Abstract
In this issue of Clinical and Vaccine Immunology, Scobie and colleagues (H. M. Scobie et al., Clin Vaccine Immunol 23:546–554, 2016, http://dx.doi.org/10.1128/CVI.00052-16) report a nationwide serosurvey of tetanus immunity in >2,000 Cambodian women of child-bearing age to monitor progress toward maternal and neonatal tetanus elimination. This commentary discusses vaccines as interventions for disease control, elimination, and eradication and emphasizes the importance of the tools needed to monitor the effectiveness of initiatives that deliver the vaccines programmatically.
TEXT
The eradication of smallpox in the late 1970s taught us many lessons, some of which have guided subsequent programs striving to achieve control, regional elimination, or global eradication of certain other infectious diseases. Three global initiatives based on the use of vaccines as the key intervention have followed in the steps of the Smallpox Eradication Program: the Global Polio Eradication Initiative (1988) (1), the Measles Initiative (2001; expanded in 2012 to the Measles & Rubella Initiative) (2), and the Maternal and Neonatal Tetanus Elimination Initiative (initially focused on neonatal tetanus [1999], this initiative expanded to include maternal tetanus as well) (3). While vaccines constitute the main intervention, surveillance is an indispensable tool for monitoring the progress of these initiatives.
Facilitating eradication of the smallpox virus, humans constituted its only reservoir, and surveillance was simplified by the distinct clinical picture of smallpox and its 100% clinical expression (i.e., lack of asymptomatic infections). The crude but highly effective vaccine used to interrupt transmission was extremely inexpensive to manufacture, and vials of lyophilized vaccine required no cold chain in the field to maintain potency, thereby facilitating field use. Moreover, the simple, practical, and effective bifurcated needle allowed minimally educated workers to quickly become competent, effective vaccinators (4). Surveillance to detect smallpox cases, the key to directing ring vaccination containment around the cases to interrupt transmission to contacts (5, 6), was practiced in an era without personal computers, the Internet, or cell phones. Once the last cases of smallpox occurred and it was clear that there was no human reservoir from which transmission could continue, within 3 years, routine smallpox vaccination was discontinued worldwide. Humans also constitute the reservoir of infection for polio and measles (7). In contrast, spores of Clostridium tetani abound in the intestines of animals and are also widely found in soil.
Despite the fact that ∼100 subclinical or nonparalytic poliovirus infections occur for each paralytic wild-type case, the Global Polio Eradication Initiative has made enormous strides and has interrupted the transmission of wild-type poliovirus type 2 (no cases since 1999) and type 3 (no cases since 2012). In all of 2015, there were only 74 cases of wild-type 1 poliovirus disease and only 14 cases as of early May 2016, all confined to Pakistan and Afghanistan (8). Similarly, the Measles Initiative has led measles deaths to plummet globally from ∼763,000 in 2000 to 114,900 in 2014, a reduction of ∼85% (9).
The Maternal and Neonatal Tetanus Elimination Initiative has also achieved extraordinary gains. In 2000, it was estimated that there were ∼800,000 neonatal tetanus deaths worldwide (6.7 deaths/1,000 live births). “Elimination” of neonatal tetanus (which many contend should more appropriately be referred to as “control,” since there will always be environmental and animal reservoirs of C. tetani spores to pose a threat and therefore vaccination cannot be abandoned) is defined as a fall in incidence to <1 case/1,000 live births. Whereas ensuring hygienic birth practices and postnatal umbilical cord care is a key component of neonatal tetanus control, it is the immunization of pregnant women with tetanus toxoid during prenatal care and of women of child-bearing age in supplementary mass immunization campaigns that have allowed many countries recently to achieve “elimination” of maternal and neonatal tetanus as a public health problem (10–14). Because C. tetani spores abound in the environment, particularly in rural areas, maintaining the incidence of neonatal and maternal tetanus disease below the threshold of elimination in recently validated countries poses a major challenge. Governments will have to ensure that high levels of tetanus toxoid vaccination coverage of adult women are maintained to sustain elimination even as future pressures build to divert limited resources to address other public health priorities.
Neonatal and maternal tetanus is a well-recognized marker of socioeconomic inequity, particularly in rural areas and urban slums, where populations often are underserved by immunization programs and face barriers to health care access. Thus, measuring a serological biomarker to monitor the prevalence of seroprotection becomes a critical tool for assessing the status of immunization programs that target pregnant women and women of child-bearing age. In this way, overlooked high-risk women can be identified and steps taken to strengthen the immunization services that are supposed to serve them.
The paper by Scobie et al. in this issue of Clinical and Vaccine Immunology (15) has many laudable features. The team performed an impressive nationwide serosurvey to measure the prevalence of protective titers of tetanus antitoxin in women aged 15 to 39 years in Cambodia, with a large sample size selected by using statistically rigorous methods. The investigators collected serum and measured tetanus antitoxin by two different methods, the double-antigen enzyme-linked immunosorbent assay (DAE, which is considered the “gold standard”) and a novel and highly sensitive multiplex bead assay (MBA) that can simultaneously test for multiple other antibodies of interest. The analyses of the tetanus antitoxin data demonstrate why such surveys are needed if neonatal and maternal tetanus cases are to be kept at very low levels. Their survey clearly identified geographic areas (in western Cambodia), age subgroups of adult women (15 to 19 and 20 to 24 years), and other groups with significantly lower rates of seroprotection. They thereby diagnosed at the population level where program strengthening is needed.
Scobie et al. (15) tout the DAE because with serum samples containing low levels of tetanus antitoxin (e.g., 0.01 to 0.14 IU/ml), this method correlates well with established in vivo assays that measure protective neutralizing antitoxin levels (16). The same is true of the toxin binding inhibition assay for tetanus antitoxin determination (17). However, these assays are not easy to set up in developing-country reference laboratories because they require careful optimization and standardized reagents that are not commercially (or otherwise easily) available. In the format originally described, they can be performed only under the auspices of the Statens Serum Institute in Copenhagen, Denmark. For these reasons, large surveys of seroprotection in other venues, in both industrialized and developing countries, have relied upon classical indirect enzyme-linked immunosorbent assays (ELISAs) (18–22). In such assays, a cutoff of 0.15 IU/ml is typically used as evidence of seroprotection, recognizing that this cutoff is 15 times the titer that is usually considered protective (0.01 IU/ml). This means that, with this ELISA, some women having titers between 0.01 and 0.14 IU/ml would be incorrectly scored as unprotected. On the other hand, for persons with titers of ≥0.15 IU/ml, one can have confidence that they are not only protected in the short term but are likely to remain protected for some period of time following the survey. Other MBAs similar to the one described by Scobie et al. (15) have been proposed as sensitive high-throughput alternatives for immune surveillance of tetanus (23) and other vaccine-preventable diseases (24–26). Similarly, the lack of commercial reagents and the requirement of a somewhat sophisticated laboratory infrastructure pose barriers for these technologies to become widely available.
Despite the multiple positive features of the survey of Scobie et al., we believe that some other survey strategies and tactics may be useful in achieving more broadly the aim of monitoring seroprotection against tetanus in developing countries, as those alternative methods are simpler, logistically more practical, and more economical and employ technologies that are easier to transfer to local institutions in developing countries.
Although serum is the “golden” fluid for determination of biomarkers, it requires the use of a sharp to obtain blood by venipuncture or by needle stick and a centrifugation step to separate serum from a clot. In addition, equipment is required, as described by Scobie et al. (15) and others (27), to maintain a reverse cold/freeze chain. The use of dried blood spots (DBS) does not obviate a needle stick but does eliminate the requirement for a cold/freeze chain (28, 29). However, once back in the reference laboratory the DBS must undergo a cumbersome elution step to obtain quasiserum for testing. The eluates contain hemoglobin and erythrocyte debris that increase background readings in traditional ELISAs, and the initial dilution required to bypass this nonspecific reactivity limits the sensitivity of this method. The convenience of ambient-temperature handling may theoretically compromise the quality of the sample in some extreme ecologies.
Oral fluid, which contains IgG-rich crevicular fluid, offers an alternative to serum or DBS collection by eliminating the need for a needle stick (20). It is easier to perform and better tolerated than blood collection (Fig. 1). Properly collected oral fluid contains ∼1% the total IgG concentration of serum, and IgG antibody titers are ∼1/100 of those measured in serum from the same individual, be that person an adult, a toddler, or an infant (20). Despite the differences in relative levels, tetanus antitoxin measured in oral fluid is an excellent predictor of serum antibody content (20); a titer ≥0.0015 IU/ml in oral fluid (1/100 of the cutoff used for serum in a standard ELISA) is considered positive and evidence of protection (20). Measurement of IgG antibody in oral fluids is useful not only for tetanus (20) but also for other vaccine antigens such as measles (30), meningococcal polysaccharides, and Haemophilus influenzae type b. Originally, oral fluids were transported by using a reverse cold chain to the reference laboratory for antibody measurements (20, 30). We propose that the final configuration of an ideal, practical antibody biomarker monitoring method, whether based on serum, blood, or oral fluid specimens, should be usable at the point of care to provide the biomarker protection results in the field within a few minutes of collection of the specimens. Kits, devices, and methods to provide such point-of-care results would enhance biomarker measurement to monitor vaccination program effectiveness, although there may be a limitation to the number of biomarkers that can be measured simultaneously.
FIG 1.
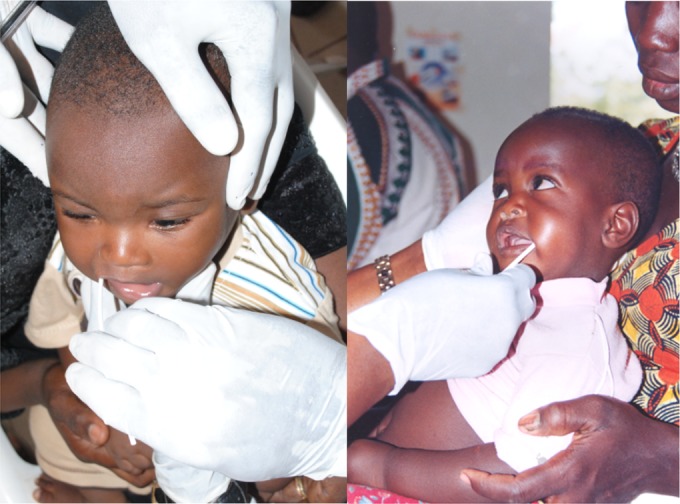
Oral fluid collection from Malian infants. Copyright Samba O. Sow, Centre pour le Développement des Vaccins du Mali (CVD-Mali), Bamako, Mali; reproduced with permission.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
For the article discussed, see doi:10.1128/CVI.00052-16.
REFERENCES
- 1.Garon JR, Cochi SL, Orenstein WA. 2015. The challenge of global poliomyelitis eradication. Infect Dis Clin North Am 29:651–665. doi: 10.1016/j.idc.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keegan R, Dabbagh A, Strebel PM, Cochi SL. 2011. Comparing measles with previous eradication programs: enabling and constraining factors. J Infect Dis 204(Suppl 1):S54–S61. doi: 10.1093/infdis/jir119. [DOI] [PubMed] [Google Scholar]
- 3.Roper MH, Vandelaer JH, Gasse FL. 2007. Maternal and neonatal tetanus. Lancet 370:1947–1959. doi: 10.1016/S0140-6736(07)61261-6. [DOI] [PubMed] [Google Scholar]
- 4.Rubin BA. 1980. A note on the development of the bifurcated needle for smallpox vaccination. WHO Chron 34:180–181. [PubMed] [Google Scholar]
- 5.Foege W. 2011. Lessons and innovations from the West and Central African smallpox eradication program. Vaccine 29(Suppl 4):D10–D12. doi: 10.1016/j.vaccine.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Foege WH, Millar JD, Henderson DA. 1975. Smallpox eradication in West and Central Africa. Bull World Health Organ 52:209–222. [PMC free article] [PubMed] [Google Scholar]
- 7.Breman JG, De Quadros CA, Dowdle WR, Foege WH, Henderson DA, John TJ, Levine MM. 2011. The role of research in viral disease eradication and elimination programs: lessons for malaria eradication. PLoS Med 8:e1000405. doi: 10.1371/journal.pmed.1000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales M, Tangermann RH, Wassilak SG. 2016. Progress toward polio eradication—worldwide, 2015–2016. MMWR Morb Mortal Wkly Rep 65:470–473. doi: 10.15585/mmwr.mm6518a4. [DOI] [PubMed] [Google Scholar]
- 9.Perry RT, Murray JS, Gacic-Dobo M, Dabbagh A, Mulders MN, Strecker T, Okwo-Bele JM, Rota PA, Goodson JL. 2015. Progress towards regional measles elimination, worldwide, 2000–2014. Wkly Epidemiol Rec. 90:623–631.26567399 [Google Scholar]
- 10.World Health Organization. 2012. Elimination of maternal and neonatal tetanus in Senegal. Wkly Epidemiol Rec. 87:253–260. [PubMed] [Google Scholar]
- 11.World Health Organization. 2012. Validation of elimination: maternal and neonatal tetanus in Burkina Faso, 2012. Wkly Epidemiol Rec. 87:450–459. [PubMed] [Google Scholar]
- 12.World Health Organization. 2013. Validation of maternal and neonatal tetanus elimination in Cameroon, 2012. Wkly Epidemiol Rec. 88:489–499. [PubMed] [Google Scholar]
- 13.World Health Organization. 2013. Validation of maternal and neonatal tetanus elimination in United Republic of Tanzania, 2012. Wkly Epidemiol Rec. 88:313–320. [PubMed] [Google Scholar]
- 14.World Health Organization. 2015. Maternal and neonatal tetanus elimination: validation surveys in Lao People's Democratic Republic, December 2013. Wkly Epidemiol Rec. 90:45–56. [PubMed] [Google Scholar]
- 15.Scobie HM, Mao B, Buth S, Wannemuehler KA, Sørensen C, Kannarath C, Jenks MH, Moss DM, Priest JW, Soeung SC, Deming MS, Lammie PJ, Gregory CJ. 2016. Tetanus immunity among women aged 15 to 39 years in Cambodia: a national population-based serosurvey, 2012. Clin Vaccine Immunol 23:546–554. doi: 10.1128/CVI.00052-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristiansen M, Aggerbeck H, Heron I. 1997. Improved ELISA for determination of anti-diphtheria and/or anti-tetanus antitoxin antibodies in sera. APMIS 105:843–853. doi: 10.1111/j.1699-0463.1997.tb05093.x. [DOI] [PubMed] [Google Scholar]
- 17.Hendriksen CF, van der Gun JW, Marsman FR, Kreeftenberg JG. 1991. The use of the in vitro toxin binding inhibition (ToBI) test for the estimation of the potency of tetanus toxoid. Biologicals 19:23–29. doi: 10.1016/1045-1056(91)90020-K. [DOI] [PubMed] [Google Scholar]
- 18.Gergen PJ, McQuillan GM, Kiely M, Ezzati-Rice TM, Sutter RW, Virella G. 1995. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med 332:761–766. doi: 10.1056/NEJM199503233321201. [DOI] [PubMed] [Google Scholar]
- 19.Redwan el-RM, Al-Awady MK. 2002. Prevalence of tetanus immunity in the Egyptian population. Hum Antibodies 11:55–59. [PubMed] [Google Scholar]
- 20.Tapia MD, Pasetti MF, Cuberos L, Sow SO, Doumbia MN, Bagayogo M, Kotloff KL, Levine MM. 2006. Measurement of tetanus antitoxin in oral fluid: a tool to conduct serosurveys. Pediatr Infect Dis J 25:819–825. doi: 10.1097/01.inf.0000232629.72160.bb. [DOI] [PubMed] [Google Scholar]
- 21.Travassos MA, Beyene B, Adam Z, Campbell JD, Mulholland N, Diarra SS, Kassa T, Oot L, Sequeira J, Reymann M, Blackwelder WC, Wu Y, Ruslanova I, Goswami J, Sow SO, Pasetti MF, Steinglass R, Kebede A, Levine MM. 2016. Immunization coverage surveys and linked biomarker serosurveys in three regions in Ethiopia. PLoS One 11:e0149970. doi: 10.1371/journal.pone.0149970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan L, Lau W, Thipphawong J, Kasenda M, Xie F, Bevilacqua J. 1997. Diphtheria and tetanus immunity among blood donors in Toronto. CMAJ 156:985–990. [PMC free article] [PubMed] [Google Scholar]
- 23.van Gageldonk PG, van Schaijk FG, van der Klis FR, Berbers GA. 2008. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J Immunol Methods 335:79–89. doi: 10.1016/j.jim.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Elberse KE, Tcherniaeva I, Berbers GA, Schouls LM. 2010. Optimization and application of a multiplex bead-based assay to quantify serotype-specific IgG against Streptococcus pneumoniae polysaccharides: response to the booster vaccine after immunization with the pneumococcal 7-valent conjugate vaccine. Clin Vaccine Immunol 17:674–682. doi: 10.1128/CVI.00408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smits GP, van Gageldonk PG, Schouls LM, van der Klis FR, Berbers GA. 2012. Development of a bead-based multiplex immunoassay for simultaneous quantitative detection of IgG serum antibodies against measles, mumps, rubella, and varicella-zoster virus. Clin Vaccine Immunol 19:396–400. doi: 10.1128/CVI.05537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Gageldonk PG, von Hunolstein C, van der Klis FR, Berbers GA. 2011. Improved specificity of a multiplex immunoassay for quantitation of anti-diphtheria toxin antibodies with the use of diphtheria toxoid. Clin Vaccine Immunol 18:1183–1186. doi: 10.1128/CVI.05081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Travassos MA, Beyene B, Adam Z, Campbell JD, Mulholland N, Diarra SS, Kassa T, Oot L, Sequeira J, Reymann M, Blackwelder WC, Pasetti MF, Sow SO, Steinglass R, Kebede A, Levine MM. 2015. Strategies for coordination of a serosurvey in parallel with an immunization coverage survey. Am J Trop Med Hyg 93:416–424. doi: 10.4269/ajtmh.15-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colson KE, Potter A, Conde-Glez C, Hernandez B, Rios-Zertuche D, Zuniga-Brenes P, Collaborators S, Iriarte E, Mokdad AH. 2015. Use of a commercial ELISA for the detection of measles-specific immunoglobulin G (IgG) in dried blood spots collected from children living in low-resource settings. J Med Virol 87:1491–1499. doi: 10.1002/jmv.24136. [DOI] [PubMed] [Google Scholar]
- 29.Colson KE, Zuniga-Brenes P, Rios-Zertuche D, Conde-Glez CJ, Gagnier MC, Palmisano E, Ranganathan D, Usmanova G, Salvatierra B, Nazar A, Tristao I, Sanchez ME, Anderson BW, Haakenstad A, Murphy T, Lim S, Hernandez B, Lozano R, Iriarte E, Mokdad AH. 2015. Comparative estimates of crude and effective coverage of measles immunization in low-resource settings: findings from Salud Mesoamerica 2015. PLoS One 10:e0130697. doi: 10.1371/journal.pone.0130697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon JK, Ramirez K, Cuberos L, Campbell JD, Viret JF, Munoz A, Lagos R, Levine MM, Pasetti MF. 2011. Mucosal IgA responses in healthy adult volunteers following intranasal spray delivery of a live attenuated measles vaccine. Clin Vaccine Immunol 18:355–361. doi: 10.1128/CVI.00354-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


