Abstract
Overexpression of IGF-1R has been demonstrated in gastrointestinal cancers, and its expression is reported as the result of the loss of tumor suppressors. IL-16 is involved in the pathophysiological process of chronic inflammatory diseases. The aim of this study is to determine the changes in the expression of IGF-1R in intestinal metaplasia (IM) and gastric cancer (GC) as well as the effect of Helicobacter pylori (H. pylori) and IL-16 on cell proliferation and IGF-1R expression in gastric cells. AGS cells were incubated with combinations of IL-16 and H. pylori. Gastric cell proliferation was studied by BrdU uptake. In H. pylori infected mucosa, IGF-1R was significantly higher in IM than chronic gastritis (CG), and also higher in GC than CG and IM. H. pylori significantly decreased BrdU uptake. IL-16 increased BrdU uptake and IGF-1R on AGS cells which had been decreased by H. pylori. Co-incubation with IL-16 increased the expression of IGF-1R mRNA in H. pylori infected cells. We conclude that the expression of IGF-1R in H. pylori infected gastric mucosa may indicate an early stage of carcinogenesis. The IL-16 secretion by H. pylori can be a trigger for the expression of IGF-1R, and it may also be a factor for gastric carcinogenesis.
Keywords: gastric cancer, IGF-1R, Helicobacter pylori, intestinal metaplasia, chronic gastritis
Introduction
A model of gastric cancer has been proposed in which superficial gastritis is followed by chronic gastritis, intestinal metaplasia and finally gastric cancer.(1,2) Gastric cancer is the second common cause of cancer related death in the world. Gastric cancers are among the most lethal neoplasms in the world. Signals from a variety of growth factor receptors are important for carcinogenesis and cancer development. These signals modify cell cycle regulation, apoptotic induction and interactions between cancer cells with their environment to promote the continuous growth potential of cancer cells.(3)
The insulin-like growth plays a cruel role in cell growth factor signaling in cell proliferation and differentiation, and it is reported that insulin-like growth factor (IGF) system is an antiapoptotic molecule, activating the phosphatidylinositol-3 kinase-AKT pathway.(4) Exogenous IGFs stimulates the gastric cell proliferation, and over expression of IGFs was recognized in the mucosa of gastric cancer.(5) IGF-1 and IGF-2 are ligands for IGF-1 receptor (IGF-1R), the complex of IGFs and IGF-1R activates signal pathways, and stimulates tumor progression and cellular differentiation.(6) They also reported that the monoclonal antibody for IGF-1R suppressed proliferation and tumorigenicity in human gastrointestinal cancer cells.
Helicobacter pylori (H. pylori) infection causes chronic gastritis, gastroduodenal ulceration and gastric adenocarcinoma. A model of gastric cancer has been proposed in which superficial gastritis is followed by chronic gastritis, intestinal metaplasia and gastric cancer.(7,8) The inflammation is a one of the risk factor for carcinogenesis, and the chronic infection and inflammation is correlated with cancer, including the patients with inflammatory bowel diseases, is a factor for colorectal carcinogenesis.(9) H. pylori infected in childhood, and the infection causes chronic inflammatory changes in gastric mucosa. These inflammatory cells infiltration is reported as a factor for gastric carcinogenesis.(10)
Interleukin-16 (IL-16), a pleiotrophic cytokine, is involved in the pathophysiological process of chronic inflammatory diseases including HIV and Crohn’s diseases. Recently, IL-16 plasma levels and tumor progression were reported for many types of cancer.(11) The serum level of IL-16 was associated gastric cancer patients compared with healthy controls.(9) But the relationship with H. pylori infection and IL-16 secretion is not clear. We studied the differences of the expression of IGF-1R in intestinal metaplasia and gastric cancer as well as the effect of H. pylori infection and IL-16 on epithelial cell proliferation and IGF-1R expression in gastric cells in vitro.
Materials and Methods
Expression of IGF-1R on gastric mucosa
The method of the immunohistological staining was described in our previous study.(12) Gastric samples were taken from biopsies through endoscopy. The diagnosis of gastric mucosa and the infection of H. pylori were diagnosed histologically. Samples were fixed, made as paraffin embedded blocks and cut into 5 µm sections. They were mounted on slides and dewaxed in xylene and sequentially dehydrated in 100%, 80% and 70% ethanol. The slides were incubated in Target Retrieval Solution (1:400 Dako, Copenhagen, Denmark) at 95°C for 5 min. The intrinsic peroxidase was blocked with 0.3% H2O2 in methanol for 30 min. Non-specific binding was blocked with PBS containing 3% skim milk (Wako, Tokyo, Japan) for 30 min. The species were incubated with anti-IGF-1R polyclonal antibody (Abcam ab39675: Cambridge, UK) overnight at 4°C. After wash with PBS PBST and PBS for 5 min, the slides were incubated with biotinylated Goat anti-rabbit antibody (1:100, Dako) for 30 min at room temperature. After slides were washed with PBS, PBST and PBS, sections were incubated with avidin-biotin complex (ABC Elite: Vector Laboratoried Inc., Burlingame, CA) and colored with DAB (Wako). Gastric biopsies were classified by histological findings as chronic gastritis (CG); either with H. pylori (CG+) or without H. pylori infection (CG−), intestinal metaplasia (IM); either with H. pylori (IM+) or without H. pylori (IM−), and gastric cancer (GC) with H. pylori (GC+). The number of samples of each group was 60 tissues. Sections were counterstained with Hematoxylin. Image Manager Software applied to a light microscope was used for computer analysis of each sample. The degree of IGF-1R positive was evaluated areas of gastric mucosa. The positive area of IGF-1R was assessed and expressed as percentage of area.
Cell culture
AGS cells (Dainippon, Tokyo, Japan) were cultured with F-12 medium (Gibco, Life-technologies, Grand Island, NY) with 10% fetal bovine serum (MP Biomedicals, Solon, OH) and anti-biotic-anti-mycotic solution (Sigma-Aldrich, St. Louis, MO) at 37°C in a humidified atmosphere of 95% air and 5% CO2. H. pylori (NCTC 11637) was cultured on Skirrow Agar with 7% horse blood (Nippon Biotest Lab., Tokyo, Japan) in atmosphere of 10% CO2 at 37°C. Colonies were harvested, 105 cfu/ml of H. pylori was resuspended in antibiotic-free F-12 medium without FBS. Experiments were performed using 6-well polypropylene tissue culture plates (NUNC, Copenhagen, Denmark) with the combination with and/or without 105 cfu/ml of H. pylori and 10−10 M or 10−9 M of recombinant human IL-16 (Pepro Tech. Inc., Rocky Hill, NJ) was added into AGS cells for 24 h.
ELISA assays
Gastric epithelial cell proliferation was studied by BrdU uptake. AGS cells were cultured on 6 well-plates (NUNC) by serum free F-12 medium without antibiotics. Cells were incubated with and/or without H. pylori and IL-16 for 24 h, additionally 1 h incubated with 10 µM BrdU (Sigma-Aldrich) solution. After 3 times wash with PBS, cells were lysed with 50 mM NaCl, 25 mM Tris-HCl, 0.5% NP-40, 0.5% sodium deoxycholate and 0.02% sodium azide. Anti-BrdU (10 µm/ml Dako) and anti-IGF-1R (Abcam) antibodies were diluted with 0.01 M phosphate buffer, 0.15 M NaCl pH 7.2, and coated each well of 96-well plates, and incubated at 4°C overnight. 100 µl of samples were pipetted into wells and incubated for 2 h at room temperature after 3 times washing with Buffer B (0.01 M phosphate buffer, 0.50 M NaCl, 0.1% Tween 20, pH 7.2). The plates were blocked with 3% skim milk (Wako) with Buffer B for 30 min after 3 times washing with Buffer B. The plates were incubated with secondary antibodies (Dako) for 1 h, and further incubated with alkaline phosphatase conjugated streptavidin (Dako) for 1 h. The expressions were followed by ampliQ detection kit (K6245, Dako) and determined by a spectrophotometer at 492 nm.
RT-PCR
Cells were transferred into 1.5 ml of microtube, and centrifuged for 10 min at 4°C. Pour of the media, and TRI Reagent and linear Acrylamide were added into microtubes. After pipetting, the samples were transferred to homogenizer and homogenized for 30 s in ice cubes, and stored for 5 min at room temperature. Add 160 µl of chloroform and stored at room temperature for 10 min. After centrifuge at 4°C for 15 min, supernatant was put into other microtubes. 400 µl of isopropyl alcohol was added and stored at −20°C overnight. Samples were centrifuged at 4°C for 20 min, and supernatant was removed. 75% ethanol was added to tubes, and tubes were dried for 5 min at room temperature after 4°C for 5 min centrifuge. RNase free water was added to the tubes, and incubated at 60°C for 10 min. The cDNA was prepared as following the protocol of High Capacity RNA-to-cDNA Master MiX (Applied Biosystems, 4390779, Foster City, CA). 1 µl of cDNA was used for PCR as following the protocol of Ampli Taq Gold with Gene Amp 10X PCR Buffer II and MgCl2 solution (Applied Biosystems, N8080241), and mixed with 5 µl of 10X PCR Buffer II, 5 µl of 10XdNTP, 5 µl of 25 mM MgCl2, 0.5 µl of 10 mM forward primer 0.5 µl of 10 µM reverse primer, 0.25 µl of Ampli Taq Gold and 32.75 µl of distilled water. The set of IGF-1R primers and length of the product were as follows: IGF-1R-s 5'GGG GAA TGG AGT GCT GTA TG-3' IGF-1R antisense 5'ATT GAG GTC ACA GAG AAC-3'.(13) The cycling program involved initial denaturtion at 95°C for 10 min, following by 35 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 45 s. PCR products were verified by 1.0% agarose gel electrophoresis (Wako) and visualized by ethidium bromide staining and ultraviolet light. All bands were analyzed by NIH image system.
Statistical analysis
Continuous variables were presented as mean ± SD, All statistical analyses were performed using Turney test, and a p value <0.05 was considered statistically significant.
Results
The expression of IGF-1R on gastric mucosa
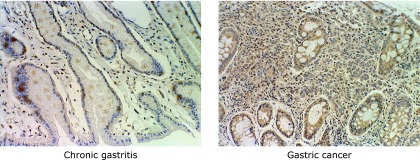
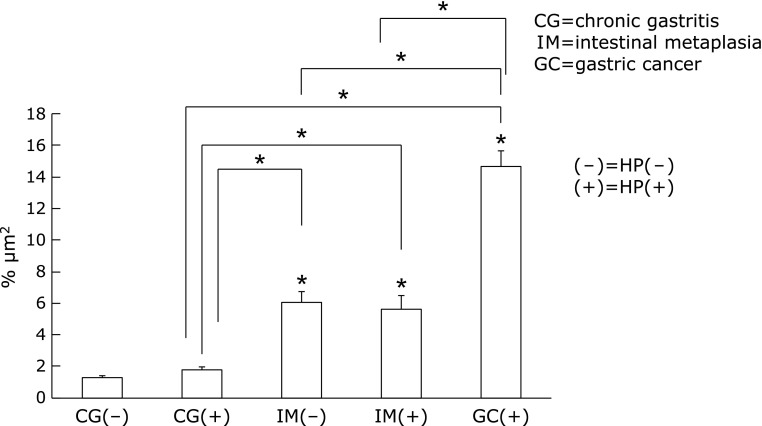
In chronic gastritis, there was no significant difference on IGF-1R protein levels between with and without H. pylori infection (H. pylori infected chronic gastritis: 0.77 ± 1.59% vs H. pylori negative chronic gastritis: 1.29 ± 0.80%). In H. pylori infected mucosa, the expression of IGF-1R was significantly higher in intestinal metaplasia than chronic gastritis (IM+: 5.64 ± 5.03% vs CG+: 0.77 ± 1.59%, p<0.01), and in H. pylori infected mucosa, IGF-1R expression was also higher in gastric cancer than chronic gastritis and intestinal metaplasia (GC+: 14.65 ± 7.37% vs CG+: 0.77 ± 1.59%, IM+: 5.64 ± 5.03%, p<0.01) (Fig. 1 and 2).
Fig. 1.
The expression of IGF-1R on gastric mucosa of chronic gastritis and gastric cancer by ABC staining. IGF-1R was staining by DAB as brown area.
Fig. 2.
The expression of IGF-1R on gastric mucosa. There was no significant difference between with and without H. pylori infection on chronic gastritis. The expression of IGF-1R was significantly increased in intestinal metaplasia and gastric cancer than chronic gastritis.
Cell Proliferation
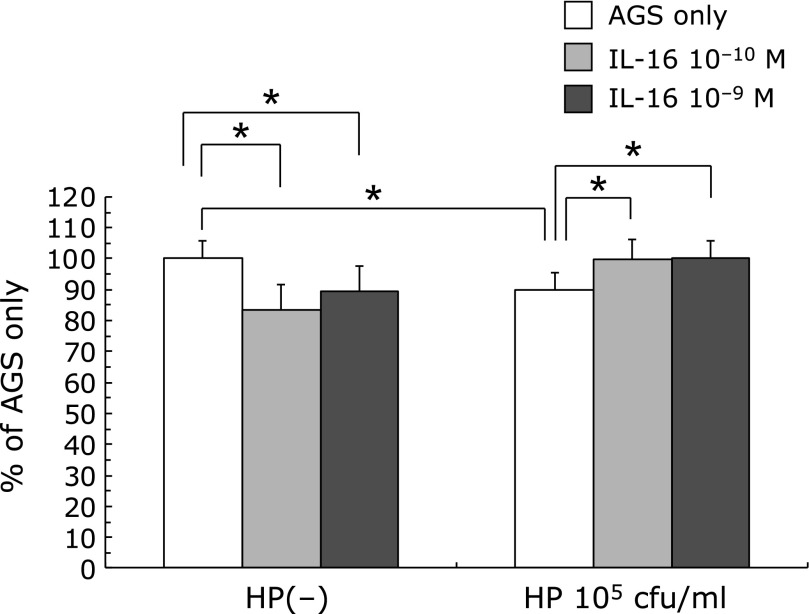
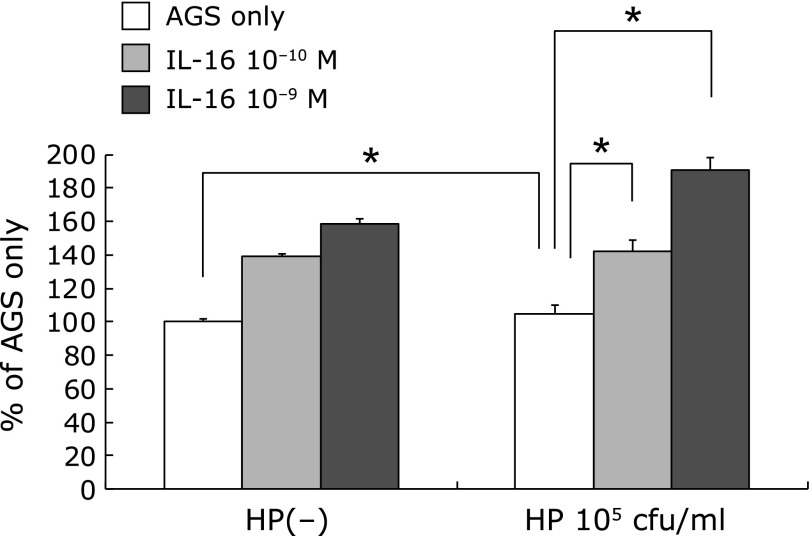
105 cfu/ml of H. pylori administration significantly decreased the BrdU uptake of AGS cells (85.76 ± 5.91% of AGS only p<0.01). 10−10 and 10−9 M of IL-16 administration significantly decreased the BrdU uptake of AGS cells. (IL-16 only: 10−10 M 84.65 ± 2.01% 10−9 M 89.50 ± 1.87%, p<0.01) Administration of 10−10 and 10−9 M of IL-16 significantly increased BrdU uptake which was decreased by 105 cfu/ml of H. pylori (H. pylori 105 cfu/ml only: 85.76 ± 5.91% vs H. pylori + IL-16 10−10 M: 100.46 ± 6.39%, H. pylori + IL-16 10−9 M: 99.97 ± 6.00%, p<0.01) (Fig. 3).
Fig. 3.
The effect of H. pylori and IL-16 on BrdU uptake on AGS cells. H. pylori infection decreased the BrdU uptake of AGS cells. The administration of IL-16 significantly increased the BrdU uptake on AGS cells which was decreased by H. pylori infection.
Expression of IGF-1R protein
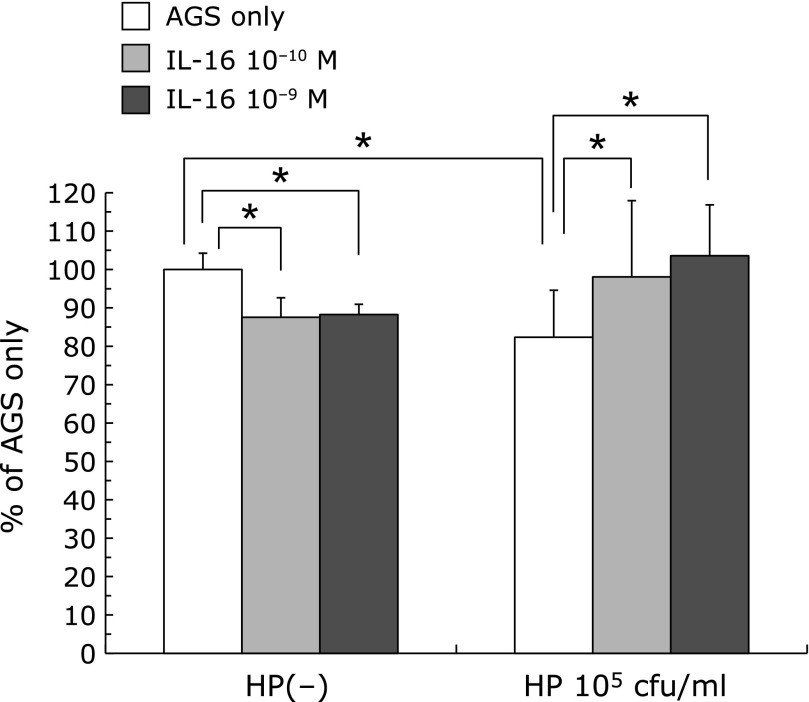
105 cfu/ml of H. pylori administration significantly decreased the IGF-1R protein level of AGS cells. (H. pylori 105 cfu/ml: 82.34 ± 12.26%, Administration of IL-16 decreased IGF-1R protein level of AGS cells but not significantly (IL-16 only: 10−10 M 87.53 ± 1.62% 10−9 M 88.27 ± 1.80%). But co-incubation with IL-16 increased the expression of IGF-1R protein which was decreased by H. pylori administration (H. pylori 105 cfu/ml only: 82.34 ± 12.26%, vs H. pylori + IL-16 10−10 M: 98.07 ± 19.88%, H. pylori+IL-16 10−9 M: 103.57 ± 13.29%, p<0.01) (Fig. 4).
Fig. 4.
The effect of H. pylori and IL-16 on IGF-1R protein levels of AGS cells. H. pylori infection decreased the expression of IGF-1R protein of AGS cells. The administration of IL-16 significantly increased the expressions of IGF-1R protein which was decreased by H. pylori infection.
The expression of IGF-1R mRNA in H. pylori infected AGS cells
As same as IGF-1R protein expression, the expression of IGF-1R was decreased by 105cfu/ml of H. pylori, (80.40 ± 19.13%) and co-administration of IL-16 increased IGF-1R mRNA expression which was decreased by H. pylori infection. (H. pylori 105cfu/ml only: 80.40 ± 19.13%, H. pylori+IL-16 10−10M: 91.38 ± 19.18%, H. pylori+IL-16 10−9M: 97.15 ± 43.95%, p<0.01) (Fig. 5).
Fig. 5.
The effect of H. pylori and IL-16 on the expression of IGF-1R mRNA of AGS cells. H. pylori infection decreased the expression of IGF-1R mRNA of AGS cells. The administration of IL-16 significantly increased the expressions of IGF-1R mRNA which was decreased by H. pylori infection.
Discussion
IGF-1R is a heterodimer of 2α- and β-subunits.(14) Binding with IGF-1R and IGF-1 and/or IGF-II, causes autophosphorylation, activates multiple signaling pathways including the mitogen-activated protein kinase (MAPK) and phosphatidylinositide 3-kinase (PI3-K)/Akt-1 pathways.(15) IGF-1R is a cell membrane receptor that is activated by its ligands, and plays critical roles in cancer cell development, proliferation and anti-apoptosis.(16) IGF-1 is a hormone similar in molecular structure to insulin.(17) The IGFs stimulate the proliferation of GI cancer cells(15) and overexpression of IGF-1R has been demonstrated in a variety of gastrointestinal cancers, and its expression is reported as the result of the loss of tumor suppressors, including wild-type p53. The expressions of IGFs and IGF-1R are showed in GI cancer and also in cancer cells, an IGF family mediated GI proliferation by paracrine and autocrine systems.(6,18) The expression of IGF-1R expression in gastric cancer with lymph node metastasis is associated poor prognosis and metastasis.(19)
Chronic inflammation is one of the factors for carcinogenesis. Some studies reported that over 20% of all cancer is associated with chronic inflammation.(20) Infection with H. pylori causes chronic gastric inflammation, and immune response of the host, is a trigger for the development of gastric cancer.(21)
The characteristic long-term chronic inflammatory reaction on gastric mucosa requires evasion of H. pylori from immune system. Epithelial cells respond to H. pylori by undergoing cellular signaling changes and by releasing cytokines into the mucosal lamina propria.(22)
In our study, on chronic gastritis, the expression of IGF-1R was not influenced by H. pylori infection only, but when the mucosa changed into intestinal metaplasia, a premalignant status, the expression of IGF-1R increased than only chronic gastritis. From this expression, infection with H. pylori only is not a necessary and sufficient condition for gastric carcinogenesis. We did not show the trigger for this increased expression of IGF-1R in this study, but the continual secretion of IGF-1R may be a factor for mucosal changing to intestinal metaplasia, and/or long-term infection with H. pylori induces intestinal metaplasia, and this mucosal change may influence the secretion of IGF-1R on gastric mucosa, and the secretion can be a factor for gastric carcinogenesis.
In this study, 105 cfu/ml of H. pylori administration decreased the BrdU uptake of AGS cells. In vivo study, it is reported that infection with H. pylori stimulates the cell proliferation on gastric mucosa.(23) The reasons for the difference between their studies are influenced the difference of gastric cancer cell lines and/or the inocubation time.
In this study, 105 cfu/ml of H. pylori inocubation decreased the BrdU uptake on AGS cells, but when we increased the number of H. pylori to 106 cfu/ml, the BrdU uptake of AGS cells was increased by H. pylori but not significantly (H. pylori 106 cfu/ml: 105.00 ± 2.21% of AGS only). From these data, the balance with the number of infected bacterium and gastric mucosa may be the important factor for gastric carcinogenesis.
H. pylori infected in childhood, especially the patient infected with H. pylori under 5 years old. From childhood, long-term infection with H. pylori causes gastric mucosal changes to chronic gastritis and intestinal metaplasia, and this change is a factor for gastric carcinogenesis. This chronic inflammation is an important factor for gastric carcinogenesis; we noticed that IL-16 is known as a chemoattractant factor of CD4+ T-cell. Recently IL-16 is not only stimulator of inflammation, IL-16 secretion is thought as a factor for carcinogenesis, and the expression of IL-16 was reported in cancer patients of bladder, ovarium and lung cancer.(11) Gao et al.(9) reported that the serum level of IL-16 was significantly higher in gastric cancer than normal mucosa. We did not show the significant difference in the expression of IL-16 on gastric mucosa of chronic gastritis, intestinal metaplasia and gastric cancer (data was not shown), but long-term infection with H. pylori causes infiltration of inflammatory cells, and this continual infiltrated inflammation by H. pylori may cause the expression of IL-16.
Also, Gao et al.(9) reported that IL-16 polymorphisms are major role in patients of gastric cancer. Zhang and Wang(24) reported that IL-16 rs4778889CC and rs11556218GG genotypes are associated with an increased risk of non-cardia gastric cancer. Not only secretion of IL-16, the polymorphisms of IL-16 is also a factor for gastric carcinogenesis. Further study of IL-16 polymorphisms in our patients need to clear the relationship of H. pylori infection and a trigger for gastric cancer.
We conclude that the expression of IGF-1R in long-term H. pylori infected gastric mucosa may indicate an early stage in carcinogenesis because it appears before the histological evident tumor. The expression of IL-16 by H. pylori infection can be a trigger for expression of IGF-1R on gastric mucosa, and the expression of IGF-1R may also a factor for gastric carcinogenesis.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Correa P. Gastric cancer: overview. Gastroenterol Clin North Am. 2013;42:211–217. doi: 10.1016/j.gtc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen TT, Kim SJ, Park JM, Hahm KB, Lee HJ. Repressed TGF-β signaling through CagA-Smad3 interaction as pathogenic mechanisms of Helicobacter pylori-associated gastritis. J Clin Biochem Nutr. 2015;57:113–120. doi: 10.3164/jcbn.15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Adachi Y, Yamamoto H, et al. Insulin-like growth factor-I receptor blockade reduces tumor angiogenesis and enhances the effects of bevacizumab for a human gastric cancer cell line, MKN45. Cancer. 2011;117:3135–3147. doi: 10.1002/cncr.25893. [DOI] [PubMed] [Google Scholar]
- 4.Ennishi D, Shitara K, Ito H, et al. Association between insulin-like growth factor-1 polymorphisms and stomach cancer risk in a Japanese population. Cancer Sci. 2011;102:2231–2235. doi: 10.1111/j.1349-7006.2011.02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ustundag Y, Sahin H, İlikhan S, Dogan BG, Kokturk F, Kar F. Helicobacter pylori eradication does not change circulating insulin-like growth factor 1 and insulin-like growth factor binding protein 3 levels in patients with and without precancerous gastric lesions. Am J Med Sci. 2013;346:381–384. doi: 10.1097/MAJ.0b013e31827beed3. [DOI] [PubMed] [Google Scholar]
- 6.Ii M, Li H, Adachi Y, et al. The efficacy of IGF-I receptor monoclonal antibody against human gastrointestinal carcinomas is independent of k-ras mutation status. Clin Cancer Res. 2011;17:5048–5059. doi: 10.1158/1078-0432.CCR-10-3131. [DOI] [PubMed] [Google Scholar]
- 7.Correa P, Piazuelo MB. Natural history of Helicobacter pylori infection. Dig Liver Dis. 2008;40:490–496. doi: 10.1016/j.dld.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuhisa T, Tsukui T. Relation between reflux of bile acids into the stomach and gastric mucosal atrophy, intestinal metaplasia in biopsy specimens. J Clin Biochem Nutr. 2012;50:217–221. doi: 10.3164/jcbn.11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao LB, Rao L, Wang YY, et al. The association of interleukin-16 polymorphisms with IL-16 serum levels and risk of colorectal and gastric cancer. Carcinogenesis. 2009;30:295–299. doi: 10.1093/carcin/bgn281. [DOI] [PubMed] [Google Scholar]
- 10.Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology. 2012;143:550–563. doi: 10.1053/j.gastro.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Richmond J, Tuzova M, Cruikshank W, Center D. Regulation of cellular processes by interleukin-16 in homeostasis and cancer. J Cell Physiol. 2014;229:139–147. doi: 10.1002/jcp.24441. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima N, Ito Y, Yokoyama K, et al. The expression of murine double minute 2 (MDM2) on Helicobacter pylori-infected intestinal metaplasia and gastric cancer. J Clin Biochem Nutr. 2009;44:196–202. doi: 10.3164/jcbn.08-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furukawa M, Raffeld M, Mateo C, et al. Increased expression of insulin-like growth factor I and/or its receptor in gastrinomas is associated with low curability, increased growth, and development of metastases. Clin Cancer Res. 2005;11:3233–3242. doi: 10.1158/1078-0432.CCR-04-1915. [DOI] [PubMed] [Google Scholar]
- 14.Adachi Y, Li R, Yamamoto H, et al. Insulin-like growth factor-I receptor blockade reduces the invasiveness of gastrointestinal cancers via blocking production of matrilysin. Carcinogenesis. 2009;30:1305–1313. doi: 10.1093/carcin/bgp134. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Adachi Y, Imsumran A, et al. Targeting for insulin-like growth factor-I receptor with short hairpin RNA for human digestive/gastrointestinal cancers. J Gastroenterol. 2010;45:159–170. doi: 10.1007/s00535-009-0151-6. [DOI] [PubMed] [Google Scholar]
- 16.Matsubara J, Yamada Y, Hirashima Y, et al. Impact of insulin-like growth factor type 1 receptor, epidermal growth factor receptor, and HER2 expressions on outcomes of patients with gastric cancer. Clin Cancer Res. 2008;14:3022–3029. doi: 10.1158/1078-0432.CCR-07-1898. [DOI] [PubMed] [Google Scholar]
- 17.Vrakas G, O'Connor M, Matsou A, et al. Markers of malnutrition after intestinal transplantation: the role of IGF-1 and calprotectin. J Clin Biochem Nutr. 2015;56:64–65. doi: 10.3164/jcbn.14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res. 1995;55:2007–2011. [PubMed] [Google Scholar]
- 19.Gryko M, Kiśluk J, Cepowicz D, et al. Expression of insulin-like growth factor receptor type 1 correlate with lymphatic metastases in human gastric cancer. Pol J Pathol. 2014;65:135–140. doi: 10.5114/pjp.2014.42678. [DOI] [PubMed] [Google Scholar]
- 20.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 21.Wroblewski LE, Peek RM, Jr. Helicobacter pylori in gastric carcinogenesis: mechanisms. Gastroenterol Clin North Am. 2013;42:285–298. doi: 10.1016/j.gtc.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sepulveda AR. Helicobacter, inflammation, and gastric cancer. Curr Pathobiol Rep. 2013;1:9–18. doi: 10.1007/s40139-013-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wroblewski LE, Piazuelo MB, Chaturvedi R, et al. Helicobacter pylori targets cancer-associated apical-junctional constituents in gastroids and gastric epithelial cells. Gut. 2015;64:720–730. doi: 10.1136/gutjnl-2014-307650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang T, Wang H. Variants of interleukin-16 associated with gastric cancer risk. Asian Pac J Cancer Prev. 2013;14:5269–5273. doi: 10.7314/apjcp.2013.14.9.5269. [DOI] [PubMed] [Google Scholar]