Abstract
To monitor progress toward maternal and neonatal tetanus elimination (MNTE) in Cambodia, we conducted a nationwide serosurvey of tetanus immunity in 2012. Multistage cluster sampling was used to select 2,154 women aged 15 to 39 years. Tetanus toxoid antibodies in serum samples were measured by gold-standard double-antigen enzyme-linked immunosorbent assay (DAE) and a novel multiplex bead assay (MBA). Antibody concentrations of ≥0.01 IU/ml by DAE or the equivalent for MBA were considered seroprotective. Estimated tetanus seroprotection was 88% (95% confidence interval [CI], 86 to 89%); 64% (95% CI, 61 to 67%) of women had antibody levels of ≥1.0 IU/ml. Seroprotection was significantly lower (P < 0.001) among women aged 15 to 19 years (63%) and 20 to 24 years (87%) than among those aged ≥25 years (96%), among nulliparous women than among parous women (71 versus 97%), and among those living in the western region than among those living in other regions (82 versus 89%). The MBA showed high sensitivity (99% [95% CI, 98 to 99%]) and specificity (92% [95% CI, 88 to 95%]) compared with DAE. Findings were compatible with MNTE in Cambodia (≥80% protection). Tetanus immunity gaps should be addressed through strengthened routine immunization and targeted vaccination campaigns. Incorporating tetanus testing in national serosurveys using MBAs, which can measure immunity to multiple pathogens simultaneously, may be beneficial for monitoring MNTE.
INTRODUCTION
Neonatal tetanus (NT), defined as tetanus occurring within the first 28 days of life, and maternal tetanus, defined as tetanus occurring during or within the first 6 weeks after pregnancy, caused an estimated >50,000 deaths worldwide in 2010 (1, 2). Since Clostridium tetani is ubiquitous in the environment, tetanus disease is not eradicable. An NT elimination goal, defined as <1 NT case/1,000 live births/year in all of the districts of a country, was adopted by World Health Organization (WHO) member countries in 1989; in 1999, the initiative was expanded to include maternal tetanus and became known as maternal and NT elimination (MNTE) (3). The target date for global MNTE was 2015, but as of August 2015, elimination had not been achieved in 21 countries (3–5).
The WHO recommends a primary series of three doses of diphtheria-tetanus-pertussis (DTP) vaccine within the first year of life and three booster doses of tetanus toxoid (TT)-containing vaccine (TTCV) in later childhood, adolescence, and adulthood to prevent tetanus in all age groups (6). In countries where maternal and neonatal tetanus remains a problem and the recommended three booster doses of TTCV are not routinely given to both sexes, the WHO recommends vaccination of pregnant women with five TTCV doses, with the first dose given at the initial antenatal care visit and the second dose given 4 weeks later (6). For MNTE, the recommended strategies include (i) vaccination of pregnant women with TTCV, (ii) providing three TTCV doses to women of reproductive age (WRA) through supplementary immunization activities (SIAs) in high-risk areas, (iii) ensuring clean delivery and umbilical cord care practices, and (iv) strengthening NT surveillance (3).
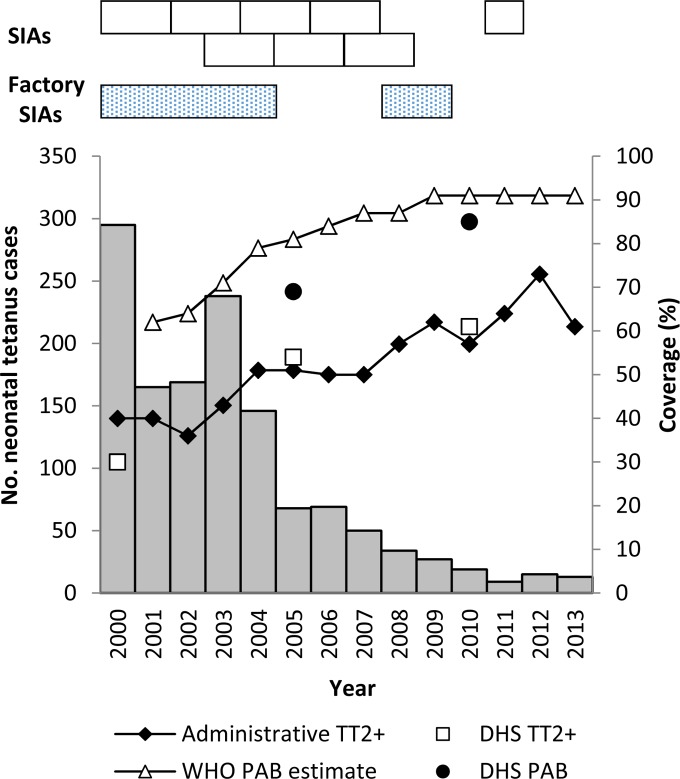
TTCV has been provided to WRA in Cambodia since 1989; intensified MNTE efforts began in 2000 (7–9). During 2000 to 2013, administrative coverage of TT, defined as the proportion of pregnant women receiving a second or subsequent dose of TT (TT2+) divided by the estimated number of live births, increased from 40 to 61% (10). During 2000 to 2011, 53 (69%) of 77 operational districts (ODs) conducted three rounds of TT SIAs; TT SIAs in garment factories occurred during 2000 to 2004, 2008, and 2009 (Fig. 1). In the 2000 and 2010 Demographic Health Surveys (DHSs) of women giving birth in the previous 5 years, point estimates increased for the proportions receiving any antenatal care from 38 to 89%, having births protected against tetanus increased from 69 to 85%, and delivering with the assistance of trained staff increased from 32 to 71% (11, 12). The reported annual number of NT cases decreased from 295 to 15 from 2000 to 2013 (Fig. 1) (13).
FIG 1.
Reported NT cases, TT vaccination coverage, and TT SIAs by year in Cambodia from 2000 to 2013. NT cases were tetanus infections that occurred within the first 28 days of life and were reported through surveillance (13). Reported annual administrative coverage of RI of pregnant women with TT2+ was calculated by dividing the total number of women who received TT2+ by the total number of live births in a year multiplied by 100 (10). DHS estimates of TT2+ coverage and the proportion of infants PAB by neonatal maternal TT immunization were determined among women with a live birth in the 5 years preceding the survey, where PAB was defined as receiving 2 TT doses during the last birth, ≥2 TT doses with the last dose ≤3 years prior to the last birth, ≥3 doses with the last dose ≤5 years prior, ≥4 doses with the last dose ≤10 years prior, or ≥5 prior doses (11, 12, 41). WHO-UNICEF annual estimates of PAB were calculated on the basis of mathematical modeling (37). During 2000 to 2011, eight phases of TT SIA targeted WRA in high-risk ODs, with each phase targeting 5 to 13 ODs. TT SIAs were also conducted in 244 garment factories during 2000 to 2004 and 402 factories in 2008 and 2009.
Serosurveys could be used to monitor progress toward the achievement and maintenance of MNTE given challenges of reliably assessing TT coverage through vaccination history (14). For in vitro assays of tetanus seroprotection, gold standards are the DAE (double-antigen enzyme-linked immunosorbent assay [ELISA]) and toxin binding inhibition (ToBI) tests, which are not commercially available (15–19). Indirect tetanus ELISAs exist, but they overestimate antibody levels in the low seroprotective range (0.01 to 0.2 IU/ml) (15, 19–21). For use in serosurveys, multiplex bead assays (MBAs) are promising, since antibody responses to many pathogens can be measured simultaneously by using minimal serum sample volumes. Tetanus MBAs have been previously validated, but only once against a gold-standard ToBI test (22–24). To our knowledge, a tetanus MBA has never been validated against the other gold standard, DAE, or used for a national serosurvey in a developing country (25, 26).
In 2012, we conducted a nationwide serosurvey to determine tetanus population immunity among Cambodian women aged 15 to 39 years. This was Cambodia's first national estimate of tetanus immunity and was relevant for the MNTE validation survey in 2015. The objectives of the tetanus serosurvey were to estimate seroprotection among WRA, identify immunity gaps, assess tetanus vaccination status during the last pregnancy, and evaluate the suitability of an MBA for tetanus serologic testing relative to that of DAE.
MATERIALS AND METHODS
Survey design.
During November and December 2012, we conducted a cross-sectional serosurvey among women aged 15 to 39 years in Cambodia. The two primary survey objectives were to estimate rubella and tetanus seroprevalence among WRA; polio, measles, and rubella serosurvey results are described elsewhere (27). The survey design and sample size were selected to provide national estimates by 5-year age strata. Cambodia was divided into five regional strata with urban and rural substrata as follows: Phnom Penh (the capital) and the western (Battambang, Kampong Chhang, Kampong Speu, Koh Kong, Pailin, Preah Sihanouk, and Pursat Provinces), northern (Banteay Mean Chey, Kampong Thom, Kratie, Mondolkiri, Otdar Mean Chey, Preah Vihear, Ratanakiri, Siem Reap, and Steung Treng Provinces), southeastern (Kampong Cham, Prey Veng, and Svay Rieng Provinces), and southwestern (Kampot, Kandal, Kep, and Takeo provinces) regions. The northern region was further divided into tetanus risk strata based on the December 2009 Cambodian NT Risk Assessment. Clusters in districts considered high and medium risk were designated “high/medium risk.” Other clusters were designated “low risk.” From the 611 clusters selected with probability proportional to size for the 2010 DHS sample, 20 clusters per region were sampled by simple random sampling; in the northern region, 15 high/medium-risk and 5 low-risk clusters were selected (11). All of the eligible women in selected households were asked to participate. After written consent was obtained, participants completed a brief questionnaire, including tetanus vaccination status and demographic information, and 5 ml of blood was collected in serum separator tubes by venipuncture. The vaccination status of parous women at the time of their last birth was assessed by recall; available cards were used to check vaccination recall. We also assessed the number of doses nulliparous women had received by the time of the survey. All doses were assumed to meet the minimum time intervals required of valid doses (6).
The serosurvey protocol was approved by the National Ethics Committee for Health Research, Cambodian Ministry of Health. This activity was approved as a public health program evaluation by the U.S. Centers for Disease Control and Prevention (CDC).
Laboratory testing.
Blood specimens were either (i) stored at 4°C for ≤96 h until delivery to the National Institute of Public Health (NIPH) laboratory in Phnom Penh, where they were centrifuged, or (ii) centrifuged in the field, and the serum was stored at 4°C. At NIPH, serum samples were stored at −80°C until shipment by air on dry ice to the CDC laboratory in Atlanta, GA. At CDC, two aliquots of serum from each participant were prepared for anti-TT IgG testing; one was shipped on dry ice to the Statens Serum Institut (SSI) in Copenhagen, Denmark.
DAE.
At the SSI, microtiter plates (MaxiSorp; Nunc, Roskilde, Denmark) were coated with 0.002 μg of purified TT as previously described (19). The plates were incubated for 2 h at room temperature (RT, 20 to 25°C) with 4-fold serial dilutions of the serum samples (1:4 to 1:65,536) in dilution buffer consisting of phosphate-buffered saline (PBS; pH 7.4), 1% (vol/vol) Triton X-100, and 1% (wt/vol) bovine serum albumin (BSA). For detection, 50 μl of biotin-labeled TT (prepared in house) diluted 1:15,000 in dilution buffer was added to each well and incubated for 1 h, followed by 30 min of incubation with 50 μl of horseradish peroxidase-conjugated streptavidin (Invitrogen) diluted 1:40,000. o-Phenylenediamine (Kem-En-Tec Diagnostics, Taastrup, Denmark) was used as the substrate, and the absorbance at 490 nm was read in a BioTek ELx808 ELISA plate reader with the KC4 Signature software. A standard curve was prepared from three independent 2-fold serial dilutions (0.01 to 0.0003125 IU/ml) of the WHO international standard for tetanus antitoxin (equine). Three independently diluted control antitoxin samples (0.0025 IU/ml) were also tested. The observed anti-TT IgG values ranged from 0.001 to 1,100 IU/ml. A result of ≥0.01 IU/ml was considered seroprotective for tetanus (19, 28).
MBA.
At the CDC, TT (Massachusetts Biologic Laboratories, Boston, MA) was coupled to SeroMap microspheres (Luminex Corp., Austin, TX) with 12.5 μg of toxoid/12.5 × 106 beads as previously described (29). Coupling buffer containing 50 mM 2-(N-morpholino)ethanesulfonic acid (MES) and 0.85% NaCl at pH 5.0 was used instead of PBS at pH 7.2 to decrease the amount of antigen required for the optimal assay signal (30). Each assay well also included a bead coupled with Schistosoma japonicum glutathione-S-transferase as a negative-control protein and 18 additional beads coupled with antigen constructs from 12 parasitic or viral diseases. These results were published recently (42).
Test serum samples, diluted 1:400 in PBS (pH 7.2) containing 0.3% Tween 20, 0.02% sodium azide, 0.5% casein, 0.5% BSA, 0.5% polyvinyl alcohol, 0.8% polyvinylpyrrolidone, and 3 μg/ml Escherichia coli extract, were assayed in duplicate for total IgG antibodies (29, 31, 32). All incubations were conducted at RT in a volume of 50 μl. Coupled beads were incubated with serum dilutions for 90 min, followed by a 45 min of incubation with 50 ng of biotinylated mouse anti-human IgG monoclonal antibody (clone H2; Southern Biotech, Birmingham, AL) per well and 40 ng of biotinylated mouse anti-human IgG4 monoclonal antibody (clone HP6025; Invitrogen, South San Francisco, CA) per well, as previously described (28). Incubation for 30 min with 250 ng of R-phycoerythrin-labeled streptavidin (Molecular Probes, Eugene, OR) per well was followed by a final washing with 0.05% Tween 20 in PBS for 30 min. Beads were suspended in 100 μl of PBS, and plates were read in a Bio-Plex 200 instrument equipped with Bio-Plex Manager 6.1 software (Bio-Rad, Hercules, CA). The average of the median fluorescent intensity values of duplicate wells minus the background fluorescence (MFI-bg) was recorded. The observed anti-TT IgG values ranged from slightly below the blank (−9.3, treated as 0) to 30,645 MFI-bg units. Positive- and negative-control serum dilutions were included on each plate to monitor assay performance. The percent coefficient of variation (%CV) of the three positive-control serum samples and one negative-control serum sample assayed on each plate (n = 51) was 4% at 27,930 MFI-bg units (>1.6 IU/ml), 7% at 3,814 MFI-bg units (0.23 IU/ml), 8% at 1,208 MFI-bg units (0.085 IU/ml), and 12% at 62 MFI-bg units (0.0066 IU/ml). To determine the seroprotection cutoff equivalent to ≥0.01 IU/ml, linear regression was performed on log-transformed DAE values between 0.004 and 1.6 IU/ml; the equation was log (MBA result) = 1.16 log (DAE result) + 4.32 (r2 = 0.91). MBA values of ≥100 MFI-bg units were considered seroprotective.
Statistical analysis.
Estimates of seroprotection and vaccination coverage with 95% (logit) confidence intervals (CIs) were calculated while accounting for the survey design by using STATA v13 (StataCorp, College Station, TX) (27). Anti-TT IgG concentrations generally correlate with the robustness and duration of seroprotection (28). Estimates of the proportions of women in different antibody level categories (<0.01, 0.01 to 0.099, ≥0.1 to 0.99, and ≥1.0 IU/ml) were calculated. Second-order Rao-Scott chi-square tests were used to test the independence of seroprotection or vaccination coverage and the factors age, region, tetanus risk strata, urban/rural residence, and parity status; for all statistically significant results (P < 0.05), we estimated the differences between the subpopulations along with the 95% CIs. Median antibody levels with interquartile ranges (IQRs) were calculated while accounting for the survey design in SUDAAN v11 (RTI International, Cary, NC). Design effect and intraclass correlation coefficients were calculated for the overall serosurvey and by region accounting for clustering only. Organ-pipe plots were constructed by plotting unweighted seroprotection by cluster (33). To estimate the accuracy of the MBA, sensitivity and specificity with standard Wilson 95% CIs for a binomial proportion were calculated for results of the MBA relative to DAE in SAS v9.3 (SAS Institute, Inc., Cary, NC).
A simplified definition was necessary to estimate the proportion of infants protected at birth (PAB) from tetanus by using the survey data, which did not include the interval of time between the pregnancy and the last/prepregnancy dose (34) (see Fig. 1 for standard PAB definition). PAB was defined as receiving the second or subsequent dose of TT during the last pregnancy, or a total of ≥2 doses before the last pregnancy; the latter part of the simplified definition was based on survey results in the Central African Republic (CAR), where 30 (94%) of 32 newborns born to mothers with ≥2 prepregnancy doses were within the period of protection afforded by these doses (35). The high/medium tetanus risk strata in the analysis phase included clusters from all of the regions designated high or medium risk in the 2009 risk assessment.
RESULTS
The survey included 2,154 women aged 15 to 39 years (response rate, 92%) (26). Vaccination cards documenting a TT vaccination history were available for 906 (42%) women. Four participants' samples had unavailable tetanus antibody test results, preventing their inclusion in these analyses.
Immunity to tetanus by DAE.
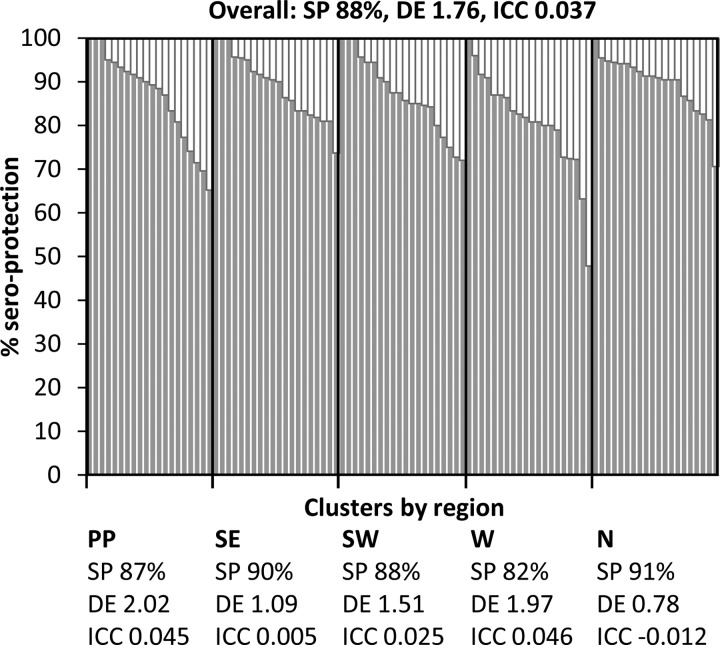
The overall estimated seroprotection of women aged 15 to 39 years in Cambodia was 88% (95% CI, 86 to 89%) (Table 1). Unweighted seroprotection varied by cluster (range, 48 to 100%), but the degree of variation among clusters was similar by region. Of the 100 total clusters, seroprotection was ≥80% in 83 (83%) clusters, 70 to 79% in 14 (14%) clusters, and <70% in three (3%) clusters in the western region and Phnom Penh (Fig. 2).
TABLE 1.
Estimated serologic protection of women aged 15 to 39 years from tetanus in Cambodia in 2012
| Population | Total no. | Seroprotecteda |
Antibody level (IU/ml) |
|||||
|---|---|---|---|---|---|---|---|---|
| No. | % | LCLb | UCLc | P value | Median | IQRe | ||
| Overall | 2,150 | 1,862 | 88 | 86 | 89 | 2.34 | 0.20–7.60 | |
| Regions | ||||||||
| Phnom Penh | 469 | 406 | 87 | 81 | 91 | 0.014d | 1.89 | 0.15–6.96 |
| Southeast | 419 | 374 | 90 | 85 | 93 | 3.39 | 0.36–8.25 | |
| Southwest | 423 | 368 | 88 | 84 | 91 | 2.26 | 0.22–8.10 | |
| West | 445 | 360 | 82 | 77 | 85 | 1.97 | 0.11–7.58 | |
| North | 394 | 354 | 91 | 85 | 94 | 2.38 | 0.10–7.45 | |
| Risk levels | ||||||||
| High/medium | 561 | 495 | 87 | 84 | 90 | 0.923 | 3.45 | 0.34–8.03 |
| Low | 1,589 | 1,367 | 88 | 85 | 90 | 2.09 | 0.14–7.54 | |
| Urban/rural | ||||||||
| Urban | 655 | 565 | 86 | 82 | 89 | 0.188 | 1.97 | 0.11–7.39 |
| Rural | 1,495 | 1,297 | 88 | 86 | 90 | 2.41 | 0.25–7.69 | |
| Age (yr) | ||||||||
| 15–19 | 435 | 261 | 63 | 58 | 68 | <0.001d | 0.03 | 0.00–1.76 |
| 20–24 | 468 | 406 | 87 | 82 | 90 | 2.06 | 0.12–8.61 | |
| 25–29 | 483 | 458 | 95 | 92 | 97 | 4.86 | 1.00–13.66 | |
| 30–34 | 449 | 437 | 96 | 93 | 98 | 4.36 | 1.25–8.06 | |
| 35–39 | 315 | 300 | 96 | 93 | 98 | 2.33 | 0.90–6.67 | |
| Parity | ||||||||
| Parous | 1,356 | 1,299 | 97 | 95 | 98 | <0.001d | 4.59 | 1.19–8.98 |
| Nulliparous | 794 | 563 | 71 | 68 | 75 | 0.20 | 0.01–3.84 | |
Tested by DAE, where seroprotected is defined as ≥0.01 IU/ml.
LCL, lower confidence limit.
UCL, upper confidence limit.
Groups were statistically significantly different by Rao-Scott chi-square test.
IQR, interquartile range.
FIG 2.
Cluster level tetanus seroprotection among women aged 15 to 39 years in Cambodia in 2012. Organ-pipe plots were constructed from unweighted seroprotection (SP) by cluster (33). Design effects (DE) and intraclass correlation coefficients (ICC) were calculated for the survey overall and by region, i.e., Phnom Penh (PP), southeast (SE), southwest (SW), west (W), and north (N).
Seroprotection differed by region (P = 0.014), age (P < 0.001), and parity (P < 0.001) (Table 1). Seroprotection was 7% (95% CI, 3 to 12%) lower in the western region (82%) than in the other regions combined (89%). Compared with women aged ≥25 years (96%), seroprotection was lower among women 15 to 19 years (63%) and 20 to 24 years (87%), with differences of 33% (95% CI, 28 to 38%) and 9% (95% CI, 5 to 14%); the difference among women aged 15 to 19 years and 20 to 24 years was 23% (95% CI, 16 to 30%). Additionally, seroprotection was 25% (95% CI, 22 to 29%) lower among nulliparous women (71%) than among parous women (97%). Among those aged 15 to 19 years, seroprotection of nulliparous (234/387 = 60%) and parous (27/48 = 56%) women was similar, but the data were too sparse for statistical analysis. No differences in seroprotection were observed by tetanus risk strata or urban/rural residence (Table 1).
Overall, the estimated median antibody level was 2.34 IU/ml (IQR, 0.20 to 7.60 IU/ml) (Table 1). An estimated 64% (95% CI, 61 to 67%) of the women had tetanus antibody levels of ≥1.0 IU/ml, 13% (95% CI, 11 to 16%) had tetanus antibody levels of 0.1 to 0.99 IU/ml, and 10% (95% CI, 8 to 13%) had tetanus antibody levels of 0.01 to 0.099 IU/ml (Table 2). The estimated median tetanus antibody levels in increasing 5-year age groups were 0.03, 2.06, 4.86, 4.36, and 2.33 IU/ml (Table 1). The proportion of women aged ≥25 years with antibody levels of ≥1.0 IU/ml (76%) was greater than that of women aged 15 to 19 years (31%) and 20 to 24 years (60%), with differences of 46% (95% CI, 38 to 54%) and 16% (95% CI, 9 to 23%). The estimated median antibody levels of nulliparous and parous women were 0.20 and 4.59 IU/ml (Table 1). The proportion of nulliparous women with antibody levels of ≥1.0 IU/ml (38%) was 40% (95% CI, 31 to 48%) lower than that of parous women (78%) (Table 2).
TABLE 2.
Estimated levels of tetanus antibodies among women aged 15 to 39 years by parity and age group in Cambodia in 2012
| Population | No. of women | % of women with tetanus antibody level (IU/ml)a of: |
|||
|---|---|---|---|---|---|
| <0.01 | 0.01–0.099 | 0.1–0.99 | ≥1.0 | ||
| Overall | 2,150 | 12 | 10 | 13 | 64 |
| Parous | 1,356 | 3 | 6 | 12 | 78 |
| Nulliparous | 794 | 29 | 17 | 16 | 38 |
| 15–19 yr | 435 | 37 | 20 | 12 | 31 |
| 20–24 yr | 468 | 13 | 10 | 16 | 60 |
| 25–29 yr | 483 | 5 | 7 | 13 | 76 |
| 30–34 yr | 449 | 4 | 5 | 12 | 80 |
| 35–39 yr | 315 | 4 | 10 | 13 | 73 |
Tested by DAE, where seroprotected is defined as ≥0.01 IU/ml.
The estimated seroprotection of nulliparous women increased with an increasing number of TT doses, as assessed by card or recall at the time of the survey, from 44% with zero doses to 95% with four doses; the estimated median antibody levels increased from 0.01 IU/ml with zero doses to 3.65 IU/ml with four doses. Among parous women whose TT vaccination status was as of their last birth, proportions seroprotected and median antibody levels by dose were higher than those of nulliparous women but exhibited a similar trend (Table 3).
TABLE 3.
Estimated serologic protection for women aged 15 to 39 years from tetanus by parity and number of tetanus toxoid doses reported in Cambodia in 2012
| Parity and no. of dosesa | Total no. | Seroprotectedb |
Antibody level (IU/ml) |
||||
|---|---|---|---|---|---|---|---|
| No. | % | LCLc | UCLd | Median | IQRe | ||
| Parous | |||||||
| 0 | 130 | 93 | 74 | 63 | 83 | 0.58 | 0.01–4.05 |
| 1 | 109 | 100 | 95 | 89 | 97 | 2.37 | 0.25–7.43 |
| 2 | 196 | 194 | 100 | 97 | 100 | 4.64 | 0.75–12.20 |
| 3 | 242 | 237 | 98 | 96 | 99 | 4.50 | 1.38–8.60 |
| 4 | 313 | 310 | 99 | 98 | 100 | 5.61 | 1.67–12.43 |
| 5+ | 365 | 364 | 100 | 98 | 100 | 5.56 | 2.04–9.95 |
| Nulliparous | |||||||
| 0 | 287 | 129 | 44 | 37 | 51 | 0.01 | 0.00–0.03 |
| 1 | 122 | 90 | 78 | 67 | 85 | 0.13 | 0.01–1.75 |
| 2 | 126 | 109 | 85 | 78 | 91 | 1.74 | 0.08–9.49 |
| 3 | 132 | 116 | 87 | 78 | 92 | 1.69 | 0.25–5.71 |
| 4 | 122 | 115 | 95 | 89 | 98 | 3.65 | 0.45–6.35 |
Total tetanus toxoid doses received until last birth if parous, or ever if nulliparous, as assessed by card or recall.
Tested by DAE, where seroprotected is defined as ≥0.01 IU/ml.
LCL, lower confidence limit.
UCL, upper confidence limit.
IQR, interquartile range.
Tetanus vaccination status during last pregnancy.
An estimated 70% (95% CI, 66 to 73%) of the parous women received TT2+ during their last pregnancy (1992 to 2012). TT2+ coverage was 18% (95% CI, 10 to 26%) lower in the northern region (56%) than in other regions (74%); TT2+ coverage among parous women aged 15 to 19 years (29%) was lower than in other age groups (range, 65 to 77%), but statistical analysis could not be performed because of sparse data (Table 4).
TABLE 4.
Estimated tetanus vaccination and protection at birth coverage among parous women aged 15 to 39 years during last pregnancy in Cambodia in 2012
| Population | Totalc | TT2+a |
Protected at birthb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | LCLf | UCLg | P value | No. | % | LCLf | UCLg | P value | ||
| Overall | 1,358 | 948 | 70 | 66 | 73 | 1,119 | 83 | 79 | 86 | ||
| Region | |||||||||||
| Phnom Penh | 261 | 192 | 72 | 60 | 83 | 0.001d | 216 | 83 | 75 | 88 | 0.001d |
| Southeast | 312 | 232 | 74 | 65 | 81 | 270 | 86 | 79 | 91 | ||
| Southwest | 261 | 205 | 77 | 68 | 84 | 236 | 90 | 79 | 95 | ||
| West | 265 | 176 | 72 | 63 | 79 | 215 | 85 | 78 | 90 | ||
| North | 259 | 143 | 56 | 49 | 64 | 182 | 71 | 63 | 78 | ||
| Risk level | |||||||||||
| High/medium | 387 | 261 | 70 | 66 | 78 | 0.968 | 296 | 78 | 68 | 86 | 0.207 |
| Low | 971 | 687 | 70 | 66 | 73 | 823 | 84 | 80 | 88 | ||
| Urban/rural | |||||||||||
| Urban | 354 | 253 | 71 | 61 | 78 | 0.811 | 298 | 84 | 74 | 90 | 0.751 |
| Rural | 1,004 | 695 | 70 | 66 | 73 | 821 | 82 | 78 | 86 | ||
| Age (yr) | |||||||||||
| 15–19 | 48 | 12 | 29 | NCe | NC | NC | 15 | 36 | NC | NC | NC |
| 20–24 | 226 | 148 | 70 | 63 | 77 | 180 | 85 | 78 | 90 | ||
| 25–29 | 395 | 294 | 71 | 64 | 76 | 344 | 87 | 82 | 91 | ||
| 30–34 | 397 | 301 | 77 | 71 | 83 | 352 | 89 | 84 | 93 | ||
| 35–39 | 292 | 193 | 65 | 56 | 73 | 228 | 74 | 64 | 82 | ||
Defined as receipt of ≥2 TT doses during last pregnancy or 1 dose during last pregnancy and ≥1 prior dose by card or recall.
Defined as receipt of ≥2 TT doses during the last pregnancy or a total of ≥2 doses prior to the last pregnancy by card or recall. The latter part of this simplified definition is based on results reported by Deming et al., where 30 (94%) of 32 newborns were born within the period of protection afforded by the prepregnancy doses (35).
Number of parous women responding about their last pregnancies, which occurred during 1992 to 2012.
Groups were statistically significantly different by Rao-Scott chi-square test.
NC, not calculated. Because of sparse data for parous women aged 15 to 19 years, 95% CIs and P values for differences across age groups were not calculated.
LCL, lower confidence limit.
UCL, upper confidence limit.
Estimated PAB coverage at the last pregnancy was 83% (95% CI, 79 to 86%). PAB coverage in the northern region (71%) was 16% (95% CI, 8 to 24%) lower than in the other regions (86%); PAB coverage among parous women aged 15 to 19 years (36%) was lower than that in the other age groups (range, 74 to 86%) (Table 4).
Accuracy of the MBA relative to DAE.
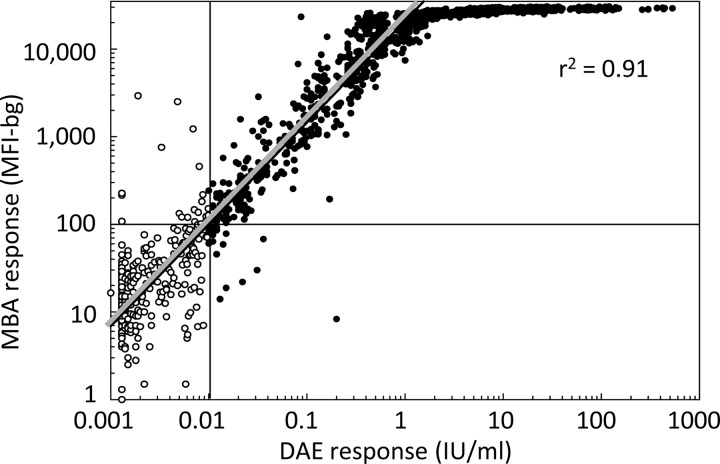
The sensitivity of the MBA relative to DAE was 99% (95% CI, 98 to 99%), and specificity was 92% (95% CI, 88 to 95%). MBA and DAE responses were highly correlated (r2 = 0.91) (Table 5 and Fig. 3). Estimates of seroprotection by MBA and DAE were similar for women aged 15 to 39 years overall and by subpopulation; all of the subpopulation differences identified by DAE were identified by MBA (Tables 1 and 6).
TABLE 5.
Concordance of tetanus serological results obtained by gold-standard DAE and a novel MBA in Cambodia in 2012
| Result | No. of specimens by DAE |
Total for MBA | |
|---|---|---|---|
| Positivea | Negative | ||
| MBA | |||
| Positiveb | 1,843 | 23 | 1,866 |
| Negative | 19 | 265 | 284 |
| Total for DAE | 1,862 | 288 | 2,150 |
Defined as ≥0.01 IU/ml.
Defined as ≥100 MFI-bg, which is equivalent to ≥0.01 IU/ml.
FIG 3.
Scatterplot of tetanus MBA responses versus DAE responses. Tetanus antibody levels of women aged 15 to 39 years in Cambodia were determined by a gold-standard DAE and a novel MBA. A DAE result of <0.01 IU/ml was considered seronegative for tetanus (white circles), and ≥0.01 IU/ml was considered seroprotective (black circles) (19). Linear regression was performed on log-transformed DAE values between 0.004 and 1.6 IU/ml, where the regression line (gray) had the equation log (MBA result) = 1.16 log (DAE result) + 4.32. On the basis of this analysis, MBA values of ≥100 MFI-bg units were considered seroprotected. The regression line goodness of fit was assessed with the r2 statistic, where 0.0 represents no linear relationship between the results and 1.0 represents a perfect linear relationship.
TABLE 6.
Estimated tetanus serologic protection of women aged 15 to 39 years by MBA in Cambodia in 2012
| Characteristic | Total no. | Seroprotecteda |
||||
|---|---|---|---|---|---|---|
| No. | % | LCLb | UCLc | P value | ||
| Overall | 2,150 | 1,866 | 87 | 85 | 89 | |
| Region | ||||||
| Phnom Penh | 469 | 407 | 87 | 81 | 90 | 0.045d |
| Southeast | 419 | 373 | 89 | 84 | 93 | |
| Southwest | 423 | 371 | 89 | 84 | 92 | |
| West | 445 | 358 | 81 | 76 | 85 | |
| North | 394 | 357 | 90 | 82 | 95 | |
| Risk level | ||||||
| High/medium | 561 | 501 | 88 | 84 | 91 | 0.616 |
| Low | 1,589 | 1,365 | 87 | 84 | 90 | |
| Urban/rural | ||||||
| Urban | 655 | 565 | 85 | 82 | 88 | 0.145 |
| Rural | 1,495 | 1,301 | 88 | 85 | 90 | |
| Age (yr) | ||||||
| 15–19 | 435 | 264 | 63 | 57 | 69 | <0.001d |
| 20–24 | 468 | 401 | 85 | 79 | 89 | |
| 25–29 | 483 | 460 | 96 | 93 | 97 | |
| 30–34 | 449 | 440 | 97 | 94 | 99 | |
| 35–39 | 315 | 301 | 96 | 94 | 98 | |
| Parity | ||||||
| Parous | 1,356 | 1,301 | 97 | 95 | 98 | <0.001d |
| Nulliparous | 794 | 565 | 71 | 66 | 75 | |
Defined as ≥100 MFI-bg, which is equivalent to ≥0.01 IU/ml by DAE.
LCL, lower confidence limit.
UCL, upper confidence limit.
Groups were statistically significantly different by Rao-Scott chi-square test.
DISCUSSION
This first national estimate of tetanus population immunity in Cambodia was 88% among WRA in 2012. However, a lower immunity level was observed among women aged 15 to 19 years (63%) and 20 to 24 years (87%) than women aged ≥25 years (96%), among nulliparous women than parous women (71 versus 97%), and among women living in the western region than women living in the other regions (82 versus 89%).
The estimated seroprotection among WRA (88%) was 15% higher than the national administrative TT2+ coverage of pregnant women in 2012 (73%), similar to serosurvey findings in Burundi and the CAR (10, 11, 35, 36). Similarly, the estimated PAB coverage (83%) in this survey was higher than the estimated TT2+ coverage (70%). TT2+ coverage underestimates the true protection from tetanus, as it excludes women unvaccinated during pregnancy but already protected through previous vaccination or who received one dose in pregnancy and had undocumented previous doses (37). Seroprotection among WRA (88%) was closer to the PAB coverage estimated by the WHO and the United Nations Children's Fund in 2012 (91%) (11, 37). PAB is the preferred indicator for monitoring MNTE but is not calculated by many country programs because it requires that vaccinators question mothers about TT vaccination history by using a definition including minimal dose intervals and the expected duration of protection for the last dose received (34, 38).
The proportions of women with tetanus seroprotection and antibody levels of ≥1.0 IU/ml were lower among those aged 15 to 19 years (63 and 31%, respectively) and 20 to 24 years (87 and 60%) than among those aged ≥25 years (96 and 76%), similar to results for polio, measles, and rubella serosurveys in Cambodia (27). The discrepancy in seroprotection noted between parous (97%) and nulliparous (71%) women and the large proportion of parous women with antibody levels of ≥1.0 IU/ml (78%) suggest effective vaccination of most pregnant women through routine immunization (RI) services. However, the low seroprotection level (63%) among parous and nulliparous women aged 15 to 19 years indicates missed TT vaccinations during first pregnancies and a lack of targeting through TT SIAs. These gaps pose a threat to future population immunity unless a greater effort is made to reach younger women through RI services and TT SIAs are continued in high-risk areas with insufficient RI coverage.
Lower tetanus seroprotection was noted in the western region (82%), despite a median PAB coverage of 89% (range, 80 to 93%) for these five provinces in the 2010 DHS (11). In the northern region, which contained the most high-risk ODs in recent tetanus risk assessments, the seroprotection was 94%, despite 75% (range, 62 to 94%) median PAB coverage among these seven provinces in the 2010 DHS (11). Increased susceptibility was not noted in the high/medium-risk areas from the 2009 risk assessment, indicating that the identified immunity gaps were closed by SIAs targeting high- and medium-risk areas (the majority of which were in the northern region) during 2009 to 2012 or that the risk assessment algorithm was insufficient in distinguishing high-risk areas. No regional differences in immunity were noted in the polio, measles, and rubella serosurveys in Cambodia (27).
Determination of true population immunity by serosurvey is helpful in light of the challenges of collecting accurate vaccination histories (14, 35). Six tetanus doses are required for lifelong immunity when vaccination is begun in infancy, but childhood vaccination records are rarely available for data abstraction in surveys of adult women (28). Nulliparous women who reported never receiving TT had higher rates of seroprotection (44%) and median antibody levels (0.01 IU/ml) than expected. This is likely because of residual immunity from infant doses and/or booster doses forgotten or unrecorded on vaccination cards, such as SIA doses. After MNTE validation, serosurveys to monitor maintenance efforts would be especially useful in countries like Cambodia, where childhood TTCV booster doses are not offered through RI services and SIAs in high-risk areas have served as the primary strategy to achieve MNTE (3).
The observed high sensitivity (99%), specificity (92%), and correlation (r2 = 0.91) of MBA relative to DAE were similar to previous findings of a tetanus MBA validation against a ToBI test (r2 = 0.96) (24). The MBA performed better than indirect ELISAs at low seroprotective antibody levels (0.01 to 0.2 IU/ml), allowing the use of a lower threshold of seroprotection (0.01 versus 0.1 to 0.2 IU/ml) (19–21, 28). The MBA and DAE identified the same subpopulations with immunity gaps, where additional strengthening of RI services or TTCV SIAs may be required. MBAs could potentially be used in nationally representative serosurveys and recurring surveys where serum samples could be collected (e.g., DHS) to assess population immunity to multiple viral, bacterial, and parasitic antigens with a small serum sample volume (1 μl). In this way, MNTE monitoring with the MBA could be integrated into existing activities, because repeated national serosurveys for individual pathogens are not logistically or financially viable.
Our evaluation had several limitations. Survey sampling was based on the 2008 census, resulting in underrepresentation of new settlements or those experiencing disproportionate growth. TT2+ and PAB coverage estimates for parous women at the time of their last births, occurring during 1992 to 2012, had limited comparability to coverage estimates from other sources or to our seroprotection estimates because of the potential for waning immunity or receipt of additional TT doses prior to the survey. The small number of clusters in high-risk areas from the 2009 tetanus risk assessment necessitated that analysis be completed for high/medium-risk areas instead. The MBA was performed at a single serum dilution chosen to be most informative across all of the antigens of interest, which prevented an assessment of exact antibody concentrations at high antitetanus levels and sacrificed some precision at values close to the seroprotection threshold, where variation in values from duplicate tests represents a larger proportion of the overall value.
Serosurvey findings are compatible with MNTE in Cambodia (≥80% TT2+/PAB coverage), which was validated in June 2015, and are useful given difficulties in assessing tetanus vaccination in adults through administrative vaccination coverage or coverage surveys (39, 40). Tetanus immunity gaps among young and nulliparous women should be addressed through strengthening RI services and the addition of childhood TTCV booster doses or continuing TTCV campaigns. Inclusion of tetanus testing in national serosurveys using MBAs, which can be tailored to evaluate the impact of multiple public health programs simultaneously, should be considered in countries where MNTE monitoring is necessary.
ACKNOWLEDGMENTS
We acknowledge the Cambodian field staff that conducted this serosurvey. We also acknowledge the assistance of Marty Roper, global tetanus subject matter expert; Penelope Campbell and Rownak Khan of UNICEF; and Minal Patel, Linda Quick, Ethleen Lloyd, and Dora Warren of the CDC.
We have no conflicts of interest to declare. The use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Footnotes
For a commentary on this article, see doi:10.1128/CVI.00259-16.
REFERENCES
- 1.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE, Child Health Epidemiology Reference Group of WHO and UNICEF . 2012. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, et al. . 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Achieving and sustaining maternal and neonatal tetanus elimination: strategic plan 2012–2015. World Health Organization, Geneva, Switzerland: http://www.who.int/immunization/diseases/MNTEStrategicPlan_E.pdf. Accessed 11 August 2014. [Google Scholar]
- 4.Hennessey K, Schluter WW, Wang X, Boualam L, Jee Y, Mendoza-Aldana J, Roesel S, Diorditsa S, Ehrenberg J. 2014. Are we there yet? Assessing achievement of vaccine-preventable disease goals in WHO's western Pacific region. Vaccine 32:4259–4266. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. 2015. Maternal and neonatal tetanus (MNT) elimination: the partnership. World Health Organization, Geneva, Switzerland: http://www.who.int/immunization/diseases/MNTE_initiative/en/index1.html Accessed 7 August 2015. [Google Scholar]
- 6.World Health Organization. 2006. Tetanus vaccine: WHO position paper. Wkly Epidemiol Rec 20:198–208. http://www.who.int/wer/2006/wer8120.pdf?ua=1. [Google Scholar]
- 7.Cambodia Ministry of Health. 2008. Cambodia national immunization program strategic plan 2008–2015. Cambodia Ministry of Health, Phnom Penh, Cambodia: http://www.wpro.who.int/health_services/cambodia_nationalhealthplan.pdf. [Google Scholar]
- 8.World Health Organization. 2012. Neonatal and child health country profile: Cambodia. World Health Organization, Geneva, Switzerland: http://www.who.int/maternal_child_adolescent/epidemiology/profiles/neonatal_child/khm.pdf?ua=1 Accessed 23 September 2014. [Google Scholar]
- 9.United Nations Children's Fund Cambodia. 2009. Progress report: maternal and neonatal tetanus elimination in Cambodia. United Nations Children's Fund Cambodia, Phnom Penh, Cambodia. [Google Scholar]
- 10.World Health Organization. 2014. WHO vaccine-preventable diseases monitoring system, global summary: coverage time series for Cambodia (KHM). World Health Organization, Geneva, Switzerland: http://apps.who.int/immunization_monitoring/globalsummary/coverages?c=KHM Accessed 14 August 2014. [Google Scholar]
- 11.Anonymous. 2011. Cambodia demographic and health survey 2010. ICF Macro, Calverton, MD: https://dhsprogram.com/pubs/pdf/FR249/FR249.pdf. [Google Scholar]
- 12.Anonymous. 2001. Cambodia demographic and health survey 2000. ORC Macro, Calverton, MD: http://dhsprogram.com/pubs/pdf/FR124/FR124.pdf. [Google Scholar]
- 13.World Health Organization. 2014. WHO vaccine-preventable diseases monitoring system, 2014 global summary: incidence time series for Cambodia (KHM). World Health Organization, Geneva, Switzerland: http://apps.who.int/immunization_monitoring/globalsummary/incidences?c=KHM Accessed 27 August 2014. [Google Scholar]
- 14.MacNeil A, Lee CW, Dietz V. 2014. Issues and considerations in the use of serologic biomarkers for classifying vaccination history in household surveys. Vaccine 32:4893–4900. doi: 10.1016/j.vaccine.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendriksen CF, vd Gun JW, Nagel J, Kreeftenberg JG. 1988. The toxin binding inhibition test as a reliable in vitro alternative to the toxin neutralization test in mice for the estimation of tetanus antitoxin in human sera. J Biol Stand 16:287–297. [DOI] [PubMed] [Google Scholar]
- 16.Hong HA, Ke NT, Nhon TN, Thinh ND, van der Gun JW, Hendriks JT, Kreeftenberg JG. 1996. Validation of the combined toxin-binding inhibition test for determination of neutralizing antibodies against tetanus and diphtheria toxins in a vaccine field study in Viet Nam. Bull World Health Organ 74:275–282. [PMC free article] [PubMed] [Google Scholar]
- 17.Sonobe MH, Trezena AG, Guilhen FB, Takano VL, Fratelli F, Sakauchi D, Morais JF, Prado SM, Higashi HG. 2007. Determination of low tetanus or diphtheria antitoxin titers in sera by a toxin neutralization assay and a modified toxin-binding inhibition test. Braz J Med Biol Res 40:69–76. [DOI] [PubMed] [Google Scholar]
- 18.Gidding HF, Backhouse JL, Burgess MA, Gilbert GL. 2005. Immunity to diphtheria and tetanus in Australia: a national serosurvey. Med J Aust 183:301–304. [DOI] [PubMed] [Google Scholar]
- 19.Kristiansen M, Aggerbeck H, Heron I. 1997. Improved ELISA for determination of anti-diphtheria and/or anti-tetanus antitoxin antibodies in sera. APMIS 105:843–853. [DOI] [PubMed] [Google Scholar]
- 20.Dokmetjian J, Della Valle C, Lavigne V, de Lujan CM, Manghi MA. 2000. A possible explanation for the discrepancy between ELISA and neutralising antibodies to tetanus toxin. Vaccine 18:2698–2703. doi: 10.1016/S0264-410X(00)00066-9. [DOI] [PubMed] [Google Scholar]
- 21.Simonsen O, Schou C, Heron I. 1987. Modification of the ELISA for the estimation of tetanus antitoxin in human sera. J Biol Stand 15:143–157. [DOI] [PubMed] [Google Scholar]
- 22.Pickering JW, Martins TB, Schroder MC, Hill HR. 2002. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for quantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae type B. Clin Diagn Lab Immunol 9:872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reder S, Riffelmann M, Becker C, Wirsing von Konig CH. 2008. Measuring immunoglobulin G antibodies to tetanus toxin, diphtheria toxin, and pertussis toxin with single-antigen enzyme-linked immunosorbent assays and a bead-based multiplex assay. Clin Vaccine Immunol 15:744–749. doi: 10.1128/CVI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Gageldonk PG, van Schaijk FG, van der Klis FR, Berbers GA. 2008. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J Immunol Methods 335:79–89. doi: 10.1016/j.jim.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Wagner KS, White JM, Andrews NJ, Borrow R, Stanford E, Newton E, Pebody RG. 2012. Immunity to tetanus and diphtheria in the UK in 2009. Vaccine 30:7111–7117. doi: 10.1016/j.vaccine.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 26.Basta NE, Borrow R, Berthe A, Onwuchekwa U, Dembele AT, Almond R, Frankland S, Patel S, Wood D, Nascimento M, Manigart O, Trotter CL, Greenwood B, Sow SO. 2015. Higher tetanus toxoid immunity 2 years after PsA-TT introduction in Mali. Clin Infect Dis 61(Suppl 5):S578–S585. doi: 10.1093/cid/civ513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao B, Chheng K, Wannemuehler K, Vynnycky E, Buth S, Soeung SC, Reef S, Weldon W, Quick L, Gregory CJ. 2015. Immunity to polio, measles and rubella in women of child-bearing age and estimated congenital rubella syndrome incidence, Cambodia, 2012. Epidemiol Infect 143:1858–1867. doi: 10.1017/S0950268814002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borrow R, Balmer P, Roper MH. 2007. The immunological basis for immunization series. Module 3: tetanus update 2006. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/43687/1/9789241595551_eng.pdf. [Google Scholar]
- 29.Moss DM, Priest JW, Boyd A, Weinkopff T, Kucerova Z, Beach MJ, Lammie PJ. 2011. Multiplex bead assay for serum samples from children in Haiti enrolled in a drug study for the treatment of lymphatic filariasis. Am J Trop Med Hyg 85:229–237. doi: 10.4269/ajtmh.2011.11-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Priest JW, Moss DM, Arnold BF, Hamlin K, Jones CC, Lammie PJ. 2015. Seroepidemiology of Toxoplasma in a coastal region of Haiti: multiplex bead assay detection of immunoglobulin G antibodies that recognize the SAG2A antigen. Epidemiol Infect 143:618–630. doi: 10.1017/S0950268814001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamlin KL, Moss DM, Priest JW, Roberts J, Kubofcik J, Gass K, Streit TG, Nutman TB, Eberhard ML, Lammie PJ. 2012. Longitudinal monitoring of the development of antifilarial antibodies and acquisition of Wuchereria bancrofti in a highly endemic area of Haiti. PLoS Negl Trop Dis 6:e1941. doi: 10.1371/journal.pntd.0001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waterboer T, Sehr P, Pawlita M. 2006. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods 309:200–204. doi: 10.1016/j.jim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. 2015. Vaccination coverage cluster surveys: reference manual, version 3, working draft (updated July). World Health Organization, Geneva, Switzerland: http://www.who.int/immunization/monitoring_surveillance/Vaccination_coverage_cluster_survey.pdf?ua=1 Accessed 23 October 2015. [Google Scholar]
- 34.World Health Organization. 2000. Protection-at-birth (PAB) method, Tunisia. Wkly Epidemiol Rec 75:203–206. [PubMed] [Google Scholar]
- 35.Deming MS, Roungou JB, Kristiansen M, Heron I, Yango A, Guenengafo A, Ndamobissi R. 2002. Tetanus toxoid coverage as an indicator of serological protection against neonatal tetanus. Bull World Health Organ 80:696–703. [PMC free article] [PubMed] [Google Scholar]
- 36.Anonymous. 1996. Expanded programme on immunization—estimating tetanus protection of women by serosurvey. Wkly Epidemiol Rec 71:117–124. [PubMed] [Google Scholar]
- 37.World Health Organization. 2014. Cambodia: WHO/UNICEF estimates of protection at birth (PAB) against tetanus (2013 revision). World Health Organization, Geneva, Switzerland: http://www.who.int/immunization/monitoring_surveillance/data/khm.pdf. Accessed 14 August 2015. [Google Scholar]
- 38.World Health Organization. 2006. Maternal immunization against tetanus: integrated management of pregnancy and childbirth. World Health Organization, Geneva, Switzerland: http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/immunization_tetanus.pdf Accessed 28 August 2014. [Google Scholar]
- 39.Stroh G, Birmingham M. 2002. Protocol for assessing neonatal tetanus mortality in the community using a combination of cluster and lot quality assurance sampling: field test version. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/67193/1/WHO_V-B_02.05_eng.pdf. [Google Scholar]
- 40.World Health Organization. 2000. Maternal and neonatal tetanus elimination by 2005: strategies for achieving and maintaining elimination. World Health Organization, Geneva, Switzerland: http://www.unicef.org/french/health/files/MNTE_strategy_paper.pdf Accessed 11 August 2014. [Google Scholar]
- 41.Anonymous. 2006. Cambodia demographic and health survey 2005. ORC Macro, Calverton, MD: https://dhsprogram.com/pubs/pdf/FR185/FR185[April-27-2011].pdf. [Google Scholar]
- 42.Priest JW, Jenks MH, Moss DM, Mao B, Buth S, Wannemuehler K, Soeung SC, Lucchi NW, Udhayakumar V, Gregory CJ, Huy R, Muth S, Lammie PJ. 2016. Integration of multiplex bead assays for parasitic diseases into a national, population-based serosurvey of women 15-39 years of age in Cambodia. PLoS Negl Trop Dis 10:e0004699. doi: 10.1371/journal.pntd.0004699. [DOI] [PMC free article] [PubMed] [Google Scholar]