Abstract
Introduction
There is little information as to what extent adverse drug reactions (ADRs) influence patients’ health-related quality of life (HR-QOL). From a pharmacovigilance perspective, capturing and making the best use of this information remains a challenge. The Netherlands Pharmacovigilance Centre Lareb received about 1800 reports after the packaging of the drug Thyrax® (levothyroxine; Aspen Pharma Trading Limited, Dublin, Ireland) changed from a brown glass bottle to a blister package in the Netherlands.
Objective
The objective of this study was to explore the impact of ADRs on HR-QOL in patients who reported a possible ADR to Lareb in relation to the change in the packaging of the drug Thyrax®. A secondary objective was to explore factors correlated with change in HR-QOL.
Methods
Patients who reported an ADR in relation to the Thyrax® packaging change were included in this study. A web-based adapted version of the COOP/WONCA questionnaire was sent to explore the HR-QOL before versus during the ADR, expressed on a 5-point scale from no impact (1) to high impact (5). Multivariable linear regression analysis was used to identify factors correlated with change in HR-QOL.
Results
Overall, 1167 patients returned the questionnaire (71.2 % response rate). The difference in HR-QOL was −0.8 for physical, −1.2 for mental, −1.4 for daily activities, −1.3 for social, and −1.3 for overall health status (p < 0.001 for each domain). Age, sex, educational level of the patient, and absence from work due to an ADR were correlated with at least one domain, while severity of the ADR was found to be correlated with all domains of HR-QOL.
Conclusion
Patients who reported possible ADRs after the Thyrax® packaging change experienced a significant decrease in HR-QOL. This impact was highest for the domains ‘daily activities’, ‘overall health status’, and ‘mental health’ and lowest for ‘physical fitness’.
Electronic supplementary material
The online version of this article (doi:10.1007/s40264-016-0422-0) contains supplementary material, which is available to authorized users.
Key Points
| In this study population, the ADRs experienced resulted in a significant decrease in all domains of health-related quality of life (HR-QOL). |
| A question about severity may be used by pharmacovigilance centers to provide a general view regarding the impact of the ADR on the patient’s HR-QOL. |
Introduction
Adverse drug reactions (ADRs) can have a great impact on a patient’s health-related quality of life (HR-QOL), i.e., the perception of physical and mental health, the perceived need for healthcare, and preferences about treatment and outcome [1]. Unfortunately, within pharmacovigilance, e.g., as part of a spontaneous ADR reporting system, systematic gathering of data on HR-QOL is still uncommon.
Information about the impact of ADRs on a patient’s HR-QOL can be useful for several purposes. Firstly, it can be used systematically during the process of signal selection. The primary aim of pharmacovigilance centers is the timely detection of unknown ADRs or new information about known ADRs. This process is also known as ‘signal detection’. In practice, a signal is a clinically important event that, if found to be drug related, might have an impact on patient management or the balance of benefits and risks [2]. In the process of selecting which potential signals deserve attention, ADR reports that are classified as ‘serious’ according the Counsel for International Organizations of Medical Sciences (CIOMS) criteria often have priority over other reports. These criteria include reactions leading to (prolongation of) hospitalization, life-threatening events, death, disabling events, congenital abnormalities, and other medically significant reactions [3]. However, non-serious ADRs, e.g., headache, itchiness or muscle pain, can have a great impact on patient’s HR-QOL. Systematically gathering this information may help to identify subgroups of patients with relatively poor HR-QOL and can in this way be used for signal prioritization.
Secondly, information about the impact of an ADR can give healthcare professionals insight into how patients feel and how satisfied they are with the treatment [4]. This can be illustrated by a study by Baiardini et al. [5] that explored HR-QOL and well-being in patients with drug-induced anaphylactic shock. That an anaphylactic shock has impact on the patient’s HR-QOL is to be expected; however, it was also found that most patients were worried about taking any medication after the ADR occurred, even those drugs that did not cause the allergic reaction. Healthcare professionals can use information on the impact of ADRs to select the most appropriate treatment strategies for the individual patient and to provide appropriate information about these ADRs.
Finally, information on the impact of ADRs can be useful in the process of patients understanding and accepting ADRs. Lorimer et al. [6] explored patients’ experiences of severe ADRs. Aside from a direct physiological effect of ADRs on a patient, emotions such as disbelief, anger, fear, frustration, and isolation were commonly expressed. Guo et al. [7], who studied ADRs in tuberculosis patients, showed that ADRs carry a higher mental well-being burden than a physical one. Van Hunsel et al. [8] demonstrated that as well altruistic motives, “I wanted to be heard” is a trigger for patients to report ADRs. The contact between the patient and their healthcare providers may also influence how patients experience the impact of ADRs on their HR-QOL. Awareness of the possible impact of ADRs on HR-QOL may help patients in the understanding and accepting of their ADRs and give them greater perspective on the burden of their disease.
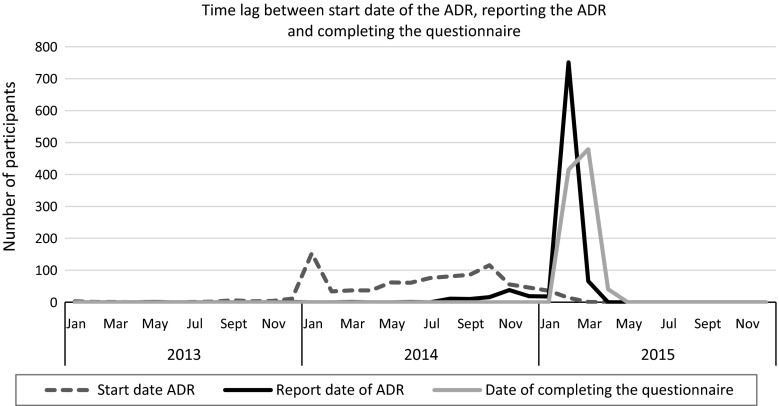
Given the relative lack of literature on how information about the impact of ADRs on patients’ HR-QOL can be captured in spontaneous ADR reporting, research is needed. Since disease type and stage influences a patient’s perception of the impact of an ADR, we considered it important to study a relatively homogenous group of patients. In the period from the end of 2013 until mid 2015, the Netherlands Pharmacovigilance Centre Lareb received about 1800 reports after the packaging was changed for the drug Thyrax® (levothyroxine; Aspen Pharma Trading Limited, Dublin, Ireland) [9]. This is a massive increase compared to the 167 reports received on levothyroxine in the period between 2006 and 2010 (an average of two to three reports per month) [10]. Thyrax® was granted marketing authorization in the Netherlands on 6 June 1980 and is indicated for the treatment of thyroid disorders [11]. At the end of 2013, the packaging changed from a bottle to a blister pack at the initiative of the Marketing Authorization Holder in order to improve protection against various environmental factors such as light, air, and humidity. According to the Marketing Authorization Holder, the formulation of the product had not been changed. Additional studies indicated that tablets from both the bottle and the blister meet the quality requirements; however, tablets from the blister has a slightly better stability [12]. Despite these findings, Lareb received lots of reports. The most reported ADRs were symptoms of hyperthyroidism including palpitations, fatigue, and headache, but symptoms of hypothyroidism were also reported as well as symptoms with no clear explanation. Most of the reports (85 %) were submitted after media attention about the Thyrax® packaging change in February 2015 (see also Fig. 1) [13]. Media attention consisted of national television coverage and reporting in newspapers [14]. The reporting pattern for this specific drug after media attention resembled the reporting pattern in New Zealand after a formulation change for the drug Eltroxin® (thyroxine; GlaxoSmithKline, Germany) [15, 16].
Fig. 1.
Time lag between start date of the adverse drug reaction, date of reporting, and completing the questionnaire. ADR adverse drug reaction
In the Netherlands, patients have been able to report ADRs to the pharmacovigilance center since 2003. The majority of the 1800 reports received on the Thyrax® packaging change were from patients (93 %). All reports were assessed on a case-by-case by a trained pharmacovigilance assessor. Feedback was sent to all patients in response to their reported ADR [17, 18]. On average, the ADRs were reported 33 (±20) weeks after the start date of the ADRs.
This study aims to explore the impact of ADRs on the HR-QOL of patients who reported a suspected ADR to Lareb in relation to the Thyrax® packaging change. We were also interested in factors that may influence the change in HR-QOL, e.g., the outcome of the ADR or its severity. Therefore, the secondary aim is to explore factors correlated with the change in HR-QOL during an ADR.
Method
Study Population
The study population consisted of all patients who experienced an ADR after the change in the packaging of Thyrax® and who reported this to Lareb up until 14 April 2015.
Measurement of Health-Related Quality of Life (HR-QOL)
In order to explore the impact of ADRs on the patients’ HR-QOL an adapted version of the COOP/WONCA charts was used. The COOP/WONCA questionnaire was developed by the Dartmouth Primary Care Cooperative Research Network (COOP) and the World Organization of National Colleges, Academics and Academic Associations of General Practitioners/Family Practitioners (WONCA). The Dutch version of COOP/WONCA has been tested in a community setting and during a hypertension screening. The validity and psychometric characteristics of the Dutch COOP/WONCA were found to be acceptable, taking into account that it concerns a generic instrument [19]. The COOP/WONCA questionnaire is a self-reported, quick and simple questionnaire consisting of single-item scales to explore HR-QOL. The following domains of the COOP/WONCA were used in this study: physical fitness, social activities, mental fitness, daily activities, and overall health status. The items were scored on a 5-level ordinal scale ranging from 1 (no impact) to 5 (high impact). HR-QOL was explored for the status at baseline (before the ADR) and during occurrence of the ADR. Subsequently, a change score in HR-QOL was calculated.
Questionnaire Development
A web-based questionnaire was designed and sent by e-mail using the SurveyMonkey® package (SurveyMonkey, Palo Alto, CA, USA) [20]. On the first question sheet of the questionnaire we asked about the five domains of HR-QOL for the situation at baseline. We then asked about the HR-QOL during the ADR on the subsequent sheet. Further questions were posted about recovery, the seriousness and severity of the ADR, if the patient was absent from work due to the ADR, if the patient was able to discuss the ADRs in a satisfying matter with their healthcare professional, and socio-demographic characteristics. Completing the questionnaire took approximately 5–10 min. See the Electronic Supplementary Material for a copy of the questionnaire.
Sending the Questionnaire
An e-mail invitation to participate in the questionnaire/study was sent to all eligible patients. A reminder was sent to all non-responders 1 week after the invitation. Collection of the responses finished 2 weeks after the first invitation was sent.
The invitation e-mail was uniquely linked to the questionnaire and the respondent’s e-mail address. Therefore, the message could not be forwarded by respondents and only one response per e-mail address was allowed. Ethics committee approval was not required as Dutch legislation does not request this for studies that do not affect the patient’s integrity [21]. Participant data were sampled and stored in accordance with privacy regulations.
Data Analysis
Overall HR-QOL and the HR-QOL change score were analyzed for each domain using descriptive statistics. A paired-sample t test was used to analyze statistically significant differences in the HR-QOL score before versus during the ADR. Multivariable linear regression analysis was carried out to explore factors correlated with changes in HR-QOL during an ADR. Potential correlating factors were the following items: recovery (yes/no); seriousness (yes/no) based on CIOMS criteria [3] and severity of the ADR (scale from 1 to 10); if the patient was absent from work due to the ADR (yes/no); if the patient was able to discuss their ADRs in a satisfying matter with their doctor and pharmacist (yes/no); age (≤20 years, 21–80 years in six equal categories in steps of 10 years, >80 years); sex; and educational level (vocational school or lower/higher professional education or higher). A backward selection procedure was used with a significance level of <0.05 to develop the model. To correct for multiple comparisons, a Bonferroni correction was conducted (corrected α = α/number of independent significance tests) [22]. It adjusted for five independent tests, leading to the corrected p value for significance of <0.01. Data were analyzed using SPSS® Statistics version 22 (IBM Corp., Armonk, NY, USA).
Results
Overall
The questionnaire was sent to 1638 patients and had a response of 71.2 % (n = 1167). The majority of respondents were female and between 41 and 60 years old (Table 1). The large majority of respondents had not recovered from the suspected ADR at the time of reporting. Only a few reports were categorized as serious. More respondents reported that they felt that they could discuss their ADRs better with their physician than with their pharmacist (Table 2). The average severity of the suspected ADRs as experienced by patients was 6.7 on a scale from 1 (no severity) to 10 (high severity). The average time between occurrence of the ADRs and reporting was 8 months (standard deviation [SD] 5 months). The average time between occurrence of the ADR and completing the questionnaire was 9 months (SD 5 months) (see also Fig. 1).
Table 1.
Respondents’ sociodemographic characteristics
| Characteristic | n | % |
|---|---|---|
| Sex | ||
| Female | 1041 | 89.2 |
| Male | 121 | 10.4 |
| Not reported | 5 | 0.4 |
| Age (years) | ||
| <20 | 14 | 1.2 |
| 21–30 | 41 | 3.5 |
| 31–40 | 104 | 8.9 |
| 41–50 | 273 | 23.4 |
| 51–60 | 377 | 32.3 |
| 61–70 | 262 | 22.5 |
| 71–80 | 54 | 4.6 |
| >80 | 7 | 0.6 |
| Not reported | 35 | 3.0 |
| Educational level | ||
| Vocational school or lower | 701 | 60.1 |
| Higher professional education or higher | 455 | 39.0 |
| Not reported | 11 | 0.9 |
Table 2.
Adverse drug reaction-related characteristics
| Characteristic | n | % |
|---|---|---|
| Recovery ADR | ||
| Yes | 179 | 15.3 |
| No | 988 | 84.7 |
| Serious ADRs | ||
| Yes | 40 | 3.4 |
| No | 1127 | 96.6 |
| Absent from work due to the ADR | ||
| Yes | 569 | 48.8 |
| No | 304 | 26.0 |
| Not reported/not applicable | 294 | 25.2 |
| Discuss the ADRs in a satisfying matter with their doctor | ||
| Yes | 809 | 69.3 |
| No | 185 | 15.9 |
| Not reported/not applicable | 173 | 14.8 |
| Discuss the ADRs in a satisfying matter with their pharmacist | ||
| Yes | 311 | 26.6 |
| No | 350 | 30.0 |
| Not reported/not applicable | 506 | 43.4 |
ADR adverse drug reaction
Quality-of-Life Scores
The overall HR-QOL at baseline ranged from 1.7 to 2.7 (Table 3). In general, patients had the perception that their HR-QOL was good at baseline. There was a statistically significant decrease in HR-QOL scores for all domains, with scores between −0.8 and −1.4 (p < 0.001). The highest decrease was observed for the domains ‘daily activities’ followed by ‘social activities’ and ‘overall health status’.
Table 3.
Health-related quality of life for the domains: physical, social, mental, daily activities, and overall health status
| Domain QOL | Before ADR | During ADR | Difference in QOL (SE) |
|---|---|---|---|
| Physical fitness | 2.3 | 3.1 | −0.8 (1.2) |
| Social activities | 1.7 | 2.9 | −1.3 (1.4)a |
| Mental fitness | 1.8 | 3.1 | −1.2 (1.3)a |
| Daily activities | 1.7 | 3.1 | −1.4 (1.2) |
| Overall health status | 2.7 | 4.0 | −1.3 (1.0) |
ADR adverse drug reaction, QOL quality of life, SE standard error
aDifference due to rounding of results
Items Correlated with Change in HR-QOL
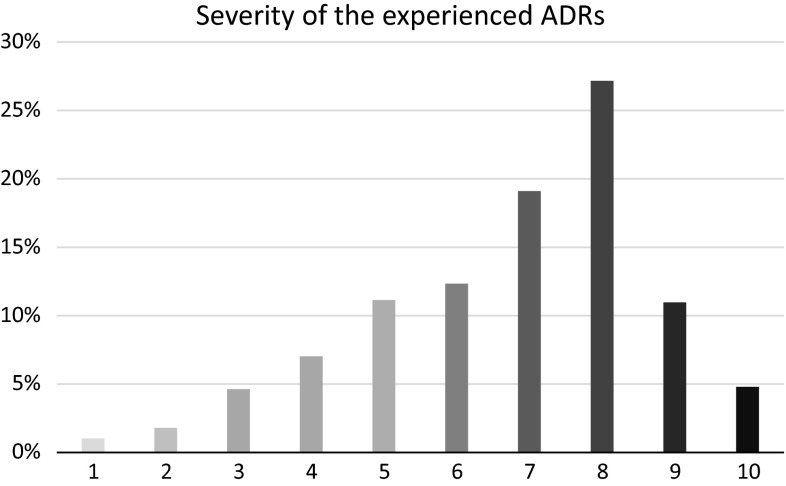
Multivariable linear regression analysis demonstrated several items that showed correlation with changes in HR-QOL (Table 4). The way the patients experienced the severity of the ADR (see Fig. 2) was found to be correlated with all domains of HR-QOL: the higher the severity, the higher the impact on the patient’s HR-QOL. Sex was found to be correlated to the domains ‘social activities’ and ‘mental fitness’. For female respondents, the ADRs had a higher impact on HR-QOL for these domains. For age, it was found that a higher age resulted in a higher impact of the ADR on HR-QOL for the domain ‘physical fitness’. Educational level was found to be correlated with the ‘physical’ domain. An educational level of maximal vocational school resulted in a higher impact on HR-QOL than an education of higher professional education/academic. Analysis further demonstrated that when patients were absent from work due to the ADR, this had a positive influence on the domain ‘overall health status’.
Table 4.
Determinants in change of quality-of-life score
| Domain QOL | Constant | Correlated items | β | 95 % CI | R 2 |
|---|---|---|---|---|---|
| Physical | 0.006 | Severity | −0.18 | −0.21 to −0.15 | 0.112 |
| Age | 0.06 | 0.02 to 0.10 | |||
| Education | 0.22 | 0.10 to 0.35 | |||
| Social | 0.634 | Severity | −0.29 | −0.33 to −0.26 | 0.188 |
| Sex | 0.31 | 0.08 to 0.54 | |||
| Mental | 0.096 | Severity | −0.24 | −0.27 to −0.20 | 0.140 |
| Sex | 0.37 | 0.14 to 0.60 | |||
| Daily activities | 0.512 | Severity | −0.28 | −0.32 to −0.25 | 0.201 |
| Overall health status | 0.107 | Severity | −0.21 | −0.24 to −0.19 | 0.190 |
| Absent from work due to the ADR | 0.003 | 0.002 to 0.004 |
ADR adverse drug reaction, QOL quality of life
Fig. 2.
Severity of the adverse drug reaction on a scale from 1 (no severity) to 10 (high severity) as experienced by patients. ADR adverse drug reaction
Discussion
In this study, we used a questionnaire to investigate the impact of ADRs on the HR-QOL of patients who reported a possible ADR to Lareb in association with the package change of the drug Thyrax®. Patients are increasingly systematically involved in the process of drug safety, from drug development to pharmacovigilance [23], and patients are now able to report ADRs directly in a growing number of countries. It remains a challenge for pharmacovigilance centers to capture some of the unique features of patient reports, such as information on HR-QOL, and to make best use of this information in a spontaneous reporting system. Since the patient is the one who actually experienced the ADR, we believe that it is best to ask them about the impact it has on their HR-QOL. In spontaneous reports, information on the impact of the ADR on daily life is present in patient reports more than in healthcare professionals’ reports [24, 25]. This study demonstrated that the reported ADRs had a significant impact on the patients’ HR-QOL. We found the highest impact on HR-QOL for the domains ‘daily activities’, ‘overall health status’, and ‘mental health’, and the lowest impact for ‘physical fitness’. The decrease in HR-QOL ranged from −0.8 to −1.4, meaning that, on average, patients’ HR-QOL dropped by one category on the 5-level ordinal scale. When interpreting the meaning of this change in HR-QOL, different perspectives have to be considered. From the point of view of the patient, a meaningful change in HR-QOL may be one that results in a considerable increase in complaints. When the patient is unable to carry out their daily business, a change of one category on the 5-level ordinal scale may be a meaningful change in HR-QOL. In contrast, a meaningful change for the healthcare professional may be one that indicates a change in the therapeutic treatment or in the prognosis of the disease [26].
Items found to be correlated with change in HR-QOL in this study were the age, sex and educational level of the patient, the severity of the ADR, and absence from work due to the ADR. Little research has been done on the perceived severity of the ADRs in relation to HR-QOL. In our study, we measured severity as a subjective representation of how patients experienced the ADRs scored on a scale from 1 (no severity) to 10 (high severity) and it was found to be correlated with all domains in HR-QOL. Studying HR-QOL in children with epilepsy, Wu et al. [27] found that patients with several different ADRs experienced lower HR-QOL. Although they did not report the severity of the ADRs, experiencing several ADRs may theoretically be related to severity.
It is important to note the difference between severity and the medical ‘seriousness’. In our study, we used CIOMS criteria to assess the seriousness of an ADR report [3]. Other studies used different criteria. For example, Guo et al. [7] used the term ‘major ADRs’, defined as ADRs requiring hospital admission, additional treatment, or discontinuation of tuberculosis medication which could be interpreted as ‘serious ADRs’. Using the Short-Form 36 questionnaire to measure HR-QOL, they found that major ADRs influenced the physical, vitality and mental health domains. However, because of the disparities in terminology, it is difficult to compare the results.
Education level was found to be correlated with ‘physical fitness’. A higher educational level resulted in a lower impact on this domain. This result is supported by a study by Davis et al. [28] exploring the extent to which treatment-related ADRs were associated with cancer-specific and general quality of life (QOL) [28]. Exploring the relationship between drug-related problems and HR-QOL in ambulatory, community-dwelling patients with musculoskeletal disorders, Ernst et al. [29] found that the level of education was positively related to the change of the mental component and not to the physical. In their study, Ernst et al. [29] also explored the impact of ‘positively addressing’ drug-related problems since this can be an important step in improving HR-QOL. This determinant can be compared with “was the patient able to discuss the ADRs in a satisfying matter with their healthcare professional” as used in our study. The present study as well as the study of Ernst et al. [29] found no statistically significant effect for this item. Somewhat surprisingly, we found that “absence from work due to the ADR” had a positive influence on the domain ‘overall health status’. An explanation could be that patients who are still working despite the ADR experience much more discomfort than those who stay at home.
HR-QOL is a psychological construct and thus an abstract concept that is not directly observable. There is no gold standard to compare against; the standardized QOL questionnaires are the best instruments that are available [30]. There are several general HR-QOL questionnaires available, but none of them was specifically developed for the pharmacovigilance setting [31]. We chose the COOP/WONCA questionnaire because it is a quick and simple, self-reporting tool that was found to be workable in this setting. In this questionnaire, each question is a single-item measurement of an aspect of functional status and it is advised not to further aggregate the item scores into one index [19]. HR-QOL was studied using patients who reported to the pharmacovigilance center. Several previous studies showed that patients consider the impact of an ADR on their HR-QOL an important subject and report about it more often than healthcare professionals [13, 24, 25, 32, 33]. This may partly explain our high response rate of 71.2 %. Furthermore, the response rate may be high due to the media attention concerning the Thyrax® packaging problem. Finally, in general, previous studies with patient questionnaires also showed that patients are willing to provide extra information [8, 17].
A strength of this study is that we included a relatively homogeneous study population of patients with a (chronic) thyroid disorder, with the majority of patients being stable on their medication before occurrence of the ADRs [13]. Our population reported a relatively high HR-QOL at baseline, but slightly lower than a population (n = 149, mean age 43.4 years, 47 % female) studied by Van Weel et al. [19] in Emmen, a rural town in the north of the Netherlands, using the COOP/WONCA questionnaire. HR-QOL at baseline was the same for the domain ‘social activities’, but slightly worse in other domains: physical fitness (2.3 vs. 1.8), mental fitness (1.8 vs. 1.5), daily activities (1.7 vs. 1.5), and overall health (2.7 vs. 2.4). More research is needed in other patient groups with higher/lower HR-QOL at baseline.
Our study has several limitations. We used spontaneous reports to the Dutch pharmacovigilance center as a basis of the study. One limitation is the period of time between onset of the ADR and the time of reporting. If patients did not remember exactly how they felt before or during the ADR it may affect the accuracy of their recall regarding the impact of the ADRs on their HR-QOL. Another consequence of measuring the impact of ADRs on the patient’s HR-QOL using data from a pharmacovigilance center is that only those patients who consider the ADRs important enough to report them will be included. A control group of patients who experienced ADRs but did not report them to the pharmacovigilance center is not available. Patients who do not report an ADR may experience a different change in HR-QOL as compared with those who did report it. Furthermore, we did not include the type of reported ADR in our analysis as a possible determinant. Since most patients reported several ADRs (average of four ADRs per report [9]), this was not considered feasible.
Practical Implications
The perceived severity of the ADR was found to be a determinant for all domains of HR-QOL. The strong relationship between severity and impact is a valuable finding from the perspective of a pharmacovigilance center. Adding HR-QOL questions to the regular ADR reporting form carries the risk that the form becomes too time-consuming to complete. If one question about severity gives a reflection of the patient’s perception of the impact of the ADRs on their HR-QOL, this question could be used on the reporting form. This aspect should be further investigated. Information regarding severity can be used in the process of signal selection and prioritization. When an ADR has a high severity in a significant share of the reports, this may be a trigger to undertake action. As already highlighted, information about the impact of ADRs can also be valuable for other stakeholders in pharmacovigilance, for example healthcare professionals and patients. Follow-up studies are needed to explore in which ways this information can best be provided and used for these stakeholders.
In order to avoid one of the main limitations of our study, namely the recall bias, follow-up studies could focus on a prospective cohort approach, for instance the Lareb Intensive Monitoring system. In this system, patients receive a questionnaire directly after the start of a new drug, followed by some follow-up questionnaires [34]. Using this method, you are able to ask patients about their HR-QOL directly after the event occurred.
Conclusion
Patients who reported possible ADRs after the Thyrax® packaging change experienced a significant decrease in HR-QOL. This impact on HR-QOL was the highest for the domains ‘daily activities’, ‘overall health status’, and ‘mental health’ and the lowest for ‘physical fitness’. Only the severity of the ADR was found to be correlated with all domains of HR-QOL.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with Ethical Standards
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Leàn Rolfes, Florence van Hunsel, Katja Taxis, and Eugene van Puijenbroek declare that they have no conflicts of interest that are directly relevant to the content of this study.
References
- 1.Centers for Disease Control and Prevention. Measuring healthy days: population assessment of health-related quality of life. Atlanta: Centers for Disease Control and Prevention; 2000. http://www.cdc.gov/hrqol/pdfs/mhd.pdf. Accessed 21 June 2015.
- 2.Waller PC, Lee EH. Responding to drug safety issues. Pharmacoepidemiol Drug Saf. 1999;8(7):535–552. doi: 10.1002/(SICI)1099-1557(199912)8:7<535::AID-PDS456>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Reporting adverse drug reactions: definitions of terms and criteria for their use. Geneva: Counsel for International Organizations of Medical Sciences (CIOMS); 1999. http://www.cioms.ch/publications/reporting_adverse_drug.pdf. Accessed 1 Aug 2015.
- 4.Carr AJ, Higginson IJ. Are quality of life measures patient centred? BMJ. 2001;322(7298):1357–1360. doi: 10.1136/bmj.322.7298.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baiardini I, Gaeta F, Molinengo G, Braido F, Canonica GW, Romano A. Quality-of-life issues in survivors to anaphylactic reactions to drugs. Allergy. 2015;70(7):877–879. doi: 10.1111/all.12610. [DOI] [PubMed] [Google Scholar]
- 6.Lorimer S, Cox A, Langford NJ. A patient’s perspective: the impact of adverse drug reactions on patients and their views on reporting. J Clin Pharm Ther. 2012;37(2):148–152. doi: 10.1111/j.1365-2710.2011.01258.x. [DOI] [PubMed] [Google Scholar]
- 7.Guo N, Marra F, Fitzgerald JM, Elwood RK, Marra CA. Impact of adverse drug reaction and predictivity of quality of life status in tuberculosis. Eur Respir J. 2010;36(1):206–208. doi: 10.1183/09031936.00159409. [DOI] [PubMed] [Google Scholar]
- 8.van Hunsel F, van der Welle C, Passier A, van Puijenbroek E, van Grootheest K. Motives for reporting adverse drug reactions by patient-reporters in the Netherlands. Eur J Clin Pharmacol. 2010;66(11):1143–1150. doi: 10.1007/s00228-010-0865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netherlands Pharmacovigilance Centre Lareb. Adverse drug reaction after packaging changes of Thyrax® [in Dutch]. 2015. http://www.lareb.nl/getmedia/e064e573-2776-402b-8661-f0c80d926a60/BCL_Rapport_Thyrax_juli_2015.pdf. Accessed 3 July 2015.
- 10.Lareb database. Thyrax (levothyroxine). 2016. http://databank.lareb.nl/Bijwerkingen/Result?formGroup=Tablet&atc=H03AA01&lang=en&drug=THYRAX%20%28LEVOTHYROXINE%29. Accessed 9 Feb 2016.
- 11.Official productinformation. Thyrax® (levothryoxine) [in Dutch]. 2014. http://db.cbg-meb.nl/Bijsluiters/h08389.pdf. Accessed 16 June 2015.
- 12.Medicines Evaluation Board. MEB assessment study quality Thyrax [in Dutch]. 2015. http://www.cbg-meb.nl/actueel/nieuws/2015/07/30/cbg-beoordeling-onderzoek-kwaliteit-thyrax. Accessed 30 July 2015.
- 13.Netherlands Pharmacovigilance Centre Lareb. Overview of reports of adverse drug reactions associated with changes of the package of Thyrax® (levothyroxine) from a bottle to a blister. 2015. http://www.lareb.nl/Signalen/KWB_2014_4_Thyrax_bottle_2. Accessed 16 June 2015.
- 14.avotros. Television broadcoast Adverse Drug Reactions of the drug Thyrax (RADAR: Bijwerkingen schildkliermedicijn Thyrax) [in Dutch]. 2015. http://www.radartv.nl/uitzending/archief/detail/aflevering/16-02-2015/bijwerkingen-schildkliermedicijn-thyrax/. Accessed 16 June 2015.
- 15.Faasse K, Cundy T, Petrie KJ. Medicine and the media. Thyroxine: anatomy of a health scare. BMJ. 2009;339:b5613. doi: 10.1136/bmj.b5613. [DOI] [PubMed] [Google Scholar]
- 16.Faasse K, Gamble G, Cundy T, Petrie KJ. Impact of television coverage on the number and type of symptoms reported during a health scare: a retrospective pre-post observational study. BMJ Open. 2012;2(4). doi:10.1136/bmjopen-2012-001607. [DOI] [PMC free article] [PubMed]
- 17.Rolfes L, van Hunsel F, van Grootheest K, van Puijenbroek E. Feedback for patients reporting adverse drug reactions; satisfaction and expectations. Expert Opin Drug Saf. 2015;14(5):625–632. doi: 10.1517/14740338.2015.1021775. [DOI] [PubMed] [Google Scholar]
- 18.Harmark L, van HF, Grundmark B. ADR reporting by the general public: lessons learnt from the Dutch and Swedish systems. Drug Saf. 2015;38(4):337–347. doi: 10.1007/s40264-015-0264-1. [DOI] [PubMed] [Google Scholar]
- 19.van Weel C, König-Zahn C, Touw-Otten FWMM, et al. Measuring functional status with the COOP/WONCA charts: a manual. 2012. http://www.gezondheidsvaardigheden.nl/wordpress/wp-content/uploads/2012/10/Handleiding_coopwonca2edruk.pdf. Accessed 18 Feb 2016.
- 20.Survey Monkey. 2015. http://www.surveymonkey.com. Accessed 1 Feb 2015.
- 21.The Central Commitee on Research Involving Human Subjects (CCMO). Guideline CCMO. 2012. http://www.ccmo.nl/. Accessed 12 Sep 2015.
- 22.Slotboom A. Statistics in words. 2. Groningen: Wolters-Noordhoff bv Groningen; 1996. p. 11. [Google Scholar]
- 23.Hoos A, Anderson J, Boutin M, Dewulf L, Geissler J, Johnston G, et al. Partnering with patients in the development and lifecycle of medicines: a call for action. Ther Innov Regul Sci. 2015;49(6):929–939. doi: 10.1177/2168479015580384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Hunsel F, Passier A, van Grootheest AC. Comparing patients’ and healthcare professionals’ ADR reports after media attention. The broadcast of a Dutch television programma about the benefits and risks of statins as an example. Br J Clin Pharmacol. 2009;67(5):558–564. doi: 10.1111/j.1365-2125.2009.03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avery AJ, Anderson C, Bond CM, Fortnum H, Gifford A, Hannaford PC, et al. Evaluation of patient reporting of adverse drug reactions to the UK ‘Yellow Card Scheme’: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess. 2011;15(20):1–iv. [DOI] [PubMed]
- 26.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395–407. doi: 10.1016/S0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 27.Wu YP, Follansbee-Junger K, Rausch J, Modi A. Parent and family stress factors predict health-related quality in pediatric patients with new-onset epilepsy. Epilepsia. 2014;55(6):866–877. doi: 10.1111/epi.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis KM, Kelly SP, Luta G, Tomko C, Miller AB, Taylor KL. The association of long-term treatment-related side effects with cancer-specific and general quality of life among prostate cancer survivors. Urology. 2014;84(2):300–306. doi: 10.1016/j.urology.2014.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ernst ME, Iyer SS, Doucette WR. Drug-related problems and quality of life in arthritis and low back pain sufferers. Value Health. 2003;6(1):51–58. doi: 10.1046/j.1524-4733.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 30.Blome C, Augustin M. Measuring change in quality of life: bias in prospective and retrospective evaluation. Value Health. 2015;18(1):110–115. doi: 10.1016/j.jval.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Dutch National Institute for Health and Environment. Dutch national puplic health compass. 2016. http://www.nationaalkompas.nl/algemeen/menu-rechts/english/. Accessed 8 Jan 2016.
- 32.Rolfes L, Wilkes S, van Hunsel F, van Puijenbroek E, van Grootheest K. Important information regarding reporting of adverse drug reactions: a qualitative study. Int J Pharm Pract. 2014;22(3):231–233. doi: 10.1111/ijpp.12056. [DOI] [PubMed] [Google Scholar]
- 33.Rolfes L, van Hunsel F, Wilkes S, van Grootheest K, van Puijenbroek E. Adverse drug reaction reports of patients and healthcare professionals-differences in reported information. Pharmacoepidemiol Drug Saf. 2015;24(2):152–158. doi: 10.1002/pds.3687. [DOI] [PubMed] [Google Scholar]
- 34.Härmark L, van Grootheest K. Web-based intensive monitoring: from passive to active drug surveillance. Expert Opin Drug Saf. 2012;11(1):45–51. doi: 10.1517/14740338.2012.629184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.