan has not yet been identified, although there are some candidates.
Many of previous reports of food factors about anti-allergic effects have suggested that effects of them were due to immune-modulatory effects. For example, flavone from Scutellariae radix has been reported to reduce IL-4 and eotaxin production and IgE production in OVA-induced allergy.(39) However, our study indicated that oral treatment with F-fucoidan prevented type I allergy through the inhibition of mast cell activation but not the inhibition of Th2 polarization. Moreover, our study suggested the inhibitory mechanism of F-fucoidan, through which it influences IECs and promotes the expression and secretion of galectin-9. Thus, increased galectin-9 in the blood binds to IgE and prevents the interaction between IgE and mast cells, followed by mast cell activation. This study showed that F-fucoidan is a novel inducer of galectin-9 and that F-fucoidan exhibits anti-allergic properties through a different mechanism from the mechanisms that have reported as effects of fucoidan previously. This anti-allergic mechanism of F-fucoidan suggested that F-fucoidan may be an effective inhibitor that can prevent symptoms in type I allergic patients even after sensitization to allergen. Therefore, anti-allergic effects of F-fucoidan should be confirmed in clinical trial in the near feature.
Acknowledgments
We would like to thank Yuki Yokoyama for her advice and technical assistance. This study received financial support from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, under Special Coordination Funds for Promoting Science and Technology, Creation of Innovative Centers for Advanced Interdisciplinary Research Areas.
Abbreviations
- DC
dendritic cell
- DC-SIGN
dendritic cell-specific intracellular adhesion molecule 3-grabbing non-integrin
- IECs
intestinal epithelial cells
- OVA
ovalbumin
- PCA
passive cutaneous anaphylaxis
- SE
standard error
- TIM-3
T-cell immunoglobulin and mucin-containing protein-3
- TNP
trinitrophenyl
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hirabayashi J, Hashidate T, Arata Y, et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572:232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 3.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 4.Wada J, Kanwar YS. Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin. J Biol Chem. 1997;272:6078–6086. doi: 10.1074/jbc.272.9.6078. [DOI] [PubMed] [Google Scholar]
- 5.Seki M, Oomizu S, Sakata K, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Dai SY, Nakagawa R, Itoh A, et al. Galectin-9 induces maturation of human monocyte-derived dendritic cells. J Immunol. 2005;175:2974–2981. doi: 10.4049/jimmunol.175.5.2974. [DOI] [PubMed] [Google Scholar]
- 7.Sziksz E, Kozma GT, Pállinger E, et al. Galectin-9 in allergic airway inflammation and hyper-responsiveness in mice. Int Arch Allergy Immunol. 2010;151:308–317. doi: 10.1159/000250439. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Song CH, Liu ZQ, et al. Intestinal epithelial cells express galectin-9 in patients with food allergy that plays a critical role in sustaining allergic status in mouse intestine. Allergy. 2011;66:1038–1046. doi: 10.1111/j.1398-9995.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima R, Miyagaki T, Oka T, et al. Elevated serum galectin-9 levels in patients with atopic dermatitis. J Dermatol. 2015;42:723–726. doi: 10.1111/1346-8138.12884. [DOI] [PubMed] [Google Scholar]
- 10.Katoh S, Ishii N, Nobumoto A, et al. Galectin-9 inhibits CD44-hyaluronan interaction and suppresses a murine model of allergic asthma. Am J Respir Crit Care Med. 2007;176:27–35. doi: 10.1164/rccm.200608-1243OC. [DOI] [PubMed] [Google Scholar]
- 11.Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 12.Niki T, Tsutsui S, Hirose S, et al. Galectin-9 is a high affinity IgE-binding lectin with anti-allergic effect by blocking IgE-Antigen complex formation. J Biol Chem. 2009;284:32344–32352. doi: 10.1074/jbc.M109.035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li B, Lu F, Wei X, Zhao R. Fucoidan: structure and bioactivity. Molecules. 2008;13:1671–1695. doi: 10.3390/molecules13081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Synytsya A, Kim WJ, Kim SM, et al. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr Polym. 2010;81:41–48. [Google Scholar]
- 15.Athukorala Y, Jung WK, Vasanthan T, Jeon YJ. An anticoagulative polysaccharide from an enzymatic hydrolysate of Ecklonia cava. Carbohydr Polym. 2006;66:184–191. [Google Scholar]
- 16.Ren R, Azuma Y, Ojima T, et al. Modulation of platelet aggregation-related eicosanoid production by dietary F-fucoidan from brown alga Laminaria japonica in human subjects. Br J Nutr. 2013;110:880–890. doi: 10.1017/S000711451200606X. [DOI] [PubMed] [Google Scholar]
- 17.Vo TS, Ngo DH, Kang KH, Jung WK, Kim SK. The beneficial properties of marine polysaccharides in alleviation of allergic responses. Mol Nutr Food Res. 2015;59:129–138. doi: 10.1002/mnfr.201400412. [DOI] [PubMed] [Google Scholar]
- 18.Oomizu S, Yanase Y, Suzuki H, Kameyoshi Y, Hide M. Fucoidan prevents Cε germline transcription and NFκB p52 translocation for IgE production in B cells. Biochem Biophys Res Commun. 2006;350:501–507. doi: 10.1016/j.bbrc.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Yanase Y, Hiragun T, Uchida K, et al. Peritoneal injection of fucoidan suppresses the increase of plasma IgE induced by OVA-sensitization. Biochem Biophys Res Commun. 2009;387:435–439. doi: 10.1016/j.bbrc.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Tokita Y, Nakajima K, Mochida H, Iha M, Nagamine T. Development of a fucoidan-specific antibody and measurement of fucoidan in serum and urine by sandwich ELISA. Biosci Biotechnol Biochem. 2010;74:350–357. doi: 10.1271/bbb.90705. [DOI] [PubMed] [Google Scholar]
- 21.de Kivit S, Saeland E, Kraneveld AD, et al. Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy. 2012;67:343–352. doi: 10.1111/j.1398-9995.2011.02771.x. [DOI] [PubMed] [Google Scholar]
- 22.Del Prete G, Maggi E, Parronchi P, et al. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988;140:4193–4198. [PubMed] [Google Scholar]
- 23.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 24.Vo TS, Kim SK. Marine-derived polysaccharides for regulation of allergic responses. Adv Food Nutr Res. 2013;73:1–13. doi: 10.1016/B978-0-12-800268-1.00001-9. [DOI] [PubMed] [Google Scholar]
- 25.Nakae S, Iikura M, Suto H, et al. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110:2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiener Z, Kohalmi B, Pocza P, et al. TIM-3 is expressed in melanoma cells and is upregulated in TGF-beta stimulated mast cells. J Invest Dermatol. 2007;127:906–914. doi: 10.1038/sj.jid.5700616. [DOI] [PubMed] [Google Scholar]
- 27.Madireddi S, Eun SY, Lee SW, et al. Galectin-9 controls the therapeutic activity of 4-1BB–targeting antibodies. J Exp Med. 2014;211:1433–1448. doi: 10.1084/jem.20132687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Luan H, Wang L, et al. Galectin-9 ameliorates anti-GBM glomerulonephritis by inhibiting Th1 and Th17 immune responses in mice. Am J Physiol Renal Physiol. 2014;306:F822–F832. doi: 10.1152/ajprenal.00294.2013. [DOI] [PubMed] [Google Scholar]
- 29.Chou FC, Shieh SJ, Sytwu HK. Attenuation of Th1 response through galectin-9 and T-cell Ig mucin 3 interaction inhibits autoimmune diabetes in NOD mice. Eur J Immunol. 2009;39:2403–2411. doi: 10.1002/eji.200839177. [DOI] [PubMed] [Google Scholar]
- 30.Bevilacqua MP, Nelson RM. Selectins. J Clin Invest. 1993;91:379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francischetti IM, Ma D, Andersen JF, Ribeiro JM. Evidence for a lectin specific for sulfated glycans in the salivary gland of the malaria vector, Anopheles gambiae. PLoS One. 2014;9:e107295. doi: 10.1371/journal.pone.0107295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gange CT, Quinn JMW, Zhou H, Kartsogiannis V, Gillespie MT, Ng KW. Characterization of sugar binding by osteoclast inhibitory lectin. J Biol Chem. 2004;279:29043–29049. doi: 10.1074/jbc.M312518200. [DOI] [PubMed] [Google Scholar]
- 33.Manne BK, Getz TM, Hughes CE, et al. Fucoidan is a novel platelet agonist for the C-type lectin-like receptor 2 (CLEC-2) J Biol Chem. 2013;288:7717–7726. doi: 10.1074/jbc.M112.424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen-Kedar S, Baram L, Elad H, Brazowski E, Guzner-Gur H, Dotan I. Human intestinal epithelial cells respond to β-glucans via Dectin-1 and Syk. Eur J Immunol. 2014;44:3729–3740. doi: 10.1002/eji.201444876. [DOI] [PubMed] [Google Scholar]
- 35.Moucadel V, Soubeyran P, Vasseur S, Dusetti NJ, Dagorn JC, Iovanna JL. Cdx1 promotes cellular growth of epithelial intestinal cells through induction of the secretory protein PAP I. Eur J Cell Biol. 2001;80:156–163. doi: 10.1078/0171-9335-00148. [DOI] [PubMed] [Google Scholar]
- 36.Akiyama Y, Oshima K, Shin K, et al. Intracellular retention and subsequent release of bovine milk lactoferrin taken up by human enterocyte-like cell lines, Caco-2, C2BBe1 and HT-29. Biosci Biotechnol Biochem. 2013;77:1023–1029. doi: 10.1271/bbb.121011. [DOI] [PubMed] [Google Scholar]
- 37.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bron PA, van Baarlen P, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol. 2011;10:66–78. doi: 10.1038/nrmicro2690. [DOI] [PubMed] [Google Scholar]
- 39.Ryu EK, Kim TH, Jang EJ, et al. Woogonin, a plant flavone from Scutellariae radix, attenuated ovalbumin induced airway inflammation in mouse model of asthma via the suppression of IL-4/STAT6 signaling. J Clin Biochem Nutr. 2015;57:105–112. doi: 10.3164/jcbn.15-45. [DOI] [PMC free article] [PubMed] [Google Scholar]