Abstract
BACKGROUND
Saphenous vein graft aneurysms (SVGAs) are rare seen issues after coronary artery bypass graft (CABG) operation which may lead to major complications including compression of adjacent structure, myocardial ischemia, rupture, and even death.
CASE REPORT
We report a patient with recurrent SVGA and its treatment by percutaneous intervention with a covered stent, the diagnostic and treatment procedure were based on contrast enhanced computed tomography and myocardial perfusion scintigraphy (MPS).
CONCLUSION
Multimodality imaging is required to demonstrate the true size and complications of the SVGA, the relationship among the adjacent structure, and to assess ischemia and size of myocardial territory supplied by the aneurysmal graft to decide treatment strategy.
Keywords: Coronary Aneurysm, Saphenous Vein, Stents, Computed Tomography, Coronary Artery Bypass Grafting
Introduction
Saphenous vein graft aneurysms (SVGAs) occur as a seldom complication of coronary artery bypass graft (CABG) with the likelihood of important consequences such as mortality.1 Diagnose with coronary artery angiography is usually definitive, but the true dimensions of the aneurysm can be obscured if a mural thrombus exists. We report a patient with recurrent SVGA treated with covered stent percutaneously; diagnose was based on myocardial perfusion scintigraphy (MPS) and contrast-enhanced computed tomography (CT).
Case Report
A 78-year-old man who had CABG operation 6 years ago admitted to our outpatient clinic with stable angina pectoris. In his history, he has had hypertension for 20 years, and abdominal aortic aneurysm (AAA) was diagnosed 4 years ago. A coronary angiography was performed while the intervention to AAA was planning; because a severe stenosis and multiple true aneurysmal dilatations (SVGAs) were detected in the aortosaphenous vein graft to the circumflex artery (Figure 1, A), polytetrafluoroethylene (PTFE)-covered stent was implanted to the graft (Figure 1, B).
Figure 1.

Coronary angiography, (A) Multiple aneurysms and stenosis on the saphenous graft vein to marginal branch of circumflex artery, (B) After polytetrafluoroethylene (PTFE)-covered stent implantation, no flow into aneurysmal graft was seen.
After a symptom-free interval of 4 years, he admitted with stable angina pectoris. During examination, chest radiography of the patient revealed an anterior mediastinal mass (Figure 2, A and B). Inferolateral wall hypokinesia with ejection fraction 45% and mild aortic insuffıciency were seen by transthoracic echocardiography. CT was performed and demonstrated a detailed anatomy of saphenous true aneurysm which is 5.9 × 4.6 cm in size including mural thrombus, especially the neck of the aneurysm and accompanying significant stenosis beyond the aneurysm (Figure 2, C and D). The newly occurred flow into aneurysmal dilatation at the distal edge of the covered stent was confirmed with coronary angiography (Figure 3, A). The MPS revealed ischemic perfusion defects on inferolateral wall. Because of the high mortality rate and Society of Thoracic Surgery score (20), treatment with the implementation of a PTFE-covered stent by percutaneous intervention again was decided. All the aneurysmal enlargements in the graft including significant stenosis were covered with the PTFE-covered stent (4.8 × 26 mm, GraftMaster, Abbott Vascular, CA, USA) deployment based on the images in the CT (Figure 3, B). CT was repeated for surveillance 6 months after the intervention. There was no more flow into the aneurysm or progressive aneurysmal dilatation, and the PTFE-covered stent was patent (Figure 3, C).
Figure 2.

Chest radiography, (A) Mediastinal mass mimicking prominence of aortic arch (posterior-anterior), (B) the anterior mediastina above the heart (left lateral), (C) contrast enhanced computed tomography (CT) confirmed true saphenous vein graft aneurysm (5.9 × 4.6 cm) with mural thrombus and significant stenosis immediately at the end of aneurysm, (D) CT showed that aneurysm is between the pulmonary artery and sternum.
Figure 3.
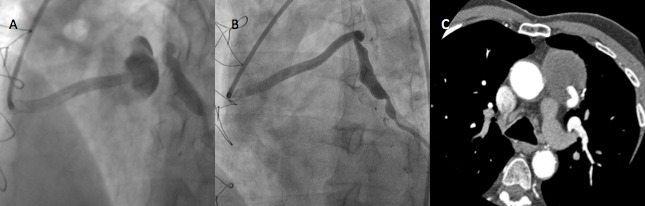
(A) At that time coronary angiography depicted flow into aneurysmal graft on the edge of previous covered stent, (B) after successful polytetrafluoroethylene (PTFE)-covered stent deployment including aneurysm and stenosis, (C) 6 months after latest intervention, contrast enhanced computed tomography showed patency of covered stent and no flow into aneurysmal graft
Discussion
Aneurysms are generally defined as a local enlargement of the vessels 1.5 times greater than the proximal reference diameter.1 SVGA is a rare complication of CABG and can be classified into different groups such as early versus late or true versus pseudoaneurysm. Late aneurysm formation, more than 5 years after CABG may occurs secondary to atherosclerotic degeneration1 and exposing high arterial pressure of the thin vein vessel wall. Early aneurysms < 12 months after surgery can occur secondary to several reasons caused by different pathophysiologies. Infection of the implanted graft,2 intrinsic weakness of the venous wall (i.e., undetected varicosities)3 and technical factors relating to conduit harvesting, preparation, and grafting, including conduit injury with or without dissection,4,5 anastomotic suture disruption,6 and failure to reverse the SVG at the time of grafting7 have been implicated in the formation of early SVGAs.
However, true graft aneurysms should be differentiated from pseudoaneurysm. Pseudoaneurysms represent a dilatation of the graft with disruption of one or more layers of its wall rather than with expansion of all layers of the wall.8 Furthermore, pseudoaneurysm are not endothelia-lined, and they represent focal distension with a hematoma. They usually occur at the proximal and distal ends of the graft.1 True aneurysms mainly involve the body of the graft and occur less common than pseudoaneurysms.
Most aneurysms are usually asymptomatic, incidentally determined, presenting as mediastinal masses on chest radiography or thoracic CT performed for unrelated causes. Symptomatic aneurysms constitute a diagnostic challenge as they may present in a variety of presumably unrelated symptoms, such as an acute coronary event or congestive heart failure.1 SVGA may lead to major complications including compression of native coronary vessels, distal embolization, myocardial ischemia and infarction, compression of the right atrium, or fistula formation and rupture into the right atrium.1 Diagnostic modalities include chest X-ray, echocardiography, thorax CT (contrast enhanced), magnetic resonance imaging angiography, and conventional angiography.7 Although coronary artery angiography is generally definitive in confirming the graft dilatation, mural thrombus can sometimes obscure the aneurysm’s true dimensions. Therefore, in our case, we planned the treatment strategy with the data obtained from both angiography and CT because of highly definitive power of CT.
The goals of treatment are to reduce the risk of complications such as rupture, mass effect, thromboembolism, and myocardial ischemia. Surgical repair may be undertaken in patients those whom are elderly and possess multiple comorbidities, favoring percutaneous approaches include covered stent grafting,9,10 coil embolization,11 vascular plug insertion,12 and alcohol injection to the graft.13
A major advantage of covered stent is the protection of distal flow patency.1 Interventions utilizing coil embolization, vascular plug insertion, or alcohol injection are reported in limited cases where graft occlusion is an acceptable outcome.
SVGAs are rare complications and cause grim consequences. The origin of this condition is unclear, but there are many possible explanations, suggest atherosclerotic process and relevant risk factors. Diagnostic approach to SVGA should include multiple imaging modalities.11 Multimodality imaging is required to demonstrate the true size and complications of the SVGA, the relationship among the adjacent structure, and to assess ischemia and size of myocardial territory supplied by the aneurysmal graft to decide treatment strategy. MPS also determines strategy about whether saphenous flow patency is important or not for myocardial territory because of showing severity of ischemia and indication of treatment. If myocardial territory supplied by saphenous graft was dispensable, it could occlude with coil or vascular plug from proximal and distal tip.
In this case, we present and discuss about saphenous graft true aneurysm; underlying mechanisms; its consequences, diagnostic challenges and its treatment options, in these cases like our, percutaneous treatment modalities should be kept in mind. Furthermore, the recurrent pattern of aneurysmal formation should be taken into consideration and should follow these patients.
Acknowledgments
None.
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Ramirez FD, Hibbert B, Simard T, Pourdjabbar A, Wilson KR, Hibbert R, et al. Natural history and management of aortocoronary saphenous vein graft aneurysms: a systematic review of published cases. Circulation. 2012;126(18):2248–56. doi: 10.1161/CIRCULATIONAHA.112.101592. [DOI] [PubMed] [Google Scholar]
- 2.Douglas BP, Bulkley BH, Hutchins GM. Infected saphenous vein coronary artery bypass graft with mycotic aneurysm. Fatal dehiscence of the proximal anastomosis. Chest. 1979;75(1):76–7. doi: 10.1378/chest.75.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Ennis BM, Zientek DM, Ruggie NT, Billhardt RA, Klein LW. Characterization of a saphenous vein graft aneurysm by intravascular ultrasound and computerized three-dimensional reconstruction. Cathet Cardiovasc Diagn. 1993;28(4):328–31. doi: 10.1002/ccd.1810280411. [DOI] [PubMed] [Google Scholar]
- 4.Kallis P, Keogh BE, Davies MJ. Pseudoaneurysm of aortocoronary vein graft secondary to late venous rupture: case report and literature review. Br Heart J. 1993;70(2):189–92. doi: 10.1136/hrt.70.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werthman PE, Sutter FP, Flicker S, Goldman SM. Spontaneous, late rupture of an aortocoronary saphenous vein graft. Ann Thorac Surg. 1991;51(4):664–6. doi: 10.1016/0003-4975(91)90335-n. [DOI] [PubMed] [Google Scholar]
- 6.de Haan HP, Huysmans HA, Weeda HW, Bosker HA, Buis B. Anastomotic pseudoaneurysm after aorto-coronary bypass grafting. Thorac Cardiovasc Surg. 1985;33(1):55–6. doi: 10.1055/s-2007-1014086. [DOI] [PubMed] [Google Scholar]
- 7.Benchimol A, Harris CL, Desser KB, Fleming H. Aneurysms of an aorto-coronary artery saphenous vein bypass graft--a case report. Vasc Surg. 1975;9(4):261–4. doi: 10.1177/153857447500900410. [DOI] [PubMed] [Google Scholar]
- 8.Stedman TL. Stedman's medical dictionary. 26th. Philadelphia, PA: Williams & Wilkins; 1995. p. p. 1450.. [Google Scholar]
- 9.Bhindi R, Newton J, Westaby S, Wilson N, Ormerod OJ, Uberoi R. Stent-graft repair of coronary vein graft aneurysm. J Vasc Interv Radiol. 2009;20(5):649–51. doi: 10.1016/j.jvir.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Rahim SA, Pitta SR, Rihal CS. Saphenous vein graft pseudoaneurysm. J Am Coll Cardiol. 2009;53(20):1918. doi: 10.1016/j.jacc.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 11.Maroo A, Rasmussen PA, Masaryk TJ, Ellis SG, Lincoff AM, Kapadia S. Stent-assisted detachable coil embolization of pseudoaneurysms in the coronary circulation. Catheter Cardiovasc Interv. 2006;68(3):409–15. doi: 10.1002/ccd.20879. [DOI] [PubMed] [Google Scholar]
- 12.Mylonas I, Sakata Y, Salinger MH, Feldman T. Successful closure of a giant true saphenous vein graft aneurysm using the Amplatzer vascular plug. Catheter Cardiovasc Interv. 2006;67(4):611–6. doi: 10.1002/ccd.20639. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenberg B, Rutledge J, Welsh RC. Occlusion of a large expanding saphenous vein bypass graft aneurysm with percutaneously injected ethylene-vinyl alcohol copolymer. JACC Cardiovasc Interv. 2010;3(10):1089–90. doi: 10.1016/j.jcin.2010.05.019. [DOI] [PubMed] [Google Scholar]


