Abstract
Aim:
Duchenne muscular dystrophy (DMD) is an X-linked genetic muscular disorder with no effective treatment at present. Mesenchymal stem cell (MSC) transplantation has been used to treat DMD, but the efficiency is low. Our previous studies show that activation of Wnt3a signaling promotes myogenic differentiation of MSCs in vitro. Here we report an effective MSC transplantation therapy in mdx mice by activation of Wnt3a signaling.
Methods:
MSCs were isolated from mouse bone marrow, and pretreated with Wnt3a-conditioned medium (Wnt3a-CM), then transplanted into mdx mice. The recipient mice were euthanized at 4, 8, 12, 16 weeks after the transplantation, and muscle pathological changes were examined. The expression of dystrophin in muscle was detected using immunofluorescence staining, RT-PCR and Western blotting.
Results:
Sixteen weeks later, transplantation of Wnt3a-pretreated MSCs in mdx mice improved the characteristics of dystrophic muscles evidenced by significant reductions in centrally nucleated myofibers, the variability range of cross-sectional area (CSA) and the connective tissue area of myofibers. Furthermore, transplantation of Wnt3a-pretreated MSCs in mdx mice gradually and markedly increased the expression of dystrophin in muscle, and improved the efficiency of myogenic differentiation.
Conclusion:
Transplantation of Wnt3a-pretreated MSCs in mdx mice results in long-term amelioration of the dystrophic phenotype and restores dystrophin expression in muscle. The results suggest that Wnt3a may be a promising candidate for the treatment of DMD.
Keywords: Wnt3a, stem cell transplantation, mesenchymal stem cells, mdx mice, dystrophin, Duchenne muscular dystrophy
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked genetic disorder that affects 1 in 3600–6000 live male births and is characterized by muscle fiber wasting, functional impairment and eventual death because of respiratory and heart failure in the second/third decade of the patient's life1. Although various therapeutic approaches have been attempted, there is no successful treatment at present2. Stem cell transplantation therapy provides a potential strategy for DMD3. Multiple stem cell populations, such as embryonic stem cells and induced pluripotent stem cells (iPS), have been tested for their capacity to treat muscular dystrophy3,4.
Mesenchymal stem cells (MSCs), which can be distinguished from hematopoietic cells on the basis of their adherence to the plastic walls of tissue culture containers, can form skeletal muscle cells, osteoblasts, chondrocytes, and adipocytes under suitable culture conditions5. MSCs can also be easily obtained from various sources and propagated manifold ex vivo in clinically relevant numbers. These features make MSCs an attractive candidate for tissue repair and gene therapy6,7.
Other groups' and our group's previous work have revealed that transplanted MSCs contribute to muscle cells and restore the sarcolemmal expression of dystrophin in models of DMD8,9,10,11,12. However, the process is rather inefficient on the basis of comparison of the number of cells transplanted with the number of formed muscle cells. A key to improve the current protocols lies in exploiting the molecular mechanisms that govern the distinct steps of myogenic differentiation in MSCs.
The Wnt signaling pathway is associated with myogenesis in embryogenesis and postnatal muscle regeneration. During embryonic development, Wnt1 and Wnt3a, which are expressed in the dorsal neural tube, and Wnt7a, which is expressed in the surface ectoderm, have been shown to activate the expression of myogenic regulatory factor genes in the paraxial mesoderm13,14. Defects in myogenesis are also present in Wnt1/Wnt3a double-knockout mouse embryos15. In postnatal muscle regeneration, Wnt proteins induce myogenic differentiation in CD45+ and AC133+ stem cells16,17. These data demonstrate the important roles of Wnt signaling in the regulation of myogenic differentiation in embryogenesis and muscle regeneration.
Our previous studies have shown that Wnt3a induces myogenic differentiation in bone marrow MSCs via the ectopic expression of activated beta-catenin or pretreatment with Wnt3a conditioned medium in vitro18,19. In the current study, we hypothesized that Wnt3a would improve the myogenic differentiation efficiency of transplanted MSCs and repair the muscle damage in vivo. To test the hypothesis, we used Wnt3a-conditioned medium to treat MSCs and transplanted the pretreated cells into mdx mice. The results demonstrated that Wnt3a-MSCs increased dystrophin expression and led to a significant morphological amelioration of the dystrophic phenotype of mdx mice. These data revealed that the transplantation of Wnt3a-MSCs may represent a potential therapeutic application for primary myopathies.
Materials and methods
Animals and MSC preparation
Mdx mice and their parental strain C57/BL10 were originally purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The colonies were subsequently established by in-house breeding at the Laboratory Animal Center of Sun Yat-sen University. The mice (including the experimental mice and breeding pairs) were housed in a specific pathogen-free animal facility. Adult male Sprague-Dawley rats (weighing 60–80 g) were obtained from the Laboratory Animal Center of Sun Yat-sen University. The primary MSCs were isolated from Sprague-Dawley rats according to the method described by Wakitani et al, with some modifications20. Briefly, the femora and tibiae of male Sprague-Dawley rats were collected; the adherent soft tissues were then carefully removed to ensure that the marrow preparations were not contaminated by myogenic precursors. Both ends of the bones were cut with bone scissors. Bone marrow plugs were hydrostatically expelled from the bones by using syringes filled with growth medium. The cells were centrifuged and resuspended twice in the growth medium. The cells were then introduced into 25-cm2 tissue culture flasks in 6 mL of growth medium. Three days later, the medium was changed, and the nonadherent cells were discarded. The medium was completely replaced every 3 d. Approximately 7–10 d after seeding, the culture flasks became nearly confluent, and the adherent cells were released from the dishes with 0.25% trypsin (Gibco Laboratories, Grand Island, NY, USA), split 1:2 or 1:3, and seeded into fresh culture flasks. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco Laboratories) and 10% fetal calf serum (FCS; Hyclone, Logan, UT, USA). Every 3 d, the medium was changed once. The cells were grown at 37 °C in a humidified atmosphere with 5% CO2. All of the animal experiments were approved by the Animal Care and Experimentation Committee of Sun Yat-sen University (Guangzhou, China).
Preparation of Wnt3a-conditioned medium
The mouse L cells were obtained from the American Type Culture Collection (CRL-2648; Manassas, VA, USA). The cells were cultured in DMEM supplemented with 10% FCS at 37 °C. pGKWnt3a and pGKneo (control) plasmids were generously provided by Prof Shinji TAKADA (Okazaki Institute for Integrative Biosciences, National Institutes of Natural Sciences, Okazaki, Aichi, Japan). The Wnt3a-conditioned medium (Wnt3a-CM) and control L-conditioned medium (L-CM) were prepared as described previously19,21. Briefly, the L cells were transfected with pGKWnt3a or pGKneo plasmids with Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). The cells were selected with G418 (0.4 g/L; Sigma, St Louis, MO, USA) for 3 weeks, and stably transfected clones were then selected. The L cells transfected with pGKWnt3a or pGKneo plasmids were termed L-Wnt3a or control L-cells, respectively. Next, 1×106L-Wnt3a or L cells were seeded in 100-mm dishes containing DMEM with 10% FCS. After 3 d of culture, the cells were cultured with fresh medium and incubated for one additional day. The medium was then collected, centrifuged at 1000×g for 10 min, and filtered through a nitrocellulose membrane. The abundance of the Wnt3a protein in Wnt3a-CM and L-CM was detected by Western blot analysis19. Wnt3a-CM was depleted of the Wnt3a protein by incubation with 4 μg/mL rabbit anti-Wnt3a antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4 °C overnight. The conditioned medium was termed Wnt3a-depleted-conditioned medium (Wnt3a depl-CM) and was used to perform antibody-blocking experiments. The conditioned medium was stored at -80 °C for further experiments.
Multilineage differentiation of Wnt3a-treated MSCs
To induce differentiation, passage 5 cells were plated at a density of 5×104 cells/mL in 6-well tissue culture plates and were allowed to adhere for 24 h at 37 °C. MSCs were induced to differentiate into myogenic, osteogenic, and adipogenic as described previously18,19. The percentage of MHC-positive cells was calculated as the ratio of positive cells to the total cells counted. Six random fields at 20-fold magnification were examined under the microscope at the indicated time points. The values represent the mean±SD.
Wnt3a-CM pretreated MSC transplantation
The female recipient mdx mice (6–8 weeks old) were preconditioned with 7 Gy of irradiation 3 d before MSC transplantation. Passage 5 MSCs were treated with 50% Wnt3a-CM or L-CM 72 h before transplantation. The cells pretreated with Wnt3a-CM or L-CM were termed Wnt3a-MSCs or L-MSCs, respectively. BrdU (10 μmol/L) was added to the medium 48 h before cell transplantation. Next, the cells were collected, counted and diluted with PBS, and 1.5×107 Wnt3a-MSCs or L-MSCs for per mdx mouse were transplanted through the tail vein. In the present study, 72 recipient mdx mice were divided into 3 groups (24 mice/group): the Wnt3a-MSCs group, L-MSCs group and PBS group. Moreover, 24 C57/BL10 mice were used as normal controls.
Histopathological analysis
The recipient mice were euthanized with an overdose of pentobarbital at 4, 8, 12, and 16 weeks after MSC transplantation. The partial left gastrocnemius muscles were homogenized in TRIzol (Invitrogen Technologies) for total RNA extraction, and the other part of the muscles were collected for Western blot analysis; the right gastrocnemius muscles were snap-frozen in cold isopentane for later processing. Five-micrometer-thick cross sections of the right gastrocnemius muscle of mdx mice were obtained as above. The sections were stained with hematoxylin and eosin (H&E; Sigma, USA) according to the manufacturer's instructions and were analyzed by microscopy. The images were captured using a digital camera at 100× magnification. The cross sectional area (CSA) was measured using ImageJ software (NIH, USA) as previously reported4. Briefly, single transverse myofibers were encircled with the freehand selection, and the fiber area was measured using the measurement analysis tool. Ten randomly selected fields (100× magnification) per gastrocnemius muscle were used, and at least 1000 myofibers per group were analyzed. The presence of adipose and connective tissue was calculated by the total image area deprived of the myofiber area. The number of fibers and centrally nucleated fibers per randomly selected field (100× magnification) was counted using the ImageJ Cell Counter plugin on the same pictures utilized for CSA determination.
Immunofluorescence analysis
Double immunofluorescence analysis was performed as previously described22. Briefly, muscle cryosections were incubated with 2 mol/L HCl for 1 h and then rinsed three times in PBS; non-specific antibody binding was blocked with 10% normal goat serum (Boster Co, China) for 1 h at room temperature. The slides were incubated overnight at 4 °C with polyclonal anti-dystrophin antibody (1:100; Santa Cruz, USA) or polyclonal anti-MyoD antibody (1:200; Santa Cruz, USA), followed by a 1-h incubation with FITC-conjugated secondary antibody (1:100; Santa Cruz, USA). The slides were then rinsed and incubated overnight at 4 °C with monoclonal anti-BrdU antibody (1:100; Santa Cruz, USA) followed by incubation with Cy3-conjugated secondary antibody for 1 h at room temperature. After being rinsed in PBS, the slides were examined by fluorescence microscopy using an Olympus DP70 scope. For each slide, the percentage of dystrophin+/BrdU+ or MyoD+/BrdU+ myofibers was calculated based on the average number of myofibers counted in 10 randomly selected fields.
RT-PCR amplification of dystrophin
The total RNA of gastrocnemius muscle was prepared using a TRIzol kit (Invitrogen, USA). One microgram of total RNA was reverse transcribed using a RevertAid™ HMinus First Strand Synthesis Kit (Fermentas, CA, USA) for 60 min at 42 °C. Complementary DNA (cDNA) was heated at 70 °C for 10 min. The first-strand synthesized cDNA was used directly for polymerase chain reaction (PCR) amplification. Each PCR reaction was carried out in 25-mL mixtures. The primer sequences of rat dystrophin were 5′-CTCGTAGTCCTGCCCAGA-3′ and 5′-GTTTGACTGCCAACCACTCG-3′. To control for the amount of RNA in different samples, GAPDH expression was used, the primers of which were 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′. The amplification products were electrophoresed on 1.5% agarose gels and visualized by ethidium bromide staining followed by densitometric analysis of the bands using UV light illumination (Bio-Rad, Hercules, CA, USA).
Immunoblot detection of dystrophin
Gastrocnemius muscle protein extracts were prepared from snap-frozen tissue muscles, homogenized in extraction buffer (75 mmol/L Tris at pH 6.8, 3.8% SDS, 4 mol/L urea, 20% glycerol, 5% beta-mercaptoethanol), heated at 95.8 °C for 5 min and centrifuged at 48 °C for 15 min. Soluble muscle protein (20 mg) was electrophoretically separated in a 6% SDS-PAGE gel and transferred to a PVDF membrane. The primary antibody was the same as that used for immunohistochemistry. The secondary antibody was a horseradish peroxidase-conjugated goat anti-rabbit antibody (1:3000; Chemicon). The chemiluminescent signal was detected using ECL reagents (Santa Cruz, USA). Immunoblotting for GAPDH, using an anti-GAPDH antibody (Sigma, USA), was performed to control for protein loading. Densitometric analysis of the bands was performed using the digital images of exposed films (MCID; Imaging Research Inc, Canada).
Detection of creatine phosphokinase (CK) activity
After deep anesthesia, blood was collected by orbital sinus bleeding using heparinized hematocrit tubes. After centrifugation at 3000 rounds per minute for 5 min and then incubation for 30 min at room temperature, the plasma CK activity was detected using CK-NAC (Sekisui Medical Co, Ltd). If the measured values deviated significantly from the average due to hemolysis, the blood samples were collected again and re-measured.
Statistical analysis
Data analysis were performed using SPSS 14. All of the values were presented as the mean±SD. The significant differences among groups were determined by one-way ANOVA followed by Bonferroni post-test to determine which groups were statistically significant. A value of P<0.05 was taken as a statistically significant difference.
Results
Improvement of muscular histopathology in mdx mice receiving MSC transplantation
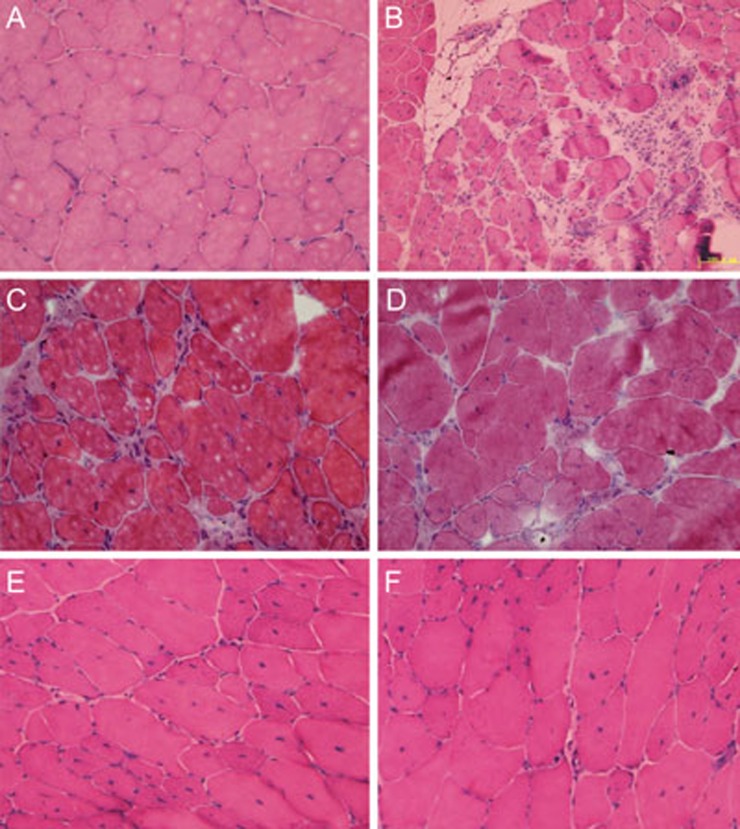
To investigate whether transplanted Wnt3a-MSCs would partly ameliorate the dystrophic phenotype of mdx, we performed histopathological evaluation of H&E-stained sections to evaluate sarcolemma damage and muscle degeneration. The muscles from control mdx mice showed characteristic signs of muscular dystrophy, including small regenerating fibers, centrally located nuclei, hypertrophic fibers, and hyperplastic connective tissue (Figure 1B). These characteristics of dystrophic muscles gradually improved in mice receiving Wnt3a-MSCtransplantation compared with control mdx mice (Figure 1D and 1F).
Figure 1.
Wnt3a-MSC transplantation ameliorated mdx muscle morphology. H&E staining of the gastrocnemius muscle from C57/BL10 (A), mdx (B), L-MSC (C, E) and Wnt3a-MSC (D, F) transplantation mice after 4 and 16 weeks transplantation. The characteristic signs of muscular dystrophy, including small regenerating fibers, centrally located nuclei, hypertrophic fibers, and hyperplastic connective tissue were present in mdx gastrocnemius muscle (B), but were completely absent from C57/BL10 mice (A). Wnt3a-MSC and L-MSC transplantation reduced these characteristics of dystrophic muscles (representative sections out of six mice examined in each group). Scale bar: 100 μm.
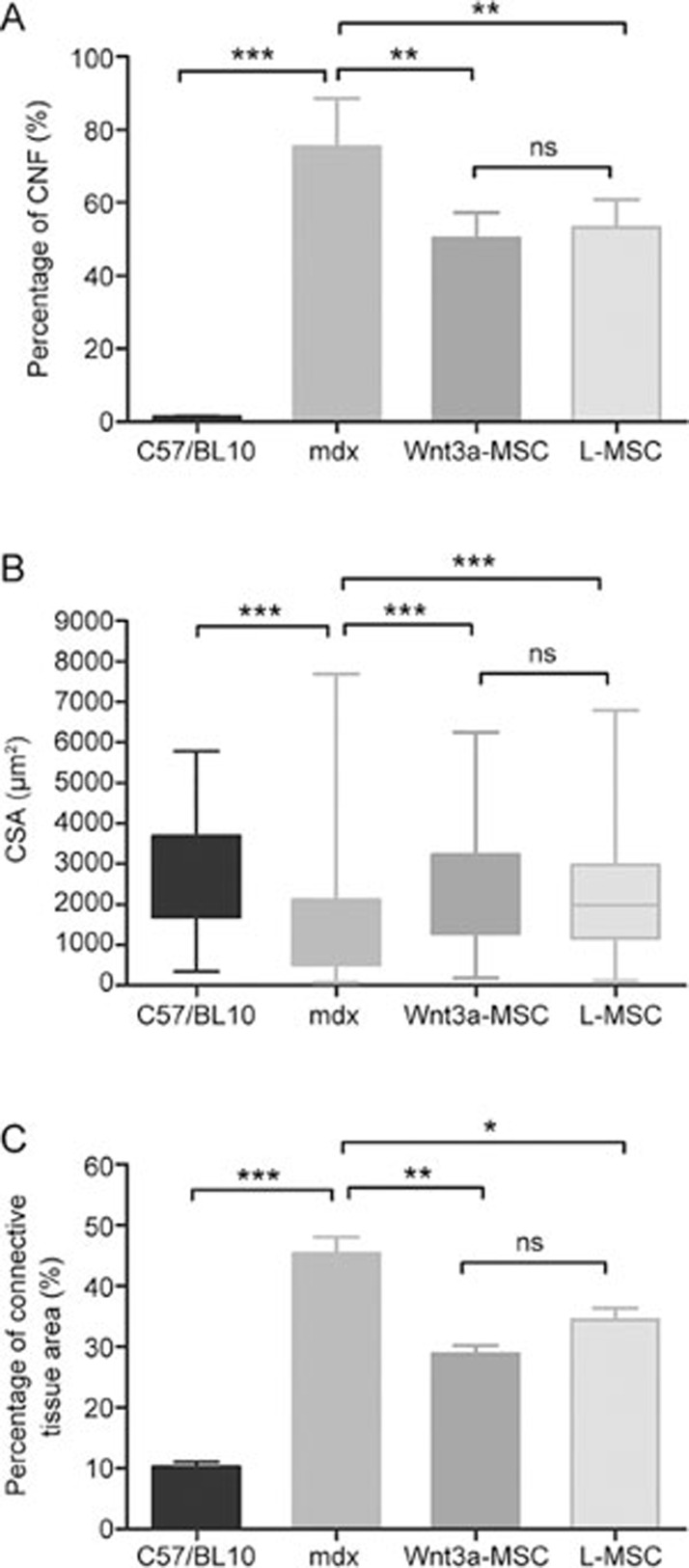
We also quantified the percentage of centrally nucleated myofibers (CNFs), fiber cross-sectional area (CSA), and percentage of the adipose and connective tissue area in muscle slices. The reduction of the percentage of CNFs in Wnt3a-MSC-transplanted mice was more obvious than that in L-MSC-transplanted mice (Figure 2A). Moreover, the measurement of myofiber CSA showed a partial normalization in Wnt3a-MSC-treated mice (Figure 2B). As shown in Figure 2B, both the variability range and extreme values of the CSA were significantly reduced in Wnt3a-MSC-engrafted mice compared with untreated mdx mice. Similarly, Wnt3a-MSC and L-MSC transplantations also led to the decrease of the adipose and connective tissue area over time (Figure 2C). In summary, these results demonstrated that the transplantation of Wnt3a-MSCs partly ameliorated the dystrophic phenotype of mdx mice compared with the L-MSC-transplanted group, although there was no statistically significant difference between the groups.
Figure 2.
Quantification of central nucleation (CNF), cross-sectional area (CSA), adipose, and connective tissue area on H&E stained sections of gastrocnemius muscle from C57/BL10, mdx, Wnt3a-MSC and L-MSC transplantation mice after 16 weeks transplantation. Six mice were analyzed in each group; at least 1000 myofibers per section were counted. All values were expressed as the mean±SD. Significance between the groups was assessed using one-way ANOVA followed by Bonferroni post test. nsP>0.05; *P<0.05; **P<0.01.
Restoration of the expression of dystrophin in MSC-transplanted mdx mouse muscle
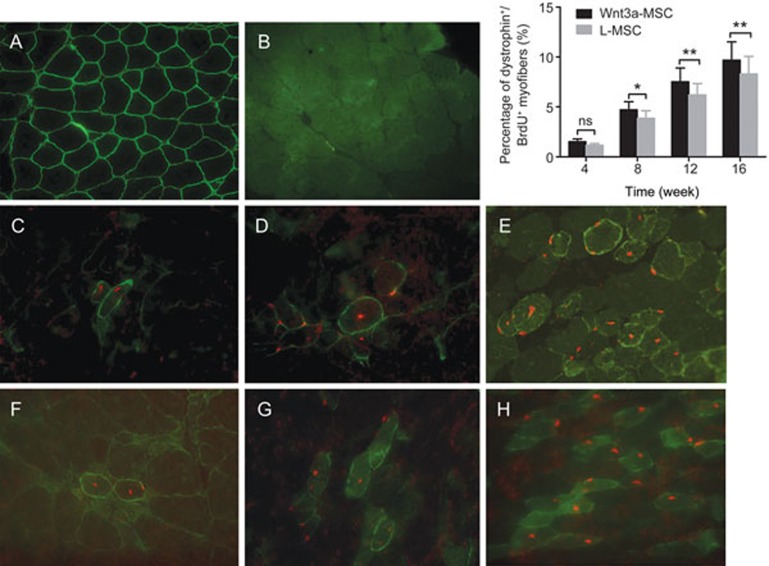
To compare the regenerative capacities of Wnt3a-MSCs and L-MSCs in vivo, the expression of dystrophin in host muscle fibers was evaluated by immunofluorescence after transplantation. The myofibers of C57/BL10 mice showed normal dystrophin immunostaining (Figure 3A). Less than 0.2% dystrophin+ fibers (revertant fibers) were present in non-grafted mdx muscles (Figure 3B). At 4 weeks after transplantation of Wnt3a-MSCs into mdx mice, 1.51%±0.29% of the gastrocnemius muscle fibers were dystrophin/BrdU positive (Figure 3F). The dystrophin/BrdU-positive myofibers increased gradually over time, reaching 4.71%±0.81% and 7.52%±1.37% at 8 and 12 weeks after transplantation, respectively (Figure 3G). The percentage of dystrophin/BrdU-positive myofibers was 9.68%±1.85% in Wnt3a-MSC-treated mice 16 weeks after engraftment (Figure 3H), whereas the percentage of dystrophin/BrdU-positive fibers was 8.31%±1.74% in L-MSC-transplanted mice (P<0.05, Figure 3E). The histograms in the uppon right corner of Figure 3 present quantification of dystrophin/BrdU-positive fibers in MSC-transplanted mdx mice showed that Wnt3a-MSCs had a significantly higher efficiency of engraftment than L-MSCs at 8, 12 and 16 weeks after transplantation (P<0.05).
Figure 3.
Dystrophin expression after MSCs transplantation. Immunofluorescence for Dystrophin (green)/BrdU (red) from the gastrocnemius muscle sections in C57/BL10 (A), mdx (B), L-MSC (C, D, E) and Wnt3a-MSC (F, G, H) transplantation mice after 4, 8, 16 weeks transplantation. Quantification of dystrophin/BrdU positive fibers in transplanted mdx mice showed that Wnt3a-MSCs had significantly higher efficiency of engraftment than L-MSCs after 8 weeks transplantation (P<0.05). All values were expressed as the mean±SD. Significance between the groups was assessed using one-way ANOVA followed by Bonferroni post test. nsP>0.05; *P<0.05; **P<0.01.
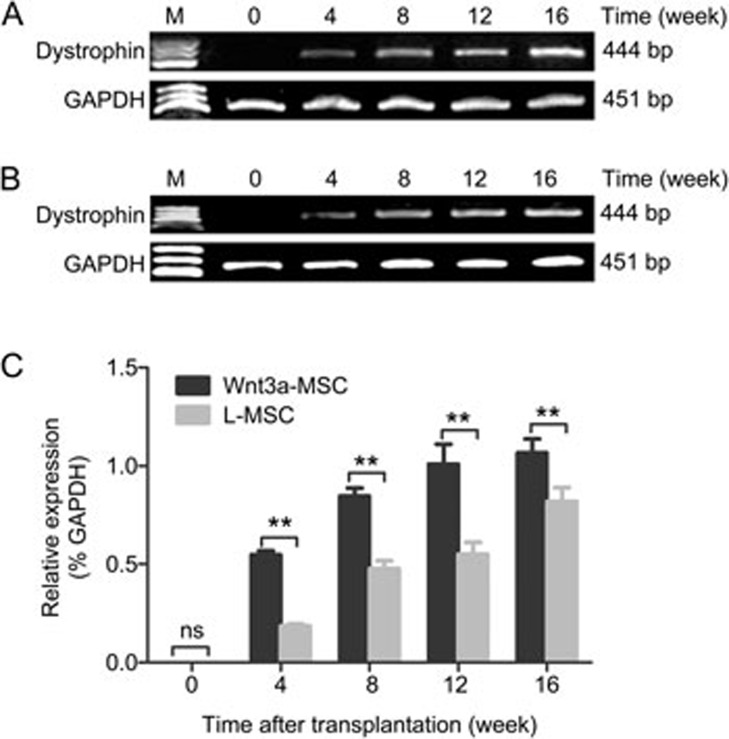
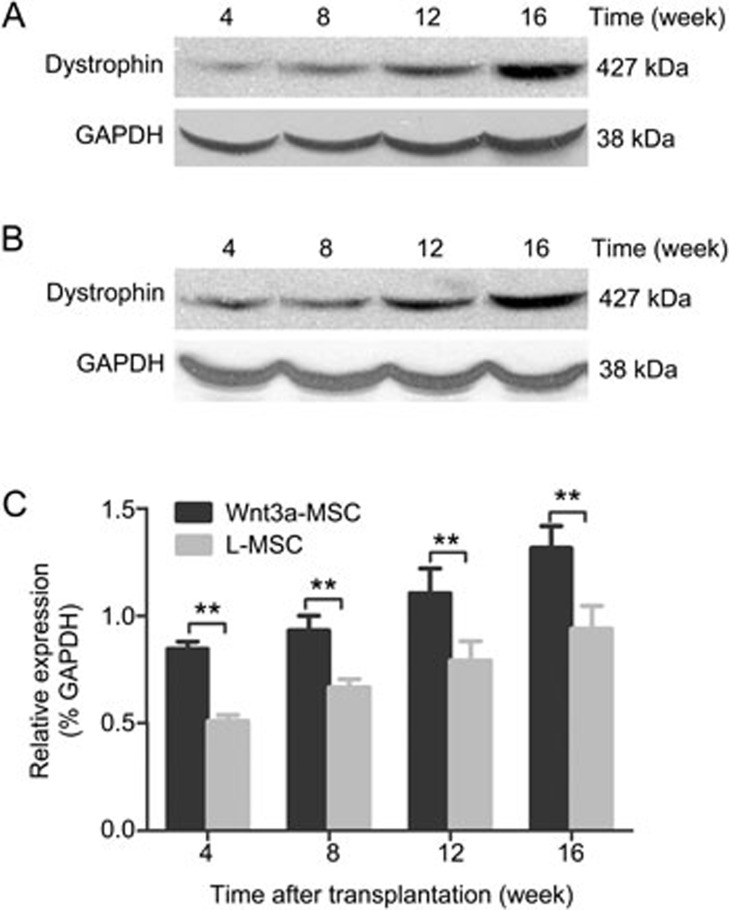
The amount of dystrophin mRNA expressed in MSC-transplanted mdx mice was quantified by RT-PCR. The results showed that dystrophin was present in the muscles of MSC-transplanted mdx mice (Figure 4A, 4B). Compared with mdx mice transplanted with L-MSCs, the expression of dystrophin mRNA after Wnt3a-MSC engraftment was higher at each time points after transplantation (Figure 4C). The expression increased over time and reached the maximum level at 16 weeks after transplantation in the present study. Furthermore, Western blot analysis was performed to confirm the presence of dystrophin in the MSC-transplanted mdx muscles. Compared with L-MSC-transplanted mice, the level of dystrophin protein in Wnt3a-MSC-transplanted mice was significantly higher than that in the L-MSC-transplanted group at each time point after transplantation (Figure 5). These results were consistent with the immunofluorescence results.
Figure 4.
RT-PCR analysis revealed the presence of dystrophin mRNA in the muscle samples collected at various time points after transplantation. The expression of dystrophin mRNA in Wnt3a-MSCs (A) engraftment was higher than that of L-MSCs (B) group at each time points after transplantation. All values were expressed as the mean±SD (C). Significance between the groups was assessed using one-way ANOVA followed by Bonferroni post test. nsP>0.05; *P<0.05; **P<0.01.
Figure 5.
Western blot detection of dystrophin expression at different time points after MSCs transplantation. The expression of dystrophin protein in Wnt3a-MSCs (A) engraftment was higher than that of L-MSCs (B) group at each time points after transplantation. All values were expressed as the mean±SD (C). Significance between the groups was assessed using one-way ANOVA followed by Bonferroni post test. **P<0.01.
These observations suggested that Wnt3a-MSCs contribute to regenerated fibers and that the engraftment of skeletal muscles is more efficient than that of L-MSCs.
Expression of MyoD in Wnt3a-MSCs after transplantation
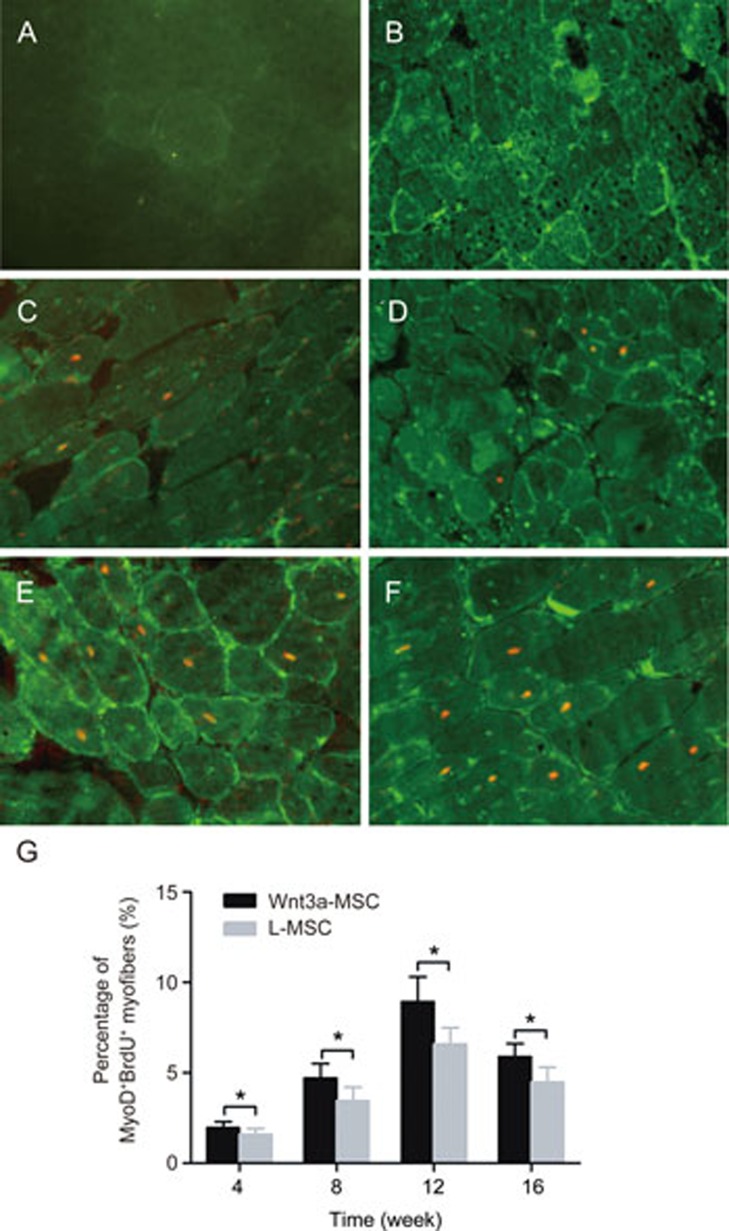
MyoD is a myogenic regulatory factor that plays an important role during myogenesis. Our previous study19 has demonstrated that Wnt3a induces the myogenic differentiation of rMSCs in vitro by triggering the expression of myogenic regulatory factors, including Pax7, MyoD, Myf5, Myf4 and myogenin. In the present study, we further examined the expression of MyoD in Wnt3a-MSCs by immunofluorescence after transplantation. No MyoD-positive myofibers were found in C57/BL10 mice (Figure 6A). Only very few cells expressing MyoD+ (<0.2%) fibers were present in non-grafted mdx mice (Figure 6B). The percentage of nuclear MyoD+/BrdU+ increased over time and peaked at 12 week after rMSC transplantation. The percentages of MyoD+/BrdU+ fibers were 1.96%±0.34%, 4.71%±0.79%, 8.93%±1.38% and 5.89%±0.73% at 4 week, 8 week, 12 week and 16 week, respectively, after transplantation in the Wnt3a-rMSC group (Figure 6D and 6F). The percentages of MyoD+/BrdU+ fibers were 1.58%±0.34%, 3.46%±0.73%, 6.58%±0.92% and 4.47%±0.85% at 4 week, 8 week, 12 week and 16 week, respectively, after transplantation in the L-CM pre-treated rMSC group (Figure 6C, 6E). The percentage of MyoD+/BrdU+ fibers was significantly higher in the Wnt3a-CM pre-treated rMSC group than in the L-CM pre-treated rMSC group at each time point (Figure 6G, P<0.05).
Figure 6.
MyoD expression after MSCs transplantation. Immunofluorescence for MyoD (green)/BrdU (red) from the gastrocnemius muscle sections in C57/BL10 (A), mdx (B), L-MSC (C, E) and Wnt3a-MSC (D, F) transplantation mice after 4 and 12 weeks transplantation. The percentage of MyoD+/BrdU+ fibers was significantly higher in Wnt3a-CM pre-treated rMSCs group than that of in L-CM pre-treated rMSCs group at each time point (G, P<0.05). All values were expressed as the mean±SD. Significance between the groups was assessed using one-way ANOVA followed by Bonferroni post test. *P<0.05.
Creatine kinase (CK) activity
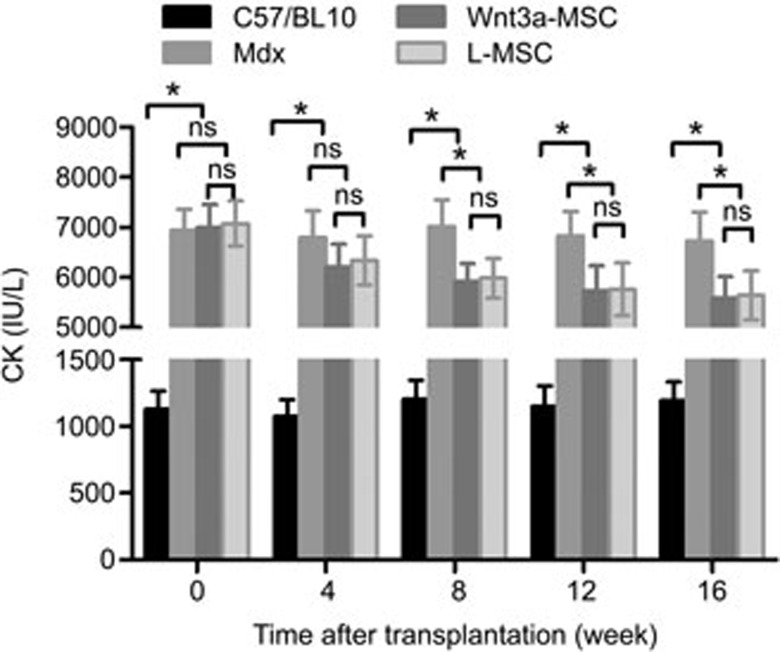
The extent to which Wnt3a-MSCs ameliorated the progression of the dystrophic phenotype was further supported biochemically by measuring the plasma CK activity. As shown in Figure 7, the plasma CK activity of untreated mdx mice was significantly higher than that of C57/BL10 mice (6942±421.8 IU/L vs 1129±138.3 IU/L, respectively; P<0.05). The levels of plasma CK in both MSC-transplanted mouse groups were significantly reduced after 8 weeks and reached the lowest level at 16 weeks after transplantation. Although the Wnt3a-MSC group had greater decreases in the CK level than did the L-MSC group, the groups showed no statistically significant differences at each time point (P>0.05).
Figure 7.
Change in CK after MSCs transplantation. The graph showed that plasma CK levels in control mdx mice were much higher than that in C57/BL10 mice. The Wnt3a-MSCs group had greater decreases in CK level compared with L-MSC group at each time points after transplantation.
Discussion
There is increasing evidence that the Wnt canonical pathway plays an important role in inducing embryonic and postnatal myogenesis. We have previously demonstrated that the activation of Wnt3a signaling promotes myogenic differentiation of bone marrow MSCs in vitro18,19. In the present study, we provide evidence for effective stem cell transplantation therapy by activation of Wnt3a signaling in dystrophic mice. The Wnt3a-pretreated MSCs retained their proliferation and multiplurity in a manner reminiscent of that of the parental population (Supplementary data). Wnt3a-MSC engraftment resulted in the amelioration of the dystrophic phenotype and restoration of the expression of dystrophin in mdx mice. The results demonstrated that Wnt3a signaling improved the regenerative capacities of MSCs and effectively repaired dystrophic muscle in primary myopathies.
MSCs can be easily obtained and differentiated into numerous mesenchymal tissue lineages. There has been much interest in these cells and their potential applications in cytotherapy and gene therapy6. Previous studies have shown that transplanted adult bone marrow cells migrate into the damaged muscle tissue and are involved in the regeneration process in vivo. Gussoni et al have documented that whole bone marrow cells have the potential to differentiate into muscle cells and express dystrophin protein in mdx mice23. They have also found that the transplanted stem cells can be converted into muscle satellite cells and are involved in the degeneration-regeneration process. Recently Fujita has found that the transplantation of Lin−/ckit−/CD106+/CD44+ MSCs successfully ameliorates muscle dysfunction by decreasing inflammation and enhancing muscle regeneration24. Our previous studies have also demonstrated that transplanted MSCs differentiate into muscle cells and express dystrophin protein in mdx mice9,22,25,26. However, the efficiency of stem cells differentiating into muscle cells could not meet the requirements for use in treatment.
Wnt signaling plays a crucial role in myogenesis in embryo development. Either Wnt1 or Wnt3a in combination with Sonic hedgehog has been shown to be sufficient for activating skeletal muscle-specific genes, such as MyoD and Myf5, in the paraxial mesoderm in coculture experiments. Wnts derived from axial structures (neural tube and notochord) preferentially activate myogenesis through a Myf5-dependent pathway, whereas Wnts derived from dorsal ectoderm preferentially activate myogenesis through a MyoD-dependent pathway. It also has been shown that the ectopic implantation of Wnt3a-expressing fibroblasts leads to increased Pax-3 expression in adjacent somites. These results support the notion that Wnt signals are involved in multiple developmental processes of progenitor cells in the developing embryo.
It has been demonstrated that the Wnt signaling pathway is necessary for postnatal myogenesis. The canonical Wnt pathway has been reported to be involved in later stages of muscle regeneration in wild-type mice27. Defective muscle regeneration, which is characterized by persistence in proliferating myoblasts and the formation of interstitial infiltrates instead of mature muscle fibers, has been observed by inhibiting Wnt activity27. Wnt molecules have been shown to activate the myogenic recruitment of CD45+ cells isolated from uninjured muscles16. Wnt signaling also induces the transcription of different skeletal muscle marker genes in muscle-derived SCA1 SP cells and circulating AC133 cells17. In previous studies, we have found that the ectopic expression of activated beta-catenin and pretreatment with Wnt3a-conditioned medium induces bone marrow MSCs to differentiate into myogenic cells18,19. These studies collectively demonstrate that Wnt signaling induces myogenesis in adult stem cells.
The Wnt signaling pathway plays an important role in regulating the degeneration and regeneration of myofibers in muscular dystrophy. Several previous studies have found that Wnt3a signaling is altered in mdx mice. By measuring the luciferase activity of the Wnt/β-catenin/T cell factor (TCF) pathway reporter constructs 8x TOPFlash, Trensz et al28 have found a 1.8-fold increase in TCF activity in the presence of serum from mdx mice vs in the presence of serum from wild-type mice. Western blot analyses have revealed that the β-catenin levels are 1.5-fold higher in dystrophic muscles than in control muscles. Biressi et al29 have shown that the distribution of increased Wnt signaling, as reflected by increased beta-galactosidase (b-Gal) activity, is not homogeneous but instead is patchy and have confirmed a similar patchy distribution of beta-catenin by immunofluorescence. Under normal circumstances, the soluble frizzled-related proteins family (SFRPs) are upregulated in the latter stages of muscle regeneration when myofibers are well formed16. Pescatori et al have found that FRZB1 and SFRP4 are progressively induced in the natural history of DMD30. The injection of sFRP2/3 into regenerating muscle markedly reduces CD45+ stem cell proliferation and myogenic specification in vivo16. One study has shown that the inhibition of MMP-9 significantly improves the engraftment of transplanted myoblasts in the skeletal muscles of mdx mice, by augmenting the expression of components of the canonical Wnt signaling and reducing the expression of genes of non-canonical Wnt signaling31. Other researchers have found that the level of expression of MiR-199a-5p is elevated in DMD dystrophic muscle, thus resulting in abnormal myofiber disruption and sarcolemmal membrane detachment through the suppression of Wnt-signaling factors32. Both the canonical Wnt signaling pathway and the noncanonical Wnt signaling pathway are involved in the proliferation and differentiation of muscle in vivo. Wnt7a treatment dramatically stimulates the symmetric expansion of satellite stem cells and results in impressive enhancement of the regeneration process during muscle regeneration. Wnt7a treatment also efficiently induces myofiber hypertrophy and a shift in the fiber type toward slow twitch in treated muscles in mdx mice and human primary myotubes33. These findings have revealed the essential role of Wnts in regenerative myogenesis in muscular dystrophy.
In conclusion, our results demonstrate that transplantation of Wnt3a-pretreated MSCs leads to the long-term amelioration of the dystrophic phenotype and the restoration of the expression of dystrophin in mdx mice. These findings make Wnt3a a promising candidate for development as an ameliorative treatment for DMD.
Author contribution
Yan-chang SHANG, Shu-hui WANG, and Cheng ZHANG designed the research; Yan-chang SHANG, Shu-hui WANG, and Fu XIONG performed the research; Fu-ning PENG and Zhen-shan LIU contributed to the immunofluorescence staining studies; Yan-chang SHANG, Shu-hui WANG and Jia GENG analyzed the data; Yan-chang SHANG and Shu-hui WANG wrote the paper.
Abbreviations
DMD, Duchenne muscular dystrophy; MSCs, mesenchymal stem cells; CNFs, centrally nucleated myofibers; CSA, cross-sectional area; CK, creatine phosphokinase.
Acknowledgments
We thank Prof Shinji TAKADA for providing the pGKWnt3a and pGKneo plasmids. The present work was supported by the grants from the National Natural Science Foundation of China (81100237 and U1032004), Natural Science Foundation of Beijing (7142042), and the Clinical Research-Supported Foundation of the Chinese People's Liberation Army General Hospital (2012FC-TSYS-3057).
Footnotes
Supplementary information is available at the Acta Pharmacologica Sinica's website.
Supplementary Information
Supplementary material
References
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010; 9: 77–93. [DOI] [PubMed] [Google Scholar]
- Ruegg UT. Pharmacological prospects in the treatment of Duchenne muscular dystrophy. Curr Opin Neurol 2013; 26: 577–84. [DOI] [PubMed] [Google Scholar]
- Farini A, Razini P, Erratico S, Torrente Y, Meregalli M. Cell based therapy for Duchenne muscular dystrophy. J Cell Physiol 2009; 221: 526–34. [DOI] [PubMed] [Google Scholar]
- Tedesco FS, Gerli MF, Perani L, Benedetti S, Ungaro F, Cassano M, et al. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci Transl Med 2012; 4: 140ra89. [DOI] [PubMed] [Google Scholar]
- Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev 2004; 13: 436–48. [DOI] [PubMed] [Google Scholar]
- Markert CD, Atala A, Cann JK, Christ G, Furth M, Ambrosio F, et al. Mesenchymal stem cells: emerging therapy for Duchenne muscular dystrophy. PM R 2009; 1: 547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Chanda D, Ponnazhagan S. Therapeutic potential of genetically modified mesenchymal stem cells. Gene Ther 2008; 15: 711–5. [DOI] [PubMed] [Google Scholar]
- Yu M, Zhang C, Zhang Y, Feng S, Yao X, Lu X. BM stem cell transplantation rescues pathophysiologic features of aged dystrophic mdx muscle. Cytotherapy 2007; 9: 44–52. [DOI] [PubMed] [Google Scholar]
- Feng SW, Chen F, Cao J, Yu MJ, Liang YY, Song XM, et al. Restoration of muscle fibers and satellite cells after isogenic MSC transplantation with microdystrophin gene delivery. Biochem Biophys Res Commun 2012; 419: 1–6. [DOI] [PubMed] [Google Scholar]
- Geng J, Peng F, Xiong F, Shang Y, Zhao C, Li W, et al. Inhibition of myostatin promotes myogenic differentiation of rat bone marrow-derived mesenchymal stromal cells. Cytotherapy 2009; 11: 849–63. [DOI] [PubMed] [Google Scholar]
- Nitahara-Kasahara Y, Hayashita-Kinoh H, Ohshima-Hosoyama S, Okada H, Wada-Maeda M, Nakamura A, et al. Long-term engraftment of multipotent mesenchymal stromal cells that differentiate to form myogenic cells in dogs with Duchenne muscular dystrophy. Mol Ther 2012; 20: 168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves MA, de Vries AA, Holkers M, van de Watering MJ, van der Velde I, van Nierop GP, et al. Human mesenchymal stem cells ectopically expressing full-length dystrophin can complement Duchenne muscular dystrophy myotubes by cell fusion. Hum Mol Genet 2006; 15: 213–21. [DOI] [PubMed] [Google Scholar]
- Munsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev 1995; 9: 2911–22. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Borello U, Vivarelli E, Kelly R, Papkoff J, Duprez D, et al. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development 1998; 125: 4155–62. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Takada S. Wnt signaling from the dorsal neural tube is required for the formation of the medial dermomyotome. Development 1998; 125: 4969–76. [DOI] [PubMed] [Google Scholar]
- Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 2003; 113: 841–52. [DOI] [PubMed] [Google Scholar]
- Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D'Antona G, et al. Human circulating AC133+ stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest 2004; 114: 182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Zhang C, Wang S, Xiong F, Zhao C, Peng F, et al. Activated beta-catenin induces myogenesis and inhibits adipogenesis in BM-derived mesenchymal stromal cells. Cytotherapy 2007; 9: 667–81. [DOI] [PubMed] [Google Scholar]
- Shang YC, Wang SH, Xiong F, Zhao CP, Peng FN, Feng SW, et al. Wnt3a signaling promotes proliferation, myogenic differentiation, and migration of rat bone marrow mesenchymal stem cells. Acta Pharmacol Sin 2007; 28: 1761–74. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 1995; 18: 1417–26. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells 1998; 3: 659–70. [DOI] [PubMed] [Google Scholar]
- Feng SW, Lu XL, Liu ZS, Zhang YN, Liu TY, Li JL, et al. Dynamic distribution of bone marrow-derived mesenchymal stromal cells and change of pathology after infusing into mdx mice. Cytotherapy 2008; 10: 254–64. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999; 401: 390–4. [DOI] [PubMed] [Google Scholar]
- Fujita R, Tamai K, Aikawa E, Nimura K, Ishino S, Kikuchi Y, et al. Endogenous mesenchymal stromal cells in bone marrow are required to preserve muscle function in mdx mice. Stem Cells 2015; 33: 962–75. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu HY, Lei QF, Zhang C, Li SN. Improved motor function in dko mice by intravenous transplantation of bone marrow-derived mesenchymal stromal cells. Cytotherapy 2011; 13: 69–77. [DOI] [PubMed] [Google Scholar]
- Xiong F, Xu Y, Zheng H, Lu X, Feng S, Shang Y, et al. Microdystrophin delivery in dystrophin-deficient (mdx) mice by genetically-corrected syngeneic MSCs transplantation. Transplant Proc 2010; 42: 2731–9. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2008; 2: 50–9. [DOI] [PubMed] [Google Scholar]
- Trensz F, Haroun S, Cloutier A, Richter MV, Grenier G. A muscle resident cell population promotes fibrosis in hindlimb skeletal muscles of mdx mice through the Wnt canonical pathway. Am J Physiol Cell Physiol 2010; 299: C939–47. [DOI] [PubMed] [Google Scholar]
- Biressi S, Miyabara EH, Gopinath SD, Carlig PM, Rando TA. A Wnt-TGFbeta2 axis induces a fibrogenic program in muscle stem cells from dystrophic mice. Sci Transl Med 2014; 6: 267ra176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescatori M, Broccolini A, Minetti C, Bertini E, Bruno C, D'Amico A, et al. Gene expression profiling in the early phases of DMD: a constant molecular signature characterizes DMD muscle from early postnatal life throughout disease progression. FASEB J 2007; 21: 1210–26. [DOI] [PubMed] [Google Scholar]
- Hindi SM, Shin J, Ogura Y, Li H, Kumar A. Matrix metalloproteinase-9 inhibition improves proliferation and engraftment of myogenic cells in dystrophic muscle of mdx mice. PLoS One 2013; 8: e72121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MS, Kawahara G, Motohashi N, Casar JC, Eisenberg I, Myers JA, et al. MicroRNA-199a is induced in dystrophic muscle and affects WNT signaling, cell proliferation, and myogenic differentiation. Cell Death Differ 2013; 20: 1194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Maltzahn J, Renaud JM, Parise G, Rudnicki MA. Wnt7a treatment ameliorates muscular dystrophy. Proc Natl Acad Sci U S A 2012; 109: 20614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material