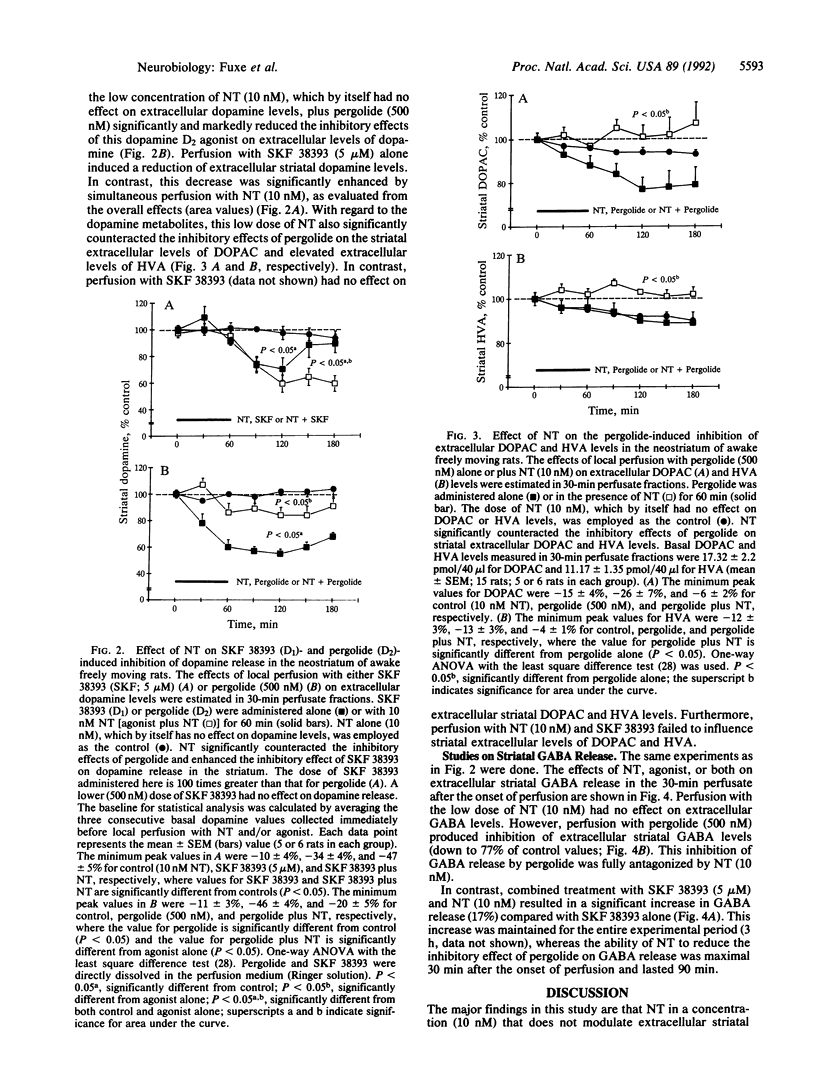
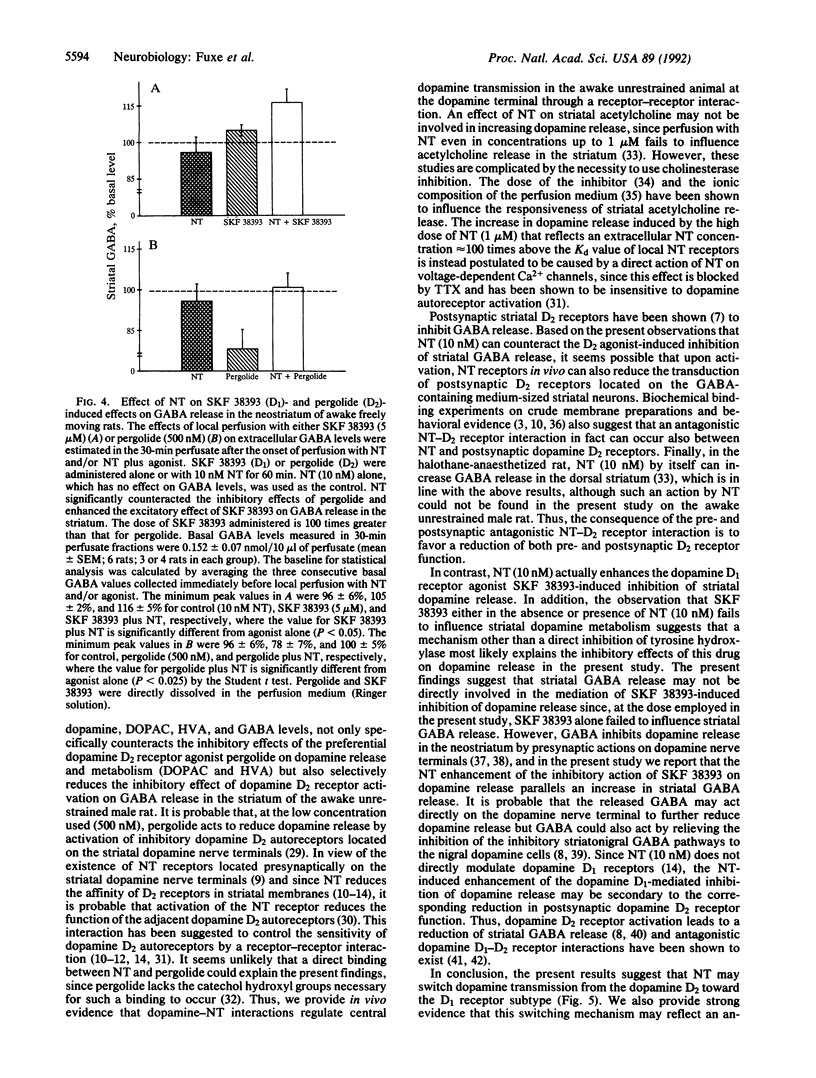
Abstract
The major mechanism underlying the neuroleptic action of the tridecapeptide neurotensin (NT) appears to be an interaction with dopamine receptor mechanisms based on biochemical binding and behavioral experiments. In vivo microdialysis was used in conscious rats to investigate the effects of local perfusion with NT on the sensitivity of striatal dopamine D1 and D2 receptors for their selective agonists by monitoring extracellular dopamine, 3,4-dihydroxyphenylacetic acid, homovanilic acid, and gamma-aminobutyric acid levels in the awake unrestrained male rat. Perfusion with NT (10 nM) counteracted the inhibitory effects of the dopamine D2 agonist pergolide (500 nM) on extracellular levels of dopamine and gamma-aminobutyric acid. In contrast, NT (10 mM) significantly enhanced the reduction of extracellular striatal levels of dopamine after perfusion with the D1 agonist SKF 38393 (5 microM), and this combined treatment also resulted in a significant increase in the extracellular striatal levels of gamma-aminobutyric acid. These results provide in vivo evidence that NT regulates central dopamine transmission by reducing pre-and postsynaptic dopamine D2 and enhancing D1 receptor sensitivity possibly through an antagonistic NT receptor-D2 receptor interaction. This heteroregulation has the potential to substantially increase the plasticity within the dopamine synapse.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi D. K., Kalivas P. W., Schenk J. O. Neurotensin binding to dopamine. J Neurochem. 1990 Apr;54(4):1321–1328. doi: 10.1111/j.1471-4159.1990.tb01965.x. [DOI] [PubMed] [Google Scholar]
- Agnati L. F., Fuxe K., Battistini N., Giardino L., Benfenati F., Martire M., Ruggeri M. Further evidence for the existence of interactions between receptors for dopamine and neurotensin. Dopamine reduces the affinity and increases the number of [3H]-neurotensin binding sites in the subcortical limbic forebrain of the rat. Acta Physiol Scand. 1985 May;124(1):125–128. doi: 10.1111/j.1748-1716.1985.tb07641.x. [DOI] [PubMed] [Google Scholar]
- Agnati L. F., Fuxe K., Benfenati F., Battistini N. Neurotensin in vitro markedly reduces the affinity in subcortical limbic 3H-N-propylnorapomorphine binding sites. Acta Physiol Scand. 1983 Dec;119(4):459–461. doi: 10.1111/j.1748-1716.1983.tb07350.x. [DOI] [PubMed] [Google Scholar]
- Agnati L. F., Fuxe K., Zoli M., Pich E. M., Benfenati F., Zini I., Goldstein M. Aspects on the information handling by the central nervous system: focus on cotransmission in the aged rat brain. Prog Brain Res. 1986;68:291–301. doi: 10.1016/s0079-6123(08)60245-9. [DOI] [PubMed] [Google Scholar]
- Bartholini G., Scatton B., Worms P., Zivkovic B., Lloyd K. G. Interactions between GABA, dopamine, acetylcholine, and glutamate-containing neurons in the extrapyramidal and limbic systems. Adv Biochem Psychopharmacol. 1981;30:119–128. [PubMed] [Google Scholar]
- Bolam J. P., Powell J. F., Wu J. Y., Smith A. D. Glutamate decarboxylase-immunoreactive structures in the rat neostriatum: a correlated light and electron microscopic study including a combination of Golgi impregnation with immunocytochemistry. J Comp Neurol. 1985 Jul 1;237(1):1–20. doi: 10.1002/cne.902370102. [DOI] [PubMed] [Google Scholar]
- Clark D., White F. J. D1 dopamine receptor--the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse. 1987;1(4):347–388. doi: 10.1002/syn.890010408. [DOI] [PubMed] [Google Scholar]
- Damsma G., de Boer P., Westerink B. H., Fibiger H. C. Dopaminergic regulation of striatal cholinergic interneurons: an in vivo microdialysis study. Naunyn Schmiedebergs Arch Pharmacol. 1990 Nov;342(5):523–527. doi: 10.1007/BF00169040. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. In-vivo brain dialysis of neurotransmitters. Trends Pharmacol Sci. 1990 Mar;11(3):116–121. doi: 10.1016/0165-6147(90)90197-g. [DOI] [PubMed] [Google Scholar]
- Drew K. L., O'Connor W. T., Kehr J., Ungerstedt U. Characterization of gamma-aminobutyric acid and dopamine overflow following acute implantation of a microdialysis probe. Life Sci. 1989;45(14):1307–1317. doi: 10.1016/0024-3205(89)90134-3. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Agnati L. F. Receptor-receptor interactions in the central nervous system. A new integrative mechanism in synapses. Med Res Rev. 1985 Oct-Dec;5(4):441–482. doi: 10.1002/med.2610050404. [DOI] [PubMed] [Google Scholar]
- Girault J. A., Spampinato U., Glowinski J., Besson M. J. In vivo release of [3H]gamma-aminobutyric acid in the rat neostriatum--II. Opposing effects of D1 and D2 dopamine receptor stimulation in the dorsal caudate putamen. Neuroscience. 1986 Dec;19(4):1109–1117. doi: 10.1016/0306-4522(86)90127-2. [DOI] [PubMed] [Google Scholar]
- Imperato A., Di Chiara G. Effects of locally applied D-1 and D-2 receptor agonists and antagonists studied with brain dialysis. Eur J Pharmacol. 1988 Nov 8;156(3):385–393. doi: 10.1016/0014-2999(88)90284-1. [DOI] [PubMed] [Google Scholar]
- Kehr J., Ungerstedt U. Fast HPLC estimation of gamma-aminobutyric acid in microdialysis perfusates: effect of nipecotic and 3-mercaptopropionic acids. J Neurochem. 1988 Oct;51(4):1308–1310. doi: 10.1111/j.1471-4159.1988.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Koller W. C., Weiner W. J., Diamond B. I., Nausieda P. A., Klawans H. L. The pharmacological evaluation of pergolide mesylate as a potential anti-parkinson agent. Neuropharmacology. 1980 Sep;19(9):831–837. doi: 10.1016/0028-3908(80)90079-9. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Inagaki S., Kito S., Wu J. Y. Dopaminergic axons directly make synapses with GABAergic neurons in the rat neostriatum. Brain Res. 1987 Mar 17;406(1-2):147–156. doi: 10.1016/0006-8993(87)90779-7. [DOI] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of the release of catecholamines. Pharmacol Rev. 1980 Dec;32(4):337–362. [PubMed] [Google Scholar]
- Nemeroff C. B. The interaction of neurotensin with dopaminergic pathways in the central nervous system: basic neurobiology and implications for the pathogenesis and treatment of schizophrenia. Psychoneuroendocrinology. 1986;11(1):15–37. doi: 10.1016/0306-4530(86)90029-6. [DOI] [PubMed] [Google Scholar]
- O'Connor W. T., Tanganelli S., Ungerstedt U., Fuxe K. The effects of neurotensin on GABA and acetylcholine release in the dorsal striatum of the rat: an in vivo microdialysis study. Brain Res. 1992 Feb 28;573(2):209–216. doi: 10.1016/0006-8993(92)90765-2. [DOI] [PubMed] [Google Scholar]
- Osborne P. G., O'Connor W. T., Drew K. L., Ungerstedt U. An in vivo microdialysis characterization of extracellular dopamine and GABA in dorsolateral striatum of awake freely moving and halothane anaesthetised rats. J Neurosci Methods. 1990 Sep;34(1-3):99–105. doi: 10.1016/0165-0270(90)90047-j. [DOI] [PubMed] [Google Scholar]
- Osborne P. G., O'Connor W. T., Drew K. L., Ungerstedt U. An in vivo microdialysis characterization of extracellular dopamine and GABA in dorsolateral striatum of awake freely moving and halothane anaesthetised rats. J Neurosci Methods. 1990 Sep;34(1-3):99–105. doi: 10.1016/0165-0270(90)90047-j. [DOI] [PubMed] [Google Scholar]
- Osborne P. G., O'Connor W. T., Kehr J., Ungerstedt U. In vivo characterisation of extracellular dopamine, GABA and acetylcholine from the dorsolateral striatum of awake freely moving rats by chronic microdialysis. J Neurosci Methods. 1991 Apr;37(2):93–102. doi: 10.1016/0165-0270(91)90119-k. [DOI] [PubMed] [Google Scholar]
- Osborne P. G., O'Connor W. T., Ungerstedt U. Effect of varying the ionic concentration of a microdialysis perfusate on basal striatal dopamine levels in awake rats. J Neurochem. 1991 Feb;56(2):452–456. doi: 10.1111/j.1471-4159.1991.tb08171.x. [DOI] [PubMed] [Google Scholar]
- Phillips A. G., Blaha C. D., Fibiger H. C., Lane R. F. Interactions between mesolimbic dopamine neurons, cholecystokinin, and neurotensin: evidence using in vivo voltammetry. Ann N Y Acad Sci. 1988;537:347–361. doi: 10.1111/j.1749-6632.1988.tb42119.x. [DOI] [PubMed] [Google Scholar]
- Quirion R., Chiueh C. C., Everist H. D., Pert A. Comparative localization of neurotensin receptors on nigrostriatal and mesolimbic dopaminergic terminals. Brain Res. 1985 Feb 18;327(1-2):385–389. doi: 10.1016/0006-8993(85)91542-2. [DOI] [PubMed] [Google Scholar]
- Reid M. S., O'Connor W. T., Herrera-Marschitz M., Ungerstedt U. The effects of intranigral GABA and dynorphin A injections on striatal dopamine and GABA release: evidence that dopamine provides inhibitory regulation of striatal GABA neurons via D2 receptors. Brain Res. 1990 Jun 11;519(1-2):255–260. doi: 10.1016/0006-8993(90)90086-q. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1(2):133–152. doi: 10.1002/syn.890010203. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Leff S. E., Creese I. Interactions of novel dopaminergic ligands with D-1 and D-2 dopamine receptors. Life Sci. 1982 Aug 16;31(7):637–645. doi: 10.1016/0024-3205(82)90764-0. [DOI] [PubMed] [Google Scholar]
- Stoof J. C., Kebabian J. W. Two dopamine receptors: biochemistry, physiology and pharmacology. Life Sci. 1984 Dec 3;35(23):2281–2296. doi: 10.1016/0024-3205(84)90519-8. [DOI] [PubMed] [Google Scholar]
- Tanganelli S., von Euler G., Fuxe K., Agnati L. F., Ungerstedt U. Neurotensin counteracts apomorphine-induced inhibition of dopamine release as studied by microdialysis in rat neostriatum. Brain Res. 1989 Nov 20;502(2):319–324. doi: 10.1016/0006-8993(89)90627-6. [DOI] [PubMed] [Google Scholar]
- Von Euler G., Fuxe K. Neurotensin reduces the affinity of D-2 dopamine receptors in rat striatal membranes. Acta Physiol Scand. 1987 Dec;131(4):625–626. doi: 10.1111/j.1748-1716.1987.tb08285.x. [DOI] [PubMed] [Google Scholar]
- Westerink B. H., Damsma G., Rollema H., De Vries J. B., Horn A. S. Scope and limitations of in vivo brain dialysis: a comparison of its application to various neurotransmitter systems. Life Sci. 1987 Oct 12;41(15):1763–1776. doi: 10.1016/0024-3205(87)90695-3. [DOI] [PubMed] [Google Scholar]
- Zetterström T., Sharp T., Ungerstedt U. Effect of dopamine D-1 and D-2 receptor selective drugs on dopamine release and metabolism in rat striatum in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1986 Oct;334(2):117–124. doi: 10.1007/BF00505810. [DOI] [PubMed] [Google Scholar]
- von Euler G. Biochemical characterization of the intramembrane interaction between neurotensin and dopamine D2 receptors in the rat brain. Brain Res. 1991 Oct 4;561(1):93–98. doi: 10.1016/0006-8993(91)90753-i. [DOI] [PubMed] [Google Scholar]
- von Euler G., Fuxe K., Benfenati F., Hansson T., Agnati L. F., Gustafsson J. A. Neurotensin modulates the binding characteristics of dopamine D2 receptors in rat striatal membranes also following treatment with toluene. Acta Physiol Scand. 1989 Apr;135(4):443–448. doi: 10.1111/j.1748-1716.1989.tb08602.x. [DOI] [PubMed] [Google Scholar]