Abstract
Enterotoxigenic Escherichia coli (ETEC) strains are a common cause of diarrhea. Extraordinary antigenic diversity has prompted a search for conserved antigens to complement canonical approaches to ETEC vaccine development. EtpA, an immunogenic extracellular ETEC adhesin relatively conserved in the ETEC pathovar, has previously been shown to be a protective antigen following intranasal immunization. These studies were undertaken to explore alternative routes of EtpA vaccination that would permit use of a double mutant (R192G L211A) heat-labile toxin (dmLT) adjuvant. Here, oral vaccination with EtpA adjuvanted with dmLT afforded significant protection against small intestinal colonization, and the degree of protection correlated with fecal IgG, IgA, or total fecal antibody responses to EtpA. Sublingual vaccination yielded compartmentalized mucosal immune responses with significant increases in anti-EtpA fecal IgG and IgA, and mice vaccinated via this route were also protected against colonization. In contrast, while intradermal (i.d.) vaccination achieved high levels of both serum and fecal antibodies against both EtpA and dmLT, mice vaccinated via the i.d. route were not protected against subsequent colonization and the avidity of serum IgG and IgA EtpA-specific antibodies was significantly lower after i.d. immunization compared to other routes. Finally, we demonstrate that antiserum from vaccinated mice significantly impairs binding of LT to cognate GM1 receptors and shows near complete neutralization of toxin delivery by ETEC in vitro. Collectively, these data provide further evidence that EtpA could complement future vaccine strategies but also suggest that additional effort will be required to optimize its use as a protective immunogen.
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) strains are among the most common causes of diarrheal illness in developing countries, where young children are most susceptible (1, 2). In addition, these pathogens are also a common cause of diarrhea in immunologically naive travelers (3) who venture to areas of endemicity where sanitation and clean water remain limited.
Given the significant impact of ETEC on global health, a vaccine to prevent these infections is a significant priority (4). However, despite decades of investigative efforts following the discovery of toxin-producing E. coli in patients with clinical illnesses indistinguishable from cholera (5), a vaccine that affords broad-based protection has yet to be developed.
All of the ETEC-specific virulence genes described to date are encoded on plasmids. These include the heat-labile and/or heat-stable enterotoxins that define this pathovar and the colonization factors (CFs). Most vaccines to date have primarily targeted these colonization factors, which include a broad array of fimbrial as well as afimbrial surface antigens thought to be essential for intestinal colonization and/or heat-labile toxin.
Unfortunately, the heterogeneity of CF antigenic structures presents a challenge to development of a broadly protective vaccine. While some antigens are more conserved and widely distributed geographically and across different phylogenic backgrounds (6), the inherent plasticity of E. coli genomes (7, 8) and the fact that many ETEC strains do not make one of the 26 different CFs described to date (9, 10) have prompted a search for additional antigens that might also be considered to complement existing vaccine development paradigms (11).
Among antigens under investigation as a putative vaccine candidate is EtpA. This plasmid-encoded secreted glycoprotein belongs to the two-partner secretion family of molecules that includes filamentous hemagglutinin (FHA), a component of acellular pertussis vaccines (12). Studies of EtpA to date have demonstrated that it plays a unique role in facilitating ETEC adhesion and toxin delivery to target intestinal epithelial cells (13). Moreover, experiments with mice have shown that EtpA promotes colonization of the small intestine and can serve as a protective antigen (13, 14).
Recent molecular characterization of strains from a variety of sources has also suggested that EtpA is sufficiently conserved to warrant further consideration as a potential vaccine antigen (7, 15, 16). Early proof-of-principal studies with EtpA were conducted using an intranasal route of vaccination with Protollin (14, 17–19) or heat-labile toxin (LT) (20). However, because LT is not safe for intranasal vaccination in humans (21, 22), and because an LT toxoid will likely be a key component of next-generation vaccines, here we investigated the immunogenicity and protective efficacy of EtpA when delivered by other routes using a double mutant (R192G L211A) heat-labile toxin (dmLT) as the adjuvant.
MATERIALS AND METHODS
Adjuvant and immunogen preparation.
The double mutant (R192G L211A) heat-labile toxin (dmLT) (23) used in these studies was manufactured by the Bioproduction Facility at Walter Reed Army Institute for Research, Silver Spring, MD (BPR-1037-00, lot no. 1735) and was stored lyophilized at −20°C prior to use. dmLT was reconstituted to 1 mg/ml in sterile phosphate-buffered saline (PBS) immediately before use and then diluted as needed with PBS. Recombinant polyhistidine-tagged EtpA glycoprotein (rEtpA) was purified as previously described using metal affinity chromatography from culture supernatants of an E. coli TOP10 strain transformed with pJL017 and pJL030 (jf1696) (Table 1) (24). Briefly, overnight cultures grown from frozen glycerol stocks maintained at −80°C were diluted 1:100 into fresh Luria broth (LB) containing final concentrations of 100 μg/ml ampicillin and 15 μg/ml chloramphenicol and grown at 37°C to an optical density at 600 nm (OD600) of ∼0.6. Arabinose (0.0002%) was then added to induce EtpA expression. After 3 h of induction, cultures were harvested at 6,000 rpm at 4°C for 10 min, and supernatant was filtered and saved for subsequent purification. Supernatant was concentrated ∼10-fold using a Pellicon concentrator with 30,000-molecular-weight (MW) cutoff (Millipore). After being loaded onto metal affinity columns (5 ml; HiTrap, GE), unbound protein was removed by washing with buffer containing 25 mM sodium phosphate, 300 mM sodium chloride (pH 7.4), and 0 to 100 mM imidazole. rEtpA was then eluted from the column in the same buffer over a gradient ranging from 100 to 600 mM imidazole. Eluate fractions containing rEtpA were pooled and then concentrated with an Amicon Ultra-15 Ultracel-100k centrifuge (Millipore), and the buffer was exchanged with PBS (pH 7.4) before being stored at −80°C. rEtpA and dmLT were combined immediately prior to vaccination.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference(s) |
|---|---|---|
| Strains | ||
| H10407 | Wild-type ETEC strain O78:H11; CFA/1 LT+ ST+ EtpA+ | 57, 58 |
| jf570 | eltAB LT deletion mutant of H10407 | 59 |
| jf876 | lacZYA::Kmr mutant of H10407 | 59 |
| jf1696 | TOP10(pJL017, pJL030) Ampr Cmr | 24 |
| TOP10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| Plasmids | ||
| pJL017 | etpBA cloned into pBAD/myc-His A, with etpA in frame with myc and 6His coding regions; Ampr | 14 |
| pJL030 | etpC gene cloned into pACYC184; Cmr | 13 |
Kmr, kanamycin resistant; Cmr, chloramphenicol resistant; Ampr, ampicillin resistant; Strr, streptomycin resistant.
Immunization protocols.
Female CD-1 mice (5 to 8 weeks old) were purchased from Charles River Laboratories. Groups of 10 to 12 mice were vaccinated on days 1, 29, and 43 via the sublingual, intradermal (i.d.), or orogastric routes, with additional vaccination given on day 57 by the sublingual route only. Before vaccination, mice were lightly anesthetized with isoflurane. For orogastric vaccination, mice were first fasted for 2 h and then gavaged with 100 μl of NaHCO3 (7.5%) to neutralize stomach acid. After 5 min, mice were then gavaged with the vaccine or controls in a final volume of 300 μl of PBS. For sublingual vaccination, 10 μl of the preparation was pipetted under the tongue, and the head was maintained upright until the mouse regained consciousness. For intradermal vaccination, an alcohol swab was used to wet and separate abdominal fur, after which 50 μl of the vaccine preparation or control solution was delivered intradermally using a 1/2-ml insulin syringe fitted with a 29-gauge needle. To confirm intradermal placement of the antigen, we performed test i.d. injections of tattoo ink in parallel sets of live CD-1 mice. Sections of skin were subsequently processed for hematoxylin and eosin staining and examination by light microscopy.
Orogastric vaccination employed 25 μg of dmLT in adjuvant-only controls and as the adjuvant with doses of either 200 or 400 μg of rEtpA. Sublingual vaccination used 5 μg of dmLT/mouse ± 10 μg of EtpA. Intradermal vaccination was done in two experiments with doses of either 100 ng of dmLT ± 250 ng of rEtpA or 1 μg of dmLT ± 2.5 μg of rEtpA.
Sample collection.
Mouse fecal samples were collected 2 weeks after each boost. Six fecal pellets from each mouse were resuspended in 1.5 ml of fecal resuspension buffer (containing 10 mM Tris base, 100 mM NaCl, 0.05% Tween 20, and 5 mM sodium azide, pH 7.4) and stored at 4°C overnight. Mouse serum samples were collected either from abdominal aorta or from terminal cardiac bleeds using 25-gauge tuberculin syringes.
Bacterial strains and growth conditions.
Strain jf876, a derivative of ETEC strain H10407 bearing a kanamycin resistance marker in the lacZYA locus (lacZYA::kan) (Table 1) was used in all colonization experiments as previously described (25). Briefly, jf876 maintained as a frozen glycerol stock at −80°C was used to inoculate sterile Luria broth (LB). After overnight growth at 37°C at 225 rpm, the culture was diluted 1:100 into fresh LB medium, grown to an OD600 of ∼0.3, and serially diluted to an inoculum of ∼105 to 106 for challenge.
Intestinal colonization studies in mice.
Intestinal colonization experiments were performed as previously described (25). Briefly, prior to challenge, mice were pretreated with streptomycin (5 g/liter) in drinking water for 24 h, followed by regular water for 12 h, and famotidine (50 mg/kg body weight) was given 1 to 3 h prior to challenge to neutralize stomach acid. Mice were then challenged with ∼105 CFU of ETEC bacteria by oral gavage (25). Twenty-four hours after challenge, mice were sacrificed and two 3-cm sections of small intestine (ileum) were collected as previously described. Following incubation in saponin (5%) for 10 min, dilutions of intestinal lysates in PBS were plated onto Luria agar plates containing kanamycin (50 μg/ml). Following overnight incubation, bacteria were enumerated by counting kanamycin-resistant colonies. All experiments with mice were performed under protocols approved by the Animal Studies Committee at Washington University School of Medicine.
Immunologic assessment.
A kinetic enzyme-linked immunosorbent assay (ELISA) was used to detect immune responses in both fecal and serum samples as previously described (15). Briefly, 96-well plates were coated with 0.1 μg/well of EtpA or GM1 gangliosides (Sigma; catalog no. G2375) in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, 0.2 g/liter NaN3, pH 8.6) overnight at 4°C. After washing, dmLT (1 μg/ml in PBS) was added to wells containing GM1 gangliosides and incubated for 1 h at 37°C. Plates were washed and blocked with 1% bovine serum albumin (BSA) for 1 h at 37°C. Plates were again washed and then incubated with fecal extracts (undiluted) or with sera (diluted 1:100 in PBS containing 1% BSA). After incubation at 37°C for 1 h, plates were washed and incubated with horseradish peroxidase (HRP)-secondary antibody conjugates for 30 min at 37°C. After washing, TMB (3,3′,5,5′-tetramethylbenzidine)-peroxidase substrate (KPL) was added, and plates were immediately read at 650 nm to collect kinetic ELISA data (26). Data were analyzed using Gen5 software (BioTek) and are reported as Vmax (milliunits per minute).
Serum EtpA antibody avidity determinations.
Avidity indexes for EtpA-specific serum IgG and IgA antibodies were determined as previously described (27). Briefly, microtiter wells were coated with EtpA as described above. After being washed and blocked, plates were incubated with sera diluted 1:10,000 at 37°C for 1 h. Plates were washed and then incubated with either 6 M urea solution or PBS for 10 min at 37°C. After being washed, plates were incubated with either anti-mouse IgA or anti-mouse IgG HRP-conjugated secondary antibody, and responses were determined by kinetic ELISA as described above. Avidity indexes were then calculated as the ratio of the Vmax (milliunits per minute) in the urea-treated wells to that in the untreated wells.
Toxin neutralization assays.
To examine the ability of antisera to prevent binding of heat-labile toxin to its receptor, plates were coated with GM1 gangliosides at a 1-μg/ml final concentration in PBS overnight at 4°C. Serum from vaccinated or control mice was diluted 1:128 in PBS and then incubated 1:1 with LT at a final concentration of 1 μg/ml at 4°C overnight. After washing of plates with PBS, LT preincubated with serum was then allowed to bind to target gangliosides for 1 h at 37°C. Plates were then washed and blocked with 1% BSA for 1 h at 37°C. Bound LT was determined by anti-LT-B kinetic ELISA. Briefly, plates were incubated using primary anti-LT-B rabbit polyclonal antiserum diluted 1:1,000 in PBS containing 1% BSA at 37°C for 1 h and washed, followed by addition of goat anti-rabbit IgG-HRP conjugate diluted 1:5,000 in 1% BSA at 37°C for 30 min. After the final washing, plates were developed with TMB substrate and data collected kinetically as described above.
To examine the ability of antisera from immunized animals to prevent effective toxin delivery by ETEC, a 96-well tissue culture plate was first seeded with Caco-2 cells and the culture was grown at 37°C in 5% CO2 until the cells formed a confluent monolayer. One microliter of a mid-logarithmic-phase Luria broth culture of H10407 was then added with 10 μl of sera from individual mice and incubated with target Caco-2 monolayers at 37°C in 5% CO2. After 2.5 h, plates were washed with preequilibrated tissue culture medium, then incubated for an additional 2 h at 37°C in 5% CO2, and finally washed 4 times with PBS. Cyclic AMP (cAMP) activation in target epithelial cells was then determined by ELISA (DetectX direct cyclic AMP kit; Arbor Assays, Ann Arbor, MI).
RESULTS
Immunogenicity and protective efficacy following oral vaccination with EtpA.
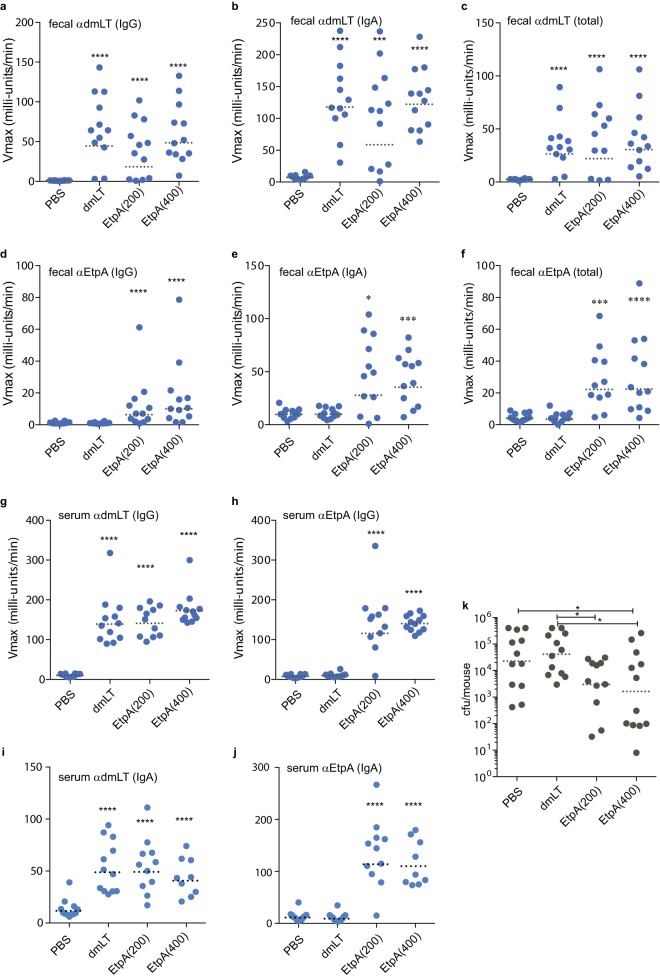
Because dmLT has been given safely to human volunteers via the oral route (28), we first examined whether EtpA could afford protection when coadministered with dmLT as the adjuvant. Following oral immunization, mice mounted robust fecal IgA, fecal IgG, and total fecal antibody responses to the dmLT adjuvant, whether administered alone or in combination with EtpA (Fig. 1a to c). Likewise, we observed significant amounts of anti-EtpA fecal antibodies compared to either dmLT-only or PBS controls (Fig. 1d to f). Oral vaccination also resulted in demonstrable increases in serum IgG and IgA antibodies against dmLT (Fig. 1g and i) and against EtpA (Fig. 1h and j).
FIG 1.
Immunogenicity and inhibition of intestinal colonization following oral immunization with EtpA adjuvanted with dmLT. (a to f) Kinetic ELISA data demonstrating fecal antibody responses to dmLT (a to c) and EtpA (d to f) following oral immunization with dmLT adjuvant alone (25 μg), 25 μg dmLT plus 200 μg rEtpA, or 25 μg dmLT plus 400 μg rEtpA. The antigen and antibody isotype tested are shown in the upper left-hand corner of each graph. Serum responses (IgG) at a dilution of 1:100 are shown for (g) dmLT and (h) EtpA, and panels i and j correspond to serum IgA responses. (k) Intestinal colonization in mice (grey symbols represent individual mice) following challenge with enterotoxigenic E. coli. Dashed horizontal lines in each figure panel represent geometric means. Comparisons to the PBS control group were made by Mann-Whitney nonparametric testing (*, P < 0.05; ***, P < 0.001; ****, P < 0.0001).
Following oral vaccination, mice in the EtpA vaccine administration group had significantly lower levels of intestinal colonization than either PBS control mice or dmLT adjuvant-only controls (Fig. 1k). While mice immunized with the highest dose of EtpA exhibited the lowest geometric mean number of CFU of ETEC in the small intestine, the difference between EtpA low- and high-dose groups was not statistically significant.
Correlation between protection and fecal antibody production.
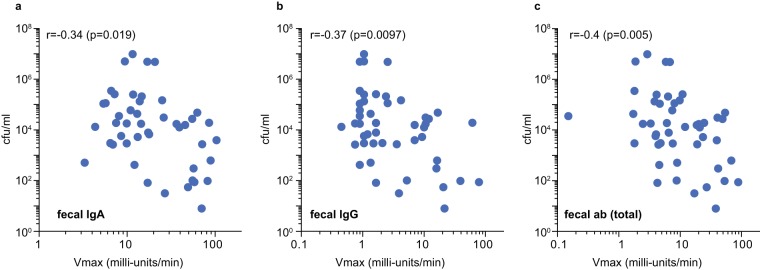
Theoretically, vaccines against enteric pathogens should engender mucosal antibodies that neutralize the ability of the pathogen to colonize the host and/or to deliver its effector molecules. Because EtpA functions as an adhesin, we examined the correlation between the levels of fecal antibody levels achieved in individual mice following vaccination and intestinal colonization. Levels of intestinal IgG (Fig. 2a) and IgA (Fig. 2b) were both related to reductions in intestinal colonization, while the strongest correlation (P = 0.005) was observed with total fecal antibody levels (Fig. 2c).
FIG 2.
Correlation of anti-EtpA fecal antibody levels and intestinal colonization in mice following oral vaccination. (a) Comparison of IgA anti-EtpA fecal antibody levels and colonization. (b) Fecal IgG versus colonization. (c) Total fecal antibody (ab) (IgG, IgM, and IgA) versus colonization. The results of Spearman (nonparametric) correlation coefficient (r) and corresponding P value are shown in the upper left-hand corner of each graph. Each point represents data from a total of 47 mice, including PBS controls (n = 12), dmLT adjuvant-only controls (n = 12), mice administered dmLT plus 200 μg EtpA (n = 11), and mice administered dmLT plus 400 μg EtpA (n = 12).
Sublingual administration of EtpA with dmLT.
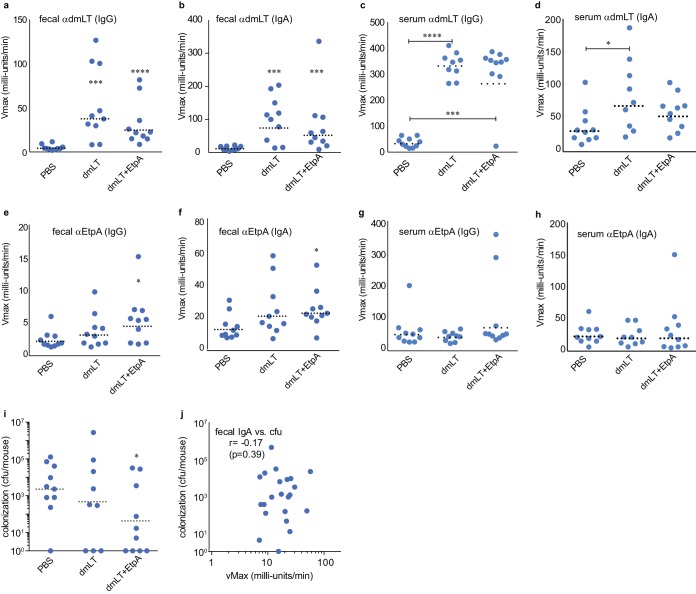
Previous studies have shown that sublingual administration of antigens can induce both systemic and mucosal antibody responses with doses that are appreciably smaller than those required for oral administration (29). Similar to oral administration, sublingual vaccination with the combination of dmLT and EtpA also stimulated production of fecal IgG (Fig. 3a) and fecal IgA (Fig. 3b) as well as serum IgG (Fig. 3c) and serum IgA (Fig. 3d) antibodies to the dmLT adjuvant. Although sublingual vaccination resulted in modest increases in responses to EtpA in feces (Fig. 3e and f), there was no significant increase in serum antibodies (Fig. 3g and h), consistent with compartmentalization of the mucosal immune response. While vaccination of mice sublingually with EtpA adjuvanted with dmLT did afford some protection against colonization relative to unvaccinated mice (Fig. 3i), we did not observe a significant correlation between fecal antibody levels and the level of intestinal colonization (Fig. 3j).
FIG 3.
Immunogenicity and inhibition of intestinal colonization following sublingual immunization with EtpA adjuvanted with dmLT. (a and b) Fecal IgG (a) and fecal IgA (b) responses to the dmLT adjuvant. (c and d) Serum IgG (c) and serum IgA (d) responses to dmLT. (e and f) Fecal IgG (e) and fecal IgA (f) responses to rEtpA. (g and h) Serum IgG (g) and serum IgA (h) responses to rEtpA. (i) Colonization of mice following vaccination with rEtpA adjuvanted with dmLT versus dmLT alone or PBS controls. (j) Correlation between fecal IgA and intestinal colonization following sublingual vaccination. Comparisons between groups were made by Mann-Whitney nonparametric testing (*, P < 0.05; ***, P < 0.001; ****, P < 0.0001).
Intradermal administration of EtpA with dmLT.
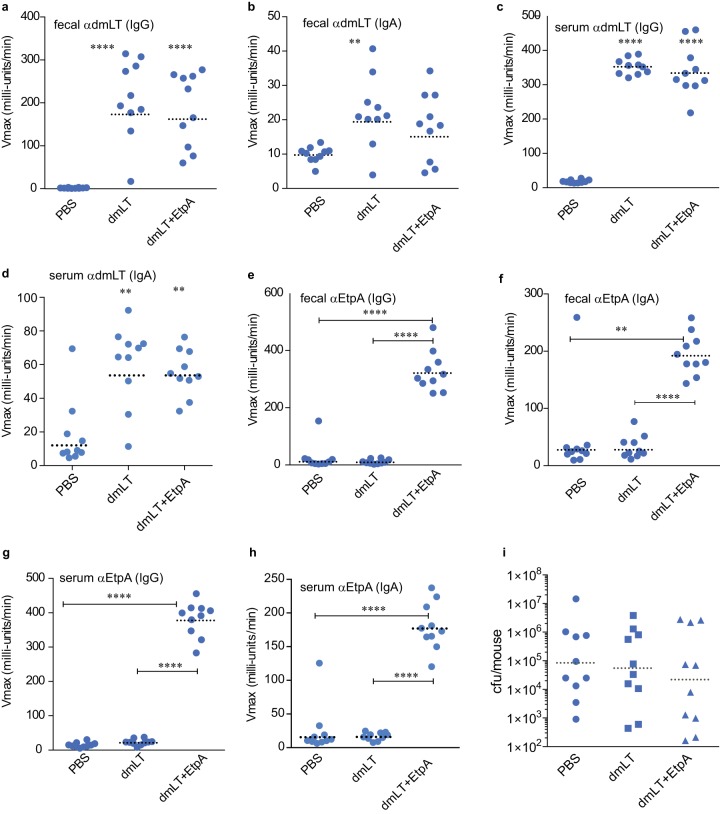
The dose of vaccine required to achieve an immunologic response is typically several orders of magnitude smaller with i.d. vaccination than that required for other routes, offering significant dose sparing. Moreover, the emergence of needle-free technologies (30, 31) could make this route feasible for deployment to developing countries, where cost and ease of administration are important considerations. Therefore, we also examined intradermal vaccination with EtpA in mice. After verification of intradermal placement of potential immunogens (see Fig. S1 in the supplemental material), we were able to achieve high titers of both serum and fecal antibodies to both the adjuvant (Fig. 4a to d) and the EtpA immunogen (Fig. 4e to h) following intradermal administration. Curiously, however, we saw no significant protection against colonization when mice were vaccinated via the i.d. route (Fig. 4g), suggesting that the route of administration could have a substantial impact on vaccine efficacy.
FIG 4.
Intradermal vaccination with EtpA results in significant increases in fecal and serum antibody without substantial protection. Shown in panels a to h are kinetic ELISA data expressed as Vmax (milliunits per minute) obtained with clarified fecal suspensions (at a 1:1 dilution) or serum (diluted 1:100). (a to d) Fecal IgG (a), IgA (b), serum IgG (c), and serum IgA (d) antibody responses to the dmLT adjuvant. (e to h) Fecal IgG (e), fecal IgA (f), serum IgG (g), and serum IgA (h) antibody responses to EtpA. (i) Mouse colonization following vaccination with EtpA and dmLT adjuvant compared to adjuvant-alone or PBS-only controls. Comparisons between groups were made by Mann Whitney (two-tailed) nonparametric testing (**, P < 0.01; ****, P < 0.0001).
Because intradermal vaccination resulted in high titers of antibody that did not protect against intestinal colonization, we questioned whether the quality of antibody from mice vaccinated intradermally differed from that of mice vaccinated orally. Antibody avidity, which examines the functional affinity of antibody-antigen interactions, has been used as marker of B cell maturation and a surrogate of protective immunity to a number of important pathogens. Importantly, antibody avidity has been shown to correlate with the presence of antigen-specific memory B cells following Vibrio cholerae infection (27), potentially permitting efficient measurement of responses that might predict protection against a number of important diarrheal pathogens. Interestingly, the serum IgG antibody avidity in the intradermally vaccinated mice was considerably lower than that in the mice vaccinated orally with EtpA (P = 0.002) (Fig. 5a), as was the EtpA-specific IgA avidity index (AI) (Fig. 5b). Collectively, these data suggest that further assessment of immunization routes, timing of administration, and doses of antigen and adjuvant will likely be important considerations in optimizing the protective efficacy of more recently described antigens, including EtpA.
FIG 5.
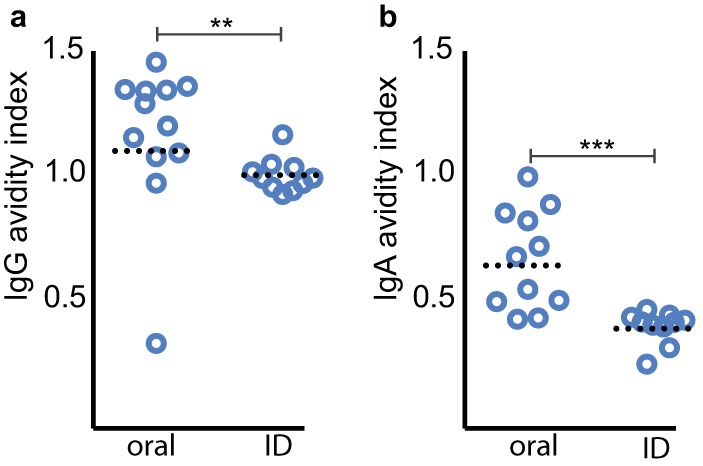
Avidity of EtpA-specific serum antibody following EtpA vaccination with dmLT varies with both dose and route of immunization. (a) EtpA-specific serum IgG avidity following oragastric immunization with rEtpA or intradermal (ID) vaccination. (b) EtpA-specific IgA avidity index after orogastric and intradermal vaccination. Statistical analysis results (**, P = 0.002; ***, P = 0.0002) reflect Mann-Whitney, two-tailed nonparametric comparisons.
Toxin neutralization by sera from vaccinated mice.
Because immunization using dmLT as the adjuvant resulted in significant production of anti-LT antibody, we also examined whether the antibodies were functionally relevant. As demonstrated in Fig. 6a, sera from mice immunized using dmLT prevented effective binding of wild-type heat-labile toxin to target GM1 gangliosides in vitro relative to sera from PBS control mice. Importantly, we found that antisera from either mice immunized with dmLT adjuvant only or from mice immunized with recombinant EtpA and dmLT yielded marked reduction in effective toxin delivery by the ETEC strain H10407, as determined by activation of cAMP in target Caco-2 epithelial cell monolayers (Fig. 6b). These data provide further evidence vaccines combining novel adhesin and toxoid approaches could offer a viable strategy to protect against ETEC.
FIG 6.
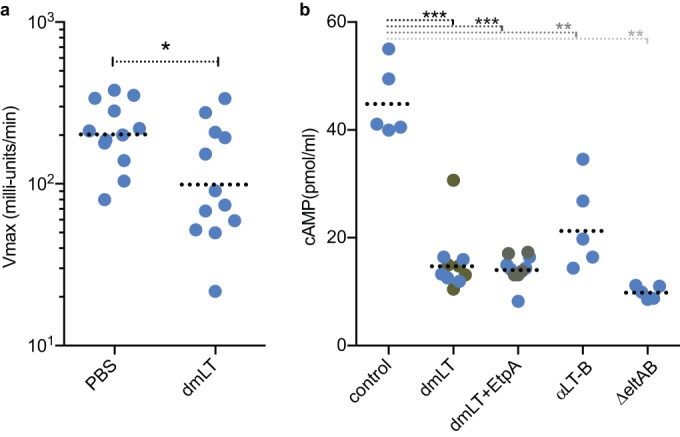
Toxin neutralization by sera from vaccinated mice. (a) Antisera from mice orally immunized with dmLT adjuvant prevent binding to GM1 gangliosides in vitro. Sera from unvaccinated (PBS-only) mice are shown as controls. All statistical comparisons were performed by Mann-Whitney 2-tailed nonparametric testing (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). (b) Sera from mice immunized with dmLT ± recombinant EtpA neutralize effective delivery of heat-labile toxin to target epithelial cells by ETEC H10407. Sera from PBS-only vaccinated control group mice are shown at left for comparison. Additional controls at right include anti-LT-B rabbit polyclonal antibody and the LT-negative (ΔeltAB) strain.
DISCUSSION
Construction of ETEC vaccines has been hampered in part by the extraordinary genetic plasticity of E. coli confounding efforts to define the ideal group of antigens that will achieve broad-based protection. Vaccines currently under study attempt to confront this challenge by using multiple live attenuated strains (32) or whole-cell killed strains that each present different CF antigens and are combined with mutant forms or subunits of heat-labile toxin (28). Interestingly, recent studies of more than 800 isolates from ETEC diarrhea cases in Bangladesh demonstrated that only approximately half of the strains expressed colonization factors (CFs) that could be identified by immunoassays (33), and new CF antigens continue to be identified by genomic sequencing (34).
The potential complexity of vaccines based exclusively on classical ETEC targets has driven investigation of other antigens common to the ETEC pathovar. Theoretically, these antigens, not currently part of ETEC vaccine platforms, could be incorporated into future iterations of vaccines to expand antigenic valency and perhaps act in concert with canonical ETEC vaccine targets to enhance efficacy. One potential alternative target is the two-partner secretion system that encodes EtpA. This system, originally discovered by transposon mutagenesis of the prototype H10407 strain (12), appears to be relatively conserved within the ETEC pathovar (7, 15, 16). EtpA-specific antibodies are present in human convalescent-phase sera, suggesting that this protein is expressed during the course of infection and is immunogenic (15, 35). These features and the molecular and functional similarity of EtpA to filamentous hemagglutinin (36), a component of acellular pertussis vaccines (37), have prompted preclinical investigation of the utility of EtpA as a protective antigen. Multiple studies with mice have now demonstrated that EtpA vaccination protects against colonization of the small intestine (14, 19, 20), thought to be a critical determinant of ETEC diarrheal illness. Another consideration driving the present studies is that ETEC and Shigella are among the most common bacterial pathogens causing serious diarrheal illness among young children in developing countries; therefore, we questioned whether subunit approaches presently being developed for Shigella effectors (38, 39) could be also applied to novel secreted ETEC antigens, including EtpA.
Because all prior studies of this target antigen to date have involved intranasal immunization, we examined whether we might be able to deliver EtpA by other means and elicit protective immune responses. These most recent studies provide further evidence that EtpA affords protection against intestinal colonization and that this antigen retains substantial immunogenicity when administered by a variety of different routes.
Perhaps not surprisingly, however, protective efficacy varied with the route of administration (40, 41), and despite substantial antibody responses following i.d. immunization, we did not achieve protection. One possible explanation for these seemingly discordant results is that the levels of quality of antibodies generated in different vaccination protocols could differ substantially. Antibody avidity is thought to represent the overall strength of binding by a polyvalent collection of antibodies to a variety of antigenic determinants and to reflect development of germinal center B cell antibody affinity maturation (42). A number of clinical studies have correlated antibody avidity with vaccine protective efficacy (43–45), and conversely low antibody avidity and poor affinity maturation have previously been associated with vaccine failures (46–50). The current studies seem to support the idea that antibody avidity could be an important parameter in evaluating antigen-specific memory responses and vaccine performance following immunization with novel and classical ETEC antigens (51).
The present data suggest that additional studies will need to take place to optimize both the route of administration, the doses of EtpA required to achieve protection, and conditions for coformulation with other antigens. Nevertheless, our studies suggest that it is possible to generate protective immune responses with the recombinant adhesin. The current studies are an extension of earlier work with Shigella subunit proteins, and no attempt was made here to optimize immune responses to EtpA. Clearly, the doses of antigen delivered orally in these experiments would be impractical for effective immunization on a large scale. However, these studies suggest that enhanced delivery through an oral vaccine strain could be of benefit (52) and that EtpA could be used to complement existing canonical approaches to development of live attenuated ETEC vaccines (32). Not unexpectedly (53, 54), some routes of immunization resulted in highly compartmentalized responses. While serum antibody responses to EtpA were low following sublingual administration of antigen, we did observe measurable increases in fecal antibody (IgG and IgA) and sublingual immunization was protective. Application of emerging methods that enhance sublingual or buccal antigen delivery (55) could accelerate development of subunit vaccines that incorporate ETEC novel antigens.
Interestingly, emerging data do suggest that intradermal vaccination with mutant LT and ETEC fimbrial tip adhesins can protect against ETEC diarrhea in a human experimental challenge model (56), and i.d. immunization with secreted Shigella effectors adjuvanted with dmLT was protective against experimental challenge in mice (39). Therefore, the i.d. route could conceivably be used as a platform for development of hybrid subunit vaccines against important enteric pathogens. Nevertheless, further effort will be required to optimize this approach and refine antigen formulations with sufficient valency to achieve broad protection against ETEC and Shigella.
Collectively, these early data with EtpA support the concept that this antigen could complement ongoing approaches to vaccine development and expand the valency of ETEC vaccines. Additional efforts will need to focus on first defining and subsequently optimizing parameters that define protection mediated by this and other novel immunogens.
Supplementary Material
ACKNOWLEDGMENTS
We thank Heather Wentzel at PATH for assistance in obtaining the double mutant LT used in these studies. We thank Suellen Greco, Director of the Division of Comparative Medicine Research Animal Diagnostic Laboratory, for kind assistance in processing and interpretation of pathological sections from vaccinated mice.
This work was supported by funding from the Department of Veterans Affairs (5I01BX001469-04), grant 2R01AI89894 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and PATH.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID, NIH, PATH, or the VA.
Funding Statement
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID, NIH, PATH, or the VA.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00248-16.
REFERENCES
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, MAL-ED Network Investigators . 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah N, DuPont HL, Ramsey DJ. 2009. Global etiology of travelers' diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg 80:609–614. [PubMed] [Google Scholar]
- 4.Svennerholm AM, Lundgren A. 2012. Recent progress toward an enterotoxigenic Escherichia coli vaccine. Expert Rev Vaccines 11:495–507. doi: 10.1586/erv.12.12. [DOI] [PubMed] [Google Scholar]
- 5.Sack RB, Gorbach SL, Banwell JG, Jacobs B, Chatterjee BD, Mitra RC. 1971. Enterotoxigenic Escherichia coli isolated from patients with severe cholera-like disease. J Infect Dis 123:378–385. doi: 10.1093/infdis/123.4.378. [DOI] [PubMed] [Google Scholar]
- 6.von Mentzer A, Connor TR, Wieler LH, Semmler T, Iguchi A, Thomson NR, Rasko DA, Joffre E, Corander J, Pickard D, Wiklund G, Svennerholm AM, Sjoling A, Dougan G. 2014. Identification of enterotoxigenic Escherichia coli (ETEC) clades with long-term global distribution. Nat Genet 46:1321–1326. doi: 10.1038/ng.3145. [DOI] [PubMed] [Google Scholar]
- 7.Sahl JW, Steinsland H, Redman JC, Angiuoli SV, Nataro JP, Sommerfelt H, Rasko DA. 2011. A comparative genomic analysis of diverse clonal types of enterotoxigenic Escherichia coli reveals pathovar-specific conservation. Infect Immun 79:950–960. doi: 10.1128/IAI.00932-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasko DA, Rosovitz MJ, Myers GS, Mongodin EF, Fricke WF, Gajer P, Crabtree J, Sebaihia M, Thomson NR, Chaudhuri R, Henderson IR, Sperandio V, Ravel J. 2008. The pangenome structure of Escherichia coli: comparative genomic analysis of E. coli commensal and pathogenic isolates. J Bacteriol 190:6881–6893. doi: 10.1128/JB.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isidean SD, Riddle MS, Savarino SJ, Porter CK. 2011. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 29:6167–6178. doi: 10.1016/j.vaccine.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 10.Peruski LF Jr, Kay BA, El-Yazeed RA, El-Etr SH, Cravioto A, Wierzba TF, Rao M, El-Ghorab N, Shaheen H, Khalil SB, Kamal K, Wasfy MO, Svennerholm AM, Clemens JD, Savarino SJ. 1999. Phenotypic diversity of enterotoxigenic Escherichia coli strains from a community-based study of pediatric diarrhea in periurban Egypt. J Clin Microbiol 37:2974–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleckenstein J, Sheikh A, Qadri F. 2014. Novel antigens for enterotoxigenic Escherichia coli vaccines. Expert Rev Vaccines 13:631–639. doi: 10.1586/14760584.2014.905745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleckenstein JM, Roy K, Fischer JF, Burkitt M. 2006. Identification of a two-partner secretion locus of enterotoxigenic Escherichia coli. Infect Immun 74:2245–2258. doi: 10.1128/IAI.74.4.2245-2258.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy K, Hilliard GM, Hamilton DJ, Luo J, Ostmann MM, Fleckenstein JM. 2009. Enterotoxigenic Escherichia coli EtpA mediates adhesion between flagella and host cells. Nature 457:594–598. doi: 10.1038/nature07568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy K, Hamilton D, Allen KP, Randolph MP, Fleckenstein JM. 2008. The EtpA exoprotein of enterotoxigenic Escherichia coli promotes intestinal colonization and is a protective antigen in an experimental model of murine infection. Infect Immun 76:2106–2112. doi: 10.1128/IAI.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Q, Qadri F, Kansal R, Rasko DA, Sheikh A, Fleckenstein JM. 2015. Conservation and immunogenicity of novel antigens in diverse isolates of enterotoxigenic Escherichia coli. PLoS Negl Trop Dis 9:e0003446. doi: 10.1371/journal.pntd.0003446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Canto F, Valenzuela P, Cantero L, Bronstein J, Blanco JE, Blanco J, Prado V, Levine M, Nataro J, Sommerfelt H, Vidal R. 2011. Distribution of classical and nonclassical virulence genes in enterotoxigenic Escherichia coli isolates from Chilean children and tRNA gene screening for putative insertion sites for genomic islands. J Clin Microbiol 49:3198–3203. doi: 10.1128/JCM.02473-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones T, Cyr S, Allard F, Bellerose N, Lowell GH, Burt DS. 2004. Protollin: a novel adjuvant for intranasal vaccines. Vaccine 22:3691–3697. doi: 10.1016/j.vaccine.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Chabot S, Brewer A, Lowell G, Plante M, Cyr S, Burt DS, Ward BJ. 2005. A novel intranasal Protollin-based measles vaccine induces mucosal and systemic neutralizing antibody responses and cell-mediated immunity in mice. Vaccine 23:1374–1383. doi: 10.1016/j.vaccine.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Roy K, Hamilton D, Ostmann MM, Fleckenstein JM. 2009. Vaccination with EtpA glycoprotein or flagellin protects against colonization with enterotoxigenic Escherichia coli in a murine model. Vaccine 27:4601–4608. doi: 10.1016/j.vaccine.2009.05.076. [DOI] [PubMed] [Google Scholar]
- 20.Roy K, Hamilton DJ, Fleckenstein JM. 2012. Cooperative role of antibodies against heat-labile toxin and the EtpA adhesin in preventing toxin delivery and intestinal colonization by enterotoxigenic Escherichia coli. Clin Vaccine Immunol 19:1603–1608. doi: 10.1128/CVI.00351-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med 350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 22.Lewis DJ, Huo Z, Barnett S, Kromann I, Giemza R, Galiza E, Woodrow M, Thierry-Carstensen B, Andersen P, Novicki D, Del Giudice G, Rappuoli R. 2009. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One 4:e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norton EB, Lawson LB, Freytag LC, Clements JD. 2011. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol 18:546–551. doi: 10.1128/CVI.00538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleckenstein JM, Roy K. 2009. Purification of recombinant high molecular weight two-partner secretion proteins from Escherichia coli. Nat Protoc 4:1083–1092. doi: 10.1038/nprot.2009.87. [DOI] [PubMed] [Google Scholar]
- 25.Allen KP, Randolph MM, Fleckenstein JM. 2006. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect Immun 74:869–875. doi: 10.1128/IAI.74.2.869-875.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsang VC, Wilson BC, Maddison SE. 1980. Kinetic studies of a quantitative single-tube enzyme-linked immunosorbent assay. Clin Chem 26:1255–1260. [PubMed] [Google Scholar]
- 27.Alam MM, Arifuzzaman M, Ahmad SM, Hosen MI, Rahman MA, Rashu R, Sheikh A, Ryan ET, Calderwood SB, Qadri F. 2013. Study of avidity of antigen-specific antibody as a means of understanding development of long-term immunological memory after Vibrio cholerae O1 infection. Clin Vaccine Immunol 20:17–23. doi: 10.1128/CVI.00521-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundgren A, Bourgeois L, Carlin N, Clements J, Gustafsson B, Hartford M, Holmgren J, Petzold M, Walker R, Svennerholm AM. 2014. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled phase I study. Vaccine 32:7077–7084. doi: 10.1016/j.vaccine.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 29.Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, Hofman P, Holmgren J, Anjuere F, Czerkinsky C. 2007. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 25:8598–8610. doi: 10.1016/j.vaccine.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 30.Lambert PH, Laurent PE. 2008. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine 26:3197–3208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 31.Kis EE, Winter G, Myschik J. 2012. Devices for intradermal vaccination. Vaccine 30:523–538. doi: 10.1016/j.vaccine.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Darsley MJ, Chakraborty S, Denearing B, Sack DA, Feller A, Buchwaldt C, Bourgeois AL, Walker R, Harro CD. 2012. ACE527 oral, live attenuated ETEC vaccine reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease. Clin Vaccine Immunol 19:1921–1931. doi: 10.1128/CVI.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begum YA, Baby NI, Faruque AS, Jahan N, Cravioto A, Svennerholm AM, Qadri F. 2014. Shift in phenotypic characteristics of enterotoxigenic Escherichia coli (ETEC) isolated from diarrheal patients in Bangladesh. PLoS Negl Trop Dis 8:e3031. doi: 10.1371/journal.pntd.0003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Mentzer A, Sjoling A, Dougan G, Svennerholm A. 2016. Whole genome sequencing of enterotoxigenic Escherichia coli (ETEC)—search for novel colonization factors, p 115–118. In 50th US-Japan Cooperative Medical Sciences Program Joint Panel Conference on Cholera and Other Bacterial Enteric Infections, Bethesda, MD. [Google Scholar]
- 35.Roy K, Bartels S, Qadri F, Fleckenstein JM. 2010. Enterotoxigenic Escherichia coli elicits immune responses to multiple surface proteins. Infect Immun 78:3027–3035. doi: 10.1128/IAI.00264-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Relman DA, Domenighini M, Tuomanen E, Rappuoli R, Falkow S. 1989. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A 86:2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward JI, Cherry JD, Chang SJ, Partridge S, Lee H, Treanor J, Greenberg DP, Keitel W, Barenkamp S, Bernstein DI, Edelman R, Edwards K. 2005. Efficacy of an acellular pertussis vaccine among adolescents and adults. N Engl J Med 353:1555–1563. doi: 10.1056/NEJMoa050824. [DOI] [PubMed] [Google Scholar]
- 38.Lee S, Picking WL, Tzipori S. 2014. The immune response of two microbial antigens delivered intradermally, sublingually, or the combination thereof. Microbes Infect 16:796–803. doi: 10.1016/j.micinf.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Heine SJ, Diaz-McNair J, Andar AU, Drachenberg CB, van de Verg L, Walker R, Picking WL, Pasetti MF. 2014. Intradermal delivery of Shigella IpaB and IpaD type III secretion proteins: kinetics of cell recruitment and antigen uptake, mucosal and systemic immunity, and protection across serotypes. J Immunol 192:1630–1640. doi: 10.4049/jimmunol.1302743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belyakov IM, Ahlers JD. 2009. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol 183:6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 41.Boyle JS, Silva A, Brady JL, Lew AM. 1997. DNA immunization: induction of higher avidity antibody and effect of route on T cell cytotoxicity. Proc Natl Acad Sci U S A 94:14626–14631. doi: 10.1073/pnas.94.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. 2010. Control systems and decision making for antibody production. Nat Immunol 11:681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 43.Goldblatt D, Vaz AR, Miller E. 1998. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J Infect Dis 177:1112–1115. doi: 10.1086/517407. [DOI] [PubMed] [Google Scholar]
- 44.Usinger WR, Lucas AH. 1999. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun 67:2366–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khurana S, Wu J, Dimitrova M, King LR, Manischewitz J, Graham BS, Ledgerwood JE, Golding H. 2013. DNA priming prior to inactivated influenza A(H5N1) vaccination expands the antibody epitope repertoire and increases affinity maturation in a boost-interval-dependent manner in adults. J Infect Dis 208:413–417. doi: 10.1093/infdis/jit178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YC, Kelly DF, Yu LM, Slack MP, Booy R, Heath PT, Siegrist CA, Moxon RE, Pollard AJ. 2008. Haemophilus influenzae type b vaccine failure in children is associated with inadequate production of high-quality antibody. Clin Infect Dis 46:186–192. doi: 10.1086/524668. [DOI] [PubMed] [Google Scholar]
- 47.Sanz-Moreno JC, Limia-Sanchez A, Garcia-Comas L, Mosquera-Gutierrez MM, Echevarria-Mayo JE, Castellanos-Nadal A, de Ory-Manchon F. 2005. Detection of secondary mumps vaccine failure by means of avidity testing for specific immunoglobulin G. Vaccine 23:4921–4925. doi: 10.1016/j.vaccine.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Narita M, Matsuzono Y, Takekoshi Y, Yamada S, Itakura O, Kubota M, Kikuta H, Togashi T. 1998. Analysis of mumps vaccine failure by means of avidity testing for mumps virus-specific immunoglobulin G. Clin Diagn Lab Immunol 5:799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. 2009. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med 15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. 2003. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med 9:1209–1213. doi: 10.1038/nm918. [DOI] [PubMed] [Google Scholar]
- 51.Alam MM, Aktar A, Afrin S, Rahman MA, Aktar S, Uddin T, Rahman MA, Al Mahbuba D, Chowdhury F, Khan AI, Bhuiyan TR, Begum YA, Ryan ET, Calderwood SB, Svennerholm AM, Qadri F. 2014. Antigen-specific memory B-cell responses to enterotoxigenic Escherichia coli infection in Bangladeshi adults. PLoS Negl Trop Dis 8:e2822. doi: 10.1371/journal.pntd.0002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Czerkinsky C, Holmgren J. 2015. Vaccines against enteric infections for the developing world. Philos Trans R Soc Lond B Biol Sci 370:20150142. doi: 10.1098/rstb.2015.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandtzaeg P. 2013. Secretory IgA: designed for anti-microbial defense. Front Immunol 4:222. doi: 10.3389/fimmu.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Czerkinsky C, Holmgren J. 2012. Mucosal delivery routes for optimal immunization: targeting immunity to the right tissues. Curr Top Microbiol Immunol 354:1–18. doi: 10.1007/82_2010_112. [DOI] [PubMed] [Google Scholar]
- 55.White JA, Blum JS, Hosken NA, Marshak JO, Duncan L, Zhu C, Norton EB, Clements JD, Koelle DM, Chen D, Weldon WC, Oberste MS, Lal M. 2014. Serum and mucosal antibody responses to inactivated polio vaccine after sublingual immunization using a thermoresponsive gel delivery system. Hum Vaccin Immunother 10:3611–3621. doi: 10.4161/hv.32253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harro C, Gutierrez R, Talaat K, Porter C, Riddle M, Maciel M, Poole S, Laird R, Savarino S. 2016. Protective efficacy of an enterotoxigenic E. coli fimbrial tip adhesin vaccine given with LTR192G by intradermal vaccination against experimental challenge with CFA/I-ETEC in adult volunteers, p 126–130. In 50th US-Japan Cooperative Medical Sciences Program Joint Panel Conference on Cholera and Other Bacterial Enteric Infections, Bethesda, MD. [Google Scholar]
- 57.Evans DG, Silver RP, Evans DJ Jr, Chase DG, Gorbach SL. 1975. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect Immun 12:656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Evans DJ Jr, Evans DG. 1973. Three characteristics associated with enterotoxigenic Escherichia coli isolated from man. Infect Immun 8:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dorsey FC, Fischer JF, Fleckenstein JM. 2006. Directed delivery of heat-labile enterotoxin by enterotoxigenic Escherichia coli. Cell Microbiol 8:1516–1527. doi: 10.1111/j.1462-5822.2006.00736.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.