Abstract
Treadmills provide a safe and efficient method for gait rehabilitation but treadmill based training paradigms have not been shown to create superior results when compared with traditional physical therapy methods such as overground training. One explanation for this may be that walking at a constant, fixed speed requires little mental engagement from the user, which has been postulated as a key factor in the success of motor learning. To increase mental engagement, we developed a user-driven treadmill control scheme. In this paper we use electroencephalography (EEG) to compare cortical activity during user-driven (active) walking with activity on a normal (passive) treadmill in nine healthy subjects. We used independent component analysis (ICA) to isolate brain activity from artifactual components. We fit equivalent dipole sources to each brain component and clustered these across subjects. Our analysis revealed that relative to the passive treadmill, active walking resulted in statistically significant decreases in spectral power, i.e. desynchronization, in the anterior cingulate, sensorimotor cortices, and posterior parietal lobe of the cortex. These results indicate that user-driven treadmills more fully engage the motor cortex and therefore could facilitate better training outcomes than a traditional treadmill.
I. Introduction
Treadmill based training is a common method of rehabilitation for individuals with a locomotor disorder. Body weight supported treadmill training is often employed because it provides a safe, convenient, and efficient method for improving lower extremity coordination and control during walking [1]. But, evidence from randomized controlled trials indicates this strategy is no more effective than traditional therapies for gait training [2]. One reason for this may be that walking on a treadmill at an imposed speed is monotonous and requires limited mental engagement of the user. Studies utilizing functional magnetic resonance imaging and transcranial magnetic stimulation have suggested that individual motivation and active user participation are crucial elements for neural plasticity and effective motor learning [3]. Thus, two important challenges are to design a novel locomotion rehabilitation paradigm that commands the user’s attention as well as a valid, reliable method to quantify engagement across locomotor tasks.
Advances in mobile functional brain imaging have made the latter task more approachable. Electroencephalography (EEG) is particularly well suited to monitor changes in cortical activity during locomotion due to its unencumbering wireless sensors, relatively dense scalp coverage, and high temporal resolution. Furthermore, advances in signal processing techniques have resulted in algorithms capable of separating brain activity from physiological and movement artifacts [4–6] and algorithms that merge noninvasively acquired EEG data with magnetic resonance based head models to estimate equivalent dipole sources of cortical activity [7]. Previous research has demonstrated the viability of EEG for studying cortical activity during walking, including extraction of visual-evoked responses during gait [8], identification of intra-stride changes in EEG spectral power that are coupled to the gait cycle [9], and extraction of kinematic information during walking from delta band EEG [10]. Collectively, these studies and others have shown that supraspinal circuits, especially those of the motor cortex, have a significant role in motor control during walking.
We have previously published a method for simulating overground locomotion on a treadmill through the use of a feedback controller that allows the user to drive it at a self-selected speed [11]. In this paper we compare brain activity, measured via EEG, during walking on a normal treadmill with the user-driven control scheme. Event related desynchronization (ERD), measured as a decrease in spectral power in the alpha (8–13 Hz) and beta (13–30 Hz) frequency bands, is a well-established movement related phenomenon [12]. Because ERD indicates a departure from baseline oscillatory activity, it is considered an electrophysiological correlate of increased cortical activation for processing of sensory information and/or production of movement [12]. A recent study showed enhanced sensorimotor desynchronization in able-bodied individuals when they walked actively in a robot-assisted treadmill trainer compared to when they passively allowed the robot to move their legs [13]. However, to our knowledge, no one has examined the differences in brain activity between standard treadmill walking and user-driven treadmill walking. We hypothesize that the user-driven scheme will induce desynchronization in the motor cortex, indicating increased engagement of the participant in the walking task. This active participation could facilitate neural plasticity and expedite the process of motor learning.
II. Methods
A. Experimental Protocol and Data Collection
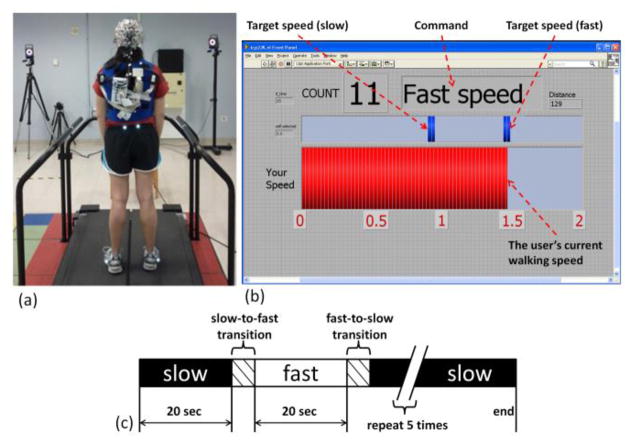
Nine healthy adults (5 female, 4 male; age: 29 ± 6 years; height 165 ± 9 cm; weight 66 ± 13 kg) with no history of neurological disease participated in this study. The experimental protocol was approved by the institutional review board of the National Institutes of Health. All subjects gave written consent prior to study participation. Prior to the walking tasks, each subject was fitted with a 64-channel active electrode, wireless EEG system (actiCHamp, Brain Products, Morrisville, NC) with sensors placed according to the 10–20 international system (Fig 1a). Reflective markers were placed on the pelvis and feet to monitor kinematics using a Vicon MX motion capture system (Vicon, Denver, CO). EEG data were collected at 500 Hz while kinematic data were collected at 120 Hz.
Fig. 1.
(a) Experimental setup including wireless EEG and treadmill. (b) Screen shown to the user during walking indicating command (slow or fast), target, and current speeds. (c) Block design of each walking trial consisting of five periods of slow and fast walking speed with transitions.
Prior to walking on the treadmill, the participant’s overground self-selected walking speed was assessed using the motion capture system. Then, slow (30% slower than self-selected) and fast (30% faster than self-selected) walking speeds were computed for each subject. Each participant completed three trials of treadmill walking in two different modes: passive walking and active walking. The order of the mode for each trial was randomized. Passive walking mode utilized typical treadmill behavior: belt speed was automatically adjusted to the target speed within each block (slow or fast) so that the walking speed was dictated by the treadmill. In the active mode, a feedback controller was implemented to allow the user to drive the speed of the treadmill [11]. Pelvis position and swing foot velocity were measured in real time by the motion capture system. These quantities were used to control the speed and acceleration of the treadmill belts so the subject’s pelvis remained in the center of the treadmill regardless of walking speed as described in [11]. In this way, the user controls the speed of the treadmill, and, in combination with the visual feedback from the screen they are able to track a target walking speed (Fig 1b). During both passive and active walking trials, the participant was instructed to minimize the difference between their walking speed and the target (slow or fast).
A treadmill walking trial consisted of ten 20 sec blocks of walking alternating between slow and fast speed (Fig 1c). Within each block of the trial, the participant was instructed to match their walking speed to the command speed (slow or fast). A screen in front of the participant displayed the target speed and the user’s current walking speed (Fig 1b). Prior to the data collection, each participant underwent 5 minutes of practice for both the passive and active walking modes.
B. EEG Signal Processing
All data analysis was performed off-line using custom software in Matlab (Mathworks, Natick, MA). Gait events (heel strike and toe-off) were determined from the foot marker and treadmill force plate data. EEG signals were high pass filtered at 1 Hz (5th order Butterworth) and time-locked to the kinematics. Noisy EEG channels, indicated by a standard deviation > 1000 μV or a kurtosis more than 5 standard deviations from the mean were removed, and the EEG was re-referenced to a common average. Next, we applied an artifact subspace reconstruction (ASR) algorithm, available as a plug-in for EEGLAB software [14], to remove high amplitude artifact from EEG collected during walking. ASR uses principal component analysis and a baseline window, collected as 1 minute of EEG during standing, to remove stereotypical (e.g. eye blinks) and non-stereotypical (e.g. head motion) artifacts from EEG recorded during walking [6]. We then ran independent component analysis (ICA) on the cleaned EEG data for each subject using an adaptive mixture algorithm [5]. We then used the dipole fitting toolbox within EEGLAB [8] to compute a single equivalent dipole for each independent component (IC) by warping the EEG electrode montage to the Montreal Neurological Institute (MNI) standard brain model. ICs whose dipole scalp projection contained greater than 20% residual variance were removed from further analysis. In a similar manner as [9], we categorized the remaining dipoles for each subject as brain or non-brain based on the dipole locations (Talairach coordinates), power spectra, and time traces of voltage. On average, we identified 7 (range: 4–10) brain dipoles for each subject. These ICs were then clustered across subjects using feature vectors formulated with dimensions for power spectral density (<100 Hz) and dipole location. Using EEGLAB functions, feature vectors were reduced to 10 principal components before being clustered across subjects using k-means (k = 7). ICs greater than 3 standard deviations from a cluster centroid were relegated to an outlier cluster and subsequently omitted from analysis.
Each time point of the IC data was marked as one of four walking commands: slow, fast, slow-to-fast transition, and fast-to-slow transition and one of two walking types: passive or active. The transitions included the six gait cycles – three before and three after – surrounding the change in command speed, a number that assured all subjects had reached steady state based on post-hoc analysis of gait speed. The slow and fast conditions included only the steady state walking at that command speed. In all subsequent analysis, only the steady state, slow command speed walking trials were analyzed. For each IC and walking mode, we computed the power spectral density (PSD) using a multitaper fast Fourier transform with discrete prolate sequences [15]. We computed the cluster grand mean PSD by averaging across all ICs within each. The non-parametric Kruskal-Wallis test was used to assess significant differences in PSD for specific frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha/mu (8–13 Hz), beta (13–30 Hz), and gamma (30–100 Hz). A value of p < 0.05 represented significance.
III. Results
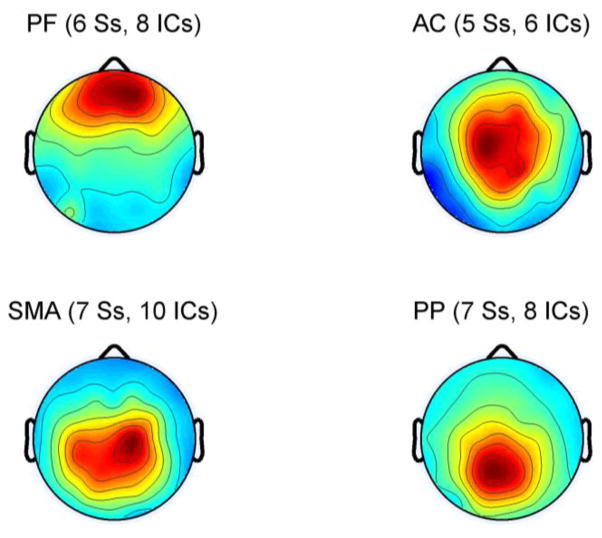
The k-means clustering resulted in seven spatially distinct groups of electrocortical dipole sources, plus one additional outlier cluster. We retained only those clusters containing dipoles from a majority of subjects for analysis. Because we did not examine within stride or unilateral measures in the present analysis, we combined the left and right sensorimotor areas into a single cluster. This resulted in clusters representing four areas of the cortex: prefrontal (PF), anterior cingulate (AC), sensorimotor area (SMA), and posterior parietal lobe (PP) (Fig 2). Using the coordinates of each dipole, we identified the Brodmann areas represented within each cluster from the Talairach atlas [16] (Table 1). Dipoles were located in a total of 9 Brodmann areas across the four clusters.
Fig. 2.
Average dipole scalp projections of the four cortical clusters: prefrontal cortex (PF), anterior cingulate cortex (AC), sensorimotor area (SMA), and posterior parietal lobe (PP). The number of subjects (S) and independent components (IC) represented in each cluster are indicated.
Table I.
Significant Differences in power spectral density by cluster and frequency band
| Cluster Name | Prefrontal (PF) | Anterior Cingulate (AC) | Sensorimotor Area (SMA) | Posterior Parietal (PP) | |
|---|---|---|---|---|---|
|
| |||||
| Brodmann Areas | 9, 10, 11 | 24, 32 | 4, 6 | 5, 7 | |
|
| |||||
| p-value (passive vs. active) | delta (1–4 Hz) | 0.1489 | 0.0243 | 0.2482 | 0.3865 |
| theta (4–8) | 0.7630 | 0.8802 | 0.4739 | 0.7919 | |
| alpha (8–13) | 0.7532 | 0.0200 | 0.0147 | 0.0000 | |
| beta (13–30) | 0.0002 | 0.0176 | 0.0218 | 0.6718 | |
| gamma (30–100) | 0.0000 | 0.0000 | 0.0001 | 0.0006 | |
Bold indicates a significant difference (p < 0.05)
All significant changes in PSD showed a desynchronization (less power) in active walking compared to passive. Furthermore, significant changes in PSD were present in all four clusters (Fig 3, Table 1). The only significant difference in delta band power was observed in the AC cluster. Theta band power was similar across both conditions for all brain regions. Desynchronization of the alpha band was present in active walking for the AC, SMA, and PP areas. Only the posterior parietal cluster did not show significance in the beta band, while significant differences in gamma band power were present in all four clusters.
Fig. 3.
Grand mean power spectral density of each cluster during passive (solid) and active (dashed) treadmill walking.
IV. Discussion
The design of a treadmill-based gait training paradigm which promotes user participation and an effective method for monitoring that participation remain active topics of research. In this study we examined brain activity during two walking conditions: typical treadmill walking (passive) and walking on a treadmill with the belt speed driven by the motion of the user (active). For a fair comparison, it is important that kinematics and kinetics of walking are similar across conditions. Our previous development of a real time controller for a user-driven treadmill resulted in similar gait patterns between conditions [11].
We used EEG to assess cortical activity of nine healthy adults while they were tracking a target speed during passive and active treadmill walking. To help control the effect of task novelty on cortical involvement, we set the command speed to be 30% less than self-selected gait speed for both passive and active conditions. We employed a two step process to clean our EEG signals. First, we applied artifact subspace reconstruction to eliminate high amplitude noise from motion artifact. We then applied adaptive mixture ICA to parse the 64-channel EEG into maximally independent components (ICs). ICs derived from scalp-recorded EEG represent the nearly synchronous activity of large groups of neurons within a relatively small cortical domain. The far-field projection of such a cortical patch of activity has been shown to be dipolar [17] and thus, we used inverse modeling combined with a boundary element model to identify a single best fitting dipole for each IC. The power spectral density of each dipole provides a measure of synchrony of this small area of the brain. To increase statistical power, we clustered ICs across subjects, resulting in groups characterizing four regions of the brain for a majority of subjects.
Our results demonstrate significant differences in cortical activity between typical and user-driven treadmill walking. It is well known that movement preparation and execution results in an event related desynchronization (ERD) of the mu rhythm contained in the alpha band (8–13 Hz) across the sensorimotor and posterior parietal cortex [12, 18]. We observed significantly more alpha band desynchronization in the AC, SMA and PP regions during active treadmill walking, indicating a further disruption of baseline oscillatory activity than is present in typical treadmill walking. This result is in agreement with a previous study that showed a decrease in mu rhythm of sensorimotor areas when individuals walked with a robotic gait trainer rather than allowing the robot to simply move their legs [13]. We also observed a power decrease in the lower beta band for the SMA cluster in agreement with prior work showing beta ERD during lower extremity movements [19].
Thus, our data may indicate that the user driven treadmill engages the motor pathways in the brain more than a typical treadmill. This finding is further supported by our observation of significant changes in gamma band activity across all four clusters, which may be important because previous work has suggested that the sensorimotor system may shift toward operating at higher (gamma) frequencies in situations requiring dynamic force output [20].
Importantly, we also observed significant changes in the PSD of the AC cluster. The dorsal portion of the anterior cingulate appears to play a prominent role in error detection [21]. In our study participants were asked to track a target speed in both the passive and active walking modes – but in the passive mode treadmill belt speed was set at the target speed, making the tracking task trivial. However, in the active mode, the treadmill reacted in real time to changes in the pelvis position and swing foot velocity of the participant, which made the tracking task more demanding. The decreased PSD at a wide range of frequency bands in the AC could be related to the increased challenge in the speed tracking task. Also, the only significant change in delta band activity was observed in the AC cluster. Previous work suggests that information pertaining to lower extremity angles and velocity may be contained in the delta band of EEG [10]. The difference in AC activity observed in the active walking may be indicative of closer tracking of limb velocity to match the target speed.
The current work examined changes in the spectral power of cortical clusters in able-bodied individuals. Future work will focus on more detailed examination of brain activity during passive and active walking, including within stride spectral perturbations, coherence, and statistical measures of connectivity and information flow with the ultimate goal of developing a treadmill based gait training paradigm to maximize cortical involvement and motor learning.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH Clinical Center (protocol number 08-CC-0205) and National Research Foundation Brain Korea 21 Plus Program.
Contributor Information
Thomas C. Bulea, Email: thomas.bulea@nih.gov, Functional and Applied Biomechanics Section at the National Institutes of Health, Bethesda, MD 20892.
Jonghyun Kim, Email: jhkim@dgist.ac.kr, Robotics Engineering Department, Daegu Gyeongbuk Institute of Science and Technology, Daegu, Republic of Korea.
Diane L. Damiano, Email: damianod@cc.nih.gov, Functional and Applied Biomechanics Section at the National Institutes of Health, Bethesda, MD 20892
Christopher J. Stanley, Email: stanleycj@cc.nih.gov, Functional and Applied Biomechanics Section at the National Institutes of Health, Bethesda, MD 20892
Hyung-Soon Park, Email: hyungspark@kaist.ac.kr, Korea Advanced Institute of Science and Technology, Daejeon, Republic of Korea.
References
- 1.Dobkin B, Barbeau H, et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized spinal cord injury locomotor trial. Neurorehabil Neural Repair. 2007;21(1):25–35. doi: 10.1177/1545968306295556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobkin BH, Duncan PW. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabil Neural Repair. 2012;26(4):308–317. doi: 10.1177/1545968312439687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lotze M, Braun C, et al. Motor learning elicited by voluntary drive. Brain. 2003;126(4):866–872. doi: 10.1093/brain/awg079. [DOI] [PubMed] [Google Scholar]
- 4.Delorme A, Sejnowski T, et al. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34(4):1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer JA, Makeig S, et al. Newton method for the ICA mixture model. Proc. 33rd IEEE Conf. Acoustics Sig. Proc; 2008. pp. 1805–1808. [Google Scholar]
- 6.Mullen T, Kothe C, et al. Real-time modeling and 3D visualization of source dynamics and connectivity using wearable EEG. Proc. 35th IEEE Conf. Eng. Med. Biol. Soc; 2013. pp. 2184–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oostenveld R, Oostendorp TF. Validating the boundary element method for forward and inverse EEG computations in the presence of a hole in the skull. Human Brain Mapping. 2002;17(3):179–192. doi: 10.1002/hbm.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gwin JT, Gramann K, et al. Removal of movement artifact from high-density EEG recorded during walking and running. J Neurophysiol. 2010;103(6):3526–3534. doi: 10.1152/jn.00105.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gwin JT, Gramann K, et al. Electrocortical activity is coupled to gait cycle phase during treadmill walking. Neuroimage. 2011;54(2):1289–1296. doi: 10.1016/j.neuroimage.2010.08.066. [DOI] [PubMed] [Google Scholar]
- 10.Presacco A, Forrester LW, Contreras-Vidal JL. Decoding intra-limb and inter-limb kinematics during treadmill walking from scalp electroencephalographic (EEG) signals. IEEE Trans Neur Sys Rehab Eng. 2012;20(2):212–219. doi: 10.1109/TNSRE.2012.2188304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Stanley CJ, et al. A user-driven treadmill control scheme for simulating overground locomotion. Proc. IEEE Conf. Eng. Med. Biol. Soc; 2012. pp. 3061–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110(11):1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 13.Wagner J, Solis-Escalante T, et al. Level of participation in robotic-assisted treadmill modulates midline sensorimotor EEG rhythms in able-bodied subjects. Neuroimage. 2012;63(3):1203–1211. doi: 10.1016/j.neuroimage.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Meth. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Thomson DJ. Spectrum estimation and harmonic analysis. Proc IEEE. 1982;70(9):1055–1095. [Google Scholar]
- 16.Lancaster JL, Woldorff MG, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delorme A, Palmer J, et al. Independent EEG sources are dipolar. PLOS One. 2012;7(2):e30135. doi: 10.1371/journal.pone.0030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babiloni C, Carducci F, et al. Human movement-related potentials vs desynchronization of EEG alpha rhythm: a high-resolution EEG study. Neuroimage. 1999;10(6):658–665. doi: 10.1006/nimg.1999.0504. [DOI] [PubMed] [Google Scholar]
- 19.Muller-Putz GR, Zimmermann D, et al. Event-related beta EEG-changes during passive and attempted foot movements in paraplegic patients. Brain Research. 2007;1137:84–91. doi: 10.1016/j.brainres.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 20.Omlor W, Patino L, et al. Gamma-range corticomuscular coherence during dynamic force output. Neuroimage. 2007;34(3):1191–1198. doi: 10.1016/j.neuroimage.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Critchley HD, Tang J, et al. Anterior cingulate activity during error and autonomic response. Neuroimage. 2005;27(4):885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]