Abstract
Introduction
Self-monitoring has been shown to be a crucial part of initial weight loss success in behavioral interventions. However, little is known about the impact of self-monitoring during the period following initial treatment.
Methods
The current study examined the role of self-monitoring on weight loss during an initial 6-month intervention period (Phase1) and a 12-month extended care period (Phase 2) in a group of 167 obese women (M±SD: BMI = 37.0±5.1 kg/m2, age = 59.9±6.2 years) enrolled in a behavioral weight loss program.
Results
Cluster analysis identified three groups of participants with low, moderate, and high rates of weight loss success during Phase 1 and Phase 2. A one-way ANOVA revealed no significant differences in self-monitoring frequency between groups during Phase 1 (p = .645), but significant differences between all three groups during Phase 2 (p = .001). High success participants completed the most self-monitoring records, followed by the moderate group. The low success group completed the least number of records. Furthermore, self-monitoring during Phase 2 significantly mediated the relationship between extended-care session attendance and percent weight change during that time (95% CI [−.004, −.001], p < .001).
Conclusion
These results highlight the importance of continuing self-monitoring after the initial phase of treatment to maintain lost weight.
Keywords: obesity, weight loss, lifestyle intervention, behavioral treatment, self-monitoring, randomized controlled trial
1. Introduction
Self-monitoring, the recording of one’s behavior, has been identified as the cornerstone of behavioral weight loss interventions. Kanfer (1970) posited that self-monitoring serves as the initial step in a feedback loop that includes (1) the observation and recording of target behaviors; (2) self-evaluation; and (3) self-reinforcement, during which the individual decides to continue with or adjust behaviors in order to align with their goals. The process allows individuals the opportunity to both establish goals for behavior change and track progress in achieving these goals (Febbraro & Clum, 1998).
In the context of behavioral weight-loss interventions, self-monitoring typically involves the tracking of food and beverage intake. Participants enrolled in behavioral programs commonly lose 8–10% of initial body weight (Butryn, Webb, & Wadden, 2011). Such results are considered favorable based on findings indicating that losses of ≥ 5% can produce positive changes in health such as reductions in triglycerides, blood glucose, and blood pressure, improved blood lipid levels, and reductions in an individual’s risk for developing type 2 diabetes (Jensen et al., 2014).
The relationship between self-monitoring and weight change within behavioral weight-loss interventions has been explored extensively within the literature. A systematic review (Burke, Wang, & Sevick, 2011) of 15 studies showed dietary self-monitoring was significantly associated with weight loss, and that weight loss was significantly greater among individuals who returned self-monitoring logs on a more consistent basis. Similarly, individuals who returned complete logs lost significantly more weight than those who had logs judged to be incomplete.
While research consistently identifies self-monitoring as a strategy associated with weight loss, the majority of studies evaluate this relationship during an initial intervention period (Burke, Wang, & Sevick, 2011). Long-term weight reductions achieved through behavioral treatment are difficult to maintain and a different set of skills may be required for success following interventions. Research findings indicate that at one year post-intervention, about one quarter of participants have maintained weight loss ≥10% of baseline weight, another quarter of participants maintained weight loss of 5–9.9% below their baseline weight, and almost 40% have only maintained weight loss of ≤4.9% below baseline. The remaining participants lost no weight or gained weight (Christian, Tsai, & Bessesen, 2010). Approximately half of participants will have returned to their baseline weight by five years, while the majority of others regain at least some of the initial weight lost (Perri & Corsica, 2002).
The findings of many typical weight loss studies are limited because they do not identify subgroups of participants with distinct response patterns. The previously mentioned review by Christian et al. (2010) sought to overcome this limitation by requesting categorical weight loss data from investigators who had conducted a 12-month behavioral weight-loss intervention for adults. However, the authors noted the review was limited by the small number of studies (n = 11) providing categorical data. The absence of studies evaluating changes in weight beyond the mean and between-group significance level represents a barrier to identifying components of treatment associated with higher rates of success. Furthermore, it limits the ability to identify behaviors associated with larger weight losses as well as maintenance of lost weight.
1.1 Current study
The current study explored patterns of weight loss as well as the short- and long-term impact of dietary self-monitoring on weight change among adults enrolled in a behavioral weight-loss intervention. It was hypothesized that participants would fall into unique clusters based on their percent weight change over time, with some participants demonstrating the pattern of weight change most commonly reported in the literature (i.e., clinically significant weight loss followed by a regain during extended care of one-third to one-half the amount initially lost), and other groups of participants showing results that are noticeably different from what is typically reported. We expected that participants in groups demonstrating greater success would also have completed more records of food and beverage intake. We hypothesized that self-monitoring would explain the relationship between treatment attendance and weight change from 0–6 months (during the intervention or Phase 1) and 6–18 months (during the extended-care phase or Phase 2).
2. Method
2.1 Lifestyle Intervention
Data for the current study was collected as part of the Treatment of Obesity in Underserved Rural Settings study, a randomized controlled trial designed to explore the effectiveness of three extended-care programs on sustained weight loss. Study design and recruitment methods, including inclusion/exclusion criteria, screening procedures, and attrition, have been previously reported (Perri et al., 2008). All included participants completed an initial 6-month lifestyle intervention for obesity (Phase 1) consisting of a low-calorie eating prescription, increased physical activity, and training in behavior modification strategies such as daily self-monitoring of food intake. After phase 1, participants were randomly assigned to one of the following three extended-care programs each lasting 12 months (Phase 2): a face-to-face maintenance program, a telephone maintenance program, or an educational control group. The face-to-face condition continued to meet in their initial weight loss groups twice per month, whereas participants in the telephone-based condition received individual telephone sessions with the same frequency. Participants assigned to the education control condition received 26 biweekly newsletters focused on tips for maintaining weight-loss but had no personal contact with the interventionists. Across each extended-care program, participants were encouraged to continue self-monitoring on three or more days per week.
2.2 Participants
Participants were women living in medically underserved rural counties in north central Florida, aged 50–75 (M ± SD age = 59.9 ± 6.2 years) with BMIs between 30 and 50 kg/m2 (BMI at baseline M ± SD = 37.0 ± 5.1 kg/m2). The study was limited to women as prior feedback from focus groups suggested that women in rural communities would feel most comfortable in groups that (a) included women only and (b) addressed issues of particular concern to women (e.g., physical appearance). Furthermore, initial recruitment response rate from men was less than 5% of potential participants.
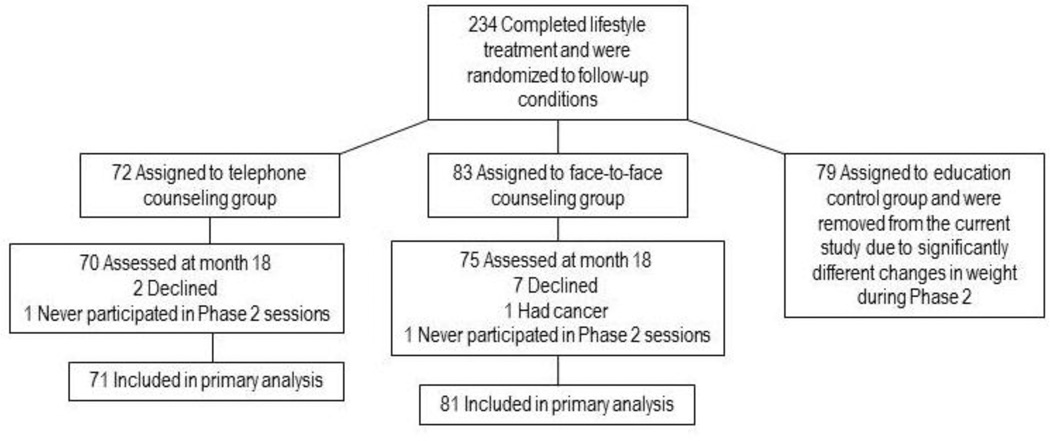
A total of 234 women completed Phase 1 of the initial study and were randomized to Phase 2. Participants randomized to the face-to-face and telephone-based condition did not demonstrate significantly different rates of weight loss in Phase 2 (p < .05). As a result, they were evaluated as one sample in the current study. The 79 participants randomized to the educational control group displayed significantly different weight change patterns from those in the other groups during Phase 2 and were not included the current study. During Phase 2, two participants did not attend group sessions and one participant was medically withdrawn. A total of 152 participants were included in the current analysis, and 145 participants completed the eighteen month assessment visit (95.4%; see Figure 2-1). For the seven participants who declined to participate in the 18 month assessment, we assumed that on average they regained 0.3kg per month after leaving the study (Wadden, Berkowitz, Sarwer, Prus-Wisniewski, & Steinberg, 2001). Baseline characteristics of the sample are summarized in Table 2-1.
Figure 2.1.
Flowchart of enrollment, randomization, and follow-up
Table 2.1.
Participant baseline characteristics
| Characteristics | |
|---|---|
| Age in years, M (SD) | 59.9 (6.2) |
| BMI in kg/m2, M (SD) | 37.0 (5.1) |
| Race/ethnicity n (%) | |
| White, Non-Hispanic | 115 (75.7) |
| Black, Non-Hispanic | 33 (21.7) |
| Hispanic/Latina | 2 (1.3) |
| American Indian or Pacific Islander | 2 (1.3) |
| Highest Level of Education, n (%) | |
| High school education only | 56 (36.8) |
| Trade, vocational, or associate’s degree | 67 (44.1) |
| Bachelor’s or advanced degree | 29 (19.1) |
| Marital Status, n (%) | |
| Never Married/Divorced/Separated | 20 (13.2) |
| Widowed | 16 (10.5) |
| Presently Married/Cohabitating | 116 (76.32) |
| Employment Category, n (%) | |
| Not working | 60 (39.5) |
| Employed, full-time or part-time | 68 (44.7) |
| More than one category/Other | 24 (15.8) |
| Total Annual Family Income, n (%) | |
| $19,999 or below | 40 (26.3) |
| $20,000–34,999 | 37 (24.3) |
| $35,000–49,999 | 29 (19.1) |
| $50,000–74,000 | 28 (18.4) |
| $75,000 or above | 15 (9.7) |
| Don’t know | 2 (1.2) |
2.3 Measures
2.3.1 Height and weight
Height was taken without shoes and measured by a stadiometer to the nearest 0.1 centimeter. Weight was measured without shoes, in light indoor clothing, and with pockets emptied, to the nearest 0.1kg using a calibrated and certified balance beam scale. Percent change in weight over time was then calculated based on measured weights at months 0, 6, and 18.
2.3.2. Dietary self-monitoring records
Participants were provided with standardized paper self-monitoring logs and instructed to record daily food and beverage consumption. During Phase 1, participants were instructed to self-monitor on a daily basis. Records were returned to group leaders and reviewed at weekly group meetings. For Phase 2, participants were asked to complete records for at least two weekdays and one weekend day every week. Both face-to-face and phone groups received stamped and addressed envelopes so food records could be returned to the group leaders. For the current study, the total number of days with written food records (i.e., days with at least two meals recorded) was summed for each participant during each phase of the study. Completed records were unable to be accessed for overall accuracy in regards to precise number of calories consumed.
3. Data analysis
The statistical software package SPSS 21.0 for Windows (SPSS Inc., IL) was used to conduct the statistical analysis for this research study.
3.1 Cluster analysis
A two-step cluster analysis was conducted to identify patterns in weight change among participants throughout the study. The number of clusters was specified a priori to examine solutions for potential patterns in weight change and a three cluster model was determined to be a best fit for the data. Clusters were analyzed based on individual participant weight change during each phase of the study. The clustering solution was determined using log-likelihood estimation and the squared Euclidean distance method was used to assess similarity between clusters in order to take into account elevation of weight change scores (Blashfield, 1980). Stability of clusters was assessed by random division of the sample into two halves, followed by repeated cluster analysis and visual inspection of the data (Clatworty, Buick, Hankins, Weinman, & Horne, 2005).
3.2 Cluster comparison
Following the cluster analysis, the resulting groups were compared based on self-monitoring frequency during each phase using a one-way Analysis of Variance (ANOVA). Normality was assessed using tests of skewness-kurtosis and visual inspection of Q-Q plots. Post-hoc tests were used to determine the significance of differences between clusters.
3.3 Mediational analysis
Significant correlations between treatment session attendance, self-monitoring frequency, and percent weight change were found during both phases of the study. Therefore, the Preacher and Hayes model was used to determine if self-monitoring frequency mediated the relationship between treatment attendance and percent weight change during each phase. The number of treatment sessions attended was entered as the independent variable and percent weight change was entered as the dependent variable. Number of self-monitoring records was entered as the potential mediating variable. The Preacher and Hayes model for mediation was chosen in order to reduce the possibility of Type I error and increase statistical power. Results were bootstrapped to 5,000 samples. Effect size was measured using ĸ2 to assess the ratio of analyzed indirect effect to maximum possible indirect effect.
4. Results
4.1 Cluster analysis
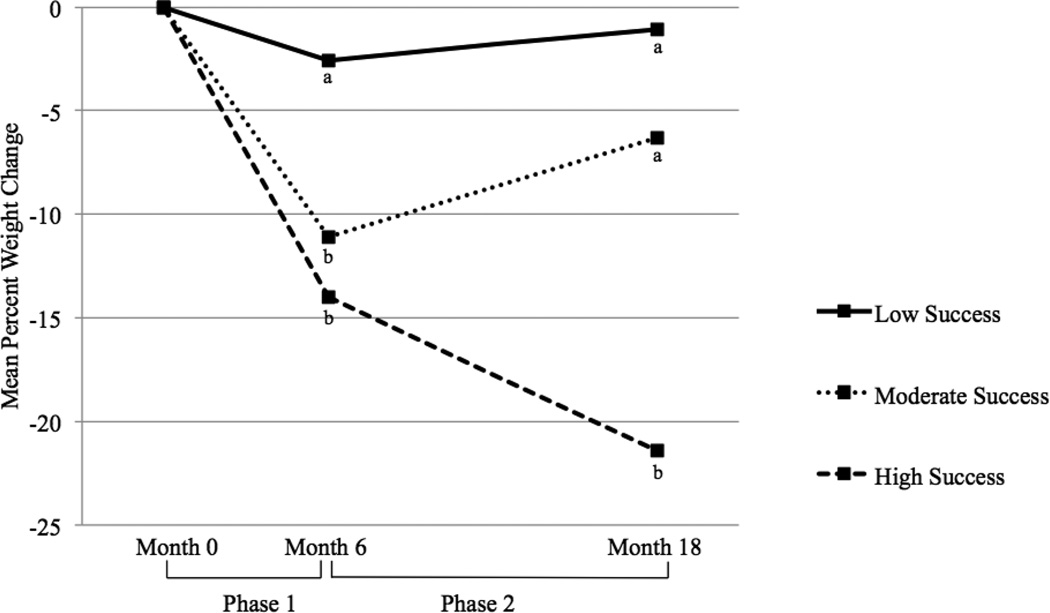
The cluster analysis of participants’ percent weight change during intervention and extended-care revealed three distinct groups of participants, based on their patterns of weight loss and potential regain over time. The largest cluster (Cluster 1) consisted of 71 participants, or 46.71% of the sample. These participants demonstrated a mean percent weight change of −11.58 ± 3.57 during Phase 1, but regained 5.11 ± 3.52 mean percent weight during Phase 2. Cluster 1 demonstrated results most typical of behavioral weight loss treatment, and was labeled the Moderate Success group. The next largest cluster (Cluster 2) evidenced low rates of achievement, and was labelled the Low Success group. Cluster 2 contained 46 participants, or 30.26% of the sample. These individuals showed a mean percent weight change of −4.46 ± 2.69 during Phase 1 and a regain of 2.21 ± 3.27 percent mean weight during Phase 2. Finally, the remaining women fell within a third cluster (Cluster 3) of 35 participants, or 23.03% of the sample, and were labelled the High Success group based on a pattern of continued weight loss over time. These participants initially showed a mean percent weight change of −14.21 ± 4.21 during Phase 1, and an additional −7.27 ± 4.01 percent mean weight change during Phase 2. Results are presented visually in Figure 4-1. Visual inspection of the scatter plots for repeated analyses using split-half data demonstrated stability of clusters. A one-way ANOVA revealed significant differences in weight change between clusters during both Phase 1[F(2, 151) = 90.45, p< .001] and Phase 2[F(2, 151) = 142.15, p<.001]. Post-hoc testing revealed each cluster differed significantly from the other two during both phases.
Figure 4.1.
Mean percent weight changes at Months 6 and 18 for participants in the Low, Moderate, and High Success clusters.
*Clusters with differing subscripts at Month 6 or Month 18 differed significantly from each other (ps ≤ .01).
4.2 Cluster comparison
During Phase 1, the number of completed self-monitoring logs ranged from 98 – 173 across groups over the six-month period. An ANOVA was used to compare weight loss clusters in regard to number of self-monitoring records completed. Intervention group was not included in the ANOVA due to finding no specific between-group differences of treatment condition on weight loss. The clusters similarly did not differ on any major demographic variables. Results of the analysis revealed significant differences between weight loss success clusters in regards to number of self-monitoring logs completed, F(2,151) = 7.45, p = .001. As Levene’s test was significant for the Phase 1 data, Games-Howell post-hoc tests were used to correct for the potential differences of variance. Follow-up testing revealed that the low success cluster completed significantly fewer food records (M ± SD = 131.85 ± 33.36) than both the moderate success cluster (148.03 ± 18.86, p = .01) and the high success cluster (150.09 ± 22.53, p = .01). The moderate and high success clusters did not differ significantly (p = .89).
ANOVA results again revealed significant differences between clusters in regards to self-monitoring records completed during Phase 2, F(2,151) = 15.42, p < .001. Games-Howell post-hoc tests were again used to determine between-group differences due to a significant Levene’s test. During this phase, the low success cluster (96.91 ± 90.75) and the moderate success cluster (108.18 ± 81.39) did not differ significantly from each other; however the high success cluster (202.54 ± 115.49) completed significantly more records than both the low (p < .001) and the moderate (p < .001) groups.
4.3 Mediational analyses
The mediational analysis examined the relationship between treatment attendance, percent weight change, and number of self-monitoring records completed. Self-monitoring frequency was used as a potential mediator as it correlated significantly with Phase 1 and Phase 2 attendance (p < .001 during both time periods) and Phase 1 and 2 percent weight change (p < .001 during both time periods). Results from Phase 1 showed the total effect of the model (p < .001) remained significant when self-monitoring frequency was added to the model as a mediator (direct effect of self-monitoring during Phase 1 = −.004, p = .0487). Moreover, the indirect effect of self-monitoring during the initial phase was significant (95% CI: −.0054, −.0005). Thus, the number of food records completed during Phase 1 partially mediated the relationship between in-person session attendance and percent weight change during this phase. The ĸ2 value (i.e., ratio of established indirect effect to maximum possible indirect effect) was .13, suggesting a small to moderate indirect effect.
Results for the Phase 2 analysis indicated the total effect of the model (p < .001) became non-significant when self-monitoring frequency was added to the model (direct effect of self-monitoring during Phase 2 = .0002, p = .80). The indirect effect of self-monitoring during this phase was found to be significant (95% CI: −.0028, −.0009), suggesting the number of food records completed during the extended-care phase fully mediated the effect of treatment attendance on percent weight change. The ĸ2 value during Phase 2 was .19, showing the effect size was larger during the extended care phase than during initial treatment, and suggesting a moderate overall indirect effect size.
5. Discussion
The current study is novel in that it examined different patterns of weight loss in response to behavioral treatment and investigated the role of self-monitoring within these categories. The most common pattern of weight loss, classified as the Moderate Success cluster and accounting for about half of all participants, demonstrated results most typically reported in the current literature (i.e., an initial clinically significant loss, followed by a regain of almost half that weight during the year after intervention; (Curioni & Lourenco, 2005; Perri & Corsica, 2002; Wadden et al., 2007). Two smaller clusters, however, demonstrated very different patterns. A Low Success cluster lost less than five percent of body weight initially, and regained half of weight lost during extended care. The smallest group, labeled the High Success cluster, demonstrated results often considered difficult to achieve in behavioral programs (Butryn et al., 2011). These participants initially lost more than 14% of their body weight and continued to lose clinically significant amounts during extended care. These results suggest there exist varied weight-loss patterns among participants and highlight the importance of exploring these differences.
The current study extends research on the role of self-monitoring in weight loss by evaluating this relationship during an initial 6- month and a 12-month extended-care period. Findings showed significant differences in the number of food records completed during both time periods between certain clusters. During Phase 1, the most successful and moderately successful participants completed a similar number of self-monitoring records that were significantly greater than those completed by the least successful participants. A different pattern was observed during Phase 2 such that participants in the high success group completed a significantly greater number of records than participants in both low and moderate success groups. Thus, only participants who reported the highest rate of food record completion continued to lose weight after the initial intervention, supporting the importance of encouraging participants to track food and beverage intake even after initial weight loss.
In addition to being associated with increased weight loss, self-monitoring frequency was found to partially explain the relationship between attendance and weight change during Phase 1 and to fully explain this relationship during Phase 2. This supports the feedback loop theory discussed previously – participants who had greater opportunity to monitor and evaluate progress most likely had increased opportunities to correct course of action or reinforce behaviors as appropriate. Considering self-monitoring had a smaller contribution to differences in weight loss during the initial program, there are likely additional factors that influence success, such as comprehension of treatment materials, adherence to other aspects of treatment, or amount of support from friends and family. Future research should focus on identifying limiting factors to weight loss early on during interventions in order address these issues in lower success participants. For these participants, it may be important to consider using pharmacological intervention as a complement to behavioral treatment (Jensen et al., 2014). Alternatively, it will be important to identify characteristics of the most successful participants that can be emphasized to help improve results of both moderate and low success participants.
Several limitations of the study are worth noting. First, the current analyses are correlational in nature. Therefore, differences in self-monitoring may be based on other aspects of treatment or individual differences yet to be explored. For example, participants in the High Success cluster may simply represent a group of individuals who are more motivated to adhere to treatment. Alternatively, a reciprocal relationship between self-monitoring and weight loss may exist, such that individuals who see early initial success are more encouraged to continue self-monitoring, whereas those participants who struggle with early weight loss might conclude self-monitoring is less effective and therefore be less motivated to continue.
Second, the mediation analysis was conducted with variables that were measured in parallel. Thus, we cannot establish the temporal precedence of self-monitoring frequency in the relationship between attendance and weight change. Third, due to the nature of pen-and-paper record keeping, there is no reliable way to determine intervals between eating and self-monitoring. Shorter intervals between eating and recording are more predictive of ultimate weight loss (Burke, Sereika, Music, Warziski, Styn, & Stone, 2008). Fourth, participants who failed to return food records were assumed to not be recording food intake. Fifth, there were seven participants who did not attend the 18 month assessment so an average rate of weight regain was used to estimate this data. Sixth, the participants in this study were all middle aged and older women living in rural communities. Therefore results cannot be generalized to other populations without further research. Finally, this study does not include a follow-up period where participants are not engaged in treatment; additional research is needed to identify weight changes following extended care.
Several implications can be drawn from the current study. During the initial intervention, almost a quarter of participants demonstrated a high degree of weight loss (i.e., 14%) and then continued to lose a clinically significant amount of weight during the subsequent 12 months. At the completion of the lifestyle intervention, these participants lost approximately 20% of their body weight, rates more closely resembling those reported for bariatric surgery recipients (Gloy et al., 2013). The unique response demonstrated by the three separate clusters suggests there is room for future studies to investigate more fully the behaviors of those in the most and least successful groups in order to determine which components of treatment are most helpful in achieving weight loss.
Collectively, the findings from the current study suggest self-monitoring can play a key role in successful long-term weight management. These initial results support previous research demonstrating self-monitoring as an effective tool to promote weight loss (Peterson et al., 2014). However, it extends beyond prior studies in illustrating the beneficial impact of self-monitoring on long-term weight loss. Future research may benefit from exploring the impact of self-monitoring on weight loss following behavioral treatment within the context of a prospective, randomized controlled trial.
Highlights.
We examined the categorical responses to weight loss treatment.
Participants showed three separate patterns of weight loss in response to treatment.
Frequent self-monitoring is associated with high rates of weight loss during behavioral treatment and extended care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: A meta-analysis of US studies. American Journal of Clinical Nutrition. 2001;74(5):579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- Blashfield RK. Propositions regarding the use of cluster analysis in clinical research. Journal of Consulting & Clinical Psychology. 1980;48(4):456–459. doi: 10.1037//0022-006x.48.4.456. [DOI] [PubMed] [Google Scholar]
- Burke LE, Sereika SM, Music E, Warziski M, Styn MA, Stone A. Using instrumented paper diaries to document self-monitoring patterns in weight loss. Contemporary Clinical Trials. 2008;29(2):182–193. doi: 10.1016/j.cct.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: A systematic review of the literature. Journal of the American Dietetic Association. 2011;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatric Clinics of North America. 2011;34(4):841–859. doi: 10.1016/j.psc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian JG, Tsai AG, Bessesen DH. Interpreting weight losses from lifestyle modification trials: Using categorical data. International Journal of Obesity (London) 2010;34(1):207–209. doi: 10.1038/ijo.2009.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy J, Buick D, Hankins M, Weinman J, Horne R. The use and reporting of cluster analysis in health psychology: A review. British Journal of Health Psychology. 2005;10:329–358. doi: 10.1348/135910705X25697. [DOI] [PubMed] [Google Scholar]
- Curioni CC, Lourenco PM. Long-term weight loss after diet and exercise: A systematic review. International Journal of Obesity Related Metabolic Disorders. 2005;29(10):1168–1174. doi: 10.1038/sj.ijo.0803015. [DOI] [PubMed] [Google Scholar]
- Febbraro GA, Clum GA. Meta-analytic investigation of the effectiveness of self-regulatory components in the treatment of adult problem behaviors. Clinical Psychology Reviews. 1998;18(2):143–161. doi: 10.1016/s0272-7358(97)00008-1. [DOI] [PubMed] [Google Scholar]
- Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. British Medical Journal. 2013;347 doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Donato KA, Apovian CM, Ard JD, Comuzzie AG, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Obesity. 2013;21(Suppl 3) [Google Scholar]
- Kanfer FH, Karoly P. Self-control: A behavioristic excursion into the lion’s den. Behavior Therapy. 1972;3(3):398–416. [Google Scholar]
- Perri MG, Corsica JA. Improving the maintenance of weight loss in behavioral treatment of obesity. In: Wadden TA, Stunkard AJ, editors. Handbook of obesity treatment. New York: Guilford Press; 2002. pp. 357–379. [Google Scholar]
- Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, Dale MS, Daniels MJ, Radcliff TA, Martin AD. Extended-care programs for weight management in rural communities: The treatment of obesity in underserved rural settings (TOURS) randomized trial. Archives of Internal Medicine. 2008;168(21):2347–2354. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson ND, Middleton KR, Nackers LM, Medina KE, Milsom VA, Perri MG. Dietary self-monitoring and long-term success with weight management. Obesity. 2014;22(9):1962–1967. doi: 10.1002/oby.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Berkowitz RI, Sarwer DB, Prus-Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity: A randomized trial. Archives of Internal Medicine. 2001;161(2):218–227. doi: 10.1001/archinte.161.2.218. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Butryn ML, Wilson C. Lifestyle modifications for the management of obesity. Gastroenterolofy. 2007;132(6):2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]