Abstract
Decreases in saliva secretion compromise food mastication and swallowing, reduce mucosal immune function, and increase the risk for oral diseases like dental caries. Chlorella is a green alga that contains a variety of nutrients including amino acids, vitamins, and minerals. In our previous study, Chlorella-derived multicomponent supplementation did not affect salivary flow rates in healthy young individuals, but Chlorella-derived supplementation attenuated a decrease in saliva secretion that was observed during a kendo training camp. Hence, we hypothesized that Chlorella-derived supplementation increases saliva secretion in individuals with lower rates of saliva flow. Sixty-four subjects took Chlorella-derived tablets for four weeks. Before and after supplementation, saliva samples were collected by chewing cotton. In the complete study group, there was no difference in saliva production before and after supplementation (1.91 ± 0.11 ml/min before vs 2.01 ± 0.12 ml/min after). Analysis of subgroups based on saliva production before supplementation found an increase in saliva secretion in the lower saliva flow group (1.18 ± 0.06 vs 1.38 ± 0.08 ml/min), but no change in the higher saliva flow group (2.63 ± 0.11 vs 2.64 ± 0.15 ml/min). These results suggest that Chlorella-derived multicomponent supplementation increases saliva production in individuals with lower levels of saliva secretion.
Keywords: Chlorella, immunoglobulin A, multicomponent supplementation, saliva secretion
Introduction
Saliva protects the oral mucosa and the teeth. Decreases in saliva secretion can compromise the mastication and swallowing of food, reduce mucosal immune function, and increase the risk for oral diseases such as dental caries. Lifestyle modifications that prevent reductions in saliva secretion would therefore be of clinical significance. A number of factors can influence salivation, including dehydration,(1) age,(2) the number of remaining teeth,(3) and bite force.(4) In addition, nutritional status is associated with saliva secretion. Dormenval et al.(5) reported that salivary flow rate was correlated with serum albumin concentrations and that the relative frequency of malnutrition was greater in subjects with lower salivary flow rates, as compared to those with higher levels of saliva secretion. Saito and coworkers reported that ingestion of astaxanthin,(6) coenzyme Q10,(7) isoflavones,(8) and quercetin(9) corrected impaired salivary secretion. Therefore, dietary modification or the use of a dietary supplement may prevent hyposalivation.
Chlorella is a unicellular green alga that grows in fresh water and contains high levels of proteins, chlorophylls, vitamins, minerals, and dietary fibers. In our previous study, Chlorella-derived multicomponent supplementation did not affect salivary flow rates in healthy young individuals, although it increased the salivary secretory immunoglobulin A (SIgA) secretion rate.(10) However, Chlorella-derived supplementation attenuated the decrease in salivary flow rate observed in university kendo athletes during a training camp.(11) Hence, we hypothesized that Chlorella-derived multicomponent supplementation may increase saliva production in individuals with lower salivary flow rates. To test this hypothesis, we compared the effects of a Chlorella-derived supplement on saliva production in subjects with lower and higher baseline salivary flow rates. In addition, we re-examined the effects of Chlorella-derived supplementation on the salivary SIgA secretion rate, because the sample size of the previous study (n = 15)(10) was small.
Materials and Methods
Subjects and experimental design
First, pre-supplementation saliva samples were obtained from 43 male and 21 female non-smokers. The mean ± SE values for the age, height, weight, and body mass index (BMI) of all subjects were 32.2 ± 2.5 years, 1.66 ± 0.01 m, 64.0 ± 1.3 kg, and 23.3 ± 0.4 kg/m2, respectively. Subjects refrained from alcohol consumption from the day before testing, and from caffeine consumption and intense physical activity on the day of testing. They then took 30 Chlorella-derived tablets per day (15 tablets twice daily, after breakfast and dinner) for four weeks, as in the previous studies.(10–14) This was consistent with the recommended dosage for Japanese consumers. The participants were asked not to modify their lifestyle during the trial period. A post-supplementation saliva sample was obtained one day after the final tablet intake.
This study was approved by the Ethics Committee of Ryutsu Keizai University and the Ethical Committee of the Institute of Health and Sport Sciences of the University of Tsukuba. The protocol of this study conformed to the principles of the Helsinki Declaration. All participants gave their written informed consent prior to study initiation.
Chlorella tablets
The Chlorella tablet used in this study (SunChlorella A; Sun Chlorella Corp., Kyoto, Japan) was the same as that used in our previous studies.(10–14) The mass of each tablet was 200 mg and dried Chlorella pyrenoidosa powder was the main ingredient. The nutritional values of these tablets (per 100 g) were: energy, 399 kcal; moisture, 5.3 g; protein, 60.8 g; lipid, 9.2 g; saccharide, 6.3 g; dietary fiber, 11.9 g; and ash, 6.5 g.
Saliva samples
Saliva samples were obtained as described previously.(15–17) The subjects rinsed their mouths with distilled water (three times for 30 s) and then rested for 5 min. Saliva production was stimulated by chewing sterilized cotton (Salivette; Sarstedt, Nümbrecht, Germany) at a frequency of 1 chew/s for 60 s. The amount of saliva (g) was converted to milliliters, assuming a saliva density of 1 g/ml. The subjects were divided into lower and higher saliva secretion groups based on the median value before supplementation (1.72 ml/min). After the saliva sample was separated from the cotton by centrifugation at 1,460 g for 15 min, the samples were frozen at –60°C. We measured salivary SIgA concentrations in duplicate using an enzyme-linked immunosorbent assay, as described previously.(15–17) The inter-assay coefficient of variation for this method was reported to be 6.2%.(16) The SIgA secretion rate was obtained from the product of the absolute SIgA concentration and the saliva flow rate.
Statistical analysis
The results are presented as means ± SEs. The paired t test was used to assess the effects of supplementation. Inter-group comparisons of patient compliance and variables prior to the supplementation period were made by unpaired t tests. The group male:female ratios were compared by chi-squared test. The analysis of covariance was used to detect group differences in the change in saliva flow rate. If a significant F value was found, a post-hoc Bonferroni/Dunn test was performed. P values <0.05 were considered statistically significant.
Results
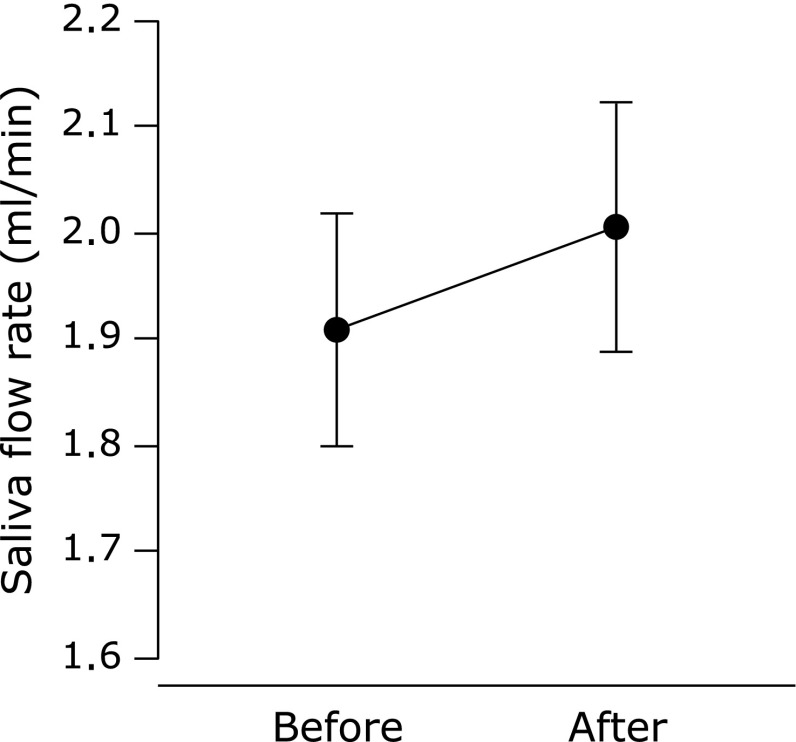
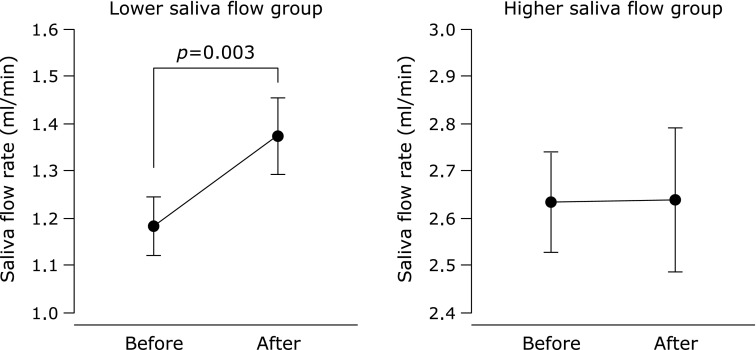
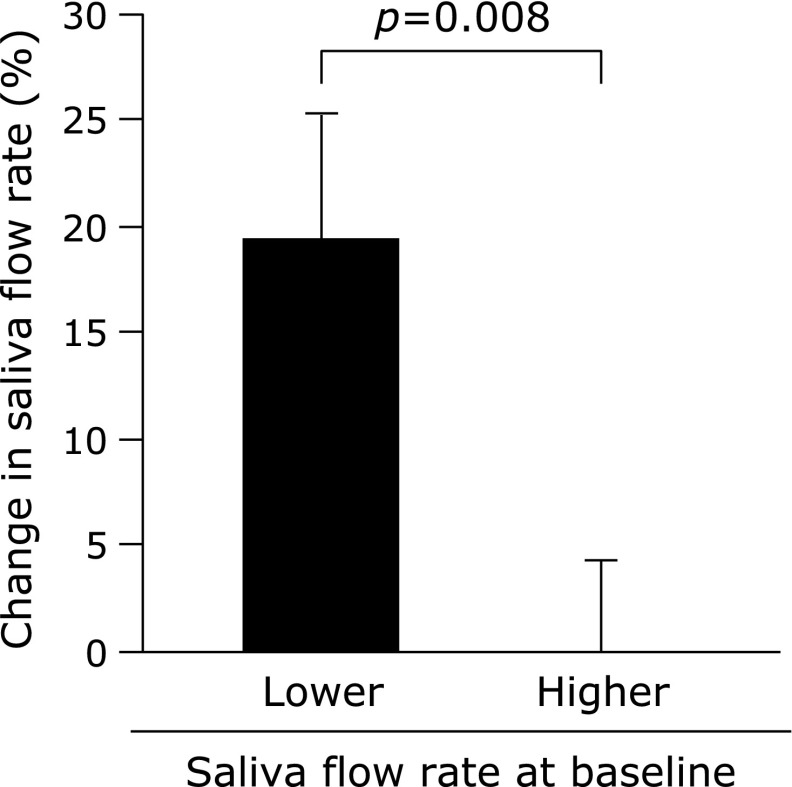
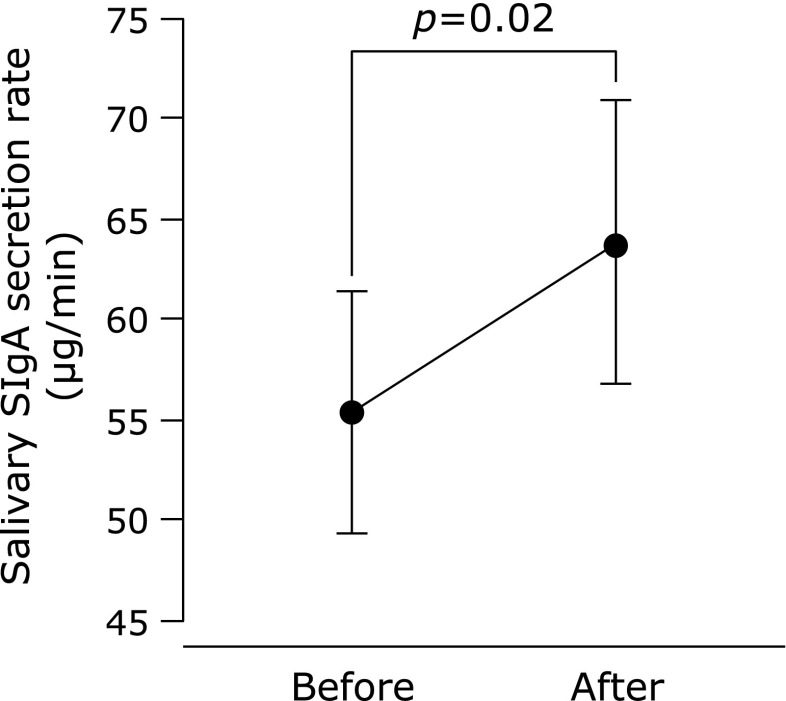
In all subjects, compliance with respect to regular tablet intake was 94.4 ± 1.1%. There was no difference between the saliva flow rate of all subjects before and after the supplementation period (p = 0.11, Fig. 1). Subjects were assigned to subgroups, depending on their salivary flow rates before supplementation. There were no significant differences in the physical characteristics of these subgroups (Table 1) or their compliance with tablet intake (lower vs higher saliva flow subgroups; 95.1 ± 1.3 vs 93.6 ± 1.8%, p = 0.50), although the differences in male:female ratio, age, and height were close to statistical significance. Saliva flow rate increased after supplementation in the lower saliva flow group, whereas there was no change in the higher group (Fig. 2). Changes in saliva flow rate before and after supplementation were greater in the lower saliva flow group than in the higher group, independent of sex (male = 0, female = 1), age, or height (Fig. 3). The salivary SIgA secretion rate increased in all subjects after supplementation, relative to the baseline (Fig. 4).
Fig. 1.
Saliva flow rate before and after Chlorella-derived multicomponent supplementation in all subjects. Values are means ± SE.
Table 1.
Characteristics of subjects
| Saliva flow | Before | After | Before vs After | |
|---|---|---|---|---|
| Sex (male/female) | Lower | 18/14 | — | — |
| Higher | 25/7 | — | — | |
| Lower vs Higher | p = 0.06 | — | — | |
| Age (years) | Lower | 36.7 ± 3.7 | — | — |
| Higher | 27.3 ± 3.1 | — | — | |
| Lower vs Higher | p = 0.06 | — | — | |
| Height (m) | Lower | 1.64 ± 0.02 | — | — |
| Higher | 1.68 ± 0.01 | — | — | |
| Lower vs Higher | p = 0.06 | — | — | |
| Body weight (kg) | Lower | 62.3 ± 1.4 | 62.4 ± 1.4 | p = 0.62 |
| Higher | 65.7 ± 2.2 | 65.8 ± 2.1 | p = 0.69 | |
| Lower vs Higher | p = 0.18 | p = 0.18 | — | |
| Body mass index (kg/m2) | Lower | 23.3 ± 0.6 | 23.4 ± 0.5 | p = 0.52 |
| Higher | 23.2 ± 0.4 | 23.3 ± 0.6 | p = 0.67 | |
| Lower vs Higher | p = 0.94 | p = 0.91 | — |
Values are means ± SE.
Fig. 2.
Saliva flow rate before and after Chlorella-derived multicomponent supplementation in the lower and higher saliva flow groups. Values are means ± SE.
Fig. 3.
Change in saliva flow rate before and after Chlorella-derived multicomponent supplementation in the lower and higher saliva flow groups. Values are means ± SE.
Fig. 4.
Salivary secretory immunoglobulin A (SIgA) secretion rate before and after Chlorella-derived multicomponent supplementation in all subjects. Values are means ± SE.
Discussion
We investigated the effects of a 4-week Chlorella-derived multicomponent supplementation on saliva flow rate. The supplement increased the saliva flow rate in the lower saliva flow group, as compared to their baseline, but not in the higher saliva flow group. These results suggest that this Chlorella-derived multicomponent supplement selectively increased saliva production in individuals with lower saliva flow rates.
There are two components of saliva secretion: the stimulated and unstimulated saliva flow rates. The stimulated flow rate reflects the ability to produce saliva during eating and speaking, whereas the unstimulated flow rate reflects the normal level of saliva in the mouth. The stimulated saliva flow rate is correlated with the unstimulated saliva flow rate.(18,19) However, it is possible that Chlorella-derived multicomponent supplementation has different effects on the stimulated and unstimulated saliva levels. For example, age is related to the unstimulated saliva flow rate,(3) but there are conflicting reports regarding the relationship between age and the stimulated saliva flow rate.(2,3,20) This study could not investigate unstimulated saliva secretion and the present findings therefore suggest that Chlorella-derived multicomponent supplementation may provide effective improvement of stimulated saliva secretion during chewing.
Chlorella-derived multicomponent supplementation increased the saliva flow rate of the lower saliva flow group. Unfortunately, this study could not identify the individual nutrients respsonsible for this increase, or the mechanisms underlying this effect. The effect of tablet caloric intake can be excluded, because subject BMI was within the normal range and did not change during the supplementation period. The National Health and Nutrition Examination Survey by the Ministry of Health, Labour, and Welfare of Japan has pointed out that the intake of vegetables by Japanese people is below the target level. An epidemiological study by Iwasaki et al.(21) reported that consumption of vegetables, fish, and shellfish, and intake of n-3 polyunsaturated fatty acid, potassium, vitamin B6, vitamin D, vitamin E, and folate were lower in individuals with hyposalivation, relative to those without hyposalivation. A previous multicomponent analysis demonstrated that the tablet used in the present study contained n-3 polyunsaturated fatty acid and many vitamins and minerals, including those mentioned above, although no vitamin D data was available.(11,12) These nutrients may be implicated in the observed increase in the saliva flow rate of individuals with lower baseline saliva production. On the other hand, the saliva flow rate in the higher saliva group did not change during the supplementation period. In this group, the baseline saliva secretion might have been close to the individual’s upper production limit. Another possibility is that sufficient nutrients had already been ingested prior to supplementation in this group.
Salivary SIgA secretion rate was previously shown to increase after Chlorella-derived multicomponent supplementation.(10) However, the sample size of this previous study was only 15. The present study investigated 64 participants and verified these previous results. In addition, this study demonstrated that saliva production increased after supplementation in the lower saliva flow group. Saliva provides a physical barrier for the mucosa and washes away viruses and bacteria. These findings indicated that Chlorella-derived supplementation may have dual benefits for mucosal immune function in individuals with low saliva secretion.
This study has several limitations. First, no placebo group was included and the results might therefore include a placebo effect. Second, we did not investigate dietary habits or collect blood samples. Further studies are needed to elucidate the mechanisms underlying the observed effect of this Chlorella-derived multicomponent supplement on saliva production. Additionally, a combination of dietary and exercise habits can affect SIgA secretion.(22) This study could not adjust for not only dietary but also exercise habits of subjects, even though the participants were asked not to modify their lifestyle during the trial period.
In conclusion, Chlorella-derived multicomponent supplementation increased the saliva flow rate of individuals with lower baseline saliva secretion.
Abbreviations
- BMI
body mass index
- SIgA
secretory immunoglobulin A
Conflict of Interest
Sun Chlorella Corp. provided funding for the study and supplied the test supplements used in this study. TO has received speaker’s honorarium from Sun Chlorella Corp. KS, AZ, and SM have no competing interests.
References
- 1.Yoshihara A, Hirotomi T, Takano N, Kondo T, Hanada N, Miyazaki H. Serum markers of chronic dehydration are associated with saliva spinability. J Oral Rehabil. 2007;34:733–738. doi: 10.1111/j.1365-2842.2007.01732.x. [DOI] [PubMed] [Google Scholar]
- 2.Toida M, Nanya Y, Takeda-Kawaguchi T, et al. Oral complaints and stimulated salivary flow rate in 1188 adults. J Oral Pathol Med. 2010;39:407–419. doi: 10.1111/j.1600-0714.2009.00852.x. [DOI] [PubMed] [Google Scholar]
- 3.Flink H, Bergdahl M, Tegelberg A, Rosenblad A, Lagerlöf F. Prevalence of hyposalivation in relation to general health, body mass index and remaining teeth in different age groups of adults. Community Dent Oral Epidemiol. 2008;36:523–531. doi: 10.1111/j.1600-0528.2008.00432.x. [DOI] [PubMed] [Google Scholar]
- 4.Yeh CK, Johnson DA, Dodds MW, Sakai S, Rugh JD, Hatch JP. Association of salivary flow rates with maximal bite force. J Dent Res. 2000;79:1560–1565. doi: 10.1177/00220345000790080601. [DOI] [PubMed] [Google Scholar]
- 5.Dormenval V, Budtz-Jørgensen E, Mojon P, Bruyère A, Rapin CH. Associations between malnutrition, poor general health and oral dryness in hospitalized elderly patients. Age Ageing. 1998;27:123–128. doi: 10.1093/ageing/27.2.123. [DOI] [PubMed] [Google Scholar]
- 6.Yamada T, Ryo K, Tai Y, et al. Evaluation of therapeutic effects of astaxanthin on impairments in salivary secretion. J Clin Biochem Nutr. 2010;47:130–137. doi: 10.3164/jcbn.10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryo K, Ito A, Takatori R, et al. Effects of coenzyme Q10 on salivary secretion. Clin Biochem. 2011;44:669–674. doi: 10.1016/j.clinbiochem.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Ryo K, Takahashi A, Tamaki Y, Ohnishi-Kameyama M, Inoue H, Saito I. Therapeutic effects of isoflavones on impaired salivary secretion. J Clin Biochem Nutr. 2014;55:168–173. doi: 10.3164/jcbn.14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi A, Inoue H, Mishima K, et al. Evaluation of the effects of quercetin on damaged salivary secretion. PLoS One. 2015;10:e0116008. doi: 10.1371/journal.pone.0116008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otsuki T, Shimizu K, Iemitsu M, Kono I. Salivary secretory immunoglobulin A secretion increases after 4-weeks ingestion of Chlorella-derived multicomponent supplement in humans: a randomized cross over study. Nutr J. 2011;10:91. doi: 10.1186/1475-2891-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otsuki T, Shimizu K, Iemitsu M, Kono I. Chlorella intake attenuates reduced salivary SIgA secretion in kendo training camp participants. Nutr J. 2012;11:103. doi: 10.1186/1475-2891-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otsuki T, Shimizu K, Iemitsu M, Kono I. Multicomponent supplement containing Chlorella decreases arterial stiffness in healthy young men. J Clin Biochem Nutr. 2013;53:166–169. doi: 10.3164/jcbn.13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otsuki T, Shimizu K, Maeda S. Changes in arterial stiffness and nitric oxide production with Chlorella-derived multicomponent supplementation in middle-aged and older individuals. J Clin Biochem Nutr. 2015;57:228–232. doi: 10.3164/jcbn.15-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umemoto S, Otsuki T. Chlorella-derived multicomponent supplementation increases aerobic endurance capacity in young individuals. J Clin Biochem Nutr. 2014;55:143–146. doi: 10.3164/jcbn.14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akimoto T, Nakahori C, Aizawa K, Kimura F, Fukubayashi T, Kono I. Acupuncture and responses of immunologic and endocrine markers during competition. Med Sci Sports Exerc. 2003;35:1296–1302. doi: 10.1249/01.MSS.0000078934.07213.25. [DOI] [PubMed] [Google Scholar]
- 16.Akimoto T, Kumai Y, Akama T, et al. Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br J Sports Med. 2003;37:76–79. doi: 10.1136/bjsm.37.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu K, Kimura F, Akimoto T, Akama T, Kuno S, Kono I. Effect of free-living daily physical activity on salivary secretory IgA in elderly. Med Sci Sports Exerc. 2007;39:593–598. doi: 10.1249/mss.0b013e318031306d. [DOI] [PubMed] [Google Scholar]
- 18.Heintze U, Birkhed D, Björn H. Secretion rate and buffer effect of resting and stimulated whole saliva as a function of age and sex. Swed Dent J. 1983;7:227–238. [PubMed] [Google Scholar]
- 19.Sreebny LM, Valdini A. Xerostomia. Part I: Relationship to other oral symptoms and salivary gland hypofunction. Oral Surg Oral Med Oral Pathol. 1988;66:451–458. doi: 10.1016/0030-4220(88)90268-x. [DOI] [PubMed] [Google Scholar]
- 20.Smith CH, Boland B, Daureeawoo Y, Donaldson E, Small K, Tuomainen J. Effect of aging on stimulated salivary flow in adults. J Am Geriatr Soc. 2013;61:805–808. doi: 10.1111/jgs.12219. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki M, Yoshihara A, Ito K, et al. Hyposalivation and dietary nutrient intake among community-based older Japanese Geriatr Gerontol Int 2015. DOI: 10.1111/ggi.12500 [DOI] [PubMed] [Google Scholar]
- 22.Shibuya T, Kaburagi T, Nagai R, Oshiro S. The effects of moderate exercise on secretory IgA production in mice depends on dietary carbohydrate intake. J Clin Biochem Nutr. 2015;57:44–49. doi: 10.3164/jcbn.15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]