Abstract
Gamma-glutamyltransferase (γ-GGT) is a membrane-bound enzyme that is involved in biotransformation, nucleic acid metabolism, and tumourigenesis. Elevated serum γ-GGT levels are related to an increased cancer risk and worse prognosis in many cancers. In the present study, we evaluated the prognostic value of preoperative serum γ-GGT in patients with hepatocellular carcinoma (HCC) who underwent liver transplantation (LT). A total of 130 HCC patients after LT were included in the study. The optimal cut-off value of γ-GGT was 128U/L by receiver operating characteristic analysis, with a sensitivity and specificity of 60.0% and 72.9%, respectively. Elevated preoperative serum γ-GGT was significantly associated with high alpha-fetoprotein (AFP), large tumor size, and macro- and micro-vascular invasion. The 1-, 3-, 5-year disease-free survival (DFS) and overall survival (OS) rates of HCC patients in the γ-GGT > 128U/L group were poorer than those in the γ-GGT ≤ 128U/L group. Stratification analysis revealed that γ-GGT exhibited a greater predictive value for DFS and OS in HCC patients beyond the Milan criteria and no macro-vascular invasion. In conclusion, elevated preoperative serum γ-GGT was significantly associated with advanced tumor stage and aggressive tumor behaviors, and serum γ-GGT can be considered as a prognostic factor for HCC patients after LT, especially for patients beyond the Milan criteria or without macro-vascular invasion.
Hepatocellular carcinoma (HCC) is the fifth-most common cancer and the second leading cause of cancer-related death worldwide, with an estimated global incidence of 782,000 new cases and nearly 746,000 deaths in 20121. Liver transplantation (LT) has been considered as an optimal radical therapy for selected patients with HCC, especially since the introduction of the Milan criteria in 19962. The Milan criteria are recognized as the standard selection criteria of transplant candidates for HCC patients. The most significant factor affecting long-term outcomes is the high postoperative recurrence rate. Despite the excellent outcomes for HCC patients fulfilling the Milan criteria, nearly 30% of patients experience tumor recurrence3. Some clinico- pathological factors, including tumor size, macro-vascular invasion, and poor differentiation as well as some molecular biomarkers, such as alpha-fetoprotein (AFP) and glypican-3 (GPC3), have been identified as prognostic predictors of HCC3,4,5,6. However, the identification of additional prognostic factors to identify patients with HCC who are at high risk of recurrence and HCC-related death is critical to improve the prognosis of these patients through novel treatments and clinical decision-making surveillance schedules.
Gamma-glutamyltransferase (γ-GGT) is a membrane-bound enzyme involved in the glutathione (GSH) metabolism7. Serum γ-GGT is a well-known marker of hepatic injury; elevated levels can result from alcohol consumption, acute and chronic liver disease and oxidative stress8,9,10. Similarly, elevated γ-GGT expression had been observed in various tumors11,12. Moreover, a study has suggested that γ-GGT plays an important role in carcinogenesis by exerting pro-oxidant effects at the membrane surface level and in the extracellular microenvironment and by promoting the release of free iron from transferrin13. GGT is involved in the prevention of apoptosis and the maintenance of proliferation through the production of hydrogen peroxide in U937 lymphoma cells14. GGT is essential to overcoming the toxicity of the drug by providing additional cysteine and can promote the drug resistance of cancer cells15. Therefore, GGT plays an important role with respect to the risk of cancer development, tumor progression, invasion and anticancer drug resistance15,16,17. Recently, serum γ-GGT has attracted attention as a prognostic marker for several human malignancies, including renal cell carcinoma, ovarian cancer and esophageal squamous cell carcinoma18,19,20. With respect to HCC, elevated serum γ-GGT has been reported to be a biomarker of poor prognosis after hepatectomy, transcatheter arterial chemoembolization (TACE) and radiofrequency ablation (RFA)21,22,23,24. However, the prognostic role of γ-GGT in HCC patients undergoing liver transplantation has not been reported thus far. We hypothesized that γ-GGT plays an important role in HCC progression and metastasis, and that it may represent a prognostic factor for HCC patients after LT. To test this hypothesis, the association of preoperative serum γ-GGT with survival was investigated in 130 HCC patients who underwent LT.
Materials and Methods
Patients
We included 130 patients with HCC who underwent liver transplantation at the Organ Transplant Center of The First Affiliated Hospital of Sun Yat-sen University between January 2008 and May 2013. The diagnosis of HCC was made based on postoperative pathological examination. Prior to LT, a complete physical examination, blood test, electrocardiograph (ECG), abdominal ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI), and chest X-ray or CT scan were performed. All patients have complete clinical, pathological, laboratory and follow-up data. All patients gave written informed consent to be in the study, and approval for the study was obtained from the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. The methods were carried out in accordance with the approved guidelines.
Measurement of serum γ-GGT levels
As part of routine clinical testing, blood samples for the evaluation of serum γ-GGT levels were collected from peripheral venous blood on the day before surgery. Serum γ-GGT concentrations were analyzed with a commercially available enzyme-coupled assay kit (Beijing Homa Biological Co., Ltd., Beijing, China) as previously described21. The normal value range of γ-GGT was between 2 and 50 U/L in our hospital.
Clinical management and follow-up
All patients were received in a classic orthotropic or modified piggyback fashion using well-described standard techniques25. An immunosuppressive regimen with anti-IL-2 receptor antibody (basiliximab) induction and tacrolimus and mycophenolate mofetil (MMF) maintenance was administered in these patients. The initial dose of tacrolimus was 0.04 mg/kg/d and the target trough tacrolimus level was 8–10 ng/ml within the first 3 months and 6–8 ng/ml thereafter. MMF was administered at 500–750 mg twice a day. A prophylactic regimen of lamivudine/entecavir and low-dose hepatitis B immunoglobulin was used to prevent hepatitis B virus (HBV) recurrence for patients with pre-transplant HBV infection. Routine tests of the concentrations of tacrolimus and MMF were performed; and immunosuppressive drugs were adjusted based on the drug concentration, liver function, serum biochemistry and blood routine during the procedure at each follow-up. Serum AFP and liver ultrasound were performed at each follow-up. Abdominal CT scan and chest X-ray was performed every 3–6 months or when recurrence was suspected. Recurrence was defined as emergence of clinical, radiological, and/or pathological diagnosis (tissues obtained by ultrasound-guided fine-needle aspiration). Once the recurrence was confirmed, patients were further treated by RFA, TACE, liver resection and/or sorafenib according to the size, number, and location of recurrent tumors as well as liver function.
Statistical analysis
The statistical analysis was conducted with Statistical Package for Social Science (SPSS) version 19.0 software (Chicago, IL, USA). All continuous variables are expressed as the mean ± standard deviation (SD). The difference between two groups were analyzed using the unpaired Student’s t or Mann-Whitney U test for continuous variables, and categorical variables were compared through the chi-square or Fisher’s exact test. A receiver operating characteristic curve (ROC) analysis was used to select the optimal cut-off value of preoperative serum γ-GGT. The survival analysis was performed by the Kaplan-Meier method and compared through the log-rank test. The Cox proportional hazard model was used to determine independent prognostic factors. The disease-free survival (DFS) time was calculated from the date of operation to the date of recurrence or the last follow-up date. The overall survival (OS) time was calculated from the date of operation to the date of death or the last follow-up date. The last follow-up date was October 31st, 2015. All statistical tests were 2-sided, and P < 0.05 was considered to be statistically significant.
Results
Clinical and pathological characteristics
A total of 130 patients were enrolled in this study, including 121 (93.1%, 121/130) male and 9 (6.9%, 9/130) female patients. The median age was 49.5 (ranging from 13 to 72) years. One-hundred and nineteen out of the 130 patients were infected by hepatitis B virus (HBV). On the basis of the Child-Pugh liver function class system, 84 patients were classified as Class A, 36 as Class B and 10 as Class C. Fifty-nine patients (45.4%, 59/130) received various therapies including hepatectomy, RFA, and TACE before transplantation. According to Edmondson-Steiner tumor differentiation stage, there were 86 (66.2%, 86/130) stage I–II and 44 (33.8%, 44/130) stage III–IV patients. Three category standards were used in this study: Milan criteria2, the University of California at San Francisco (UCSF) criteria26, and Hangzhou criteria27. For these patients, 46 (35.4%, 46/130) patients fulfilled the Milan criteria, whereas, 69 (53.1%, 69/130) and 81 (62.3%, 81/130) patients fulfilled the UCSF criteria and Hangzhou criteria, respectively.
Determination of the cut-off value for elevated γ-GGT by ROC curve
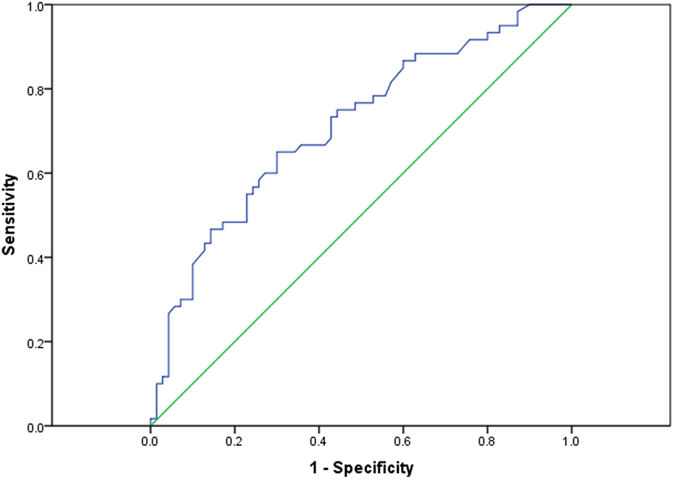
The ROC curve analysis indicated that the optimal cut-off value of serum γ-GGT was 128 U/L for predicting recurrence. The area under the ROC curve was 0.712, with a 95% confidential interval (CI) of 0.623–0.801 (Fig. 1). It presented a sensitivity of 60.0% and a specificity of 72.9% and was related to the highest Youden Index (sensitivity+specificity-1). Subsequently, all patients were classified into either the γ-GGT ≤ 128 U/L (n = 77) group or the γ-GGT > 128U/L group (n = 53).
Figure 1. Receiver operating characteristic curves for the determination of the cut-off value for preoperative serum γ-GGT in patients with HCC after liver transplantation.
Association of preoperative serum γ-GGT with clinicopathological features
The association between preoperative serum γ-GGT and clinicopathological features is summarized Table 1. Preoperative serum γ-GGT increased with AFP > 400 ng/ml (P = 0.002), larger tumor (P < 0.001), and macro- (P < 0.001) and micro-vascular invasion (P = 0.001). A higher proportion of patients with γ-GGT > 128U/L were beyond the Milan criteria (52.4%, 44/84), UCSF criteria (54.1%, 33/61) and Hangzhou criteria (63.3%, 31/49) than those with γ-GGT ≤ 128 U/L (all P < 0.001). However, there was no association of γ-GGT levels with gender (P = 0.241), age (P = 0.538), hepatitis B surface antigen (HBsAg) (P = 0.756), Child-Pugh stage (P = 0.097), preoperative tumor therapy (P = 0.985), tumor number (P = 0.249) or Edmondson grading (P = 0.248).
Table 1. Relationship between preoperative serum γ-GGT levels and clinicopathological characteristics.
| Category | Subcategory | Cases | γ-GGT (U/L) |
P value | |
|---|---|---|---|---|---|
| ≤128(n = 77) | >128(n = 53) | ||||
| Gender | Male | 121 | 70 | 51 | |
| Female | 9 | 7 | 2 | 0.241 | |
| Age (years) | ≤50 | 68 | 42 | 26 | |
| <50 | 62 | 35 | 27 | 0.538 | |
| HBsAg | Positive | 119 | 70 | 49 | |
| Negative | 11 | 7 | 4 | 0.756 | |
| Child-Pugh stage | A | 84 | 46 | 38 | |
| B | 36 | 22 | 14 | ||
| C | 10 | 9 | 1 | 0.097 | |
| Preoperative tumor therapy | Yes | 59 | 35 | 24 | |
| No | 71 | 42 | 29 | 0.985 | |
| AFP (ng/ml) | ≤400 | 82 | 57 | 25 | |
| >400 | 48 | 20 | 28 | 0.002 | |
| Size of largest tumor (cm) | ≤5 | 74 | 55 | 19 | |
| 5 to 8 | 22 | 10 | 12 | ||
| >8 | 34 | 12 | 22 | <0.001 | |
| Tumor number | ≤3 | 93 | 58 | 35 | |
| >3 | 37 | 19 | 18 | 0.249 | |
| Edmondson grading | I–II | 86 | 54 | 32 | |
| III–IV | 44 | 23 | 21 | 0.248 | |
| Macro-vascular invasion | Yes | 29 | 7 | 22 | |
| No | 101 | 70 | 31 | <0.001 | |
| Micro-vascular invasion | Yes | 20 | 5 | 15 | |
| No | 110 | 72 | 38 | 0.001 | |
| Milan criteria | Within | 46 | 37 | 9 | |
| Beyond | 84 | 40 | 44 | <0.001 | |
| UCSF criteria | Within | 69 | 49 | 20 | |
| Beyond | 61 | 28 | 33 | 0.004 | |
| Hangzhou criteria | Within | 81 | 59 | 22 | |
| Beyond | 49 | 18 | 31 | <0.001 | |
γ-GGT, γ-Gamma-glutamyltransferase; HBsAg, Hepatitis B surface antigen; AFP, Alpha fetoprotein.
DFS and OS according to γ-GGT levels
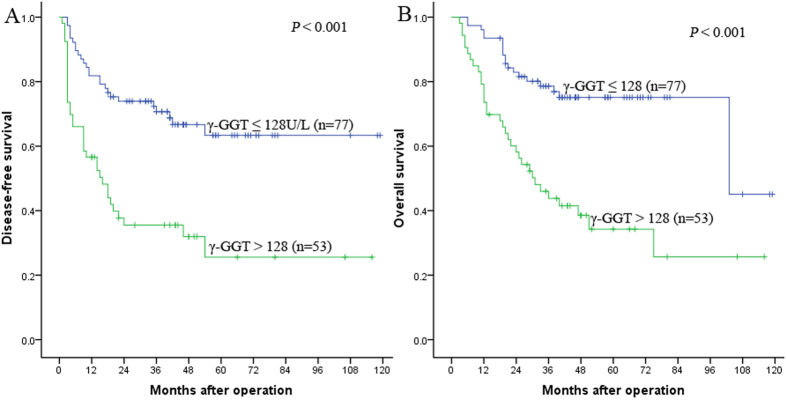
The influence of preoperative serum γ-GGT levels on prognosis was analyzed. The results showed that the 1-, 3-, and 5-year DFS rates were 81.8%, 70.7%, and 63.4% in the γ-GGT ≤ 128 U/L group, and 56.6%, 35.5%, and 25.6% in the γ-GGT > 128 U/L group, respectively (P < 0.001, Fig. 2A). Correspondingly, the 1-, 3-, and 5-year OS rates were 93.5%, 78.6%, and 75.1% in the γ-GGT ≤ 128 U/L group, and 73.6%, 43.8%, and 34.3% in the γ-GGT > 128 U/L group, respectively (P < 0.001, Fig. 2B). The preoperative serum γ-GGT > 128 U/L was a risk factor for HCC patients who underwent liver transplantation.
Figure 2.
Kaplan-Meier survival curves demonstrating that patients with γ-GGT ≤ 128 U/L exhibited shorter DFS (A) and OS (B) rates than those with γ-GGT > 128 U/L (all P < 0.001, log-rank).
Risk factors for HCC patients’ prognosis
After a median follow-up period of 40.3 (range 3–119) months, 60 (60/130) patients experienced recurrence and 53 (53/130) patients died. For all patients included in this study, the 1-, 3-, and 5-year DFS rates were 71.5%, 56.7%, and 48.7%, respectively. For the same period, the 1-, 3-, and 5-year OS rates were 85.4%, 64.3%, and 58.0%, respectively. A univariate analysis identified the Child-Pugh stage, tumor number, size of largest tumor, macro-vascular invasion, micro-vascular invasion, AFP and γ-GGT as significant risk factors associated with DFS. Furthermore, tumor number, size of largest tumor, macro-vascular invasion, micro-vascular invasion, AFP and γ-GGT were significant risk factors associated with OS (Table 2). Multivariate Cox proportional hazards regression model revealed that Child-Pugh stage, tumor number, size of largest tumor, AFP and γ-GGT were independent risk factors of DFS, and tumor number, size of largest tumor, and γ-GGT were independent risk factors of OS (Table 3).
Table 2. Influence of clinicopathological characteristics on patient prognosis.
| Variables | n | DFS |
OS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1-yr | 3-yr | 5-yr | P | 1-yr | 3-yr | 5-yr | P | ||
| Gender | |||||||||
| Male | 121 | 71.1% | 56.0% | 47.2% | 85.1% | 63.4% | 56.5% | ||
| Female | 9 | 77.8% | 66.7% | 66.7% | 0.382 | 88.9% | 77.8% | 77.8% | 0.539 |
| Age (years) | |||||||||
| ≤50 | 68 | 64.7% | 52.1% | 42.6% | 80.9% | 57.7% | 49.0% | ||
| >50 | 62 | 79.0% | 61.8% | 54.9% | 0.152 | 90.3% | 71.7% | 67.2% | 0.060 |
| HBsAg | |||||||||
| Positive | 119 | 72.3% | 57.9% | 49.1 | 85.7% | 65.6% | 58.5% | ||
| Negative | 11 | 63.6% | 42.4% | 42.4% | 0.402 | 81.8% | 63.6% | 54.5% | 0.780 |
| Child-Pugh stage | |||||||||
| A | 84 | 66.7% | 48.7% | 39.3% | 83.3% | 60.9% | 56.0% | ||
| B | 36 | 80.6% | 69.0% | 63.3% | 74.1% | 67.7% | 54.4% | ||
| C | 10 | 80.0% | 80.0% | 80.0% | 0.037 | 80.0% | 80.0% | 80.0% | 0.364 |
| Preoperative tumor therapy | |||||||||
| Yes | 59 | 72.9% | 53.1% | 50.7% | 86.4% | 66.3% | 52.6% | ||
| No | 71 | 70.4% | 59.8% | 52.0% | 0.580 | 84.5% | 62.8% | 60.9% | 0.960 |
| AFP (ng/ml) | |||||||||
| ≤400 | 82 | 84.1% | 72.5% | 60.7% | 92.7% | 77.5% | 70.8% | ||
| >400 | 48 | 75.0% | 29.6% | 26.3% | <0.001 | 72.9% | 41.7% | 36.4% | <0.001 |
| Size of largest tumor (cm) | |||||||||
| ≤5 | 74 | 86.5% | 75.3% | 71.2% | 95.9% | 84.2% | 78.6% | ||
| 5 to 8 | 22 | 68.2% | 38.4% | 25.6% | 77.3% | 54.2% | 36.9% | ||
| >8 | 34 | 41.2% | 26.7% | 16.7% | <0.001 | 67.6% | 29.4% | 29.4% | <0.001 |
| Tumor number | |||||||||
| ≤3 | 93 | 81.7% | 69.1% | 59.8% | 87.1% | 77.1% | 68.3% | ||
| >3 | 37 | 45.9% | 25.3% | 20.2% | <0.001 | 81.1% | 31.3% | 31.3% | <0.001 |
| Edmondson grading | |||||||||
| I–II | 86 | 73.3% | 55.1% | 46.4% | 87.2% | 65.9% | 55.7% | ||
| III–IV | 44 | 68.2% | 59.1% | 51.7% | 0.812 | 81.8% | 61.1% | 61.1% | 0.799 |
| Macro-vascular invasion | |||||||||
| Yes | 29 | 44.8% | 8.0% | 4.0% | 72.4% | 23.6% | 19.7% | ||
| No | 101 | 79.2% | 70.7% | 61.7% | <0.001 | 89.1% | 76.7% | 69.6% | <0.001 |
| Micro-vascular invasion | |||||||||
| Yes | 20 | 50.0% | 20.8% | 13.9% | 75.0% | 27.3% | 21.8% | ||
| No | 110 | 75.5% | 63.0% | 54.7% | <0.001 | 87.3% | 71.1% | 64.7% | <0.001 |
| γ-GGT (U/L) | |||||||||
| ≤128 | 77 | 81.8% | 70.7% | 63.4% | 93.5% | 78.6% | 75.1% | ||
| >128 | 53 | 56.6% | 35.5% | 32.0% | <0.001 | 73.6% | 43.8% | 34.3% | <0.001 |
| Milan criteria | |||||||||
| Within | 46 | 93.5% | 88.7% | 85.3% | 97.8% | 95.6% | 89.9% | ||
| Beyond | 84 | 59.5% | 38.5% | 28.5% | <0.001 | 78.6% | 47.3% | 40.8% | <0.001 |
| UCSF criteria | |||||||||
| Within | 69 | 91.3% | 80.4% | 78.1% | 94.2% | 89.5% | 81.9% | ||
| Beyond | 61 | 49.2% | 29.3% | 15.9% | <0.001 | 75.4% | 35.8% | 31.3% | <0.001 |
| Hangzhou criteria | |||||||||
| Within | 81 | 87.7% | 80.9% | 71.8% | 92.6% | 84.8% | 79.9% | ||
| Beyond | 49 | 44.9% | 16.3% | 10.2% | <0.001 | 73.5% | 31.4% | 24.2% | <0.001 |
DFS, disease-free survival; OS, overall survival; Other abbreviations as in Table 1.
Table 3. Prognostic factors for DFS and OS by multivariate Cox proportional hazards regression model.
| Variables | DFS |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Child-Pugh stage | 0.563 | 0.327–0.970 | 0.038 | |||
| Tumor number | 2.632 | 1.541–4.494 | <0.001 | 2.032 | 1.125–3.671 | 0.019 |
| Size of largest tumor | 1.461 | 1.058–2.016 | 0.021 | 1.906 | 1.373–2.647 | <0.001 |
| AFP | 1.965 | 1.093–3.533 | 0.024 | |||
| γ-GGT | 2.000 | 1.160–3.448 | 0.013 | 2.239 | 1.251–4.006 | 0.007 |
Prognostic value of preoperative serum γ-GGT in HCC patients without macro-vascular invasion
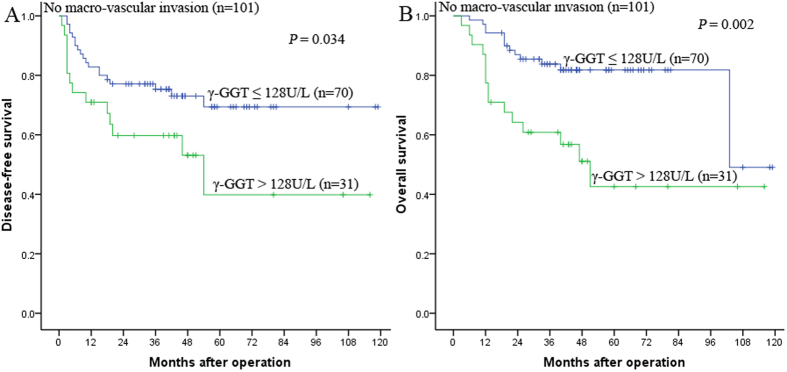
We further clarified the prognostic value of preoperative serum γ-GGT in HCC patients without macro-vascular invasion. Our results demonstrated that in the patients without macro-vascular invasion subgroup, the 1-, 3-, and 5-year DFS rates were 82.9%, 75.3%, and 69.4% for patients with γ-GGT ≤ 128 U/L and 71.0%, 59.8%, and 39.8% for patients with γ-GGT > 128 U/L, respectively (P = 0.034, Fig. 3A), and the 1-, 3-, and 5-year OS rates were 94.3%, 83.8%, and 81.8% for patients with γ-GGT ≤ 128 U/L and 77.4%, 60.8%, and 42.6% for patients with γ-GGT > 128 U/L, respectively (P = 0.002, Fig. 3B).
Figure 3.
Kaplan-Meier survival curves demonstrating that patients with γ-GGT > 128 U/L exhibited shorter DFS (A) and OS (B) rates than those with γ-GGT ≤ 128 U/L in the no macro-vascular invasion subgroup (A: P = 0.034; B: P = 0.002, log-rank).
Prognostic value of preoperative serum γ-GGT in HCC patients beyond the Milan criteria
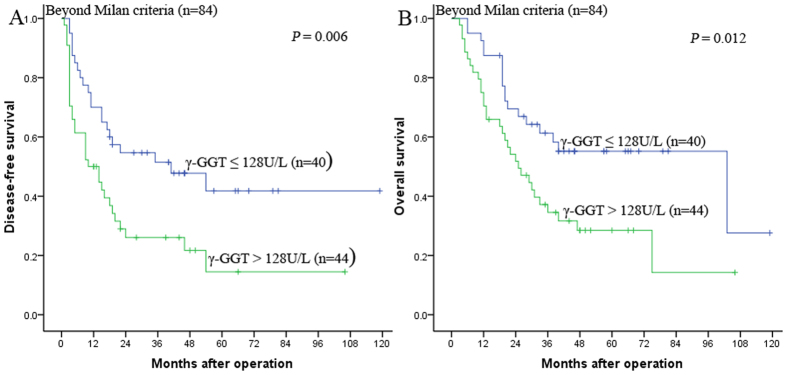
The Milan criteria are recognized as the golden candidate selection criteria for HCC patients who receive liver transplantation. However, the Milan criteria are too restrictive and insufficient for the increasing list of candidates, and many patients beyond the Milan criteria receive LT. We further clarified the prognostic value of preoperative serum γ-GGT in HCC patients beyond the Milan criteria. Our results demonstrated that in the subgroup of patients beyond the Milan criteria, the 1-, 3-, and 5-year DFS rates were 70.0%, 51.4%, and 41.8% for patients with γ-GGT ≤ 128 U/L and 50.0%, 26.1%, and 14.5% for patients with γ-GGT > 128 U/L, respectively (P = 0.006, Fig. 4A), and the 1-, 3-, 5-year OS rates were 87.5%, 61.3%, and 55.2% for patients with γ-GGT ≤ 128 U/L and 70.5%, 34.5%, and 28.5% for patients with γ-GGT > 128 U/L, respectively (P = 0.012, Fig. 4B).
Figure 4.
Kaplan-Meier survival curves demonstrating that patients with γ-GGT ≤ 128 U/L exhibited longer DFS (A) and OS (B) rates than those with γ-GGT > 128 U/L in the beyond the Milan criteria subgroup (A: P = 0.006; B: P = 0.012, log-rank).
Discussion
Liver transplantation is considered a curative treatment for HCC patients, especially those with underlying cirrhotic liver disease who are not eligible for hepatectomy. Unfortunately, HCC patients undergoing LT, which is designed to completely remove the tumor and the underlying liver disease, have a high recurrence rate due to the deposition of the recipient’s circulating tumor cells in the donor liver, lung, bone and subsequent HCC recurrence28. Hence, it is important to identify the risk factors for tumor recurrence. Several clinicopathological features including poor liver function, high serum AFP levels, large tumor size, multiple tumors, and macro and micro-vascular invasion have been previously reported to be indicators for poor prognosis in HCC patients6,29. The results of our study evidently demonstrate that elevated preoperative serum γ-GGT is a predictor of survival for HCC patients after LT.
γ-GGT is a crucial enzyme of glutathione (GSH) metabolism, and it is related to biotransformation, nucleic acid metabolism, and tumorigenesis22. γ-GGT has been widely used as a marker enzyme for several cancers, including ovarian tumors and renal cell carcinoma18,19. Recently, serum γ-GGT has been identified as a useful risk predictor in addition to traditional risk factors for cancer because it is a marker of oxidative stress30. Some studies initially focused on the possible association between serum γ-GGT and the incidence of various cancers31,32. More recently, elevated serum γ-GGT has been associated with a worse prognosis in many cancers, including renal cell carcinoma, esophageal squamous cell carcinoma and endometrial cancer20,33,34.
With respect to HCC, Zhang et al. firstly reported in 2011 that GGT levels were an important prognostic factor for patients with intermediate HCC treated with TACE in 2011, especially within the normal AFP subgroup22. Later, Guiu et al. also reported that GGT was an independent predictor of outcome after TACE for HCC in a European population23. Ma et al. reported that baseline serum GGT levels were a simple serum marker that may be used for prognosis in HCC treated by RFA24. We demonstrated that serum γ-GGT was a promising and reliable prognostic biomarker in HCC patients after hepatic resection, especially for patients with small HCC or AFP ≤ 200 ng/mL21. However, the prognostic value of serum γ-GGT in HCC patients treated by LT has not previously been reported. In the present study, we determined that the appropriate cut-off value of serum γ-GGT was 128 U/L for predicting recurrence. In the correlation analysis, we observed that preoperative serum γ-GGT > 128 U/L exhibited a strong connection with AFP > 400 ng/ml, larger tumor, and macro and micro-vascular invasion. The results predicted that elevated preoperative serum γ-GGT was significantly associated with advanced tumor stage and aggressive tumor behaviors in HCC. Our results indicated that the 1-, 3-, and 5-year DFS and OS rates in patients with γ-GGT ≤ 128 U/L group were higher than those with the γ-GGT > 128 U/L group, respectively. Preoperative serum γ-GGT was a risk factor for HCC patients who underwent liver transplantation. Furthermore, the multivariaret Cox proportional hazards model identified γ-GGT as an independent risk factor for HCC patients.
Macro-vascular invasion (portal or hepatic vein tumor thrombi) is a risk factor of poor prognosis in HCC patients21,29. However, some patients with no macro-vascular invasion suffer tumor recurrence shortly after LT. Therefore, it is important to identify simple and effective serum markers to predict the prognosis of HCC patients with no macro-vascular invasion. In the study, we determined that the prognosis of patients with γ-GGT > 128 U/L was worse than those with γ-GGT ≤ 128 U/L in the no macro-vascular invasion subgroup by stratification analysis. The result reveals that preoperative serum γ-GGT may predict the prognosis of HCC patients without macro-vascular invasion after LT, and suggests that HCC patients without macro-vascular invasion are more suitable for LT if they exhibited preoperative serum γ-GGT ≤ 128 U/L.
It is well known that the Milan criteria is the golden candidate selection criteria for HCC patients treated by LT2. However, it has been proven to be restrictive and far away from stratifying the growing candidate list. A large proportion of HCC patients beyond the Milan criteria have a potential curative chance and good outcome after LT. The primary cause of failure of the pretransplant diagnosis and staging by Milan criteria is the failure to account for tumor biology. In this study, stratification analysis of the Milan criteria revealed that the DFS and OS rates in the patients with γ-GGT ≤ 128 U/L were greater than those in the patients with γ-GGT > 128 U/L in the beyond the Milan criteria subgroup. The result suggests that preoperative serum γ-GGT levels may be used as a simple and effective marker for selecting HCC patients who are planning to undergo liver transplantation and are beyond the Milan criteria.
Several additional limitations of the study merit mention. Due to its retrospective nature and the relatively small size in a single center, we couldn’t divide the data into a training set and a test set for statistical validation. Further multicenter, large prospective studies are needed to confirm our findings. Although elevated preoperative serum γ-GGT predicted poor prognosis, we were not able to select liver transplantation candidates according to preoperative serum γ-GGT levels, which we hope to demonstrate in the future clinical trials.
In conclusion, our results demonstrate that elevated preoperative serum γ-GGT is significantly associated with advanced tumor stage and aggressive tumor behaviors, and that preoperative serum γ-GGT can be considered a prognostic factor for HCC patients after LT, especially for patients beyond the Milan criteria and with no macro-vascular invasion. Based on our findings, preoperative serum γ-GGT levels can be considered a simple and effective marker for the selection of HCC patients, who are candidates for liver transplantation, particular those beyond the Milan criteria.
Additional Information
How to cite this article: Fu, S.-J. et al. Elevated Preoperative Serum Gamma-glutamyltranspeptidase Predicts Poor Prognosis for Hepatocellular Carcinoma after Liver Transplantation. Sci. Rep. 6, 28835; doi: 10.1038/srep28835 (2016).
Acknowledgments
This study was supported by the National High Technology Research and Development Program of China (863 Program) (2012AA021007 & 2012AA021008), the Key Clinical Project from the Ministry of Health (2010159), the National Natural Science Foundation of China (81373156 and 81471583), the Special Fund for Science Research by Ministry of Health (201302009), the Guangdong Provincial Key Laboratory Construction Projection on Organ Donation and Transplant Immunology (2013A061401007), the Guangdong Provincial international Cooperation Base of Science and Technology (Organ Transplantation)(2015B050501002), Pearl River Nova Program of Guangzhou (201506010014), Guangdong Provincial Natural Science Funds for Distinguished Young Scholars (2015A030306025), and the China Postdoctoral Science Foundation (2015M582474). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Professor Jian Zhang from State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University for statistical help.
Footnotes
Author Contributions S.-J.F., Q.Z., Z.-Y.G. and X.-S.H. were the primary authors of the manuscript. These authors were involved in the conception, design and coordination of the study as well as in the data analysis, interpretation of the results and drafting the manuscript. X.-S.H. and Z.-Y.G. were in charge of all experimental procedures. F.J., M.-G.C., L.-W.W. and Q.-Q.R. participated in the experimental procedures and critically revised the content of the manuscript. All authors contributed to the interpretation of the data and critically revised the manuscript. All authors read and approved the final manuscript.
References
- World Health Organization. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/factsheetscancer.aspx (accessed May 3, 2014).
- Mazzaferro V. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334, 693–699 (1996). [DOI] [PubMed] [Google Scholar]
- Xu X. et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut doi: 10.1136/gutjnl -2014–308513 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W. J. et al. Risk factors for early recurrence of HBV-related hepatocellular carcinoma meeting Milan criteria after curative resection. Asian Pac J Cancer Pre 14, 7101–7106 (2013). [DOI] [PubMed] [Google Scholar]
- Yang S. L. et al. Preoperative serum α-fetoprotein and prognosis after heaptecotomy for hepatocellular carcinoma. Br J Surg. doi: 10.1002/bjs (2016). [DOI] [PubMed] [Google Scholar]
- Fu S. J. et al. Glypican-3 is a potential prognostic biomarker for hepatocellular carcinoma after curative resection. Surgery 153, 536–544 (2013). [DOI] [PubMed] [Google Scholar]
- Orlowski M. & Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci USA 67, 1248–1255 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschke R. et al. Alcoholic liver disease associated with increased gammaglutamyltransferase activities in serum and liver. Adv Exp Med Biol 132, 647–654 (1980). [DOI] [PubMed] [Google Scholar]
- Lee D. H. et al. Gamma-glutamyltransferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab 89, 5410–5414 (2004). [DOI] [PubMed] [Google Scholar]
- Ishizaka N. et al. Association between gamma- glutamyltransferase levels and insulin resistance according to alcohol consumption and number of cigarettes smoked. J Atheroscler Thromb 17, 476–485 (2010). [DOI] [PubMed] [Google Scholar]
- Franzini M. et al. Modulation of cell growth and cisplatin sensitivity by membrane. Gamma -glutamyltransferase in melanoma cells. Eur J Cancer 42, 2623–2630 (2006). [DOI] [PubMed] [Google Scholar]
- Hanigan M. H. et al. Gammaglutamyl transpeptidase accelerates tumour growth and increases the resistance of tumors to cisplatin in vivo. Carcinogenesis 20, 553–559 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici S. et al. Possible role of membrane gamma-glutamyltransferase activity in the facilitation of transferrin-dependent and –independent iron uptake by cancer cells. Cancer Cell Int 3, 7 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Bello, B. et al. Hydrogen peroxide produced during gamma- glutamyltransferase activity is involved in prevention of apotosis and maintainance of proliferation in U937 cells. FASEB J 13, 9–79 (1999). [DOI] [PubMed] [Google Scholar]
- Corti A. et al. Gammaglutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res 30, 1169–1181 (2010). [PubMed] [Google Scholar]
- Whitfield J. B. Gamma glutamyl transferase. Crit Rev Clin Lab Sci 38, 263–355 (2001). [DOI] [PubMed] [Google Scholar]
- Pompella A. et al. Expression of -glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol 71, 231–238 (2006). [DOI] [PubMed] [Google Scholar]
- Hofbauer S. L. et al. Pretherapeutic gamma- glutamyltransferase is an independent prognostic factor for patients with renal cell carcinoma. Br J Cancer 1118, 1526–1531 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C. et al. Association of gamma-glutamyltransferase with severity of disease at diagnosis and prognosis of ovarian cancer. Br J Cancer 109, 610–614 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F. et al. Prognostic significance of gamma- glutamyltransferase in patients with resectable esophageal squamous cell carcinoma. Dis Esophagus 285, 496–504 (2015). [DOI] [PubMed] [Google Scholar]
- Fu S. J. et al. Prognostic value of preoperative serum gamma-glutamyl- transpeptidase in patients with hepatocellular carcinoma after hepatectomy. Tumour Biol PMID:26449826 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang J. B. et al. Prognostic significance of serum gamma- glutamyltransferase in patients with intermediate hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol 23, 787–793 (2011). [DOI] [PubMed] [Google Scholar]
- Guiu B. et al. Serum gamma-glutamyl-transferase independently predicts outcome after transarterial chemoembolization of hepatocellular carcinoma: external validation. Cardiovasc Intervent Radiol 35, 1102–1108 (2012). [DOI] [PubMed] [Google Scholar]
- Ma H. et al. γ- Glutamyl-transpeptidase is a prognostic marker of survival and recurrence in radiofrequency-ablation treatment of hepatocellular carcinoma. Ann Surg Oncol 21, 3084–3089 (2014). [DOI] [PubMed] [Google Scholar]
- Han M. et al. Liver transplantation using organs from deceased organ donors: a single organ transplant center experience. HBPD INT 13, 409–415 (2014). [DOI] [PubMed] [Google Scholar]
- Yao F. Y. et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33, 1394–1403 (2001). [DOI] [PubMed] [Google Scholar]
- Zheng S. S. et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 85, 1726–1732 (2008). [DOI] [PubMed] [Google Scholar]
- Li Q. G., et al. Disseminated tumor cells homing into rats’ liver: a new possible mechanism of HCC recurrence. World J Gastroenterol 10, 903–905 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S. J. et al. Prognostic value of preoperative peripheral neutrophilto- lymphocyte ratio in patients with HBV-associated hepatocellular carcinoma after radical hepatectomy. Med Oncol 30, 721 (2013). [DOI] [PubMed] [Google Scholar]
- Reuter S. et al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biol Med 49, 1603–1616 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hemelrijck M. et al. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons—the Swedish AMORIS study. Eur J Cancer 47, 2033–2041 (2011). [DOI] [PubMed] [Google Scholar]
- Mok Y. et al. γ-Glutamyltransferase and cancer risk: The Korean cancer prevention study. Int J Cancer doi: 10.1002/ijc.29659 (2015). [DOI] [PubMed] [Google Scholar]
- Dalpiaz O. et al. Preoperative serum-gamma- glutamyltransferase (GGT) does not represent an independent prognostic factor in a European cohort of patients with non-metastatic renal cell carcinoma. J Clin Pathol 68, 547–551 (2015). [DOI] [PubMed] [Google Scholar]
- Seebacher V. et al. Prognostic significance of gammaglutamyltransferase in patients with endometrial cancer: a multi-centre trial. Br J Cancer 106, 1551–1555 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]