Extracellular vesicles (EVs) are produced by virtually all cell types. Within the past few years, work in this field has revealed more information about fungal EVs. Fungal EVs have been shown to carry proteins, lipids, pigments, polysaccharides, and RNA; these components are known virulence factors, a fact which supports the hypothesis that fungal EVs concentrate pathogenic determinants.
KEYWORDS: Fungal pathogenesis, vesicles, ceramide, fungi, glycolipids, sphingolipids
ABSTRACT
Extracellular vesicles (EVs) are produced by virtually all cell types. Within the past few years, work in this field has revealed more information about fungal EVs. Fungal EVs have been shown to carry proteins, lipids, pigments, polysaccharides, and RNA; these components are known virulence factors, a fact which supports the hypothesis that fungal EVs concentrate pathogenic determinants. Additionally, recent studies have demonstrated that fungal EVs stimulate the host immune system. In this review, putative roles of fungal EVs are discussed, including their potential as vaccination tools and their possible contribution to pathogenesis in invasive fungal diseases.
INTRODUCTION
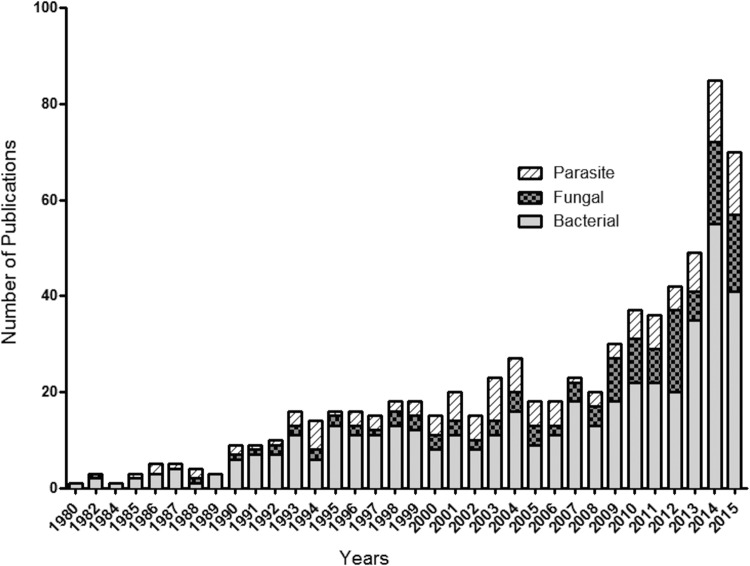
Extracellular vesicles (EVs) are membranous compartments that are released by all living cells investigated so far. EVs play important roles in nutrition, physiopathogenesis, and cell-to-cell communication (1). EVs are now considered key mediators of immunopathogenesis in bacteria, fungi, and protozoa, which has encouraged research activity in this field (Fig. 1). Currently, the majority of the literature concerning EVs is specific to bacterial cells.
FIG 1 .
Numbers of publications (peer-reviewed articles, book chapters, reviews, notes, conference reviews) related to EVs produced by bacterial, fungal, and parasite organisms. The Scopus database was used to track microbial EV-related publications from 1980 to 2015. The keywords “fungal/fungi/yeast extracellular vesicles,” “bacterial/bacteria extracellular vesicles,” and “parasite/protozoan/protozoa extracellular vesicles” were used for article searches in titles, abstracts, and keywords.
EVs are composed of lipid bilayers, and they range in size from 30 to 1,000 nm (2–5). The dimensions and composition of EVs are determined by their mechanisms of biogenesis, which are classified into compartments: exosomes, microvesicles (also called ectosomes), and apoptotic bodies (6). Exosomes originate from the endocytic pathway and are the smallest vesicles, being approximately 30 to 100 nm in size. Microvesicles are released from cells through membrane shedding, and their dimensions range from 100 to 1,000 nm (6, 7). Lastly, apoptotic bodies are shed from the plasma membrane of cells during programmed death and usually are the largest EVs, ranging from 800 to 1,000 nm in size (8). This review discusses the relationship between the composition and biological functions of fungal EVs and addresses the roles of EVs in fungal pathogenesis and their potential biological activity as therapeutic and prophylactic tools.
MICROBIAL EXTRACELLULAR VESICLES
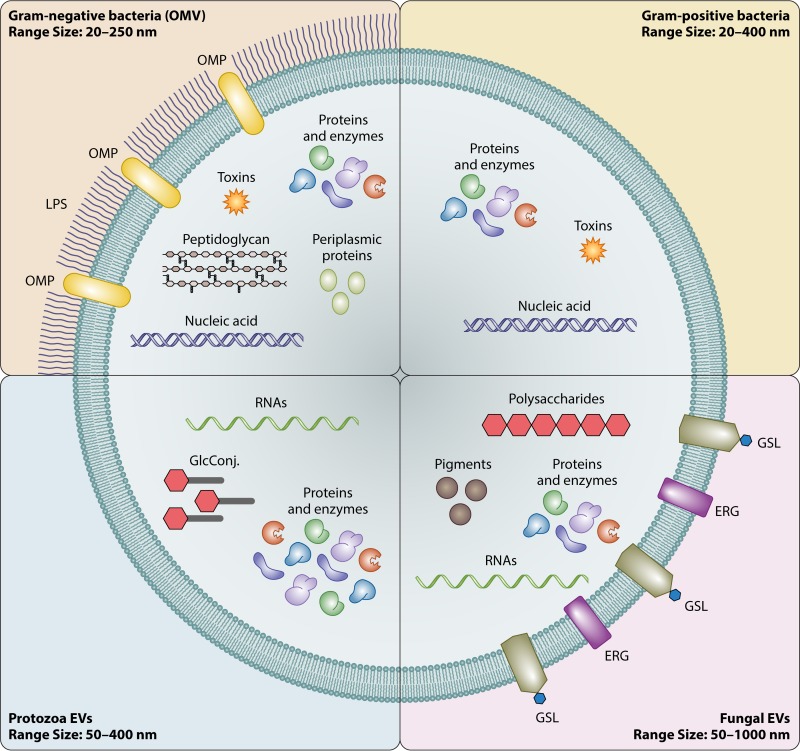
It is still unclear how EVs are released by cell wall-containing microorganisms, including Gram-positive bacteria, mycobacteria, and fungi (9, 10). EV cargo in these microorganisms includes enzymes, nucleic acids, polysaccharides, pigments, lipoproteins, and toxins (2, 11–14). Both shared EV cargo and organism-specific EV cargo have been characterized, in association with organism-related differences in dimensions (Fig. 2).
FIG 2 .
Schematic presentation of EVs produced by different microbial cells. OMP, outer membrane protein; GlcConj., glycoconjugates; GSL, glycosphingolipids; ERG, ergosterol; LPS, lipopolysaccharide.
BACTERIAL VESICLES
In bacteria, EVs have been most well studied in Gram-negative cells. The first reports of EV characterization were in Escherichia coli in the 1960s (15–18). Gram-negative EVs are also known as outer membrane vesicles (OMVs), due to their site of biogenesis (11, 19). OMVs can increase bacterial resistance to antibiotics and phages by serving as decoy targets for these molecules (19). They can also transfer DNA between cells and carry enzymes that degrade antibiotics (19). Gram-negative OMVs participate in the interaction of bacteria with host cells during infection. They deliver virulence factors, such as toxins, into host cells, including immune cells (19). OMV production is also known to be important for stress responses and nutrient acquisition for bacteria (11). While OMVs have been shown to play a role in bacterial virulence, they can also be harnessed as a potential vaccine tool. Some applications of OMVs as vaccines have already been approved for use in humans or are in clinical trials (20–23).
EVs released by Gram-positive bacteria differ from those released by Gram-negative EVs cells due to the lack of outer membranes and the presence of a thicker peptidoglycan layer (24). The mechanisms by which Gram-positive EVs are released are still not clear, but it is known that they carry cell wall hydrolases and peptidoglycan-degrading enzymes, suggesting that gaps might be formed in cell wall layers in order to release EVs (12, 25–27). Gram-positive species releasing EVs include Staphylococcus aureus (28), Bacillus subtilis (29), Streptococcus pneumoniae (26), Bacillus anthracis (12), and Listeria monocytogenes (30). Virulence factors of Gram-positive bacteria, including enzymes (β-lactamases, hemolysin, and coagulase) and toxins (12, 28, 31), are also released into the extracellular milieu through EVs. Work done with B. anthracis EVs efficiently illustrates how toxin components essential for virulence are released into host cells inside vesicles (12).
EVs released by Gram-positive bacteria are potentially emerging as vaccine components. Rivera and colleagues have shown that toxins released inside B. anthracis EVs induced robust immune responses in BALB/c mice that led to higher survival rates in animals challenged with the pathogen (12). Similar results were observed with Mycobacterium tuberculosis EVs (32). Mice immunized with mycobacterial EVs induced strong T helper type 1 cell responses (Th1), elicited antibody production, and reduced bacterial burden (32).
PROTOZOAN VESICLES
Parasite EVs were first described more than 20 years ago, although their relevance for secretory mechanisms has been realized only recently (33). In Leishmania spp. and Trypanosoma cruzi, EVs play important roles in protein export pathways, macrophage communication, and inflammatory responses (34–36). Proteomic analysis in T. cruzi has shown the presence of a great number of proteins containing nucleic acid-binding sites and ribosomal proteins within EVs (37). Different types of small RNAs were also detected in parasite EVs (38). Additionally, there were found to be differences between small RNAs packaged in EVs in each parasite developmental form (38). It has been demonstrated that T. cruzi (39) and Plasmodium falciparum (40) can transfer genetic information between parasites and from parasites to mammalian host cells. More recently, Fernandez-Calero and colleagues suggested that, under conditions of nutritional stress, a specific type of small RNA is released into EVs that may play a role in parasite-host interaction (41). In Leishmania spp., EV release is considered the most important mechanism of protein secretion and mediates delivery of parasite proteins into macrophages to cause production of interleukin-8 (IL-8) (35). Accordingly, EVs may increase parasite virulence; thus, pretreatment of mice with T. cruzi EVs followed by intraperitoneal parasite inoculation resulted in mortality indices that were higher than those seen with untreated mice (34). Moreover, EVs induced increased heart parasitism and inflammation through enhanced IL-10 and IL-4 production (34). These data suggested that T. cruzi EVs could facilitate parasite dissemination and pathogenic mechanisms (34). More recently, Szempruch and colleagues demonstrated that African trypanosomes release EVs through flagellum-derived nanotube formation, resulting in vesicular fusion with mammalian erythrocytes, membrane disruption, and anemia (42). Together, these results indicate that parasite EVs might participate in mechanisms of either virulence or immune response modulation in parasitic infections.
FUNGAL VESICLES
EVs produced by fungal cells are peculiar because, like bacterial EVs, fungal EVs must traverse a cell wall in order to be released. The mechanisms of EV release across the complex molecular network of the fungal cell wall are still unknown. Wolf and colleagues have utilized electron microscopy techniques to suggest that EVs interact with cell wall components (43). They showed that single and multiple vesicles simultaneously gain access to the extracellular milieu by crossing of the cell wall (43). The cellular origin of fungal EVs remains unknown. Rodrigues and colleagues suggested that EVs could originate from cytoplasmic subtractions (44). Other studies indicated that multivesicular bodies and membrane budding may also participate in EV formation (43, 45). These mechanisms are compatible with the presence of cytoplasmic proteins lacking secretory signals in EVs (44). The mechanisms of passage through the cell wall are similarly unknown. Proteomic analysis of EVs from Saccharomyces cerevisiae, Histoplasma capsulatum, Paracoccidioides brasiliensis, Candida albicans, and Cryptococcus neoformans revealed the presence of cell wall-degrading enzymes, suggesting that EV-associated molecules could be present in vesicular membranes and thus exposed, catalyzing cell wall crossing by hydrolysis of structural components (13, 46–49).
EVs transport virulence-associated components to the extracellular medium, suggesting that they are required for fungal pathogenesis (2, 13, 47, 48, 50). In contrast, EVs have been shown to induce protection in experimental models of fungal infection (13). In the following sections, possible roles of fungal EVs as “virulence bags” and their potential as vaccine tools are discussed.
EV CARGO IN FUNGI
Components of fungal EVs, including lipids (neutral glycolipids, sterols, and phospholipids), polysaccharides (glucuronoxylomannan [GXM] and α-galactosyl epitopes and mannose and N-acetylglucosamine residues), proteins (lipases, proteases, phosphatase, urease, laccase, and many others), and nucleic acids (different RNA species) have been described as virulence factors in different fungal species (2, 13, 14, 47–52). In C. neoformans and C. albicans, mass spectrometry and high-performance thin-layer chromatography (HPTLC) analysis revealed the presence of glucosylceramide (GlcCer) and sterols in EVs (2, 13). In addition, lipidomic analysis of P. brasiliensis EVs revealed the presence of GlcCer, brassica sterol, ergosterol, and lanosterol in different strains (53). GlcCer is a well-known virulence determinant of C. neoformans, since mutant cells lacking GlcCer synthase were avirulent in a murine model of cryptococcosis (54). C. albicans mutants lacking GlcCer biosynthesis were hypovirulent in BALB/c mice (55). In other fungi, such as Aspergillus nidulans and Fusarium graminearum, GlcCer is essential for hyphal growth and spore germination (56, 57), highlighting the role of GlcCer in both yeast and filamentous fungi.
Complex carbohydrates, such as GXM, are also exported in fungal EVs (48). In P. brasiliensis, EVs contain antigenic α-galactopyranosyl epitopes (50). Peres da Silva and colleagues have shown that residues of mannose and N-acetylglucosamine are present at the surface of P. brasiliensis EVs, where they mediate recognition by innate immune receptors (14). These observations might suggest a role for P. brasiliensis EVs in pathogen-host communication with the innate immune system, but it remains to be confirmed (14).
Fungal pigments are also found in and exported extracellularly by fungal EVs (48, 52). Melanin granules have dimensions that are comparable to those of EVs (52). In addition, melanin ghosts stained with lipophilic dyes suggested the presence of associated lipids. In fact, purified C. neoformans EVs contain electron-dense and dark granules (48) and are able to catalyze melanin synthesis in the presence of l-3,4-dihydroxyphenylalanine (L-DOPA) (52), strongly suggesting that melanin synthesis occurs inside fungal vesicles for further transport to the cell wall (52).
RNA-containing vesicles have been characterized in C. neoformans, C. albicans, S. cerevisiae, and P. brasiliensis (51). In these organisms, EVs carry small RNA molecules that are protected by EV membranes against exogenous RNase degradation. Mature tRNAs and mRNAs and noncoding RNAs are also present in fungal EVs (51). Although little is known about the function of RNAs in fungal EVs, it has been suggested that these molecules could participate in cell-to-cell communication, including host cells (51). This is supported by studies in T. cruzi, in which tRNA induces changes in host cells, making them more susceptible to infection (58).
Proteomic analysis of fungal EVs revealed the presence of a complex protein composition with multiple functions, including sugar metabolism, cell wall architecture, cell signaling, lipid metabolism, cell growth/division, and virulence (13, 46–49). Although many of these proteins have been shown to be shared in C. neoformans, P. brasiliensis, C. albicans, H. capsulatum, and S. cerevisiae EVs, species-specific protein molecules have also been abundantly observed (13, 46–49). For instance, enzymes essential to glucuronic acid metabolism were found in C. neoformans EVs but not in those of other species (48). EVs produced by fungi contain proteins such as plasma membrane ATPases, cytoskeleton proteins, heat shock proteins (HSP70 and HSP90), adhesins, and antioxidant proteins, including superoxide dismutase and catalase B (2, 13, 47, 48). The activity of urease and phosphatase was also detected in C. neoformans EVs (48). Urease is known to be an important virulence factor of C. neoformans in enhancing the invasion of host central nervous system (59). Phosphatases, which are surface components in different fungal species (60–62), were detected in other fungal EVs (48, 49).
In the context of all the EV cargo mentioned above, some examples of the involvement of mutants in vesicle formation can help understanding of the importance of fungal EVs. In C. neoformans, a Sec6 RNA interference (RNAi) mutant accumulates EVs inside the cells and the levels of some virulence factors, such as those involved in laccase, urease, and polysaccharide secretion, are significantly decreased. As a consequence, its virulence is attenuated (63). This was the first evidence correlating fungal EVs with virulence. Also, in C. neoformans, a secretion mutant called sav1, lacking a Sec4 GTPase homolog protein of post-Golgi secretion, accumulated EVs inside the cells and showed reduced secretion of proteins (64). In C. albicans, phosphatidylserine synthase (CHO1) and phosphatidylserine decarboxylase (PSD1 and PSD2) mutants also showed differences in the structures and constitution of EVs (65), but whether this phenotype affects Candida virulence is not known. There are some secretion-defective mutants that have been reported in the literature, but the correlation of defects in secretion of EVs with fungal virulence has been studied by our group.
FUNGAL EVs AS POTENTIAL VACCINES
There are many practical advantages to the general use of EVs as potential vaccine tools. As discussed by Schorey and coworkers (7), EVs offer more-stable conformational conditions for protein components, are able to circulate in body fluids, and show efficient association with antigen-presenting cells (7). However, fungal EVs manifest important particularities. For instance, C. neoformans EVs are unstable in the presence of host serum proteins (66).
Studies using EVs as vaccine tools initially included dendritic cell vesicles used for tumor control (67). Recent studies have demonstrated that EV prophylaxis induced increased survival and slower tumor development in mice (68). Development of vaccines using pathogen-derived EVs first included Gram-negative OMVs produced by Neisseria meningitidis serogroup B (69–71). Four licensed OMV vaccines are now available (70–72), each of them having been developed for a specific outbreak. Meningococcal group B vaccine (Bexsero), the most recently licensed OMV vaccine, was designed to provide broad protection through combining multiple antigens (72). These EV vaccines were associated with high effectiveness in regions where the circulating and vaccine strains were the same (22, 70, 71, 73). Promising studies focusing on other bacterial species are available in the current literature (20, 21, 74, 75).
Studies on the immunobiological activity of fungal EVs have been performed more recently. In 2011, it was shown that EVs in Malassezia sympodialis carry antigens and allergens that modulate the immune system through in vivo stimulation of IL-4 and tumor necrosis factor alpha (TNF-α) (76), suggesting an involvement of EVs with atopic eczema (76). Oliveira and colleagues showed that C. neoformans EVs are biologically active in vitro (77): they stimulate nitric oxide, cytokine (TNF-α, IL-10, and transforming growth factor β [TGF-β]), and fungicidal activity in macrophages (77). This observation might be related to the detection of key immunogens in C. neoformans EVs, including heat-shock proteins and GlcCer (78). In fact, human antibodies against C. neoformans GlcCer were demonstrated to be fungistatic (45, 78). Similarly, P. brasiliensis EVs also stimulated cytokine production in macrophages (14).
Although some reports suggest a protective role of C. neoformans EVs, other reports indicate that these compartments can enhance pathogenesis of this fungus. Huang and colleagues have demonstrated that C. neoformans EVs are able to cross the brain-blood barrier and accumulate in lesion sites of brain infection (79). In an in vitro model of C. neoformans infection, EVs facilitated adhesion and transcytosis of C. neoformans during interaction with human brain microvascular endothelial cells (79), leading to increased brain infectivity. Finally, pretreatment of mice with C. neoformans EVs rendered the mice more susceptible to cryptococcosis (79). Therefore, it is still unclear how C. neoformans EVs interfere with either fungal virulence or host stimulation.
There are no fungal vaccines in the clinic, although some animal studies suggest that a fungal vaccine in the clinic may be closer than ever. Wormley and colleagues have suggested the potential protection of using a gamma interferon (IFN-γ)-producing C. neoformans strain in a mouse pulmonary infection model (80). Mice were able to resolve the primary infection and showed complete protection against second pulmonary challenge with the C. neoformans strain (80). This finding, which was based on utilization of engineered fungi to produce host cytokines in order to induce a protective host immune response, was an important advance in the vaccine field. Another example of a potential fungal vaccine is the C. neoformans knockout strain lacking sterylglucosidase 1 (Sgl1), an enzyme responsible for degradation of sterylglucosides. The Δsgl1 strain was nonpathogenic in an infection model and, interestingly, acted as a vaccine strain, protecting mice when administered prior to challenge with wild-type C. neoformans or C. gattii (81). This protection may be ascribed to a dramatic accumulation of sterol glucosides (SGs) in the Δsgl1 mutant (81), and these fungal SGs may act as stronger immunostimulators than plant SGs (82–85). The most interesting observation was that the host was protected against primary and secondary infection even when CD4+ T cells were deficient. These data suggest the potential protective effect of an attenuated fungal strain in immunodeficient hosts, such as HIV-positive patients, where cryptococcosis most frequently occurs. Interestingly, if SGs are found in the vesicles of the Δsgl1 strain, this not only might significantly contribute to the protective effect but also could increase interest in using EVs instead of the live attenuated mutant as a vaccine formulation.
Recently, Vargas and colleagues demonstrated that EVs obtained from C. albicans cultures are immunologically active (13). Exposure of macrophages and dendritic cells to purified EVs resulted in nitric oxide production and release of IL-12, TGF-β, and IL-10 in macrophages and of IL-10 and TNF-α in dendritic cells (13). The ability of C. albicans EVs to stimulate host cells depended on lipid composition, since EVs from phospholipid synthase mutants differentially stimulated cytokine production in macrophages (65). Treatment of Galleria mellonella with EVs before infection with C. albicans reduced fungal burden (13). These data indicate the ability of C. albicans EVs to modulate the innate immune response and the potential of EVs to interfere with fungal pathogenesis in vivo in favor of infection control (13). Together, these results strongly suggest that fungal EVs activate host immunity by multiple mechanisms that may favor the host during fungal infections, reinforcing their potential use as a vaccination strategy.
CONCLUSION
Fungal diseases are a global public health problem, especially for immunocompromised individuals, such as those affected by HIV infection and cancer (86) (http://www.cdc.gov/fungal/global/index.html). It is estimated that millions of people die every year due to invasive fungal infection (86–88), with a mortality rate comparable to that of malaria (89). The current available antifungal therapies have significant disadvantages: polyene are toxic, azoles induce the development of resistance and are notorious for drug-drug interactions, and echinocandins have a narrow spectrum of activity (86, 90). Thus, there is a need for research and development (R&D) of new antifungals as well as new, alternative preventive strategies to combat fungal diseases.
As discussed above, fungal EVs may represent this new, alternative strategy once we fully understand their cargo and their role as virulence bags. Although there are a variety of examples of the role of fungal EVs in carrying many virulence factors, the relevance of their cargo in vivo is incompletely understood. On the other hand, fungal EVs are promising in vitro activators of the host immune system against pathogens that operate by inducing cytokine release by innate immune cells, suggesting that they may be a potent tool for vaccine development. Nevertheless, their role in in vivo protection is still under study. In bacteria, exciting results that have shown some examples of successful EV vaccines support the idea that fungal EVs could also be used as a protective tool against the most deadly fungal infections. In addition, the concept of therapeutic fungal vaccines is gaining more traction, since there are currently fungal proteins in preclinical phase I studies for vaccine development (91, 92). More studies and more effort are clearly necessary for assessing the role of fungal EVs in the fungus-host interaction and to decipher the mechanisms by which they might stimulate a protective host response.
ACKNOWLEDGMENTS
This work was supported by grants from Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), by NIH grants AI56168, AI100631, and AI116420, and by Merit Review grant I01BX002624 from the Veterans Affairs Program in Biomedical Laboratory Research and Development to M.D.P. M.D.P. is a Burroughs Wellcome Investigator in Infectious Diseases. M.L.R. also acknowledges support from the Instituto Nacional de Ciência e Tecnologia de Inovação em Doenças Negligenciadas (INCT-IDN). M.L.R. is the recipient of a Pathfinder Award from the Wellcome Trust (United Kingdom; grant no. WT103212MF).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We are very grateful to Rodrigo Pinheiro and Arielle Bryan for valuable suggestions and English proofreading.
REFERENCES
- 1.Deatherage BL, Cookson BT. 2012. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun 80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, Alvarez M, Nakouzi A, Feldmesser M, Casadevall A. 2007. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell 6:48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellen AF, Zolghadr B, Driessen AM, Albers SV. 2010. Shaping the archaeal cell envelope. Archaea 2010:608243. doi: 10.1155/2010/608243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JH, Lee J, Park J, Gho YS. 2015. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol 40:97–104. doi: 10.1016/j.semcdb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Marcilla A, Martin-Jaular L, Trelis M, de Menezes-Neto A, Osuna A, Bernal D, Fernandez-Becerra C, Almeida IC, Del Portillo HA. 2014. Extracellular vesicles in parasitic diseases. J Extracell Vesicles 3:25040. doi: 10.3402/jev.v3.25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. 2015. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schorey JS, Cheng Y, Singh PP, Smith VL. 2015. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep 16:24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Théry C, Ostrowski M, Segura E. 2009. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 9.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. 2015. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 13:620–630. doi: 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigues ML, Godinho RMC, Zamith-Miranda D, Nimrichter L. 2015. Traveling into outer space: unanswered questions about fungal extracellular vesicles. PLoS Pathog 11:e1005240. doi: 10.1371/journal.ppat.1005240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulp A, Kuehn MJ. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol 64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera J, Cordero RJ, Nakouzi AS, Frases S, Nicola A, Casadevall A. 2010. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc Natl Acad Sci U S A 107:19002–19007. doi: 10.1073/pnas.1008843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vargas G, Rocha JD, Oliveira DL, Albuquerque PC, Frases S, Santos SS, Nosanchuk JD, Gomes AM, Medeiros LC, Miranda K, Sobreira TJ, Nakayasu ES, Arigi EA, Casadevall A, Guimaraes AJ, Rodrigues ML, Freire-de-Lima CG, Almeida IC, Nimrichter L. 2015. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell Microbiol 17:389–407. doi: 10.1111/cmi.12374. [DOI] [PubMed] [Google Scholar]
- 14.Peres da Silva R, Heiss C, Black I, Azadi P, Gerlach JQ, Travassos LR, Joshi L, Kilcoyne M, Puccia R. 2015. Extracellular vesicles from Paracoccidioides pathogenic species transport polysaccharide and expose ligands for DC-SIGN receptors. Sci Rep 5:14213. doi: 10.1038/srep14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishop DG, Work E. 1965. An extracellular glycolipid produced by Escherichia coli grown under lysine-limiting conditions. Biochem J 96:567–576. doi: 10.1042/bj0960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Work E, Knox KW, Vesk M. 1966. The chemistry and electron microscopy of an extracellular lipopolysaccharide from Escherichia coli. Ann N Y Acad Sci 133:438–449. doi: 10.1111/j.1749-6632.1966.tb52382.x. [DOI] [PubMed] [Google Scholar]
- 17.Knox KW, Vesk M, Work E. 1966. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol 92:1206–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knox KW, Cullen J, Work E. 1967. An extracellular lipopolysaccharide-phospholipid-protein complex produced by Escherichia coli grown under lysine-limiting conditions. Biochem J 103:192–201. doi: 10.1042/bj1030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwechheimer C, Kuehn MJ. 2015. Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol 13:605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acevedo R, Fernández S, Zayas C, Acosta A, Sarmiento ME, Ferro VA, Rosenqvist E, Campa C, Cardoso D, Garcia L, Perez JL. 2014. Bacterial outer membrane vesicles and vaccine applications. Front Immunol 5:121. doi: 10.3389/fimmu.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WH, Choi HI, Hong SW, Kim KS, Gho YS, Jeon SG. 2015. Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Exp Mol Med 47:e183. doi: 10.1038/emm.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold R, Galloway Y, McNicholas A, O’Hallahan J. 2011. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine 29:7100–7106. doi: 10.1016/j.vaccine.2011.06.120. [DOI] [PubMed] [Google Scholar]
- 23.Holst J, Oster P, Arnold R, Tatley MV, Næss LM, Aaberge IS, Galloway Y, McNicholas A, O’Hallahan J, Rosenqvist E, Black S. 2013. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum Vaccin Immunother 9:1241–1253. doi: 10.4161/hv.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shockman GD, Barren JF. 1983. Structure, function, and assembly of cell walls of gram-positive bacteria. Annu Rev Microbiol 37:501–527. doi: 10.1146/annurev.mi.37.100183.002441. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Kim SH, Choi DS, Lee JS, Kim DK, Go G, Park SM, Kim SH, Shin JH, Chang CL, Gho YS. 2015. Proteomic analysis of extracellular vesicles derived from Mycobacterium tuberculosis. Proteomics 15:3331–3337. doi: 10.1002/pmic.201500037. [DOI] [PubMed] [Google Scholar]
- 26.Olaya-Abril A, Prados-Rosales R, McConnell MJ, Martín-Peña R, González-Reyes JA, Jiménez-Munguía I, Gómez-Gascón L, Fernández J, Luque-García JL, García-Lidón C, Estévez H, Pachón J, Obando I, Casadevall A, Pirofski LA, Rodríguez-Ortega MJ. 2014. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J Proteomics 106:46–60. doi: 10.1016/j.jprot.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Scheurwater EM, Burrows LL. 2011. Maintaining network security: how macromolecular structures cross the peptidoglycan layer. FEMS Microbiol Lett 318:1–9. doi: 10.1111/j.1574-6968.2011.02228.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP, Gho YS. 2009. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 29.Brown L, Kessler A, Cabezas-Sanchez P, Luque-Garcia JL, Casadevall A. 2014. Extracellular vesicles produced by the Gram-positive bacterium Bacillus subtilis are disrupted by the lipopeptide surfactin. Mol Microbiol 93:183–198. doi: 10.1111/mmi.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JH, Choi CW, Lee T, Kim SI, Lee JC, Shin JH. 2013. Transcription factor sigmaB plays an important role in the production of extracellular membrane-derived vesicles in Listeria monocytogenes. PLoS One 8:e73196. doi: 10.1371/journal.pone.0073196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Lee EY, Kim SH, Kim DK, Park KS, Kim KP, Kim YK, Roh TY, Gho YS. 2013. Staphylococcus aureus extracellular vesicles carry biologically active beta-lactamase. Antimicrob Agents Chemother 57:2589–2595. doi: 10.1128/AAC.00522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prados-Rosales R, Carreño LJ, Batista-Gonzalez A, Baena A, Venkataswamy MM, Xu J, Yu X, Wallstrom G, Magee DM, LaBaer J, Achkar JM, Jacobs WR Jr., Chan J, Porcelli SA, Casadevall A. 2014. Mycobacterial membrane vesicles administered systemically in mice induce a protective immune response to surface compartments of Mycobacterium tuberculosis. mBio 5:e01921-14. doi: 10.1128/mBio.01921-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torrecilhas AC, Schumacher RI, Alves MJ, Colli W. 2012. Vesicles as carriers of virulence factors in parasitic protozoan diseases. Microbes Infect 14:1465–1474. doi: 10.1016/j.micinf.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Trocoli Torrecilhas AC, Tonelli RR, Pavanelli WR, da Silva JS, Schumacher RI, de Souza W, E Silva NC, de Almeida Abrahamsohn I, Colli W, Manso Alves MJ. 2009. Trypanosoma cruzi: parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect 11:29–39. doi: 10.1016/j.micinf.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Silverman JM, Clos J, Horakova E, Wang AY, Wiesgigl M, Kelly I, Lynn MA, McMaster WR, Foster LJ, Levings MK, Reiner NE. 2010. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J Immunol 185:5011–5022. doi: 10.4049/jimmunol.1000541. [DOI] [PubMed] [Google Scholar]
- 36.Gonçalves MF, Umezawa ES, Katzin AM, de Souza W, Alves MJ, Zingales B, Colli W. 1991. Trypanosoma cruzi: shedding of surface antigens as membrane vesicles. Exp Parasitol 72:43–53. doi: 10.1016/0014-4894(91)90119-H. [DOI] [PubMed] [Google Scholar]
- 37.Bayer-Santos E, Aguilar-Bonavides C, Rodrigues SP, Cordero EM, Marques AF, Varela-Ramirez A, Choi H, Yoshida N, da Silveira JF, Almeida IC. 2013. Proteomic analysis of Trypanosoma cruzi secretome: characterization of two populations of extracellular vesicles and soluble proteins. J Proteome Res 12:883–897. doi: 10.1021/pr300947g. [DOI] [PubMed] [Google Scholar]
- 38.Bayer-Santos E, Lima FM, Ruiz JC, Almeida IC, da Silveira JF. 2014. Characterization of the small RNA content of Trypanosoma cruzi extracellular vesicles. Mol Biochem Parasitol 193:71–74. doi: 10.1016/j.molbiopara.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Silva MR, das Neves RF, Cabrera-Cabrera F, Sanguinetti J, Medeiros LC, Robello C, Naya H, Fernandez-Calero T, Souto-Padron T, de Souza W, Cayota A. 2014. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol Res 113:285–304. doi: 10.1007/s00436-013-3655-1. [DOI] [PubMed] [Google Scholar]
- 40.Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, Baum J, Cowman AF. 2013. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 153:1120–1133. doi: 10.1016/j.cell.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Calero T, Garcia-Silva R, Pena A, Robello C, Persson H, Rovira C, Naya H, Cayota A. 2015. Profiling of small RNA cargo of extracellular vesicles shed by Trypanosoma cruzi reveals a specific extracellular signature. Mol Biochem Parasitol 199:19–28. doi: 10.1016/j.molbiopara.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Szempruch AJ, Sykes SE, Kieft R, Dennison L, Becker AC, Gartrell A, Martin WJ, Nakayasu ES, Almeida IC, Hajduk SL, Harrington JM. 2016. Extracellular vesicles from Trypanosoma brucei Mediate virulence factor transfer and cause host anemia. Cell 164:246–257. doi: 10.1016/j.cell.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf JM, Espadas-Moreno J, Luque-Garcia JL, Casadevall A. 2014. Interaction of Cryptococcus neoformans extracellular vesicles with the cell wall. Eukaryot Cell 13:1484–1493. doi: 10.1128/EC.00111-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues ML, Franzen AJ, Nimrichter L, Miranda K. 2013. Vesicular mechanisms of traffic of fungal molecules to the extracellular space. Curr Opin Microbiol 16:414–420. doi: 10.1016/j.mib.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigues ML, Travassos LR, Miranda KR, Franzen AJ, Rozental S, de Souza W, Alviano CS, Barreto-Bergter E. 2000. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect Immun 68:7049–7060. doi: 10.1128/IAI.68.12.7049-7060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliveira DL, Nakayasu ES, Joffe LS, Guimarães AJ, Sobreira TJ, Nosanchuk JD, Cordero RJ, Frases S, Casadevall A, Almeida IC, Nimrichter L, Rodrigues ML. 2010. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One 5:e11113. doi: 10.1371/journal.pone.0011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albuquerque PC, Nakayasu ES, Rodrigues ML, Frases S, Casadevall A, Zancope-Oliveira RM, Almeida IC, Nosanchuk JD. 2008. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol 10:1695–1710. doi: 10.1111/j.1462-5822.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodrigues ML, Nimrichter L, Oliveira DL, Nosanchuk JD, Casadevall A. 2008. Vesicular trans-cell Wall transport in fungi: A mechanism for the Delivery of virulence-associated macromolecules? Lipid Insights 2:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vallejo MC, Nakayasu ES, Matsuo AL, Sobreira TJ, Longo LV, Ganiko L, Almeida IC, Puccia R. 2012. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. J Proteome Res 11:1676–1685. doi: 10.1021/pr200872s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vallejo MC, Matsuo AL, Ganiko L, Medeiros LC, Miranda K, Silva LS, Freymüller-Haapalainen E, Sinigaglia-Coimbra R, Almeida IC, Puccia R. 2011. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic alpha-galactosyl epitopes. Eukaryot Cell 10:343–351. doi: 10.1128/EC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peres da Silva R, Puccia R, Rodrigues ML, Oliveira DL, Joffe LS, César GV, Nimrichter L, Goldenberg S, Alves LR. 2015. Extracellular vesicle-mediated export of fungal RNA. Sci Rep 5:7763. doi: 10.1038/srep07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisenman HC, Frases S, Nicola AM, Rodrigues ML, Casadevall A. 2009. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology 155:3860–3867. doi: 10.1099/mic.0.032854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallejo MC, Nakayasu ES, Longo LV, Ganiko L, Lopes FG, Matsuo AL, Almeida IC, Puccia R. 2012. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS One 7:e39463. doi: 10.1371/journal.pone.0039463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rittershaus PC, Kechichian TB, Allegood JC, Merrill AH Jr., Hennig M, Luberto C, Del Poeta M. 2006. Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J Clin Invest 116:1651–1659. doi: 10.1172/JCI27890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noble SM, French S, Kohn LA, Chen V, Johnson AD. 2010. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat Genet 42:590–598. doi: 10.1038/ng.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rittenour WR, Chen M, Cahoon EB, Harris SD. 2011. Control of glucosylceramide production and morphogenesis by the Bar1 ceramide synthase in Fusarium graminearum. PLoS One 6:e19385. doi: 10.1371/journal.pone.0019385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levery SB, Momany M, Lindsey R, Toledo MS, Shayman JA, Fuller M, Brooks K, Doong RL, Straus AH, Takahashi HK. 2002. Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS Lett 525:59–64. doi: 10.1016/S0014-5793(02)03067-3. [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Silva MR, Cabrera-Cabrera F, das Neves RF, Souto-Padrón T, de Souza W, Cayota A. 2014. Gene expression changes induced by Trypanosoma cruzi shed microvesicles in mammalian host cells: relevance of tRNA-derived halves. BioMed Res Int 2014:305239. doi: 10.1155/2014/305239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR. 2000. Urease as a virulence factor in experimental cryptococcosis. Infect Immun 68:443–448. doi: 10.1128/IAI.68.2.443-448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnold WN, Mann LC, Sakai KH, Garrison RG, Coleman PD. 1986. Acid phosphatases of Sporothrix schenckii. J Gen Microbiol 132:3421–3432. doi: 10.1099/00221287-132-12-3421. [DOI] [PubMed] [Google Scholar]
- 61.Mahvi TA, Spicer SS, Wright NJ. 1974. Cytochemistry of acid mucosubstance and acid phosphatase in Cryptococcus neoformans. Can J Microbiol 20:833–838. doi: 10.1139/m74-128. [DOI] [PubMed] [Google Scholar]
- 62.Bernard M, Mouyna I, Dubreucq G, Debeaupuis JP, Fontaine T, Vorgias C, Fuglsang C, Latgé JP. 2002. Characterization of a cell-wall acid phosphatase (PhoAp) in Aspergillus fumigatus. Microbiology 148:2819–2829. doi: 10.1099/00221287-148-9-2819. [DOI] [PubMed] [Google Scholar]
- 63.Panepinto J, Komperda K, Frases S, Park YD, Djordjevic JT, Casadevall A, Williamson PR. 2009. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol Microbiol 71:1165–1176. doi: 10.1111/j.1365-2958.2008.06588.x. [DOI] [PubMed] [Google Scholar]
- 64.Yoneda A, Doering TL. 2006. A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol Biol Cell 17:5131–5140. doi: 10.1091/mbc.E06-08-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolf JM, Espadas J, Luque-Garcia J, Reynolds T, Casadevall A. 2015. Lipid biosynthetic genes affect Candida albicans extracellular vesicle morphology, Cargo, and immunostimulatory properties. Eukaryot Cell 14:745–754. doi: 10.1128/EC.00054-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolf JM, Rivera J, Casadevall A. 2012. Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles. Cell Microbiol 14:762–773. doi: 10.1111/j.1462-5822.2012.01757.x. [DOI] [PubMed] [Google Scholar]
- 67.Vader P, Breakefield XO, Wood MJ. 2014. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med 20:385–393. doi: 10.1016/j.molmed.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Näslund TI, Gehrmann U, Gabrielsson S. 2013. Cancer immunotherapy with exosomes requires B-cell activation. Oncoimmunology 2:e24533. doi: 10.4161/onci.24533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frasch CE, Robbins JD. 1978. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J Exp Med 147:629–644. doi: 10.1084/jem.147.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, Casanueva GV, Rico CO, Rodriguez CR, Terry MH. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann 14:195–207; discussion: 208–210. [PubMed] [Google Scholar]
- 71.Holst J, Martin D, Arnold R, Huergo CC, Oster P, O’Hallahan J, Rosenqvist E. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27(Suppl 2):B3–12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 72.Bai X, Findlow J, Borrow R. 2011. Recombinant protein meningococcal serogroup B vaccine combined with outer membrane vesicles. Expert Opin Biol Ther 11:969–985. doi: 10.1517/14712598.2011.585965. [DOI] [PubMed] [Google Scholar]
- 73.Rosenqvist E, Høiby EA, Wedege E, Bryn K, Kolberg J, Klem A, Rønnild E, Bjune G, Nøkleby H. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect Immun 63:4642–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernández S, Fajardo EM, Mandiarote A, Año G, Padrón MA, Acosta M, Cabrera RA, Riverón LA, Álvarez M, Blaín K, Fariñas M, Cardoso D, García LG, Campa C, Pérez JL. 2013. A proteoliposome formulation derived from Bordetella pertussis induces protection in two murine challenge models. BMC Immunol 14(Suppl 1):S8. doi: 10.1186/1471-2172-14-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Camacho AI, Irache JM, Gamazo C. 2013. Recent progress towards development of a Shigella vaccine. Expert Rev Vaccines 12:43–55. doi: 10.1586/erv.12.135. [DOI] [PubMed] [Google Scholar]
- 76.Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg L, Gabrielsson S, Scheynius A. 2011. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses—novel mechanisms for host-microbe interactions in atopic eczema. PLoS One 6:e21480. doi: 10.1371/journal.pone.0021480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, Casadevall A, Rodrigues ML, Nimrichter L. 2010. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect Immun 78:1601–1609. doi: 10.1128/IAI.01171-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodrigues ML, Nakayasu ES, Almeida IC, Nimrichter L. 2014. The impact of proteomics on the understanding of functions and biogenesis of fungal extracellular vesicles. J Proteomics 97:177–186. doi: 10.1016/j.jprot.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang SH, Wu CH, Chang YC, Kwon-Chung KJ, Brown RJ, Jong A. 2012. Cryptococcus neoformans-derived microvesicles enhance the pathogenesis of fungal brain infection. PLoS One 7:e48570. doi: 10.1371/journal.pone.0048570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wormley FL Jr., Perfect JR, Steele C, Cox GM. 2007. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect Immun 75:1453–1462. doi: 10.1128/IAI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rella A, Mor V, Farnoud AM, Singh A, Shamseddine AA, Ivanova E, Carpino N, Montagna MT, Luberto C, Del Poeta M. 2015. Role of Sterylglucosidase 1 (Sgl1) on the pathogenicity of Cryptococcus neoformans: potential applications for vaccine development. Front Microbiol 6:836. doi: 10.3389/fmicb.2015.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Donald PR, Lamprecht JH, Freestone M, Albrecht CF, Bouic PJ, Kotze D, van Jaarsveld PP. 1997. A randomised placebo-controlled trial of the efficacy of beta-sitosterol and its glucoside as adjuvants in the treatment of pulmonary tuberculosis. Int J Tuberc Lung Dis 1:518–522. [PubMed] [Google Scholar]
- 83.Lee JH, Han Y. 2006. Ginsenoside Rg1 helps mice resist to disseminated candidiasis by Th1 type differentiation of CD4+ T cell. Int Immunopharmacol 6:1424–1430. doi: 10.1016/j.intimp.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 84.Lee JH, Lee JY, Park JH, Jung HS, Kim JS, Kang SS, Kim YS, Han Y. 2007. Immunoregulatory activity by daucosterol, a beta-sitosterol glycoside, induces protective Th1 immune response against disseminated candidiasis in mice. Vaccine 25:3834–3840. doi: 10.1016/j.vaccine.2007.01.108. [DOI] [PubMed] [Google Scholar]
- 85.Bouic PJ, Etsebeth S, Liebenberg RW, Albrecht CF, Pegel K, Van Jaarsveld PP. 1996. Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: implications for their use as an immunomodulatory vitamin combination. Int J Immunopharmacol 18:693–700. doi: 10.1016/S0192-0561(97)85551-8. [DOI] [PubMed] [Google Scholar]
- 86.Rodrigues ML. 2016. Funding and innovation in diseases of neglected populations: the paradox of cryptococcal meningitis. PLoS Negl Trop. Drosophila Inf Serv 10:e0004429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bassetti M, Righi E. 2015. Overview of fungal infections—the Italian experience. Semin Respir Crit Care Med 36:796–805. doi: 10.1055/s-0035-1562890. [DOI] [PubMed] [Google Scholar]
- 88.Denning DW, Bromley MJ. 2015. Infectious disease. How to bolster the antifungal pipeline. Science 347:1414–1416. doi: 10.1126/science.aaa6097. [DOI] [PubMed] [Google Scholar]
- 89.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 90.Vandeputte P, Ferrari S, Coste AT. 2012. Antifungal resistance and new strategies to control fungal infections. Int J Microbiol 2012:713687. doi: 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmidt CS, White CJ, Ibrahim AS, Filler SG, Fu Y, Yeaman MR, Edwards JE Jr., Hennessey JP Jr.. 2012. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 30:7594–7600. doi: 10.1016/j.vaccine.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Bernardis F, Amacker M, Arancia S, Sandini S, Gremion C, Zurbriggen R, Moser C, Cassone A. 2012. A virosomal vaccine against candidal vaginitis: immunogenicity, efficacy and safety profile in animal models. Vaccine 30:4490–4498. doi: 10.1016/j.vaccine.2012.04.069. [DOI] [PubMed] [Google Scholar]