Abstract
A promising concept for human immunodeficiency virus (HIV) vaccines focuses immunity on the highly conserved transition state structures and epitopes that appear when the HIV glycoprotein gp120 binds to its receptor, CD4. We are developing chimeric antigens (full-length single chain, or FLSC) in which gp120 and CD4 sequences are flexibly linked to allow stable intrachain complex formation between the two moieties (A. DeVico et al., Proc Natl Acad Sci U S A 104:17477–17482, 2007, doi:10.1073/pnas.0707399104; T. R. Fouts et al., J Virol 74:11427–11436, 2000, doi:10.1128/JVI.74.24.11427-11436.2000). Proof of concept studies with nonhuman primates show that FLSC elicited heterologous protection against simian-human immunodeficiency virus (SHIV)/simian immunodeficiency virus (SIV) (T. R. Fouts et al., Proc Natl Acad Sci U S A 112:E992–E999, 2016, doi:10.1073/pnas.1423669112), which correlated with antibodies against transition state gp120 epitopes. Nevertheless, advancement of any vaccine that comprises gp120-CD4 complexes must consider whether the CD4 component breaks tolerance and becomes immunogenic in the autologous host. To address this, we performed an immunotoxicology study with cynomolgus macaques vaccinated with either FLSC or a rhesus variant of FLSC containing macaque CD4 sequences (rhFLSC). Enzyme-linked immunosorbent assay (ELISA) binding titers, primary CD3+ T cell staining, and temporal trends in T cell subset frequencies served to assess whether anti-CD4 autoantibody responses were elicited by vaccination. We find that immunization with multiple high doses of rhFLSC did not elicit detectable antibody titers despite robust responses to rhFLSC. In accordance with these findings, immunized animals had no changes in circulating CD4+ T cell counts or evidence of autoantibody reactivity with cell surface CD4 on primary naive macaque T cells. Collectively, these studies show that antigens using CD4 sequences to stabilize transition state gp120 structures are unlikely to elicit autoimmune antibody responses, supporting the advancement of gp120-CD4 complex-based antigens, such as FLSC, into clinical testing.
INTRODUCTION
Protection against human immunodeficiency virus (HIV) infection demands persistent humoral responses against the viral envelope glycoprotein that provide sterilizing immunity against a broad diversity of viral strains (1). These demands guide current efforts toward developing antibody-based HIV vaccines. One prominent approach seeks to develop vaccines that generate neutralizing antibodies specific for conserved epitopes on HIV envelope antigens gp120 and gp41 as they are configured on free virions (2). This goal has been elusive given the genetic plasticity of the HIV type 1 (HIV-1) env gene, which propagates escape variants to every known broadly neutralizing domain (3–8), coupled with the immunological complexities of generating broadly neutralizing human antibodies (9).
An alternative vaccine approach is to generate broadly protective antibody responses against indispensable epitopes that are exposed on gp120 once it binds to the host cell receptor, CD4, and establishes a key transition state structure (10–16). This highly conserved structure is absolutely required by all HIV strains for both coreceptor engagement and viral entry (16). Epitopes on transition state gp120, including those designated CD4 induced (CD4i), are now established as targets for potent Fc receptor-dependent humoral effector functions against cell-bound virions (13, 17, 18) or infected cells (19, 20). Humoral responses to CD4i gp120 epitopes have been linked with vaccine-mediated protection in nonhuman primate (NHP) challenge models with simian-human immunodeficiency virus (SHIV) or simian immunodeficiency virus (SIV) (21–24), control of HIV infection (25), and with reduced risk in the RV144 clinical trial (26, 27).
One biologically relevant approach toward developing transition state HIV vaccines is to use portions of human CD4 to bind, constrain, and stabilize gp120 (10, 12, 28, 29). Our approach tethers the D1D2 domains of human CD4 to the full-length HIV-1(BaL) gp120 via a flexible amino acid linker (Fig. 1A) (12, 21). A chimeric antigen with this design, termed full-length single chain (FLSC), forms a stable intramolecular complex that elicits anti-gp120 antibody responses against conserved transition state (CD4i) epitopes as well as other domains (e.g., V3, V1V2) believed to be important targets for protective immunity (21, 27, 30–33). We also developed a rhesus variant of FLSC (rhFLSC) in which the human CD4 D1D2 region (GenBank accession no. NP_000607.1 [34]) of FLSC was replaced with a macaque CD4 D1D2 sequence (Fig. 1B) that is 100% conserved in Macaca mulatta (GenBank accession no. D63347.1) and Macaca fascicularis (GenBank accession no. D63349.1) (Fig. 2). Thus, the use of rhFLSC in either macaque species is fully analogous to the application of FLSC in humans. In previous studies, we showed that rhFLSC can induce concurrent and balanced anti-gp120 antibody and T cell responses that correlate with protection of rhesus macaques against rectal challenge with heterologous SHIV162P3 or SIV in both single-high-dose (10, 35, 36) and multiple-low-dose (21) challenge models.
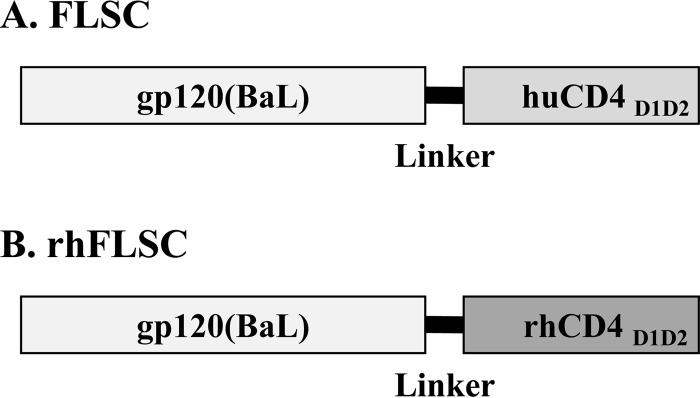
FIG 1.
Schematic diagram of the FLSC and rhFLSC proteins. (A) FLSC is comprised of gp120 from HIV-1(BaL) genetically linked to the D1 and D2 regions of human CD4 (huCD4D1D2; GenBank accession no. NP_000607.1) by 20 amino acids (glycines and serines). (B) In the rhesus version of FLSC, rhFLSC, the huCD4D1D2 has been replaced with the D1 and D2 regions of rhesus CD4 (rhCD4D1D2; GenBank accession no. D63347.1).
FIG 2.

Amino acid sequence comparison between the D1D2 regions of cynomolgus, rhesus, and human CD4. The amino acid sequences of the D1D2 regions (amino acids 26 to 203) of cynomolgus (cyno), rhesus, and human CD4 (GenBank accession nos. D63349.1, D63347.1, and NP_000607.1 (34), respectively) are shown. Dots depict sequence identity to the cynomolgus sequence on the top line, while boldface letters depict changes differing from the cynomolgus sequence. The rhesus and human sequences are identical to the CD4 D1D2 components contained in rhFLSC and FLSC (Fig. 1), respectively. The D1 region is shaded in light gray, while the D2 region is shaded in dark gray.
Any HIV vaccine deliberately comprising of or allowing gp120-CD4 interactions carries the potential for eliciting anti-CD4 autoantibody responses with potentially “immunotoxic” effects. This concern immediately applies to FLSC in humans, but it also extends to any HIV envelope-based vaccine construct (2) with CD4 binding capacity. It has already been shown that macaques vaccinated with unliganded HIV envelope antigens sporadically exhibit immunological evidence of gp120 binding to host cell CD4 during vaccination (37, 38). Under typical circumstances, subjects are expected to be immunologically tolerant to self-antigens, such as CD4. However, autologous antibodies can be induced against a variety of fully human biological agents that are used for therapy (39–46). Depending on their epitope specificity, autoreactive anti-CD4 antibodies have been shown to mediate CD4+ T cell depletion, suppression of T cell-dependent immune responses, or various other forms of immune dysregulation (47–54), effects that define safety concerns for HIV vaccine testing.
Whether gp120-CD4 complexes might raise autoimmune responses in humans, deleterious or otherwise, is difficult to predict from available information. Insights from natural HIV infection, where gp120-CD4 interactions are guaranteed, are limited. It is estimated that 10% of HIV-positive (HIV+) individuals develop anti-CD4 autoantibodies for unknown reasons. Any role for these responses in HIV infection or pathogenesis remains equivocal (55–67). Thus, comprehensive analyses in relevant experimental models are needed to answer this question.
Owing to its design, rhFLSC can be exploited to assess whether gp120-CD4 antigens cause a deleterious immune response to CD4 resulting in loss of immunological tolerance in the autologous host. Here we describe an immunotoxicity study using cynomolgus macaques that specifically addresses this question. The cynomolgus macaque model was selected because it can provide a reasonable measure of useful clinical biomarkers to assess immunotoxicity (68–73). Using multiple parameters, we assessed whether immunization with rhFLSC or FLSC induces anti-CD4 autoantibodies after vaccination with repeated multiple high or low doses. Our results suggest that the CD4 D1D2 moiety of gp120-CD4 complexes is not immunogenic in the autologous host, consistent with the clinical development of FLSC and related concepts in human trials.
MATERIALS AND METHODS
Vaccines.
HEK-293 cells stably expressing FLSC or rhFLSC were used in a high cell density fed-batch fermentation process to produce large quantities of FLSC or rhFLSC protein. The proteins were purified using lectin affinity chromatography using Galantahus nivalis lectin (GNL) coupled to 4% agarose beads (12, 74) followed by ion exchange and hydrophobic interaction chromatography. The proteins were concentrated and diafiltered into formulation buffer (5 mM sodium acetate [NaOAc], 40 mg/ml mannitol, pH 6.2). Prior to fill/finish, the proteins were adjusted to 0.3 mg/ml in formulation buffer and then formulated with aluminum phosphate (alum; Catalent Pharma Solutions, Middleton, WI) by adding 20 mg/ml alum in 0.8% NaCl to a final concentration of 2.4 mg/ml (Catalent Pharma Solutions).
Animals and vaccinations.
The cynomolgus macaque studies were performed at BIOQUAL, Inc. (Rockville, MD) and Advanced Biosciences Laboratory Inc. (Rockville, MD). The animal study protocols were approved by the IACUC committee at BIOQUAL, Inc. This study followed the guidelines outlined in BIOQUAL's Environment Enhancement Plan to promote the physical and behavioral health as well as overall well-being of the nonhuman primates (NHPs) involved in these studies. The animals were housed at BIOQUAL in a facility that is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International), USDA Registered, and has a category 1 assurance from the Office of Laboratory Animal Welfare (assurance A3086-01).
Animals were observed twice daily for overall health and well-being. The animals were observed for any notable changes in stool condition, overall food consumption, evidence of trauma, signs of pain or distress, and the appearance of any clinical signs or symptoms of poor health. Additionally, blood was analyzed at regular intervals for any abnormal hematological changes. Any abnormal observations were conveyed to the attending BIOQUAL veterinarian for further assessment and, if necessary, treatment.
Multiple high- and low-dose FLSC and rhFLSC vaccinations.
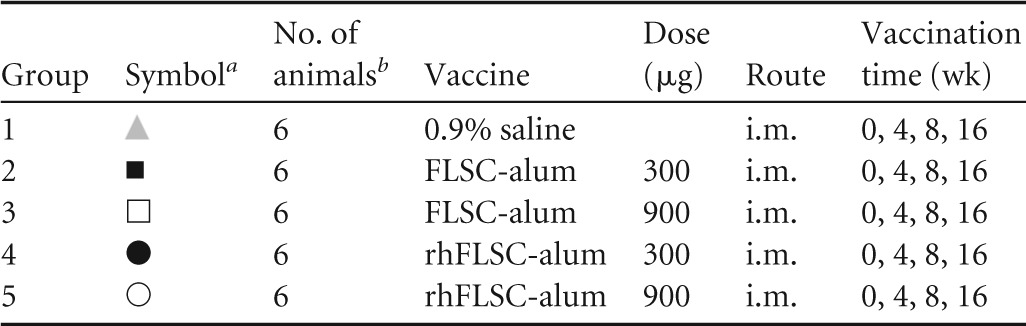
The study design is shown in Table 1. Cynomolgus macaques (three per gender per group) were injected intramuscularly (i.m.) in the quadriceps with 300 or 900 μg of FLSC or rhFLSC or 0.9% saline as a control. The immunizations were administered four times throughout the study on weeks 0, 4, 8, and 16. One week after the third and fourth immunizations, 10 mg keyhole limpet hemocyanin (KLH) was administered subcutaneously (s.c.) between the scapulae. KLH was administered to study T cell-dependent antibody responses, which will be presented in a subsequent article. Peripheral blood was collected from each animal every 1 to 2 weeks and processed (e.g., sera, peripheral blood mononuclear cells [PBMCs]) for subsequent assays as indicated. Animals were observed daily for abnormal clinical signs and symptoms. Physical examinations, body temperatures, and weights were obtained at least weekly. Body temperatures were also obtained 24 h after each immunization.
TABLE 1.
Vaccination groups used in this study
T cell counts.
A TruCount immunophenotyping method (BD Biosciences, San Jose, CA) was used to quantify absolute counts and percentages of the subpopulation of cells, including CD3+ CD4+ T cells, CD3+ CD8+ T cells, CD20+ CD3− B cells, and total CD45+ CD14− leukocytes. For the fluorescence-activated cell sorting (FACS) analysis of the data, it was ensured that a minimum of 5,000 CD45+ leukocytes were acquired and that multicheck and multicheck low-positive control (stabilized human blood) samples yielded results consistent with expected values.
Anti-FLSC or anti-rhFLSC capture ELISAs.
High-binding 96-well Immulon 2HB microtiter plates (Thermo Fisher Scientific) were coated overnight at 4°C with 50 μl/well of a capture antibody specific for the D7 epitope of HIV gp120 (i.e., D7324 affinity-purified sheep anti-HIV gp120 antibody; Aalto Bio Reagents, Dublin, Ireland) at 2 μg/ml in 1× phosphate-buffered saline (PBS). The plates were washed three times with 400 μl/well of 1× Tris-buffered saline with Tween 20 (TBST) (0.05% Tween 20 in 1× Tris-buffered saline) and blocked for 1 h with 330 μl/well of Blotto buffer (5% [wt/vol] nonfat milk in 1× TBST). The plates were washed again, and then 100 μl/well of purified antigen (e.g., FLSC, rhFLSC) at 1 μg/ml in Blotto buffer was added to each plate and allowed to bind for 1 h. Control and test samples serially diluted in Blotto buffer were added at 100 μl/well to the washed antigen-coated plates and allowed to bind for 1 h. After another washing, 50 μl/well of horseradish peroxidase (HRP)-conjugated goat anti-monkey IgG (Kirkegaard & Perry Laboratories [KPL], Gaithersburg, MD) diluted 1:1,000 in Blotto buffer was then added and incubated for 1 h. The plates were washed as described above, and the amount of peroxidase bound on the plate was determined by a colorimetric reaction using the SureBlue 3,3′,5,5′-tetramethylbenzidine (TMB) one-component microwell HRP substrate (KPL) at 100 μl/well. The reaction was stopped with 50 μl of 1 N H2SO4, and the absorbance was measured at 450 nm with a SpectraMax Plus384 microplate reader (Molecular Devices, Sunnyvale, CA). Data were evaluated using SoftMaxPro v 5.4 (Molecular Devices) and Excel v 2010 (Microsoft) software. Prior to assessing test samples, assay/batch controls were established, and methods were verified.
Cross-competition ELISAs.
Competition enzyme-linked immunosorbent assays (ELISAs) were performed as previously described (10, 27). Briefly, high-binding 96-well Immulon 2HB microtiter plates (Thermo Fisher Scientific) were coated with the D7324 capture antibody specific for the D7 epitope of HIV gp120, and purified antigen (e.g., FLSC, rhFLSC) was bound to the capture antibody-coated plates as described above. Biotinylated human monoclonal antibodies (2X) specific for CD4i epitopes (i.e., A32 [15, 16, 75–79], 17b [15, 77–79], 19e [36]; obtained from the Institute of Human Virology, Baltimore, MD) were mixed 1:1 with serially diluted test sera or unbiotinylated antibody (i.e., control) in Blotto buffer and incubated for 10 min. Fifty microliters of the antibody mixtures were added to washed D7324 captured antigen plates and allowed to bind for 1 h. After another washing, 50 μl/well of HRP-conjugated polystreptavidin (Thermo Fisher Scientific) diluted 1:5,000 in Blotto buffer was then added and incubated for 1 h. The plates were washed, the amount of peroxidase bound on the plate was determined by a colorimetric reaction, and data were analyzed as described above. Prior to assessing test samples, assay/batch controls were established, and the competitive ELISA methods were verified.
Anti-CD4 solid-phase ELISAs.
Anti-CD4 ELISAs were performed using a qualified in-house optimized assay described below. Briefly, high-binding 96-well Immulon 2HB microtiter plates (Thermo Fisher Scientific) were coated overnight at 4°C with 50 μl/well of one of the following recombinant CD4 proteins at 1 μg/ml in 1× PBS: (i) histidine (His)-tagged cynomolgus CD4 (cyCD4; Sino Biological, Beijing, China); (ii) His-tagged rhesus CD4 (rhCD4; eEnzyme, Gaithersburg, MD); or (iii) Leu3-His-tagged human CD4 (Ectodomain) (huCD4; Sino Biological). The plates were washed four times with 400 μl/well of 1× TBST and blocked for 1 h with 330 μl/well of bovine serum albumin (BSA) buffer (2% [wt/vol] BSA in 1× TBST). The plates were washed again, and anti-CD4 antibody control (CD4R1; NIH Nonhuman Primate Reagent Resource, Boston, MA [R24 OD010976 and NIAID contract HHSN 272201300031C]) and test samples serially diluted in BSA buffer were added at 100 μl/well to the washed antigen-coated plates and allowed to bind for 1 h. After another washing, 50 μl/well of HRP-conjugated mouse anti-monkey IgG antibody (KPL) diluted 1:5,000 in BSA buffer was added and incubated for 1 h. The plates were washed as described above and detected and evaluated as described above for anti-FLSC capture ELISAs. Prior to assessing test samples, assay/batch controls were established, and methods were verified.
Flow cytometry.
Briefly, naive cynomolgus PBMCs were incubated with test serum samples. Binding of antibodies to the PBMCs was assessed by flow cytometry. The results are presented as serum binding as a percentage of CD3+ T cells. A “fluorescence minus one” (FMO) background reference was used as a negative control, while anti-CD4 and anti-CD8 antibodies were used as positive controls for binding.
RESULTS
FLSC vaccination induces robust antigen-specific antibody responses in cynomolgus macaques.
Cynomolgus macaques (six animals/group) were immunized i.m. with either FLSC or rhFLSC (Fig. 1) formulated in alum or a saline control four times on weeks 0, 4, 8, and 16 (Table 1). The FLSC and rhFLSC vaccines were administered at either 300 or 900 μg per dose (Table 1), matching planned clinical applications. Sera, collected approximately every 2 weeks, were subjected to ELISAs and assessed for the presence of antibodies specific for FLSC. After the first immunization with FLSC or rhFLSC, all groups had significant levels of anti-FLSC antibody titers with less than a half-log-unit difference between the high-dose (900-μg) and low-dose (300-μg) groups (Fig. 3A, open versus closed symbols). After boosting with a second FLSC or rhFLSC dose on week 4, the anti-FLSC titers increased by ∼2 log units by week 6 with no significant difference between the high- and low-dose groups (Fig. 3A). A third immunization with FLSC or rhFLSC on week 8 did not boost the anti-FLSC titers further.
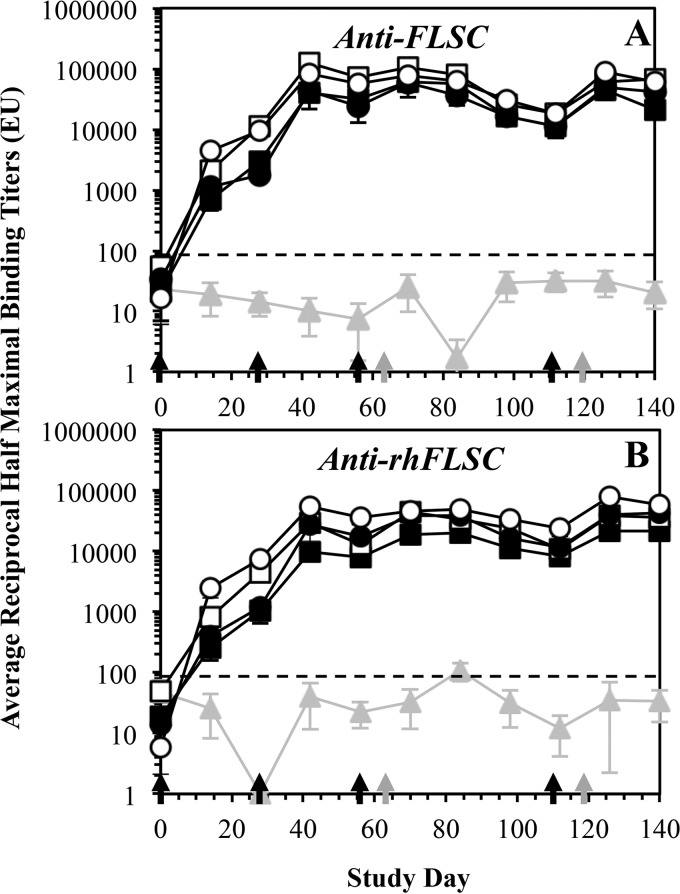
FIG 3.
Anti-FLSC and anti-rhFLSC titers in cynomolgus macaques vaccinated with multiple high or low doses of FLSC or rhFLSC. Cynomolgus macaques (six per group) were immunized i.m. four times with FLSC (squares) or rhFLSC (circles) at 300 μg (solid symbols) or 900 μg (open symbols) per dose or 0.9% saline (gray triangles and gray lines) as a control. Immunization days are indicated by black arrows along the x axis. Gray arrows along the x axis indicate days of KLH (10 mg s.c.) administration. Sera collected (every ∼2 weeks) from each animal at the indicated time points were subjected to ELISAs. The average anti-FLSC (A) or anti-rhFLSC (B) reciprocal half-maximal titers (ELISA units [EU]) are shown. Values are means ± standard errors of the means (SEMs) (error bars). In these assays, reciprocal half-maximal titers of <100 are considered negative as indicated by the black dashed lines.
These high titers of anti-FLSC antibodies were relatively stable and dropped by only about a half log unit by week 16. Following a fourth immunization on week 16, the anti-FLSC titers returned to their peak levels (e.g., week 6 levels) (Fig. 3A). The anti-FLSC antibody titers were robust, since by 9 months after the first immunization, the average titers dropped by approximately 1 log unit from the peak time points for each immunized group (data not shown). These data demonstrate that the anti-FLSC antibody response is long-lasting and comparable between the high-dose (900-μg) and low-dose (300-μg) groups (Fig. 3A, open versus closed symbols). Immunization with rhFLSC also generated antibodies that were cross-reactive to FLSC (Fig. 3A, circles) and were similar in magnitude to those elicited by vaccination with FLSC (Fig. 3A, squares). Furthermore, no significant gender-related differences in the anti-FLSC antibody responses were observed between any of the groups (data not shown).
Rhesus FLSC vaccination induces antibody responses comparable to those induced by FLSC vaccination in cynomolgus macaques.
The sera, collected approximately every 2 weeks, were subjected to additional ELISAs and assessed for the presence of antibodies specific for rhFLSC. The anti-rhFLSC titers (Fig. 3B) were similar in magnitude and durability to those elicited against FLSC (Fig. 3A). After the first immunization with FLSC or rhFLSC, all groups had significant levels of anti-rhFLSC antibodies with approximately a half-log-unit difference between the high-dose (900-μg) and low-dose (300-μg) groups (Fig. 3B, open versus closed symbols), which were boosted after a second immunization on week 4 (Fig. 3B). The third and fourth immunizations of FLSC or rhFLSC on weeks 8 and 16, respectively, did not significantly boost the anti-rhFLSC titers further (Fig. 3B). These data show that the anti-rhFLSC antibody response (Fig. 3B) is as robust as the anti-FLSC antibody response (Fig. 3A) and comparable among the groups. In this regard, sera from FLSC-immunized animals (Fig. 3B, squares) had cross-reactive titers to rhFLSC that were similar in magnitude to the titers to FLSC (Fig. 3A), indicating that antibodies generated against human CD4 do not substantially contribute to the measured titers to FLSC. Furthermore, no significant gender-related differences were observed in the anti-rhFLSC antibody responses (data not shown).
Vaccination elicits CD4i antibody responses.
Previously we have shown that immunization with FLSC (12) and rhFLSC induced CD4i antibodies (16, 21), which correlated with (i) postinfection control of viremia in rhesus macaques after high-dose challenge with SHIV162P3 (16) and (ii) protection from acquisition after repeated low-dose challenges with SHIV162P3 or SIVmac251 in three independent rhesus macaque studies (21). Anti-FLSC and anti-rhFLSC antibodies were robustly induced after our multiple high- or low-dose vaccination regimen (Fig. 3). To confirm that this regimen elicits the expected humoral response to conserved CD4i epitopes, we performed cross-competition ELISAs using the human monoclonal anti-CD4i antibodies, A32 (15, 16, 75–79), 17b (15, 77–79), and 19e (36). FLSC and rhFLSC induced antibodies cross-competitive with A32 (Fig. 4A), 17b (Fig. 4B), and 19e (Fig. 4C) in a dose-dependent manner.
FIG 4.
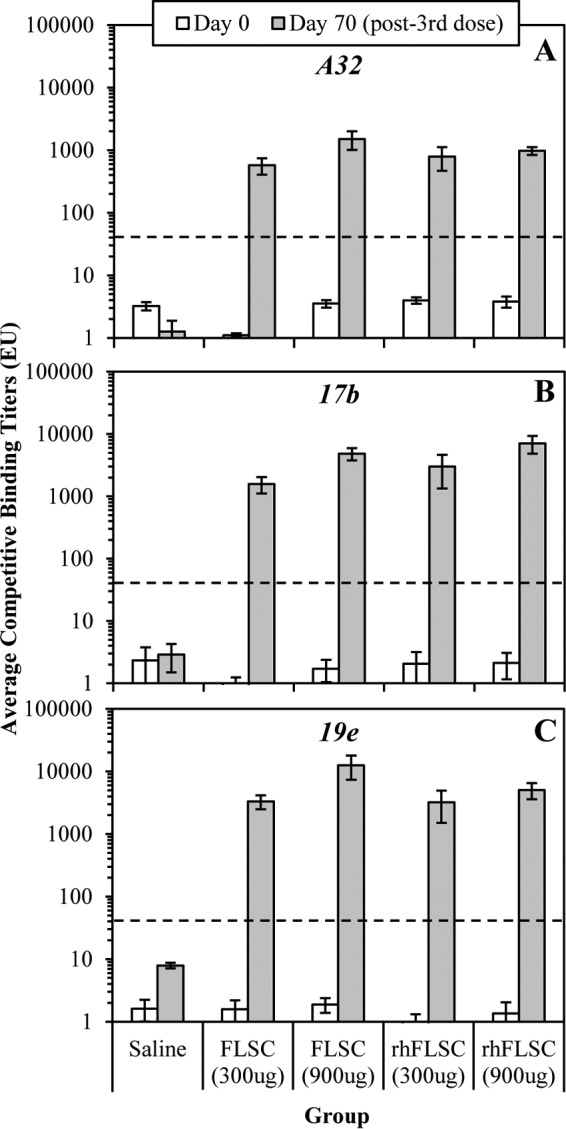
Vaccination induces antibodies reactive to CD4i epitopes. Immune sera collected from cynomolgus macaques immunized with FLSC or rhFLSC (Fig. 3) on days 0 and 70 (week 10 [i.e., 2 weeks after the third dose]) were subjected to cross-competition ELISAs with anti-CD4i human monoclonal antibodies specific for A32 (A), 17b (B), or 19e (C). Values are means ± SEMs (error bars). In these assays, binding titers of <30 are considered negative as indicated by the black dashed lines.
Vaccination with FLSC and rhFLSC do not alter circulating leukocyte frequencies.
The FDA's Guidance for Industry: S8 Immunotoxicity Studies for Human Pharmaceuticals (80) suggests that nonfunctional assays, such as immunophenotyping of leukocyte populations, “might provide useful clinical biomarkers” to assess immunotoxicity. To this end, cell populations in the blood from each vaccinated macaque were assessed every 1 to 2 weeks, including 24 h postimmunization. Cell populations evaluated include but are not limited to lymphocytes, platelets, white blood cells (e.g., neutrophils, monocytes, eosinophils, basophils), and red blood cells. Circulating T cell counts in blood from immunized animals were also assessed. The mean CD3+ CD4+ (Fig. 5A) and CD3+ CD8+ (Fig. 5B) T cell counts in the FLSC (Fig. 5, squares) or rhFLSC (Fig. 5, circles) immunization groups were similar to those of the saline control group (Fig. 5, gray triangles) at each time point assessed. Additionally, there was no measurable impact on CD20+ CD3− B cell and CD45+ CD14− leukocyte cell counts throughout the course of the study in any immunization group (data not shown). No clinically significant vaccine-related changes in any other cell population were noted at any point throughout this study (data not shown).
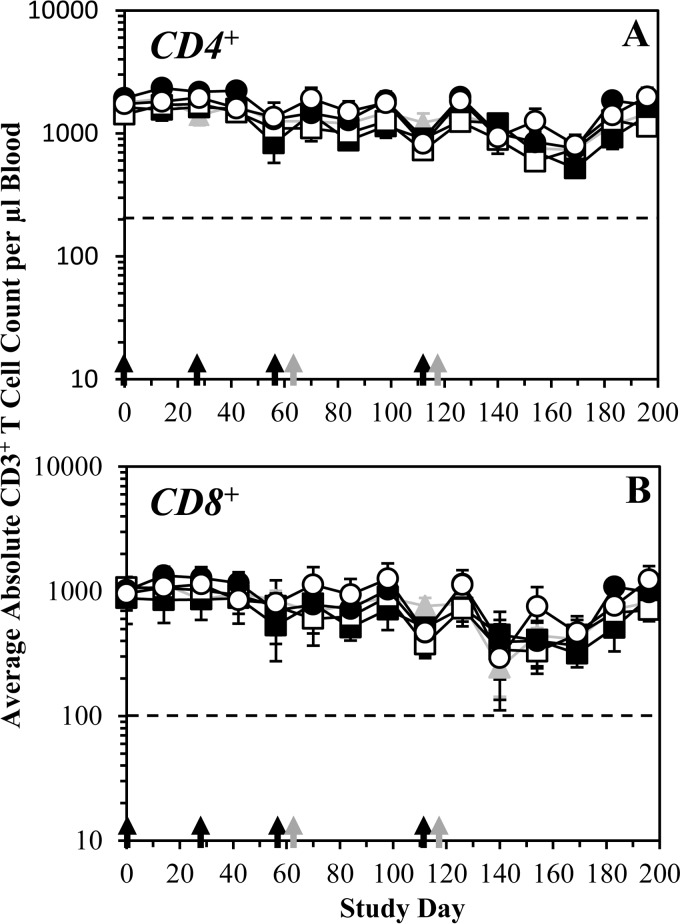
FIG 5.
Multiple high or low doses of FLSC or rhFLSC do not impact CD4+ or CD8+ T cell counts. Cynomolgus macaques (six per group) were immunized i.m. four times with FLSC (squares) or rhFLSC (circles) at 300 μg (solid symbols) or 900 μg (open symbols) per dose or 0.9% saline (gray triangles and lines) as a control. Immunization days are indicated by black arrows along the x axis. Gray arrows along the x axis indicate days of KLH (10 mg s.c.) administration. CD3+ CD4+ (A) and CD3+ CD8+ (B) T cells were counted using a FACS TruCount protocol (BD Biosciences) from PBMCs collected from each animal at the indicated time points. The average absolute counts per microliter of blood are shown. Values are means ± SEMs (error bars). In these assays, counts below the dashed lines (<200 in panel A and <100 in panel B) are considered clinically significant.
Among individual animals, three animals had absolute CD3+ CD4+ T cell counts that sporadically dipped below 200 (i.e., 130 to 168), each occurring only at a single time point with no apparent temporal trend: animal T011 from the saline control group on day 169, animal T018 from the rhFLSC high-dose (i.e., 900-μg) group on day 56, and animal R992 from the rhFLSC high-dose (i.e., 900-μg) group on day 169. All of these events occurred ≥4 weeks after the last immunization dose. The absolute CD3+ CD4+ T cell counts at the time points before and after these below normal counts in each of these animals were in the normal range (≥200).
Seven animals had absolute CD3+ CD8+ T cell counts below 100 (i.e., 20 to 87) and only on day 140: animals R986 and R996 in the saline control group, animals R995 and R997 in the FLSC low-dose (i.e., 300-μg) group, animals R993 and R998 in the FLSC high-dose (i.e., 900-μg) group, and animal R994 in the rhFLSC low-dose (i.e., 300-μg) group. This time point (day 140) was ≥4 weeks after the fourth and final immunization dose. The absolute CD3+ CD8+ T cell counts at the time points before and after these lower counts in each of these animals were in the normal range (≥100).
Such transient declines in individual CD3+ CD4+ and CD3+ CD8+ T cell counts were considered incidental and unrelated to FLSC or rhFLSC vaccination and were not considered significant, as they did not occur over more than one consecutive time point or immediately after immunization, nor were the declines uniform among the macaques or groups. Therefore, we conclude that there was no notable impact on the CD4+ and CD8+ T cell counts in any immunized animal throughout the course of the study.
Anti-CD4 antibodies are elicited only against heterologous CD4, not “self” CD4, after FLSC or rhFLSC vaccination, respectively.
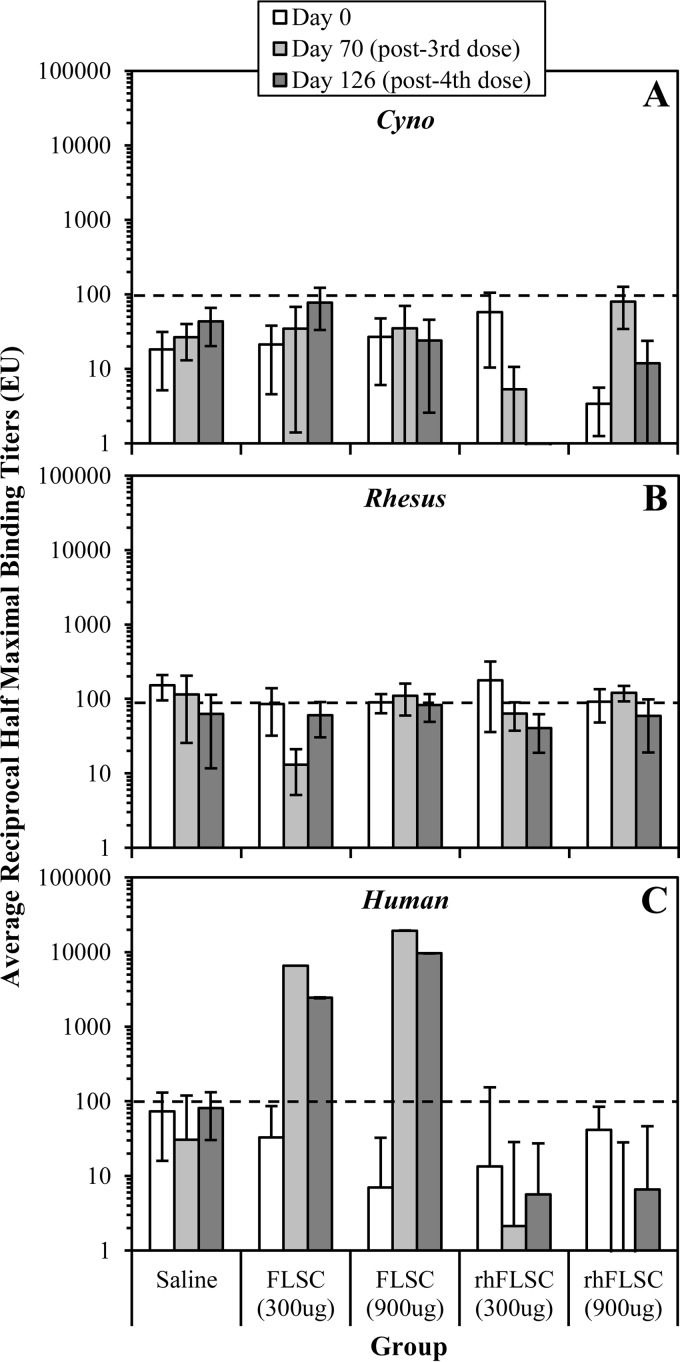
The FLSC immunogen is a human vaccine containing the D1D2 region of human CD4 (huCD4). Therefore, it was expected that cynomolgus macaques immunized with FLSC, which contains the huCD4 D1D2, would likely generate an antibody response against huCD4 because the huCD4 D1D2 region (amino acids 26 to 203; GenBank accession no. NP_000607) is only 88% identical to the CD4 D1D2 region in cynomolgus macaques (cyCD4; GenBank accession no. D63349) (Fig. 2). In order to accurately assess an autoimmune response to a self-antigen, we also vaccinated cynomolgus macaques with the rhesus version of FLSC, rhFLSC. Based on 100% amino acid sequence identity between cyCD4 and rhesus macaque CD4 (rhCD4; GenBank accession no. D63347) (Fig. 2), it was anticipated that the cynomolgus macaques vaccinated with rhFLSC would not generate an autoantibody response against either cyCD4 or rhCD4.
We assessed the sera collected 2 weeks after vaccination with the third doses (week 10 [i.e., day 70]) and fourth doses (week 18 [i.e., day 126]) of FLSC or rhFLSC for the presence of antibodies specific for the homologous cyCD4 and rhCD4 CD4 or the heterologous huCD4. Anti-CD4 autoantibody titers specific for cyCD4 or rhCD4 (Fig. 6A and B, respectively) were not detected in any of the immunized animals. However, heterologous anti-CD4 antibody titers specific for huCD4 were detected, but only in animals immunized with FLSC (Fig. 6C). In some groups, represented by error bars (standard errors of the means [SEMs]) above the lower limit for positivity (Fig. 6A and B), a few animals had titers returned by the ELISA software as >100; however, upon visual inspection of the ELISA curves, all curves were flat with little to no absorbance, indicating that no binding/titers were present. No significant gender-related differences were observed (data not shown). The results show that rhFLSC, which contains the rhCD4 D1D2 region and is homologous to cyCD4, did not elicit an autoimmune response to cyCD4 or rhCD4 (Fig. 6A and B) in the cynomolgus macaques, suggesting that FLSC is unlikely to induce an autoimmune response to huCD4 in humans.
FIG 6.
Multiple high or low doses of FLSC or rhFLSC do not elicit anti-CD4 autoimmune antibodies. Cynomolgus macaques (six per group) were immunized i.m. four times with FLSC or rhFLSC at 300 μg or 900 μg per dose or 0.9% saline as a control. Sera collected from each animal on day 0, 70 (week 10 [i.e., 2 weeks after the third vaccination]), or 126 (week 18 [i.e., 2 weeks after the fourth vaccination]) were subjected to anti-cyCD4 (A), anti-rhCD4 (B), or anti-huCD4 (C) ELISAs. The average anti-CD4 reciprocal half-maximal titers (EU) are shown. Values are means ± SEMs (error bars). In these assays, reciprocal half-maximal titers of <100 are considered negative as indicated by the black dashed lines.
Immune sera from FLSC- or rhFLSC-vaccinated animals do not bind primary naive macaque T cells.
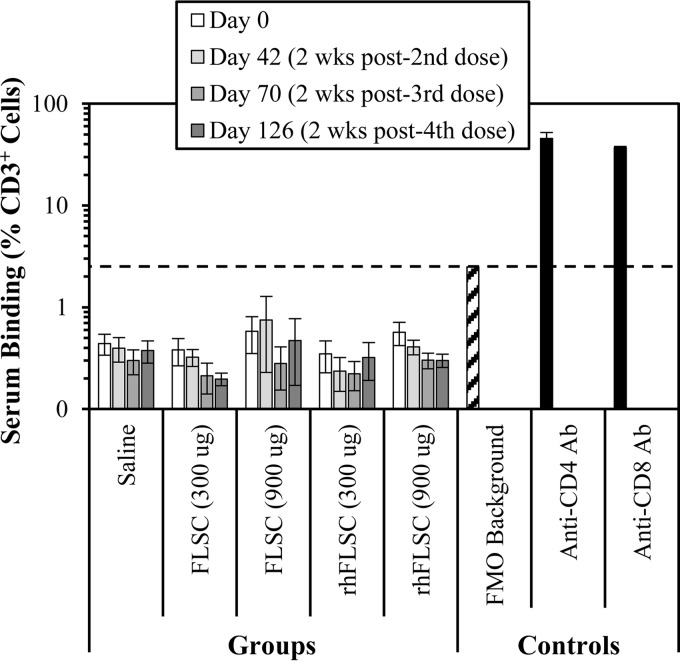
We used flow cytometry to determine whether antibodies undetectable by ELISA were present in the immune sera that could bind to T cells, which could potentially be capable of depleting CD4+ cells. All serum samples tested from days 0 (preimmune), 40 (2 weeks after the second dose), 70 (2 weeks after the third dose), and 126 (2 weeks after the fourth dose) were negative for binding to CD3+ T cells. The average percent binding in each group was below the “fluorescence minus one” (FMO) negative reference control and significantly lower than the positive anti-CD4 and anti-CD8 antibody controls (Fig. 7). Two animals had levels slightly above a blank background, but below the FMO negative control: animal R990 (in the rhFLSC, 900-μg group) had a background response of 1.25% on day 0 (preimmune) but had no binding at any later time points, and animal R998 (in the FLSC, 900-μg group) had background responses of 1.56% and 1.97% on days 0 and 70, respectively, but had no binding on days 40 and 126. One animal had levels slightly above the FMO negative control at a single time point: animal T006 (in the FLSC, 900-μg group) had a response of 3.36% on day 40 but had no binding on days 0, 70, and 126. All of these responses were lower than or comparable to the FMO negative-control sample of 2.94%, significantly lower than the values for the positive controls (38.7 to 52.3%), and therefore were not considered positive for binding.
FIG 7.
Immune sera do not bind to CD3+ T cells. Cynomolgus macaques (six per group) were immunized i.m. four times with FLSC or rhFLSC at 300 μg or 900 μg per dose or 0.9% saline as a control. Anti-CD4 or anti-CD8 antibodies (black bars) or immune sera collected from each animal on day 0, 42 (week 6 [i.e., 2 wks post-2nd dose]), 70 (week 10 [i.e., 2 wks post-3rd dose]), or 126 (week 18 [i.e., 2 wks post-4th dose]) were mixed with naive cynomolgus macaque PBMCs. Antibody binding to CD3+ cells was assessed by flow cytometry. The percentages of CD3+ cells bound by the immune sera or positive-control anti-CD4 and anti-CD8 antibodies are shown. Values are means ± SEMs (error bars). In these assays, levels below the fluorescence minus one (FMO) background control (hatched bar) are considered negative as indicated by the black dashed line.
DISCUSSION
Immunogens based on HIV gp120 remain viable candidates for HIV vaccine development. Lead concepts include “native-like” envelopes presenting neutralizing epitopes extant on free virions or conserved transition state structures embodied by FLSC that occur after attachment and elicit cross-protective immunity (10, 12, 21). The latter case involves gp120-CD4 complex formation by design, the former by sporadic circumstance as was shown in rhesus macaques (37, 38). Overall, the risk of breaking tolerance via ligating human CD4 to gp120 is an obvious safety concern for the clinical testing of HIV envelope-based vaccines.
In natural HIV infection, where gp120-CD4 complexes frequently form, putative anti-CD4 autoantibodies have been detected in roughly 10% of subjects studied (55–67). These antibodies seem directed against the D3 and D4 regions of CD4 (59, 61, 66, 67), are not immunoreactive with cell surface CD4 (56, 58, 59, 66), and lack detectable biological activity in vitro (58, 66). However, the provenance of these responses is unclear. Although the presence of anti-CD4 antibodies may correlate with declines in CD4+ T cells (63–65, 81, 82) during HIV infection, there is no clear causal link between such autoreactivity and AIDS. Moreover, anti-CD4 autoantibody responses are not unique to HIV infection, as they have been detected in HIV-exposed but uninfected individuals (63–65, 81, 82) as well as in preparations of “normal” immunoglobulin (83). Overall, available evidence that gp120-CD4 complexes cause deleterious autoimmune responses in the autologous host to CD4 remains equivocal.
The availability of a well-characterized antigen containing macaque CD4 D1D2 (rhFLSC) provides an opportunity to test this question in cynomolgus macaques, which possess species-matched CD4 sequences. In the protocols described here, vaccination with either FLSC or rhFLSC elicited robust cognate (Fig. 3) and anti-CD4i (Fig. 4) immune responses. In contrast, there was no evidence that the responses included antibodies autoreactive with macaque CD4 as detected by ELISA (Fig. 6A and B) or flow cytometric assays of primary naive T cells (Fig. 7). This outcome agreed with the absence of detectable autoreactive responses in rhFLSC-immunized rhesus macaques (10). In accordance with the ELISA measures, immune sera from either FLSC- or rhFLSC-immunized cynomolgus macaques did not bind to autologous primary CD3+ T cells determined by flow cytometry (Fig. 7). A similar lack of immunoreactivity with primary cells was observed with immune sera from rhesus macaques vaccinated with rhFLSC (10).
Previous reports showed that immunization of rhesus macaques with soluble extracellular human CD4 (D1D4) in the absence of gp120 elicited antibodies that cross-reacted with macaque cells (84, 85). Chimpanzees immunized with soluble human CD4 D1D4 developed antibodies reactive with digitonin-treated, but not untreated, chimpanzee lymphocytes (86). In either case, the specificities of the putative autoreactive antibodies were not detailed. Our results suggest that gp120-bound human CD4 (D1D2) does not elicit such cross-reactive responses in macaques using an immunization protocol similar to those employed for gp120-based immunogens in humans. Alternatively, the macaque-reactive antibodies previously observed may have been induced against human CD4 D3D4 that was included in the human CD4 immunogen but is absent in FLSC and rhFLSC. We did find that vaccination of macaques with FLSC raised antibodies reactive with human CD4 in the absence of reactivity with rhesus or cynomolgus macaque CD4 (Fig. 6). We predict that these antibodies are specific to the sequences that differ between human and macaque CD4, which are 88% homologous in their respective D1D2 domains (Fig. 2). Efforts to explore this in detail are ongoing.
Regulatory guidelines recommend the use of functional immunotoxicity assays that are relevant and specific to the mechanism of action of the pharmaceutical or biotherapeutic of interest. Assays that can improve clinical trial design and decisions are preferred, as they can provide endpoints for evaluating immune function and deficiencies. Accordingly, we examined the impact of FLSC vaccination on T cell subset frequencies. On the basis of past reports (47–54), we defined one indication of anti-CD4 immunotoxicity as a decline of CD4+ cell counts to <200 cells/ml over two consecutive time points after administration of vaccine antigen in any single animal. According to this definition, there was no consistent impact on circulating cynomolgus CD4+ or CD8+ T cell populations in vaccinated animals during the course of the immunization protocol (Fig. 5). These observations agree with our previous studies of rhFLSC-vaccinated rhesus macaques (10, 21), in which no perturbations of T cell subsets were noted.
Collectively, our immunotoxicity assessments in cynomolgus macaques, coupled with previous assessments in rhesus macaques, provide redundant evidence that gp120-bound CD4 is not immunogenic in the autologous host during an otherwise robust response to vaccination involving gp120-CD4 complexes.
ACKNOWLEDGMENTS
We thank Jacqueline Martin and Gurjinder Jandu from Profectus Biosciences, Inc., for technical support. We also kindly thank Kenneth Bagley (Profectus Biosciences, Inc.) and Melanie Hartsough (Biologics Consulting Group, Alexandria, VA) for editorial assistance.
Funding Statement
This work was funded by HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID) (R44AI091567), the Bill and Melinda Gates Foundation (OPP1017606 and OPP41351), and the Henry M. Jackson Foundation (HJF) (715319).
REFERENCES
- 1.Lewis GK, DeVico AL, Gallo RC. 2014. Antibody persistence and T-cell balance: two key factors confronting HIV vaccine development. Proc Natl Acad Sci U S A 111:15614–15621. doi: 10.1073/pnas.1413550111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sliepen K, Sanders RW. 2016. HIV-1 envelope glycoprotein immunogens to induce broadly neutralizing antibodies. Expert Rev Vaccines 15:349–365. doi: 10.1586/14760584.2016.1129905. [DOI] [PubMed] [Google Scholar]
- 3.D'Costa S, Slobod KS, Webster RG, White SW, Hurwitz JL. 2001. Structural features of HIV envelope defined by antibody escape mutant analysis. AIDS Res Hum Retroviruses 17:1205–1209. doi: 10.1089/088922201316912808. [DOI] [PubMed] [Google Scholar]
- 4.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 5.Landais E, Huang X, Havenar-Daughton C, Murrell B, Price MA, Wickramasinghe L, Ramos A, Bian CB, Simek M, Allen S, Karita E, Kilembe W, Lakhi S, Inambao M, Kamali A, Sanders EJ, Anzala O, Edward V, Bekker LG, Tang J, Gilmour J, Kosakovsky-Pond SL, Phung P, Wrin T, Crotty S, Godzik A, Poignard P. 2016. Broadly neutralizing antibody responses in a large longitudinal sub-Saharan HIV primary infection cohort. PLoS Pathog 12:e1005369. doi: 10.1371/journal.ppat.1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krumm SA, Mohammed H, Le KM, Crispin M, Wrin T, Poignard P, Burton DR, Doores KJ. 2016. Mechanisms of escape from the PGT128 family of anti-HIV broadly neutralizing antibodies. Retrovirology 13:8. doi: 10.1186/s12977-016-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, Wang C, O'Dell S, Li Y, Keele BF, Yang Z, Imamichi H, Doria-Rose N, Hoxie JA, Connors M, Shaw GM, Wyatt RT, Mascola JR. 2012. Selection pressure on HIV-1 envelope by broadly neutralizing antibodies to the conserved CD4-binding site. J Virol 86:5844–5856. doi: 10.1128/JVI.07139-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo D, Shi X, Arledge KC, Song D, Jiang L, Fu L, Gong X, Zhang S, Wang X, Zhang L. 2012. A single residue within the V5 region of HIV-1 envelope facilitates viral escape from the broadly neutralizing monoclonal antibody VRC01. J Biol Chem 287:43170–43179. doi: 10.1074/jbc.M112.399402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynes BF, Moody MA, Alam M, Bonsignori M, Verkoczy L, Ferrari G, Gao F, Tomaras GD, Liao HX, Kelsoe G. 2014. Progress in HIV-1 vaccine development. J Allergy Clin Immunol 134:3–10. doi: 10.1016/j.jaci.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeVico A, Fouts T, Lewis GK, Gallo RC, Godfrey K, Charurat M, Harris I, Galmin L, Pal R. 2007. Antibodies to CD4-induced sites in HIV gp120 correlate with the control of SHIV challenge in macaques vaccinated with subunit immunogens. Proc Natl Acad Sci U S A 104:17477–17482. doi: 10.1073/pnas.0707399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devico AL, Fouts TR, Shata MT, Kamin-Lewis R, Lewis GK, Hone DM. 2002. Development of an oral prime-boost strategy to elicit broadly neutralizing antibodies against HIV-1. Vaccine 20:1968–1974. doi: 10.1016/S0264-410X(02)00080-4. [DOI] [PubMed] [Google Scholar]
- 12.Fouts TR, Tuskan R, Godfrey K, Reitz M, Hone D, Lewis GK, DeVico AL. 2000. Expression and characterization of a single-chain polypeptide analogue of the human immunodeficiency virus type 1 gp120-CD4 receptor complex. J Virol 74:11427–11436. doi: 10.1128/JVI.74.24.11427-11436.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mengistu M, Ray K, Lewis GK, DeVico AL. 2015. Antigenic properties of the human immunodeficiency virus envelope glycoprotein gp120 on virions bound to target cells. PLoS Pathog 11:e1004772. doi: 10.1371/journal.ppat.1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu L, Gerard NP, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso AA, Desjardin E, Newman W, Gerard C, Sodroski J. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 15.Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184–186. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 16.DeVico AL. 2007. CD4-induced epitopes in the HIV envelope glycoprotein, gp120. Curr HIV Res 5:561–571. doi: 10.2174/157016207782418560. [DOI] [PubMed] [Google Scholar]
- 17.Lewis GK, Finzi A, DeVico AL, Pazgier M. 2015. Conformational masking and receptor-dependent unmasking of highly conserved Env epitopes recognized by non-neutralizing antibodies that mediate potent ADCC against HIV-1. Viruses 7:5115–5132. doi: 10.3390/v7092856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acharya P, Tolbert WD, Gohain N, Wu X, Yu L, Liu T, Huang W, Huang CC, Kwon YD, Louder RK, Luongo TS, McLellan JS, Pancera M, Yang Y, Zhang B, Flinko R, Foulke JS Jr, Sajadi MM, Kamin-Lewis R, Robinson JE, Martin L, Kwong PD, Guan Y, DeVico AL, Lewis GK, Pazgier M. 2014. Structural definition of an antibody-dependent cellular cytotoxicity response implicated in reduced risk for HIV-1 infection. J Virol 88:12895–12906. doi: 10.1128/JVI.02194-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollara J, Bonsignori M, Moody MA, Pazgier M, Haynes BF, Ferrari G. 2013. Epitope specificity of human immunodeficiency virus-1 antibody dependent cellular cytotoxicity [ADCC] responses. Curr HIV Res 11:378–387. doi: 10.2174/1570162X113116660059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, Kappes JC, Shaw GM, Hoxie JA, Robinson JE, Haynes BF. 2011. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol 85:7029–7036. doi: 10.1128/JVI.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fouts TR, Bagley K, Prado IJ, Bobb KL, Schwartz JA, Xu R, Zagursky RJ, Egan MA, Eldridge JH, LaBranche CC, Montefiori DC, Le Buanec H, Zagury D, Pal R, Pavlakis GN, Felber BK, Franchini G, Gordon S, Vaccari M, Lewis GK, DeVico AL, Gallo RC. 2015. Balance of cellular and humoral immunity determines the level of protection by HIV vaccines in rhesus macaque models of HIV infection. Proc Natl Acad Sci U S A 112:E992–E999. doi: 10.1073/pnas.1423669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, Shields J, Parenteau L, Whitney JB, Abbink P, Ng'ang'a DM, Seaman MS, Lavine CL, Perry JR, Li W, Colantonio AD, Lewis MG, Chen B, Wenschuh H, Reimer U, Piatak M, Lifson JD, Handley SA, Virgin HW, Koutsoukos M, Lorin C, Voss G, Weijtens M, Pau MG, Schuitemaker H. 2015. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 349:320–324. doi: 10.1126/science.aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, Dugast AS, Alter G, Ferguson M, Li W, Earl PL, Moss B, Giorgi EE, Szinger JJ, Eller LA, Billings EA, Rao M, Tovanabutra S, Sanders-Buell E, Weijtens M, Pau MG, Schuitemaker H, Robb ML, Kim JH, Korber BT, Michael NL. 2013. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155:531–539. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeVico A. 2013. Editorial: Fc receptor-mediated effector functions in the humoral control of HIV-1 infection. Curr HIV Res 11:343–344. doi: 10.2174/1570162X113116660056. [DOI] [PubMed] [Google Scholar]
- 26.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao HX, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fouts T, Godfrey K, Bobb K, Montefiori D, Hanson CV, Kalyanaraman VS, DeVico A, Pal R. 2002. Crosslinked HIV-1 envelope-CD4 receptor complexes elicit broadly cross-reactive neutralizing antibodies in rhesus macaques. Proc Natl Acad Sci U S A 99:11842–11847. doi: 10.1073/pnas.182412199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devico A, Silver A, Thornton AM, Sarngadharan MG, Pal R. 1996. Covalently crosslinked complexes of human immunodeficiency virus type 1 (HIV-1) gp120 and CD4 receptor elicit a neutralizing immune response that includes antibodies selective for primary virus isolates. Virology 218:258–263. doi: 10.1006/viro.1996.0188. [DOI] [PubMed] [Google Scholar]
- 30.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, Wenschuh H, Zerweck J, Greene K, Gao H, Berman PW, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Robb ML, Michael NL, Kim JH, Zolla-Pazner S, Haynes BF, Mascola JR, Self S, Gilbert P, Montefiori DC. 2013. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One 8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. 2014. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 6:228ra39. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connell RJ, Kim JH, Excler JL. 2014. The HIV-1 gp120 V1V2 loop: structure, function and importance for vaccine development. Expert Rev Vaccines 13:1489–1500. doi: 10.1586/14760584.2014.951335. [DOI] [PubMed] [Google Scholar]
- 33.Andrabi R, Voss JE, Liang CH, Briney B, McCoy LE, Wu CY, Wong CH, Poignard P, Burton DR. 2015. Identification of common features in prototype broadly neutralizing antibodies to HIV envelope V2 apex to facilitate vaccine design. Immunity 43:959–973. doi: 10.1016/j.immuni.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang JH, Yan YW, Garrett TP, Liu JH, Rodgers DW, Garlick RL, Tarr GE, Husain Y, Reinherz EL, Harrison SC. 1990. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature 348:411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
- 35.Barnett SW, Burke B, Sun Y, Kan E, Legg H, Lian Y, Bost K, Zhou F, Goodsell A, Zur Megede J, Polo J, Donnelly J, Ulmer J, Otten GR, Miller CJ, Vajdy M, Srivastava IK. 2010. Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J Virol 84:5975–5985. doi: 10.1128/JVI.02533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, Delaporte E, Peeters M, Derdeyn CA, Allen S, Hunter E, Saag MS, Hoxie JA, Hahn BH, Kwong PD, Robinson JE, Shaw GM. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med 201:1407–1419. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forsell MN, Dey B, Morner A, Svehla K, O'Dell S, Hogerkorp CM, Voss G, Thorstensson R, Shaw GM, Mascola JR, Karlsson Hedestam GB, Wyatt RT. 2008. B cell recognition of the conserved HIV-1 co-receptor binding site is altered by endogenous primate CD4. PLoS Pathog 4:e1000171. doi: 10.1371/journal.ppat.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douagi I, Forsell MN, Sundling C, O'Dell S, Feng Y, Dosenovic P, Li Y, Seder R, Lore K, Mascola JR, Wyatt RT, Karlsson Hedestam GB. 2010. Influence of novel CD4 binding-defective HIV-1 envelope glycoprotein immunogens on neutralizing antibody and T-cell responses in nonhuman primates. J Virol 84:1683–1695. doi: 10.1128/JVI.01896-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamberlain P. 2013. Assessing immunogenicity of biosimilar therapeutic monoclonal antibodies: regulatory and bioanalytical considerations. Bioanalysis 5:561–574. doi: 10.4155/bio.13.6. [DOI] [PubMed] [Google Scholar]
- 40.Kelley M, Ahene AB, Gorovits B, Kamerud J, King LE, McIntosh T, Yang J. 2013. Theoretical considerations and practical approaches to address the effect of anti-drug antibody (ADA) on quantification of biotherapeutics in circulation. AAPS J 15:646–658. doi: 10.1208/s12248-013-9468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kloks C, Berger C, Cortez P, Dean Y, Heinrich J, Bjerring Jensen L, Koppenburg V, Kostense S, Kramer D, Spindeldreher S, Kirby H. 2015. A fit-for-purpose strategy for the risk-based immunogenicity testing of biotherapeutics: a European industry perspective. J Immunol Methods 417:1–9. doi: 10.1016/j.jim.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Schaeverbeke T, Truchetet ME, Kostine M, Barnetche T, Bannwarth B, Richez C. 2016. Immunogenicity of biologic agents in rheumatoid arthritis patients: lessons for clinical practice. Rheumatology (Oxford) 55:210–220. doi: 10.1093/rheumatology/kev277. [DOI] [PubMed] [Google Scholar]
- 43.Tatarewicz SM, Mytych DT, Manning MS, Swanson SJ, Moxness MS, Chirmule N. 2014. Strategic characterization of anti-drug antibody responses for the assessment of clinical relevance and impact. Bioanalysis 6:1509–1523. doi: 10.4155/bio.14.114. [DOI] [PubMed] [Google Scholar]
- 44.Tovey MG, Legrand J, Lallemand C. 2011. Overcoming immunogenicity associated with the use of biopharmaceuticals. Expert Rev Clin Pharmacol 4:623–631. doi: 10.1586/ecp.11.39. [DOI] [PubMed] [Google Scholar]
- 45.van Schie KA, Wolbink GJ, Rispens T. 2015. Cross-reactive and pre-existing antibodies to therapeutic antibodies—effects on treatment and immunogenicity. MAbs 7:662–671. doi: 10.1080/19420862.2015.1048411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin L, Chen X, Vicini P, Rup B, Hickling TP. 2015. Therapeutic outcomes, assessments, risk factors and mitigation efforts of immunogenicity of therapeutic protein products. Cell Immunol 295:118–126. doi: 10.1016/j.cellimm.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Larche M, Robinson DS, Kay AB. 2003. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol 111:450–463. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 48.Mason U, Aldrich J, Breedveld F, Davis CB, Elliott M, Jackson M, Jorgensen C, Keystone E, Levy R, Tesser J, Totoritis M, Truneh A, Weisman M, Wiesenhutter C, Yocum D, Zhu J. 2002. CD4 coating, but not CD4 depletion, is a predictor of efficacy with primatized monoclonal anti-CD4 treatment of active rheumatoid arthritis. J Rheumatol 29:220–229. [PubMed] [Google Scholar]
- 49.Schulze-Koops H, Lipsky PE. 2000. Anti-CD4 monoclonal antibody therapy in human autoimmune diseases. Curr Dir Autoimmun 2:24–49. doi: 10.1159/000060506. [DOI] [PubMed] [Google Scholar]
- 50.VanderBorght A, Geusens P, Raus J, Stinissen P. 2001. The autoimmune pathogenesis of rheumatoid arthritis: role of autoreactive T cells and new immunotherapies. Semin Arthritis Rheum 31:160–175. doi: 10.1053/sarh.2001.27736. [DOI] [PubMed] [Google Scholar]
- 51.Delmonico FL, Cosimi AB, Kawai T, Cavender D, Lee WH, Jolliffe LK, Knowles RW. 1993. Nonhuman primate responses to murine and humanized OKT4A. Transplantation 55:722–728. doi: 10.1097/00007890-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Mourad GJ, Preffer FI, Wee SL, Powelson JA, Kawai T, Delmonico FL, Knowles RW, Cosimi AB, Colvin RB. 1998. Humanized IgG1 and IgG4 anti-CD4 monoclonal antibodies: effects on lymphocytes in the blood, lymph nodes, and renal allografts in cynomolgus monkeys. Transplantation 65:632–641. doi: 10.1097/00007890-199803150-00006. [DOI] [PubMed] [Google Scholar]
- 53.Powelson JA, Knowles RW, Delmonico FL, Kawai T, Mourad G, Preffer FK, Colvin RB, Cosimi AB. 1994. CDR-grafted OKT4A monoclonal antibody in cynomolgus renal allograft recipients. Transplantation 57:788–793. doi: 10.1097/00007890-199403270-00002. [DOI] [PubMed] [Google Scholar]
- 54.Wee SL, Stroka DM, Preffer FI, Jolliffe LK, Colvin RB, Cosimi AB. 1992. The effects of OKT4A monoclonal antibody on cellular immunity of nonhuman primate renal allograft recipients. Transplantation 53:501–507. doi: 10.1097/00007890-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Keay S, Tacket CO, Murphy JR, Handwerger BS. 1992. Anti-CD4 anti-idiotype antibodies in volunteers immunized with rgp160 of HIV-1 or infected with HIV-1. AIDS Res Hum Retroviruses 8:1091–1098. doi: 10.1089/aid.1992.8.1091. [DOI] [PubMed] [Google Scholar]
- 56.Chams V, Jouault T, Fenouillet E, Gluckman JC, Klatzmann D. 1988. Detection of anti-CD4 autoantibodies in the sera of HIV-infected patients using recombinant soluble CD4 molecules. AIDS 2:353–361. doi: 10.1097/00002030-198810000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Wilks D, Walker LC, Habeshaw JA, Youle M, Gazzard B, Dalgleish AG. 1990. Anti-CD4 autoantibodies and screening for anti-idiotypic antibodies to anti-CD4 monoclonal antibodies in HIV-seropositive people. AIDS 4:113–118. doi: 10.1097/00002030-199002000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Chams V, Idziorek T, Klatzmann D. 1991. Biological properties of anti-CD4 autoantibodies purified from HIV-infected patients. AIDS 5:565–569. doi: 10.1097/00002030-199105000-00014. [DOI] [PubMed] [Google Scholar]
- 59.Callahan LN, Roderiquez G, Mallinson M, Norcross MA. 1992. Analysis of HIV-induced autoantibodies to cryptic epitopes on human CD4. J Immunol 149:2194–2202. [PubMed] [Google Scholar]
- 60.Keiser P, Keay S, Wasserman S, Wecksler W. 1992. Anti-CD4 antibodies are associated with HIV-1 seroconversion and may be detectable before anti-HIV-1 antibodies. The Multicenter AIDS Cohort Study. AIDS Res Hum Retroviruses 8:1919–1927. doi: 10.1089/aid.1992.8.1919. [DOI] [PubMed] [Google Scholar]
- 61.Martin L, Idziorek T, Lehen A, Gluckman JC, Klatzmann D. 1994. Anti-CD4 autoantibodies in HIV-infected individuals: detection by two immunoenzymatic techniques. AIDS 8:389–390. doi: 10.1097/00002030-199403000-00017. [DOI] [PubMed] [Google Scholar]
- 62.Muller C, Kukel S, Bauer R. 1994. Antibodies against CD4+ lymphocytes in plasma of HIV-infected patients are related to CD4 cell depletion in vivo. Immunol Lett 41:163–167. doi: 10.1016/0165-2478(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 63.Burastero SE, Gaffi D, Lopalco L, Tambussi G, Borgonovo B, De Santis C, Abecasis C, Robbioni P, Gasparri A, Lazzarin A, Celada F, Siccardi AG, Beretta A. 1996. Autoantibodies to CD4 in HIV type 1-exposed seronegative individuals. AIDS Res Hum Retroviruses 12:273–280. doi: 10.1089/aid.1996.12.273. [DOI] [PubMed] [Google Scholar]
- 64.Lopalco L, Magnani Z, Confetti C, Brianza M, Saracco A, Ferraris G, Lillo F, Vegni C, Lazzarin A, Siccardi AG, Burastero SE. 1999. Anti-CD4 antibodies in exposed seronegative adults and in newborns of HIV type 1-seropositive mothers: a follow-up study. AIDS Res Hum Retroviruses 15:1079–1085. doi: 10.1089/088922299310377. [DOI] [PubMed] [Google Scholar]
- 65.Lopalco L, Pastori C, Cosma A, Burastero SE, Capiluppi B, Boeri E, Beretta A, Lazzarin A, Siccardi AG. 2000. Anti-cell antibodies in exposed seronegative individuals with HIV type 1-neutralizing activity. AIDS Res Hum Retroviruses 16:109–115. doi: 10.1089/088922200309458. [DOI] [PubMed] [Google Scholar]
- 66.Sekigawa I, Groopmen JE, Allan JD, Ikeuchi K, Biberfield G, Takatsuki K, Byrn RA. 1991. Characterization of autoantibodies to the CD4 molecule in human immunodeficiency virus infection. Clin Immunol Immunopathol 58:145–153. doi: 10.1016/0090-1229(91)90156-5. [DOI] [PubMed] [Google Scholar]
- 67.Thiriart C, Goudsmit J, Schellekens P, Barin F, Zagury D, De Wilde M, Bruck C. 1988. Antibodies to soluble CD4 in HIV-1-infected individuals. AIDS 2:345–351. doi: 10.1097/00002030-198810000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Boelsterli UA. 2003. Animal models of human disease in drug safety assessment. J Toxicol Sci 28:109–121. doi: 10.2131/jts.28.109. [DOI] [PubMed] [Google Scholar]
- 69.Ebeling M, Kung E, See A, Broger C, Steiner G, Berrera M, Heckel T, Iniguez L, Albert T, Schmucki R, Biller H, Singer T, Certa U. 2011. Genome-based analysis of the nonhuman primate Macaca fascicularis as a model for drug safety assessment. Genome Res 21:1746–1756. doi: 10.1101/gr.123117.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamperschroer C, Kaur A, Lebrec H. 2012. A summary of meeting proceedings for ‘Measuring immune responses in non-human primates for drug development—opportunities and challenges for predicting human efficacy and immunotoxicity’. J Immunotoxicol 9:108–120. doi: 10.3109/1547691X.2011.631610. [DOI] [PubMed] [Google Scholar]
- 71.Ponce R, Abad L, Amaravadi L, Gelzleichter T, Gore E, Green J, Gupta S, Herzyk D, Hurst C, Ivens IA, Kawabata T, Maier C, Mounho B, Rup B, Shankar G, Smith H, Thomas P, Wierda D. 2009. Immunogenicity of biologically-derived therapeutics: assessment and interpretation of nonclinical safety studies. Regul Toxicol Pharmacol 54:164–182. doi: 10.1016/j.yrtph.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 72.Shen S, Pyo CW, Vu Q, Wang R, Geraghty DE. 2013. The essential detail: the genetics and genomics of the primate immune response. ILAR J 54:181–195. doi: 10.1093/ilar/ilt043. [DOI] [PubMed] [Google Scholar]
- 73.Vugmeyster Y, Xu X, Theil FP, Khawli LA, Leach MW. 2012. Pharmacokinetics and toxicology of therapeutic proteins: advances and challenges. World J Biol Chem 3:73–92. doi: 10.4331/wjbc.v3.i4.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilljam G. 1993. Envelope glycoproteins of HIV-1, HIV-2, and SIV purified with Galanthus nivalis agglutinin induce strong immune responses. AIDS Res Hum Retroviruses 9:431–438. doi: 10.1089/aid.1993.9.431. [DOI] [PubMed] [Google Scholar]
- 75.Finnegan CM, Berg W, Lewis GK, DeVico AL. 2001. Antigenic properties of the human immunodeficiency virus envelope during cell-cell fusion. J Virol 75:11096–11105. doi: 10.1128/JVI.75.22.11096-11105.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guan Y, Pazgier M, Sajadi MM, Kamin-Lewis R, Al-Darmarki S, Flinko R, Lovo E, Wu X, Robinson JE, Seaman MS, Fouts TR, Gallo RC, DeVico AL, Lewis GK. 2013. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc Natl Acad Sci U S A 110:E69–E78. doi: 10.1073/pnas.1217609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ho DD, McKeating JA, Li XL, Moudgil T, Daar ES, Sun NC, Robinson JE. 1991. Conformational epitope on gp120 important in CD4 binding and human immunodeficiency virus type 1 neutralization identified by a human monoclonal antibody. J Virol 65:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sullivan N, Sun Y, Sattentau Q, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. 1998. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J Virol 72:4694–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thali M, Furman C, Ho DD, Robinson J, Tilley S, Pinter A, Sodroski J. 1992. Discontinuous, conserved neutralization epitopes overlapping the CD4-binding region of human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol 66:5635–5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.US Food and Drug Administration. 2006. Guidance for Industry: S8 immunotoxicity studies for human pharmaceuticals. US Food and Drug Administration, Rockville, MD. [Google Scholar]
- 81.Furci L, Beretta A, Siccardi A, Lazzarin A, Confetti C, Magnani Z, Scarpellini P, Lopalco L, Burastero SE. 1997. Human immunodeficiency virus type 1 glycoprotein 120-specific T lymphocytes provide intermolecular help for anti-CD4 autoantibody production in exposed uninfected subjects. AIDS Res Hum Retroviruses 13:1461–1469. doi: 10.1089/aid.1997.13.1461. [DOI] [PubMed] [Google Scholar]
- 82.Beretta A, Furci L, Burastero S, Cosma A, Dinelli ME, Lopalco L, DeSantis C, Tambussi G, Carrow E, Sabbatani S, Clerici M, Lazzarin A, Siccardi AG. 1996. HIV-1-specific immunity in persistently seronegative individuals at high risk for HIV infection. Immunol Lett 51:39–43. doi: 10.1016/0165-2478(96)02553-9. [DOI] [PubMed] [Google Scholar]
- 83.Hurez V, Kaveri SV, Mouhoub A, Dietrich G, Mani JC, Klatzmann D, Kazatchkine MD. 1994. Anti-CD4 activity of normal human immunoglobulin G for therapeutic use (intravenous immunoglobulin, IVIg). Ther Immunol 1:269–277. [PubMed] [Google Scholar]
- 84.Watanabe M, Levine CG, Shen L, Fisher RA, Letvin NL. 1991. Immunization of simian immunodeficiency virus-infected rhesus monkeys with soluble human CD4 elicits an antiviral response. Proc Natl Acad Sci U S A 88:4616–4620. doi: 10.1073/pnas.88.11.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watanabe M, Chen ZW, Tsubota H, Lord CI, Levine CG, Letvin NL. 1991. Soluble human CD4 elicits an antibody response in rhesus monkeys that inhibits simian immunodeficiency virus replication. Proc Natl Acad Sci U S A 88:120–124. doi: 10.1073/pnas.88.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watanabe MJ, Boyson JE, Lord CI, Letvin NL. 1992. Chimpanzees immunized with recombinant soluble CD4 develop anti-self CD4 antibody responses with anti-human immunodeficiency virus activity. Proc Natl Acad Sci U S A 89:5103–5107. doi: 10.1073/pnas.89.11.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]