Abstract
Shikonin, an anti-inflammatory compound of “Shikon”, inhibits the neutrophil superoxide (O2•−) generation by NADPH oxidase 2 (Nox2); however, the mechanisms of how shikonin affects Nox2 activity remained unclear. We aimed to elucidate the relationship between the inhibition of Nox2 activity and influences on intracellular Ca2+ concentration ([Ca2+]i) by shikonin. For this purpose, we used a simultaneous monitoring system for detecting changes in [Ca2+]i (by fluorescence) and O2•− generation (by chemiluminescence) and evaluated the effects of shikonin on neutrophil-like HL-60 cells stimulated with N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP). Since fMLP activates Nox2 by elevation in [Ca2+]i via fluxes such as inositol 1,4,5-trisphosphate-induced Ca2+ release (IICR) and store-operated Ca2+ entry (SOCE), we also evaluated the effects of shikonin on IICR and SOCE. Shikonin dose-dependently inhibited the fMLP-induced elevation in [Ca2+]i and O2•− generation (IC50 values of 1.45 and 1.12 µM, respectively) in a synchronized manner. Analyses of specific Ca2+ fluxes showed that shikonin inhibits IICR and IICR-linked O2•− generation (IC50 values: 0.28 and 0.31 µM for [Ca2+]i and O2•−, respectively), as well as SOCE and SOCE-linked O2•− generation (IC50 values: 0.39 and 0.25 µM for [Ca2+]i and O2•−, respectively). These results suggested that shikonin inhibits the O2•− generation by Nox2 in fMLP-stimulated neutrophils by targeting Ca2+ fluxes such as IICR and SOCE.
Keywords: simultaneous detection, intracellular calcium, superoxide, NADPH oxidase, shikonin
Introduction
Anti-inflammatory compounds can act by suppressing cellular pro-inflammatory responses and oxidative stress. In the latter case, the anti-inflammatory compound can be an antioxidant by itself or an inhibitor of reactive oxygen species (ROS)-producing enzymes by inhibiting enzymatic activity directly or upstream events needed for enzymatic activation such as Ca2+ fluxes. We have previously developed a simultaneous monitoring system for intracellular Ca2+ concentration ([Ca2+]i) and superoxide anion (O2•−) generation by measuring changes in fluorescence and chemiluminescence, respectively,(1–3) using N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP)-stimulated neutrophils as a tool to evaluate anti-inflammatory properties of test compounds. The fMLP-stimulated O2•− generation by NADPH oxidase 2 (Nox2) in cells depends on Ca2+-regulated pathways;(4–10) therefore, impairment of O2•− generation in parallel with altered [Ca2+]i profiles will indicate that Ca2+ fluxes are likely target candidates. We have previously found that ibuprofen is an anti-inflammatory compound showing impaired O2•− generation due to altered Ca2+ fluxes in neutrophil-like cells.(3) In contrast, if O2•− scavenging occurs without alterations in [Ca2+]i profiles as in the case of ascorbic acid,(3) one can recognize it as an antioxidant (scavenger of O2•−) not affecting upstream pathways for Nox2 activation, including Ca2+ fluxes. Therefore, the simultaneous monitoring system is especially useful in elucidating such different anti-inflammatory mechanisms of test compounds.
We have also been investigating the anti-inflammatory effects of shikonin, the major active substance of the herbal medicine “Shikon” (Lithospermum erythrorhizon),(11–13) for its therapeutic potential in inflammatory diseases. Shikonin scavenges ROS such as O2•−,(14–16) hydroxyl radical,(15,17) singlet oxygen,(15) and alkyl-oxy radical(16). Moreover, shikonin also inhibits the activities of enzymes that produce ROS such as Nox2(16,18,19) and nitric oxide synthase(20) by mechanisms likely independent of ROS-scavenging effects. The inhibition of Nox2 by shikonin (IC50 range, 0.4~2 µM) was reported to occur before activation of the enzyme,(16,18,19) a process that requires assembly of cytosolic and membrane components to form a complex with O2•−-generating activity.(21) Although the inhibition targets are believed to be processes and/or molecules relevant to enzyme activation, such targets remain unknown and might differ depending on the stimulant used for activation of Nox2.
Here, we aimed to elucidate the effects of shikonin on the fMLP-stimulated O2•− generation by Nox2 linked with Ca2+ fluxes in neutrophils. Ca2+ entry into neutrophils results from the stimulation of cells by agonists such as fMLP.(4,22) Agonist interaction with G-protein linked receptors on the cell membrane causes the formation of phospholipase C (PLC)-mediated inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG).(4,22) IP3 activates its receptor on the endoplasmic reticulum (ER), causing the release of stored Ca2+ from the ER to the cytosol, which is termed IP3-induced calcium release (IICR). The emptying of the ER via IICR is then followed by an influx of external Ca2+ across the plasma membrane, through capacitative or store-operated calcium entry (SOCE).(4,23–25) Another route for Ca2+ entry through plasma membranes is via PLC-linked receptor occupation by agonists, but is store-independent (i.e., non-SOCE); it is called receptor-operated calcium entry (ROCE).(4,24,26)
Presently, the effects of shikonin on the fMLP-induced O2•− generation and Ca2+ fluxes in neutrophil-like HL-60 cells were investigated. We first verified that the direct scavenging of O2•− by shikonin can be considered negligible under the assay conditions herein. Cells pretreated with shikonin had synchronized changes in [Ca2+]i levels and O2•− generation in response to fMLP. The inhibition of O2•− generation by shikonin was linked to the inhibition of the cellular Ca2+ fluxes, IICR and SOCE.
Material and Methods
Reagents
Shikonin and sterile-filtered, cell-culture grade DMSO were purchased from Wako Pure Chem. Ind., Ltd. (Osaka, Japan). fMLP, ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA: a chelating agent with high specificity for Ca2+), hypoxanthine, xanthine oxidase from bovine milk, N-{4-[3,5-bis (trifluoromethyl)-1H-pyrazol-1-yl]phenyl}-4-methyl-1,2,3-thiazole-5-carboxamide (BTP2: an inhibitor of SOCE)(27,28) and thapsigargin (TG: an irreversible inhibitor of sarco/endoplasmic reticulum membrane Ca2+-ATPase (SERCA) that pumps Ca2+ into the stores; besides inhibiting SERCA, TG passively depletes Ca2+ stores thus allowing SOCE upon Ca2+ addition)(29–31) were purchased from Sigma-Aldrich (St. Louis, MO). The following reagents were from the sources in parentheses: 1-[2-amino-5-(2,7-dichloro-6-acetoxymethoxy-3-oxo-9-xanthenyl)phenoxy]-2-(2-amino-5-methylphenoxy)ethane-N,N,N',N'-tetraacetic acid, tetra (acetoxymethyl) ester (fluo-3 AM; Dojindo Laboratories Kumamoto, Japan) and 2-methyl-6-phenyl-3,7-dihydroimidazo [1, 2-a] pyrazin-3-one (CLA; Tokyo Kasei, Tokyo, Japan).
Stock solutions were prepared in the solvents indicated and stored at −20°C: BTP2 (10 mM, DMSO); CLA (50 µM, MilliQ water); EGTA (100 mM; MilliQ water, pH adjusted to 8.1); fMLP (1 mM, DMSO); TG (1 mM, DMSO); and shikonin (30 mM, DMSO). The stock solutions of fMLP and TG were diluted 10 times in 1:3 (v/v) DMSO and Ringer-Hepes buffer (RH buffer: 154 mM NaCl, 5.6 mM KCl, and 10 mM Hepes, pH 7.4) before use. The stock solution of shikonin was diluted with DMSO to 3.3, 1.0, 0.33, 0.1, and 0.033 mM solutions, of which aliquots of 1/1,000 of the assay volume were used. In assays with BTP2, a 1/1,000 assay volume of the stock solution was used. The final concentration of DMSO in the assays depended on the number of lipophilic reagents added, but did not exceed 0.3% (v/v), a level that was verified to have no influence on cellular fluorescence and chemiluminescence responses.
Analytical apparatus
The analyses were carried out using a simultaneous monitoring system for fluorescence and chemiluminescence, the CFL-C2000 (Hamamatsu Photonics K.K., Hamamatsu, Japan). The system used is the same as previously described(3) with the exception of a more sensitive photomultiplier tube (H10682-210, Hamamatsu Photonics K.K.) as a detector. This detector enables measurements with higher sensitivity than the previous model. In brief, by repeatedly turning on and off the LED excitation light at high speed, two kinds of light signals, fluorescence (from fluo-3 AM preloaded within cells; excitation at 500 nm and emission at 523 nm) and chemiluminescence (from the reaction of CLA with O2•− generated upon fMLP-stimulation of cells) can be detected by a single detector.
Chemical scavenging of O2•− by shikonin
The O2•−-scavenging ability of shikonin was tested in the previously described hypoxanthine-xanthine oxidase assay,(3) using the CFL-C2000 system. The difference was that CLA was used as the O2•−-sensitive reagent because its chemiluminescence is detected at 380 nm, which corresponds to a region of low absorption in the visible region of the shikonin spectrum (the absorbance increases above 450 nm and peaks at 520 nm). Briefly, the reaction mixture (0.1 µM hypoxanthine, 1 µM CLA, shikonin or DMSO alone in RH buffer; assay volume: 1.5 ml) was placed in a disposable polymethylmethacrylate cuvette (1-cm light path) and pre-incubated for 5 min at 37°C in a dedicated incubator (PI6100-prototype, Hamamatsu Photonics K.K.) with mild stirring at approximately 150 rotations/min, with a cross-head magnetic stirrer (9-mm diameter, 6-mm height) placed in the bottom of the cuvette. Then, the cuvette containing the mixture was transferred to the sample holder of the CFL-C2000 under the same temperature and stirring conditions as in the pre-incubation step. After baseline acquisition for 50 s, xanthine oxidase (final, 2.4 × 10−3 U/ml) was added and chemiluminescence monitoring continued. The O2•−-scavenging ability was determined by decreases in the peak area under the curve (AUC) of the responses as previously described,(3,32) and expressed as ratios relative to control without shikonin.
Preparation of neutrophil-like cells from HL-60 cells
The human HL-60 acute promyelocytic leukemia cell line was obtained from the American Type Culture Collection (Manassas, VA), and cultured in GIT® medium (Wako Pure Chem. Ind., Ltd.) at 37°C in a humidified atmosphere containing 5% CO2. The medium was replaced with fresh GIT medium containing 1.3% (v/v) DMSO to induce differentiation of the HL-60 cells into neutrophil-like cells, as previously described.(3) After culture for 96 h, the resulting neutrophil-like cells were used in all cell assays. The neutrophil-like cells were washed with RH buffer and loaded with fresh GIT medium containing 3 µM fluo-3 AM for 45 min at 37°C and 5% CO2-atmosphere. The cells were then washed twice with RH buffer, suspended, and maintained in RH buffer on ice. Cell count was performed in Trypan Blue.
Assessment of O2•− generation and [Ca2+]i levels associated with full cellular responses to fMLP
The effects of shikonin on O2•− generation and [Ca2+]i levels of fMLP-stimulated cells were verified using the CFL-C2000 (Analytical apparatus), as follows. Assays contained 1 × 105 fluo-3 AM loaded cells/ml (Preparation of neutrophil-like cells from HL-60 cells; total volume: 1.5 ml throughout) in RH buffer with 1 µM CLA supplemented with 1 mM CaCl2, unless otherwise stated. Cuvettes and apparatus for pre-incubation were the same as described in Chemical scavenging of O2•− by shikonin. Briefly, shikonin or DMSO as a vehicle was added to the cells suspended in RH buffer with CLA and Ca2+, and incubated for 7 min at 37°C with stirring before transferring the cuvette containing the mixture to the CFL-C2000. After acquisition of chemiluminescence (O2•−) and fluorescence ([Ca2+]i) baselines for 50-s, the cells were stimulated by injection of fMLP (final, 1 µM), and data were acquired under the same temperature and stirring conditions as in the pre-incubation step. The order and timing for when the test compounds were added are detailed in the legend of Fig. 2.
The chemiluminescence and fluorescence intensities were monitored and recorded as time courses of the cellular responses. These responses were quantified by AUCs with appropriate settings made in the software of the detection system. Data were expressed as a function of shikonin concentration after calculation of ratios relative to control assays without shikonin.
Assessment of Ca2+ fluxes: separate assays for IICR and SOCE
For verification of the effects of shikonin on IICR, fluo-3 AM loaded cells (1 × 105 cells/ml; described in Preparation of neutrophil-like cells from HL-60 cells) were maintained for 1 min in 1 mM CaCl2-containing RH buffer to replenish Ca2+ stores depleted during cell isolation and washings, and then assayed in the presence of 5 mM EGTA. EGTA chelates extracellular Ca2+, preventing both SOCE and ROCE; therefore, stimulation of cells with fMLP in the presence of EGTA allows only IICR-mediated elevation of [Ca2+]i (i.e., due to release of Ca2+ from intracellular stores). Both O2•− generation and changes in [Ca2+]i due to IICR were monitored using the CFL-C2000 as described in the section before this one. The incubation periods, additions and periods of data acquisition are detailed in the legend of Fig. 3. Addition of EGTA did not change the pH of the reaction, which remained constant at pH 7.4.
To evaluate the effect of shikonin on Ca2+ flux from the extracellular environment via SOCE, Ca2+ entry after the addition of 1 mM Ca2+ was verified in cells pretreated with TG under Ca2+-free conditions (RH buffer with 0.1 mM EGTA and 1 µM CLA). TG has been used to investigate SOCE because it selectively interferes with intracellular Ca2+ pools by depleting IP3-sensitive Ca2+ stores without generation of IP3.(29,30) The depletion of Ca2+ stores then provokes the entry of Ca2+ via SOCE if Ca2+ is added to the extracellular buffer. The effects of shikonin on SOCE were verified by monitoring O2•− generation and [Ca2+]i levels as described in the legend of Fig. 4. BTP2 was used as a standard inhibitor of SOCE.
Statistical analysis
Experiments were repeated at least 3 times and measurements for each concentration of test compound were done in triplicate or more. Data are expressed as means ± SD, as indicated in the legends. Effects of different shikonin concentrations were analyzed using one-way analysis of variance (ANOVA). When ANOVA showed significant differences, post hoc analysis was performed with the Dunnett’s test. Statistical significance was set at a confidence level of 1%, and refers to a two-sided probability.
Results
Shikonin is a chemical scavenger of O2•−
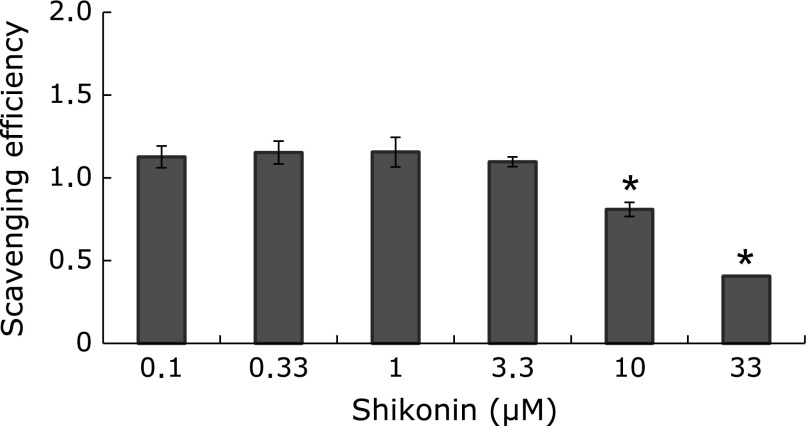
As we(16) and others(14,15) have previously reported, shikonin scavenges O2•−, with IC50 values of 2.9~17 µM depending on the source, assay conditions and O2•− detection system used. To know the contribution of the chemical scavenging of O2•− by shikonin in RH buffer with 1 µM CLA used in assays with cells, we first investigated the quenching of O2•−-produced chemiluminescence of the hypoxanthine-xanthine oxidase reaction(3) in the CFL-C2000 (Fig. 1). The scavenging activity was not noted until a concentration of 3.3 µM shikonin, but it significantly increased at higher concentrations: the IC50 was 24.6 ± 3.7 µM (Fig. 1: mean ± SD, n = 3). These results show that the chemical scavenging of O2•− can be considered negligible below 3.3 µM in the buffer conditions of the following assays with cells, where the effects of shikonin appear at concentrations lower than 1.0 µM (Fig. 2–4).
Fig. 1.
Direct scavenging of O2•− by shikonin. Shikonin (final, 0.1–33.0 µM) was added to cuvettes containing 0.1 µM hypoxanthine, 1 µM CLA and 1 mM CaCl2 in RH buffer and then preincubated at 37°C for 5 min before setting in CFL-C2000. The chemiluminescence baseline was monitored for 50 s before addition of xanthine oxidase (final, 2.4 × 10−3 U/ml) to produce O2•−. Scavenging efficiencies were expressed as decreases in the chemiluminescence response (ratios of the areas under the curve, AUCs, of assays with shikonin relative to DMSO-control assays) and shown as a function of shikonin concentration. Values are means ± SD (n = 3). Statistically significant difference between all treatments was determined by one-way ANOVA followed by Dunnett’s test: *p<0.01 vs DMSO-controls.
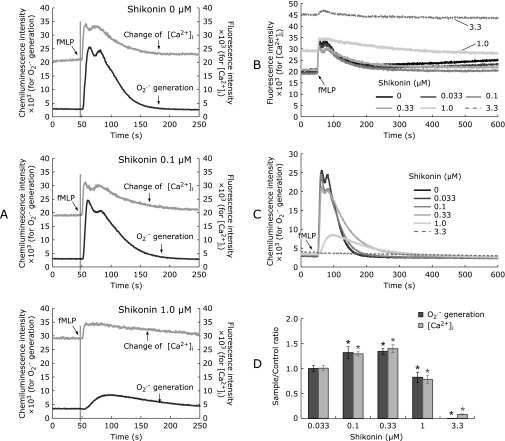
Fig. 2.
Synchronized inhibition of fMLP-elicited changes in [Ca2+]i and O2•− generation by shikonin. Fluo-3 AM-preloaded neutrophil-like cells (1 × 105/ml) were incubated at 37°C in the absence (DMSO) or presence of shikonin (0.033–3.3 µM) under low-rotation stirring in a dedicated incubator for 7 min before transferring of the assay cuvette to CFL-C2000. The assay buffer was RH buffer with 1 µM CLA and 1 mM CaCl2 without EGTA added. After 50-s baselines of fluorescence and chemiluminescence were obtained, cells were stimulated with fMLP (1 µM), as indicated (panels A, B and C). Representative assays of 0, 0.1 and 1.0 µM shikonin focused on the initial 250-s periods (panel A), and superimposed charts for comparison of changes in [Ca2+]i (panel B) and O2•− generation (panel C) are shown. The responses (AUCs) of shikonin-treated cells were shown as ratios relative to DMSO-control assays (panel D). Values are means ± SD (n = 6). Statistically significant difference between all treatments was determined by one-way ANOVA followed by Dunnett’s test: *p<0.01 vs DMSO-controls.
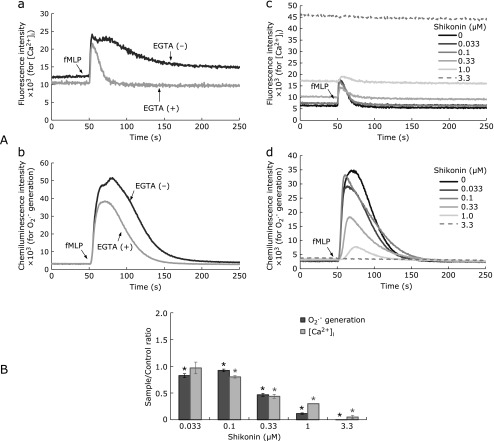
Fig. 3.
Inhibition of IICR and subsequent O2•− generation by shikonin. Fluo-3 AM-preloaded neutrophil-like cells (1 × 105/ml RH buffer, 1 µM CLA) were incubated with 1 mM CaCl2 for 1 min in a 37°C-preincubator before addition of 5 mM EGTA. Shikonin (0.033–3.3 µM) was added 1 min after EGTA, and pre-incubation was continued for a further 5 min before transferring of the assay cuvette to the CFL-C2000 to start simultaneous monitoring of chemiluminescence and fluorescence. Stimulation with fMLP (1 µM) was done after 50 s, and changes in [Ca2+]i levels and O2•− generation were further monitored. Panel A: Charts of changes in [Ca2+]i (a) and O2•− generation (b) of control assays without shikonin in the presence (grey lines) or absence (black lines) of EGTA are shown, with focus on the initial 250-s periods. The effects of increasing concentrations of shikonin on IICR-originated changes in [Ca2+]i (c) and O2•− generation (d) are shown superimposed for comparison. Panel B: Shikonin effects were shown as ratios of the responses (AUC) relative to DMSO-control assays. The AUCs were calculated by integrated signals in the period between the addition of fMLP until complete baseline decay is observed. Periods were the followings, for assays with and without EGTA, respectively: 110 and 700 s (fluorescence); 267 and 773 s (chemiluminescence). Values are means ± SD (n = 3). Statistically significant difference between all treatments was determined by one-way ANOVA followed by Dunnett’s test: *p<0.01 vs DMSO-controls.
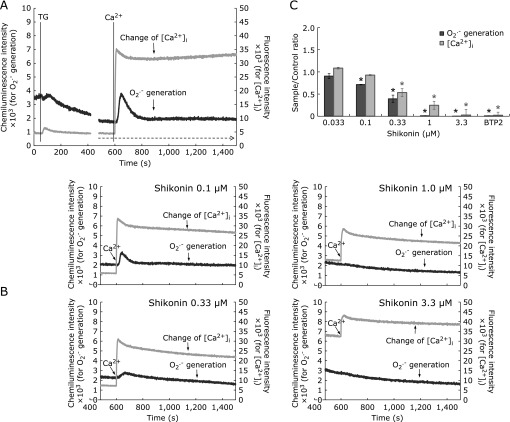
Fig. 4.
Inhibition of SOCE by shikonin. Panel A: SOCE triggered by TG was checked in the absence of shikonin, as follows. Neutrophil-like cells (1 × 105/ml), preloaded with fluo-3 AM and washed as described in Materials and Methods, were suspended in Ca2+-free buffer (RH buffer, 0.1 mM EGTA, 1 µM CLA), warmed for 3 min in a 37°C-preincubator, and then set in CFL-C2000 for monitoring of chemiluminescence and fluorescence. Addition of TG 1 min later (final 1 µM; left vertical line) causes a transient elevation of [Ca2+]i due to release of Ca2+ from stores. After the release of Ca2+ from stores is ended, addition of external Ca2+ (1 mM; vertical line near 600 s) causes SOCE, detected as a sharp jump in the fluorescence signal followed by a peak, and finally, stabilization of [Ca2+]i at a relatively high level (grey lines). O2•− generation following such changes in [Ca2+]i is shown by simultaneously monitored chemiluminescence (black lines). Panel B: Charts of TG and shikonin added cells show the responses before and after addition of external Ca2+; only responses corresponding to the latter part of the reaction in Panel A (i.e., part indicated by the dotted line arrow) are shown. The order of additions and incubation times were as follows: cells, pre-incubated for 3 min as in Panel A and kept in the pre-incubator, received additions of TG (1 µM) at 4 min, and shikonin (0.1–3.3 µM), BTP2 (10 µM) or DMSO at 6 min, and were further incubated for 5 min before transferring of the assay cuvette to the CFL-2000. After 2 min of monitoring of fluorescence and chemiluminescence, Ca2+ (final 1 mM) was added to the reaction mixture. Panel C: Inhibition of O2•− generation associated with SOCE ([Ca2+]i) is shown as a function of shikonin concentration by AUC ratios relative to DMSO control assays. The AUCs corresponding to SOCE were calculated from integrated signal responses in the period of 900 s after Ca2+ addition, from assays exemplified in panel B, as follows: parallel controls without TG were performed for each shikonin concentration, and these backgrounds were subtracted from respective assays with TG. Data are means ± SD (n = 3). Statistically significant difference between all treatments was determined by one-way ANOVA followed by Dunnett’s test: *p<0.01 vs DMSO-controls.
Shikonin elicits synchronized changes in [Ca2+]i levels and O2•− generation of cells
fMLP induced a biphasic elevation of [Ca2+]i as indicated by the fluo-3 AM signal within cells (Fig. 2A, control: 0 µM shikonin). The rapid transient elevation of [Ca2+]i at 6 s after fMLP stimulation reflects the release of Ca2+ from intracellular Ca2+-stores (IICR). This initial response was followed by a second peak of elevation of [Ca2+]i at around 25 s of fMLP stimulation due to an overlapped influx of extracellular Ca2+ via SOCE and ROCE. This influx of extracellular Ca2+ decreased in approximately 4~5 min after fMLP stimulation to a level higher than the initial baseline (Fig. 2A top, grey chart). We assigned the two peaks as IICR, and SOCE plus ROCE, respectively, based on comparison of the fMLP-stimulation chart profiles in the presence and absence of EGTA (e.g., Fig. 3A subpanel a; single-peaked chart with EGTA vs two-peaked chart without EGTA). As such, the second peak corresponding to SOCE plus ROCE disappears when extracellular Ca2+ is chelated. Shikonin-induced inhibition of [Ca2+]i elevation differed depending on the concentration used: at lower concentrations (0.033 to 0.33 µM), it gradually decreased the peak intensities, especially of the second peak. However, the two-peaked appearance of the responses was kept similar to the DMSO-control assay (Fig. 2A: 0.1 µM, and 2B). In contrast, high concentrations of shikonin (greater than 1.0 µM) caused severe inhibition of Ca2+ fluxes with a different pattern. Specifically, the response appeared as a single, low and broad peak that decayed slowly (Fig. 2A and B, 1.0 µM). It was also noted that the baseline of fluorescence increased at concentrations of shikonin greater than 1.0 µM. The increase in baseline fluorescence was seen in the absence of cells, indicating that it could be originated from shikonin itself. Indeed, shikonin has an intrinsic fluorescent emission that increases in the range of 570–700 nm upon excitation at 500 nm (data not shown), which is the excitation wavelength used in the assay.
Coincident with the above changes in [Ca2+]i responses, the chemiluminescence charts also show a two-peaked response, indicating two detectable waves of O2•− generation (Fig. 2A and C: 0 µM) with maximum points delayed by 6.5 s from those of the [Ca2+]i peaks. These results support the fact that Nox2 activation depends on [Ca2+]i-mediated responses. Owing to the lack of specific granules in neutrophil-like cells originated from HL-60 cells,(33) the O2•− generation detected in the assay could be attributed to Nox2 assembled at the plasma membranes (i.e., to O2•− produced in the extracellular space).
The generation of O2•− was gradually inhibited in the presence of shikonin. At shikonin concentrations of up to 0.1 µM, inhibition of the second peak due to reduced O2•− generation was more pronounced than the inhibition seen for the first peak, as judged by peak heights (Fig. 2C). At 1.0 µM shikonin, the shape of the O2•− generation peak changed to a single, low and broad one owing to disappearance of the first peak (Fig. 2A and C); the activity also decayed slowly. Peaks became broader when 0.1 µM or more shikonin was used and at 3.3 µM, the generation of O2•− was completely inhibited (Fig. 2C).
The graphs showing O2•− generation and [Ca2+]i responses (Fig. 2D), expressed as AUC ratios relative to DMSO-control assays, were very coincident, suggesting that the effects of shikonin on fMLP-induced Nox2 activity are based on changes in [Ca2+]i.
Unexpectedly, in assays with shikonin concentrations up to 0.33 µM, the AUC ratios relative to DMSO-controls showed a consistent elevation for both [Ca2+]i and O2•− generation (Fig. 2D). The increase was likely caused by slower decays of [Ca2+]i; although peaks became shorter in height, the responses were prolonged and thus gave greater areas than DMSO-controls. In contrast, at shikonin concentrations of 1.0 µM or higher, pretreatment with shikonin caused a significant decrease in the AUC ratios of [Ca2+]i levels and O2•− generation (Fig. 2D). The decreases in [Ca2+]i levels and O2•− generation were paralleled until a concentration of 1.0 µM shikonin; at 3.3 µM shikonin, O2•− generation was completely lost although [Ca2+]i still showed a small response (charts in Fig. 2B and C). The IC50 values for the [Ca2+]i response and O2•− generation were 1.45 ± 0.06 (n = 6) and 1.12 ± 0.03 µM (n = 6), respectively.
In order to elucidate the inhibition of individual pathways involved in the shikonin-induced changes in [Ca2+]i and O2•− generation, we performed experiments under conditions that allowed evaluation of distinct Ca2+ fluxes such as IICR and SOCE.
Shikonin inhibits IICR
The effects of shikonin on IICR were evaluated in the presence of EGTA (Fig. 3). In these assays, changes in [Ca2+]i following fMLP stimulation occur only due to IICR. The IICR under such conditions corresponded to approximately 8.5% (based on peak AUCs; Fig. 3A, grey chart in subpanel a) of the total elevation in [Ca2+]i seen in the controls in the presence of Ca2+ (i.e., fMLP stimulation of cells in RH with 0.1 mM EGTA, 1 mM CaCl2). The IICR is followed by a peak of O2•− generation accounting for approximately 50.2% of the activity measured in the control assays with Ca2+ (Fig. 3A, grey chart in subpanel b).
Shikonin affected the IICR-mediated responses, as determined by the gradual decreases in both peaks of [Ca2+]i and O2•− generation (Fig. 3A, subpanels c and d). The respective AUCs relative to DMSO-controls showed roughly coincident inhibition of [Ca2+]i levels and O2•− generation until 0.33 µM shikonin (Fig. 3B). Above this concentration, both of the AUC ratios continued to decrease. However, the AUC ratio of O2•− generation decreased more dramatically than that of the [Ca2+]i levels. At concentrations above 0.33 µM, shikonin likely impairs Nox2 activity either by direct suppression or by inhibiting events involved in enzyme activation besides events related to IICR.
Shikonin inhibits SOCE
The effects of shikonin on SOCE that occurs when external Ca2+ is added to cells pretreated with TG under Ca2+-free conditions are shown in Fig. 4. As described in Reagents, TG allows observation of Ca2+ entry via SOCE upon addition of Ca2+ to the reaction mixture.(29–31)
Treatment of cells with TG (1 µM) under Ca2+-free conditions causes a transient elevation of [Ca2+]i at around 20 s after the addition of TG (Fig. 4A), reflecting the release of stored Ca2+. After that elevation, the [Ca2+]i levels return to baseline by around 280 s (Fig. 4A). After Ca2+ stores are depleted, addition of Ca2+ to the extracellular buffer results in a rapid entry of Ca2+ through the plasma membrane. This is demonstrated by a sudden jump in the intensity of fluorescent signals followed by a peak that decays in ~120 s to a stable but higher [Ca2+]i level compared to the Ca2+-free conditions (Fig. 4A). This Ca2+ entry occurs because empty stores activate SOCE through the plasma membrane using the same channels that work for Ca2+ entry following fMLP stimulation.(31)
The depletion of Ca2+ stores by TG was accompanied by O2•− generation both before and after the addition of Ca2+ to the reaction mixture (Fig. 4A). The amount of O2•− produced by the release of Ca2+ from stores before addition of external Ca2+ was around 1/11 of that produced after the addition of Ca2+ (i.e., that of SOCE-associated response). The TG-induced O2•− generating activities including the SOCE-associated responses corresponded to approximately 3.4% of the full-reaction with fMLP stimulation in Ca2+-containing buffer. It was previously unknown that a small O2•− generation response occurs upon TG-induced release of Ca2+ stores, even under Ca2+-free conditions;(5,34) the disagreement in study results could be owing to the high sensitivity of the present system. However, the entry of extracellular Ca2+ to cells through SOCE resulting in a much higher generation of O2•− after TG-treatment is in agreement with previous reports.(5,34,35)
The reaction described above was used to investigate whether shikonin inhibits SOCE. Shikonin was added 2 min after TG, i.e., at a time when the TG-induced release of Ca2+ stores is ended (Fig. 4A), and cells were further incubated for 5 min in the presence of both compounds; the responses were monitored with the CFL-C2000. BTP2 was used as a positive control for SOCE inhibition.(28) Shikonin inhibited SOCE as shown by the gradually diminishing peak heights and AUCs of [Ca2+]i in the charts of Fig. 4B (0.1 to 3.3 µM) and the bar graph in panel C. After correction for backgrounds in the absence of TG ([Ca2+]i and O2•−), it was noted that the inhibition of SOCE was roughly parallel with the decrease in O2•− generation until a shikonin concentration of 0.33 µM (Fig. 4C); however, with 1.0 µM or more shikonin, the O2•− generation zeroed whereas SOCE still remained observable. These results suggested the possibility that shikonin as high as 1.0 µM or above could directly affect Nox2 enzyme or its activation, in addition to effects on SOCE. At 3.3 µM shikonin, both O2•− generation and SOCE were completely inhibited, similar to the inhibition seen with BTP2.
These results show that the inhibition of SOCE by shikonin is also involved in the suppression of O2•− generation.
Discussion
Based on the knowledge that shikonin suppresses O2•− generation in neutrophils, the present study aimed to elucidate the effects of shikonin on the cellular Ca2+ fluxes that lead to the generation of O2•−. For this purpose, we investigated whether inhibition of O2•− generation by shikonin is attributed to changes in [Ca2+]i, by measuring [Ca2+]i levels and O2•− generation with the CFL-C2000 system, in fMLP-stimulated neutrophil-like cells. This system simultaneously monitors both [Ca2+]i levels and O2•− generation within the same cell sample, and thus is highly applicable for screening the effects of compounds on cellular [Ca2+]i associated with O2•− generation.
Previous studies have indicated that shikonin chemically scavenges ROS such as O2•−(14–16) and hydroxyl radical,(15,17) and biologically behaves as an anti-inflammatory compound by directly targeting these oxidative molecules. The effects of shikonin on gene expression of key molecules involved in the inflammatory response have also been described;(11,13) however, the mechanisms of how shikonin suppresses O2•− generation of cells remained unclear. Under the assay conditions described here, it is likely that the O2•−-scavenging activity of shikonin has no significant role in the inhibition of the respiratory burst from fMLP-stimulated cells, since the IC50 values differed by more than 20-fold (Results, Shikonin is a chemical scavenger of O2•− and Shikonin elicits synchronized changes in [Ca2+]i levels and O2•− generation of cells: 24.6 µM for chemical scavenging vs 1.12 µM for O2•− generation; Fig. 1 and 2D, respectively). This difference in the IC50 values indicated that the inhibition mechanisms of shikonin include more sensitive intracellular targets than the direct scavenging of O2•−. We herein found a likely target candidate to be the cellular Ca2+ fluxes.
Our data indicate that shikonin inhibits the fMLP-stimulated O2•− generation through inhibition of cellular Ca2+ fluxes (Fig. 2), such as IICR (Fig. 3) and SOCE (Fig. 4). The apparent IC50 values for the fMLP-stimulated responses in Ca2+-containing buffer for [Ca2+]i levels and O2•− generation are 1.45 and 1.12 µM, respectively (Fig. 2D). In contrast, the IC50 values for IICR and SOCE were one order of magnitude lower (for [Ca2+]i and O2•−: 0.28 and 0.31 µM for IICR in Fig. 3B; and 0.39 and 0.25 µM for SOCE, estimated from Fig. 4C, respectively), indicating that very sensitive molecule(s) or reactions in the IICR and SOCE are targeted by shikonin. Therefore, the gradual inhibition of the two-peaked responses of both [Ca2+]i levels and O2•− generation seen in the fMLP-stimulated cells (Fig. 2B and C: peak height decreases by shikonin in the range of 0.1–0.33 µM) might be owned to the influences of shikonin on IICR and SOCE.
The target of shikonin when inhibiting IICR might be on IP3 formation, since it has been reported that a shikonin derivative, acetylshikonin, inhibits IP3 formation via impairment of PLC activity in fMLP-stimulated rat neutrophils.(36) This observation was concomitant with inhibition of Ca2+ release from internal stores (IC50: ~5 µM, in the presence of 1 mM EDTA),(36) suggesting that Ca2+-related events could be involved in the inhibition of Nox2 activity in fMLP-stimulated cells. Here, we obtained direct proof for this link because our monitoring system simultaneously examines changes in [Ca2+]i levels and O2•− generation: with increasing shikonin, both chart profiles had similar shapes and were almost synchronous (Fig. 2).
It is still difficult to discuss about possible components of SOCE that are targeted by shikonin because all molecules involved in phagocytic Ca2+ fluxes have yet to be determined. However, molecules known to be involved in SOCE such as the [Ca2+]i-sensor protein stromal interacting molecule 1 (STIM1)(37) expressed in ER membranes, the plasma membrane-located calcium release-activated Ca2+ channel protein 1 (Orai1), and members of the transient receptor potential channels (TRPC)(4,23,25,38) might be considered as targets of shikonin. Among these molecules, STIM1 and Orai proteins might be potential targets because these proteins are thought to be regulated by critical cysteine modifications in a redox-dependent manner,(39) and shikonin reacts with thiols.(40) Knockdown of STIM1 has been shown to abrogate SOCE and Nox2 activity in murine neutrophils.(10) In addition, Nox2 itself requires cysteines for its activity, providing a likely target for shikonin.(41)
Our findings indicate that the inhibition of Nox2 activity by shikonin in fMLP-stimulated cells can be explained in great part by suppression of IICR and SOCE. Former studies have shown the importance of extracellular Ca2+ entry for Nox2 activation (reviews(4,25,38)). We did not directly evaluate whether shikonin has any influence on ROCE. This possibility exists and remains to be elucidated. The existence and importance of ROCE in neutrophils have been suggested,(42–44) however, current molecular and mechanistic understanding cannot delineate its role in Nox2 activation. It has been reported that O2•− generation by Nox2 at plasma membranes (i.e., O2•− release to the extracellular space) requires Ca2+ entry from outside the cell since studies have shown that Nox2 activity decreases in the presence of EGTA(4,5,45) or depends on extracellular Ca2+ concentrations.(10)
Our results show a slight O2•− generation followed by TG-induced depletion of intracellular stores under Ca2+-free conditions that is enhanced to around 11-fold after the addition of Ca2+ (Fig. 4A). These results are in agreement with previous neutrophil studies(6,35) showing O2•− generation with 1 µM TG in Ca2+-containing buffer detected by the superoxide dismutase-sensitive reduction of cytochrome c. In our study, the total TG-induced response (before and after the addition of Ca2+; Fig. 4A) accounted for 3.4% of the usual activity with fMLP stimulation in the presence of Ca2+. When fMLP is used as a stimulator, the activation signal spreads from the G-protein coupled receptors linked to heterotrimeric G-proteins into multiple pathways including the interaction of Gα and Gβγ with not only PLC but also phosphoinositide-3-kinase and p21-activated kinase, whose downstream effects lead to Nox2 activation.(22) This might explain the difference between TG- and fMLP-stimulated O2•− generation. These results support the view that both intracellular and extracellular Ca2+ are required for fMLP-stimulated O2•− generation.(46)
The present results suggested that the relevance of targets of shikonin other than Ca2+ fluxes to the inhibition of Nox2 activity appeared only at concentrations above 1.0 µM (Fig. 3B and 4C). These results do not exclude, however, the existence of inhibition sites that directly affect Nox2 enzyme activity or its assembly steps. Previous studies with acetylshikonin reported an impaired translocation of the Nox2 cytosolic component p47phox to membranes with concentrations above 3.0 µM.(19)
In summary, we show that shikonin affects the fMLP-elicited O2•− generation of neutrophil-like cells by targeting Ca2+ fluxes such as SOCE and IICR. The use of a simultaneous monitoring system and proper selection of assay conditions discriminating for specific Ca2+ fluxes is a valuable strategy for elucidating the role of Ca2+ fluxes in the ROS-generating activity of cells.
Acknowledgments
We would like to thank Dr. Shigetoshi Okazaki, Dr. Mitsuo Hiramatsu and Dr. Fumihiko Ikemoto for helpful discussions and Mr. Takashi Koike, for valuable technological support.
Abbreviations
- AUC
area under the curve
- BTP2
N-{4-[3,5-bis(trifluoromethyl)-1H-pyrazol-1-yl]phenyl}-4-methyl-1,2,3-thiazole-5-carboxamide
- [Ca2+]i
cytosolic, intracellular Ca2+ concentration
- CLA
2-methyl-6-phenyl-3,7-dihydroimidazo [1, 2-a] pyrazin-3-one
- DAG
diacylglycerol
- DMSO
dimethylsulfoxide
- ER
endoplasmic reticulum
- EGTA
ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid
- fluo-3 AM
1-[2-amino-5-(2,7-dichloro-6-acetoxymethoxy-3-oxo-9-xanthenyl) phenoxy]-2-(2-amino-5-methylphenoxy) ethane-N,N,N',N'-tetraacetic acid, tetra (acetoxymethyl) ester
- fMLP
N-formyl-l-methionyl-l-leucyl-l-phenylalanine
- IC50
50% inhibitory concentration
- IICR
inositol 1,4,5-trisphosphate-induced calcium release
- IP3
inositol 1,4,5-trisphosphate
- Nox2
NADPH oxidase 2
- O2•−
superoxide anion
- RH
Ringer-Hepes buffer
- ROCE
receptor-operated calcium entry
- ROS
reactive oxygen species
- SERCA
sarco/endoplasmic reticulum membrane Ca2+-ATPase
- SOCE
store-operated calcium entry
- STIM1
[Ca2+]i-sensor protein stromal interacting molecule 1
- TG
thapsigargin
- TRPC
transient receptor potential channel
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ishibashi K, Okazaki S, Hiramatsu M. Simultaneous measurement of superoxide generation and intracellular Ca2+ concentration reveals the effect of extracellular Ca2+ on rapid and transient contents of superoxide generation in differentiated THP-1 cells. Biochem Biophys Res Commun. 2006;344:571–580. doi: 10.1016/j.bbrc.2006.02.173. [DOI] [PubMed] [Google Scholar]
- 2.Satozono H, Kazumura K, Okazaki S, Hiramatsu M. Simultaneous measurement of superoxide generation and intracellular calcium ion of neutrophil-like culture cells. Luminescence. 2006;21:69–71. doi: 10.1002/bio.882. [DOI] [PubMed] [Google Scholar]
- 3.Kazumura K, Sato Y, Satozono H, et al. Simultaneous monitoring of superoxides and intracellular calcium ions in neutrophils by chemiluminescence and fluorescence: evaluation of action mechanisms of bioactive compounds in foods. J Pharm Biomed Anal. 2013;84:90–96. doi: 10.1016/j.jpba.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Bréchard S, Tschirhart EJ. Regulation of superoxide production in neutrophils: role of calcium influx. J Leukoc Biol. 2008;84:1223–1237. doi: 10.1189/jlb.0807553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foyouzi-Youssefi R, Petersson F, Lew DP, Krause KH, Nüsse O. Chemoattractant-induced respiratory burst: increases in cytosolic Ca2+ concentrations are essential and synergize with a kinetically distinct second signal. Biochem J. 1997;322:709–718. doi: 10.1042/bj3220709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiszt M, Szebérenyi JB, Káldi K, Ligeti E. Role of different Ca2+ sources in the superoxide production of human neutrophil granulocytes. Free Rad Biol Med. 1999;26:1092–1099. doi: 10.1016/s0891-5849(98)00283-4. [DOI] [PubMed] [Google Scholar]
- 7.Valentin F, Bueb J, Capdeville-Atkinson C, Tschirhart E. Rac-1-mediated O2− secretion requires Ca2+ influx in neutrophil-like HL-60 cells. Cell Calcium. 2001;29:409–415. doi: 10.1054/ceca.2001.0203. [DOI] [PubMed] [Google Scholar]
- 8.Granfeldt D, Samuelsson M, Karlsson A. Capacitative Ca2+ influx and activation of the neutrophil respiratory burst. Different regulation of plasma membrane- and granule-localized NADPH-oxidase. J Leukoc Biol. 2002;71:611–617. [PubMed] [Google Scholar]
- 9.Suda T, Suzuki Y, Matsui T, et al. Dapsone suppresses human neutrophil superoxide production and elastase release in a calcium-dependent manner. Br J Dermatol. 2005;152:887–895. doi: 10.1111/j.1365-2133.2005.06559.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Clemens RA, Liu F, et al. STIM1 calcium sensor is required for activation of the phagocyte oxidase during inflammation and host defense. Blood. 2014;123:2238–2249. doi: 10.1182/blood-2012-08-450403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Yang L, Oppenheim JJ, Howard MZ. Cellular pharmacology studies of shikonin derivatives. Phytother Res. 2002;16:199–209. doi: 10.1002/ptr.1100. [DOI] [PubMed] [Google Scholar]
- 12.Papageorgiou VP, Assimopoulou AN, Ballis AC. Alkannins and shikonins: a new class of wound healing agents. Curr Med Chem. 2008;15:3248–3267. doi: 10.2174/092986708786848532. [DOI] [PubMed] [Google Scholar]
- 13.Andüjar I, Rios JL, Giner RM, Recio MC. Pharmacological properties of shikonin - a review of literature since 2002. Planta Med. 2013;79:1685–1697. doi: 10.1055/s-0033-1350934. [DOI] [PubMed] [Google Scholar]
- 14.Sekine T, Masumizu T, Maitani Y, Nagai T. Evaluation of superoxide anion radical scavenging activity of shikonin by electron spin resonance. Int J Pharm. 1998;174:133–139. [Google Scholar]
- 15.Gao D, Kakuma M, Oka S, Sugino K, Sakurai H. Reaction of beta-alkannin (shikonin) with reactive oxygen species: detection of beta-alkannin free radicals. Bioorg Med Chem. 2000;8:2561–2569. doi: 10.1016/s0968-0896(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida LS, Kohri S, Tsunawaki S, et al. Evaluation of radical scavenging properties of shikonin. J Clin Biochem Nutr. 2014;55:90–96. doi: 10.3164/jcbn.13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekine T, Masumizu T, Maitani Y, Takayama K, Kohno M, Nagai T. Effect of shikonin and alkannin on hydroxyl radical generation system concerned with iron ion. Yakugaku Zasshi. 1998;118:609–615. doi: 10.1248/yakushi1947.118.12_609. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 18.Kawakami N, Koyama Y, Tanaka J, Ohara A, Hayakawa T, Fujimoto S. Inhibitory effect of acetylshikonin on the activation of NADPH oxidase in polymorphonuclear leukocytes in both whole cell and cell-free systems. Biol Pharm Bull. 1996;19:1266–1270. doi: 10.1248/bpb.19.1266. [DOI] [PubMed] [Google Scholar]
- 19.Wang JP, Tsao LT, Raung SL, Hsu MF, Kuo SC. Investigation of the inhibition by acetylshikonin of the respiratory burst in rat neutrophils. Br J Pharmacol. 1997;121:409–416. doi: 10.1038/sj.bjp.0701147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida LS, Kawada T, Irie K, et al. Shikonin directly inhibits nitric oxide synthases: possible targets that affect thoracic aorta relaxation response and nitric oxide release from RAW 264.7 macrophages. J Pharmacol Sci. 2010;112:343–351. doi: 10.1254/jphs.09340fp. [DOI] [PubMed] [Google Scholar]
- 21.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4:a006049. doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smyth JT, Hwang SY, Tomita T, DeHaven WI, Mercer JC, Putney JW. Activation and regulation of store-operated calcium entry. J Cell Mol Med. 2010;14:2337–2349. doi: 10.1111/j.1582-4934.2010.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salmon MD, Ahluwalia J. Pharmacology of receptor operated calcium entry in human neutrophils. Int Immunopharmacol. 2011;11:145–148. doi: 10.1016/j.intimp.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Burgos RA, Conejeros I, Hidalgo MA, Werling D, Hermosilla C. Calcium influx, a new potential therapeutic target in the control of neutrophil-dependent inflammatory diseases in bovines. Vet Immunol Immunopathol. 2011;143:1–10. doi: 10.1016/j.vetimm.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 26.Dietrich A, Kalwa H, Rost BR, Gudermann T. The diacylgylcerol-sensitive TRPC3/6/7 subfamily of cation channels: functional characterization and physiological relevance. Pflugers Arch. 2005;451:72–80. doi: 10.1007/s00424-005-1460-0. [DOI] [PubMed] [Google Scholar]
- 27.Zitt C, Strauss B, Schwarz EC, et al. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J Biol Chem. 2004;279:12427–12437. doi: 10.1074/jbc.M309297200. [DOI] [PubMed] [Google Scholar]
- 28.Steinckwich N, Frippiat JP, Stasia MJ, et al. Potent inhibition of store-operated Ca2+ influx and superoxide production in HL60 cells and polymorphonuclear neutrophils by the pyrazole derivative BTP2. J Leukoc Biol. 2007;81:1054–1064. doi: 10.1189/jlb.0406248. [DOI] [PubMed] [Google Scholar]
- 29.Jackson TR, Patterson SI, Thastrup O, Hanley MR. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988;253:81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inesi G, Sagara Y. Thapsigargin, a high affinity and global inhibitor of intracellular Ca2+ transport ATPases. Arch Biochem Biophys. 1992;298:313–317. doi: 10.1016/0003-9861(92)90416-t. [DOI] [PubMed] [Google Scholar]
- 31.Demaurex N, Lew DP, Krause KH. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992;267:2318–2324. [PubMed] [Google Scholar]
- 32.Hirayama O, Takagi M, Hukumoto K, Katoh S. Evaluation of antioxidant activity by chemiluminescence. Anal Biochem. 1997;247:237–241. doi: 10.1006/abio.1997.2053. [DOI] [PubMed] [Google Scholar]
- 33.Fontana JA, Wright DG, Schiffman E, Corcoran BA, Deisseroth AB. Development of chemotactic responsiveness in myeloid precursor cells: studies with a human leukemia cell line. Proc Natl Acad Sci U S A. 1980;77:3664–3668. doi: 10.1073/pnas.77.6.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nüsse O, Serrander L, Foyouzi-Youssefi R, Monod A, Lew DP, Krause KH. Store-operated Ca2+ influx and stimulation of exocytosis in HL-60 granulocytes. J Biol Chem. 1997;272:28360–28367. doi: 10.1074/jbc.272.45.28360. [DOI] [PubMed] [Google Scholar]
- 35.Kano S, Iizuka T, Ishimura Y, Fujiki H, Sugimura T. Stimulation of superoxide anion formation by the non-TPA type tumor promoters palytoxin and thapsigargin in porcine and human neutrophils. Biochem Biophys Res Commun. 1987;143:672–677. doi: 10.1016/0006-291x(87)91406-9. [DOI] [PubMed] [Google Scholar]
- 36.Wang JP, Kuo SC. Impairment of phosphatidylinositol signaling in acetylshikonin-treated neutrophils. Biochem Pharmacol. 1997;53:1173–1177. doi: 10.1016/s0006-2952(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 37.Bréchard S, Plançon S, Melchior C, Tschirhart EJ. STIM1 but not STIM2 is an essential regulator of Ca2+ influx-mediated NADPH oxidase activity in neutrophil-like HL-60 cells. Biochem Pharmacol. 2009;78:504–513. doi: 10.1016/j.bcp.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Clemens RA, Lowell CA. Store-operated calcium signaling in neutrophils. J Leukoc Biol. 2015;98:497–502. doi: 10.1189/jlb.2MR1114-573R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nunes P, Demaurex N. Redox regulation of store-operated Ca2+ entry. Antioxid Redox Signal. 2014;21:915–932. doi: 10.1089/ars.2013.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao D, Hiromura M, Yasui H, Sakurai H. Direct reaction between shikonin and thiols induces apoptosis in HL60 cells. Biol Pharm Bull. 2002;25:827–832. doi: 10.1248/bpb.25.827. [DOI] [PubMed] [Google Scholar]
- 41.Dahan I, Smith SM, Pick E. A Cys-Gly-Cys triad in the dehydrogenase region of Nox2 plays a key role in the interaction with p67phox. J Leukoc Biol. 2015;98:859–874. doi: 10.1189/jlb.4A0315-107R. [DOI] [PubMed] [Google Scholar]
- 42.Itagaki K, Kannan KB, Hauser CJ. Lysophosphatidic acid triggers calcium entry through a non-store-operated pathway in human neutrophils. J Leukoc Biol. 2005;77:181–189. doi: 10.1189/jlb.0704390. [DOI] [PubMed] [Google Scholar]
- 43.Bréchard S, Melchior C, Plançon S, Schenten V, Tschirhart EJ. Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium. 2008;44:492–506. doi: 10.1016/j.ceca.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Salmon MD, Ahluwalia J. Actions of calcium influx blockers in human neutrophils support a role for receptor-operated calcium entry. Cell Immunol. 2010;262:6–10. doi: 10.1016/j.cellimm.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Gallois A, Bueb JL, Tschirhart E. Effect of SK&F 96365 on extracellular Ca2+-dependent O2− production in neutrophil-like HL-60 cells. Eur J Pharmacol. 1998;361:293–298. doi: 10.1016/s0014-2999(98)00728-6. [DOI] [PubMed] [Google Scholar]
- 46.Kim-Park WK, Moore MA, Hakki ZW, Kowolik MJ. Activation of the neutrophil respiratory burst requires both intracellular and extracellular calcium. Ann N Y Acad Sci. 1997;832:394–404. doi: 10.1111/j.1749-6632.1997.tb46267.x. [DOI] [PubMed] [Google Scholar]