Abstract
Introduction
Investigations of the effects of occupational exposure to lead on the concentrations of thyroid hormones in the blood have not produced consistent results. A meta-analysis was performed to assess the effect of occupational exposure to lead on thyroid hormone concentrations using the results from published studies.
Methods
Group means from studies of the thyroid function of persons occupationally exposed to lead were used in a meta-analysis. Differences between the control and exposed groups, and the slopes between thyroid hormone concentrations and log10 blood lead concentrations or duration of exposure to lead were estimated using mixed models. The hormones analyzed were thyroid stimulating hormone, total and free thyroxine, and total and free triiodothyronine.
Results
No differences in mean thyroid hormone concentrations were found between the exposed and control groups. No relationships were found between blood lead or the duration of exposure to lead and thyroid hormone concentrations.
Conclusion
The results of the analysis do not provide evidence for an effect of occupational lead exposure on thyroid function in men.
Keywords: blood lead, thyroid stimulating hormone, thyroxine, triiodothryonine
INTRODUCTION
There are few reviews and no meta-analyses of the effects of occupational exposure to lead on the thyroid and on the concentrations of thyroid hormones in the blood.
An editorial on occupational and environmental diseases of the endocrine system [Cohen and Felig 1984] indicated that occupational exposure to lead may induce hypothyroidism by acting on the hypothalamus and pituitary, and that the effect of lead may vary by race. These conclusions were based on one study [Robins, et al. 1983].
In a review of the effect of lead on the thyroid, Łasisz, Zdrojewicz, and Marcinkowski [1992] concluded that the hypothyroidism found in workers exposed to lead may be evidence of a negative effect of lead on thyroid function.
Doumouchtsis, Doumouchtsis, Doumouchtsis, and Perrea [2009] reviewed occupational and animal studies, and concluded that lead appears to affect the hypothalamic-pituitary-thyroid axis and that lead influences thyroid hormone kinetics in a pattern that is inconclusive.
In a review of the effect of environmental pollutants on the thyroid, Pearce and Braverman [2009], citing studies of occupational exposures, concluded that lead may adversely affect the pituitary–thyroid axis by way of an unknown mechanism.
Investigations of the effects of occupational exposure to lead on the concentrations of thyroid hormones in the blood have not produced consistent results. For example, Robins, Cullen, Connors, and Kayne [1983] found an inverse relationship between free thyroxine and blood lead concentrations in 47 men that worked in a brass foundry, while Refowitz [1984] found no such relationship in 58 men working at a secondary copper smelter.
The purpose of the meta-analysis reported here was to assess the effect of occupational exposure to lead on thyroid hormone concentrations. The results from published occupational studies were used. Differences between control and exposed groups, and slopes between thyroid hormone concentrations and the log base 10 blood lead concentrations or duration of exposure to lead were estimated. Means from published studies were used. The hormones that were analyzed included thyroid stimulating hormone, total and free thyroxine, and total and free triiodothyronine
MATERIALS AND METHODS
Studies
Studies were identified by searching the reference sections of review articles and the papers describing individual studies. Searches were made on PubMed combining the terms ‘blood lead’ and either ‘thyroid’, ‘thyroxine’, or ‘triiodothyronine’. The last search was made in April 3, 2014. Data from 16 studies published in journal articles were used [Andrzejak, et al. 1996, Bielecka, et al. 1987, Bledsoe, et al. 2011, Cullen, et al. 1984, Dursun and Tutus 1999, Erfurth, et al. 2001, Gennart, et al. 1992, Gustafson, et al. 1989, Horiguchi, et al. 1987, Liang, et al. 2003, López, et al. 2000, Robins, et al. 1983, Schumacher, et al. 1998, Singh, et al. 2000, Tuppurainen, et al. 1988, Yılmaz, et al. 2012]. All the studies that were included had subjects that were exposed to lead. All of the exposures were occupational. Persons in the control groups were not occupationally exposed to lead. The sample size of a control or exposed group had to be greater than one for a group to be included. Case reports of an individual were not included. Studies of children or adolescents were not included. Not all studies had measurements of all the thyroid hormones. Eight studies did not have a control group. Two studies had more than one exposed group. Demographic information by study is presented in a supplementary table (Table SI).
Blood Lead Measurements
Blood lead was measured in samples of whole blood by atomic absorption spectrophotometry in all but one study [Horiguchi, et al. 1987] which used anodic stripping voltammetry.
Thyroid Hormone Measurements
The thyroid hormones measured in the studies include thyroid stimulating hormone, total and free thyroxine, and total and free triiodothyronine.
Free thyroxine is a measure of the thyroxine in the blood that is not bound to binding proteins, primarily thyroxine-binding globulin, transthyretin (prealbumin), and albumin [Schussler 2000]. Total thyroxine is a measure of the bound and unbound thyroxine.
Free triiodothyronine is a measure of the triiodothyronine in the blood that is not bound to binding proteins, also primarily thyroxine-binding globulin, transthyretin, and albumin [Bartalena and Robbins 1993]. Total triiodothyronine is a measure of the bound and unbound triiodothyronine.
Thyroid stimulating hormone, total and free thyroxine, and total and free triiodothyronine were all measured in serum by immunoassays, usually from kits supplied by manufacturers. In some instances, free thyroxine was not directly measured, but was calculated as the estimated free thyroxine [Cullen, et al. 1984, Robins, et al. 1983, Schumacher, et al. 1998] or the free thyroxine index [Gennart, et al. 1992].
Statistical Analysis
The data that were analyzed consisted of group means that were reported in a paper or that were calculated from the results of individuals that were reported in a paper. Mixed linear models were used to analyze the data. Study was a random variable. A classification variable for exposure status or the log base 10 of the mean blood lead concentration was included as a fixed effect.
The model used to compare exposed and control groups was
yijk is the mean thyroid hormone measurement. µ is the grand mean. τi is the effect of exposure, i = 1 = control, i = 2 = exposed. δj is the random effect of study j. εijk is a random error. For most cells, k = 1, however, in some studies there was more than one exposed group, so k was greater than one.
The model used for blood lead concentration was
yij is the mean thyroid hormone measurement. α is the intercept. β is the slope. xi is the log base 10 of the mean blood lead concentration of group i. Values from the control and exposed groups were included. δj is the random effect of study j. εij is a random error.
The model used for duration of exposure to lead was
yi is the mean thyroid hormone measurement. α is the intercept. β1 is the slope for duration of exposure. x1i is the mean duration of exposure in years for group i. Only the exposed groups had values. β2 is the slope for age. x2i is the mean age in years for group i. A random effect of study was not included because there was not enough data to estimate the effect. εi is a random error.
All calculations were done with SAS® (Release 9.3, SAS Institute, Inc., Cary, North Carolina). The MIXED procedure was used to estimate the models. The method of estimation was residual maximum likelihood.
SigmaPlot® (Version 11.0, Systat Software, Inc., Chicago, Illinois) was used to make the graph. Simple linear least squares regression was used to fit the data in the graphs.
RESULTS
In the 16 studies, there were 366 subjects in the control groups (228 males, 0 females) and 1,372 subjects in the exposed groups (1,280 males, 15 females). The sex of the subjects was not always reported. Race was reported in studies done in the United States, for the control groups (51 white, 32 black) and the exposed groups (62 white, 93 black).
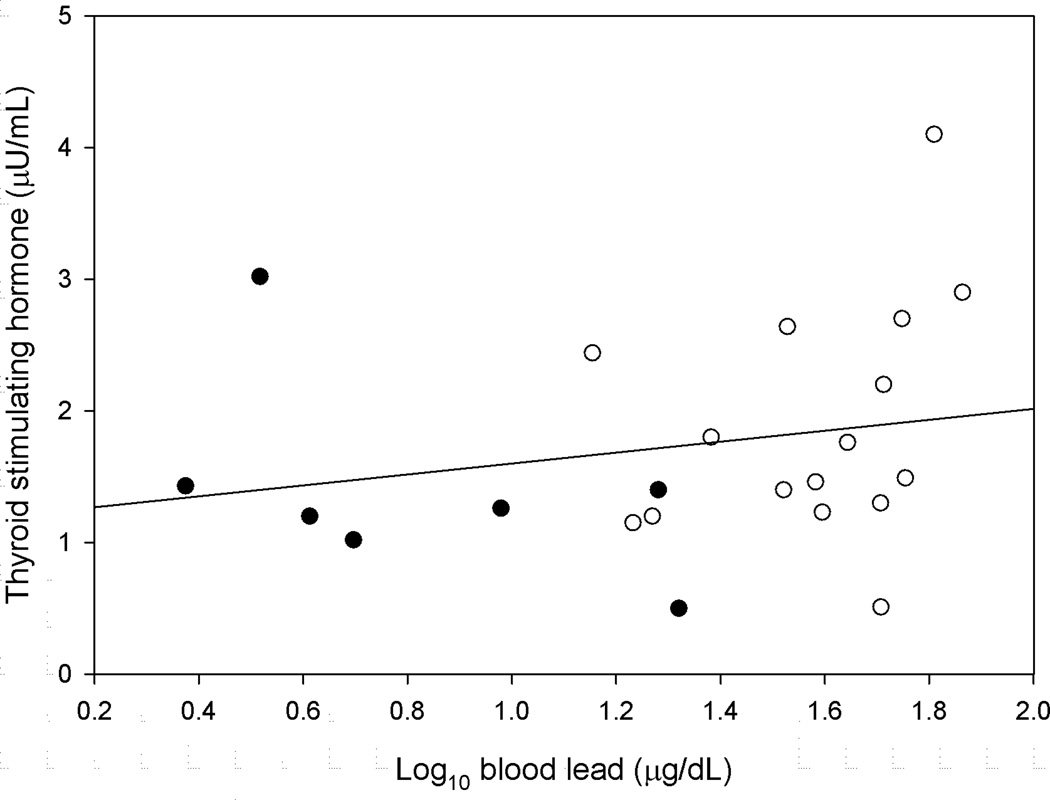
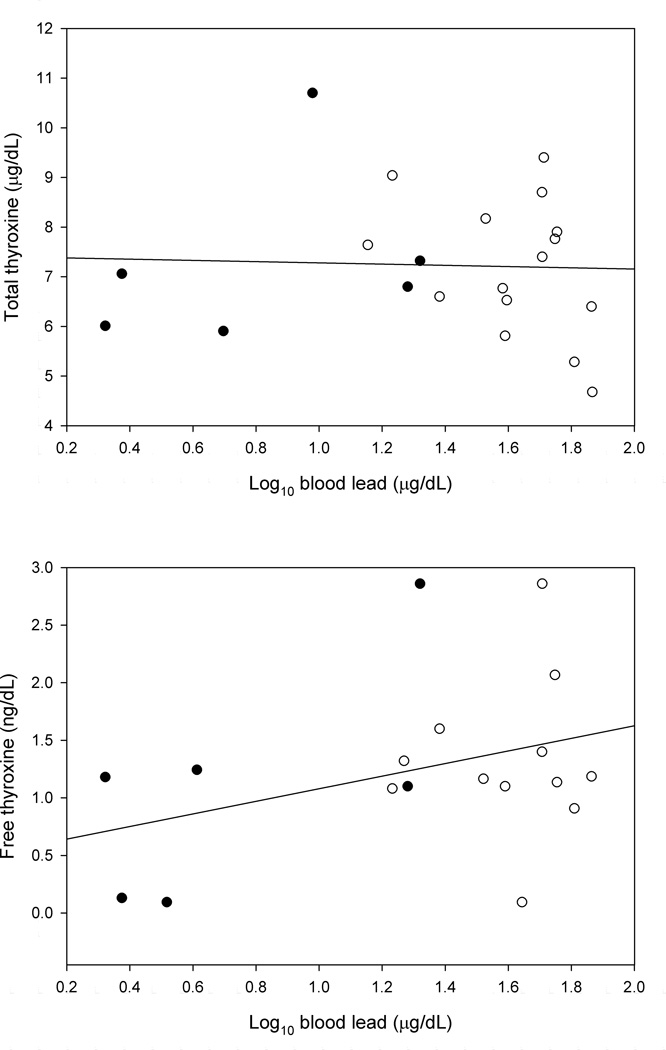
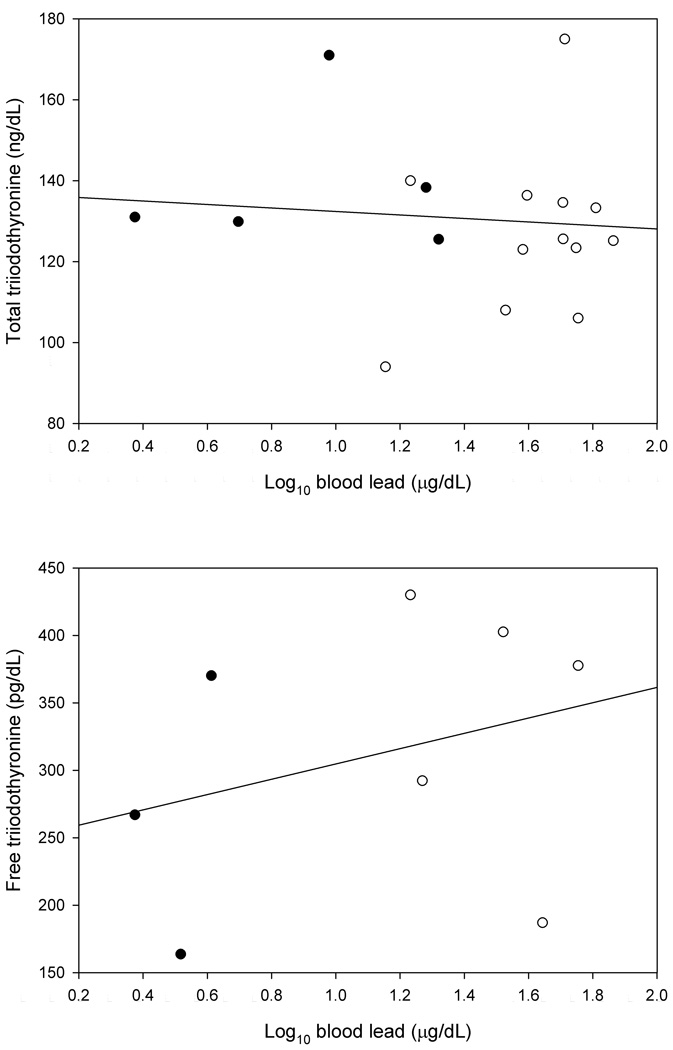
Table I shows the means of age, duration of exposure, blood lead, and the thyroid hormone measurements for the exposed and control groups. Table II shows the results of the mixed models comparing the means of the thyroid hormone measurements of the exposed and control groups. No statistically significant differences were found. Table III shows the results of the mixed models used to estimate the slopes between the blood lead concentration and the thyroid hormone measurements. No statistically significant relationships were found. Table IV shows the results of the models used to estimate the slopes between the duration of exposure to lead and the thyroid hormone measurements. No statistically significant relationships were found.
Table I.
Control Groups and Exposed Groups Summary Statistics
| Control groups |
Exposed groups |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | n | n | |||||||||
| Variable | Studies | Groups | Mean | STD | Min | Max | Studies | Groups | Mean | STD | Min | Max |
| Age (years) | 7 | 7 | 37.64 | 6.09 | 28.80 | 43.90 | 15 | 17 | 39.43 | 8.81 | 30.50 | 69.00 |
| Duration of exposure (years) | 0 | 0 | 14 | 16 | 9.79 | 5.67 | 4.20 | 24.00 | ||||
| Blood lead (µg/dL) | 8 | 8 | 8.30 | 7.60 | 2.10 | 20.90 | 16 | 18 | 43.27 | 18.06 | 14.28 | 73.53 |
| Thyroid stimulating hormone (µU/mL) | 7 | 7 | 1.40 | 0.78 | 0.50 | 3.02 | 14 | 16 | 1.89 | 0.89 | 0.51 | 4.10 |
| Total thyroxine (µg/dL) | 6 | 6 | 7.30 | 1.76 | 5.91 | 10.70 | 14 | 15 | 7.21 | 1.36 | 4.68 | 9.40 |
| Free thyroxine (ng/dL) | 6 | 6 | 1.10 | 1.01 | 0.09 | 2.86 | 11 | 12 | 1.33 | 0.67 | 0.09 | 2.86 |
| Total triiodothyronine (ng/dL) | 5 | 5 | 139.13 | 18.40 | 125.50 | 171.00 | 11 | 12 | 127.03 | 20.41 | 94.00 | 175.00 |
| Free triiodothyonine (pg/dL) | 3 | 3 | 266.92 | 103.25 | 163.64 | 370.13 | 4 | 5 | 337.88 | 98.87 | 187.01 | 430.00 |
STD: standard deviation
Min: minimum
Max: maximum
Table II.
Estimated Differences between the Control Groups and Exposed Groups Means
| n | n | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Studies | Groups | Difference | SE | DF | t | p | LCL | UCL |
| Thyroid stimulating hormone (µU/mL) | 14 | 23 | 0.05 | 0.21 | 8 | 0.24 | 0.8129 | −0.44 | 0.55 |
| Total thyroxine (µg/dL) | 14 | 21 | 0.31 | 0.46 | 6 | 0.68 | 0.5240 | −0.81 | 1.42 |
| Free thyroxine (ng/dL) | 11 | 18 | 0.19 | 0.14 | 6 | 1.33 | 0.2308 | −0.16 | 0.54 |
| Total triiodothyronine (ng/dL) | 11 | 17 | 2.16 | 3.40 | 5 | 0.63 | 0.5535 | −6.59 | 10.91 |
| Free triiodothyonine (pg/dL) | 4 | 8 | 57.14 | 54.67 | 3 | 1.05 | 0.3727 | −116.84 | 231.12 |
Difference = exposed groups mean – control groups mean
SE: standard error
DF: denominator degrees of freedom
LCL: 95% lower confidence limit
UCL: 95% upper confidence limit
Table III.
Estimated Slopes between the Thyroid Hormone Variables and Log10 Blood Lead (µg/dL)
| n | n | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Studies | Groups | Slope | SE | DF | t | p | LCL | UCL |
| Thyroid stimulating hormone (µU/mL) | 14 | 23 | 0.02 | 0.26 | 8 | 0.09 | 0.9307 | −0.57 | 0.62 |
| Total thyroxine (µg/dL) | 14 | 21 | 0.19 | 0.53 | 6 | 0.35 | 0.7353 | −1.10 | 1.48 |
| Free thyroxine (ng/dL) | 11 | 18 | 0.21 | 0.17 | 6 | 1.28 | 0.2487 | −0.19 | 0.62 |
| Total triiodothyronine (ng/dL) | 11 | 17 | 7.01 | 3.76 | 5 | 1.86 | 0.1215 | −2.66 | 16.67 |
| Free triiodothyonine (pg/dL) | 4 | 8 | 59.99 | 52.80 | 3 | 1.14 | 0.3384 | −108.05 | 228.04 |
SE: standard error
DF: denominator degrees of freedom
LCL: 95% lower confidence limit
UCL: 95% upper confidence limit
Table IV.
Estimated Slopes between the Thyroid Hormone Variables and Duration of Exposure to Lead (Years)
| n | n | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Studies | Groups | Slope | SE | DF | t | p | LCL | UCL |
| Thyroid stimulating hormone (µU/mL) | 13 | 15 | −0.02 | 0.07 | 12 | −0.31 | 0.7635 | −0.17 | 0.13 |
| Total thyroxine (µg/dL) | 13 | 14 | 0.13 | 0.08 | 11 | 1.52 | 0.1576 | −0.06 | 0.31 |
| Free thyroxine (ng/dL) | 10 | 11 | 0.02 | 0.06 | 8 | 0.29 | 0.7773 | −0.13 | 0.17 |
| Total triiodothyronine (ng/dL) | 11 | 12 | 2.43 | 1.48 | 9 | 1.64 | 0.1360 | −0.93 | 5.78 |
| Free triiodothyronine (pg/dL) | 3 | 4 | 7.75 | 6.04 | 1 | 1.28 | 0.4214 | −68.97 | 84.47 |
The slopes were adjusted for age.
SE: standard error
DF: denominator degrees of freedom
LCL: 95% lower confidence limit
UCL: 95% upper confidence limit
Figure 1 shows the relationship between the concentration of thyroid stimulating hormone and the blood lead concentration. Figure 2 shows the relationships between the concentrations of total and free thyroxine and the blood lead concentration. Figure 3 shows the relationships between the concentrations of total and free triiodothyronine and the blood lead concentration.
FIGURE 1.
The Thyroid Stimulating Hormone Concentration as a Function of the Log10 Blood Lead Concentration (Control Groups: Filled circle, Exposed Groups: Open Circle)
FIGURE 2.
The Total and Free Thyroxine Concentrations as a Function of the Log10 Blood Lead Concentration (Control Groups: Filled Circle, Exposed Groups: Open Circle)
FIGURE 3.
The Total and Free Triiodothyronine Concentrations as a Function of the Log10 Blood Lead Concentration (Control Groups: Filled Circle, Exposed Groups: Open Circle)
DISCUSSION
No differences in mean thyroid hormone concentrations were found between the lead exposed and control groups. No relationships were found between mean blood lead and mean thyroid hormone concentrations. No relationships were found between mean duration of exposure to lead and mean thyroid hormone concentrations. Only the exposed groups had duration of exposure data. No detected difference or relationship does not necessarily mean no effect of lead. It may mean that the number of studies was too small to detect an effect.
Cumulative blood lead concentrations were estimated in four studies [Bledsoe, et al. 2011, Erfurth, et al. 2001, Robins, et al. 1983, Schumacher, et al. 1998]. There was not enough data to do an analysis of this variable. Bledsoe, Pinkerton, Silver, Deddens, and Biagini [2011] found an inverse relationship between the free thyroxine and cumulative blood lead concentrations. None of the other studies found a relationship between the cumulative blood lead concentration and a thyroid hormone concentration.
Case Studies
There are case studies of lead poisoning occurring with thyroid dysfunction. Thyroid dysfunction may affect the blood lead concentration by affecting bone turnover. In the case of hyperthyroidism, lead is probably being released from bone into blood. Thyrotoxicosis results in an imbalance between bone resorption and bone formation and results in net bone loss [Duncan Bassett and Williams 2003], while hypothyroid patients can have low bone turnover and increased cortical thickness [Mosekilde and Melsen 1978]. Lower bone mineral density [Nash, et al. 2004] and higher bone turnover [Machida, et al. 2009] have been associated with higher blood lead concentrations. Descriptions of the case studies follow.
Kremer and Frank [1955] reported the case of a patient with coexisting myxedema and lead poisoning occurring 36 years following exposure to lead. The patient’s blood lead measurements were 0.1 mg % (100 µg/dL) and 0.11 mg % (110 µg/dL) on separate days prior to being treated for lead poisoning.
Cagin, Diloy-Puray, and Westerman [1978] reported the case of a patient with a retained bullet who developed lead poisoning in association with thyrotoxicosis. The patient’s blood lead concentration was 107 µg/dL.
Tiwari, Timms, and Rothe [1985] reported the case of a euthyroid woman with lead poisoning and hyperthyroxinaemia. Her thyroxine and free thyroxine levels were abnormally high and decreased as her blood lead concentration decreased. Her thyroid stimulating hormone level and thyroid stimulating hormone response to thyrotropin releasing hormone were always normal, as were her concentrations of thyroxine binding globulin and tiiodothyronine. The woman’s blood lead level was 64 µg/L.
Goldman, White, Kales, and Hu [1994] reported the case of a woman with thyrotoxicosis and an elevated blood lead concentration (53 µg/dL) with no current source of lead exposure. She had an elevated bone lead level and increased bone turnover. Her lead poisoning was attributed to the release of lead from bone due to hyperthyroidism.
Klein, Barbé, Pascal, Weryha, and Leclère [1998] reported two cases of hyperthyroidism, an 82 year old woman with a toxic multinodular thyroid enlargement and a 46 year old man with Graves’s disease, both with lead poisoning. Treatment with radioactive iodine cured the hypothyroidism and lead poisoning in both patients. They attributed the lead poisoning to increased bone turnover and the release of lead from bone due to the hyperthyroidism.
Clinical Studies
Three clinical studies have led to hypotheses about where and how lead affects thyroid function. Two of these studies were used in the meta-analysis.
Sandstead, Stant, Bertram Brill, Arias, and Terry [1969] examined 24 patients with lead poisoning, 3 with industrial saturnism and 21 with lead intoxication due to the ingestion of illicitly distilled whiskey. They concluded that there was an injury to the iodine trapping and concentrating mechanism of the thyroid in these patients. The baseline urine lead concentrations of the patients ranged from 10 to 480 µg/L.
Robins, Cullen, Connors, and Kayne [1983] examined 12 patients for lead intoxication at their occupational medicine clinic. Seven of those examined had low serum values of estimated free thyroxine (< 1.0 ng/dL) and six had low serum values of thyroxine (< 4.6 µg/dL). None of the patients had characteristic physical findings of myxedema. The blood lead levels of the patients ranged from 28 to 117 µg/dL. The seven patients with low estimated free thyroxine values were tested one to eight months later after being removed from the source of lead exposure or receiving chelation therapy. The blood lead levels of these patients decreased 16 to 77 µg/dL. Their estimated free thyroxine levels increased 0.0 to 0.3 ng/dL and remained below normal.
Cullen, Kayne, and Robins [1984] evaluated seven men with symptomatic lead intoxication. The blood lead concentrations of the men ranged from 66 to 139 µg/dL. Two of the men had subnormal serum thyroxine and estimated free thyroxine concentrations and one of the men had an elevated concentration of thyroid binding globulin and no thyroid stimulating hormone response to stimulation by thyroid releasing hormone. The authors concluded that the men had defects in thyroid function originating in the pituitary and hypothalamus.
Environmental and Population Studies
The results of environmental and population studies provide evidence for a decrease in the concentrations of serum thyroid stimulating hormone and serum total thyroxine as the blood lead concentration increases. The results are not consistent. The average blood lead concentration in the studies discussed in this section ranged from 1.3 to 3.1 µg/dL.
Abdelouahab et al. [2008] studied a group of 124 men and 87 women who lived in lakeside communities and ate freshwater fish. They found a statistically significant inverse relationship between the serum concentration of thyroid stimulating hormone and the whole blood lead concentration in women but not in men. Meeker et al. [2009] found that the serum concentration of thyroid stimulating hormone decreased as the whole blood lead concentration increased in 219 men participating in a study of environmental influences on male reproductive health.
Studies using the population data from the National Health and Nutrition Examination Survey have investigated the relationships between the serum concentrations of thyroid stimulating hormone, total thyroxine, free thyroxine, total triiodothyronine, and free triiodothyronine and the blood lead concentration. The studies used data from the same (2007–2008) or overlapping (2007–2010) years.
Yorita Christensen [2013] found a statistically significant inverse relationship between the serum concentration of total thyroxine and the blood lead concentration in adults. Mendy, Gasana, and Vieira [2013] found a statistically significant inverse relationship between the serum concentration of total thyroxine and the blood lead concentration in women, but not in men. Luo and Hendryx [2014] found that the concentration of total thyroxine decreased as the blood lead concentration increased in men, but not in women. They also found that the concentration of free triiodothyronine increased as the blood lead concentration increased in men, but not in women. Chen, Kim, Chung, and Dietrich [2013] found no statistically significant relationships.
With regard to total thyroxine, Mendy, Gasana, and Vieira [2013] hypothesized that lead may reduce the number of proteins that bind to thyroxine and enhance its clearance from the blood. Luo and Hendryx [2014] hypothesized that a decrease in total thyroxine may be due to lead enhancing the activity of an enzyme, type I deiodinase, which converts thyroxine to triiodothyronine.
CONCLUSION
In the present study, no differences in mean thyroid hormone concentrations were found between the lead exposed and control groups. No relationships were found between mean blood lead or duration of exposure to lead and mean thyroid hormone concentrations. The results of this meta-analysis do not provide evidence for an effect of occupational lead exposure on thyroid function in men. Few women were included in the studies, so that a conclusion about them is not warranted.
Supplementary Material
Footnotes
Conflict of interest statement:
Edward F. Krieg, Jr. does not have any competing interests or conflicts of interest.
Author contributions statement:
Edward F. Krieg, Jr. collected and analyzed the data and wrote the manuscript.
DISCLAIMERS
The findings and conclusions in this report are those of the author and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Mention of company names or products does not constitute endorsement by the National Institute for Occupational Safety and Health.
REFERENCES
- Abdelouahab N, Mergler D, Takser L, Vanier C, St-Jean M, Baldwin M, Spear PA, Chan HM. Gender differences in the effects of organochlorines, mercury, and lead on thyroid hormone levels in lakeside communities of Quebec (Canada) Environ Res. 2008;107:380–392. doi: 10.1016/j.envres.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Andrzejak R, Antonowicz J, Bolanowska B, Hebdziński L, Kabacińska-Knapik D, Smolik R. Thyroid function in smelters after long-term exposure to heavy metals. Med Pr. 1996;47:253–258. [PubMed] [Google Scholar]
- Bartalena L, Robbins J. Thyroid hormone transport proteins. Clin Lab Med. 1993;13:583–598. [PubMed] [Google Scholar]
- Bielecka W, Frydrych J, Wojtas A. Thyroxine (T4) levels in workers exposed to lead. Med Pr. 1987;38:40–44. [PubMed] [Google Scholar]
- Bledsoe ML, Pinkerton LE, Silver S, Deddens JA, Biagini RE. Thyroxine and free thyroxine levels in workers occupationally exposed to inorganic lead. Environ Health Insights. 2011;5:55–61. doi: 10.4137/EHI.S7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagin CR, Diloy-Puray M, Westerman MP. Bullets, lead poisoning, and thyrotoxicosis. Ann Intern Med. 1978;89:509–511. doi: 10.7326/0003-4819-89-4-509. [DOI] [PubMed] [Google Scholar]
- Chen A, Kim SS, Chung E, Dietrich KN. Thyroid hormones in relation to lead, mercury, and cadmium exposure in the National Health and Nutrition Examination Survey, 2007–2008. Environ Health Perspect. 2013;121:181–186. doi: 10.1289/ehp.1205239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen K, Felig P. Occupational and other environmental diseases of the endocrine system. Arch Intern Med. 1984;144:469–471. [PubMed] [Google Scholar]
- Cullen MR, Kayne RD, Robins JM. Endocrine and reproductive dysfunction in men associated with occupational inorganic lead intoxication. Arch Environ Health. 1984;39:431–440. doi: 10.1080/00039896.1984.10545877. [DOI] [PubMed] [Google Scholar]
- Doumouchtsis KK, Doumouchtsis SK, Doumouchtsis EK, Perrea DN. The effect of lead intoxication on endocrine functions. J Endocrinol Invest. 2009;32:175–183. doi: 10.1007/BF03345710. [DOI] [PubMed] [Google Scholar]
- Duncan Bassett JH, Williams GR. The molecular actions of thyroid hormone in bone. Trends Endocrinol Metab. 2003;14:356–364. doi: 10.1016/s1043-2760(03)00144-9. [DOI] [PubMed] [Google Scholar]
- Dursun N, Tutus A. Chronic occupational lead exposure and thyroid function. The Journal of Trace Elements in Experimental Medicine. 1999;12:45–49. [Google Scholar]
- Erfurth EM, Gerhardsson L, Nilsson A, Rylander L, Schütz A, Skerfving S, Börjesson J. Effects of lead on the endocrine system in lead smelter workers. Arch Environ Health. 2001;56:449–455. doi: 10.1080/00039890109604481. [DOI] [PubMed] [Google Scholar]
- Gennart J-P, Bernard A, Lauwerys R. Assessment of thyroid, testes, kidney and autonomic nervous system function in lead-exposed workers. Int Arch Occup Environ Health. 1992;64:49–57. doi: 10.1007/BF00625951. [DOI] [PubMed] [Google Scholar]
- Goldman RH, White R, Kales SN, Hu H. Lead poisoning from mobilization of bone stores during thyrotoxicosis. Am J Ind Med. 1994;25:417–424. doi: 10.1002/ajim.4700250309. [DOI] [PubMed] [Google Scholar]
- Gustafson Å, Hedner P, Schütz A, Skerfving S. Occupational lead exposure and pituitary function. Int Arch Occup Environ Health. 1989;61:277–281. doi: 10.1007/BF00381426. [DOI] [PubMed] [Google Scholar]
- Horiguchi S, Endo G, Kiyota I. Measurement of total triiodothyronine (T3), total thyroxine (T4) and thyroid-stimulating hormone (TSH) levels in lead-exposed workers. Osaka City Med J. 1987;33:51–56. [PubMed] [Google Scholar]
- Klein M, Barbé F, Pascal V, Weryha G, Leclère J. Lead poisoning secondary to hyperthyroidism: report of two cases. Eur J Endocrinol. 1998;138:185–188. doi: 10.1530/eje.0.1380185. [DOI] [PubMed] [Google Scholar]
- Kremer HU, Frank MN. Coexisting myxedema and chronic plumbism. Ann Intern Med. 1955;42:1130–1136. doi: 10.7326/0003-4819-42-5-1130. [DOI] [PubMed] [Google Scholar]
- Łasisz B, Zdrojewicz Z, Marcinkowski Z. Effect of lead on thyroid function. Wiad Lek. 1992;45:116–119. [PubMed] [Google Scholar]
- Liang Q, Liao R, Su S, Huang S, Pan R, Huang J. Effects of lead on thyroid function of occupationally exposed workers. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2003;21:111–113. [PubMed] [Google Scholar]
- López CM, Piñeiro AE, Núñez N, Avagnina AM, Villaamil EC, Roses OE. Thyroid hormone changes in males exposed to lead in the Buenos Aires area (Argentina) Pharmacol Res. 2000;42:599–602. doi: 10.1006/phrs.2000.0734. [DOI] [PubMed] [Google Scholar]
- Luo J, Hendryx M. Relationship between blood cadmium, lead, and serum thyroid measures in US adults - the National Health and Nutrition Examination Survey (NHANES) 2007–2010. Int J Environ Health Res. 2014;24:125–136. doi: 10.1080/09603123.2013.800962. [DOI] [PubMed] [Google Scholar]
- Machida M, Sun S-J, Oguma E, Kayama F. High bone matrix turnover predicts blood levels of lead among perimenopausal women. Environ Res. 2009;109:880–886. doi: 10.1016/j.envres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, Paneth N, Wirth JJ. Multiple metals predict prolactin and thyrotropin (TSH) levels in men. Environ Res. 2009;109:869–873. doi: 10.1016/j.envres.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy A, Gasana J, Vieira ER. Low blood lead concentrations and thyroid function of American adults. Int J Environ Health Res. 2013;23:461–473. doi: 10.1080/09603123.2012.755155. [DOI] [PubMed] [Google Scholar]
- Mosekilde L, Melsen F. Morphometric and dynamic studies of bone changes in hypothyroidism. Acta Pathol Microbiol Scand A. 1978;86:56–62. doi: 10.1111/j.1699-0463.1978.tb02012.x. [DOI] [PubMed] [Google Scholar]
- Nash D, Magder LS, Sherwin R, Rubin RJ, Silbergeld EK. Bone density-related predictors of blood lead level among peri- and postmenopausal women in the United States: The third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2004;160:901–911. doi: 10.1093/aje/kwh296. [DOI] [PubMed] [Google Scholar]
- Pearce EN, Braverman LE. Environmental pollutants and the thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23:801–813. doi: 10.1016/j.beem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Refowitz RM. Thyroid function and lead: no clear relationship. J Occup Med. 1984;26:579–583. doi: 10.1097/00043764-198408000-00012. [DOI] [PubMed] [Google Scholar]
- Robins JM, Cullen MR, Connors BB, Kayne RD. Depressed thyroid indexes associated with occupational exposure to inorganic lead. Arch Intern Med. 1983;143:220–224. [PubMed] [Google Scholar]
- Sandstead HH, Stant EG, Bertram Brill A, Arias LI, Terry RT. Lead intoxication and the thyroid. Arch Intern Med. 1969;123:632–635. [PubMed] [Google Scholar]
- Schumacher C, Brodkin CA, Alexander B, Cullen M, Rainey PM, van Netten C, Faustman E, Checkoway H. Thyroid function in lead smelter workers: absence of subacute or cumulative effects with moderate lead burdens. Int Arch Occup Environ Health. 1998;71:453–458. doi: 10.1007/s004200050305. [DOI] [PubMed] [Google Scholar]
- Schussler GC. The thyroxine-binding proteins. Thyroid. 2000;10:141–149. doi: 10.1089/thy.2000.10.141. [DOI] [PubMed] [Google Scholar]
- Singh B, Chandran V, Bandhu HK, Mittal BR, Bhattacharya A, Jindal SK, Varma S. Impact of lead exposure on pituitary-thyroid axis in humans. Biometals. 2000;13:187–192. doi: 10.1023/a:1009201426184. [DOI] [PubMed] [Google Scholar]
- Tiwari I, Timms P, Rothe P. Lead poisoning and euthyroid hyperthyroxinaemia. Lancet. 1985;1:1508–1509. doi: 10.1016/s0140-6736(85)92282-2. [DOI] [PubMed] [Google Scholar]
- Tuppurainen M, Wägar G, Kurppa K, Sakari W, Wambugu A, Fröseth B, Alho J, Nykyri E. Thyroid function as assessed by routine laboratory tests of workers with long-term lead exposure. Scand J Work Environ Health. 1988;14:175–180. doi: 10.5271/sjweh.1934. [DOI] [PubMed] [Google Scholar]
- Yılmaz H, Keten A, Karacaoğlu E, Tutkun E, Akçan R. Analysis of the hematological and biochemical parameters related to lead intoxication. J Forensic Leg Med. 2012;19:452–454. doi: 10.1016/j.jflm.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Yorita Christensen KL. Metals in blood and urine, and thyroid function among adults in the United States 2007–2008. Int J Hyg Environ Health. 2013;216:624–632. doi: 10.1016/j.ijheh.2012.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.