Abstract
Bacteria are able to de-epoxidize or epimerize deoxynivalenol (DON), a mycotoxin, to deepoxy-deoxynivalenol (deepoxy-DON or DOM-1) or 3-epi-deoxynivalenol (3-epi-DON), respectively. Using different approaches, the intestinal toxicity of 3 molecules was compared and the molecular basis for the reduced toxicity investigated. In human intestinal epithelial cells, deepoxy-DON and 3-epi-DON were not cytotoxic, did not change the oxygen consumption or impair the barrier function. In intestinal explants, exposure for 4 hours to 10 μM DON induced intestinal lesions not seen in explants treated with deepoxy-DON and 3-epi-DON. A pan-genomic transcriptomic analysis was performed on intestinal explants. 747 probes, representing 323 genes, were differentially expressed, between DON-treated and control explants. By contrast, no differentially expressed genes were observed between control, deepoxy-DON and 3-epi-DON treated explants. Both DON and its biotransformation products were able to fit into the pockets of the A-site of the ribosome peptidyl transferase center. DON forms three hydrogen bonds with the A site and activates MAPKinases (mitogen-activated protein kinases). By contrast deepoxy-DON and 3-epi-DON only form two hydrogen bonds and do not activate MAPKinases. Our data demonstrate that bacterial de-epoxidation or epimerization of DON altered their interaction with the ribosome, leading to an absence of MAPKinase activation and a reduced toxicity.
Mycotoxins are toxic secondary metabolites produced by various molds, such as Aspergillus, Penicillium and Fusarium which may contaminate food and feed at all stages of the food/feed chain1,2. Despite the improvement of agricultural and manufacturing practices, mycotoxin contamination cannot be avoided and still represents a permanent health risk for both humans and animals. It is thus important to develop decontamination strategies3. Among mycotoxins, deoxynivalenol (DON) produced by Fusarium species, is commonly detected in cereal crops, including wheat, barley, and maize. It is the most abundant trichothecene in food with a frequent occurrence at toxicologically relevant concentrations worldwide4,5.
DON causes acute and chronic disorders in humans and animals, with the gastrointestinal tract being an organ sensitive to its adverse effects6. DON affects the intestinal histomorphology, impairs barrier function and nutrient absorption7,8. DON also disrupts the local intestinal immune response; it triggers and potentiates intestinal inflammation9,10. At the cellular and subcellular level, DON binds to the ribosome, inhibits protein and nucleic acid synthesis and triggers ribotoxic stress11,12,13 leading to the activation of kinases, MAPKs and their downstream signaling pathways14.
Several strategies have been developed to limit DON toxicity15, among them, bacterial biotransformation which depends on the ability of microorganisms to generate DON metabolites with reduced toxicity. De-epoxidation is a reductive chemical reaction opening the 12,13-epoxy ring transforming DON into its de-epoxide metabolite de-epoxy-deoxynivalenol (deepoxy-DON or DOM-1) (Supplementary Figure S1)16. Several microbial strains are capable of DON de-epoxidation15,17. Several in vitro studies demonstrated the reduced toxicity of deepoxy-DON. In vivo trials on farm animals receiving feed contaminated with DON have also shown a beneficial effect of the bacteria able to de-epoxidize DON, according to zootechnical parameters and immune response18. The hydroxyl on carbon 3 also seems to be significant for the toxic activity of DON and a detoxification strategy targeting this part of the C3-OH, leading to the formation of 3-epi-DON, was recently proposed19. Four bacterial strains, all isolated from soil, have been described to epimerize DON into 3-epi-DON20,21. Only one paper has investigated the effect of 3-epi-DON and demonstrated the lack of toxicity, both in vitro and in vivo, of this DON metabolite22.
The aim of the current study was to assess the efficacy of microbial transformation through analysis of the intestinal toxicity of deepoxy-DON and 3-epi-DON. Using physiological, histological and transcriptomic analysis, we have observed reduced toxicity of deepoxy-DON and 3-epi-DON, both for human intestinal epithelial cells and pig intestinal explants. We have further demonstrated that these microbial metabolites of DON fit into the ribosome pocket but do not elicit ribotoxic stress or activate the MAPKinase pathway. Our paper provides the first molecular insight for the reduced toxicity of deepoxy-DON and 3-epi-DON.
Results
Deepoxy-DON and 3-epi-DON do not impair proliferation of human intestinal cells
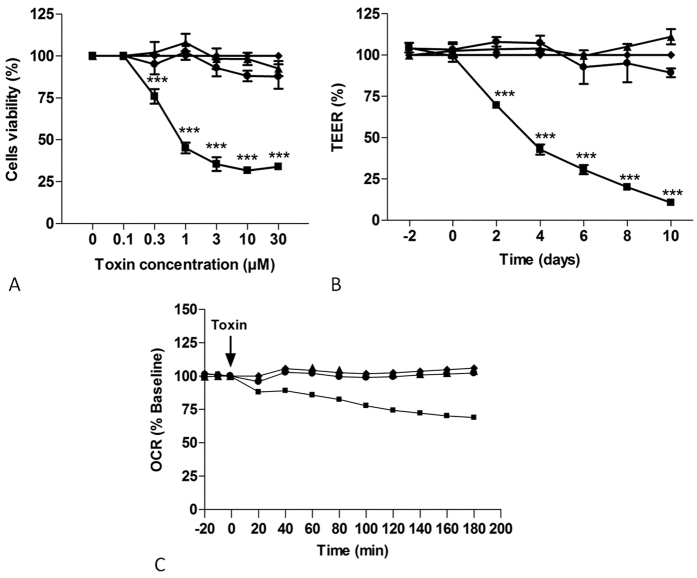
Comparative effects of deepoxy-DON, 3-epi-DON and DON were first evaluated on proliferating human Caco-2 cells. The cell viability was assessed by the quantification of ATP using the luminescent cell viability assay. As shown in Fig. 1, Panel A, 48 hours exposure to deepoxy-DON or 3-epi-DON at concentrations up to 30 μM had no significant impact on cell viability. By contrast, DON markedly decreased the viability of proliferating cells in a dose-dependent manner; exposure to 10 μM of DON for 48 hours reduced cell viability by approximately 70%. The IC50 was calculated at 1.30 μM.
Figure 1. Effects of deepoxy-DON or 3-epi-DON on human intestinal epithelial cells.
Activation of cytotoxicity (Panel A), TEER (Panel B) and OCR (Panel C). (Panel A): Proliferative Caco-2 cells were incubated with increasing concentrations of diluent (♦), DON (◾), deepoxy-DON (▴) or 3-epi-DON (●) for 48 hours. Cell viability evaluated by measurement of ATP, is expressed as % of control cells. (Panel B): Caco-2 cells, differentiated on inserts, were treated with 10 μM of diluent (♦), DON (◾), deepoxy-DON (▴) or 3-epi-DON (●) and TEER was measured. (Panel C): After establishment of baseline oxygen consumption rate in proliferated Caco-2 cells seeded to 1.5 × 104 cells/well, diluent (♦), DON (◾), deepoxy-DON (▴) or 3-epi-DON (●), was injected at final concentration of 10 μM as indicated by the arrow. The rate of oxygen consumption was then measured for the indicated time. For visual clarity, statistical indicators were omitted from the graph. The OCR values are shown as the percent of baseline for each group. Results are expressed as mean ± SEM of 3–4 independent experiments, ***p < 0.001.
Deepoxy-DON and 3-epi-DON do not impair cell viability and TEER of differentiated human intestinal cells
The comparative toxicity of deepoxy-DON, 3-epi-DON and DON was also performed on differentiated Caco-2 cells through the measurement of TEER. As already described23, differentiated cells are more resistant to DON and at least 30 μM are needed to induce a significant decrease in viability. At 10 μM of DON, a significant decrease of TEER in differentiated Caco-2 cells at a non-cytotoxic dose was observed (Fig. 1, Panel B). The decrease was time-dependent and reached 25% after 2 days and about 90% at 10 days. By contrast, cells treated with 10 μM deepoxy-DON or 3-epi-DON didn’t show any decrease in TEER.
Deepoxy-DON and 3-epi-DON do not affect oxygen consumption in Caco-2 cells
The impact of deepoxy-DON, 3-epi-DON and DON on bioenergetic function in Caco-2 cells was evaluated using extracellular flux analyses. As shown in Fig. 1, Panel C, DON linearly decreased the rate of oxygen consumption in a time-dependent manner starting at the 40 minute stage. Approximately 3 hours after DON exposure, oxygen consumption values were 32% less than the base value. By contrast, deepoxy-DON and 3-epi-DON had no effect on the cellular oxygen consumption of proliferating Caco-2 cells and displayed bioenergetics profiles comparable to that of control cells.
Deepoxy-DON and 3-epi-DON do not induce histological alterations of intestinal explants
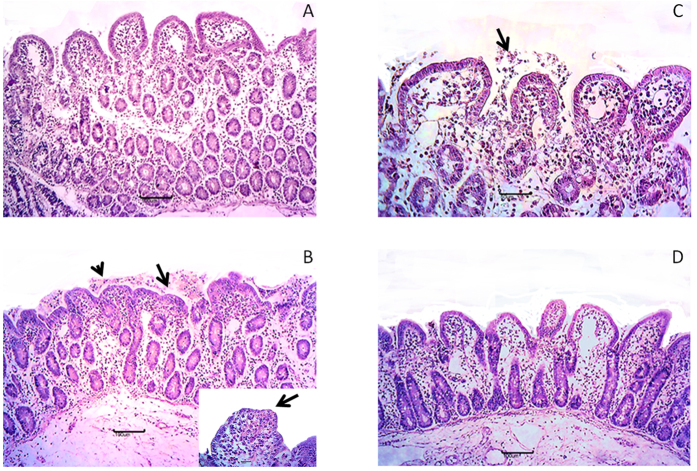
In order not to restrict the observations to an intestinal cell line, experiments were also performed on jejunal explants, a model developed to assess short-term effects of mycotoxins24. The effects on intestines of deepoxy-DON, 3-epi-DON and DON were first compared with histology (Fig. 2). Lymphatic vessel dilation was observed at different intensities in all groups. Control explants displayed normal villi lined with columnar enterocytes (Fig. 2, Panel A). Explants exposed to deepoxy-DON (Fig. 2, Panel C) and 3-epi-DON (Fig. 2, Panel D) presented similar features but mild interstitial edema and cell debris on apical surface (arrow) were also observed. By contrast, multifocal to diffuse villi atrophy, multifocal villi fusion (arrows), necrosis of apical enterocytes and cellular debris (arrowhead, Fig. 2, Panel B) were observed after 4 hours of explant incubation with 10 μM of DON.
Figure 2. Comparative effects of deepoxy-DON and 3-epi-DON and DON on morphology of intestinal explants.
Jejunal explants from 4 different animals were exposed for 4 hours, to diluent or 10 μM toxins and stained with HE for histological analysis. Normal villi lined with columnar enterocytes were observed on control explants (Panel A) multifocal villi atrophy (arrow) and cell debris (arrowhead), apical necrosis (insert) on DON explants (Panel B) histological aspects similar to control group on deepoxy-DON (Panel C) or 3-epi-DON (Panel D) explants. Bar 100 μm; insert bar 20 μm.
Deepoxy-DON and 3-epi-DON do not induce intestinal inflammation
To complete the analysis of the intestinal toxicity of deepoxy-DON and 3-epi-DON, their effects on the expression of inflammatory genes were analyzed by RT-qPCR. As already described9, a strong intestinal inflammatory response was observed in jejunal explants in the presence of DON and a significant increase in expression of IL-1α, TNFA, IL-1β, IL-8, IL-12p40, Il-17A and IL-22 was also observed (Table 1). By contrast, no induction in the expression of these genes was observed in deepoxy-DON and 3-epi-DON treated explants, demonstrating that microbial transformation of DON to deepoxy-DON or 3-epi-DON led to decreased inflammatory response in intestinal explants (Table 1).
Table 1. DON but not deepoxy-DON & 3-epi-DON up-regulated mRNA relative expression levels of pro-inflammatory cytokines and chemokines in pig jejunal explants.
| Cytokines | Explant treatments |
|||
|---|---|---|---|---|
| Control | DON | Deepoxy-DON | 3-epi-DON | |
| IL1B | 1.00 ± 0.40a | 17.4 ± 5.1b | 0.7 ± 0.2a | 0.8 ± 0.3a |
| IL1A | 1.00 ± 0.30a | 3.9 ± 1.4b | 0.9 ± 0.2a | 0.9 ± 0.2a |
| IL8 | 1.00 ± 0.20a | 4.5 ± 1.2b | 1 ± 0.1a | 0.9 ± 0.2a |
| IL12p40 | 1.00 ± 0.31a | 2.3 ± 0.4b | 1.2 ± 0.2a | 0.9 ± 0.2a |
| IL17A | 1.00 ± 0.50a | 15.8 ± 5.6b | 0.8 ± 0.1a | 1.3 ± 0.4a |
| IL22 | 1.00 ± 0.30a | 7.9 ± 1.3b | 1.3 ± 0.5a | 1.4 ± 0.5a |
| TNFA | 1.00 ± 0.30a | 3.5 ± 0.5b | 1.1 ± 0.4a | 1.1 ± 0.3a |
Notes: results are expressed in arbitrary units relative to control group. Results are mean ± SEM of 6 animals. Means in a row without a common letter differ (Newman-Keuls test, P < 0.05).
DON but not deepoxy-DON and 3-epi-DON changes gene expression profile in intestinal explants
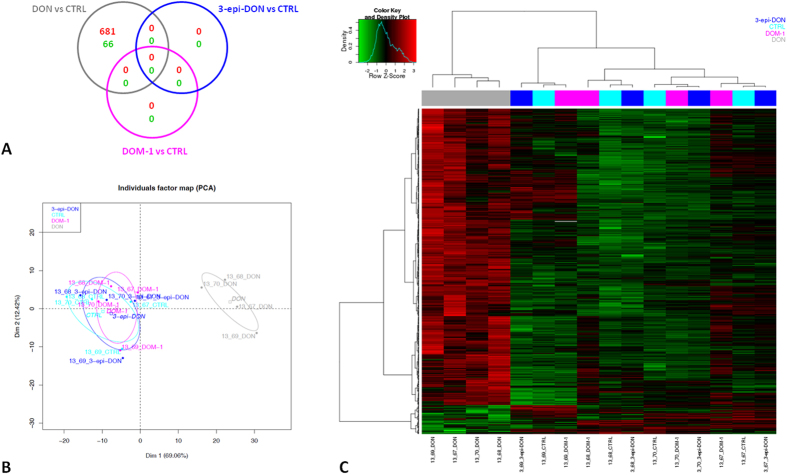
The effects of DON, deepoxy-DON and 3-epi-DON were investigated beyond the inflammatory response, through a genome wide transcriptomic analysis. Exposure to DON resulted in differential expression of 747 probes; 681 and 66 probes corresponding to 303 and 33 genes were up- and down-regulated respectively (Fig. 3, Panel A). By contrast, no genes were differentially expressed in deepoxy-DON and 3-epi-DON treated explants when compared to control explants, indicating that microbial transformation of DON to deepoxy-DON or 3-epi-DON abolishes the toxicity of the mycotoxin.
Figure 3. Gene expression profile of intestinal explants exposed to deepoxy-DON, 3-epi-DON or DON.
Jejunal explants from 4 different animals were exposed for 4 hours, to diluent or 10 μM toxins and gene expression was analyzed with a 60 K microarray. (Panel A): Venn diagram illustrating the overlaps between the probes significantly up- or down-regulated in response to DON, deepoxy-DON (DOM-1) and 3-epi-DON treatment. (Panel B): Principal Component Analysis of differentially expressed probes between DON, deepoxy-DON (DOM-1), 3-epi-DON and control (747 with BH adjusted p-value < 0.05). (Panel C): Heat map representing differentially expressed probes between DON, deepoxy-DON (DOM-1), 3-epi-DON and control explant. Red and green colors indicate values above and below the mean (average Z-score) respectively. Black color indicates values close to the mean.
DON differentially expressed genes were then selected to perform principal component analysis (PCA) (Fig. 3, Panel B) and cluster analysis (Fig. 3, Panel C). Two clusters were distinguished, indicating up-regulated and down-regulated genes (Supplementary Table S2). The most significantly up-regulated genes in DON-treated explants, with a change of more than 2.4 fold compared to control, were immune genes such as CCL20, CXCL2, PRDM1, AREG, CSF2, FOSL1 (Table 2). As expected, the 6 pro-inflammatory cytokines already tested in RT-qPCR analysis were also up-regulated in the DNA array analysis; a strong correlation between the two methods of analysis was observed (coefficient R2 = 0.96). DON also increased the expression of the ER heat shock protein HSP70 gene (HSPA2), genes of ubiquitination pathway (HSPA2, BIRC2, NEDD4L, BIRC3) and genes of metallothioneins (MT1A, MT1M and MT2B). DON decreased expression of the CHAC1 gene, genes for molecular transport including ABCC2, SLC15A1, SLC9A2, the CCL24 gene which is thought to play a role in the immune response, the MLEC gene which is connected to protein misfolding under conditions of endoplasmic reticulum (ER) stress, and other genes (Table 2).
Table 2. Top scored differentially expressed genes in DON treated porcine jejunal explants.
| Gene symbol | Gene name | −log (p-value) | Ratio |
|---|---|---|---|
| a. Up-regulated genes | |||
| IL1B | interleukin 1 beta | 4.428 | 1.29E-11 |
| CCL20 | chemokine (C-C motif) ligand 20 | 3.481 | 1.79E-06 |
| IL1A | interleukin 1. alpha | 3.207 | 6.46E-09 |
| CXCL2 | chemokine (C-X-C motif) ligand 2 | 3.129 | 1.87E-04 |
| IL22 | interleukin 22 | 2.955 | 1.13E-07 |
| PRDM1 | PR domain containing 1 with ZNF domain | 2.793 | 4.76E-06 |
| AREG/AREGB | amphiregulin | 2.662 | 1.94E-11 |
| CSF2 | colony stimulating factor 2 (granulocyte-macrophage) | 2.593 | 1.86E-05 |
| IL8 | interleukin 8 | 2.585 | 1.25E-06 |
| FOSL1 | FOS-like antigen 1 | 2.447 | 4.22E-04 |
| IER3 | immediate early response 3 | 2.446 | 1.95E-04 |
| CCR7 | chemokine (C-C motif) receptor 7 | 2.325 | 1.79E-08 |
| CALCB | calcitonin-related polypeptide beta | 2.313 | 9.03E-11 |
| GADD45A | growth arrest and DNA-damage-inducible alpha | 2.270 | 5.61E-08 |
| TNFAIP3 | tumor necrosis factor alpha-induced protein 3 | 2.260 | 1.36E-08 |
| RND1 | Rho family GTPase 1 | 2.255 | 3.24E-06 |
| IER2 | immediate early response 2 | 2.227 | 3.44E-06 |
| CD83 | CD83 molecule | 2.207 | 1.10E-05 |
| PLAUR | plasminogen activator. urokinase receptor | 2.085 | 9.86E-04 |
| BTG2 | BTG family member 2 | 2.073 | 1.25E-06 |
| IFRD1 | interferon-related developmental regulator 1 | 2.025 | 1.14E-08 |
| RGS1 | regulator of G-protein signaling 1 | 2.020 | 3.24E-06 |
| GEM | GTP binding protein overexpressed in skeletal muscle | 2.013 | 4.52E-05 |
| CCL4 | chemokine (C-C motif) ligand 4 | 2.004 | 6.44E-04 |
| STX11 | syntaxin 11 | 1.989 | 4.27E-05 |
| GADD45G | growth arrest and DNA-damage-inducible gamma | 1.881 | 2.26E-06 |
| GADD45B | growth arrest and DNA-damage-inducible beta | 1.873 | 9.01E-04 |
| NEDD9 | neural precursor cell expressed developmentally down-regulated 9 | 1.870 | 1.15E-10 |
| LAMA3 | laminin. alpha 3 | 1.858 | 2.07E-05 |
| CD274 | CD274 molecule | 1.846 | 8.75E-11 |
| IL17A | interleukin 17A | 1.844 | 2.11E-11 |
| b. Down-regulated genes | |||
| CHAC1 | cation transport regulator homolog 1 (E. coli) | −1.696 | 9.18E-04 |
| ABCC2 | ATP-binding cassette sub-family C (CFTR/MRP) member 2 | −1.015 | 2.45E-06 |
| SLC15A1 | solute carrier family 15 (oligopeptide transporter) member 1 | −0.851 | 3.56E-05 |
| SLC9A2 | solute carrier family 9 subfamily A (NHE2 cation proton antiporter 2) member 2 | −0.804 | 9.09E-06 |
| CCL24 | chemokine (C-C motif) ligand 24 | −0.784 | 8.75E-04 |
| MTTP | microsomal triglyceride transfer protein | −0.755 | 3.26E-05 |
| DMBT1 | deleted in malignant brain tumors 1 | −0.666 | 2.67E-04 |
| MLEC | Malectin | −0.654 | 9.50E-04 |
| SSH1 | slingshot protein phosphatase 1 | −0.628 | 1.06E-03 |
| VPS26B | vacuolar protein sorting 26 homolog B (S. pombe) | −0.610 | 1.04E-03 |
| ACE2 | angiotensin I converting enzyme 2 | −0.607 | 7.35E-04 |
| SCGB2A1 | secretoglobin. family 2A member 1 | −0.594 | 2.74E-04 |
| MYEOV | myeloma overexpressed | −0.592 | 1.58E-04 |
| NPR3 | natriuretic peptide receptor 3 | −0.582 | 8.74E-04 |
| CBL | Cbl proto-oncogene. E3 ubiquitin protein ligase | −0.574 | 3.70E-04 |
| PLOD2 | procollagen-lysine 2-oxoglutarate 5-dioxygenase 2 | −0.547 | 3.98E-05 |
| C4BPA | complement component 4 binding protein Alpha | −0.525 | 1.04E-03 |
| ARHGEF37 | Rho guanine nucleotide exchange factor (GEF) 37 | −0.521 | 9.19E-04 |
| DESI2 | desumoylating isopeptidase 2 | −0.501 | 3.04E-04 |
| STOML3 | stomatin (EPB72)-like 3 | −0.487 | 8.33E-04 |
| UNC119B | unc-119 homolog B (C elegans) | −0.467 | 2.42E-04 |
| ZER1 | zyg-11 related. cell cycle regulator | −0.455 | 4.92E-04 |
| EGLN1 | egl-9 family hypoxia-inducible factor 1 | −0.443 | 2.26E-04 |
| TCAP | titin-cap | −0.441 | 8.90E-04 |
| PECAM1 | platelet/endothelial cell adhesion molecule 1 | −0.431 | 1.43E-04 |
| ZCCHC14 | zinc finger CCHC domain containing 14 | −0.430 | 5.16E-04 |
| GALNT4 | polypeptide N-acetylgalactosaminyltransferase 4 | −0.395 | 6.91E-04 |
| ANKRD13A | ankyrin repeat domain 13A | −0.388 | 8.85E-04 |
| UNC45A | unc-45 homolog A (C. elegans) | −0.377 | 7.64E-04 |
| TPP1 | tripeptidyl peptidase I | −0.375 | 5.37E-04 |
| OSBPL7 | oxysterol binding protein-like 7 | −0.349 | 1.05E-03 |
Pathway analysis of differentially expressed genes exposed to DON was performed using Ingenuity Pathway Analysis software (IPA). The top 10 scored pathways are listed in Table 3, the entire list can be found as Supplementary Table S3. DON disturbed pathways related to immunity/inflammation, such as cytokines regulations (IL-17 axis, IL-10 signaling), leukocytes functions (diapedesis), iNOS and NFkB signaling. DON affected other pathways associated with cell cycle regulation, apoptosis and ER stress response. Moreover, the results underlined the effects of DON on PXR/RXR, FXR/RXR signaling pathways and mitochondrial L-carnitine Shuttle Pathways.
Table 3. Ten top scored canonical pathways differentially regulated in 10 μM DON treated porcine jejuna explants and list of genes in each pathway.
| Ingenuity Canonical Pathways | −log (p-value) | Ratio | Molecules |
|---|---|---|---|
| a. Up-regulated pathways | |||
| Granulocyte Adhesion and Diapedesis | 1.18E01 | 1.1E-01 | CCL3,IL1B,MMP12,EZR,CCL20,CLDN4,CCL3L1/CCL3L3,SELE,MMP13,CXCL2,VCAM1,CXCL8, CXCR4, IL18, IL1RN,TNF,CXCR2,ICCL4,XCL1 |
| Agranulocyte Adhesion and Diapedesis | 1.13E01 | 1.04E-01 | CCL3,IL1B,MMP12,EZR,CCL20,CLDN4,CCL3L1/CCL3L3,SELE,MMP13,CXCL2,VCAM1,CXCL8, CXCR4,IL18,IL1RN,TNF,CXCR2,IL1A,CCL4,XCL1 |
| Glucocorticoid Receptor Signaling | 1.11E01 | 7.69E-02 | HSPA2,NFKB1,CCL3,CSF2,JAK2,IL1B,PLAU,SELE,NFKBIE,NFATC1,NFKBIA,VCAM1,CXCL8, FOS,IL1RN,DUSP1,SGK1,NR3C1,TNF,SMAD3,CDKN1A,IL10,FOXO3 |
| Differential Regulation of Cytokine Production in Intestinal Epithelial Cells by IL-17A and IL-17F | 1.07E01 | 3.91E-01 | CCL3,CSF2,IL1B,TNF,IL1A,IL17A,IL10,CCL4,IL17F |
| Communication between Innate and Adaptive Immune Cells | 1.01E01 | 1.25E-01 | CCR7,CCL3,CSF2,IL1B,CCL3L1/CCL3L3,CD40,CXCL8,CD83,IL18,IL1RN,TNF,IL1A,IL10,CCL4 |
| Differential Regulation of Cytokine Production in Macrophages and T Helper Cells by IL-17A and IL-17F | 1.01E01 | 4.44E-01 | CCL3,CSF2,IL1B,TNF,IL17A,IL10,CCL4,IL17F |
| Hepatic Fibrosis/Hepatic Stellate Cell Activation | 9.55E00 | 1.03E-01 | CCR7,NFKB1,IL1B,EDNRB,MMP13,CD40,VCAM1,CXCL8,IFNGR1,TNF,SMAD3,IL1A,EDN1,IL4R, IL10,TIMP |
| IL-10 Signaling | 9.48E00 | 1.54E-01 | FOS,IL18,NFKB1,IL1RN,IL1B,SOCS3,TNF,IL1A,IL4R,IL10,NFKBIE,NFKBIA |
| T Helper Cell Differentiation | 9.41E00 | 1.67E-01 | GATA3,IFNGR1,BCL6,IL18,STAT4,TNF,ICOSLG/LOC102723996,IL17A,IL4R,IL10,CD40,IL17F |
| Atherosclerosis Signaling | 9.4E00 | 1.08E-01 | NFKB1,IL1B,SELE,MMP13,CD40,VCAM1,CXCL8,CXCR4,IL18,IL1RN,TNF,IL1A,F3,TNFRSF12A, PLA2G4A |
| b. Down-regulated pathways | |||
| PXR/RXR Activation | 2.28E00 | 2.17E-02 | ABCC2, CPT1A |
| FXR/RXR Activation | 2.07E00 | 1.82E-02 | ABCC2, MTTP |
| Mitochondrial L-carnitine Shuttle Pathway | 1.57E00 | 4.55E-02 | CPT1A |
| Granulocyte Adhesion and Diapedesis | 1.49E00 | 1.1E-02 | CCL24, PECAM1 |
| Agranulocyte Adhesion and Diapedesis | 1.44E00 | 1.04E-02 | CCL24, PECAM1 |
| LPS/IL-1 Mediated Inhibition of RXR Function | 1.31E00 | 8.16E-03 | ABCC2, CPT1A |
| Complement System | 1.28E00 | 2.86E-02 | C4BPA |
| Erythropoietin Signaling | 9.87E-01 | 1.27E-02 | CBL |
| Chemokine Signaling | 9.75E-01 | 1.33E-02 | CCL24 |
| Ephrin B Signaling | 9.46E-01 | 1.22E-02 | CBL |
In silico analysis of the interaction of deepoxy-DON and 3-epi-DON with ribosomes and their inability to activate MAPKs
The above data indicate that microbial transformation of DON into deepoxy-DON or 3-epi-DON abrogates its toxicity. The last step of this study was to investigate the underlying mechanism and more specifically to determine the ability of DON, deepoxy-DON and 3-epi-DON to bind to the A site of the ribosome peptidyl transferase center and to activate the MAPKs.
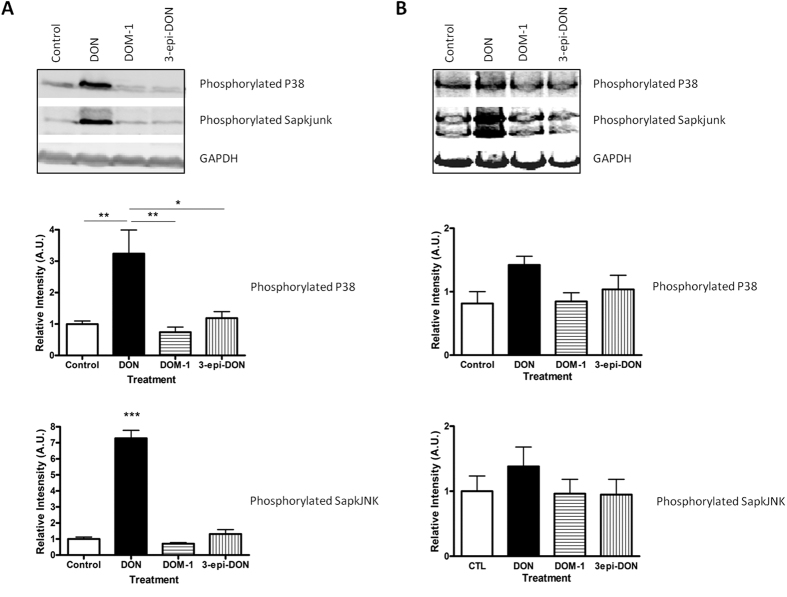
As expected, after 1 hour of exposure to 10 μM DON, MAPKs were activated in both differentiated monolayers Caco-2 cells and jejunal explants (Fig. 4). In cells, DON significantly increased phosphorylated p38 (3.24 ± 0.75 vs. 1 ± 0.09) compared to the control (relative intensity in arbitrary unit (A.U.); p < 0.05; n = 3) and phosphorylated SapKjunk (7.28 ± 0.64 vs. 1 ± 0.12 for control A.U.; p < 0.05; n = 3). By contrast, deepoxy-DON and 3-epi-DON were not able to activate these MAPKs in Caco-2 cells (Fig. 4 panel A). Similar trends were observed in jejunal explants (Fig. 4 panel B).
Figure 4. Effects of deepoxy-DON or 3-epi-DON on activation of MAPK on human intestinal epithelial cells.
(Panel A): Caco-2 cells, differentiated on inserts. (Panel B): Jejunal explants. Samples were treated for 1 h with 10 μM toxins and analyzed by western blot for expression of phosphorylated P38, phosphorylated JNK and GAPDH, used as a protein loading control. Representative immunoblots and normalized expression graph. Results are expressed as mean ± SEM of 3–4 independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001.
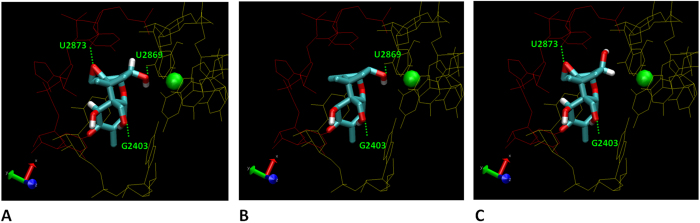
The last step was to investigate the ability of DON and its bacterial metabolites, deepoxy-DON and 3-epi-DON, to bind to the 60S subunit inside the A-site of the peptidyl transferase center of the ribosome. The crystallographic data (4U53.pdb) obtained for DON and yeast ribosomes were used13. As shown in Fig. 5, (panel A) DON is able to fit in the pocket of the A-site of the ribosome 60S subunit. Within the pocket, the 3-hydroxyl group of DON is associated with a magnesium atom and stabilized by other nucleotides. In this position, DON forms 3 hydrogen bonds with the A-site. The first one is between the oxygen of the DON epoxy group on C12 and one hydrogen of the sugar of the uracil U2873; the second one is between the oxygen of the C15 group CH2OH and one hydrogen of the guanine basis G2403; and the last one is between the hydrogen of the C3 group and one oxygen of the uracil U2869. The in silico analysis revealed that both deepoxy-DON and 3-epi-DON were also able to fit into the pocket of the peptidyl transferase center of the ribosome (Fig. 5, Panel B and C). However, because of the absence of the epoxy group or the isomeric change, these two metabolites were only able to form 2 hydrogen bonds with the A sites of the peptidyl transferase center. Deepoxy-DON and 3-epi-DON did not form the bond with U2873 and U2869, respectively.
Figure 5. Interaction between the Ribosome 60S PTC subunit binding site and deepoxy-DON, 3-epi-DON or DON.
Both sides of A site of the yeast ribosome 60S PTC subunit are colored in red and yellow respectively. Hydrogen and oxygen atoms are represented in white and red respectively. (Panel A): detailed views of the co-crystal (4UJX) of DON inside the A-site. (Panel B): detailed view of deepoxy-DON modeling inside the A-site. (Panel C): detailed views of 3-epi-DON modeling inside the A-site. The magnesium atom inside the A-site pocket has been pointed out in green.
Discussion
Despite good agricultural practices, contamination by mycotoxins cannot be avoided. Several strategies have been developed to reduce mycotoxin exposure. Among them, microbial transformation is of interest but requires demonstration of the absence of toxicity of the metabolites produced. The aims of the present study were (i) to analyze the intestinal toxicity of two bacterial metabolites of DON, deepoxy-DON and 3-epi-DON and (ii) to investigate the molecular basis for their reduced toxicity.
Through the action of bacteria, DON can be epimerized on the hydroxyl group of C3 or de-epoxidized on the C12-C13 epoxide19. Epimerization is an aerobic irreversible transformation that may require two enzymatic activities of partially overlapping substrate specificity which occur together in sequence: oxidation of DON to 3-keto-DON and then conversion to 3-epi-DON. To date only four bacterial strains have been described to epimerize DON to 3-epi-DON19. A very recent paper shows that this bacterial metabolite is substantially less toxic than DON when tested in vitro on proliferating human Caco2 cells, as well as in vivo when given orally to mice for 14 days22. Deepoxy-DON is obtained after de-epoxidation of DON; several bacterial species and enzymes are able to catalyze this reaction19. The lower toxicity of deepoxy-DON, as compared to DON has been demonstrated in vitro on Swiss mouse 3T3 fibroblasts16, lymphocytes17 and brine shrimp25. In vivo supplementation of DON-contaminated feed, with bacteria and/or an enzyme able to de-epoxidize DON, induced a reduction of the toxicity as shown by measurement of zootechnical or immune parameters18,26,27.
In the present study, we observed reduced intestinal toxicity of 3-epi-DON and deepoxy-DON when compared to DON. The toxicity of DON and its bacterial metabolites was first investigated on proliferating and differentiated Caco-2 cells. As already demonstrated, DON induced a significant decrease in Caco-2 cell proliferation, reduced their barrier function and altered their respiratory capacities28,29,30. This is the first investigation of the toxicity of deepoxy-DON on human intestinal epithelial cells, although the absence of toxicity of 3-epi-DON on Caco-2 cells has been recently demonstrated22.
The toxicity of DON and its bacterial metabolites on intestinal tissues was further evaluated. Because of the difficulties accessing human intestinal samples, the study was performed on porcine intestinal explants. Indeed, pigs are very sensitive to DON and can be considered good models for extrapolation to humans, with a digestive physiology very similar to that of humans8,31. Histological assessment showed normal villi lined with columnar enterocytes, mild interstitial edema and cell debris on the apical surface for the control, 3-epi-DON and deepoxy-DON treated explants. This is in accordance with the absence of histopathological lesions observed in mice after a 14 day oral exposure to 25 or 100 mg 3-epi-DON/Kg bw (body weight)22. Effects of purified deepoxy-DON on the intestine have never been tested, however nutritional strategies including bacteria/enzyme transforming DON to deepoxy-DON have reduced the occurrence and extent of intestinal lesions18 and showed the same zootechnical performance as in control animals26,27. By contrast, as already shown, treatment with 10 μM of DON induces intestinal damage indicated by villi atrophy and villi fusion24. To confirm that the two microbial transformation products of DON were not toxic, a pan-genomic analysis using a DNA array containing 62,976 probes was performed on jejunal explants. It revealed that no probes were differentially expressed between control explants and the ones treated with either deepoxy-DON or 3-epi-DON. To the best of our knowledge this is the first genome wide analysis performed for deepoxy-DON and 3-epi-DON.
The global transcriptomic analysis of the effect of DON on the intestine indicated that DON does not only interfere with genes involved in the immune response. As already described for human and murine thymus cells32,33,34, DON exposure also targets ER (endoplasmatic reticulum) stress, protein synthesis, oxidative stress, cell cycle regulation and apoptosis in intestinal tissues. The strong alteration of the gene MLEC implicated in misfolded glycoprotein quality control observed herein is likely due to the arrest of translation induced by ribotoxic stress. This leads to less protein entering the ER to temper the unfolded protein response and therefore protein synthesis33. The increased gene expression of the ER heat shock protein HSP70 could also reduce the accumulation of unfolded protein in ER lumen. An increased expression of some genes involved in the ubiquitination pathway was observed in the presence of DON. This result could indicate that the presence of DON may induce the increase in proteins involved in protein degradation33,35,36. Our data also underline the decrease of the unfolded protein response pro-apoptotic gene CHAC1. The CHAC1 protein seems to play a role in glutathione degradation37. ER stress could also induce leakage of calcium from the reticulum leading to activation of NFkB, NRF2-mediated oxidative stress response and apoptosis33. The present work emphasizes the effect of DON on metallothioneins MT1A, MT1M and MT2B. A relationship between metallothionein protein levels, used as a marker of oxidative stress, and mycotoxins in the liver of rats fed on naturally contaminated wheat has been reported38. Therefore, it could be assumed that MTs are associated with pathways protecting the intestine against DON toxicity. The present study underlines the effect of DON on the genes of intestinal transporters. DON decreases the expression of the solute carrier SLC15A1 and SLC9A2 involved in proton-coupled oligopeptides transporter PepT1 and a Na+/H+ exchanger, respectively39,40. Similar effects on other mRNA expression transporters as sugars transporters were described in the jejunum and to a lesser extent in the liver of broilers exposed to DON41. Accordingly, it has been experimentally shown that DON decreases the intestinal uptake of various nutrients in human epithelial intestinal cell line HT-29-D442. This effect is likely due to a specific modulation of intestinal transporters expression, rather than a consequence of cell damage. The transcriptomic analysis demonstrates that DON down-regulates the expression of ABCC2 gene that encodes for MRP2, a protein involved in efflux of DON and other mycotoxins and also in the transport of a wide range of organic anions including bile salt flow43. An action of DON on mitochondrial dysfunction, attested to by the down-regulation of CPT1A mRNA was also observed in this study. CPT1A encodes for a key regulatory enzyme of β-oxidation and is required for transport of long chain fatty acids into mitochondria44. The modulation of β-oxidation in addition to the modulation of intestinal transporters could explain the energy failure reported after DON exposure42. It is now necessary to investigate these changes at the protein level.
The use of bacteria is a promising approach to DON decontamination. In the present study we observed that deepoxy-DON and 3-epi-DON were devoid of intestinal toxicity. The underlying mechanism was further investigated. DON is known to develop its toxic potential by interacting with the peptidyl transferase at the 60S ribosomal subunit level, blocking the protein synthesis at the elongation step, inducing a ribotoxic stress and activating MAPKinases7,12,13. In accordance with literature, we observed that DON induced phosphorylation of JNK and p38 proteins24,45. By contrast, deepoxy-DON and 3-epi-DON did not active these signaling pathways, suggesting an absence of ribotoxic stress. To further the analysis, a modeling of deepoxy-DON and 3-epi-DON in the ribosome peptidyl transferase center was performed. DON and its bacterial metabolites fit into the A-site pocket, however whereas DON binds to the peptidyl transferase center with three hydrogen bonds, only two hydrogen bonds were identified between deepoxy-DON or 3-epi-DON and the peptidyl transferase center. This suggests that the absence of the epoxy group or the isomeric transformation decreases the affinity of these latter metabolites for the active site pocket A of the ribosome and prevents the induction of ribotoxic stress. In silico modeling revealed that a third hydrogen bond (the one between the oxygen of the C15 group CH2OH and the hydrogen of the guanine base G2403) could be involved in the interaction of DON with the ribosome. It would be of interest to establish whether this hydrogen bond is necessary for the toxicity of DON. Unfortunately we were not able to identify a proper DON metabolite or another fusariotoxin metabolite to confirm the involvement of this hydrogen bond in the structure-toxicity relationship. A more thorough analysis, with additional trichothecenes would be needed to fully determine the molecular basis of toxicity.
In conclusion, the present study confirms that the toxicity of DON is not only linked to the epoxy group but is also influenced by the C3 group16,19,46. It demonstrates that microbial biotransformation of DON into deepoxy-DON or 3-epi-DON decreases the intestinal toxicity of this mycotoxin. The underlying metabolism causes decreased affinity of the metabolites to the ribosome and the lack of MAPKinases activation. These data significantly increase the current knowledge of intestinal toxicity of DON, deepoxy-DON and 3-epi-DON and contribute to the evaluation of the effectiveness of the microbial biotransformation strategies in the fight against mycotoxins.
Methods
Toxins
Purified DON was purchased from Sigma-Aldrich (St Louis, MO, USA). Deepoxy-DON was obtained by transformation of crystalline DON (Romer Labs, Tulln, Austria), dissolved in medium 1047 at a concentration of 2 mg/ml, by inoculation with BBSH 797, Gen. nov. sp. nov. of family Coriobacteriaceae in sterile medium, at 37 °C for six days. Biotransformation of DON to deepoxy-DON was confirmed by LC-MS/MS, and deepoxy-DON was purified by solid phase extraction and preparative HPLC48. The purity of the deepoxy-DON preparation was 99%, based on chromatograms recorded at 220 nm. The identity of deepoxy-DON was confirmed by comparison of enhanced product ion spectra of the preparation used in the present study with a reference standard (Romer Labs, Tulln, Austria) recorded on a 4000 QTrap mass spectrometer at a collision energy of 30 eV (Supplementary Figure S2).
3-epi-DON was produced by microbial transformation of DON21. Briefly, DON was co-incubated with the bacterial strain, Devosia mutans 17-2-E-8, in corn meal broth medium at 28 °C for 48 h. High-speed counter-current chromatography (HSCCC) and preparative high performance liquid chromatography (prep-LC) were applied to separate 3-epi-DON. The obtained product was analyzed by liquid chromatography (LC) and identified by congruent retention time and UV/Vis spectrum and mass spectrometric (MS) data. Nuclear magnetic resonance (NMR) experiments such as correlation spectroscopy (COSY), heteronuclear single quantum coherence (HSQC) and nuclear overhauser effect (NOE) were conducted for structural characterization of 3-epi-DON. Purification and structure identification of 3-epi-DON was already demonstrated in a previous publication21. The 3-epi-DON used in the experiment had a purity of 96.8%.
Toxins were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) and stored at −20 °C until use.
Caco-2 cell culture
Caco-2 cells (passages 99 - 106) obtained from the TC7 were cultured in 75-cm2 culture flasks (Cellstar cell culture flasks, Sigma-Aldrich) in Dulbecco’s Modified Eagle Medium enriched with glutamine (Gibco, Cergy-Pontoise, France), supplemented with 10% of heat inactivated fetal bovine serum, 0.5% of gentamycine (Eurobio, Courtaboeuf, France) and 1% of non-essential amino acids (Sigma-Aldrich). Cells were maintained at 37 °C in an atmosphere of 5% CO2 and 90% relative humidity. The medium was changed every 2 days. Cells were passaged once a week. The partially confluent cell monolayers were trypsinized with Trypsin-EDTA (Eurobio).
Cell viability assay
Cell viability assay was performed with the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, USA) according to manufacturer’s instruction. This test measures the quantity of ATP, proportional to the quantity of cells. Cells were seeded at the density of 1.56 × 105 cells/cm2 in 96-well microtiter plates. Cells were grown for 24 hours and exposed to DON, deepoxy-DON or 3-epi-DON, or corresponding concentrations of DMSO, for 48 hours. Luminescence was measured with a spectrophotometer (TECAN Infinite M200, Männedorf, Switzerland).
Trans-epithelial electrical resistant measurements
To assess the integrity of individual monolayers, trans-epithelial electrical resistance (TEER) was measured as already described49,50. Cells (1.34 × 105 cells/cm2) were grown until differentiation on polyethylene terephthalate membrane inserts (surface area 0.3 cm2, pore size 0.4 μm) in 24-well format (Becton Dickinson, Pont de Claix, France). The medium was changed every two days. Differentiated cells were exposed to 10 μM of diluent or toxins, DON, deepoxy-DON or 3-epi-DON. The culture medium in the apical side of differentiated cells in each well was replaced every two days with medium containing toxin. The TEER was measured for each well daily for 11 days using a Millicell-ERS Voltohmmeter (Millipore, Saint-Quentin-en-Yvelines, France). TEER values were expressed as % of initial values.
Oxygen consumption measurements
The acute effect of toxins on oxygen consumption of Caco-2 cells was assessed using an XF24 extracellular flux analyzer (Seahorse Bioscience, North Billerica, USA). The procedure was performed according to the manufacturer’s instructions. Briefly, cells were cultured in XF24 cell culture microplates at 1.5 × 104 cells per well and maintained as described above. Oxygen consumption rates (OCR) were measured in proliferated Caco-2 cells in non-buffered DMEM (Seahorse Bioscience) supplemented with 10 mM glucose, 2 mM sodium pyruvate (Sigma-Aldrich) and 2 mM glutamine (Eurobio) and adjusted to pH 7.4. OCR was monitored every 20 minutes before (basal level) and after injection of diluent or toxins (10 μM). OCR values from each well were normalized against viable cell counts (calculated with a Malassez cell) and expressed as a percentage of the baseline value.
Intestinal jejunal explants
Jejunal explants were obtained from 5 week old crossbred castrated male piglets as described previously24. The experiment was conducted under the guidelines of the French Ministry of Agriculture for animal research. All animal experimentation procedures were approved by the Ethics committee of Pharmacology-Toxicology of Toulouse-Midi-Pyrénées in animal experimentation (Toxcométhique), N°: TOXCOM/0017/IO PP, in accordance with the European Directive on the protection of animals used for scientific purposes (Directive 2010/63/EU). Two authors (I.P.O. and A.P.) have an official agreement with the French Veterinary Services permitting animal experimentation. Explants were treated for 4 hours at 39 °C with 10 μM of toxins (DON, deepoxy-DON or 3-epi-DON) or diluent (DMSO) in complete medium. After incubation, treated explants were fixed in 10% formalin (Sigma-Aldrich) for histological analysis or stored at −80 °C for RNA extraction.
Histological assessment
Explants fixed with 10% formalin for 24 hours were dehydrated and embedded in paraffin wax (Labonord, Templemars, France) according to standard histological procedures. Sections of 5 μm were stained with haematoxylin and eosin (Sigma-Aldrich) for histopathological assessment. Histological findings were scored based on histological changes and the severity of lesions as previously described24.
Gene expression analysis of explants by RT-qPCR
For the gene expression analysis, total RNAs were extracted in lysing matrix D tubes (MP Biomedicals, Illkirch, France) containing guanidine thiocyanate-acid phenol (Eurobio). The quality of these RNA was assessed (Agilent RNA 6000 Nano Kit Quick, Agilent Bioanalyzer 2100); the mean RNA Integrity Number (RIN) of these mRNA preparations was 6.32 ± 0.83.
Reverse transcription and RT-qPCR steps were performed as already described51,52 with previously published primers (Supplementary Table S1)23. Amplification efficiency and initial fluorescence were determined by the ΔCt method. Obtained values were normalized using two reference genes, ribosomal protein L32 (RPL32) and cyclophilin A (CycloA). Gene expression levels of treated explants were expressed relative to the mean of the control explants.
Gene expression analysis by microarray
The microarray GPL16524 (Agilent technology, 8 × 60 K) used in this experiment consisted of 43,603 spots derived from the 44 K (V2:026440 design) Agilent porcine specific microarray. This was enhanced with 9,532 genes from adipose tissue, 3,776 genes from the immune system and 3,768 genes from skeletal muscle23,53. For each sample, Cyanine-3 (Cy3) labeled cRNA was prepared from 200 μg of total RNA using the One-Color Quick Amp Labeling kit (Agilent) according to the manufacturer’s instructions, followed by Agencourt RNAClean XP (Agencourt Bioscience Corporation, Beverly, Massachusetts). About 600 ng of Cy3-labelled cRNA were hybridized on SurePrint G3 Porcine GE microarray (8×60 K) following the manufacturer’s instructions. Slides were scanned immediately, after washing on an Agilent G2505C Microarray Scanner with Agilent Scan Control A.8.5.1 software. All experimental details are available in the Gene Expression Omnibus (GEO) database under accession GSE66918 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=olixoose dvmdnqr&acc=GSE66918).
The differentially expressed (DE) genes (adjusted p-value ≤ 0.05) were hierarchically clustered and visualized in heat maps. Functional analysis of DE genes was performed using the Ingenuity pathway Analysis tool (IPA, http://www.ingenuity.com) to identify pathways and processes affected by toxins.
Immunoblotting
Expression of the phosphorylated MAPK p38 and JNK (Jun amino-terminal kinases) was assessed on differentiated Caco-2 cells and jejunal explants by immunoblotting as previously described49,54. Cells differentiated on 24-well inserts or explants were treated with 10 μM of diluent (DMSO) or toxins, DON, deepoxy-DON or 3-epi-DON for 1 hour. Proteins were extracted, quantified and a total of 15 μg of protein was separated by SDS-PAGE. The membranes were probed with rabbit antibodies (Cell Signaling Technology, Danvers, USA) specific for: phospho-SPAK/JNK or phospho-p38 diluted at 1:500 or GAPDH diluted at 1:1000. After washing, the membranes were incubated with 1:10,000 CFTM770 goat anti-rabbit IgG (Biotium, Hayward, USA) for the detection. Antibody detection was performed using an Odyssey Infrared Imaging Scanner (Li-Cor Science Tec, les Ulis, France) with the 770 nm channel. The expression of the proteins was estimated after normalization with GAPDH signal.
Molecular modeling
The yeast 80S ribosome X-ray structure, determined at a resolution of 3.3 Å (PDB code 4U5313), was used in all docking calculation. The whole chain A of the ribosome peptidyl transferase center was taken into account. The atomic coordinates of the PDB file were used for docking calculations using NAMD under VMD1.955. VMD was used as visualization tools for various tasks (alignments, ligands location). The molecular structures of deepoxy-DON and 3-epi-DON were built using molefacture from the DON structure included in PDB-file 4U53, after adding hydrogen and after verification of the chirality and the type of each atoms. Ligand structures were minimized by NAMD using AMBER force field topology and parameter files. Molecular docking experiments of deepoxy-DON and 3-epi-DON were done in VMD based on the superposition of the carbon backbone of DON. To relax structures and to evaluate the interaction of hydrogen bonds between ribosome and ligand, a semi-rigid energy minimization was calculated by NAMD using AMBER topology and parameter files with a fixed ribosome structure and a flexible structure for the ligand. Associated to PDB-file 4U53 of DON, PDB-file 4U53 including structures of deepoxy-DON and 3-epi-DON are available in Supplementary Data (SupplementaryData_S1_4U53wDON, Supplementary Data_S2_4U53wdeepoxyDON and SupplementaryData_S3_4U53w3EpiDON).
Statistical analysis
Data are expressed as a mean ± SEM of values. The results were analyzed using the Fisher test on equality of variances, one-way ANOVA and Bonferroni as a test post-hoc; p-values < 0.05 were considered significant. Microarray data from Feature Extraction software was analyzed with R using Bioconductor packages and the limma lmFit function as previously described23. Probes with adjusted p-value ≤ 0.05 were considered differentially expressed between treated and control conditions. Hierarchical clustering was applied to the samples and the probes using 1-Pearson correlation coefficient as distance and Ward’s criterion for agglomeration.
Additional Information
How to cite this article: Pierron, A. et al. Microbial biotransformation of DON: molecular basis for reduced toxicity. Sci. Rep. 6, 29105; doi: 10.1038/srep29105 (2016).
Supplementary Material
Acknowledgments
The authors are grateful to A.M. Cossalter, J. Laffitte and P. Pinton (INRA Toxalim) for sample collection and western-blot experiments. The authors thank Drs L. Huc (INRA, Toxalim), F. Smih-Rouet and M. Barutaut (I2MC INSERM UMR 1048) for Bioenergetic measurements and H.E. Schwartz-Zimmermann for preparation and characterization of deepoxy-DON. Thanks are also due to C.A. Moll for language editing. A. Pierron and L.S. Murate were supported by fellowships from CIFRE (2012/0572, jointly financed by the BIOMIN Holding GmbH, Association Nationale de la Recherche Technique and INRA) and CAPES (Brazil) respectively.
Footnotes
Author Contributions A.P. and S.M. performed in vitro, ex vivo, Q-PCR experiments and IPA analysis. A.P., F.L.B. and L.S.M. performed histological analysis. L.S.M. performed western analysis and helped with in vitro experiments. Y.L. and N.L. performed microarray analysis and in silico modeling respectively. W.-D.M. and G.S. characterized, purified and provided the deepoxy-DON, J.W.H. and T.Z. characterized, purified and provided the 3-epi-DON. I.P.O., G.S. and W-D.M. provided conception and design of research. A.P., S.M. and I.P.O. wrote the manuscript.
References
- Bennett J. W. & Klich M. Mycotoxins. Clin Microbiol Rev 16, 497 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad J. C., Nielsen K. F. & Samson R. A. Recommendations concerning the chronic problem of misidentification of mycotoxigenic fungi associated with foods and feeds. Adv Exp Med Biol 571, 33 (2006). [DOI] [PubMed] [Google Scholar]
- Awad W. A., Ghareeb K., Bohm J. & Zentek J. Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 27, 510 (2010). [DOI] [PubMed] [Google Scholar]
- EFSA, Deoxynivalenol in food and feed: occurrence and exposure. EFSA Journal 11, 3379 (2013). [Google Scholar]
- CAST, Mycotoxins: Risks in Plant, Animal, and Human Systems. Council for Agricultural Science and Technology-Potential economic costs of mycotoxins in United States Task Force Rep. 138 (Iowa, USA), 136 (2003). [Google Scholar]
- Pestka J. J. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84, 663 (2010). [DOI] [PubMed] [Google Scholar]
- Maresca M. From the gut to the brain: journey and pathophysiological effects of the food-associated mycotoxin Deoxynivalenol. Toxins 5, 784 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P. & Oswald I. P. Effect of deoxynivalenol and other Type B trichothecenes on the intestine: a review. Toxins 6, 1615 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano P. M. et al. Deoxynivalenol as a new factor in the persistence of intestinal inflammatory diseases: an emerging hypothesis through possible modulation of Th17-mediated response. PLoS One 8, e53647 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke V. et al. The mycotoxin deoxynivalenol potentiates intestinal inflammation by salmonella typhimurium in porcine ileal loops. PLoS One 6, e23871 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin V. I. & Anderson P. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J Biol Chem 274, 13985 (1999). [DOI] [PubMed] [Google Scholar]
- Pestka J. J., Zhou H. R., Moon Y. & Chung Y. J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unraveling a paradox. Toxicol Lett 153, 61 (2004). [DOI] [PubMed] [Google Scholar]
- Garreau de Loubresse N. et al. Structural basis for the inhibition of the eukaryotic ribosome. Nature 513, 517 (2014). [DOI] [PubMed] [Google Scholar]
- Pestka J. J. Deoxynivalenol-induced proinflammatory gene expression: mechanisms and pathological sequelae. Toxins 2, 1300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., He J. & Gong J. Microbial transformation of trichothecene mycotoxins. World Mycotoxin J 1, 23 (2008). [Google Scholar]
- Sundstol Eriksen G., Pettersson H. & Lundh T. Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food Chem Toxicol 42, 619 (2004). [DOI] [PubMed] [Google Scholar]
- Schatzmayr G. et al. Microbiologicals for deactivating mycotoxins. Mol Nutr Food Res 50, 543 (2006). [DOI] [PubMed] [Google Scholar]
- Grenier B. et al. Biotransformation approaches to alleviate the effects induced by fusarium mycotoxins in swine. J Agric Food Chem 61, 6711 (2013). [DOI] [PubMed] [Google Scholar]
- Karlovsky P. Biological detoxification of the mycotoxin deoxynivalenol and its use in genetically engineered crops and feed additives. Appl Microbiol Biotechnol 91, 491 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiller F. et al. Masked mycotoxins: a review. Mol Nutr Food Res 57, 165 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. W. et al. An epimer of deoxynivalenol: purification and structure identification of 3-epi-deoxynivalenol. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32, 1523 (2015). [DOI] [PubMed] [Google Scholar]
- He J. W. et al. Toxicology of 3-epi-deoxynivalenol, a deoxynivalenol-transformation product by Devosia mutans 17-2-E-8. Food Chem Toxicol 84, 250 (2015). [DOI] [PubMed] [Google Scholar]
- Pierron A. et al. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-beta-D-glucoside. Arch Toxicol in press (2016). [DOI] [PubMed] [Google Scholar]
- Lucioli J. et al. The food contaminant deoxynivalenol activates the mitogen activated protein kinases in the intestine: Interest of ex vivo models as an alternative to in vivo experiments. Toxicon 66, 31 (2013). [DOI] [PubMed] [Google Scholar]
- Swanson S. P., Rood H. D. Jr., Behrens J. C. & Sanders P. E. Preparation and characterization of the deepoxy trichothecenes: deepoxy HT-2, deepoxy T-2 triol, deepoxy T-2 tetraol, deepoxy 15-monoacetoxyscirpenol, and deepoxy scirpentriol. Appl Environ Microbiol 53, 2821 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. Z. et al. Efficacy of detoxification of deoxynivalenol-contaminated corn by Bacillus sp. LS100 in reducing the adverse effects of the mycotoxin on swine growth performance. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 28, 894 (2011). [DOI] [PubMed] [Google Scholar]
- He P., Young L. G. & Forsberg C. Microbially detoxified vomitoxin-contaminated corn for young pigs. J Anim Sci 71, 963 (1993). [DOI] [PubMed] [Google Scholar]
- Alassane-Kpembi I. et al. New insights into mycotoxin mixtures: The toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol Appl Pharmacol 272, 191 (2013). [DOI] [PubMed] [Google Scholar]
- Akbari P. et al. Deoxynivalenol: a trigger for intestinal integrity breakdown. FASEB J 28, 2414 (2014). [DOI] [PubMed] [Google Scholar]
- Bin-Umer M. A. et al. Elimination of damaged mitochondria through mitophagy reduces mitochondrial oxidative stress and increases tolerance to trichothecenes. Proc Natl Acad Sci USA 111, 11798 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejdfors P., Ekelund M., Jeppsson B. & Westrom B. R. Mucosal in vitro permeability in the intestinal tract of the pig, the rat, and man: species- and region-related differences. Scand J Gastroenterol 35, 501 (2000). [DOI] [PubMed] [Google Scholar]
- van Kol S. W., Hendriksen P. J., van Loveren H. & Peijnenburg A. The effects of deoxynivalenol on gene expression in the murine thymus. Toxicol Appl Pharmacol 250, 299 (2011). [DOI] [PubMed] [Google Scholar]
- Katika M. R. et al. Transcriptome analysis of the human T lymphocyte cell line Jurkat and human peripheral blood mononuclear cells exposed to deoxynivalenol (DON): New mechanistic insights. Toxicol Appl Pharmacol 264, 51 (2012). [DOI] [PubMed] [Google Scholar]
- Mishra Sakshi, Dwivedi, Premendra D., Pandey, Haushila P. & Das Mukul Role of oxidative stress in Deoxynivalenol induced toxicity. Food Chem Toxicol 72, 20 (2014). [DOI] [PubMed] [Google Scholar]
- Shen Y. et al. ER stress differentially regulates the stabilities of ERAD ubiquitin ligases and their substrates. Biochem Biophys Res Commun 352, 919 (2007). [DOI] [PubMed] [Google Scholar]
- Osman A. M. et al. Protein expression profiling of mouse thymoma cells upon exposure to the trichothecene deoxynivalenol (DON): implications for its mechanism of action. J Immunotoxicol 7, 147 (2010). [PubMed] [Google Scholar]
- Kumar A. et al. Mammalian proapoptotic factor ChaC1 and its homologues function as gamma-glutamyl cyclotransferases acting specifically on glutathione. EMBO Rep 13, 1095 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasatkova A. et al. Changes in metallothionein level in rat hepatic tissue after administration of natural mouldy wheat. Int J Mol Sci 10, 1138 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein C. et al. Tissue distribution of Na+/H+ exchanger isoforms NHE2 and NHE4 in rat intestine and kidney. Am J Physiol 273, C1496 (1997). [DOI] [PubMed] [Google Scholar]
- Smith D. E., Clemencon B. & Hediger M. A. Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol Aspects Med 34, 323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich B., Neuenschwander S., Bucher B. & Wenk C. Fusarium mycotoxin-contaminated wheat containing deoxynivalenol alters the gene expression in the liver and the jejunum of broilers. Animal 6, 278 (2012). [DOI] [PubMed] [Google Scholar]
- Maresca M., Mahfoud R., Garmy N. & Fantini J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J Nutr 132, 2723 (2002). [DOI] [PubMed] [Google Scholar]
- Videmann B., Tep J., Cavret S. & Lecoeur S., Epithelial transport of deoxynivalenol: involvement of human P-glycoprotein (ABCB1) and multidrug resistance-associated protein 2 (ABCC2). Food Chem Toxicol 45, 1938 (2007). [DOI] [PubMed] [Google Scholar]
- Nakamura M. T., Yudell B. E. & Loor J. J. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res 53, 124 (2014). [DOI] [PubMed] [Google Scholar]
- Sergent T. et al. Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations. Toxicol Lett 164, 167 (2006). [DOI] [PubMed] [Google Scholar]
- Sato I. & Ueno Y. Comparative toxicities of trichothecenes. In Rodricks J. V., Hesseltine C. W., Mehlman M. A. (eds) Mycotoxins in human and animal health Pathotox Publishers, Park Forest South, IL, 295 (1977). [Google Scholar]
- Caldwell D. R. & Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol 14, 794 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz-Zimmermann H. E. et al. Deoxynivalenol (DON) sulfonates as major DON metabolites in rats: from identification to biomarker method development, validation and application. Anal Bioanal Chem 406(30), 7911 (2014). [DOI] [PubMed] [Google Scholar]
- Pinton P. et al. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol Sci 130, 180 (2012). [DOI] [PubMed] [Google Scholar]
- Loiseau N. et al. Fumonisin B1 exposure and its selective effect on porcine jenunal segment: sphingolipids, glycolipids and transepithelial-passage disturbance. Biochem. Pharmacol. 74, 144 (2007). [DOI] [PubMed] [Google Scholar]
- Halloy D. J., Gustin P. G., Bouhet S. & Oswald I. P. Oral exposure to culture material extract containing fumonisins predisposes swine to the development of pneumonitis caused by Pasteurella multocida. Toxicology 213, 34 (2005). [DOI] [PubMed] [Google Scholar]
- Gourbeyre P. et al. Pattern recognition receptors in the gut: analysis of their expression along the intestinal tract and the crypt/villus axis. Physiol Rep 3(2), e12225 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. et al. Transcriptome analysis of porcine PBMC after stimulation by LPS or PMA/ionomycin using an expression array targeting the pig immune response. BMC genomic 11, 292 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissonnier G. M. et al. Subclinical doses of T-2 toxin impair acquired immune response and liver cytochrome P450 in pigs. Toxicology. 247, 46 (2008). [DOI] [PubMed] [Google Scholar]
- Humphrey W., Dalke A. & Schulten K. VMD: visual molecular dynamics. J Mol Graph 14, 33 (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.