Abstract
We aimed to assess and compare the expression of Dickkopf homolog 3 (DKK3), a possible tumor suppressor gene (TSG), in oral leukoplakia (OLK) and oral submucous fibrosis (OSF) using immunohistochemistry. Seventy-five cases of normal oral mucosa (NOM), OLK, OSF, and squamous cell carcinoma (OSCC) were studied. DKK3 was expressed in all cases of NOM, OLK and OSCC. There was steady increases in the percentage of the positive cells progressing toward OSCC. The expression was localized in the cytoplasm and cell membrane of cell affected by OLK with mild dysplasia and OLK with severe dysplasia. No significant association was observed between DKK3 expression and dysplastic status of OLK. Loss of DKK3 expression was observed in 15 of 30 cases in the OSF group, which was significantly associated with histological grade of OSF (P<0.0001). The percentage of positive cells gradually declined with the increasing severity of epithelial atrophy. A significant difference (P<0.01) was observed when comparing DKK3 expression among different groups of OLK and OSF cases. DKK3 may have diverse expressions in oral premalignant lesions. Loss of DKK3 expression in dysplastic/advanced stage of OSF may imply a high risk of progression to oral cancer.
Key words: Oral leukoplakia, oral submucous fibrosis, DKK3, immunohistochemistry
Introduction
Despite the improvement in the diagnostic and therapeutic strategies, oral squamous cell carcinoma (OSCC) remains a major public health problem; it is the eighth most common cancer worldwide. The five-year survival rate of patients with OSCC remains almost unchanged.1 Oral leukoplakia (OLK) and oral submucous fibrosis (OSF) are potentially malignant disorders with a high risk of transitioning to OSCC. Dysplasia occurs in 25% of OLK cases and 26% of OSF cases.2-4 The potential for malignant transformation of OLK and OSF lesions with evidence of epithelial dysplasia ranges from 5%-15% and 3%-19%, respectively.3,5 Therefore, attempts are being made to identify specific molecular biomarkers to predict which lesions have the potential to be malignant. The Wnt pathway plays a role in cell adhesion, proliferation, and differentiation.6 Activation of the canonical Wnt pathway occurs by binding extracellular ligands to the frizzled receptor and the low-density lipoprotein (LRP5/6) co-receptors, which ultimately leads to stabilization of β-catenin. Consequently, the cytoplasmic level of β-catenin increases. β-catenin translocates to the nucleus, interacts with Tcf/LEF family, and activates transcription by replacing repressor complexes like the Groucho repressor complex.6 The human Dickkopf family comprises four members (DKK1, 2, 3, and 4),7 which function as inhibitors of the canonical Wnt pathway. DKK3 is a potential tumor suppressor gene (TSG) that is localized on 11p15, a locus often deleted in cancerous cells. 8 DKK3 interacts with the Krm receptors intracellularly,9,10 which exerts its inhibitory effect on Wnt signaling either by binding to bTrCP, thus blocking β-catenin from entering the nucleus, 11 or by increasing the localization of β-catenin in the cell membrane.12 DKK3 has been shown to be downregulated in various cancer tissues,13-16 suggesting that this gene may be a TSG, while hypermethylation of its promoter correlates with the occurrence of cancers.17,18 However, its overexpression has been reported to inhibit cell proliferation.12,19
Since the expression of DKK3 in potentially premalignant disorders has not been examined, in this study we aim to assess and compare immunohistochemical expression of DKK3 in OLK and OSF as well as correlation with clinicopathological features.
Materials and Methods
Patients and tissues
Seventy-five formalin-fixed paraffin-embedded blocks with histologically proven NOM, OSF, OLK, and OSCC were collected retrospectively. The samples consisted of 5 NOM, 14 OSF without dysplasia, 16 OSF with dysplasia, 18 OLK with mild dysplasia, 12 OLK with moderate/severe dysplasia, and 10 OSCC. All patients in this study provided informed consent, and the study was approved by the School of Medicine, Central South University. Thorough clinicopathological characteristics were recorded. OSCC was diagnosed according to WHO criteria of 2005.20 According to Pindborg’s criteria,21 OSF cases were further categorized as early stage (n=5), moderately advanced (n=16), or advanced (n=9). NOM samples were obtained from the buccal mucosa during the surgical removal of third molars.
Immunohistochemical staining
The biopsy samples were fixed in 10% neutral buffered formalin, left 24 h at room temperature, routinely processed for histology and embedded in paraffin wax. Four-micrometer-thick sections were cut, dewaxed in xylene, and dehydrated in a graded alcohol. After the slides were rinsed, endogenous peroxidase activity was blocked by treatment with 3% H2O2 for 15 min at room temperature. The slides were pretreated with citrate buffer (10% citrate buffer stock in distilled water, pH 6.0) and microwaved for 20 min to retrieve the antigen. Non-reactive staining was blocked by 1.0% goat serum in Tris-buffered saline (pH 6.0) applied for 3 min. Primary rabbit polyclonal antibodies against human DKK3 (AP1523a, Abgent, San Diego, CA, USA; 1:50) were diluted in phosphate-buffered saline (PBS, pH 7.4) and then incubated for 1 h at 37°C in a humidified chamber. After the slides were rinsed with PBS, they were incubated with a secondary antibody (biotinylated polylink; Dako, Hamburg, Germany) for 30 min at 37°C in a humidified chamber and then rinsed with PBS. DKK3 expression was detected by a diaminobenzidine chromogen system (Dako). The sections were counterstained with hematoxylin, dehydrated, and then mounted.
Image analysis
DKK3 expression and epithelial thickness was measured in all tissue samples using ImageJ, Java-based image processing software (National Institute of Mental Health, Bethesda, MD, USA). DKK3 expression was scored as previously described by Carmona et al.22 The mean values for each group of measurements were compared using the Student’s t-test.
Statistical analysis
Statistical analysis was performed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA). The chi-square test was used to determine the relationship between DKK3 expression and clinicopathological parameters. DKK3 expression was compared between different OLK and OSF groups using the Mann-Whitney U test. Differences with a probability value of less than 0.05 were considered to be statistically significant.
Results
DKK3 staining was observed in 60 of the samples and was lost in 15. In NOM, DKK3 was localized only in the cell membrane of suprabasal cell layers, whereas the basal cell layer was negatively stained except for some scattered spots of weak DKK3 expression. (Figure 1 A,B). DKK3 expression was observed in all OLK cases (Table 1). It was localized in both the cell membrane and cytoplasm. However, the percentage of cytoplasmic expression in cases of OLK with severe dysplasia was higher compared to OLK with mild dysplasia. In contrast, the membranous expression of the suprabasal cells was higher in OLK with mild dysplasia compared to OLK with severe dysplasia (Figure 1 C-F). No statistical difference was observed (P=0.323) (Table 1).
Figure 1.
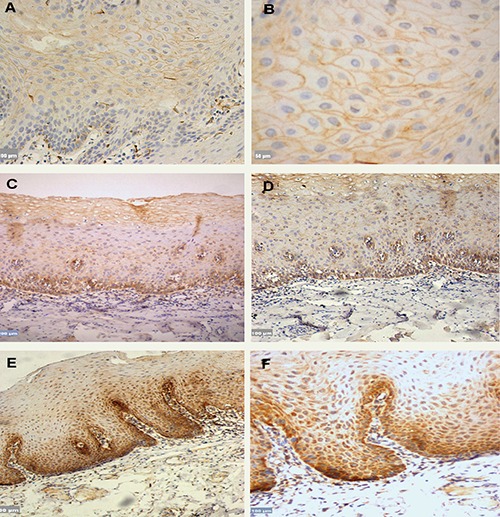
A,B) DKK3 expression in NOM, which was localized in the cell membrane of suprabasal cell layers, however absence of staining was detected in the basal layer; scale bars: A) 100 μm; B) 50 μm. C,D) OLK Mild dysplasia, DKK3 staining was localized in the cytoplasm of the basal layer and in the cell membranes of suprabasal layer; scale bars: C) 200 μm; D) 100 μm. E,F) OLK severe dysplasia, the staining was localized in the cytoplasm as in mild dysplasia; however, the percentage of cytoplasmic positive cells were higher in severe dysplasia compared with mild dysplasia; scale bars: E) 200 μm; F) 100 μm.
Table 1.
Clinicopathological characteristics and DKK3 expression in patients with oral leukoplakia.
| No. of patients (n=30) (%) | DKK3 expression | χ2 | P value | ||
|---|---|---|---|---|---|
| +(%) | ++(%) | ||||
| Sex | 0.139 | 0.709 | |||
| Female | 3(10) | 1 (33.3) | 2 (66.7) | ||
| Male | 27(90) | 17 (63.0) | 10 (37.0) | ||
| Age (years) | 0.362 | 0.547 | |||
| <46 | 17(60) | 11 (64.7) | 6 (35.3) | ||
| >46 | 13 (43.3) | 7 (53.8) | 6 (46.2) | ||
| Dysplastic status | 0.978 | 0.323 | |||
| Mild dysplasia | 18(60) | 9 (50.0) | 9 (50.0) | ||
| Severe dysplasia | 12(40) | 9 (75.0) | 3 (25.0) | ||
| Habits | 0.536 | 0.464 | |||
| Alcohol | 21(70) | 14 (66.7) | 7 (33.3) | ||
| Alcohol and smoking | 9(30) | 4 (44.4) | 5 (55.6) | ||
DKK3 expression was detected in 15 of 30 tissue samples of patients with OSF (Table 2). DKK3 expression was detected in 13 cases of OSF without dysplasia and was lost in 1, whereas DKK3 was detected in 2 cases of OSF with dysplasia and was lost in 14. A significant difference was observed when comparing DKK3 staining among cases of OSF with and without dysplasia (Figure 2 A-F). DKK3 expression was observed in 4 out of 5 patients in the early stage, 9 out of 16 in the moderately advanced stage, and 2 out of 9 in the advanced stage. However, it was lost in 1, 7, and 7cases, respectively. There was a gradual decline in DKK3 expression while epithelial atrophy progressed, but this was not statistically significant. No DKK3 expression was detected in the cell membrane of any OSF case. In the early stage of OSF, DKK3 expression was localized in the cytoplasm of the basal and suprabasal layers, but it was only localized in the cytoplasm of the basal layer in the moderately advanced and advanced stages (Figure 2 A-F). Cytoplasmic DKK3 expression was detected in all OSCC cases (Figure 3 A,B). The relationship between DKK3 expression and the various clinicopathological characteristics of OLK and OSF was studied. Only the dysplastic status of OSF, which shows significant correlation with DKK3 expression, other shows no significance (Tables 1 and 2). When DKK3 expression was compared between OLK groups and OSF groups, significant differences were observed (P<0.05) (Table 3).
Table 2.
Clinicopathological characteristics and DKK3 expression in patients with oral submucous fibrosis.
| No. of patients | DKK3 expression | χ2 | P value | ||
|---|---|---|---|---|---|
| +(%) | -(%) | ||||
| Sex | 0.000 | 1.000 | |||
| Male | 26 | 13(50) | 13(50) | ||
| Female | 4 | 1(25) | 3(75) | ||
| Age (years) | 2.143 | 0.143 | |||
| <31 | 14 | 5 (35.7) | 9 (64.3) | ||
| >31 | 16 | 10 (62.5) | 6 (37.5) | ||
| Grade | 4.563 | 0.130 | |||
| Early | 5 | 4(80) | 1(20) | ||
| Moderate | 16 | 9 (56.3) | 7 (43.7) | ||
| Advanced | 9 | 2 (22.2) | 7 (77.8) | ||
| Dysplasia status | 19.286 | 0.0001* | |||
| Dysplasia | 16 | 2 (12.5) | 14 (87.5) | ||
| No dysplasia | 14 | 13 (92.9) | 1 (7.1) | ||
| Habit | 0.556 | 0.456 | |||
| Betel nut | 18 | 10 (55.6) | 8 (44.4) | ||
| Betel nut and smoking | 12 | 5 (41.7) | 7 (58.3) | ||
| Duration | 0.133 | 0.715 | |||
| <8 years | 15 | 8 (53.3) | 7 (46.7) | ||
| >8 years | 15 | 7 (46.7) | 8 (53.3) | ||
| Mouth opening | 0.635 | 0.426 | |||
| <28 | 9 | 3 (33.3) | 6 (77.7) | ||
| >28 | 21 | 12 (57.1) | 9 (42.9) | ||
| Stage/atrophy° | 0.362 | 0.547 | |||
| Early stage/atrophic | 2 | 2 | 0 | ||
| Moderate stage/atrophic | 7 | 4 | 3 | ||
| Sever stage/atrophic | 9 | 2 | 7 | ||
*Statistically significant at P<0.05;
°relation between epithelial atrophy and DKK3 expression according to Pindborg’s criteria.
Figure 2.
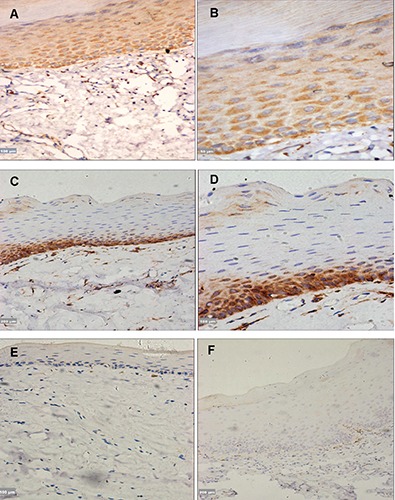
A,B) OSF without dysplasia/early stage, DKK3 expression was localized in the cytoplasm of basal and suprabasal layers; scale bars: A) 100 μm; B) 50 μm. C,D) OSF without dysplasia/moderately advanced, DKK3 was localized in the cytoplasm of basal layer; however, negative staining was observed in the suprabasal layers; scale bars: C) 200 μm; D) 100 μm. E) OSF advanced stage, complete loss of DKK3 expression in the basal and suprabasal layer; scale bar: 100 μm. F) OSF with dysplasia/ moderately advanced stage, showing absence of DKK3 staining; scale bar: 200 μm.
Figure 3.
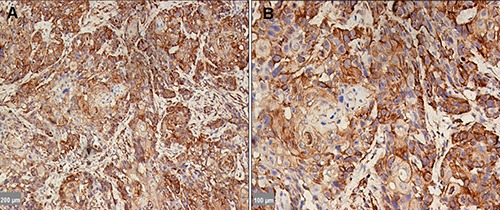
DKK3 expression in OSCC, which was localized in the cytoplasm; however, the staining was completely absent in the nucleus. Scale bars: A) 200 μm; B) 100 μm.
Table 3.
A comparative account of DKK3 expression in different stages of oral leukoplakia and oral submucous fibrosis.
| Groups | Rate (%) | χ2 (Mann-Whitney test) | Z | P value |
|---|---|---|---|---|
| OSFWD | 16 (26.7) | OSFWD vs OLKWSD | 4.970 | 0.0001* |
| OSFWTD | 14 (23.3) | OSFWD vs OLKWMD | 4.476 | 0.0001* |
| OLKWSD | 18 (30.0) | OSFWTD vs OLKWSD | 1.899 | 0.058* |
| OLKWMD | 12 (20.0) | OSFWTD vs OLKWMD | 2.512 | 0.012* |
| OSFWD vs OSFWTD | 4.543 | 0.0001* |
OSFWTD, oral submucous fibrosis without dysplasia; OSFWD, oral submucous fibrosis with dysplasia; OLKWMD, oral leukoplakia with mild dysplasia; OLKWD, oral leukoplakia with moderate/sever dysplasia.
*Statistically significant at P<0.05.
Discussion
An important aim of this study was to assess and compare DKK3 expression in OLK and OSF patients. The current study is the first to investigate DKK3 expression in both OLK and OSF. In NOM, DKK3 was expressed on the cell membrane at suprabasal layers and was absent at the basal layer. In OLK, the percentage of DKK3-positive cytoplasmic expression in mild dysplasia was less compared with severe dysplasia. However, this was not statistically significant (P=0.323). Further, the staining was localized in the cytoplasm of the basal layer in both mild and severe dysplasia. Our finding was in line with the discovery23 that DKK3 expression gradually increases from NOM to OLK and from OLK to OSCC. Additionally, DKK3 was only expressed in the cytoplasm in OSF cases. This was in agreement with Fujii et al.23 findings regarding the location of the expression either in the cell membrane of the NOM or in the cytoplasm of the OLK and OSF. The precise mechanism by which DKK3 expression shifts from cell membrane to the cytoplasm is not fully clear. However, this does not appear to be through inhibition of the canonical Wnt/β-catenin signaling pathway.23 The most striking finding in the present study was the loss of DKK3 expression in 50% of OSF cases. In contrast to OLK cases, the percentage of positive cytoplasmic expression was higher in cases of OSF without dysplasia than in cases of OSF with dysplasia (P=0.0001).
DKK3 downregulation by CpG methylation has been reported in various cancer types.17,24-26 It is suggested that DKK3 is involved in SCC carcinogenesis in the head, neck, and oral regions.23,27,28 However, its specific function has yet to be investigated. Pannone et al.27 reported that DKK3 is epigenetically inactivated in oral cancer. Katase et al.28 showed that by performing a genome-wide loss of heterozygosity (LOH) analysis. An allele at the DKK3 locus is frequently deleted in head and neck SCC suggesting that DKK3 functions as a TSG. DKK3 knockdown in OSCC-derived cells resulted in reduced cell migration and invasion and may have an oncogenic function that is independent of Wnt signaling.29 An intriguing question was raised: Why was DKK3 expressed in all OLK cases, but loss of protein expression was reported in 50% of OSF cases? To answer this question, we should first understand the cause of OSF development, which is still not clear. Many studies show a close association between chewing areca nuts and OSF development.30 Therefore, it is tempting to hypothesize that there may be underlying factors responsible for loss of DKK3 expression in OSF with dysplasia. The possible explanation for aberrant DKK3 expression may be the chemicals in areca nuts: arecoline, arecaidine, guvacine, and guvacoline. These compounds have been proposed to be highly cytotoxic and genotoxic to cultured human buccal epithelial cells.31 Teh et al.32 demonstrated that genomic instability is due to LOH in several chromosomal loci containing known oncogenes and TSGs associated with head and neck carcinogenesis in OSF tissues. Whether this reflects that DKK3 is instable or hypermethylation occurs in the promoter remains to be confirmed by molecular biology techniques. Surprisingly, a gradual loss/reduced expression of DKK3 in OSF cases, from overexpression in the early stage to partial loss in the moderately advanced stage, and then to severe loss in the advanced stage was observed. In other words, DKK3 expression progressively decreased with increased severity of epithelial atrophy from the early to advanced stages of OSF. However, the results were not significant (P=0.547). In their investigation in colorectal cancer, Zitt et al. reported that DKK3 positive samples showed a higher mean microvessel count than did DKK3 negative samples. Suggesting that DKK3 can be considered a putative pro-angiogenic protein in colorectal cancer.33 Since OSF occurs because of the stromal changes, which undergo progressive hyalinization, decrease in vascularity and cellularity.34 We hypothesized that DKK3 expression in OSF samples may decrease with the decrease in vascularity of the stromal layer. Recently, DKK3 is the clinical target of gene therapy in prostate,19 gastric scirrhous35 and renal fibrosis.36 Federico et al. demonstrated that DKK3 might serve as a diagnostic marker to identify the degree of atrophy and fibrosis in human patients with different types of chronic kidney diseases.36 Based on the Federico et al. findings as well as our study findings, DKK3 may serve as a diagnostic marker to identify different stages of OSF based on the severity of epithelial atrophy. Further, it may also help to assess the OSF carcinogenesis based on the dysplastic status of OSF, as most predictions about the onset of malignancy are currently based on the severity of dysplasia.37,38
We note several limitations to this study. The number of patients was relatively small; only single antibody was investigated. Therefore, further well-designed study with large sample size and multiple markers are needed.
Acknowledgments
The authors would like to thank Prof. Li Jiang, Dr. Xia Ronghui, and Dr. Soher Jayash for technical help.
References
- 1.Peterson PE. Strengthening the prevention of oral cancer: the WHO perspective. Community Dent Oral Epidemiol 2005;33:397-99. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 2007;36:575-80. [DOI] [PubMed] [Google Scholar]
- 3.Axéll T, Pindborg JJ, Smith CJ, Waal IV. Oral white lesions with special reference to precancerous and tobacco-related lesions: conclusions of an international symposium held in Uppsala, Sweden, May 18-21 1994. International Collaborative Group on Oral White Lesions. J Oral Pathol Med 1996;25:49-54. [DOI] [PubMed] [Google Scholar]
- 4.Pindborg JJ, Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Mehta FS. Oral submucous fibrosis as a precancerous condition. Scand J Dent Res 1984;92:2249. [DOI] [PubMed] [Google Scholar]
- 5.Murti PR, Bhonsle RB, Pindborg JJ, Daftary DK, Gupta PC, Mehta FS. Malignant transformation rate in oral submucous fibrosis over a 17-year period. Community Dent Oral Epidemiol 1985;13:340-1. [DOI] [PubMed] [Google Scholar]
- 6.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol 2009;129:1614-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006;25:7469-81. [DOI] [PubMed] [Google Scholar]
- 8.Tsuji T, Nozaki I, Miyazaki M, Sakaguchi M, Pu H, Hamazaki Y, et al. Antiproliferative activity of REIC/Dkk-3 and its significant down-regulation in non-small-cell lung carcinomas. Biochem Biophys Res Commun 2001;289:257-63. [DOI] [PubMed] [Google Scholar]
- 9.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature 2002;417:664-7. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura RE, Hackam AS. Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth Factors 2010;28:232-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee EJ, Jo M, Rho SB, Park K, Yoo YN, Park J, et al. Dkk3, downregulated in cervical cancer, functions as a negative regulator of β-catenin. Int J Cancer 2009;124:287-97. [DOI] [PubMed] [Google Scholar]
- 12.Hoang BH, Kubo T, Healey JH, Yang R, Nathan SS, Kolb EA, et al. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-β-catenin pathway. Cancer Re. 2004;64:2734-9. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh SY, Hsieh PS, Chiu CT, Chen WY. Dickkopf-3/REIC functions as a suppressor gene of tumor growth. Oncogene 2004;23:9183-9. [DOI] [PubMed] [Google Scholar]
- 14.Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y, Namba M. A REIC gene shows down-regulation in human immortalized cells and human tumor-derived cell lines. Biochem Biophys Res Commun 2000;268:20-4. [DOI] [PubMed] [Google Scholar]
- 15.Nozaki I, Tsuji T, Iijima O, Ohmura Y, Andou A, Miyazaki M, et al. Reduced expression of REIC/Dkk-3 gene in non-small cell lung cancer. Int J Oncol 2001;19:117-21. [DOI] [PubMed] [Google Scholar]
- 16.Kurose K, Sakaguchi M, Nasu Y, Ebara S, Kaku H, Kariyama R, et al. Decreased expression of REIC/Dkk-3 in human renal clear cell carcinoma. J Urol 2004;171:1314-8. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Ouchida M, Tsuji T, Hanafusa H, Miyazaki M, Namba M, et al. Reduced expression of the REIC/Dkk-3 gene by promoter-hypermethylation in human tumor cells. Gene 2002;282:151-8. [DOI] [PubMed] [Google Scholar]
- 18.Lodygin D, Epanchintsev A, Menssen A, Diebold J, Hermeking H. Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res 2005;65:4218-27. [DOI] [PubMed] [Google Scholar]
- 19.Abarzua F, Sakaguchi M, Takaishi M, Nasu Y, Kurose K, Ebara S, et al. Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Res 2005;65:9617-22. [DOI] [PubMed] [Google Scholar]
- 20.Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. IARC Press, Lyon: 2005. [Google Scholar]
- 21.Pindborg JJ, Sirsat SM. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol 1966;22:764-79. [DOI] [PubMed] [Google Scholar]
- 22.Carmona R, Macías D, Guadix JA, Portillo V, Pérez-Pomares JM, Muñoz-Chápuli R. A simple technique of image analysis for specific nuclear immunolocalization of proteins. J Microsc 2007;225:96-9. [DOI] [PubMed] [Google Scholar]
- 23.Fujii M, Katase N, Lefeuvre M, Gunduz M, Buery RR, Tamamura R, et al. Dickkopf (Dkk)-3 and β-catenin expressions increased in the transition from normal oral mucosal to oral squamous cell carcinoma. J Mol Histol 2011;42:499-504. [DOI] [PubMed] [Google Scholar]
- 24.Queimado L, Obeso D, Hatfield MD, Yang Y, Thompson DM, Reis AM. Dysregulation of Wnt pathway components in human salivary gland tumors. Arch Otolaryngol Head Neck Surg 2008;134:94-101. [DOI] [PubMed] [Google Scholar]
- 25.Urakami S, Shiina H, Enokida H, Kawakami T, Kawamoto K, Hirata H, et al. Combination analysis of hypermethylated Wnt-antagonist family genes as a novel epigenetic biomarker panel for bladder cancer detection. Clin Cancer Res 2006;12:2109-16. [DOI] [PubMed] [Google Scholar]
- 26.Maehata T, Taniguchi H, Yamamoto H, Nosho K, Adachi Y, Miyamoto N, et al. Transcriptional silencing of Dickkopf gene family by CpG island hypermethylation in human gastrointestinal cancer. World J Gastroenterol 2008;14:2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pannone G, Bufo P, Santoro A, Franco R, Aquino G, Longo F, et al. WNT pathway in oral cancer: epigenetic inactivation of WNT-inhibitors. Oncol Rep 2010;24:1035-41. [DOI] [PubMed] [Google Scholar]
- 28.Katase N, Gunduz M, Beder L, Gunduz E, Lefeuvre M, Hatipoglu OF, et al. Deletion at Dickkopf (dkk)-3 locus (11p15. 2) is related with lower lymph node metastasis and better prognosis in head and neck squamous cell carcinomas. Oncol Res 2008;17:273-82. [DOI] [PubMed] [Google Scholar]
- 29.Katase N, Lefeuvre M, Tsujigiwa H, Fujii M, Ito S, Tamamura R, et al. Knockdown of Dkk-3 decreases cancer cell migration and invasion independently of the Wnt pathways in oral squamous cell carcinoma derived cells. Oncol Rep 2013;29:1349-55 [DOI] [PubMed] [Google Scholar]
- 30.Gupta PC, Sinor PN, Bhonsle RB, Pawar VS, Mehta HC. Oral submucous fibrosis in India: a new epidemic. Natl Med J 1998;1:113-4. [PubMed] [Google Scholar]
- 31.Sundqvist K, Liu Y, Nair J, Bartsch H, Arvidson K, Grafström RC. Cytotoxic and genotoxic effects of areca nut-related compounds in cultured human buccal epithelial cells. Cancer Res 1989;49:5294-8. [PubMed] [Google Scholar]
- 32.Teh MT, Tilakaratne WM, Chaplin T, Young BD, Ariyawardana A, Pitiyage G, et al. Fingerprinting genomic instability in oral submucous fibrosis. J Oral Pathol Med 2008;37:430-6. [DOI] [PubMed] [Google Scholar]
- 33.Zitt M, Untergasser G, Amberger A, Moser P, Stadlmann S, Zitt M, et al. Dickkopf-3 as a new potential marker for neoangiogenesis in colorectal cancer: expression in cancer tissue and adjacent non-cancerous tissue. Dis Markers 2008;24:101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajalalitha P, Vali S. Molecular pathogenesis of oral submucous fibrosis - a collagen metabolic disorder. J Oral Pathol Med 2005;34:321-8. [DOI] [PubMed] [Google Scholar]
- 35.Than SS, Kataoka K, Sakaguchi M, Murata H, Abarzua F, Taketa C, et al. Intraperitoneal administration of an adenovirus vector carrying REIC/Dkk-3 suppresses peritoneal dissemination of scirrhous gastric carcinoma. Oncol Rep 2011;25:989-95. [DOI] [PubMed] [Google Scholar]
- 36.Federico G, Meister M, Mathow D, Heine GH, Moldenhauer G, Popovic ZV, et al. Tubular Dickkopf-3 promotes the development of renal atrophy and fibrosis. JCI Insight 2016;1:e84916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowan CG, Gregg TA, Napier SS, McKenna SM, Kee F. Potentially malignant oral lesions in Northern Ireland: a 20-year population-based perspective of malignant transformation. Oral Dis 2001;7:18-24. [PubMed] [Google Scholar]
- 38.Reibel J. Prognosis of oral pre-malignant lesions: significance of clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med 2003;14:47. [DOI] [PubMed] [Google Scholar]


